Abstract
The metabolic compartmentalization enabled by mitochondria is key feature of many cellular processes such as energy conversion to ATP production, redox balance, and the biosynthesis of heme, urea, nucleotides, lipids, and others. For a majority of these functions, metabolites need to be transported across the impermeable inner mitochondrial membrane by dedicated carrier proteins. Here, we examine the substrates, structural features, and human health implications of four mitochondrial metabolite carrier families: the SLC25A family, the mitochondrial ABCB transporters, the mitochondrial pyruvate carrier (MPC), and the sideroflexin proteins.
Keywords: metabolism, metabolite carriers, mitochondria, transporters
Subject Categories: Membrane & Intracellular Transport, Metabolism
Mitochondrial carriers play an essential role in the metabolic compartmentalization by mitochondria. This review discusses the substrates, structural features, and human health implications of mitochondrial metabolite carriers.
Glossary
- ABCB
ATP‐binding cassette B class
- ADP
adenosine diphosphate
- Akt
protein kinase B
- ANT
adenine nucleotide translocator
- ATP
adenosine triphosphate
- BAT
brown adipose tissue
- BCAA
branched‐chain amino acids
- C4 metabolites
four‐carbon dicarboxylate intermediates
- CIC
tricarboxylate carrier
- CoA
coenzyme A
- DIC
dicarboxylate carrier
- dPCoA
3′‐dephosphocoenzyme A
- EF‐hand
Ca2+ binding motif
- ETC
electron transport chain
- GDC
Graves’ disease carrier
- HHH Syndrome
hyperammonemia‐hyperornithinemia‐homocitrullinuria
- IMM
inner mitochondrial membrane
- ISC
iron–sulfur cluster
- LCFA
long‐chain fatty acid
- MPC
mitochondrial pyruvate carrier
- NADH
reduced nicotinamide adenine dinucleotide
- NBD
nucleotide binding domain
- OGC
oxoglutarate carrier
- OMM
outer mitochondrial membrane
- ORNT
ornithine carrier
- PAP
3′‐phosphoadenosine 5′‐phosphate
- PiC‐A/B
mitochondrial phosphate carrier isoforms
- ROS
reactive oxygen species
- SFXN
sideroflexin
- SLC1
solute carrier family 1
- SLC25A
solute carrier family 25
- TCA
tricarboxylic acid cycle
- TMD
transmembrane domain
- UCP
uncoupling protein
Introduction
Cellular compartmentalization into organelles provides the cell with a unique opportunity to segregate specialized processes, with the benefit of enabling mutually exclusive reactions, reactions that require a specialized environment or reactions with particular substrate requirements. One such organelle, the mitochondrion, performs a variety of cellular tasks from the biosynthesis of heme, nucleotides, urea, lipids, and other metabolites to ATP generation, redox balance, and others. In order to perform these various functions, mitochondria need to import and export a very diverse set of solutes and metabolites. While the outer mitochondrial membrane (OMM) appears to be quite permeable, a significant challenge arises for molecules at the inner mitochondrial membrane (IMM). The IMM is intrinsically impermeable, which enables the mitochondrial matrix to have an environment that is isolated and distinct from the environment in the cytosol. This is in contrast to the nucleus, which freely exchanges molecules with the cytosol through the nuclear pore complex. As such, mitochondria employ a variety of highly specific transporters to support these specialized functions and processes. Moreover, by controlling solute transport, mitochondria exert profound control over all of cellular metabolism. In this review, we will explore the diversity of substrates, structures, and health implications of the four characterized mitochondrial metabolite transporter families: the SLC25A family, mitochondrial ABCB transporters, the mitochondrial pyruvate carrier (MPC), and the sideroflexin carriers.
SLC25 mitochondrial solute carriers
Comprising 53 family members, most members of the SLC25 carrier family transport solutes across the IMM as part of a variety of distinct metabolic processes. Of note, one SLC25 member is peroxisomal (SLC25A17) and two others appear to be OMM transporters (SLC25A46 and SLC25A50). Each SLC25 member is characterized by a three‐domain structure in which each domain comprises two alpha helices, connected by a loop‐helix‐loop (Fig 1) 1, 2, 3, 4. Interestingly, the C‐terminus of odd‐numbered helices contains a signature PX[DE]XX[KR] motif that forms salt bridges to prevent entrance from the matrix when the transporter is open on the cytoplasmic side 4, 5, 6, 7.
Figure 1. The four families of mitochondrial metabolite transporters.
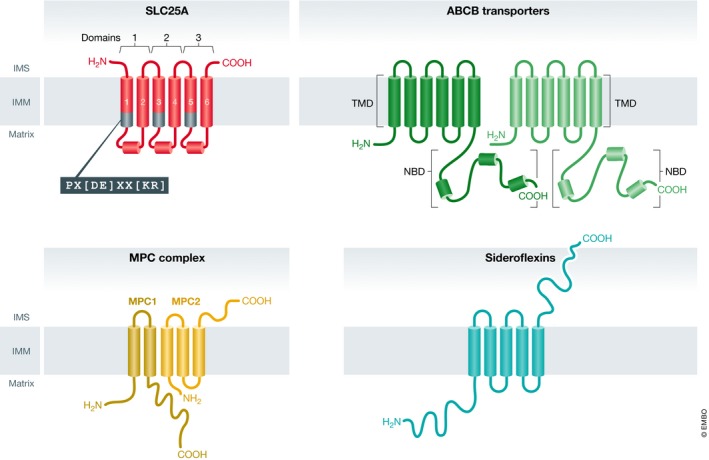
Illustrations of the four classes of mitochondrial metabolite carriers: SLC25A, ABCB transporters, mitochondrial pyruvate carrier (MPC) complex, and the sideroflexin proteins. Each SLC25A member is a pseudo‐symmetrical threefold structure with each domain containing two alpha helices connected by a soluble loop‐helix‐loop. The end of each odd‐numbered helix contains a signature carrier motif: PX[DE]XX[KR]. ABCB transporters of the mitochondria contain twelve membrane‐spanning helices regulated by nucleotide binding domains (NBDs). The MPC complex consists of two proteins: MPC1, a two‐pass transmembrane protein, and MPC2, believed to be a three‐pass transmembrane protein. These two individual units interact as a functional heterodimer. Sideroflexins are five‐pass transmembrane proteins.
This general structural similarity and limited conserved sequence is essentially where the commonalities end. The SLC25 family of solute carriers is diverse with some unique structural features, such as the eight EF‐hands of SLC25A12 and SLC25A‐13 and four EF‐hands and amphipathic α‐helix of ATP‐Mg/Pi carriers 8, 9, substrates, requirement for calcium, and tissue expression pattern. A majority of carriers antiport substrates between the intermembrane space and matrix, with a smaller number acting as uniporters or symporters 10. As would be expected given these important but varied roles, mutations affecting SLC25 proteins can lead to a variety of debilitating diseases and metabolic disorders, some of which are fatal. In this section, we will review many members of the mitochondrial SLC25 solute carrier family and their roles in mitochondrial metabolism. For additional discussion of the SLC25 family, we recommend these reviews: for a comprehensive biochemical review of the SLC25s 10; for an introduction to SLC25 biogenesis 11; for a thorough structural perspective 12; and for detailed metabolic integration and crosstalk with the MPC complex 13.
SLC25 members in the TCA cycle and respiratory metabolism
Compartmentalizing metabolism into the mitochondrial matrix is essential for the progression of the TCA cycle and oxidative phosphorylation. Indeed, several SLC25 family members are needed to carry metabolites and coenzymes related to the TCA cycle into or out of the mitochondrial matrix (Fig 2).
Figure 2. Interplay of mitochondrial metabolite transporters in the TCA cycle and oxidative phosphorylation.
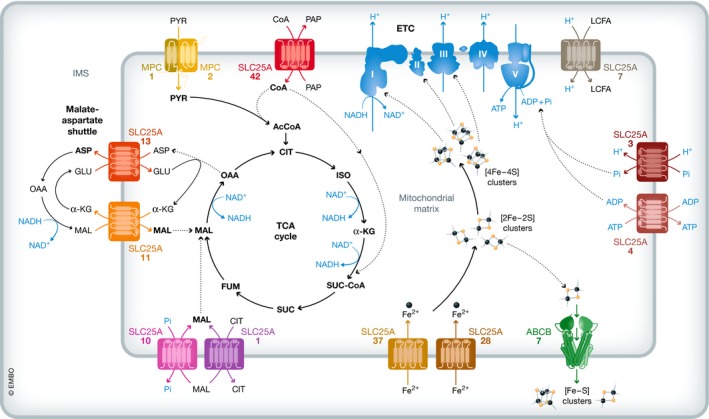
The MPC complex transports pyruvate and SLC25A42 transports CoA into the matrix where pyruvate dehydrogenase catalyzes the decarboxylation and thioesterification of pyruvate into acetyl‐CoA. Acetyl‐CoA then donates a two‐carbon unit to the TCA cycle. The TCA cycle intermediate malate can also be transported into the matrix by SLC25A11, which also participates in the malate–aspartate redox shuttle with SLC25A13, or by either SLC25A10 or SLC25A1. Ultimately, NADH molecules produced by the TCA cycle serve as electron donors to the ETC, which features multiple iron–sulfur clusters in complexes I–III derived from the iron imported by SLC25A28 and SLC25A37. These iron–sulfur clusters are also exported into the cytosol for iron–sulfur assembly by ABCB7. ETC complexes I–IV create a proton gradient required for the generation of ATP by complex V, which also requires ADP transported into the matrix by SLC25A4 and phosphate transported by SLC25A3. To generate heat instead of ATP energy, SLC25A7 transports protons into the matrix, dissipating the proton gradient established by complexes I–IV.
While conventional mitochondrial carbohydrate metabolism begins with pyruvate flux into the mitochondrial matrix (see Mitochondrial pyruvate carrier below), the availability of coenzyme A (CoA) is required for the first step in its metabolism. Carrying of CoA into the mitochondrial matrix is performed by SLC25A42, which exchanges adenosine nucleotides or 3′‐phosphoadenosine 5′‐phosphate (PAP) for CoA, dPCoA, or deoxyA(X)P 14. Pyruvate dehydrogenase irreversibly catalyzes the decarboxylation and thioesterification of pyruvate into acetyl‐CoA, which then feeds the two carbons into the TCA cycle. Instrumental to this reaction is a thiamine pyrophosphate coenzyme, which is exchanged into the mitochondrial matrix by SLC25A19 for thiamine monophosphate 15, 16. Inversely, pyruvate dehydrogenase is inhibited by branched‐chain amino acids (BCAAs) 17 which are carried into the mitochondrial matrix by SLC25A44 18. SLC25A42‐imported CoA is also required for a subsequent step of the TCA cycle catalyzed by α‐ketoglutarate dehydrogenase, wherein α‐ketoglutarate and CoA are conjugated to succinyl‐CoA. Not surprisingly, mutations in the SLC25A42 gene that impair mitochondrial CoA import result in mitochondrial encephalomyopathies, lactic acidosis, developmental delays, muscle weakness, and epilepsy. The best‐characterized SLC25A42 mutation, N291D, likely impairs CoA transport by increasing negative charge in the phosphate subgroup binding pocket via its close proximity, thereby inhibiting proper substrate binding and transport 19, 20, 21.
