Summary
Aim: As antibiotics are generally not recommended for the treatment of acute sore throat, the availability of clinically efficacious, over‐the‐counter (OTC) treatment alternatives is becoming increasingly important. This study was designed to determine the analgesic properties of amylmetacresol and 2,4‐dichlorobenzyl alcohol (AMC/DCBA) throat lozenges (Strepsils®) in the relief of acute sore throat caused by upper respiratory tract infections.
Methods: Patients (n = 310) were randomly assigned to receive AMC/DCBA throat lozenges (n = 155) or non‐medicated placebo lozenges (n = 155). After baseline assessments, patients completed three rating assessments at 10 timepoints from 5 to 20 min after first dose. Subsequent lozenges were taken as required, and assessments were made at the end of Day 1, 24 h after first dose, and at the end of Days 2 and 3. Analgesic properties were assessed by comparing severity of throat soreness and sore throat relief ratings. Difficulty in swallowing and functional impairment scores were also assessed.
Results: Amylmetacresol/DCBA throat lozenges reduced throat soreness at 5 min after first dose, which persisted for 2 h and was significantly different vs. non‐medicated lozenges at all assessment timepoints for the duration of the 3‐day study. Similar significant effects were observed with sore throat relief, easing of difficulty with swallowing and functional impairment scores. There were no differences in adverse events reported between treatment groups.
Conclusion: Amylmetacresol/DCBA throat lozenges provide rapid analgesic effects that last for 2 h, providing ongoing relief long after the lozenge has dissolved. The superior analgesic effects and improvements in functional impairment scores observed with AMC/DCBA throat lozenges translate into pain relief benefits that are clinically meaningful and are thus a suitable OTC treatment option for patients in the self‐management of acute sore throat.
What’s known
Owing to the predominantly viral causes of acute sore throats, antibiotics are ineffective and generally not recommended for the treatment of acute sore throat. Therefore, clinically proven over‐the‐counter options for the rapid, safe and effective treatment of sore throat symptoms are becoming increasingly important.
What’s new
This study clearly demonstrates significant analgesic effects and improvements in functional impairment scores with the use of amylmetacresol and 2,4‐dichlorobenzyl alcohol throat lozenges (Strepsils®), over and above the demulcent effects of non‐medicated lozenges, thus rendering these lozenges as a valuable treatment option in the self‐management of acute sore throat.
Introduction
On average, an adult will suffer from a sore throat 2–3 times per year, with children being prone to more frequent episodes of sore throat than adults (1), because of their immunological naivety. Acute sore throat is one of the most common complaints associated with upper respiratory tract infections (URTIs) that result in presentation at general practice surgeries (2) or local pharmacy stores for treatment. Approximately a quarter of the population in England and Wales will consult their physician each year because of a respiratory tract infection (3). Respiratory tract infections that include URTIs and lower respiratory tract infections are the reason for 60% of all antibiotic prescribing in general practice, resulting in significant costs to the healthcare system (4).
Over the years, our understanding of the aetiology of sore throat has diversified beyond bacterial causes. Contrary to commonly held historical beliefs, bacterial infections are not the most common cause of sore throats. The most common bacterial cause, group A β‐haemolytic streptococcus (Streptococcus pyogenes), only account for approximately 20% of all sore throats in adults and young children (5). In fact, up to 80% of sore throats in adults are caused by viruses (5, 6), such as influenza A, respiratory syncytial virus, severe acute respiratory syndrome coronavirus and rhinovirus (7). Thus, antibiotics are generally not suitable for the treatment of acute sore throats (8). Many country guidelines, including the National Institute for Health and Clinical Excellence guidelines in the UK, have advised against the use of antibiotics for minor ailments including acute sore throat, for fear of antibiotic resistance and unnecessary exposure to potential adverse effects (4). A no or delayed antibiotic prescribing strategy was recommended for adults and children, except for those patients who are at high risk of associated complications, such as quinsy (4). Therefore, the availability of over‐the‐counter (OTC) treatments is becoming increasingly important and plays an important role in the self‐management of acute sore throat, especially the availability of clinically efficacious, well‐tolerated, fast‐ and long‐acting products.
There are many OTC treatments available for the self‐management of sore throats, including throat lozenges and other topical treatments, such as pastilles, sprays and gargles, but there is a lack of recent evidence in the literature to support the efficacy of the latter three product types. Although medicated topical treatments act to relieve sore throats primarily by delivering the active ingredients directly to the areas affected, gargles are only able to deliver the active ingredients to the anterior oral cavity and not the palatine tonsils or the pharynx (9). Conversely, medicated throat lozenges have the added advantage over sprays and gargles of being slow‐releasing (10), ensuring continuous delivery of the active ingredients to all affected areas of the throat and over a prolonged period of time (11).
Strepsils® (Reckitt Benckiser Healthcare International, Hull, UK) lozenges are medicated lozenges that contain the antibacterial (12, 13, 14) and antiviral (7) ingredients amylmetacresol (AMC; 0.6 mg) and 2,4‐dichlorobenzyl alcohol (DCBA; 1.2 mg). From here on in this study, Strepsils lozenges will be referred to by its generic name ‘AMC/DCBA throat lozenges’. Several studies exist to support the efficacy and safety of AMC/DCBA throat lozenges in the relief of acute sore throat pain (15, 16, 17, 18, 19). However, many of these studies were performed on small number of patient and were insufficiently powered to draw strong statistical conclusions. In addition, to date, no studies have examined the effect of throat lozenges on everyday activities impaired by sore throat‐related pain/symptoms.
Therefore, this study was conducted in a larger group of patients to further examine the analgesic and functional benefits of AMC/DCBA throat lozenges vs. non‐medicated placebo lozenges in patients with acute sore throat over a period of 3 days.
Methods
Patient selection
Patients with sore throat because of URTIs were screened and enrolled into this study between November 2007 and February 2008 from eight Primary Care Investigational Sites in Northern Ireland. Only patients who met the following inclusion criteria were included in the study: men or women aged between 18 and 75 years; primary diagnosis of sore throat with a recent onset within the past 4 days (i.e. ≤ 4 days) because of URTI; baseline sore throat score of ≥ 6 on the Throat Soreness Scale (TSS); objective findings confirming the presence of tonsillopharyngitis [i.e. ≥ 5 points on the expanded 21‐point Tonsillopharyngitis Assessment (TPA)] (20); and written informed consent for study participation.
Main exclusion criteria included: history of allergy or known intolerance to the study or rescue medication (paracetamol) and their ingredients; sore throat present for more than 4 days; evidence of severe coughing or mouth breathing; history of disease that could compromise breathing; use of any medicated confectionery or any products with demulcent properties, such as boiled sweets, within the previous 2 h; use of any analgesic, antipyretic or ‘cold’ medication within the previous 8 h; and use of a longer‐acting or slow‐release analgesic during the previous 24 h.
