Introduction: The Many Levels of Circadian Genetics
The topic, “Genetics of Circadian Rhythms” could be a consideration of a number of questions. Are differences between individuals in their circadian rhythmicity a result of differences in their genes? If so, what genes? What genes encode the “clock” mechanism? How can genes form a clock? Does the circadian clock mechanism affect gene activity? If so, how?
Here, we describe the developing appreciation of the multiple interconnections of circadian rhythmicity with genetics in mammals. As illustrated in Figure 1, a core set of genes function as a clock in every cell as a feedback loop of gene transcription and translation-- providing a means for circadian rhythms to persist independently in each cell. However, the clocks within the cell can be influenced by a variety of interacting pathways and influences ranging from the intracellular (e.g., redox state) to nutritional, endocrine and behavioral. Similarly, a variety of genetic polymorphisms in other genes appear to influence the expression of circadian rhythms in individuals. The genetic circadian clock regulates rhythms of gene expression via direct transcriptional regulation, via histone acetylation/deacetylation. Despite the many genes and mechanisms, such results point to a very central role of circadian clock genes in regulating biochemical, metabolic and physiological processes at many different levels of organization.
Figure 1. Multidimensional genetic clockwork.
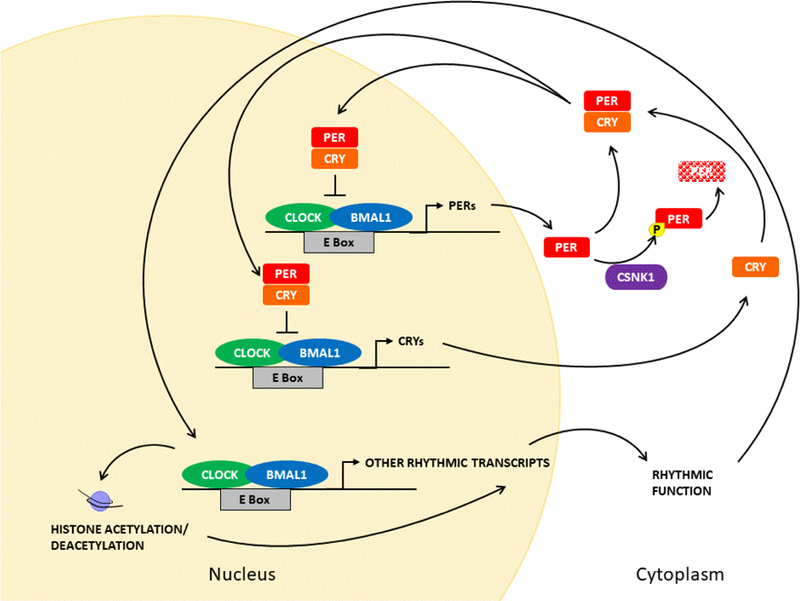
The core clock of animals has the proteins CLOCK and BMAL1 functioning as positive regulators, binding to E-box regulatory elements and transactivating the transcription of the per and Cry genes, as well as multiple other rhythmically expressed genes. Phosphorylation of PER by CSNK1 can lead to its degradation. PER and CRY proteins form dimers and enter the nucleus to repress the CLOCK:BMAL1 complex. Rhythmic transcription leads to rhythms in key regulator genes and cellular functions that can feedback to alter core clock function. In addition, CLOCK affects histone acetylation, another mechanism by which gene activity can be modulated.
Circadian Rhythms are Heritable
One of the first questions to consider is one of “Nature or Nurture”. To what extent are attributes of circadian rhythms determined by one’s genes (Nature) as opposed to one’s environment (Nurture)? A variety of experimental approaches to this question have been employed over the years, all supporting the role of genetics. For example, attempts to alter the endogenous period of mice by gestation and rearing under non-24 hour days (either 20hr or 28hr light-dark cycles) failed to have lasting effects on free-running period in the mice as adults1. Mice of an inbred strain, which are effectively genetically identical to one another, but genetically distinct from mice of another strain, can be compared. Significant differences between strains in circadian rhythm properties have been noted2–6, that are above and beyond the differences between individuals of the same strain.
Similarly, studies of human twins have provided a means to estimate the “heritability” (proportion of phenotypic variance attributable to genetic variance) of circadian rhythms. The extent to which monozygotic (identical) twins are more similar than dizygotic twins can be used to estimate heritability. Hence, significant heritability has been described for the timing and pattern of serum cortisol rhythms7–8 and of chronotype (morningness vs. eveningness)9–10 by twin comparisons.
Evidence of heritability does not provide an indication of the number or identity of the genes at play, however. Genes contributing to one aspect of circadian rhythmicity (the endogenous, or “free-running” periodicity, for example) may be different from those involved in another (such as morning vs. evening preference). Further, variants in genes involved in critical functions may be deleterious and thus eliminated by natural selection-- meaning that the allelic polymorphisms naturally present in populations (and represented in strain or twin comparisons) may be in different genes from those essential for generation of circadian timing. Hence, there are many reasons to expect the existence of multiple types of circadian rhythm genes.