While an important TCA cycle intermediate, malate is also essential for mitochondrial redox homeostasis. Malate can be exchanged into or out of the mitochondrial matrix by three distinct carriers: SLC25A10 (DIC or dicarboxylate carrier), SLC25A11 (OGC or oxoglutarate carrier), and SLC25A1 (CIC or tricarboxylate transport protein). SLC25A10 and SLC25A11 exchange malate for phosphate and α‐ketoglutarate, respectively 22, 23, 24, 25, while SLC25A1 exchanges citrate for malate 26, 27, 28, 29. Significantly, biallelic mutations in the SLC25A10 gene result in epileptic encephalopathies similar to SLC25A42 mutations and complete loss of SLC25A10 or SLC25A11 results in mitochondrial respiration defects and cell death 30, 31.
SLC25A11, along with SLC25A12 (aralar) and SLC25A13 (citrin), plays key roles in the malate–aspartate shuttle, which enables the net flow of electrons across the IMM, which is impermeant to the NADH electron carrier (Fig 2) (see below) 32. Both SLC25A12, expressed in the central nervous system and skeletal muscle, and SLC25A13, highly expressed in the liver, contain eight N‐terminal EF‐hand calcium binding motifs exposed to the intermembrane space 33, 34, 35. Upon binding of a calcium ion to the EF‐hand, the N‐terminal gate opens, allowing translocation of aspartate out and glutamate in 9.
In the typical “forward” direction of the malate–aspartate shuttle, malate is oxidized to oxaloacetate by malate dehydrogenase in the mitochondrial matrix, coupled with NAD+ reduction to NADH, which donates the electron to the electron transport chain (ETC). Aspartate transaminase then converts oxaloacetate and glutamate to aspartate and α‐ketoglutarate. The reverse reactions happen outside of the mitochondrial matrix, enabling the capture and mitochondrial import of electrons from NADH. Thus, the central components of this system are the three transporters where SLC25A12 and SLC25A13 appear to both catalyze the exchange of aspartate and glutamate and SLC25A11 exchanges malate for α‐ketoglutarate. Furthermore, this shuttle system exemplifies how SLC25 carriers SLC25A11–13 provide layers of regulation on compartmentalized metabolism.
A problematic byproduct of oxidative respiration is the production of reactive oxygen species (ROS). To balance sufficient ATP production while reducing ROS, mitochondria may have evolved to regulate the mitochondrial membrane potential and ATP production using uncoupling proteins, a six‐member subclass: SLC25A7 (UCP1), SLC25A8 (UCP2), SLC25A9 (UCP3), SLC25A14 (UCP5), SLC25A27 (UCP4), and SLC25A30 (UCP6). The best‐studied member of this family is UCP1, which is expressed exclusively in thermogenic brown and beige adipocytes. It functions as a specific proton carrier that dissipates the proton gradient produced by the ETC releasing the energy as heat 36, 37, 38, 39. Not surprisingly, the loss of UCP1 drastically reduces the thermogenic capacity and energy dissipation of adipose tissue and results in cold intolerance and predisposition for obesity 40. To aid in transport, a long‐chain fatty acid (LCFA) binds within UCP1 38. Rather than the LCFA actually being “transported”, it might simply serve as a cofactor in the proton transport process. Similarly, literature suggests that UCP3 41, which is expressed in BAT and skeletal muscle, also carries protons and LCFAs, yet contrasting evidence suggests LCFAs either are exchanged or serve as cofactors for proton transport 42, 43, 44. Interestingly, UCP3 overexpression can also inhibit tumorigenesis, perhaps by exchanging LCFAs out of the mitochondria encouraging increased lipid catabolism and changes in membrane composition, which might block pro‐growth/survival Akt signaling by preventing Akt recruitment to regions of the plasma membrane 45.
In contrast, UCP2, which is highly expressed in skeletal muscle and the immune system, exchanges C4 metabolites (i.e., malate, oxaloacetate, aspartate) for phosphate and protons 46, 47. Interestingly, UCP2 along with UCP3 is weakly expressed on the IMM and may only be activated by increased ROS derivatives and excess matrix fatty acids 48. UCP4 and UCP5, which are primarily found in the central nervous system, appear to exchange anions for protons 49, 50. Interestingly, UCP2–4 may regulate mitochondrial calcium homeostasis, as their overexpression reduces mitochondrial calcium storage 51, 52. UCP4 overexpression is neuroprotective by reducing overload of mitochondrial calcium and ROS 53, 54. Interestingly, the last member of the uncoupling protein subfamily, UCP6, also known as kidney mitochondrial carrier protein‐1 can exchange sulfur oxyanions, phosphate, dicarboxylates, and tricarboxylates for proton flux into the mitochondria 50. While its ability to decrease the mitochondrial membrane potential is less than UCP1, it may play a protective role in the kidney similar to UCP4 in the brain as its expression is upregulated in the presence of oxidative stress 55. How all of these observations can be harmonized into an understanding of UCP6 function remains to be determined.
While the UCP subfamily are classically considered to be inhibitory to ATP production, many other members of the SLC25A family play a positive role. These include SLC25A4 (adenine nucleotide translocator, ANT1), SLC25A5 (adenine nucleotide translocator 2, ANT2), SLC25A6 (adenine nucleotide translocator 3, ANT3), SLC25A31 (adenine nucleotide translocator 4, ANT4), SLC25A3 (mitochondrial phosphate carrier, isoforms PiC‐A and PiC‐B), and iron carriers SLC25A28 and SLC25A37 (mitoferrin‐2 and mitoferrin‐1, respectively). First, iron–sulfur clusters are synthesized in the mitochondrial matrix and are necessary for aconitase activity and complex I, II, and III formation of the ETC in the mitochondria as well as very important non‐mitochondrial functions. The import of mitochondrial iron to feed iron–sulfur cluster biogenesis is dependent on SLC25A28 and SLC25A37, which uniport iron into the mitochondrial matrix 56, 57. This transported iron is incorporated into iron–sulfur clusters by a dedicated and complex system of ISC proteins as well as being used in other scenarios 58.
Establishment of the mitochondrial membrane potential via the ETC complexes I–IV and the consumption of that potential by complex V powers the generation of ATP from ADP and phosphate. As a key part of this system, the most abundant protein on the mitochondrial inner membrane, ANT1, exchanges ADP in for ATP out 2, 59, 60, 61, 62. Not surprisingly, ANT1 is highly expressed in tissues with elevated energetic demand such as skeletal muscle, heart, and brain. Disruptive missense mutations R80H and R235G reduce the positive charge within the pore, leading to decreased ADP/ATP exchange flux. Indeed, these mutations lead to severe congenital hypotonia, muscle weakness, and early childhood death 63. Additional characterized mutations (i.e., A90D, D104G, L98P) cause progressive mitochondrial diseases such as external ophthalmoplegia 64, 65, 66. Other adenine nucleotide translocators (ANT2–4) are differentially expressed in liver, kidney, brain, with ANT4 being expressed in germ cells 59, 67, 68, 69, yet the function of exchanging ADP and ATP appears to be similar for all of the isoforms.
The two alternatively spliced isoforms of SLC25A3 (mitochondrial phosphate carrier, PiC‐A and PiC‐B) symport phosphate and protons into the mitochondrial matrix for use in ATP synthesis by complex V 70, 71, 72. Similar to ANT1 mutations, SLC25A3 mutations cause a disorder of oxidative phosphorylation characterized by lactic acidosis, hypertrophic cardiomyopathy, and muscular hypotonia 73.
As described above, members of the SLC25 family facilitate the transport of metabolites and coenzymes for a variety of TCA cycle and oxidative phosphorylation reactions. For a majority of these reactions, their compartmentalization in the mitochondrial matrix or IMM enables these metabolic processes to proceed in an organized manner. Most significantly, the aforementioned SLC25A carriers provide regulation on these events, as their expression and activity can dictate downstream metabolic progression.
SLC25s in the urea, fatty acid, and heme synthesis pathways
In addition to the TCA cycle and respiratory metabolism, mitochondrial SLC25 family members play essential and complementary roles in mitochondrial and cytosolic cooperative metabolism, including the biosynthesis of heme, fatty acids, as well participation in the urea cycle and fatty acid β‐oxidation. For example, iron carried by SLC25A28 and SLC25A37 (mitoferrin‐2 and mitoferrin‐1, respectively) is not only used for iron–sulfur cluster biogenesis but also for iron‐containing heme cofactor as well as iron‐containing proteins, including those in lipid biosynthesis 56, 74.
SLC25A1 (tricarboxylate carrier) exchanges malate for citrate and a proton 26. While the imported malate can enter the TCA cycle and be converted to oxaloacetate, the exported citrate can be used by ATP citrate lyase to generate cytosolic acetyl‐CoA. Not only is this a very important reaction because acetyl‐CoA is the primary building block for cytosolic fatty acid biosynthesis, but it also shows how compartmentalizing cellular reactions and the carrying function of the SLC25 family change cellular metabolism. Conversely, the catabolism of fatty acids through β‐oxidation is a robust means of mitochondrial ATP generation through supplying the TCA cycle with acetyl‐CoA. Here, fatty acids that are intrinsically unable to cross the OMM and IMM are conjugated to carnitine by carnitine palmitoyltransferase I. SLC25A20 (carnitine‐acylcarnitine translocase or CACT) then exchanges the fatty acylcarnitine into the mitochondrial matrix for carnitine 75, 76, 77. Within the matrix, carnitine palmitoyltransferase II catalyzes the reconjugation of fatty acids to CoA, releasing free carnitine. Such acyl‐CoA molecules are the immediate substrate for β‐oxidation and generation of acetyl‐CoA to supply the TCA cycle. Not surprisingly, mutations that impair SLC25A20 function result in infantile coma with hypoketonemia as patients are unable to generate usable energy from fatty acid oxidation 78.