Study design
This multicentre, randomised, double‐blind, parallel‐group, placebo‐controlled, multiple‐dose study was conducted in accordance with the Declaration of Helsinki, and complied with International Conference on Harmonisation, Good Clinical Practice and applicable regulatory requirements. Eligible patients were randomised into two treatment groups according to a randomised block design. One group received AMC/DCBA throat lozenges (active lozenges; Strepsils®) and the other group received non‐medicated sugar‐based lozenges (placebo lozenges). The investigators and the patients were blinded to the study treatment. Both lozenge treatments were identical in appearance, with the same colour, size and shape, but AMC/DCBA throat lozenges contained the active ingredients, AMC (0.6 mg) and DCBA (1.2 mg), whereas the non‐medicated placebo lozenges contained only sugar and glucose. Drug supplies were packed and labelled by the Investigational Material Supplies Unit (IMSU), according to a computer‐generated randomisation schedule provided by a statistician not involved with the study analysis.
At screening, patients were allocated a unique patient (screening) number and at randomisation, study patients were allocated a randomisation number in numerical sequence. Treatment allocation was performed on a by‐centre basis.
The IMSU and statistician held the master randomisation list. Investigators were supplied with the randomisation code for each of their patients as code break envelopes that were only broken in the event of an emergency.
Objectives
The primary objective of this study was to determine the analgesic properties of AMC/DCBA throat lozenges compared with non‐medicated lozenges, in patients with sore throat because of URTIs.
The secondary objectives were to determine any additional patient benefits provided by active lozenges compared with placebo lozenges, including the effects on functional impairment scores and the type of relief experienced within the mouth.
Efficacy end‐points
The primary efficacy end‐point for this study was the change from baseline in severity of throat soreness for the AMC/DCBA throat lozenges group vs. the non‐medicated lozenges group at 2 h after first dose.
The secondary efficacy end‐points were:
-
•
Area under the curve (AUC) from baseline to 2 h for the change from baseline in throat soreness;
-
•
The change from baseline in severity of throat soreness at the end of Day 1, at 24 h after first dose and at the end of Days 2 and 3;
-
•
Sore throat relief at 2 h after first dose and at the end of Day 1, at 24 h after first dose and at the end of Days 2 and 3;
-
•
Total sum of pain relief ratings: AUC from baseline to 2 h after first dose for sore throat relief;
-
•
Onset of analgesia, defined as time first to report ‘moderate pain relief’ (i.e. the mid‐point on the seven‐point sore throat relief scale);
-
•
The change from baseline in difficulty in swallowing at 2 h after first dose and at the end of Day 1, at 24 h after first dose and at the end of Days 2 and 3;
-
•
AUC from baseline to 2 h for the change from baseline in difficulty in swallowing;
-
•
The number of patients who were symptom free at the end of Day 1, at 24 h after first dose and at the end of Days 2 and 3;
-
•
Overall treatment rating at 2 h and at the end of Day 3;
-
•
Overall lozenge consumption as recorded in the patient diary up to the end of Day 3;
-
•
Overall rescue medication (paracetamol) consumption as recorded in the patient diary up to the end of Day 3;
-
•
Responses to consumer questionnaire relating to patients’ opinions on pain relief, what the relief felt like and how their sore throat affects their daily activities;
-
•
The overall proportion of patients with adverse events (AEs) and serious AEs, i.e. safety and tolerability.
Assessments
At screening, oral temperature, size of tonsils, oropharyngeal colour, number of oropharyngeal enanthems, and size, number and tenderness of the anterior cervical lymph nodes were scored 0–3 according to an expanded TPA. Demographical information, including gender, race, age, height and weight, in addition to medical history, current medical status and concomitant medication were also collected.
At baseline assessment, patients assessed how sore their throat was (using the 11‐point TSS, which ranged from 0 = not sore to 10 = very sore) and recorded difficulty in swallowing [using a horizontal 100 mm visual analogue scale (VAS), which ranged from 0 = not difficult to 100 = very difficult]. Under supervision within the investigative sites, patients took the first of their randomly assigned test lozenge, sucking it slowly until it had dissolved without chewing or crunching. Patients completed self‐assessments of throat soreness, sore throat relief (using a sore throat relief scale: a seven‐point category scale, ranging from 0 = no relief to 6 = complete relief) and difficulty in swallowing at the following timepoints: 5, 10, 15, 30, 45, 60, 75, 90, 105, 120 min after first dose, at the end of Day 1, 24 h after first dose, at the end of Days 2 and 3. During the initial 2‐h period, patients complied with ‘nil‐by‐mouth’ and smoking cessation requirements. At 2 h after first dose and at the end of Day 3, patients also completed the overall treatment rating on an 11‐point ordinal scale ranging from 0 = poor to 10 = excellent.
After completion of the initial 2‐h assessment, patients were given enough study medication for the duration of the study period (one lozenge every 2–3 h, as required), rescue medication (paracetamol 500 mg tablets) and patient diaries to take home. Patients could take paracetamol, two tablets, up to four times a day as required, but paracetamol‐containing products were contraindicated during the study.
Patient diaries were completed at the end of Day 1, at 24 h after first dose and at the end of Days 2 and 3, and returned along with any unused lozenges and rescue medication within 7 days after the first dose of the lozenge was taken. The number of patients reporting freedom from symptoms was also explored, defined as complete sore throat relief (on a seven‐point scale, where 0 = no relief and 6 = complete relief) and scoring either 0 or 1 on the TSS (using an 11‐point scale, where 0 = not sore and 10 = very sore) at various timepoints over the course of 3 days.
Treatment compliance with first lozenge administration was monitored by site‐staff mouth inspections, and overall treatment compliance was determined by counting the medication returned at the end of the study and checked against patient diaries, which documented the time and date when each lozenge was taken.
During the study, patients also completed a two‐part consumer questionnaire consisting of 16 questions. The first part of the questionnaire relating to pain relief was completed at 5 min and other pain relief questions completed at 2 h after first dose. The second part of the consumer questionnaire relating to functional impairment was completed at baseline and repeated at the end of Day 3 using an 11‐point functional impairment scale. To minimise variability in the application of the questionnaire, the study nurse or Investigator at each site instructed the patients on how to complete the questionnaire according to a script.
Any spontaneously reported AEs were recorded, with any ongoing AEs followed up. Changes in concomitant medication, study medication discontinuation and reasons for discontinuation were recorded at the follow‐up assessment, which took place between 1 and 4 days after Day 3.
Sample size
The sample size of the study was predetermined using results from an earlier preliminary study conducted in 1996 (19). In that earlier study, the difference between AMC/DCBA throat lozenges and non‐medicated lozenges in the mean change from baseline in the severity of throat soreness at 2 h was 0.7 ± 1.9. Sample size calculations were based on the assumption that the variability in the mean change from baseline in the severity of throat soreness in this study was similar in magnitude. Therefore, 155 patients were needed per group to provide 90% power to detect a statistically significant difference in mean change from baseline of 0.7.
Statistical analyses
All statistical tests were performed using a two‐tailed 5% overall significance level. The null hypothesis at all times was that the two treatments were equivalent. The primary end‐point was analysed by analysis of covariance (ANCOVA) with baseline throat soreness severity as a covariate and factors for the treatment group and centre. Centres recruiting less than eight subjects were pooled for analysis purposes. Treatment group differences were estimated from the ANCOVA model. All other efficacy end‐points and the supportive analyses were considered as descriptive evidence of efficacy and were analysed without any procedures to account for multiple comparisons.