The Core Circadian Clock
Early evidence suggested a genetic mechanism lay at the core generator of self-sustained circadian rhythms. In 1961, Colin Pittendrigh11 noted that the clock mechanism could be contained in single cells and was considered unlikely to be simply a chemical reaction since it was relatively insensitive to being sped up or slowed down by changes in temperature. Further evidence that gene transcription and translation was involved included demonstration that inhibition of protein synthesis could reset, or temporarily stop, the circadian clock12–14. To identify what the genes and proteins comprising the circadian clock were, induction of mutations and screening for abnormal circadian rhythms proved the most effective approach-- in both Drosophila melanogaster15 and in mice16. Mutagenesis and phenotypic screening has also been successful in identifying circadian clock genes in other organisms such as the fungus Neurospora crassa17, plants18 and cyanobacteria19.
Described here are the identification of the transcription-translation feedback genes now known to form the core circadian clock. These are the “positive” elements CLOCK and BMAL1 that drive the transcription of the “negative” elements PER and CRY that in turn feedback to inhibit CLOCK:BMAL1.
Period
The first identified clock component gene, period (denoted per) was discovered in 1971 in Drosophila by a “forward genetic” approach consisting of chemically inducing random mutations in the genome, and detecting those mutations that affect circadian rhythms by screening the progeny of the mutagenized individuals for altered rhythmicity15. This approach has the advantage that no assumptions are made about the nature of the genes or gene products involved, but is based on the presumption that there exist single genes that, when mutated, will alter rhythms in a detectable manner. At the time, this presumption of the existence of single genes that could regulate a complex behavior was considered radical or absurd, but now appears to have been foresight.
Initially, three alleles of the per gene were identified by the process of mutagenesis and screening. Flies carrying these alleles had either no apparent rhythm in eclosion (emergence from the pupal case) or locomotion, or had either long (approximately 29 hours) or short periods (approximately 19 hours) for the rhythms of eclosion and locomotor activity15.
Confirmation of the importance of the per gene as a central circadian clock component was possible upon its molecular identification, for which the 2017 Nobel Prize was awarded to Hall, Rosbash, and Young. The gene’s function was demonstrated by rescue of the arrhythmic mutant phenotype after introduction of the wild-type allele of the per gene into mutant flies20,21. The level of the mRNA transcript encoded by the per gene was shown to oscillate in a circadian fashion [40] as a result of transcriptional regulation23, and the levels of the PER protein were shown to lag the per mRNA levels24. In fact, shifts in the circadian phase can be evoked by the induction of PER protein under the control of a non-circadian promoter25. Thus, many lines of evidence indicate that the per gene encodes a protein that is a clock component. Three orthologs of the per gene, Per1, Per2, and Per3, have now been identified in mammals and the levels of their mRNA have also been shown to oscillate with a circadian period26–30.
Clock
In the early 1990s, no genes in mammals had been identified as even possible candidate circadian clock genes, leading to an effort to again apply chemical mutagenesis and phenotypic screening, this time in mice. In a screen of over 300 first-generation progeny of mutagen-treated mice (potential heterozygous mutants), Vitaterna and colleagues found one animal that had a free- running period of about 24.8 hours, more than six standard deviations longer than the mean of ~24.7 hours16. In homozygotes, this mutation results in a dramatic lengthening of the period to about 28 hours which is usually followed by the eventual loss of circadian rhythmicity (i.e., arrhythmicity) after about 1–3 weeks in DD. The affected gene was named Clock (an acronym for Circadian locomotor output cycles kaput16) and mapped to mouse chromosome 516,31. The Clock gene was cloned by a combination of genetic rescue and positional cloning. Clock/Clock mutant mice were phenotypically rescued by a bacterial artificial chromosome (BAC) transgene that contained the Clock gene, allowing for functional identification of the gene32. The Clock gene encodes a transcriptional regulatory protein having a basic helix-loop-helix DNA-binding domain, a PAS dimerization domain and a Q-rich transactivation domain. The mutant form of the CLOCK protein (CLOCK-∆19) lacks a portion of the activation domain found in wild-type protein, and thus, while it is capable of protein dimerization, transcriptional activation is diminished or lost. The protein dimerization domain is named PAS because the genes originally identified with it were, per, ARNT and sim. Clock mRNA is expressed in the SCN as well as other tissues, but it does not oscillate in a circadian manner in all tissues33.
Bmal1
The presence of the PAS dimerization domain in CLOCK protein led to a screen for potential partners for the CLOCK protein. A protein of unknown function, BMAL1 (Brain and Muscle ARNT-Like 1, now denoted ARNT-Like or Arntl), was able to dimerize with the CLOCK protein34. Creation of mice with a null allele of Bmal1 demonstrated the critical role of this gene in circadian rhythm generation. These mutant mice, while displaying light-dark responsive differences in activity level, become arrhythmic immediately upon release in constant darkness.