The urea cycle is another metabolic system that coordinates across the cytosolic and mitochondrial compartments via SLC25 carriers, including SLC25A15 (ornithine carrier 1, ORNT1), SLC25A2 (ornithine carrier 2, ORNT2), SLC25A12 (aspartate/glutamate carrier 1, aralar), and SLC25A13 (aspartate/glutamate carrier 2, citrin). Citrulline, which is produced in the mitochondrial matrix, is exchanged into the cytosol where argininosuccinate synthase reacts it with ATP and aspartate to generate argininosuccinate and begin the cytosolic portion of the cycle 79, 80, 81, 82. Common missense mutations (i.e., E180K and F188Δ) in SLC25A15 and SLC25A2 (ORNT1–2) carriers cause hyperammonemia‐hyperornithinemia‐homocitrullinuria (HHH syndrome) resulting in a significant buildup of ammonia in the blood due to failure of the urea cycle 82, 83, 84.
Importantly, a key contributor to the cytosolic part of the urea cycle, aspartate, is exchanged into the cytosol for glutamate by SLC25A12 and SLC25A13, calcium‐dependent carriers that were described previously 32. As expected, inactivating mutations in SLC25A13 (i.e., 851del4, 1638Ins23) cause type II citrullinemia, a disorder with high levels of ammonia in the blood similar to that of HHH syndrome described above 34.
Unknown depths of the SLC25 family
While many SLC25 family members have been characterized for their structure, substrate specificity, and health implications, some carriers remain completely unannotated. In addition, five other SLC25 proteins still have ambiguous substrate specificity including SLC25A16 (Graves’ disease carrier, GDC), SLC25A30 (UCP6), SLC25A38, SLC25A39, and SLC25A40. For example, while expression of human SLC25A16 can complement a yeast leu5Δ mutant, lacking the yeast CoA carrier, SLC25A16 has not been shown to transport in CoA 85, 86, 87. Redundant roles exist for many of the SLC25 carriers, yet, SLC25A16 and SLC25A42 only share 39% protein sequence homology. Interestingly, a N291D mutation in SLC25A42 impairs CoA transport into mitochondria and this N291 residue is conserved in SLC25A16. These two carriers might serve redundant CoA transport functions, yet it remains unclear as to why both carriers are highly expressed in the same tissues 19.
The current literature is very sparse about the poorly characterized SLC25A38–40 carriers. SLC25A38 is highly expressed in transferrin receptor‐positive (CD71+) erythroid cells. Consistent with this expression pattern, zebrafish SLC25A38 knockouts are anemic and phenocopy non‐syndromic congenital sideroblastic anemia 88. While a yeast orthologue, Hem25p, transports glycine into the mitochondrial matrix, the function and substrates of human SLC25A38 remain unknown. Similarly, SLC25A39 is highly expressed in hematopoietic tissues and the central nervous system, but zebrafish knockouts do not exhibit anemia or porphyria 89, 90. A clue to the transport function of SLC25A39 comes from its yeast homologue Mtm1p, which interacts with mitoferrins and the mtm1Δ mutant can be complemented with the zebrafish SLC25A39 gene. Moreover, the Drosophila melanogaster homologue, Shawn, is necessary for neural development and knockouts cause imbalances in metal localization and homeostasis, with deficient manganese, iron overload, and mitochondria‐specific calcium overload 91. Shawn is also predicted to be homologous to SLC25A40, which is also highly expressed in central nervous system tissue. While some mutations in the SLC25A40 gene cause a form of pontocerebellar hypoplasia called cerebellar atrophy with progressive microcephaly, a specific Y125C missense mutation, occurring adjacent to the second transmembrane region, causes hypertriglyceridemia 92, 93. In spite of these intriguing observations, these SLC25 transporters have unassigned substrates and transport mechanisms.
In addition to the six incompletely characterized carriers described above, another 10 SLC25 members remain completely orphaned. These include SLC25A34, SLC25A35 and SLC25A43, SLC25A45, SLC25A47–53. What are their functions, substrates, and regulatory mechanism? How do they contribute to compartmentalized metabolism? Are they highly expressed in specific tissues? And what are the health implications of missense or null mutations of each? Some of these orphan carriers may serve functions outside of metabolite transport. For example, SLC25A46 does not contain the SLC25A consensus sequence which is essential for metabolite transport 94. Interestingly, SLC25A46 was discovered to be the mammalian orthologue to Ugo1, a mitofusin‐1 and mitofusin‐2 regulator that is required for inner and outer membrane fusion. Not surprisingly, SLC25A46 −/− mice display a severe ataxic phenotype due to highly dysfunctional mitochondria in cerebellar and Purkinje neurons 95, 96. Furthermore, SLC25A46 mutations, such as T142I and L341P, both of which destabilize the protein, cause mitochondrial hyperfusion resulting in pontocerebellar hypoplasia, Leigh syndrome, and atrophy spectrum disorder 94, 97, 98. Similarly, SLC25A50, another uncharacterized carrier, forms high‐molecular‐weight complexes on the OMM and is important for mitochondrial morphology and transport 99, 100. Indeed, SLC25s have roles outside of canonical metabolite transport, an interesting feature that may have been overlooked for the aforementioned transporters.
Unexpected carriers of the mitochondria
Surprisingly, even with 53 distinct SLC25 carriers, additional non‐canonical carriers have evolved to fulfill specific metabolite transport functions. For instance, three members of the ATP‐binding cassette (ABC) transporter superfamily are definitively mitochondrially localized and pump metabolites in support of cytosolic and mitochondrial metabolism. More surprisingly, research over the past decade has unveiled two more mitochondrial carrier families: the MPC and the sideroflexins. These novel carrier families are distinct from each other and from the SLC25 family in their structures and substrates (Figs 1 and 2). In this section, we will uncover features of the structures, functions, and substrates of the mitochondrial ABCB, MPC, and sideroflexin families, while also discussing their unique and cooperative roles in compartmentalized mitochondrial metabolism and homeostasis.
ABCB transporters of the mitochondria
The ABC transporter superfamily encompasses 48 ATP‐dependent solute carriers that span seven subfamilies 101. All members typically contain two nucleotide binding domains (NBD) and two transmembrane domains (TMD) with each TMD featuring six to 10 membrane‐spanning α‐helices (Fig 1) 102. In humans, three members of the ABCB subclass are putatively localized to mitochondria: ABCB7, ABCB8, and ABCB10, with a fourth member, ABCB6, having a controversial cellular localization. While similar in structure and having related roles in heme synthesis and iron transport, these mitochondrial carriers exhibit differences in substrates, localization and expression patterns, and health implications.
ABCB7 is a putative IMM transporter, which is homologous to the yeast Atm1p carrier that is well characterized to transport mitochondrial iron–sulfur (Fe–S) clusters into the cytosol, where these clusters bind to respective proteins for diverse functions (Fig 2) 103, 104. Structurally, after Fe–S binding to the matrix surface of ABCB7, ATP binding causes a major conformational shift, with the transport channel becoming outward‐facing and allowing for substrate release into the intermembrane space 105. ABCB7 has a binding site for reduced glutathione and is predicted to transport a reduced glutathione‐coordinated 2Fe‐2S cluster as a substrate 106. Indeed, ABCB7 mutants mimic the massive decrease in cytosolic Fe–S availability observed in atm1Δ yeast cells. Furthermore, null mutations in ABCB7 result in X‐linked sideroblastic anemia and refractory anemia with ring sideroblasts, which are anemias wherein excess mitochondrial iron appears to be pathogenic 107, 108.
Similarly, knockouts of ABCB8 also lead to decreases in cytosolic Fe–S clusters and excess mitochondrial iron 109. ABCB8 is an IMM ABC transporter that exports an unknown substrate from the mitochondrial matrix 110, 111. ABCB8 is ubiquitously expressed with highest expression in the heart, where it is essential for proper cardiac contractility, mitochondrial structure, and iron flux 109. Interestingly, ABCB8 can exist as a “half molecule” ABC, with only one TMD and NBD, which is unique among the ABCB transporter subfamily. This “half molecule” structure may provide an explanation into its newly discovered role as part of the ATP‐sensitive mitochondrial potassium channel, wherein four ABCB8 molecules coordinate with four MITOK channels to form a channel 112.
Like the other mitochondrial ABC transporters, ABCB10, an IMM carrier, also appears to be important for iron homeostasis since mutants lacking ABCB10 exhibit mitochondrial iron deficiency 113, 114, 115. ABCB10 features conserved motifs: (N/IXXR) on TM‐helix2 and NXXDGXR on TM‐helix 3B, which appear to form a patch that can bind an amphipathic substrate 116. Interestingly, ABCB10 interacts with SLC25A37/mitoferrin‐1 and supports mitochondrial iron transport 117. While its substrate remains unknown, an ABCB10 null mutation also reduces the abundance of the aminolevulinic acid synthase that catalyzes the rate‐limiting step in heme biosynthesis, which has led to the hypothesis that ABCB10 transports an intermediate in the heme biosynthesis pathway 113.
ABCB6 is a member of the family with unclear subcellular localization. It has been reported to be an OMM nutrient carrier, but has also been shown to localize to the plasma membrane, lysosomal membrane, or in the case of ABCB6 mutants, to the Golgi 118, 119, 120, 121, 122, 123. We will focus on its reported mitochondrial function. Highly upregulated in erythroid cells, fetal liver, and bone marrow, ABCB6 imports porphyrins, which include heme, into the intermembrane space and if localized on the plasma membrane exports porphyrins into the extracellular milieu 124, 125. In the heme biosynthesis pathway, ABCB6 coordinates the entrance of coproporphyrinogen III into the mitochondria, after multiple coordinated cytosolic events, where sequential enzymatic reactions, high iron concentration, and 2Fe‐2S availability allow for ferrochelatase to convert protoporphyrin IX into heme. Interestingly, ABCB6 is upregulated in the presence of high intracellular porphyrins as well as during erythroid differentiation 124. Given its role in heme transport, it is surprising that humans lacking ABCB6, referred to as Langereis [Lan(−)] blood type, do not present any signs of impaired heme delivery 126, 127. Similarly, abcb6 −/− mice appear to compensate for the loss of ABCB6 by increasing the heme biosynthetic pathway 128. As such, these results may indicate that ABCB6 may not properly localize to the mitochondria for porphyrin transport or may be compensated by another transporter.
Mitochondrial pyruvate carrier
A major step in central carbon metabolism is the transport of cytosolic pyruvate into the mitochondria where it can feed the TCA cycle. In 2012, Bricker et al, and Herzig et al, 129, 130 discovered the proteins that comprise the MPC: MPC1 and MPC2 are two broadly conserved IMM transmembrane proteins that form a heterodimeric complex to enable the transport of pyruvate (Figs 1 and 2) 131. Yeast have an MPC2 paralog, known as MPC3, which can partially compensate for the loss of MPC2. While the exact pyruvate transport mechanism remains elusive, transport likely involves either pyruvate symport with a proton or antiport with a hydroxide ion, as proteoliposome‐based in vitro transport assays rely on pHinterior to be around ~ 8.0 and pHexterior to be 6.5 131. Previous studies of intact mitochondria similarly showed that mitochondrial pyruvate import is dependent upon the proton gradient 132, 133, 134.