The changes from baseline in difficulty in swallowing at 2 hours post first dose, at the end of Day 1, at 24 hours post first dose and at the end of Days 2 and 3 were analysed by ANCOVA with factors for treatment group, centre and covariates for the baseline value from difficulty in swallowing and baseline throat soreness severity. The AUC for change from baseline to 2 hours post first dose in difficulty in swallowing was similarly analysed.
All other secondary variables were analysed using the same ANCOVA model as for the primary end‐point. Differences between treatment groups in the proportion of patients reporting treatment‐emergent AEs were compared using the chi‐square test.
The number of patients who reported being free from symptoms was compared between treatment groups using a logistic regression model with factors for treatment group and centre, and a continuous covariate for baseline throat soreness severity.
For the questionnaire survey, questions with binary responses were analysed using a logistic regression model with factors for treatment group and centre, and a covariate for baseline throat soreness severity. Questions with non‐binary responses were analysed by ANCOVA with the same factors as for the binary data. The change from predose to the end of Day 3 in the functional impairment scale (each component and overall total score) was analysed by ANCOVA with factors for treatment group, centre and covariates for the baseline throat soreness and the relevant baseline functional impairment score.
Results
Participant flow/recruitment
From a total of 314 patients who were screened, 310 patients were enrolled into the study and randomised to receive AMC/DCBA throat lozenges (n = 155) or non‐medicated lozenges (n = 155) (Figure 1). Three patients withdrew from the study in the AMC/DCBA throat lozenges group, one because of an AE (mouth ulcer), one was lost to follow‐up and the third withdrew for other reasons (the patient could not stay in the clinic and only provided data up to the 45‐min assessment and withdrew 1 h postdosing). Two patients withdrew from the study in the placebo group, both because of AEs (i.e. vomiting and an increase in severity of throat soreness after first dose).
Figure 1.
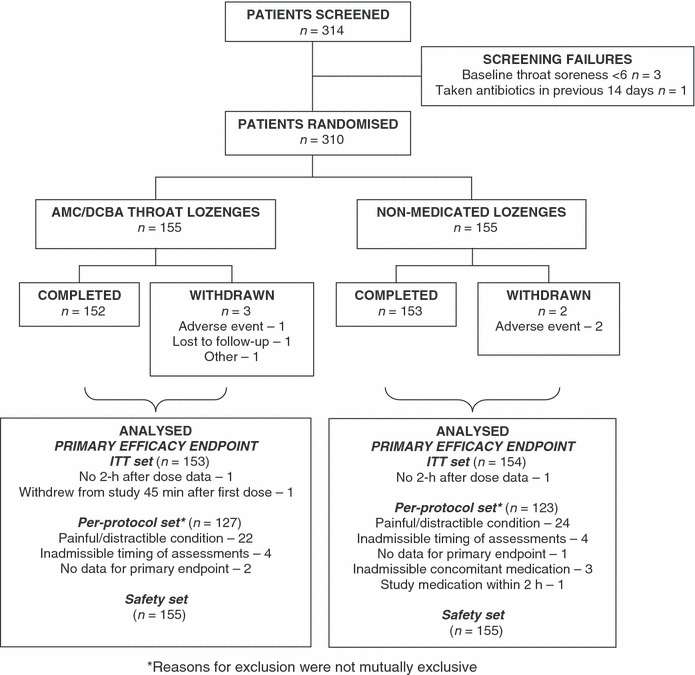
Patient flow for selection, randomisation and analysis
Baseline data and medical history
Baseline demographic data (Table 1) and medical histories of patients were generally well matched between the two treatment groups. The mean age of all patients was 36.1 ± 14.1 years (range 18–76 years). At screening, the mean TPA score in both treatment groups was similar [i.e. 8.8 ± 2.8 (standard deviation; SD) and 9.1 ± 2.6 in the AMC/DCBA throat lozenge and non‐medicated lozenge group respectively]. The mean throat soreness score in each treatment group was 7.1 ± 1.0 and 7.2 ± 1.1 respectively, and the mean difficulty in swallowing score was 62.6 ± 19.6 and 62.5 ± 20.3 respectively.
Table 1.
Baseline demographical data – ITT population
| AMC/DCBA throat lozenges | Non‐medicated lozenges | Overall | |
|---|---|---|---|
| Number of patients (n) | 155 | 155 | 310 |
| Age, years [mean (SD)] | 36.3 (14.0) | 35.9 (14.2) | 36.1 (14.1) |
| Gender (% men) | 32.3 | 32.3 | 32.3 |
| Race (% Caucasian) | 100.0 | 95.5 | 97.7 |
| Height, cm [mean (SD)] | 167.3 (9.2) | 167.7 (9.0) | 167.5 (9.1) |
| Weight, kg [mean (SD)] | 74.5 (16.5) | 77.0 (18.9) | 75.7 (17.8) |
| BMI, kg/m2 [mean (SD)] | 26.6 (5.4) | 27.3 (6.0) | 26.9 (5.7) |
AMC/DCBA, amylmetacresol and 2,4‐dichlorobenzyl alcohol; BMI, body mass index; ITT, intention‐to‐treat; SD, standard deviation.
Twenty‐eight percentage of patients reported a previous medical condition and 56% of patients reported an ongoing medical condition (21% had psychiatric conditions and 21% had gastrointestinal conditions).
Data set analyses
Three analysis sets were used in the data analyses of this study (Figure 1). The ‘intention‐to‐treat (ITT) set’ consisted of all patients who were randomised to the study and who took at least one dose of study medication. The ‘per‐protocol (PP) set’ consisted of all patients who satisfied all of the inclusion/exclusion criteria and who correctly received the treatment to which they were randomised and successfully completed the assessments up to the 2 h after first dose. Therefore, the only variables assessed using the PP set were the primary efficacy end‐point, the AUC from baseline to 2 h after first dose for pain relief and the AUC for the change from baseline in throat soreness. The ‘safety set’ consisted of all patients who took at least one dose of study medication and was analysed as treated.
Efficacy data
Primary outcome
The primary efficacy end‐point was significantly different in the AMC/DCBA throat lozenges group compared with the non‐medicated lozenges group. The difference between least squares (LS) mean values for change from baseline in severity of throat soreness at 2 h after first dose was −1.21 [95% confidence interval (CI): −1.59, −0.82; p < 0.0001 for AMC/DCBA throat lozenges vs. non‐medicated lozenges group; ITT population; Table 2]. The statistical conclusions for the PP population were qualitatively identical to the ITT population [i.e. difference between LS mean values −1.01 (95% CI: −1.38, −0.63); p < 0.0001 for active vs. placebo group]. This was true for the other efficacy measures assessed using the PP data set, and so only the ITT population data will be discussed from here on in this study.
Table 2.