Additional actions of the CLOCK:BMAL1 heterodimer have become clear. While Clock mRNA does not oscillate, its protein’s nuclear vs. cytoplasmic localization does35. By studying the intracellular localization of CLOCK and BMAL1 in fibroblasts of mouse embryos with mutations in different clock genes, and ectopically expressing the proteins, it was found that nuclear accumulation of CLOCK was dependent on formation of the CLOCK:BMAL1 dimer, as was phosphorylation of the complex and its degradation35. Other PAS domain containing proteins failed to affect the localization of CLOCK, indicating that these posttranslational events are specific to the CLOCK:BMAL1 dimer.
Following the identification of CLOCK:BMAL1 dimerization, the ability of this heterodimer to regulate transcription was tested using a reporter construct based on the upstream regulatory elements of the per gene. The per gene of Drosophila contains an upstream regulatory element, the “clock control region”, within which is contained a sequence needed for positive regulation of transcription, the E-box element (CACGTG)36. CLOCK-BMAL1 heterodimers were found to activate transcription of the mPer gene in a process that requires binding to the E- box element34. However, CLOCK-∆19 mutant protein was not able to activate transcription, consistent with the finding that exon 19, which is skipped in Clock mutants33, is necessary for transactivation. Thus, CLOCK protein interacts with the regulatory regions of the per gene to allow transcription of the per mRNA and eventual translation of PER protein. However, this positive regulation alone will not produce an oscillation in per mRNA levels, which is known to be responsible for the oscillation in PER protein levels23. Findings that the Clock mutation dramatically decreases per genes’ expression also confirms the positive regulation of CLOCK:BMAL1 on per transcription in situ37,38. Mice with null mutations of Per1, Per2, or Per3 alone display altered circadian periods39,40, while mice with both Per1 and Per2 null mutations lose rhythmicity. Per3 null mutant mice exhibit only a subtle alteration in rhythmicity and Per1/Per3 or Per2/Per3 double mutants are not substantially distinct from the Per1 or Per2 single mutants. These findings suggest there may be some functional redundancy among the different mammalian per genes, but raise the question of the role of Per3 in mammalian circadian rhythms.
Cryptochromes
Cryptochromes are blue light responsive flavoprotein photopigments related to photolyases, so named because their function was cryptic when first identified. In mammals, two cryptochrome genes, Cry1 and Cry2 have been identified, and were found to be highly expressed in the ganglion cells and inner nuclear layer of the retina as well as the SCN41, and their mRNA expression levels oscillate in these tissues. Targeted mutant mice lacking Cry2 exhibit a lengthened circadian period, while mice lacking Cry1 have shortened circadian period; mice with both mutations have immediate loss of rhythmicity upon transfer to constant darkness42–45. Thus, like the mammalian period genes, the cryptochrome genes appear to have both distinct (given their opposite effects on circadian period) and compensatory (given that either gene can sustain rhythmicity in the absence of the other) functions.
Further evidence for a central clock function is the finding that the cryptochromes appear to share a number of regulatory features with the period genes. In Clock mutant mice, the mRNA levels of Cry1 and Cry2 are reduced in the SCN and in skeletal muscle46, suggesting that the cryptochromes also are induced by CLOCK:BMAL1 transactivation. Using mammalian cell lines, CRY1 and CRY2 were found by coimmunoprecipitation to interact with PER1, PER2, and PER3, leading to nuclear localization of the CRY:PER dimer as indicated by co-transfection assays with epitope-tagged proteins46. Either CRY:CRY or CRY:PER complexes were capable of inhibiting CLOCK:BMAL1 transactivation of Per1 or vasopressin transcription46. Thus, the CRYs as well as the PERs are capable of a negative feedback function, inhibiting CLOCK:BMAL1-induced transcription.
Casein Kinase 1
The tau mutation of the hamster arose spontaneously in a laboratory stock47. The mutation is semi-dominant and shortens the period from 24 to 22 hours in heterozygotes, and 20 hours in homozygotes. The mutation pre-dated the Clock mutation and demonstrated that single gene mutations could profoundly alter48 the circadian clock in mammals, just as in flies and Neurospora. Unfortunately, the genetic tools needed for cloning this important and interesting gene were not available for the hamster, and thus its molecular identity could not be determined by conventional genetic mapping/positional cloning approaches.
Lowrey and colleagues were able to identify a genomic region of conserved synteny (a grouping of genes together on a chromosome) in hamsters, mice, and humans, that encompassed the tau mutation49. Tau was thus identified as being a mutation in the Casein Kinase 1 epsilon (CK1e) gene, the mammalian ortholog of the Drosophila doubletime gene. Sequencing of the gene identified a point mutation, which leads to altered enzyme dynamics and autophosphorylation state. CK1e can phosphorylate PER proteins, and the tau mutant enzyme is deficient in this ability. Thus, CK1e may lead to degradation of PERs, slowing the accumulation of PER in the nucleus and thus repression of CLOCK:BMAL1. Casein Kinase 1 delta (CK1d) has also been implicated in mammalian circadian rhythmicity50–53.
Interacting Clock Pathways
Although not depicted in figure 1, a number of genes have been identified as key regulators of the core circadian clock genes. These genes form addition feedback loops to the genetic clock, and are described here.