Upon discovery of the genes that encode the MPC, human MPC1 mutations that impair mitochondrial pyruvate import have been identified. The A58G and L79H MPC1 mutations massively impair mitochondrial pyruvate import 135, 136. The A58G mutation leads to the truncation of MPC1, thereby destabilizing MPC1/MPC2 complexes and impairing pyruvate transport. The existing A58G patients also have an additional R97W mutation. Two siblings with these mutations presented with hypotonia, hyperlactacidemia, and other metabolic irregularities, and died in early childhood 129, 135, 136. Interestingly, the R97W mutation appears to not directly impair pyruvate transport but leads to less stable MPC1/MPC2 complexes. The MPC1 L79H mutant protein forms stable complexes with MPC2 but they have impaired pyruvate transport function 136. Not surprisingly, patients with the L79H mutation often present with mild hyperlactacidemia, hyperpyruvicemia, and developmental delays 136. Loss of MPC1 or MPC2 has been studied in a variety of model systems and typically exhibits the expected decrease in pyruvate oxidation, which is frequently compensated by increased fatty acid or glutamine consumption 137, 138, 139, 140, 141, 142. It has been recently proposed that acetylation of MPC1 and MPC2 might regulate pyruvate transport, which would add an additional layer of control on central carbon metabolism in the mitochondria 143, 144, 145.
Decreased or absent MPC activity causes a metabolic phenotype that is reminiscent of the Warburg effect, a metabolic adjustment to perform aerobic glycolysis instead of oxidative phosphorylation for ATP synthesis that was first described in cancer cells 146, 147, 148. This metabolic program has been shown to support biosynthetic metabolism and biomass accumulation to enable rapid cellular proliferation as well as immune evasion 149. Over the past several years, multiple groups have shown that decreased MPC function either correlates with or causes increased tumor growth and poor patient prognosis 137, 146, 150, 151. Interestingly, some cancer types appear to require MPC function for tumor establishment and maintenance 152, 153.
Sideroflexins
Sideroflexins are five‐pass IMM proteins, whose functions as mitochondrial transporters were only recently discovered (Fig 1). Kory et al 154 convincingly demonstrated sideroflexin 1 (SFXN1) to be the mitochondrial serine transporter, a key metabolic regulator for one‐carbon metabolism. Prior to this pivotal finding, knowledge of SFXN1 was limited to its fairly broad expression that is enriched in kidney, liver, spleen, blood, and intestine. Previously, mutations in SFXN1 were shown to result in flex‐tailed mice that display major axial skeletal abnormalities and anemia 155. Using a CRISPR‐based screen, Kory et al found SFXN1, an IMM protein with previously unknown function, to be synthetic lethal with loss of serine hydroxymethyl transferase‐1. In media lacking serine, SFXN1 −/− cells exhibited slowed proliferation and accumulation of purine synthesis intermediates. These phenotypes are similar to defects found upon serine hydroxymethyl transferase‐2 mutation and are indicative of defects in mitochondrial serine transport and one‐carbon metabolism 154. Importantly, these authors were able to demonstrate that SFXN1 is sufficient for serine transport. In vitro proteoliposome transport assays using purified SFXN1 displayed serine transport along with a few other amino acids like alanine, cysteine, and glycine 154.
Interestingly, expression of SFXN3, the closest homologue to SFXN1, rescued proliferation of SFXN1 −/− cells 154. SFXN3 shares 77% amino acid sequence identity to SFXN1 and it is also ubiquitously expressed, with its highest expression in the cortex and hippocampus areas of the brain 155, 156. Not surprisingly, SFXN1 −/− SFXN3 −/− cells massively accumulate purine synthesis intermediates, suggesting redundant roles for these two related proteins in serine transport and one‐carbon metabolism 154.
Currently, less is known about the three additional sideroflexins. Sideroflexin 2, 4, and 5 only share 56, 22, and 39% sequence homology to SFXN1, respectively. Zebrafish lacking SFXN4 exhibit reduced globin+ cells, respiration defects, anemia, and erythropoiesis defects 157. A patient bearing a homozygous P78L mutation in SFXN4, which truncates the protein before the five transmembrane regions and is therefore likely to be a null mutation, caused macrocytic anemia and severe complex I deficiencies 157. Another patient, who had heterozygous R247K and T158M mutations in SFXN4, presented with similar lactic acidosis and complex I deficiencies 157. More recently, the heterozygous mutations Q323H and Q388X were shown to phenocopy the above patient mutations. If SFXN4 is also a metabolite transporter, as seems very likely, it appears that its transport substrate is a molecule that is required for complex I assembly, either directly or indirectly. In contrast, our current SFXN2 and SFXN5 knowledge is mostly limited to expression patterns as their substrates and human health implications are essentially unknown.
Conclusions and future directions
Researchers have defined many of the functions, structures, substrates, and human health implications of members of the four mitochondrial solute carrier families. We know substrates for 37 SLC25 proteins, two mitochondrial ABCB transporters, the MPC, and two of the sideroflexins. This leaves sixteen SLC25 members with unknown substrates, 10 of which are completely orphaned. Two ABCB transporters and three sideroflexins have poorly defined substrates, functions, and regulation. We suggest that it is critical that we develop the means to define the substrates and physiological functions of mitochondrial metabolite carriers. If we do so, we are very likely to find that several of them have important biomedical impact, including as potential therapeutic targets.
Of significance, there are compelling arguments that metabolite transporters might be safer and more effective metabolic modulators than drugs directly targeting metabolic enzymes. In cortical neuron cultures, MPC inhibition by UK‐5099 was non‐toxic and cells remained viable. In contrast, treatment with antimycin A, an inhibitor of cytochrome c reductase, resulted in cellular toxicity and death 158. As many mitochondrial carriers are upregulated in cancer, discovering inhibitors could be beneficial for patient outcomes. For example, knockdown of SLC25A10 (DIC) rendered adenocarcinomic cells more susceptible to cisplatin, a common chemotherapeutic 159. Given carrier promiscuity, targeting the central carrier for a given substrate may not completely eliminate transport of that substrate. This promiscuity proves beneficial in the above situations, as cells can remain viable while also becoming more susceptible to adjuvant therapies.
Moving forward, the major challenge of defining substrates and inhibitors for mitochondrial carriers, which is similar for transporters from other cellular locations, is the availability of a robust assay that can measure transport of any metabolite with reasonable throughput. Unfortunately, the current state‐of‐the‐art is to employ radiolabeled substrates in in vitro proteoliposome transport assays. This technique is low throughput, is reliant on access to a labeled version of the substrate to be tested, and is subject to issues of reverse transport and relationship with the pH gradient. More recently, several groups have developed high‐throughput thermostability shift assays to determine novel substrates and regulators of carriers 160, 161, 162. Development of substrate‐agnostic, higher‐throughput, and robust methodologies such as those above will have a transformative impact on our ability to identify substrates, agonists, antagonists, as well as allosteric and orthosteric regulators.
With the recent discoveries of the sideroflexins and MPCs as mitochondrial solute carriers, we must be willing to look beyond conventional carrier structures to identify new metabolite carriers. Sideroflexins and the heterodimeric MPC are predicted to be five‐pass transmembrane solute carriers, differing from the canonical SLC25s which have six helical transmembrane regions. Accordingly, the nature of structures can convey metabolites across the mitochondrial inner membrane. As is the case of MPC, two or more subunits may be needed for function. In addition, there are likely to be many scenarios that require cooperation between two or more transport proteins, such as for the recently identified mitochondrial ATP‐sensitive potassium channel—a coordination between ABCB8 and the MITOK cation channel 112. Even more strikingly, a splice variant of SLC1A5 was determined to be the mitochondrial glutamine transporter, showcasing that alternative splicing may indeed play a role in creating unorthodox mitochondrial carriers 163. The intelligent use of CRISPR‐based screening, development of new higher‐throughput methods of substrate identification, and thoughtful multi‐disciplinary collaborations promises to shed light on this critically important, but still mysterious, group of metabolic regulators.
Conflict of interest
The authors declare that they have no conflict of interest.
In need of answers
What are the substrates and physiological functions of the orphaned SLC25A, ABCB, and sideroflexin transporters?
Are mitochondrial transporters subject to orthosteric and allosteric regulators? How do these regulators bind and change transport function? How does this integrate with post‐translational modification?
Are there splice variants of other carrier families, such as the recently discovered SLC1A5 mitochondrial glutamine carrier, that reveal a canonical or non‐canonical mitochondrial targeting sequence?
Are there other non‐canonical carrier subclasses like the MPC family and sideroflexins? Are there additional novel assemblies like the newly identified mitochondrial ATP‐sensitive potassium channel?
How do we develop more robust, high‐throughput, and substrate‐agnostic assays of mitochondrial (and other) metabolite transporters?
Acknowledgements
We would like to thank Eric Taylor (University of Iowa) and Gregory Ducker (University of Utah) for their feedback and reviews. We would also like to thank members of the Rutter laboratory for helpful suggestions. J.R. is an investigator of the Howard Hughes Medical Institute.
EMBO Reports (2020) 21: e50071
See the Glossary for abbreviations used in this article.
