Abstract
Synthetic biology needs to adopt sound scientific and industry‐like standards in order to achieve its ambitious goals of efficient and accurate engineering of biological systems.
Subject Categories: Synthetic Biology & Biotechnology, Methods & Resources, S&S: Ethics
Standards are the basis of technology: they allow rigorous description and exact measurement of properties, reliable reproducibility and a common “language” that enables different communities to work together. Molecular biology was in part created by physicists; yet, the field did not inherit the focus on the quantitation, the definition of system boundaries and the robust, unequivocal language that is characteristic of the other natural sciences. However, synthetic biology (SynBio) increasingly requires scientific, technical, operational and semantic standards for the field to become a full‐fledged engineering discipline with a high level of accuracy in the design, manufacturing and performance of biological artefacts. Although the benefits of adopting standards are clear, the community is still largely reluctant to accept them, owing to concerns about adoption costs and losses in flexibility.
… Synthetic Biology (SynBio) increasingly requires scientific, technical, operational and semantic standards for the field to become a full‐fledged engineering discipline …
What standards are good for
In science and technology, the terms standard and standardisation describe different things: shared semantic and graphical languages for annotating the nature and the properties of systems and their components; the definition of units of relevant properties and parameters along with methods to calculate them; specifications of properties and arrangements for the physical assembly of the components of a system; and unambiguous protocols for the construction of objects. Such standards enable an abstract and precise description of a system with a suitable—also standardised—quantitative language or equivalent methods of representation.
Beyond their important role in the natural sciences, standards were also one of the key drivers for the industrial revolution as they enabled a seamless integration of product design, fabrication of its components and the final assembly—let alone tracing parts and helping to sort out matters of safety and intellectual property. Standards are for instance imperative for designing electronic circuits built from well‐defined, universal simple components, such as resistors, diodes and transistors, or for software engineering that uses precompiled modules and functions. Standards enabled the rapid rise of the personal computer industry in the 1980s and 1990s by interlinking standard components such as hard discs, memory or keyboards through standardised interfaces and protocols.
… standards were also one of the key drivers for the industrial revolution as they enabled a seamless integration of product design, fabrication of its components and the final assembly…
From software to nuts and bolts, the concept of a universally usable toolbox of parts to assemble more complex systems is typical for every discipline of engineering: electronics, software, mechanical design, architecture, chemical synthesis and so on. Standards enable people to work together through interoperability, coordination of labour, reproducibility and reuse of other people's efforts and achievements.
Standards must be reliable, robust and affordable, but, first and foremost, they must be agreed on by their users. Indeed, standardisation—the process of implementing and developing technical standards—requires the consensus of many different parties, such as private and public companies, organisations and policy makers. Standardisation can be driven by public acceptance/market forces (de facto standards), directly ordained by law (de jure standards) or, most commonly, arise from the combination of legal/technical requirements and recognition by potential operators since, in general, the broader the applicability of a format, the greater its market 1.
Standards in the life sciences
That said, the core standardisation process in many scientific and engineering disciplines took place decades to centuries ago, but it is still in its infancy in the life sciences. Interestingly, it is still a bottleneck for even well‐developed technologies: smartphones, for instance, still lack standard key components such as batteries or electric charger cables (see e.g. https://ec.europa.eu/info/law/better-regulation/initiatives/ares-2018-6427186_en).
In this context, the conceptual frame of synthetic biology aims to making biology easier to engineer by applying principles such as modularity, orthogonality, chain production and reproducibility. Moreover, the rapid advances in wet and computational tools for genome editing, metabolic design and in silico modelling are opening new opportunities for genetic programming that could not have been anticipated even just a few years ago, and allow engineers to tackle increasingly complex engineering objectives. The growing demand for scaling up such technologies raises the issue of what is needed to make them work at an industrial scale 2. Following the path of other branches of engineering, the establishment of standards appears among the key objectives of contemporary SynBio—and eventually of the life sciences as a whole—as a prerequisite for applications such as bioremediation, biomedicine, bioenergy, novel chemicals, innovative materials and cellular factories.
Although standards in SynBio have contributed to successes such as the synthesis of artemisinin or morphine (both in yeast), the problem of defining common standards is still far from being resolved. The reusability patterns of the iGEM parts database 3, the context dependence of biological components 4, the variable behaviour among strains, genetic stability or even the contested philosophical analogy between cells and machines are by no means solved issues at this point. However, there is no doubt that even partial progress on standardisation would have major consequences for bioengineering.