Rev-erb alpha and ROR
While the negative feedback of PER and CRY proteins on their own CLOCK:BMAL1-induced transcription constitutes a form of negative feedback, and may be sufficient to explain the oscillations in expression of Per and Cry genes, the rhythmic expression of Bmal1 with an opposite phase was not explained by this feedback. Rev-erbα, an orphan nuclear receptor (now denoted Nr1d1), was the missing link. Its promoter region contains 3 E-boxes and transcription is thus positively regulated by CLOCK and BMAL154. Its transcription is negatively regulated by PER and CRYs and is at a minimum when mPER2 is at a maximum, and it is constitutively expressed at intermediate levels in Cry1/Cry2 or Per1/Per2 double knockouts. REV-ERBα protein appears to drive the circadian oscillation in Bmal1 transcription: the Bmal1 promoter includes two RORE sequences (enhancer sequences that recognize members of the REV-ERB and ROR orphan nuclear receptor families) and Bmal1 expression is drastically reduced in Rev- erba null mutants54. Thus Rev-erbα may act to link the positive and negative regulatory signals of other clock genes to the transcription of Bmal1 and to cellular metabolism. CLOCK:BMAL1 activity is suppressed by a NAD+ dependent deacetylase SIRT1, which is in turn regulated by the circadian control of NAD+ biosynthesis55–58.
Rev-erba also contributes to the differences between the phase of Cry1 mRNA rhythms relative to other clock genes whose transcription is enhanced by CLOCK:BMAL binding to E- boxes. The Cry1 gene has three candidate REV-ERB/ROR binding sites59; in vitro assays indicate that REV-ERBα binds to two of these sites. Luciferase reporter assays indicate that REV-ERBα protein can inhibit transcription of Cry1 through binding at these two sites. REV- ERBβ also appears to share some functional redundancy with REV-ERBα60.
Fbxl3 and Fbxl21
In addition to Rev-erb, other genes modulate the Cryptochromes. The Overtime mutation in mice was identified in a mutagenesis screen based on a lengthened free-running circadian period61. The responsible mutation was ultimately identified as being in a known gene encoding the F-box protein Fbxl3, but a gene previously unknown to be involved in circadian rhythmicity. Fbxl3OVTM mutant appear to be functionally comparable to null mutants. FBXL3 protein leads to degradation of CRY1, while the FBXL3-OVTM mutant protein is less effective in this capacity. Thus, the period lengthening may be a direct result of a delay in degradation of CRY, effectively preventing the core cycle from restarting. The closely related protein FBXL2162 has been found to function in a similar manner63,64.
NPAS2
NPAS2 (neuronal PAS family member 2) shares the closest homology with CLOCK of all identified bHLH-PAS family members. Null mutants of this gene have altered circadian activity patterns, noteably the absence of a “siesta” in later subjective night, but no dramatic alterations in circadian free-running period or persistence65. However, when null mutants of the Clock had less dramatic phenotypes than the dominant-negative Δ19 mutant31,66, the role of NPAS2 was re- examined. In the absence of functioning CLOCK, NPAS2 appears to be able to partially compensate67.
Dec1 and Dec2
Like other clock genes, Dec1 and Dec2 are basic helix-loop-helix transcription factors that bind to E-boxes. DEC1 and DEC2 have been found to inhibit transactivation of Per by CLOCK and BMAL168. DEC1 and DEC2 form dimers69. The inhibition of CLOCK and BMAL1 transactivation may be related to interactions with BMAL1, but can also be attributed to binding to (and thus possibly competition for) E-boxes70. A human allelic variant in Dec2 has been linked with total sleep time71. This functional relationship has been confirmed in transgenic mice expressing the human Dec2 allele.
Polygenic Circadian Traits
A few spontaneous allelic variants in core clock genes have been identified from a familial pattern that suggests a single gene pattern of inheritance. For example, a familial form of Delayed Sleep Wake Phase Disorder (DSWPD, the most common circadian sleep-wake disorder) has been identified, associated with a mutation in the CRY1 gene72. Individuals with Advanced Sleep Wake Phase Disorder (ASWPD) fall asleep several hours earlier than the general population, but again if allowed to sleep during their desired time window, sleep quality and duration are normal for age73. There is a strong familial component to ASWPD, with mutations demonstrated in casein kinase Id51, and the casein kinase Ie (CKIe) binding region of PER274 and of CRY275. These mutations result in a more rapid progression through the transcription/ translation feedback loop, leading to an overall shorter intrinsic circadian period76. However, in the majority of cases, traits such as intrinsic circadian period are continuous or quantitative (rather than discrete or qualitative), indicative of multiple genetic loci influencing the trait.
Two general approaches to the identification of genes underlying heritability of circadian rhythms in mammals have been taken. In mice, the approach of identification of Quantitative Trait Loci (QTLs) uses linkage mapping to find genes or genomic regions that contribute to quantitative differences in circadian traits. In humans, the approach of Genome-Wide Association Studies uses statistical association of circadian phenotypic traits to genetic polymorphisms in populations.