Effect of AMC/DCBA throat lozenges vs. non‐medicated lozenges on various parameters over the 3‐day study period – ITT population
| 2 h after first dose | End of Day 1 | 24 h after first dose | End of Day 2 | End of Day 3 | |
|---|---|---|---|---|---|
| Severity of throat soreness (measured on a 11‐point scale where 0 = not sore, 10 = very sore) | |||||
| AMC/DCBA throat lozenges (n = 147–153) | |||||
| LS mean | −2.06 | −1.57 | −2.43 | −3.06 | −4.02 |
| Non‐medicated lozenges (n = 150–154) | |||||
| LS mean | −0.85 | −0.74 | −1.17 | −1.61 | −2.15 |
| Difference between LS mean* (95% CI) | −1.21 (−1.59, −0.82) | −0.83 (−1.21, −0.46) | −1.25 (−1.69, −0.82) | −1.45 (−1.93, −0.96) | −1.87 (−2.4, −1.34) |
| p‐Value for treatment† | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| Sore throat relief (measured on a 7‐point scale where 0 = No relief, 1 = Slight relief, 2 = Mild relief, 3 = Moderate relief, 4 = Considerable relief, 5 = Almost complete relief, 6 = Complete relief) | |||||
| AMC/DCBA throat lozenges (n = 147–153) | |||||
| LS mean | 1.93 | 1.97 | 2.42 | 2.84 | 3.37 |
| Non‐medicated lozenges (n = 150–154) | |||||
| LS mean | 0.84 | 1.01 | 1.28 | 1.49 | 1.79 |
| Difference between LS mean‡ (95% CI) | 1.09 (0.78, 1.40) | 0.95 (0.67, 1.23) | 1.14 (0.81, 1.48) | 1.35 (0.97, 1.73) | 1.58 (1.15, 2.01) |
| p‐Value for treatment† | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 |
| Difficulty in swallowing (measured on a 100 mm VAS, where 0 mm = Not difficult, 100 mm = Very difficult) | |||||
| AMC/DCBA throat lozenges (n = 142–150) | |||||
| LS mean | −15.0 | −10.7 | −17.9 | −23.5 | −33.1 |
| Non‐medicated lozenges (n = 147–150) | |||||
| LS mean | −3.8 | −3.8 | −8.9 | −11.6 | −15.9 |
| Difference between LS mean* (95% CI) | −11.1 (−15.0, −7.3) | −6.9 (−10.6, −3.3) | −11.9 (−16.7, −7.1) | −11.9 (−16.7, −7.1) | −17.2 (−22.4, −12.0) |
| p‐Value for treatment¶ | < 0.0001 | = 0.0002 | < 0.0001 | < 0.0001 | < 0.0001 |
*AMC/DCBA throat lozenges minus non‐medicated lozenges. A negative difference favours AMC/DCBA throat lozenges. †Estimated from ANCOVA model with factors for treatment and centre and a covariate for baseline throat soreness. ‡AMC/DCBA throat lozenges minus non‐medicated lozenges. A positive difference favours AMC/DCBA throat lozenges. §Estimated from ANCOVA model with factors for treatment and centre and covariates for baseline throat soreness and baseline score for difficulty in swallowing. AMC/DCBA, amylmetacresol and 2,4‐dichlorobenzyl alcohol; ANCOVA, analysis of covariance; CI, confidence interval; LS, least squares; ITT, intention‐to‐treat.
Secondary outcomes
Severity of throat soreness. Compared with non‐medicated lozenges, AMC/DCBA throat lozenges induced significantly different mean changes from baseline in severity of throat soreness at all assessment timepoints from 5 to 120 min; all p < 0.0001 (Figure 2). The maximum mean change from baseline following administration of AMC/DCBA throat lozenges was achieved at 75 min after first dose.
Figure 2.
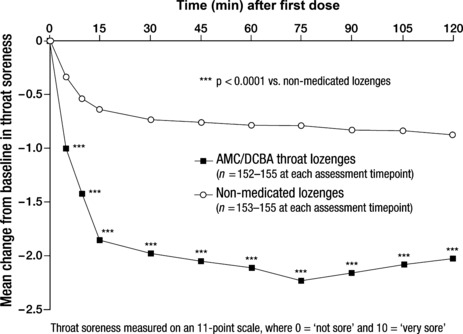
Effect of amylmetacresol and 2,4‐dichlorobenzyl alcohol (AMC/DCBA) throat lozenges on throat soreness after first dose – intention‐to‐treat (ITT) population
The AUC from baseline to 2 h after first dose for the change from baseline in severity of throat soreness was significantly different in the AMC/DCBA throat lozenges group compared with the non‐medicated lozenges group [difference between LS mean values −1.26 (95% CI: −1.54, −0.97); p < 0.0001 vs. non‐medicated lozenges group].
The mean change from baseline in severity of throat soreness data obtained at the end of Day 1, at 24 h after first dose and at the end of Days 2 and 3 supported the superiority of AMC/DCBA throat lozenges over non‐medicated lozenges (all p < 0.0001; Table 2). The difference between treatments in mean change from baseline in throat soreness gradually increased over the 3‐day study period (Figure 3).
Figure 3.
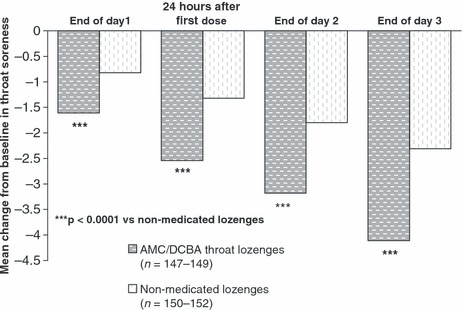
Effect of amylmetacresol and 2,4‐dichlorobenzyl alcohol (AMC/DCBA) throat lozenges on throat soreness over the 3‐day study period – intention‐to‐treat (ITT) population
Sore throat relief. Sore throat relief was observed as early as 5 min after first dose with an AMC/DCBA throat lozenge, which differed significantly from that achieved with non‐medicated lozenges and persisted for 2 h (p < 0.0001 vs. placebo for all assessment timepoints; Figure 4). Similar to the change in throat soreness data, maximum sore throat relief following the consumption of AMC/DCBA throat lozenges was achieved at 75 min after first dose (Figure 4).
Figure 4.
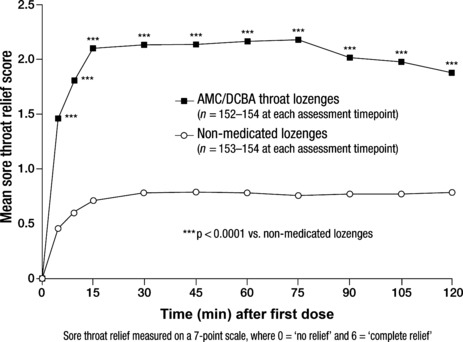
Effect of amylmetacresol and 2,4‐dichlorobenzyl alcohol (AMC/DCBA) throat lozenges on sore throat relief after first dose – intention‐to‐treat (ITT) population. Each activity was measured on an 11‐point scale where 0 = would not interfere at all and 10 = would completely interfere
The mean AUC from baseline to 2 h after first dose for sore throat relief yielded significant differences between treatment groups in favour of AMC/DCBA throat lozenges, i.e. difference between LS mean values 1.28 (95% CI: 1.04, 1.52); p < 0.0001 vs. non‐medicated lozenges group. The mean sore throat relief scores at 2 h after first dose, at the end of Day 1, at 24 h after first dose and at the end of Days 2 and 3 were significantly greater for AMC/DCBA throat lozenges group compared with the non‐medicated lozenges group (all p < 0.0001; Table 2).