One bottleneck is the widespread and incorrect assumption among many researchers in the life sciences that standards may increase interoperability but necessarily limit flexibility—which is obviously important for any creative research. Rather, good standards will increase people's flexibility and creativity because it will make it easier for them to achieve their scientific objectives. A separate challenge is identifying specific systems and operations that need to be standardised, and then navigating the minefield of personal interests that typically inhibit agreement on a given format or language. As Murray Gell‐Mann quipped, “a scientist would rather use someone else's toothbrush than someone else's nomenclature”. Scientists and engineers will adopt standards only when they add value to their efforts to overcome the often steep costs of adoption.
Standards for engineering biology
While a number of SynBio standards have already been developed and await adoption by the broader community of users 5, others touch on core biological questions that are by no means solved from a scientific point of view. There is a legitimate concern that we still need to know more fundamental facts before we can describe engineered biosystems with a formal, unequivocal language. One typical case involves the design of genetic circuits, an archetypal product of SynBio endeavours. Habitual practices include directly transplanting toolkit for building electronic logic gates and related information‐processing devices into the biological domain. However, one must be honest about how far these abstractions and their accompanying theoretical framework reflect biological reality. Boolean logic relies on values that are either true or false. In electronics, this is readily implemented using voltage levels that are separated by a larger amount than the expected noise to faithfully represent the state of the gate. In contrast, biological implementations of circuits tend to have a much higher noise‐to‐signal ratio, which makes it difficult to effectively distinguish true and false states and strongly limits the design of logic circuits. One way to alleviate this problem is by redesigning regulatory components to behave more digitally, but ultimately, we may need to revisit information processing in/by biological systems with other formalisms, either existing or yet to be developed, that go beyond Boolean logic 6.
Scientists and engineers will adopt standards only when they add value to their efforts to overcome the often steep costs of adoption.
The same theory/implementation conundrum might be true for biological metrology, one of the main tenets of SynBio. Electronic circuits crucially rely on a clear definition of potential and current, their description in volts and amperes, and methods to measure these. By the same token, it is difficult to think about genetic circuits without robust measures of signal transmission through the regulation of gene expression or other core cellular processes. The concepts of RNA polymerase per second (PoPS; 7) and ribosome per second (RiPS) as biological counterparts of current were conceptualised early in the history of SynBio. Alas, very little has been done to further develop these units as practicable indicators of genetic circuit performance, perhaps due to the difficulties of measuring them accurately.
These examples showcase how developing standards for biological engineering still requires addressing a number of core scientific and technological gaps that have been left behind in the ongoing frenzy of application‐focused development. Yet, such unsolved issues may strike back when the field continues to move from largely academic endeavours towards industrial realisation.
…developing standards for biological engineering still requires addressing a number of core scientific and technological gaps that have been left behind in the ongoing frenzy of application‐focused development.
Key actors in the standards conversation
International discussions about SynBio standards, mostly with US and EU stakeholders, have been going on since before 2010. Under the umbrella of the BIOROBOOST Project (http://standardsinsynbio.eu), the conversation now incorporates key actors of SynBio from Europe, North America and Asia. Much of the discussions deal with identifying key challenges for the development, promulgation and adoption of standards, and identifying stakeholders in academia, industry, research centres and politics.
The most conspicuous technical challenges include standardising simple biological parts, devices and circuits, chassis, metrology, descriptive languages (including graphical representations) and software tools. But the complexity of the endeavour also asks for the creation of a network of SynBio practitioners that share and evolve these standards together. While this is reminiscent of earlier Computational Modeling in Biology Network (COMBINE, http://co.mbine.org/), the focus of these SynBio networks needs to go beyond academic interests to include industry and commerce, and to develop strategies for educating a new generation of synthetic biologists who routinely use standards.
From the regulatory, technical and societal point of view, the challenge is complex. For example, there are practical questions such as the level of detail required in a given biological standard, which can go from light to very deep. As indicated above, standard is an umbrella concept, which includes a number of different approaches to harmonisation. These range from agreeing on metrology units and best practices to measure them, to developing standardised functional chassis—specific, formatted biological hosts for specific applications—to data formats, to safety criteria for approval by regulatory agencies and to ISO‐approved reports and technical specifications.