Quantitative Trait Loci
Genetic heterogeneity underlies many phenotypic variations observed in circadian rhythmicity. The complex nature of circadian behavior is reflected in the phenotypic variability observed in mammalian species; this has been described particularly well in mice. Significant differences in circadian behavior have been demonstrated when comparing multiple inbred strains of mice2–5. In particular, when endogenous period has been measured, differences of nearly a full hour have been observed among the most divergent strains. Strain differences are also present in other aspects of circadian behavior, including entrainment to light-dark cycles and the robustness of the rhythm. Crosses among such inbred strains have indicated a polygenic basis for these differences in circadian behavior.
The first comprehensive quantitative trait locus (QTL) analysis of circadian behavior in mammals (mouse) and the first to use genetic interaction analysis to identify QTLs was conducted in 200177. In this work, 14 QTLs in the mouse genome associated with five different phenotypic traits underlying circadian behavior. Importantly, all but one of these QTLs did not overlap with known circadian clock genes, demonstrating that many allelic variants in genes beyond the canonical circadian clock mechanism must exist. Although severe disruption of circadian rhythms may be caused by mutations in core clock genes, it is possible that the broad variety of circadian behavior observed in mammalian species, including humans, is the result of polymorphisms in multiple, interacting loci.
Molecular identification of interacting QTL
Quantitative genetic approaches were used to identify modifiers of the original Clock mutation, Clock∆19, in the genetic backgrounds of C3H/HeJ and BALB/cJ inbred mouse strains. In C3H/HeJ, genetic suppression of circadian phenotype in Clock∆19 mutant mice is mediated via the melatonin biosynthetic pathway78. Two major QTLs were mapped on chromosomes 11 and X in which genes encoding melatonin biosynthetic enzymes, Aanat (aralkylamine N- acetyltransferase) and Asmt (acetylserotonin O-methyltransferase) are mapped, respectively. It has been known that most laboratory mouse strains, except C3H/HeJ and CBA/J, do not make detectable level of melatonin due to the mutations on Aanat and Asmt79,80. Melatonin and the selective melatonin agonist, ramelteon, indeed can phenocopy the genetic suppression of the Clock∆19 mutation in the suprachiasmatic nucleus (SCN). The Clock∆19 mutation has also been shown to be associated with metabolic syndrome in mice81. It is therefore interesting that the melatonin receptor 1B (MTNR1B) gene has been strongly associated with fasting serum glucose levels in humans82–84. Perhaps, the suppressive effects of melatonin on circadian rhythm may also have relevance to other phenotypes such as metabolism.
On the other hand, the gene responsible for suppression of the Clock∆19 mutant phenotypic in the BALB/cJ strain background is a transcription factor, Usf1, occurring as a consequence of a single-nucleotide change in the promoter region that increases mRNA and protein expression in BALB/cJ mice85. USF1 and CLOCK:BMAL1 compete for the same E-box regulatory sites in circadian genes, such as Per1,Per2 or Dbp. The Clock∆19 mutation significantly reduces the affinity of CLOCK:BMAL1 binding to E-box sites. The reduced affinity of the mutant CLOCK:BMAL1 complex permits USF1 to compete more effectively for the same E-box sites, and the transcriptional activation potential of USF1 can then rescue the reduced transcriptional activation of the CLOCK∆19 mutant protein. USF1 and CLOCK:BMAL1 DNA binding were examined on a genome-wide basis using ChIP-Seq technology. This revealed that the Clock∆19 mutant causes dramatic increases in USF1 binding genome-wide and also reveals that the USF1 and CLOCK:BMAL1 transcriptional networks interact extensively. This has ramifications for understanding the shared role of USF1 and CLOCK:BMAL1 in the regulation of metabolism and lipid biosynthetic pathways.
Genome-Wide Association Studies
Genome wide association studies (GWAS) are used to identify associations between chromosomal regions (loci) and trait of interest in human. Since genomic regions of linkage disequilibrium are much smaller in human populations (<100kb) than in experimental mouse cross (40–80Mb), the mapping resolution in GWAS is much higher than mouse QTL so that very limited number of candidate genes are going to be identified. Several large scale GWAS were conducted for circadian amplitude and for chronotype (such as morningness and eveningness) recently88–88. These studies identified a large number of loci, most of which are not canonical clock genes.
These human findings coincide with the previous QTL mapping in mouse crosses77 and supports the notion that the broad variety of circadian behavior observed in humans in phase and in amplitude is the result of polymorphisms in multiple, interacting loci. The number of loci detected in these GWAS was much higher than the mouse study. This may be because the number of samples for GWAS are 2–3 log unit higher than mouse study, so that detection of loci associating with circadian phenotype is much more sensitive. Natural variations in human circadian rhythms are subject to complex, polygenic regulation, with allelic variants at large numbers of loci affecting the circadian phenotypes. With the possibility of a hundred or more loci influencing the phenotype, the relative contribution of each genetic locus may be very small.