Onset of analgesia. Sixty‐three percentage of patients in the AMC/DCBA throat lozenges group reported moderate pain relief compared with 22% in the non‐medicated lozenges group. The Kaplan–Meier median time to report moderate pain relief was 45 min (95% CI: 15; 780 min) in the AMC/DCBA throat lozenges group compared with an equivalent mean value of greater than 3600 min in the non‐medicated lozenges group.
Difficulty in swallowing. At each assessment timepoint after first dose, mean changes from baseline in difficulty in swallowing were significantly greater in the AMC/DCBA throat lozenges group than in the non‐medicated lozenges group; all p < 0.0001 (Figure 5). As with pain relief and changes in throat soreness, the maximum mean reduction from baseline in difficulty in swallowing following the consumption of the first AMC/DCBA throat lozenge was achieved at 75 min after dose (Figure 5). At 2 h after first dose, the difference between LS mean values for change from baseline in difficulty in swallowing was −11.1 (95% CI: −15.0, −7.3); p < 0.0001 vs. non‐medicated lozenges group (Table 2).
Figure 5.
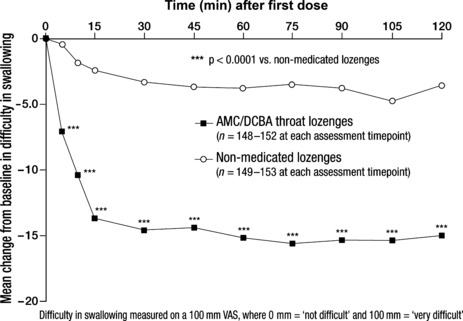
Effect of amylmetacresol and 2,4‐dichlorobenzyl alcohol (AMC/DCBA) throat lozenges on difficulty in swallowing after first dose – intention‐to‐treat (ITT) population
The difference between LS mean values for AUC for the change from baseline in difficulty in swallowing at 2 h after first dose was −10.6 (−13.4, −7.8) in the AMC/DCBA throat lozenges group compared with the non‐medicated lozenges group (p < 0.0001).
Difficulty in swallowing was significantly eased at the end of Day 1, at 24 h after first dose and at the end of Days 2 and 3 (Table 2). Similar to the sore throat relief and change in throat soreness data, the difference between treatments in change from baseline in difficulty in swallowing gradually increased over the 3‐day study period.
Freedom from symptoms. For the analysis relating to the number of patients who reported being free from symptoms, a small percentage of patients were symptom free at the end of Day 1 and 24 h after first dose in both treatment groups, but this was not significantly different. However, there was a significant difference between treatment groups in favour of the AMC/DCBA throat lozenges at the end of Days 2 and 3. At the end of Day 2, 16% of patients who took the AMC/DCBA throat lozenges became symptom free compared with 6% of patients who took the non‐medicated lozenges [p < 0.01; odds ratio (OR): 3.39; 95% CI: 1.48, 7.77]. At the end of Day 3, this number increased to 35% and 10% of patients, respectively (p < 0.0001; OR: 6.26; 95% CI: 3.15, 12.43).
Overall treatment rating at 2 h and at the end of Day 3. The overall treatment rating at 2 h was significantly greater in the AMC/DCBA throat lozenges group vs. non‐medicated lozenges group [difference between LS mean values 2.74 (95% CI: 2.15, 3.32); p < 0.0001]. This was similarly the case at the end of Day 3 [i.e. 2.83 (95% CI: 2.23, 3.43); p < 0.0001 vs. non‐medicated lozenges group].
Improvement in functional impairment. A total of 296 patients (145 in the AMC/DCBA throat lozenges group and 151 in the non‐medicated lozenges group) provided baseline and Day 3 data for the functional impairment scale.
The baseline mean scores obtained for each of the eight functions/activities assessed were similar in both of the treatment groups. The combined total baseline mean scores (±SD) obtained for all patients in each of the eight functions/activities were: swallowing (7.24 ± 1.79), talking (6.03 ± 2.33), eating a meal (5.72 ± 2.28), sleeping (4.34 ± 3.16), working (3.92 ± 3.18), concentrating (2.97 ± 2.71), reading (1.50 ± 2.16) and driving a car (0.96 ± 1.87).
The analyses for change from predose to the end of Day 3 in the functional impairment scale for each of the eight activities for the ITT population are presented in Figure 6.
Figure 6.
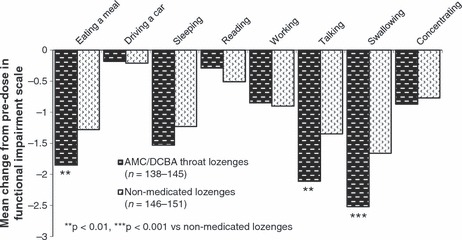
Change from predose to the end of Day 3 for each activity in the functional impairment scale – intention‐to‐treat (ITT) population. Each activity was measured on an 11‐point scale where 0 = would not interfere at all and 10 = would completely interfere
Mean reductions in functional impairment scores for all eight activities favoured AMC/DCBA throat lozenges, with statistically significant differences for the three activities most impaired at baseline: swallowing [difference between LS mean values −1.11 (95% CI: −1.75, −0.47)], eating a meal [−0.86 (95% CI: −1.45, −0.26)] and talking [−1.00 (95% CI: −1.61, −0.39)]; p = 0.0007, p = 0.005 and p = 0.002, respectively vs. placebo. The mean total score summing up all eight responses was significantly different between the treatment groups [i.e. difference between LS mean values −3.9 (95% CI: −7.3, −0.5); p = 0.03 for AMC/DCBA throat lozenges vs. non‐medicated lozenges group].
Relief experienced from the moment the lozenge was consumed, and whether the patient felt better when asked at 2 h after first dose. When asked at 5 min after first dose whether any relief was felt from the moment the lozenge was consumed, 101/154 (66%) patients in the AMC/DCBA throat lozenges group reported experiencing relief compared with 23/147 (16%) in the non‐medicated lozenges group; this difference was statistically significant (p < 0.0001). When asked at 2 h after first dose whether they felt any better than before the lozenge was taken, 98/151 (65%) patients in the AMC/DCBA throat lozenges group said yes compared with 40/152 (26%) patients in the non‐medicated lozenges group (p < 0.0001).
Type of pain relief experienced. In answer to the question ‘How can you describe the type of relief this lozenge gave you?’, the most popular terms give by the patients for the type of relief experienced with the AMC/DCBA throat lozenges were ‘soothing relief’, ‘soreness relief’ and ‘coating relief’. Significantly more subjects in the AMC/DCBA throat lozenges group gave more favourable responses for all seven categories of relief reported in Table 3. When asked about the importance of each of the types of relief descriptions, the majority of patients in the study, i.e. 228/308 (74%), rated ‘soothing relief’ as being very or extremely important to them.
Table 3.