It is necessary to distinguish between biological standards that could be similar to physics and engineering counterparts, such as the PoPS or RiPS units discussed above, and standard operating procedures (SOPs), which help users to carry out routine operations with efficiency, consistent quality and performance, and are compliant with regulations. For instance, the composition and preparation of the M9 medium would be an SOP, while the metrics for calculating containment of a given SynBio agent when released in the environment could become a biological standard. There are, of course, many grey zones between these two—for instance, formats for enabling communication between unrelated software, cloning methods, CRISPR‐based editing and so on—that will hopefully be solved through conversations between stakeholders in the various forums just mentioned. The question remains, however, whether the wider community of potential users will see the value of adopting standards in their daily practice. Today, SynBio and systems biology practitioners are widely using the Synthetic Biology Open Language SBOL 8 and SBOL visual for describing vectors and constructs 9, and there is a great consensus on the need to go beyond the state of the art and further advance towards the standardisation of biological systems 5, 10.
Stages of adoption
Is there a take‐home lesson from the history of technology adoption that we can learn from for popularising biological standards? In fact, the trajectory of acceptance in the realm of engineering typically involves several stages: from an innovator phase to adoption by even the most recalcitrant laggards (Fig 1). Using this frame, it seems that most of the SynBio's standards developments are still in the innovator phase.
Figure 1. Adoption curve of/for biological standards.
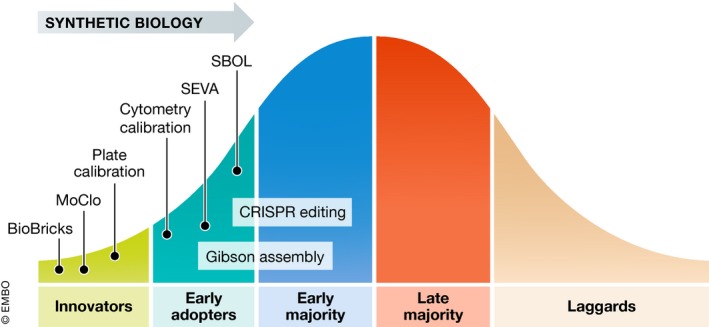
Illustrative examples of the position of SynBio standards along the technology adoption curve: SynBio standards are largely still in the innovator phase but with a few examples having progressed to the early adopters or early majority segments. SBOL, Synthetic Biology Open Language; SEVA, Standard European Vector Architecture; MoClo, modular cloning.
Many developments, even if critical for the early years in SynBio, never left the innovator state and are now outdated; advances in cloning and DNA synthesis have for instance replaced BioBricks. Others, such as SBOL 8 or the Standard European Vector Architecture (SEVA; 9) are increasingly successful as interim formats in the early adopter stage. Yet, these may or may not become generally adopted depending on success stories and potential alternative scientific and technical solutions. Such progress will be determined by the combination of a bottom‐up demand for interoperability and collaboration and a top‐down implementation and enforcement by official agencies. Journal editors also have a role to play as well as reviewers of journal articles and grant proposals in insisting on the use of standards to improve reproducibility and reuse. Generally, it is important to realise that standards are ultimately social constructs to represent norms, objects or procedures, and that they become accepted by a group of individuals for practical reasons.
… standards are ultimately social constructs to represent norms, objects or procedures, and that they become accepted by a group of individuals for practical reasons.
Low‐hanging fruits
Despite the difficulties, it should be possible to come up with science‐based standardisation proposals in SynBio that work across the biological, the digital and the social realms. The already existing ones at hand involve simple biological parts: devices such as promoters and other regulatory nodes and simple circuits—for instance, inverters, basic gates—such as those deposited in the repository of biological parts and other curated collections. The next stage involves definition and adoption of SynBio chassis other than laboratory bacteria or yeast strains. Not every species or strain that can host recombinant DNA can be considered a chassis, and this effort requires establishment of a map of requirements and functional relationships between industrially relevant practical applications and different biological platforms. Finally, standardisation would need to address the issue of metrology through the gene expression flow including fundamental units and the technologies and references to measure them, as well as computational language and software tools for easing collaborations between different actors. The main efforts to collect such low‐hanging fruits would be greatly facilitated by biofoundries with good connections to policy makers with the objective of making the whole endeavour more appealing for the industrial sector.
.. the key to success is the merger of technical consistency and scientific soundness with legal requirements and consensus among end users.
The academic community cannot be a mere observer of these developments. In fact, there is much to do for endowing biological standards with a solid scientific basis, including the definition of each level of biological complexity amenable to standardisation. But the role in promoting standards is not only technical. There is ample room for networks of practitioners involving industrial players, who can provide information on how biological properties and processes could improve product development, manufacturability and consumer confidence. This could create a framework for identifying and monitoring standardisation requirements and maintaining an evolving list of scientific and industrial priorities. Ideally, such priority lists should also be considered by funding bodies to help in developing and driving adoption of standards. Relevant regulatory bodies should be involved to adapt or ease rules on the management of GMOs and/or SynBio agents. The same academic–industrial networks could also strengthen ongoing public outreach and citizen involvement to help overcoming the negative perception of genetic engineering in general.