The Circadian Transcriptome
As described above, the molecular circadian clock, at its core, is a transcriptional and translational feedback loop, a direct cellular output of which is waves of oscillatory gene expression driven by the rhythmic transcriptional activity of the clock. It has been a central question to understand the program and functional importance of the circadian transcriptome in gene expression profile studies, in which gene expression levels in the SCN and peripheral tissues are accessed at regular time-points under entrained (e.g., 12hr Light:12hr Dark) or free- running (e.g., constant dark) conditions. Early studies have estimated that roughly 30% of the genome is under circadian control89–92. This number has been increasing since due to technical advances in gene expression profiling techniques and larger sample sizes used in subsequent studies. A comprehensive study in mice sampled 12 tissues and found that collectively the expression of 43% of all protein-coding genes showed a circadian rhythmicity93. A recent study found an even higher number of genes whose expression were rhythmic in at least one tissue of a diurnal primate94. These gene expression-profiling studies have revealed a complex circadian transcriptome. First, the circadian transcriptome is highly tissue-specific, and only a small fraction of clock-regulated genes are rhythmic across different tissues91–95. This tissue specificity appears to be associated with the particular function and metabolic need of the tissue91–96. For example, genes rhythmic in the SCN are involved in neuropeptide metabolism and are known for regulating the circadian locomotor activity, while genes cycling in the liver are important for nutrient metabolisms and regulation of metabolic intermediates91. In addition, the phases (e.g., peak time) of the circadian gene expression in a tissue distribute across the entire 24-hour day, as opposed to be restricted to the peak of transcriptional activity of the clock in the tissue, although tissue-specific “rush hours” (times when a large proportion of rhythmic genes have peak transcription) can be observed93. Furthermore, even for a gene that are cycling across multiple tissues, the peak of the oscillation can vary by hours in one tissue compared to another93. The complexity of circadian transcriptomic oscillations across multiple tissues raises an intriguing implication that the circadian transcriptomic program must be under intricate orchestration throughout the body for an overall optimal functional output and health outcome.
A number of mechanisms may contribute to this complexity of circadian transcriptome. First, the tissue-specific circadian program in the transcriptome is also likely to be associated with tissue-specific configurations of the epigenome, the chemical modifications to the DNA and histone proteins that regulate the binding of transcription factors to regulatory regions of genes and thus the transcriptional activity. Tissue-specific epigenome configurations are critical to establishing cell identity. The epigenome also exhibits clock-regulated circadian oscillations, which correlate with the rhythms in the transcriptome97–100. Interestingly, CLOCK shows histone acetyltransferase activity, which modifies chromatin to allow for the accessibility of regulatory elements of genes by transcription factors101, a required step for clock-driven circadian transcription activation102. Secondly, in addition to interacting with each other to regulate gene expression, each of the clock proteins also interacts with other transcriptional regulators with distinctive profiles of transcriptional target genes103. For example, CLOCK in flies interacts with a range of partner transcription factors and binds to cis-regulatory motifs of genes in a tissue- specific manner, which is associated with the tissue-specific program of circadian transcriptome104. Furthermore, by comparing oscillatory patterns of total mRNA to pre-mRNA or nascent mRNA, recent studies suggest that rhythmic transcription activation may only contribute to a portion or even a small portion (e.g., 20%−30%) of the rhythms observed in the transcriptome and that post-transcriptional mechanisms are involved103,105,106. Consistent with these observations, the transcript levels of hundreds of non-coding regulatory RNAs are also rhythmic93,99.
In addition to these cellular mechanisms, the circadian transcriptome is also regulated by systemic signals either directly from the central brain clock in the suprachiasmatic nuclei (SCN) or indirectly from SCN output rhythms. When the clock machinery was arrested in the liver by tissue-specific inhibition of Bmal1 expression, 10% of circadian transcriptome remained cycling, suggested a strong contribution from the central clock96. Time of feeding, an output rhythm driven by the SCN clock, together with the SCN clock itself, has been shown to drive the rhythms in the hepatic transcriptome in mice107,108. Similarly, when mice were sleep deprived at different times of the day so that their sleep debt no longer varied across the circadian cycle, the rhythmic pattern of gene expression in the brain are largely diminished, indicating circadian rhythmic gene expression in the brain are largely dependent on the sleep/wake rhythms109. Consistent observations have also been reported in human peripheral blood110,111. Finally, body temperature rhythms also reset the phase of the circadian clock in peripheral tissues112.
The combination of cellular and systemic mechanisms in shaping the peripheral circadian transcriptomic profiles has important health implications. Under conditions such as jet lag and shift work, the timing signals driven by the SCN clock, behavioral activities, and the local clock are no longer in concert, leading to a state known as the “internal desynchrony”. Internal desynchrony is associated with disrupted circadian transcriptome, altered functional outputs, and often disorders. As an example, feeding at a “wrong” time of the day, a condition associated with disrupted metabolic output and deregulation of body weight113, creates desynchrony between the transcriptional output of the local clock and systemic signals from the master clock and a reprogrammed circadian transcriptome107. Another important health implication of the circadian transcriptome can be drawn from the finding that many genes encoding drug targets or metabolizing enzymes are expressed rhythmically93. Thus, the efficacy and toxicity of a drug, particularly one with a short half-life, may be influenced by the time when the drug is taken, a phenomenon that has been reported in circadian pharmacokinetics studies114,115.