References
- 1. Saraste M, Walker JE (1982) Internal sequence repeats and the path of polypeptide in mitochondrial ADP/ATP translocase. FEBS Lett 144: 250–254 [DOI] [PubMed] [Google Scholar]
- 2. Aquila H, Misra D, Eulitz M, Klingenberg M (1982) Complete amino acid sequence of the ADP/ATP carrier from beef heart mitochondria. Hoppe Seylers Z Physiol Chem 363: 345–349 [PubMed] [Google Scholar]
- 3. Kunji ER, Harding M (2003) Projection structure of the atractyloside‐inhibited mitochondrial ADP/ATP carrier of Saccharomyces cerevisiae . J Biol Chem 278: 36985–36988 [DOI] [PubMed] [Google Scholar]
- 4. Pebay‐Peyroula E, Dahout‐Gonzalez C, Kahn R, Trezeguet V, Lauquin GJ, Brandolin G (2003) Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426: 39–44 [DOI] [PubMed] [Google Scholar]
- 5. Nelson DR, Felix CM, Swanson JM (1998) Highly conserved charge‐pair networks in the mitochondrial carrier family. J Mol Biol 277: 285–308 [DOI] [PubMed] [Google Scholar]
- 6. Ruprecht JJ, Hellawell AM, Harding M, Crichton PG, McCoy AJ, Kunji ER (2014) Structures of yeast mitochondrial ADP/ATP carriers support a domain‐based alternating‐access transport mechanism. Proc Natl Acad Sci USA 111: E426–E434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robinson AJ, Overy C, Kunji ER (2008) The mechanism of transport by mitochondrial carriers based on analysis of symmetry. Proc Natl Acad Sci USA 105: 17766–17771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harborne SP, King MS, Crichton PG, Kunji ER (2017) Calcium regulation of the human mitochondrial ATP‐Mg/Pi carrier SLC25A24 uses a locking pin mechanism. Sci Rep 7: 45383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thangaratnarajah C, Ruprecht JJ, Kunji ER (2014) Calcium‐induced conformational changes of the regulatory domain of human mitochondrial aspartate/glutamate carriers. Nat Commun 5: 5491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Palmieri F, Monne M (2016) Discoveries, metabolic roles and diseases of mitochondrial carriers: a review. Biochim Biophys Acta 1863: 2362–2378 [DOI] [PubMed] [Google Scholar]
- 11. Ferramosca A, Zara V (2013) Biogenesis of mitochondrial carrier proteins: molecular mechanisms of import into mitochondria. Biochim Biophys Acta 1833: 494–502 [DOI] [PubMed] [Google Scholar]
- 12. Ruprecht JJ, Kunji ERS (2020) The SLC25 mitochondrial carrier family: structure and mechanism. Trends Biochem Sci 45: 244–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor EB (2017) Functional properties of the mitochondrial carrier system. Trends Cell Biol 27: 633–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fiermonte G, Paradies E, Todisco S, Marobbio CM, Palmieri F (2009) A novel member of solute carrier family 25 (SLC25A42) is a transporter of coenzyme A and adenosine 3′,5′‐diphosphate in human mitochondria. J Biol Chem 284: 18152–18159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lindhurst MJ, Fiermonte G, Song S, Struys E, De Leonardis F, Schwartzberg PL, Chen A, Castegna A, Verhoeven N, Mathews CK et al (2006) Knockout of Slc25a19 causes mitochondrial thiamine pyrophosphate depletion, embryonic lethality, CNS malformations, and anemia. Proc Natl Acad Sci USA 103: 15927–15932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang J, Samuels DC (2008) The evidence that the DNC (SLC25A19) is not the mitochondrial deoxyribonucleotide carrier. Mitochondrion 8: 103–108 [DOI] [PubMed] [Google Scholar]
- 17. Li T, Zhang Z, Kolwicz SC Jr, Abell L, Roe ND, Kim M, Zhou B, Cao Y, Ritterhoff J, Gu H et al (2017) Defective branched‐chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia‐reperfusion injury. Cell Metab 25: 374–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki H, Igarashi K, Dai Z, White PJ, McGarrah RW, Ilkayeva OR et al (2019) BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572: 614–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shamseldin HE, Smith LL, Kentab A, Alkhalidi H, Summers B, Alsedairy H, Xiong Y, Gupta VA, Alkuraya FS (2016) Mutation of the mitochondrial carrier SLC25A42 causes a novel form of mitochondrial myopathy in humans. Hum Genet 135: 21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Almannai M, Alasmari A, Alqasmi A, Faqeih E, Al Mutairi F, Alotaibi M, Samman MM, Eyaid W, Aljadhai YI, Shamseldin HE et al (2018) Expanding the phenotype of SLC25A42‐associated mitochondrial encephalomyopathy. Clin Genet 93: 1097–1102 [DOI] [PubMed] [Google Scholar]
- 21. Iuso A, Alhaddad B, Weigel C, Kotzaeridou U, Mastantuono E, Schwarzmayr T, Graf E, Terrile C, Prokisch H, Strom TM et al (2019) A homozygous splice site mutation in SLC25A42, encoding the mitochondrial transporter of coenzyme A, causes metabolic crises and epileptic encephalopathy. JIMD Rep 44: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fiermonte G, Palmieri L, Dolce V, Lasorsa FM, Palmieri F, Runswick MJ, Walker JE (1998) The sequence, bacterial expression, and functional reconstitution of the rat mitochondrial dicarboxylate transporter cloned via distant homologs in yeast and Caenorhabditis elegans . J Biol Chem 273: 24754–24759 [DOI] [PubMed] [Google Scholar]
- 23. Bisaccia F, Indiveri C, Palmieri F (1988) Purification and reconstitution of two anion carriers from rat liver mitochondria: the dicarboxylate and the 2‐oxoglutarate carrier. Biochim Biophys Acta 933: 229–240 [DOI] [PubMed] [Google Scholar]
- 24. Palmieri L, Palmieri F, Runswick MJ, Walker JE (1996) Identification by bacterial expression and functional reconstitution of the yeast genomic sequence encoding the mitochondrial dicarboxylate carrier protein. FEBS Lett 399: 299–302 [DOI] [PubMed] [Google Scholar]
- 25. Runswick MJ, Walker JE, Bisaccia F, Iacobazzi V, Palmieri F (1990) Sequence of the bovine 2‐oxoglutarate/malate carrier protein: structural relationship to other mitochondrial transport proteins. Biochemistry 29: 11033–11040 [DOI] [PubMed] [Google Scholar]
- 26. Kaplan RS, Mayor JA, Johnston N, Oliveira DL (1990) Purification and characterization of the reconstitutively active tricarboxylate transporter from rat liver mitochondria. J Biol Chem 265: 13379–13385 [PubMed] [Google Scholar]
- 27. Stipani I, Kramer R, Palmieri F, Klingenberg M (1980) Citrate transport in liposomes reconstituted with triton extracts from mitochondria. Biochem Biophys Res Commun 97: 1206–1214 [DOI] [PubMed] [Google Scholar]
- 28. Stipani I, Palmieri F (1983) Purification of the active mitochondrial tricarboxylate carrier by hydroxylapatite chromatography. FEBS Lett 161: 269–274 [DOI] [PubMed] [Google Scholar]
- 29. Bisaccia F, De Palma A, Palmieri F (1989) Identification and purification of the tricarboxylate carrier from rat liver mitochondria. Biochim Biophys Acta 977: 171–176 [DOI] [PubMed] [Google Scholar]
- 30. Punzi G, Porcelli V, Ruggiu M, Hossain MF, Menga A, Scarcia P, Castegna A, Gorgoglione R, Pierri CL, Laera L et al (2018) SLC25A10 biallelic mutations in intractable epileptic encephalopathy with complex I deficiency. Hum Mol Genet 27: 499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baulies A, Montero J, Matias N, Insausti N, Terrones O, Basanez G, Vallejo C, Conde de La Rosa L, Martinez L, Robles D et al (2018) The 2‐oxoglutarate carrier promotes liver cancer by sustaining mitochondrial GSH despite cholesterol loading. Redox Biol 14: 164–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palmieri L, Pardo B, Lasorsa FM, del Arco A, Kobayashi K, Iijima M, Runswick MJ, Walker JE, Saheki T, Satrustegui J et al (2001) Citrin and aralar1 are Ca2+‐stimulated aspartate/glutamate transporters in mitochondria. EMBO J 20: 5060–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Begum L, Jalil MA, Kobayashi K, Iijima M, Li MX, Yasuda T, Horiuchi M, del Arco A, Satrustegui J, Saheki T (2002) Expression of three mitochondrial solute carriers, citrin, aralar1 and ornithine transporter, in relation to urea cycle in mice. Biochim Biophys Acta 1574: 283–292 [DOI] [PubMed] [Google Scholar]
- 34. Kobayashi K, Sinasac DS, Iijima M, Boright AP, Begum L, Lee JR, Yasuda T, Ikeda S, Hirano R, Terazono H et al (1999) The gene mutated in adult‐onset type II citrullinaemia encodes a putative mitochondrial carrier protein. Nat Genet 22: 159–163 [DOI] [PubMed] [Google Scholar]
- 35. del Arco A, Satrustegui J (1998) Molecular cloning of Aralar, a new member of the mitochondrial carrier superfamily that binds calcium and is present in human muscle and brain. J Biol Chem 273: 23327–23334 [DOI] [PubMed] [Google Scholar]
- 36. Kozak LP, Britton JH, Kozak UC, Wells JM (1988) The mitochondrial uncoupling protein gene. Correlation of exon structure to transmembrane domains. J Biol Chem 263: 12274–12277 [PubMed] [Google Scholar]
- 37. Aquila H, Link TA, Klingenberg M (1985) The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. EMBO J 4: 2369–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fedorenko A, Lishko PV, Kirichok Y (2012) Mechanism of fatty‐acid‐dependent UCP1 uncoupling in brown fat mitochondria. Cell 151: 400–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bouillaud F, Ricquier D, Thibault J, Weissenbach J (1985) Molecular approach to thermogenesis in brown adipose tissue: cDNA cloning of the mitochondrial uncoupling protein. Proc Natl Acad Sci USA 82: 445–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matthias A, Ohlson KB, Fredriksson JM, Jacobsson A, Nedergaard J, Cannon B (2000) Thermogenic responses in brown fat cells are fully UCP1‐dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid‐induced thermogenesis. J Biol Chem 275: 25073–25081 [DOI] [PubMed] [Google Scholar]
- 41. Boss O, Samec S, Paoloni‐Giacobino A, Rossier C, Dulloo A, Seydoux J, Muzzin P, Giacobino JP (1997) Uncoupling protein‐3: a new member of the mitochondrial carrier family with tissue‐specific expression. FEBS Lett 408: 39–42 [DOI] [PubMed] [Google Scholar]
- 42. Costford SR, Seifert EL, Bézaire V, Gerrits M F, Bevilacqua L, Gowing A, Harper ME (2007) The energetic implications of uncoupling protein‐3 in skeletal muscle. Appl Physiol Nutr Metab 32: 884–894 [DOI] [PubMed] [Google Scholar]