In sum, we argue that the promise of SynBio for the benefit of global society and industry will only be met if significant advances are achieved on the standardisation front. To this end, it is not only essential to overcome national/political barriers and particular interests of given research groups, but also to gather key players in a permanent forum with the aim of making biological standards one of the ingredients of the 4th Industrial Revolution. Standards in biology will be used provided that they have intrinsic properties such as robustness, ease of use and context independence. But the key to success is the merger of technical consistency and scientific soundness with legal requirements and consensus among end users. This goes beyond the realm of research and tackles sociological and cultural issues that have been traditionally alien to the conversation. If this can be achieved, the benefits for SynBio and for society at large will be great.
Further reading
Standardisation initiatives for Biology and Biotechnology
Xie Z, Hall J, McCarthy IP, Skitmore M, Shen L. Standardization efforts: The relationship between knowledge dimensions, search processes and innovation outcomes. Technovation 2016, 48–49: 69–78
Beal J. Bridging the Gap: A Roadmap to Breaking the Biological Design Barrier. Front Biotechnol 2014, 2, 87
Knight T. Idempotent vector design for standard assembly. MIT artificial intelligent laboratory communications. https://doi.org/hdl.handle.net/1721.1/21168
Schreiber F, Sommer B, Bader GD, Gleeson P, Golebiewski M, Hucka M, Keating SM, König M, Myers C, Nickerson D, Waltemath D. Specifications of Standards in Systems and Synthetic Biology: Status and Developments in 2019. J Integr Bioinform 2019, 16, 20190035
Myers CJ, Beal J, Gorochowski TE, Kuwahara H, Madsen C, McLaughlin JA, et al (2017). A standard‐enabled workflow for synthetic biology. Biochem Soc Trans 45, 793–803
Roehner N, Beal J, Clancy K, Bartley B, Misirli G, Grunberg R, Oberortner E, Pocock M, Bissell M, Madsen C, Nguyen T, Zhang M, Zhang Z, Zundel Z, Densmore D, Gennari J, Wipat A, Sauro H, Myers C. Sharing structure and function in biological design with SBOL 2.0”, ACS Syn Biol 2016, 5(6): 498–506
Beal J, Nguyen T, Gorochowski TE, Goñi‐Moreno A, Scott‐Brown J, McLaughlin JA, Madsen C, Aleritsch B, Bartley B, Bhakta S, Bissell M, Castillo HS, Clancy K, Luna A, Le Novère N, Palchick Z, Pocock M, Sauro H, Sexton JT, Tabor JJ, Voigt CA, Zundel Z, Myers C, Wipat A. Communicating structure and function in synthetic biology diagrams. ACS Synth Biol 2019, 8, 8, 1818–1825
Madsen C, Goni‐Moreno A, Palchick ZPU, Roehner N, Bartley B, Bhatia S et al Synthetic Biology Open Language Visual (SBOL Visual) Version 2.1. J Integr Bioinform 2019, 16, 20180101
Quinn JY, Cox RS III, Adler A, Beal J, Bhatia S, Cai Y, et al (2015) SBOL Visual: A Graphical Language for Genetic Designs. PLoS Biol 13(12):e1002310
Walsh DI 3rd, Pavan M, Ortiz L, Wick S, Bobrow J, Guido NJ, Leinicke S, Fu D, Pandit S, Qin L, Carr PA, Densmore D. Standardizing automated DNA Assembly: best practices, metrics, and protocols using robots. SLAS Technol 2019, 24, 282–290
Synthetic Biology success stories
Ro D, Paradise E, Ouellet M et al Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006, 440, 940–943
DeLoache W, Russ Z, Narcross L et al An enzyme‐coupled biosensor enables (S)‐reticuline production in yeast from glucose. Nat Chem Biol 2015, 11, 465–471
Fernández‐Rodríguez J, Yang L, Gorochowski TT, Gordon DB and Voigt CA. Memory and combinational logic based on DNA inversions: dynamics and evolutionary stability. ACS Synth Biol 2015, 4, 12, 1361–1372
Weinberg BH, Cho JH, Agarwal Y, Pham NTH, Caraballo LD, Walkosz M, Ortega C, Trexler M, Tague N, Law B, Benman WKJ, Letendre J, Beal J, Wong WW. High‐performance chemical –and light‐inducible recombinases in mammalian cells and mice. Nat Commun 2019, 10, 4845