A challenge of circadian-based drug treatment is the lack of biomarkers of internal timing, which is characteristic to individuals and is highly variable in human populations116. Although in principle, internal timing can be inferred using transcriptomic and metabolomic data at only one time point117,118, or with only a few markers in combination with advanced statistical methods119, its utility remains to be tested under disease conditions, which typically disrupts circadian transcriptomic patterns, such as seen in the brain of depression patients120. In addition, given the complexity of the tissue-specific circadian program, methodologies capable of inferring tissue-specific circadian timing and internal desynchrony are needed. Finally, future studies are needed to demonstrate whether the circadian transcriptome can be used as a disease signature to aid the disease diagnosis, or even as a reference point to treat the disease by restoring the “optimal” cycling pattern. Indeed, these are very challenging tasks at present, particularly given the circadian transcriptome is also plastic to environmental inputs, such as diet121, and are modulated by aging122. Nevertheless, with the advances in circadian metabolomics123 and proteomics124–128, we are now beginning to gain an even more detailed understanding of the tissue-specific circadian program and its role in health and disease, building a knowledge base that will eventually enable circadian-based medicine.
Conclusions: Clock genes are everywhere
The core circadian oscillator can be found in individual cells. The basis for this oscillation in mammals, as in other organisms, lies in rhythmic feedback regulation of transcription of clock genes. The levels of the PER and CRY proteins alter the rate of transcription of their own genes. This alteration is achieved by inhibition of the enhancement of transcription that results from binding of the CLOCK-BMAL1 heterodimer to the E-box element of the promoter region of the Per and Cry genes. Additional interactions between circadian clock proteins may slow the time course of this feedback, achieving the near-24-hour interval: the phosphorylation of PER by CKIε may lead to its degradation, and the association with BMAL1 appears needed for CLOCK to be present in the nucleus. Mutations in core clock genes are involved in the pathogenesis of some familial circadian rhythm sleep-wake disorders. Beyond these core clock genes, rhythmic transcription programs regulate gene expression and gene activity throughout the genome. Finally, it appears that much of the heritability of circadian rhythm traits in mammals stems from polymorphisms in numerous loci beyond the set of core clock genes, with implications for a wide range of neurologic disorders. As computational and molecular techniques mature, future studies are expected to synthesize an understanding of the circadian genetic program at a multi-scale level, with important implications for circadian medicine.
Key Points.
Circadian rhythms are generated by a genetic mechanism: a feedback loop of gene transcription and translation of core “clock genes”. This genetic clock mechanism functions in diverse cell and tissue types throughout the body.
Gene and polymorphisms in both core clock genes and interacting genes contribute to individual differences in the expression and properties of circadian rhythms.
The circadian clock profoundly influences the patterns of gene activity in nearly all tissues, indicating that genetic alterations lead to expression and development of disease.
Acknowledgements
Preparation of this manuscript was in part supported by NIH PO1 AG 11412.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis FC and Menaker M. 1981. Development of the mouse circadian pacemaker: Independence from environmental cycles. Journal of comparative physiology Volume 143, Issue 4, pp 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz WJ, Zimmerman P. Circadian timekeeping in BALB/c and C57BL/6 inbred mouse strains. J Neurosci 1990;10(11):3685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Possidente B, Hegmann JP, Carlson L, Elder B. Pigment mutations associated with altered circadian rhythms in mice. Physiol Behav 1982;28(3):389–92. [DOI] [PubMed] [Google Scholar]
- 4.Ebihara S, Tsuji K, Kondo K. Strain differences of the mouse’s free-running circadian rhythm in continuous darkness. Physiol Behav 1978;20(6):795–9. [DOI] [PubMed] [Google Scholar]
- 5.Jiang P, Striz M, Wisor JP, O’Hara BF. 2011. Behavioral and genetic dissection of a mouse model for advanced sleep phase syndrome. Sleep 2011. January 1;34(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang P, Franklin KM, Duncan MJ, O’Hara BF, Wisor JP. 2012. Distinct phase relationships between suprachiasmatic molecular rhythms, cerebral cortex molecular rhythms, and behavioral rhythms in early runner (CAST/EiJ) and nocturnal (C57BL/6J) mice. Sleep 2012. October 1;35(10):1385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linkowski P, Van Onderbergen A, Kerkhofs M, Bosson D, Mendlewicz J, and Van Cauter E 1993. Twin study of the 24-h cortisol profile: evidence for genetic control of the human circadian clock. AJP Endocrinology and Metabolism 264(2): E173–E181. [DOI] [PubMed] [Google Scholar]
- 8.Linkowski P 1994. Genetic influences on EEG sleep and the human circadian clock. A twin study. Pharmacopsychiatry 1994. January;27(1):7–10. [DOI] [PubMed] [Google Scholar]