- 43. Schrauwen P, Hesselink M (2002) UCP2 and UCP3 in muscle controlling body metabolism. J Exp Biol 205: 2275–2285 [DOI] [PubMed] [Google Scholar]
- 44. Pohl EE, Rupprecht A, Macher G, Hilse KE (2019) Important trends in UCP3 investigation. Front Physiol 10: 470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nowinski SM, Solmonson A, Rundhaug JE, Rho O, Cho J, Lago CU, Riley CL, Lee S, Kohno S, Dao CK et al (2015) Mitochondrial uncoupling links lipid catabolism to Akt inhibition and resistance to tumorigenesis. Nat Commun 6: 8137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vozza A, Parisi G, De Leonardis F, Lasorsa FM, Castegna A, Amorese D, Marmo R, Calcagnile VM, Palmieri L, Ricquier D et al (2014) UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci USA 111: 960–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi‐Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D et al (1997) Uncoupling protein‐2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet 15: 269–272 [DOI] [PubMed] [Google Scholar]
- 48. Esteves TC, Brand MD (2005) The reactions catalysed by the mitochondrial uncoupling proteins UCP2 and UCP3. Biochim Biophys Acta 1709: 35–44 [DOI] [PubMed] [Google Scholar]
- 49. Mao W, Yu XX, Zhong A, Li W, Brush J, Sherwood SW, Adams SH, Pan G (1999) UCP4, a novel brain‐specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Lett 443: 326–330 [DOI] [PubMed] [Google Scholar]
- 50. Gorgoglione R, Porcelli V, Santoro A, Daddabbo L, Vozza A, Monne M, Di Noia MA, Palmieri L, Fiermonte G, Palmieri F (2019) The human uncoupling proteins 5 and 6 (UCP5/SLC25A14 and UCP6/SLC25A30) transport sulfur oxyanions, phosphate and dicarboxylates. Biochim Biophys Acta Bioenerg 1860: 724–733 [DOI] [PubMed] [Google Scholar]
- 51. Trenker M, Malli R, Fertschai I, Levak‐Frank S, Graier WF (2007) Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol 9: 445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chan SL, Liu D, Kyriazis GA, Bagsiyao P, Ouyang X, Mattson MP (2006) Mitochondrial uncoupling protein‐4 regulates calcium homeostasis and sensitivity to store depletion‐induced apoptosis in neural cells. J Biol Chem 281: 37391–37403 [DOI] [PubMed] [Google Scholar]
- 53. Liu D, Chan SL, de Souza‐Pinto NC, Slevin JR, Wersto RP, Zhan M, Mustafa K, de Cabo R, Mattson MP (2006) Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromolecular Med 8: 389–414 [DOI] [PubMed] [Google Scholar]
- 54. Cho I, Hwang GJ, Cho JH (2016) Uncoupling protein, UCP‐4 may be involved in neuronal defects during aging and resistance to pathogens in Caenorhabditis elegans . Mol Cells 39: 680–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haguenauer A, Raimbault S, Masscheleyn S, Gonzalez‐Barroso Mdel M, Criscuolo F, Plamondon J, Miroux B, Ricquier D, Richard D, Bouillaud F et al (2005) A new renal mitochondrial carrier, KMCP1, is up‐regulated during tubular cell regeneration and induction of antioxidant enzymes. J Biol Chem 280: 22036–22043 [DOI] [PubMed] [Google Scholar]
- 56. Shaw GC, Cope JJ, Li L, Corson K, Hersey C, Ackermann GE, Gwynn B, Lambert AJ, Wingert RA, Traver D et al (2006) Mitoferrin is essential for erythroid iron assimilation. Nature 440: 96–100 [DOI] [PubMed] [Google Scholar]
- 57. Li FY, Nikali K, Gregan J, Leibiger I, Leibiger B, Schweyen R, Larsson C, Suomalainen A (2001) Characterization of a novel human putative mitochondrial transporter homologous to the yeast mitochondrial RNA splicing proteins 3 and 4. FEBS Lett 494: 79–84 [DOI] [PubMed] [Google Scholar]
- 58. Braymer JJ, Lill R (2017) Iron‐sulfur cluster biogenesis and trafficking in mitochondria. J Biol Chem 292: 12754–12763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li K, Warner CK, Hodge JA, Minoshima S, Kudoh J, Fukuyama R, Maekawa M, Shimizu Y, Shimizu N, Wallace DC (1989) A human muscle adenine nucleotide translocator gene has four exons, is located on chromosome 4, and is differentially expressed. J Biol Chem 264: 13998–14004 [PubMed] [Google Scholar]
- 60. Pfaff E, Heldt HW, Klingenberg M (1969) Adenine nucleotide translocation of mitochondria. Kinetics of the adenine nucleotide exchange. Eur J Biochem 10: 484–493 [DOI] [PubMed] [Google Scholar]
- 61. Pfaff E, Klingenberg M (1968) Adenine nucleotide translocation of mitochondria. 1. Specificity and control. Eur J Biochem 6: 66–79 [DOI] [PubMed] [Google Scholar]
- 62. Duee ED, Vignais PV (1969) Kinetics and specificity of the adenine nucleotide translocation in rat liver mitochondria. J Biol Chem 244: 3920–3931 [PubMed] [Google Scholar]
- 63. Thompson K, Majd H, Dallabona C, Reinson K, King MS, Alston CL, He L, Lodi T, Jones SA, Fattal‐Valevski A et al (2016) Recurrent de novo dominant mutations in SLC25A4 cause severe early‐onset mitochondrial disease and loss of mitochondrial DNA copy number. Am J Hum Genet 99: 860–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Deschauer M, Hudson G, Muller T, Taylor RW, Chinnery PF, Zierz S (2005) A novel ANT1 gene mutation with probable germline mosaicism in autosomal dominant progressive external ophthalmoplegia. Neuromuscul Disord 15: 311–315 [DOI] [PubMed] [Google Scholar]
- 65. Komaki H, Fukazawa T, Houzen H, Yoshida K, Nonaka I, Goto Y (2002) A novel D104G mutation in the adenine nucleotide translocator 1 gene in autosomal dominant progressive external ophthalmoplegia patients with mitochondrial DNA with multiple deletions. Ann Neurol 51: 645–648 [DOI] [PubMed] [Google Scholar]
- 66. Napoli L, Bordoni A, Zeviani M, Hadjigeorgiou GM, Sciacco M, Tiranti V, Terentiou A, Moggio M, Papadimitriou A, Scarlato G et al (2001) A novel missense adenine nucleotide translocator‐1 gene mutation in a Greek adPEO family. Neurology 57: 2295–2298 [DOI] [PubMed] [Google Scholar]
- 67. Stepien G, Torroni A, Chung AB, Hodge JA, Wallace DC (1992) Differential expression of adenine nucleotide translocator isoforms in mammalian tissues and during muscle cell differentiation. J Biol Chem 267: 14592–14597 [PubMed] [Google Scholar]
- 68. Brower JV, Rodic N, Seki T, Jorgensen M, Fliess N, Yachnis AT, McCarrey JR, Oh SP, Terada N (2007) Evolutionarily conserved mammalian adenine nucleotide translocase 4 is essential for spermatogenesis. J Biol Chem 282: 29658–29666 [DOI] [PubMed] [Google Scholar]
- 69. Dolce V, Scarcia P, Iacopetta D, Palmieri F (2005) A fourth ADP/ATP carrier isoform in man: identification, bacterial expression, functional characterization and tissue distribution. FEBS Lett 579: 633–637 [DOI] [PubMed] [Google Scholar]
- 70. Fiermonte G, Dolce V, Palmieri F (1998) Expression in Escherichia coli, functional characterization, and tissue distribution of isoforms A and B of the phosphate carrier from bovine mitochondria. J Biol Chem 273: 22782–22787 [DOI] [PubMed] [Google Scholar]
- 71. Dolce V, Fiermonte G, Palmieri F (1996) Tissue‐specific expression of the two isoforms of the mitochondrial phosphate carrier in bovine tissues. FEBS Lett 399: 95–98 [DOI] [PubMed] [Google Scholar]
- 72. Dolce V, Iacobazzi V, Palmieri F, Walker JE (1994) The sequences of human and bovine genes of the phosphate carrier from mitochondria contain evidence of alternatively spliced forms. J Biol Chem 269: 10451–10460 [PubMed] [Google Scholar]
- 73. Mayr JA, Merkel O, Kohlwein SD, Gebhardt BR, Bohles H, Fotschl U, Koch J, Jaksch M, Lochmuller H, Horvath R et al (2007) Mitochondrial phosphate‐carrier deficiency: a novel disorder of oxidative phosphorylation. Am J Hum Genet 80: 478–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wiesenberger G, Link TA, von Ahsen U, Waldherr M, Schweyen RJ (1991) MRS3 and MRS4, two suppressors of mtRNA splicing defects in yeast, are new members of the mitochondrial carrier family. J Mol Biol 217: 23–37 [DOI] [PubMed] [Google Scholar]
- 75. Indiveri C, Iacobazzi V, Giangregorio N, Palmieri F (1998) Bacterial overexpression, purification, and reconstitution of the carnitine/acylcarnitine carrier from rat liver mitochondria. Biochem Biophys Res Commun 249: 589–594 [DOI] [PubMed] [Google Scholar]
- 76. Indiveri C, Tonazzi A, Palmieri F (1990) Identification and purification of the carnitine carrier from rat liver mitochondria. Biochim Biophys Acta 1020: 81–86 [DOI] [PubMed] [Google Scholar]
- 77. Indiveri C, Iacobazzi V, Giangregorio N, Palmieri F (1997) The mitochondrial carnitine carrier protein: cDNA cloning, primary structure and comparison with other mitochondrial transport proteins. Biochem J 321(Pt 3): 713–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Stanley CA, Hale DE, Berry GT, Deleeuw S, Boxer J, Bonnefont JP (1992) Brief report: a deficiency of carnitine‐acylcarnitine translocase in the inner mitochondrial membrane. N Engl J Med 327: 19–23 [DOI] [PubMed] [Google Scholar]
- 79. Bradford NM, McGivan JD (1980) Evidence for the existence of an ornithine/citrulline antiporter in rat liver mitochondria. FEBS Lett 113: 294–298 [DOI] [PubMed] [Google Scholar]
- 80. Indiveri C, Tonazzi A, Palmieri F (1992) Identification and purification of the ornithine/citrulline carrier from rat liver mitochondria. Eur J Biochem 207: 449–454 [DOI] [PubMed] [Google Scholar]
- 81. Indiveri C, Tonazzi A, Stipani I, Palmieri F (1997) The purified and reconstituted ornithine/citrulline carrier from rat liver mitochondria: electrical nature and coupling of the exchange reaction with H+ translocation. Biochem J 327(Pt 2): 349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Camacho JA, Obie C, Biery B, Goodman BK, Hu CA, Almashanu S, Steel G, Casey R, Lambert M, Mitchell GA et al (1999) Hyperornithinaemia‐hyperammonaemia‐homocitrullinuria syndrome is caused by mutations in a gene encoding a mitochondrial ornithine transporter. Nat Genet 22: 151–158 [DOI] [PubMed] [Google Scholar]
- 83. Camacho JA, Rioseco‐Camacho N (2009) The human and mouse SLC25A29 mitochondrial transporters rescue the deficient ornithine metabolism in fibroblasts of patients with the hyperornithinemia‐hyperammonemia‐homocitrullinuria (HHH) syndrome. Pediatr Res 66: 35–41 [DOI] [PubMed] [Google Scholar]