Results obtained in answer to the question ‘How can you describe the type of relief this lozenge gave you?’
| AMC/DCBA throat lozenges (n = 155; %) | Non‐medicated lozenges (n = 155; %) | p‐Value* | |
|---|---|---|---|
| Soothing relief | 97 (63) | 42 (27) | < 0.0001 |
| Soreness relief | 63 (41) | 19 (12) | < 0.0001 |
| Coating relief | 48 (31) | 24 (15) | 0.0006 |
| Pain relief | 47 (30) | 6 (4) | < 0.0001 |
| Relief from burning | 22 (14) | 8 (5) | 0.008 |
| No relief | 17 (11) | 80 (52) | < 0.0001 |
| Relief from swelling | 11 (7) | 1 (1) | 0.02 |
*Estimated from a logistic regression model with factors for treatment and centre and a covariate for baseline throat soreness. AMC/DCBA, amylmetacresol and 2,4‐dichlorobenzyl alcohol.
Throat‐related aspects of relief experienced. AMC/DCBA throat lozenges were considered by patients to provide relief deeper within the throat and to be more moisturising/lubricating, soothing and coating than the non‐medicated lozenges. The differences between treatment groups were statistically significant (all p < 0.0001; Table 4).
Table 4.
Results from answering throat‐related questions – ITT population
| n | Mean (SD) | LS mean* | Difference between LS means† | SE | 95% CI | p‐Value for treatment* | |
|---|---|---|---|---|---|---|---|
| How deep down within the throat was the relief felt? (Measured on 10‐point scale 1 = not at all deep, 10 = very deep in the throat) | |||||||
| AMC/DCBA throat lozenges | 153 | 5.03 (2.09) | 5.00 | 2.17 | 0.24 | 1.70, 2.64 | < 0.0001 |
| Non‐medicated lozenges | 154 | 2.86 (2.10) | 2.83 | ||||
| How deep down within the throat do you think this lozenge coats the throat? (Measured on 10‐point scale 1 = not at all deep, 10 = very deep in the throat) | |||||||
| AMC/DCBA throat lozenges | 153 | 5.01 (2.12) | 4.89 | 2.29 | 0.24 | 1.83, 2.76 | < 0.0001 |
| Non‐medicated lozenges | 154 | 2.71 (2.02) | 2.60 | ||||
| Please tell us your overall opinion of how moisturising/lubricating this lozenge is? (Measured on 10‐point scale 1 = not moisturising/lubricating at all, 10 = very moisturising/lubricating) | |||||||
| AMC/DCBA throat lozenges | 153 | 5.41 (2.10) | 5.53 | 1.85 | 0.27 | 1.32, 2.38 | < 0.0001 |
| Non‐medicated lozenges | 154 | 3.56 (2.59) | 3.68 | ||||
| How soothing do you think this lozenge is? (Measured on 10‐point scale 1 = not at all soothing, 10 = very soothing) | |||||||
| AMC/DCBA throat lozenges | 153 | 5.56 (1.99) | 5.74 | 2.24 | 0.25 | 1.74, 2.74 | < 0.0001 |
| Non‐medicated lozenges | 154 | 3.32 (2.46) | 3.49 | ||||
| How much do you think this lozenge coats the throat? (Measured on 10‐point scale 1 = not at all coating, 10 = very coating) | |||||||
| AMC/DCBA throat lozenges | 154 | 5.13 (1.97) | 5.03 | 2.26 | 0.23 | 1.81, 2.71 | < 0.0001 |
| Non‐medicated lozenges | 154 | 2.86 (2.03) | 2.77 | ||||
*Estimated from ANCOVA model with factors for treatment and centre and a covariate for baseline throat soreness. †AMC/DCBA throat lozenges minus non‐medicated lozenges. AMC/DCBA, amylmetacresol and 2,4‐dichlorobenzyl alcohol; ANCOVA, analysis of covariance; CI, confidence interval; LS, least squares; SD, standard deviation; SE, standard error.
Overall lozenge consumption up to the end of Day 3. There was no statistical difference between treatment groups in terms of the mean total number of lozenges consumed. The difference between LS mean values was −1.18 (95% CI: −2.50, 0.14); p = 0.08 for the AMC/DCBA throat lozenges vs. non‐medicated lozenges group.
Overall rescue medication (paracetamol) consumption. During the first 24 h after first dose, patients in both treatment groups consumed approximately two doses of rescue medication, which increased to approximately five doses by Day 3. There was no significant difference between the treatment groups at both timepoints (p = 0.21 and p = 0.41, respectively).
Concomitant medication ongoing at randomisation and started during study. Fifty‐two percentage of patients in the AMC/DCBA throat lozenges group compared with 60% in the non‐medicated lozenges group were taking concomitant medication (e.g. anti‐acne preparations, anti‐emetics, anti‐epileptics, anti‐histamines, diuretics and antibacterials for systemic use, etc.) at randomisation; 0.6% (n = 1) of whom from each group was taking a systemic antibacterial medication. During the course of the study, i.e. after receiving the lozenges, an additional five patients from the AMC/DCBA throat lozenges group (i.e. total 3.2%) and an additional seven patients from the non‐medicated throat lozenges group (i.e. total 4.5%) were started on a systemic antibacterial medication.
Adverse events. There was no difference between the AMC/DCBA throat lozenges and non‐medicated lozenges group in relation to the proportion of patients reporting AEs, i.e. 16% vs. 16%, respectively. A total of 43 events were reported in the AMC/DCBA throat lozenges group vs. 41 events in the non‐medicated lozenges group. In both treatment groups, the majority of AEs reported were mild with only five treatment‐emergent events classified as severe. Most AEs were events related to the patients’ URTIs, such as headache, cough, chills and pyrexia. The most common AE reported was headache, with 13 (8%) patients reporting 17 occurrences in the AMC/DCBA throat lozenges group and nine (6%) reporting nine occurrences in the non‐medicated lozenges group.
In both treatment groups, none of the reported events was definitely related to treatment, one event was probably related (i.e. severe mouth ulceration by patient in AMC/DCBA throat lozenges), and a further five events of possible relationship were reported [four from the non‐medicated lozenge group: two of whom reported tongue disorders, one nausea, one tongue ulceration and one from the AMC/DCBA throat lozenges group (mouth ulceration)].
Discussion
Summary of main findings
The superiority of AMC/DCBA throat lozenges in the relief of sore throat over non‐medicated lozenges was clearly apparent in this study. Statistically significant differences were obtained for all variables related to sore throat relief, throat soreness, difficulty in swallowing and the overall treatment rating. The results were robust with identical conclusions drawn from the equivalent PP analyses.