Open questions in biological standards
Vilanova C, Tanner K, Dorado‐Morales P, Villaescusa P, Chugani D, Frías A, Segredo E, Molero X, Fritschi M, Morales L, Ramón D, Peña C, Peretó J, Porcar M. Standards not that standard. J Biol Eng 2015, 9, 17
Nicholson DJ. Is the cell really a machine? J Theor Biol 2019, 477, 108–126
Beal J. Signal‐to‐Noise Ratio Measures Efficacy of Biological Computing Devices and Circuits. Front Bioeng Biotechnol. 2015, 3, 93
Hallinan JJ, Wipat A, Kitney R, Woods S, Taylor K and Goñi‐Moreno, A. Future‐proofing synthetic biology: educating the next generation. Eng Biology 2019, 3, 25–31
Schmidt M. A metric space for semantic containment: Towards the implementation of genetic firewalls. Biosystems, 2019, 185, 104015
Kitney R, Adeogun M, Fujishima Y, Goñi‐Moreno A, Johnson R, Maxon M, Steedman S, Ward S, Winickoff D, Philp J. Enabling the advanced bioeconomy through public policy supporting biofoundries and engineering biology. Trends in Biotechnol 2019, 37, 917–920
Acknowledgements
Mireia Alonso is gratefully acknowledged for help in formatting the different versions of this manuscript. This work was funded by the European Union through the BioRoboost Project, H2020‐NMBP‐TR‐IND‐2018‐2020/BIOTEC‐01‐2018 (CSA), Project ID 820699. Jake Beal was also supported in part by NSF Expeditions in Computing Program Award #1522074. This document does not contain technology or technical data controlled under either US International Traffic in Arms Regulation or US Export Administration Regulations. The information and views set out in this article are those of the authors and do not necessarily reflect the official opinion of the European Commission.
EMBO Reports (2020) 21: e50521
References
- 1. Dan SM (2019) How interface formats gain market acceptance: the role of developers and format characteristics in the development of de facto standards. Technovation 88: 102054 [Google Scholar]
- 2. Beal J, Haddock‐Angelli T, Farny N, Reggberg R (2018) Time to get serious about measurement in synthetic biology. Trends Biotechnol 36: 869–871 [DOI] [PubMed] [Google Scholar]
- 3. Vilanova C, Porcar M (2014) IGEM 2.0–refoundations for engineering biology. Nat Biotechnol 32: 420–424 [DOI] [PubMed] [Google Scholar]
- 4. Carr SB, Beal J, Densmore DM (2017) Reducing DNA context independence in bacterial promoters. PLoS ONE 12: e0176013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de Lorenzo V, Schmidt M (2018) Biological standards for the knowledge‐Based BioEconomy: what is at stake. New Biotechnol 40: 170–180 [DOI] [PubMed] [Google Scholar]
- 6. Grozinger L, Amos M, Gorochowski TT, Carbonell P, Oyarzún DA, Stoof R, Fellermann H, Zuliani P, Tas H, Goñi‐Moreno A (2019) Pathways to cellular supremacy in Biocomputing. Nat Com 10: 5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Endy D (2005) Foundations for engineering biology. Nature 438: 449–453 [DOI] [PubMed] [Google Scholar]
- 8. Galdzicki M, Clancy K, Oberortner E, Pocock M, Quinn J, Rodriguez C, Roehner N, Wilson M, Adam L, Anderson JV et al (2014) SBOL: a community standard for communicating designs in synthetic biology. Nat Biotechnol 32: 545–550 [DOI] [PubMed] [Google Scholar]
- 9. Martínez‐García E, Goñi‐Moreno A, Bartley B, McLaughlin J, Sánchez‐Sampedro L, Pascual Del Pozo H, Prieto Hernández C, Marletta AS, De Lucrezia D, Sánchez‐Fernández G et al (2020) SEVA 3.0: An update of the Standard European Vector Architecture for enabling portability of genetic constructs among diverse bacterial hosts. Nucleic Acids Res 8: D1164–D1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schreiber F, Sommer B, Bader GD, Gleeson P, Golebiewski M, Hucka M, Keating SM, König M, Myers C, Nickerson D et al (2019) Specifications of standards in systems and synthetic biology: status and developments in 2019. J Integr Bioinform 16: 20190035 [DOI] [PMC free article] [PubMed] [Google Scholar]