- 84. Camacho JA, Rioseco‐Camacho N, Andrade D, Porter J, Kong J (2003) Cloning and characterization of human ORNT2: a second mitochondrial ornithine transporter that can rescue a defective ORNT1 in patients with the hyperornithinemia‐hyperammonemia‐homocitrullinuria syndrome, a urea cycle disorder. Mol Genet Metab 79: 257–271 [DOI] [PubMed] [Google Scholar]
- 85. Prohl C, Pelzer W, Diekert K, Kmita H, Bedekovics T, Kispal G, Lill R (2001) The yeast mitochondrial carrier Leu5p and its human homologue Graves’ disease protein are required for accumulation of coenzyme A in the matrix. Mol Cell Biol 21: 1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zarrilli R, Oates EL, McBride OW, Lerman MI, Chan JY, Santisteban P, Ursini MV, Notkins AL, Kohn LD (1989) Sequence and chromosomal assignment of a novel cDNA identified by immunoscreening of a thyroid expression library: similarity to a family of mitochondrial solute carrier proteins. Mol Endocrinol 3: 1498–1505 [DOI] [PubMed] [Google Scholar]
- 87. Fiermonte G, Runswick MJ, Walker JE, Palmieri F (1992) Sequence and pattern of expression of a bovine homologue of a human mitochondrial transport protein associated with Grave's disease. DNA Seq 3: 71–78 [DOI] [PubMed] [Google Scholar]
- 88. Guernsey DL, Jiang H, Campagna DR, Evans SC, Ferguson M, Kellogg MD, Lachance M, Matsuoka M, Nightingale M, Rideout A et al (2009) Mutations in mitochondrial carrier family gene SLC25A38 cause nonsyndromic autosomal recessive congenital sideroblastic anemia. Nat Genet 41: 651–653 [DOI] [PubMed] [Google Scholar]
- 89. Nilsson R, Schultz IJ, Pierce EL, Soltis KA, Naranuntarat A, Ward DM, Baughman JM, Paradkar PN, Kingsley PD, Culotta VC et al (2009) Discovery of genes essential for heme biosynthesis through large‐scale gene expression analysis. Cell Metab 10: 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Haitina T, Lindblom J, Renstrom T, Fredriksson R (2006) Fourteen novel human members of mitochondrial solute carrier family 25 (SLC25) widely expressed in the central nervous system. Genomics 88: 779–790 [DOI] [PubMed] [Google Scholar]
- 91. Slabbaert JR, Kuenen S, Swerts J, Maes I, Uytterhoeven V, Kasprowicz J, Fernandes AC, Blust R, Verstreken P (2016) Shawn, the Drosophila homolog of SLC25A39/40, is a mitochondrial carrier that promotes neuronal survival. J Neurosci 36: 1914–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rosenthal EA, Ranchalis J, Crosslin DR, Burt A, Brunzell JD, Motulsky AG, Nickerson DA, Project NGES, Wijsman EM, Jarvik GP (2013) Joint linkage and association analysis with exome sequence data implicates SLC25A40 in hypertriglyceridemia. Am J Hum Genet 93: 1035–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Durmaz B, Wollnik B, Cogulu O, Li Y, Tekgul H, Hazan F, Ozkinay F (2009) Pontocerebellar hypoplasia type III (CLAM): extended phenotype and novel molecular findings. J Neurol 256: 416–419 [DOI] [PubMed] [Google Scholar]
- 94. Janer A, Prudent J, Paupe V, Fahiminiya S, Majewski J, Sgarioto N, Des Rosiers C, Forest A, Lin ZY, Gingras AC et al (2016) SLC25A46 is required for mitochondrial lipid homeostasis and cristae maintenance and is responsible for Leigh syndrome. EMBO Mol Med 8: 1019–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li Z, Peng Y, Hufnagel RB, Hu YC, Zhao C, Queme LF, Khuchua Z, Driver AM, Dong F, Lu QR et al (2017) Loss of SLC25A46 causes neurodegeneration by affecting mitochondrial dynamics and energy production in mice. Hum Mol Genet 26: 3776–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yang L, Slone J, Li Z, Lou X, Hu YC, Queme LF, Jankowski MP, Huang T (2020) Systemic administration of AAV‐Slc25a46 mitigates mitochondrial neuropathy in Slc25a46‐/‐ mice. Hum Mol Genet 29: 649–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Abrams AJ, Hufnagel RB, Rebelo A, Zanna C, Patel N, Gonzalez MA, Campeanu IJ, Griffin LB, Groenewald S, Strickland AV et al (2015) Mutations in SLC25A46, encoding a UGO1‐like protein, cause an optic atrophy spectrum disorder. Nat Genet 47: 926–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Steffen J, Vashisht AA, Wan J, Jen JC, Claypool SM, Wohlschlegel JA, Koehler CM (2017) Rapid degradation of mutant SLC25A46 by the ubiquitin‐proteasome system results in MFN1/2‐mediated hyperfusion of mitochondria. Mol Biol Cell 28: 600–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bahat A, Goldman A, Zaltsman Y, Khan DH, Halperin C, Amzallag E, Krupalnik V, Mullokandov M, Silberman A, Erez A et al (2018) MTCH2‐mediated mitochondrial fusion drives exit from naive pluripotency in embryonic stem cells. Nat Commun 9: 5132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ruggiero A, Aloni E, Korkotian E, Zaltsman Y, Oni‐Biton E, Kuperman Y, Tsoory M, Shachnai L, Levin‐Zaidman S, Brenner O et al (2017) Loss of forebrain MTCH2 decreases mitochondria motility and calcium handling and impairs hippocampal‐dependent cognitive functions. Sci Rep 7: 44401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wilkens S (2015) Structure and mechanism of ABC transporters. F1000Prime Rep 7: 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rees DC, Johnson E, Lewinson O (2009) ABC transporters: the power to change. Nat Rev Mol Cell Biol 10: 218–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Srinivasan V, Pierik AJ, Lill R (2014) Crystal structures of nucleotide‐free and glutathione‐bound mitochondrial ABC transporter Atm1. Science 343: 1137–1140 [DOI] [PubMed] [Google Scholar]
- 104. Kispal G, Csere P, Guiard B, Lill R (1997) The ABC transporter Atm1p is required for mitochondrial iron homeostasis. FEBS Lett 418: 346–350 [DOI] [PubMed] [Google Scholar]
- 105. Dawson RJ, Locher KP (2006) Structure of a bacterial multidrug ABC transporter. Nature 443: 180–185 [DOI] [PubMed] [Google Scholar]
- 106. Li J, Cowan JA (2015) Glutathione‐coordinated [2Fe‐2S] cluster: a viable physiological substrate for mitochondrial ABCB7 transport. Chem Commun (Camb) 51: 2253–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Allikmets R, Raskind WH, Hutchinson A, Schueck ND, Dean M, Koeller DM (1999) Mutation of a putative mitochondrial iron transporter gene (ABC7) in X‐linked sideroblastic anemia and ataxia (XLSA/A). Hum Mol Genet 8: 743–749 [DOI] [PubMed] [Google Scholar]
- 108. Boultwood J, Pellagatti A, Nikpour M, Pushkaran B, Fidler C, Cattan H, Littlewood TJ, Malcovati L, Della Porta MG, Jadersten M et al (2008) The role of the iron transporter ABCB7 in refractory anemia with ring sideroblasts. PLoS ONE 3: e1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ichikawa Y, Bayeva M, Ghanefar M, Potini V, Sun L, Mutharasan RK, Wu R, Khechaduri A, Jairaj Naik T, Ardehali H (2012) Disruption of ATP‐binding cassette B8 in mice leads to cardiomyopathy through a decrease in mitochondrial iron export. Proc Natl Acad Sci USA 109: 4152–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hogue DL, Liu L, Ling V (1999) Identification and characterization of a mammalian mitochondrial ATP‐binding cassette membrane protein. J Mol Biol 285: 379–389 [DOI] [PubMed] [Google Scholar]
- 111. Ardehali H, Xue T, Dong P, Machamer C (2005) Targeting of the mitochondrial membrane proteins to the cell surface for functional studies. Biochem Biophys Res Commun 338: 1143–1151 [DOI] [PubMed] [Google Scholar]
- 112. Paggio A, Checchetto V, Campo A, Menabo R, Di Marco G, Di Lisa F, Szabo I, Rizzuto R, De Stefani D (2019) Identification of an ATP‐sensitive potassium channel in mitochondria. Nature 572: 609–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Seguin A, Takahashi‐Makise N, Yien YY, Huston NC, Whitman JC, Musso G, Wallace JA, Bradley T, Bergonia HA, Kafina MD et al (2017) Reductions in the mitochondrial ABC transporter Abcb10 affect the transcriptional profile of heme biosynthesis genes. J Biol Chem 292: 16284–16299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Shirihai OS, Gregory T, Yu C, Orkin SH, Weiss MJ (2000) ABC‐me: a novel mitochondrial transporter induced by GATA‐1 during erythroid differentiation. EMBO J 19: 2492–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Zhang F, Hogue DL, Liu L, Fisher CL, Hui D, Childs S, Ling V (2000) M‐ABC2, a new human mitochondrial ATP‐binding cassette membrane protein. FEBS Lett 478: 89–94 [DOI] [PubMed] [Google Scholar]
- 116. Shintre CA, Pike AC, Li Q, Kim JI, Barr AJ, Goubin S, Shrestha L, Yang J, Berridge G, Ross J et al (2013) Structures of ABCB10, a human ATP‐binding cassette transporter in apo‐ and nucleotide‐bound states. Proc Natl Acad Sci USA 110: 9710–9715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Chen W, Paradkar PN, Li L, Pierce EL, Langer NB, Takahashi‐Makise N, Hyde BB, Shirihai OS, Ward DM, Kaplan J et al (2009) Abcb10 physically interacts with mitoferrin‐1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc Natl Acad Sci USA 106: 16263–16268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Haffke M, Menzel A, Carius Y, Jahn D, Heinz DW (2010) Structures of the nucleotide‐binding domain of the human ABCB6 transporter and its complexes with nucleotides. Acta Crystallogr D Biol Crystallogr 66: 979–987 [DOI] [PubMed] [Google Scholar]
- 119. Paterson JK, Shukla S, Black CM, Tachiwada T, Garfield S, Wincovitch S, Ernst DN, Agadir A, Li X, Ambudkar SV et al (2007) Human ABCB6 localizes to both the outer mitochondrial membrane and the plasma membrane. Biochemistry 46: 9443–9452 [DOI] [PubMed] [Google Scholar]
- 120. Andolfo I, Alper SL, Delaunay J, Auriemma C, Russo R, Asci R, Esposito MR, Sharma AK, Shmukler BE, Brugnara C et al (2013) Missense mutations in the ABCB6 transporter cause dominant familial pseudohyperkalemia. Am J Hematol 88: 66–72 [DOI] [PubMed] [Google Scholar]