Single‐dose data indicated that the effects of AMC/DCBA throat lozenges on throat soreness, pain relief and difficulty in swallowing were evident as early as 5 min and lasted for at least 2 h. The rapid analgesic effects were also supported by the patient questionnaire survey component of this study. Peak effects of all three variables were observed 75 min after the first dose, which suggests that the analgesic benefits provided by AMC/DCBA throat lozenges were not restricted to only the time that the lozenge was present in the mouth, and that relief benefits continued long after the lozenge had dissolved. As earlier studies have shown, the mean time for an AMC/DCBA throat lozenge to dissolve in the mouth is 6.77 ± 2.01 min (10). Furthermore, release of the active ingredients AMC and DCBA into the mouth was demonstrated to begin almost immediately after the lozenge was administered, with measureable quantities present in the saliva at 1 min and peak salivary concentrations at 4 min, followed by a steady decline (10). This time‐course release of active ingredients coincides with the findings of this study, because significant analgesic effects on throat soreness and sore throat relief, as well as on difficulty in swallowing were observed at the 5‐min assessment timepoint; patients expressed a sense of relief from the moment they took a lozenge. More importantly, data from this study support the notion that AMC/DCBA throat lozenges have a faster onset of analgesic action than non‐medicated lozenges, as indicated by the greater than 80‐times shorter median time first to report of ‘moderate’ pain relief data.
The multiple‐dose data for changes in sore throat severity, difficulty in swallowing and sore throat relief supported the freedom from symptoms data and in that the improvements observed with AMC/DCBA throat lozenges compared with non‐medicated lozenges increased over the 3‐day study period. The greatest differences were observed at the end of Day 3, which suggests that continued use of AMC/DCBA throat lozenges over a 3‐day period can significantly benefit patients, with more patients likely to experience greater improvements in throat soreness, faster pain relief, greater easing of difficulty in swallowing and freedom from symptoms than those on non‐medicated lozenges.
The three activities/functions considered to be most impaired by acute sore throat patients were swallowing, talking and eating a meal. AMC/DCBA throat lozenges significantly improved the functional impairment scores for all three activities by the end of the 3‐day study period compared with non‐medicated throat lozenges. Acute sore throat was not considered by patients to have as much impact on the other five activities examined (driving a car, working, concentrating, reading and sleeping), as indicated by the relatively low scores given at baseline. However, a trend in improvement in the latter four activities was reported by all patients regardless of which treatment lozenge was taken, and in particular the ability to sleep was most improved.
A significantly greater proportion of patients who received AMC/DCBA throat lozenges than those who received non‐medicated lozenges reported experiencing pain relief from the moment they took the lozenge. This pain relief element of the questionnaire provides further support for the pain relief findings reported by patients who experienced pain relief at 5 min after first lozenge consumption and demonstrates an instant action of AMC/DCBA throat lozenges.
The most commonly used terms to describe the types of pain relief provided were ‘soothing relief’, ‘soreness relief’ and ‘coating relief’, where ‘soothing action’ was voted by the majority of AMC/DCBA throat lozenge‐treated patients as being very or extremely important. In addition, relief provided by the active lozenges was reported to be deeper within the throat, more moisturising/lubricating, soothing and coating than the non‐medicated lozenges.
The same number of patients within each treatment group reported at least one treatment‐emergent AE, which was mostly mild and related to the patient’s URTI. In keeping with previous findings (16, 17, 18, 19), AMC/DCBA throat lozenges are well‐tolerated with only a few AEs that are possibly or probably related to the lozenge.
Strengths and limitations of the study
The assessments of analgesic properties were made using standard, published and reliable methodologies and subjective rating scales, i.e. ordinal scales, a 100 mm VAS scale and a categorical scale. Different from other studies, throat soreness and pain relief were also analysed over the first 2‐h period using AUC data instead of the sum of the pain intensity or pain relief scores. This was in accordance with published literature that suggests this to be a more appropriate way of handling serial measurement data (21, 22). An advantage of AUC analyses is that it is based on actual rather than scheduled timings, which allowed for the uneven time interval between assessments.
This study is the largest study to date involving AMC/DCBA throat lozenges, with over 300 sore throat patients participating in the study. Aside from the benefits of a larger patient number, which reduces any variations in the results obtained, especially those associated with VAS measures, another advantage of this study was that it was multi‐centred, thus allowing centre variations to be accounted for.
Comparison with existing literature
In keeping with previous findings (19), this larger study demonstrated significant reductions in the severity of throat soreness at 2 h after first dose with AMC/DCBA throat lozenges compared with non‐medicated lozenges, i.e. the mean difference in treatment effect was −1.21 in this study vs. −0.7 in Wade et al.’s study.
Based on the work of Salaffi et al. and Farrar et al., a minimum clinically significant improvement in sore throat could be defined as a reduction of 1 or 2 on the Sore Throat pain intensity Scale (23, 24, 25). Therefore, the reduction in the severity of throat soreness induced by AMC/DCBA throat lozenges, as demonstrated in this study, is deemed clinically meaningful compared with non‐medicated lozenges. Patients experienced a clinically meaningful reduction of ≥ 1 in their sore throat score at 5 min after first dose, which continued to increase to −4.11 at the end of Day 3. As expected, a ‘placebo effect’ was observed with the non‐medicated lozenges, which reached −2.31 at the end of Day 3. This finding can be explained by the self‐limiting nature of acute sore throats, where patients naturally feel better over the course of time without any treatment. However, as results from this study show, treatment with AMC/DCBA throat lozenges provides added benefits to the patients that are over and above the demulcent properties of non‐medicated lozenges, enabling patients to feel better faster, to experience pain relief more quickly and to carry out everyday activities with greater ease.
Similar to previous findings, pain relief was reported by patients soon after lozenge consumption (17, 18, 19), but in this study, pain relief was observed more rapidly than reported previously (i.e. at 5 min and not 15 min after first dose). This was because 5‐min assessment timepoints were not included in previous studies, and so it was not possible until now to observe any effect at that earlier timepoint.
This is the first study to investigate the impact of throat lozenges on functional impairment scores of everyday activities in adult patients with acute sore throat. The other studies that have been conducted that also include functional activity as an outcomes measure compared orally administered analgesics, and not throat lozenges. For example, a study conducted by Weckx et al. in 2002 examined the effects of celecoxib vs. diclofenac on the symptoms of viral pharyngitis and included quality‐of‐life outcome measures, such as patient’s global assessment of disease activity and patient’s functional activity (26). Whereas functional activity in the study by Weckx et al. was assessed using a categorical scale ranging from 0 (able to work and function normally in all activities) to 3 (working, studying or housekeeping activities severely impaired/unable to perform; and requiring bed rest) (26), this study utilised a different functional impairment scale that ranged from 0 = would not interfere at all to 10 = would completely interfere and assessed eight specific daily activities. Similar to the findings of Weckx et al. (26), an overall positive outcome in functional impairment scores was demonstrated in this study in patients treated with the active lozenge at the end of Day 3.
Implications for future research or clinical practice
In conclusion, AMC/DCBA throat lozenges provide fast, safe and effective relief for sore throats because of URTIs. Patients can feel the lozenge working soon after first lozenge administration. The analgesic effects continue long after the lozenge has dissolved, with additional functional benefits in swallowing being experienced by patients.
Furthermore, the results of this survey highlight the debilitating effects of sore throat on the everyday lives of patients, including basic activities, such as eating a meal, swallowing and talking. Results demonstrated herein show significant improvements in functional impairment scores of these daily activities that are most affected by sore throat in patients treated with AMC/DCBA throat lozenges compared with non‐medicated throat lozenges. Thus, AMC/DCBA throat lozenges not only provide clinically meaningful pain relief, but they also allow patients to re‐engage in their everyday activities and carry on with their daily lives, thereby further supporting this treatment as a suitable treatment option in the self‐management of acute sore throat.