- 121. Kiss K, Brozik A, Kucsma N, Toth A, Gera M, Berry L, Vallentin A, Vial H, Vidal M, Szakacs G (2012) Shifting the paradigm: the putative mitochondrial protein ABCB6 resides in the lysosomes of cells and in the plasma membrane of erythrocytes. PLoS ONE 7: e37378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Zhang C, Li D, Zhang J, Chen X, Huang M, Archacki S, Tian Y, Ren W, Mei A, Zhang Q et al (2013) Mutations in ABCB6 cause dyschromatosis universalis hereditaria. J Invest Dermatol 133: 2221–2228 [DOI] [PubMed] [Google Scholar]
- 123. Mitsuhashi N, Miki T, Senbongi H, Yokoi N, Yano H, Miyazaki M, Nakajima N, Iwanaga T, Yokoyama Y, Shibata T et al (2000) MTABC3, a novel mitochondrial ATP‐binding cassette protein involved in iron homeostasis. J Biol Chem 275: 17536–17540 [DOI] [PubMed] [Google Scholar]
- 124. Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa‐Pineda B, Murti KG, Schuetz JD (2006) Identification of a mammalian mitochondrial porphyrin transporter. Nature 443: 586–589 [DOI] [PubMed] [Google Scholar]
- 125. Fukuda Y, Cheong PL, Lynch J, Brighton C, Frase S, Kargas V, Rampersaud E, Wang Y, Sankaran VG, Yu B et al (2016) The severity of hereditary porphyria is modulated by the porphyrin exporter and Lan antigen ABCB6. Nat Commun 7: 12353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Helias V, Saison C, Ballif BA, Peyrard T, Takahashi J, Takahashi H, Tanaka M, Deybach JC, Puy H, Le Gall M et al (2012) ABCB6 is dispensable for erythropoiesis and specifies the new blood group system Langereis. Nat Genet 44: 170–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Peyrard T (2013) The LAN blood group system: a review. Immunohematology 29: 131–135 [PubMed] [Google Scholar]
- 128. Ulrich DL, Lynch J, Wang Y, Fukuda Y, Nachagari D, Du G, Sun D, Fan Y, Tsurkan L, Potter PM et al (2012) ATP‐dependent mitochondrial porphyrin importer ABCB6 protects against phenylhydrazine toxicity. J Biol Chem 287: 12679–12690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N et al (2012) A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 337: 96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ER, Martinou JC (2012) Identification and functional expression of the mitochondrial pyruvate carrier. Science 337: 93–96 [DOI] [PubMed] [Google Scholar]
- 131. Tavoulari S, Thangaratnarajah C, Mavridou V, Harbour ME, Martinou JC, Kunji ER (2019) The yeast mitochondrial pyruvate carrier is a hetero‐dimer in its functional state. EMBO J 38: e100785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Papa S, Guerrieri F, Lorusso M, Quagliariello E (1970) On the proton translocation system of the inner mitochondrial membrane. FEBS Lett 10: 295–298 [DOI] [PubMed] [Google Scholar]
- 133. Halestrap AP (1978) Stimulation of pyruvate transport in metabolizing mitochondria through changes in the transmembrane pH gradient induced by glucagon treatment of rats. Biochem J 172: 389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Halestrap AP (1978) Pyruvate and ketone‐body transport across the mitochondrial membrane. Exchange properties, pH‐dependence and mechanism of the carrier. Biochem J 172: 377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Brivet M, Garcia‐Cazorla A, Lyonnet S, Dumez Y, Nassogne MC, Slama A, Boutron A, Touati G, Legrand A, Saudubray JM (2003) Impaired mitochondrial pyruvate importation in a patient and a fetus at risk. Mol Genet Metab 78: 186–192 [DOI] [PubMed] [Google Scholar]
- 136. Oonthonpan L, Rauckhorst AJ, Gray LR, Boutron AC, Taylor EB (2019) Two human patient mitochondrial pyruvate carrier mutations reveal distinct molecular mechanisms of dysfunction. JCI Insight 4: e126132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bensard CL, Wisidagama DR, Olson KA, Berg JA, Krah NM, Schell JC, Nowinski SM, Fogarty S, Bott AJ, Wei P et al (2020) Regulation of tumor initiation by the mitochondrial pyruvate carrier. Cell Metab 31: 284–300.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, Sudderth J, Calvaruso MA, Lumata L, Mitsche M et al (2014) Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell 56: 414–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Vacanti NM, Divakaruni AS, Green CR, Parker SJ, Henry RR, Ciaraldi TP, Murphy AN, Metallo CM (2014) Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol Cell 56: 425–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sharma A, Oonthonpan L, Sheldon RD, Rauckhorst AJ, Zhu Z, Tompkins SC, Cho K, Grzesik WJ, Gray LR, Scerbo DA et al (2019) Impaired skeletal muscle mitochondrial pyruvate uptake rewires glucose metabolism to drive whole‐body leanness. Elife 8: e45873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Gray LR, Sultana MR, Rauckhorst AJ, Oonthonpan L, Tompkins SC, Sharma A, Fu X, Miao R, Pewa AD, Brown KS et al (2015) Hepatic mitochondrial pyruvate carrier 1 is required for efficient regulation of gluconeogenesis and whole‐body glucose homeostasis. Cell Metab 22: 669–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. McCommis KS, Chen Z, Fu X, McDonald WG, Colca JR, Kletzien RF, Burgess SC, Finck BN (2015) Loss of mitochondrial pyruvate carrier 2 in the liver leads to defects in gluconeogenesis and compensation via pyruvate‐alanine cycling. Cell Metab 22: 682–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Vadvalkar SS, Matsuzaki S, Eyster CA, Giorgione JR, Bockus LB, Kinter CS, Kinter M, Humphries KM (2017) Decreased mitochondrial pyruvate transport activity in the diabetic heart: role of mitochondrial pyruvate carrier 2 (MPC2) acetylation. J Biol Chem 292: 4423–4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Liang L, Li Q, Huang L, Li D, Li X (2015) Sirt3 binds to and deacetylates mitochondrial pyruvate carrier 1 to enhance its activity. Biochem Biophys Res Commun 468: 807–812 [DOI] [PubMed] [Google Scholar]
- 145. Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 105: 14447–14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Schell JC, Olson KA, Jiang L, Hawkins AJ, Van Vranken JG, Xie J, Egnatchik RA, Earl EG, DeBerardinis RJ, Rutter J (2014) A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell 56: 400–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM (2004) ONCOMINE: a cancer microarray database and integrated data‐mining platform. Neoplasia 6: 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Cancer Genome Atlas N (2012) Comprehensive molecular characterization of human colon and rectal cancer. Nature 487: 330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Liberti MV, Locasale JW (2016) The Warburg effect: how does it benefit cancer cells? Trends Biochem Sci 41: 211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Chai Y, Wang C, Liu W, Fan Y, Zhang Y (2019) MPC1 deletion is associated with poor prognosis and temozolomide resistance in glioblastoma. J Neurooncol 144: 293–301 [DOI] [PubMed] [Google Scholar]
- 151. Tang XP, Chen Q, Li Y, Wang Y, Zou HB, Fu WJ, Niu Q, Pan QG, Jiang P, Xu XS et al (2019) Mitochondrial pyruvate carrier 1 functions as a tumor suppressor and predicts the prognosis of human renal cell carcinoma. Lab Invest 99: 191–199 [DOI] [PubMed] [Google Scholar]
- 152. Tompkins SC, Sheldon RD, Rauckhorst AJ, Noterman MF, Solst SR, Buchanan JL, Mapuskar KA, Pewa AD, Gray LR, Oonthonpan L et al (2019) Disrupting mitochondrial pyruvate uptake directs glutamine into the TCA cycle away from glutathione synthesis and impairs hepatocellular tumorigenesis. Cell Rep 28: 2608–2619.e2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Bader DA, Hartig SM, Putluri V, Foley C, Hamilton MP, Smith EA, Saha PK, Panigrahi A, Walker C, Zong L et al (2019) Mitochondrial pyruvate import is a metabolic vulnerability in androgen receptor‐driven prostate cancer. Nat Metab 1: 70–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Kory N, Wyant GA, Prakash G, Uit de Bos J, Bottanelli F, Pacold ME, Chan SH, Lewis CA, Wang T, Keys HR et al (2018) SFXN1 is a mitochondrial serine transporter required for one‐carbon metabolism. Science 362: eaat9528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Fleming MD, Campagna DR, Haslett JN, Trenor CC 3rd, Andrews NC (2001) A mutation in a mitochondrial transmembrane protein is responsible for the pleiotropic hematological and skeletal phenotype of flexed‐tail (f/f) mice. Genes Dev 15: 652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Rivell A, Petralia RS, Wang YX, Mattson MP, Yao PJ (2019) Sideroflexin 3 is a mitochondrial protein enriched in neurons. Neuromolecular Med 21: 314–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Hildick‐Smith GJ, Cooney JD, Garone C, Kremer LS, Haack TB, Thon JN, Miyata N, Lieber DS, Calvo SE, Akman HO et al (2013) Macrocytic anemia and mitochondriopathy resulting from a defect in sideroflexin 4. Am J Hum Genet 93: 906–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Divakaruni AS, Wallace M, Buren C, Martyniuk K, Andreyev AY, Li E, Fields JA, Cordes T, Reynolds IJ, Bloodgood BL et al (2017) Inhibition of the mitochondrial pyruvate carrier protects from excitotoxic neuronal death. J Cell Biol 216: 1091–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhou X, Paredes JA, Krishnan S, Curbo S, Karlsson A (2015) The mitochondrial carrier SLC25A10 regulates cancer cell growth. Oncotarget 6: 9271–9283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Majd H, King MS, Palmer SM, Smith AC, Elbourne LD, Paulsen IT, Sharples D, Henderson PJ, Kunji ER (2018) Screening of candidate substrates and coupling ions of transporters by thermostability shift assays. Elife 7: e38821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Huber KV, Olek KM, Muller AC, Tan CS, Bennett KL, Colinge J, Superti‐Furga G (2015) Proteome‐wide drug and metabolite interaction mapping by thermal‐stability profiling. Nat Methods 12: 1055–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Nji E, Chatzikyriakidou Y, Landreh M, Drew D (2018) An engineered thermal‐shift screen reveals specific lipid preferences of eukaryotic and prokaryotic membrane proteins. Nat Commun 9: 4253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Yoo HC, Park SJ, Nam M, Kang J, Kim K, Yeo JH, Kim JK, Heo Y, Lee HS, Lee MY et al (2020) A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab 31: 267–283.e212 [DOI] [PubMed] [Google Scholar]