Author contributions
All authors have read and approved the manuscript. Damien McNally: concept/design, study conduction, data collection, data interpretation, article development, approval of article. Michele Simpson: concept/design, study conduction, data collection, data analyses/interpretation. Christopher Morris: data interpretation, review of article, approval of article. Adrian Shephard: concept/design, data interpretation, article development, review of article, approval of article. Michael Goulder: study design, statistical/data analyses, review of article, approval of article.
Acknowledgements
The research was funded by Reckitt Benckiser Healthcare International. The authors would like to thank Quyen Chu, PhD (Sudler & Hennessey, London), who is a member of the European Medical Writers Association, for medical writing/editorial support, which was funded by Reckitt Benckiser Healthcare International.
Disclosures Reckitt Benckiser Healthcare International is the manufacturer of AMC/DCBA throat lozenges (Strepsils®). Christopher Morris and Adrian Shephard are employees of Reckitt Benckiser Healthcare International. Damien McNally was the lead investigator of this study. Michele Simpson was the contract clinical project manager and Michael Goulder was the lead statistician for this study.
References
- 1. Marshall S. Giving advice on sore throats. Pharm J 2008; 280: 127–30. [Google Scholar]
- 2. Little P, Williamson I, Warner G, Gould C, Gantley M, Kinmonth AL. Open randomised trial of prescribing strategies in managing sore throat. BMJ 1997; 314: 722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ashworth M, Charlton J, Ballard K, Latinovic R, Gulliford M. Variations in antibiotic prescribing and consultation rates for acute respiratory infection in UK general practices 1995–2000. Br J Gen Pract 2005; 55: 603–8. [PMC free article] [PubMed] [Google Scholar]
- 4. UK NICE guidelines on ‘Respiratory tract infections – antibiotic prescribing: prescribing of antibiotics for self‐limiting respiratory tract infections in adults and children in primary care’ 2008. http://www.nice.org.uk/nicemedia/pdf/CG69FullGuideline.pdf (accessed October 2009). [PubMed]
- 5. Summers A. Sore throats. Accid Emerg Nurs 2005; 13: 15–7. [DOI] [PubMed] [Google Scholar]
- 6. Worrall GJ. Acute sore throat. Can Fam Physician 2007; 53: 1961–2. [PMC free article] [PubMed] [Google Scholar]
- 7. Oxford JS, Lambkin R, Gibb I, Balasingam S, Chan C, Catchpole A. A throat lozenge containing amylmetacresol and dichlorobenzyl alcohol has a direct virucidal effect on respiratory syncytial virus, influenza A and SARs‐CoV. Antivir Chem Chemother 2005; 16: 129–34. [DOI] [PubMed] [Google Scholar]
- 8. Del Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev 2006; 18: CD000023. [DOI] [PubMed] [Google Scholar]
- 9. Becker W, Naumann HH, Pfaltz CR. Chapter 3: Mouth and pharynx. In: Buckingham RA, ed. Ear, Nose and Throat Diseases – A Pocket Reference, 3rd edn Stultgart: Georg Thieme Verlag, 1986: p.367 [of 1989 English version translated from German book] [Google Scholar]
- 10. Wade AG, Marshall LE, Simpson M, Shephard A. Bioavailability and efficacy of active lozenges in the relief of sore throat pain. Poster presented at the Annual Scientific Meeting of the British Pain Society. Glasgow, UK. 24–27 April 2007.
- 11. Limb M, Connor A, Pickford M et al. Scintigraphy can be used to compare efficacy of sore throat formulations. Int J Clin Pract 2009; 63: 606–12. [DOI] [PubMed] [Google Scholar]
- 12. Richards RME. Inhibitory activity of lozenges on oral bacteria. Pharmacotherapy 1988; 2: 142 (abstract 168). [Google Scholar]
- 13. Richards RME, Cowie G, McCague GJ. In vivo investigations of the antibacterial activity of lozenges and mouthwashes on the aerobic bacterial flora of the mouth and throat. Pharm J 1989; 242: 659–63. [Google Scholar]
- 14. Richards RME, Xing DKL. In vitro evaluation of the antimicrobial activities of selected lozenges. J Pharm Sci 1993; 82: 1218–20. [DOI] [PubMed] [Google Scholar]
- 15. Bergener B, Hobon J, Elmgren J, Thorén PA. Clinical and bacteriological testing of Strepsils. Farmacevtisk Revy 1972; 72: 782–5. [Google Scholar]
- 16. Scollo GL, Rocca M. Open clinical study of the efficacy and tolerability of the combination 2‐4 dichlorobenzyl alcohol, amylmetacresol and vitamin C. Therapy 1985; 144: 593–5. [Google Scholar]
- 17. Berry P. Rapid Relief of Acute Sore Throat With Strepsils Lozenges: A Single‐Blind, Comparative Study. London: Royal Society of Medicine Press, 2008; ISBN: 978‐1‐85315‐869‐8. [Google Scholar]
- 18. Marazzi PJ. Strepsils Anaesthetic Lozenges Versus Control Strepsils Lozenges in the Relief of Moderate‐to‐Severe Sore Throat: A Double‐Blind, Crossover, Multiple‐Dose, Randomized Study. London: Royal Society of Medicine Press, 2008; ISBN: 978‐1‐85315‐869‐8. [Google Scholar]
- 19. Wade AG. A Randomized, Double‐Blind, Parallel‐Group, Placebo‐Controlled, Multiple‐Dose Study of the Efficacy of Strepsils Lozenges in the Relief of Acute Sore Throat. London: Royal Society of Medicine Press, 2008; ISBN: 978‐1‐85315‐869‐8. [Google Scholar]
- 20. Watson N, Nimmo WS, Christian J, Charlesworth A, Speight J, Miller K. Relief of sore throat with the anti‐inflammatory throat lozenge flurbiprofen 8.75mg: a randomised, double‐blind placebo‐controlled study of efficacy and safety. Int J Clin Pract 2000; 54: 490–6. [PubMed] [Google Scholar]
- 21. Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990; 300: 230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Max MB. Chapter 1: The design of clinical trials for treatment of pain. In: Max MB. and Lynn J.eds. Online Interactive Textbook on Clinical Symptom Research. National Institute of Dental and Craniofacial Research, NIH; http://symptomresearch.nih.gov . (accessed October 2009) [Google Scholar]
- 23. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimum clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004; 8: 283–91. [DOI] [PubMed] [Google Scholar]
- 24. Farrar JT, Berlin JA, Strom BL. Clinically important changes in acute pain outcome measures: a validation study. J Pain Symptom Manage 2003; 25: 406–11. [DOI] [PubMed] [Google Scholar]
- 25. Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11‐point numerical pain rating scale. Pain 2001; 94: 149–58. [DOI] [PubMed] [Google Scholar]
- 26. Weckx LLM, Ruiz JE, Duperly J et al. Efficacy of celecoxib in treating symptoms of viral pharyngitis: a double‐blind, randomised study of celecoxib versus diclofenac. J Int Med Res 2002; 30: 185–94. [DOI] [PubMed] [Google Scholar]