- 9.Toomey R1, Panizzon MS, Kremen WS, Franz CE, Lyons MJ. 2015. A twin-study of genetic contributions to morningness-eveningness and depression. Chronobiol Int 2015. April;32(3):303–9. doi: 10.3109/07420528.2014.971366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vink JM1, Groot AS, Kerkhof GA, Boomsma DI. 2001. Genetic analysis of morningness and eveningness. Chronobiol Int 2001. September;18(5):809–22. [DOI] [PubMed] [Google Scholar]
- 11.Pittendrigh CS, Circadian Rhythms and the Circadian Organization of Living Systems. Cold Spring Harb Symp Quant Biol, 1960. 25(0): p. 159–184. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi JS, Molecular neurobiology and genetics of circadian rhythms in mammals. Annual Review of Neuroscience, 1995. 18: p. 531–553. [DOI] [PubMed] [Google Scholar]
- 13.Inouye SIT, et al. , Inhibitor of protein synthesis phase shifts a circadian pacemaker in the mammalian SCN. American Journal of Physiology, 1988. 255: p. R1055–R1058. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe K, et al. , Anisomycin induces phase shifts of circadian pacemaker in primary cultures of rat suprachiasmatic nucleus. Brain Research, 1995. 684(2): p. 179–184. [DOI] [PubMed] [Google Scholar]
- 15.Konopka RJ and Benzer S, Clock mutants of Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America, 1971. 68(9): p. 2112–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vitaterna MH, et al. , Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science, 1994. 264(5159): p. 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunlap JC, Genetics and molecular analysis of circadian rhythms. Annual Review of Genetics, 1996. 30: p. 579–601. [DOI] [PubMed] [Google Scholar]
- 18.Millar AJ, et al. , Circadian clock mutants in Arabidopsis identified by luciferase imaging. Science, 1995. 267(5201): p. 1161–1163. [DOI] [PubMed] [Google Scholar]
- 19.Kondo T, et al. , Circadian clock mutants of cyanobacteria. Science, 1994. 266(5188): p. 1233–1236. [DOI] [PubMed] [Google Scholar]
- 20.Bargiello TA, Jackson FR, and Young MW, Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature, 1984. 312(5996): p. 752–754. [DOI] [PubMed] [Google Scholar]
- 21.Zehring WA, et al. , P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell, 1984. 39(2 Pt 1): p. 369–376. [DOI] [PubMed] [Google Scholar]
- 22.Hardin PE, Hall JC, and Rosbash M, Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature, 1990. 343(6258): p. 536–540. [DOI] [PubMed] [Google Scholar]
- 23.Hardin PE, Hall JC, and Rosbash M, Circadian oscillations in period gene mRNA levels are transcriptionally regulated. Proceedings of the National Academy of Sciences of the United States of America, 1992. 89(24): p. 11711–11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edery I, et al. , Temporal phosphorylation of the Drosophila period protein. Proceedings of the National Academy of Sciences of the United States of America, 1994. 91(6): p. 2260–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edery I, Rutila JE, and Rosbash M, Phase shifting of the circadian clock by induction of the Drosophila period protein. Science, 1994. 263(5144): p. 237–240. [DOI] [PubMed] [Google Scholar]
- 26.Albrecht U, et al. , A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell, 1997. 91(7): p. 1055–1064. [DOI] [PubMed] [Google Scholar]
- 27.Shearman LP, et al. , Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron, 1997. 19(6): p. 1261–1269. [DOI] [PubMed] [Google Scholar]
- 28.Sun ZS, et al. , RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell, 1997. 90(6): p. 1003–1011. [DOI] [PubMed] [Google Scholar]
- 29.Tei H, et al. , Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature, 1997. 389(6650): p. 512–516. [DOI] [PubMed] [Google Scholar]
- 30.Zylka MJ, et al. , Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron, 1998. 20(6): p. 1103–10. [DOI] [PubMed] [Google Scholar]
- 31.King DP, et al. , The mouse Clock mutation behaves as an antimorph and maps within the W19H deletion, distal of Kit. Genetics, 1997. 146(3): p. 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antoch MP, et al. , Functional identification of the mouse circadian Clock gene by transgenic BAC rescue. Cell, 1997. 89(4): p. 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King DP, et al. , Positional cloning of the mouse circadian clock gene. Cell, 1997. 89(4): p. 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gekakis N, et al. , Role of the CLOCK protein in the mammalian circadian mechanism. Science, 1998. 280(5369): p. 1564–1569. [DOI] [PubMed] [Google Scholar]
- 35.Kondratov RV, et al. , BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev, 2003. 17(15): p. 1921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hao H, Allen DL, and Hardin PE, A circadian enhancer mediates PER-dependent mRNA cycling in Drosophila melanogaster. Molecular & Cellular Biology, 1997. 17(7): p. 3687–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bunger MK, et al. , Mop3 is an essential component of the master circadian pacemaker in mammals. Cell, 2000. 103(7): p. 1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shearman L and Weaver D, Photic induction of Period gene expression is reduced in Clock mutant mice. Neuroreport, 1999. 10(3): p. 613–8. [DOI] [PubMed] [Google Scholar]
- 39.Bae K, et al. , Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron, 2001. 30(2): p. 525–36. [DOI] [PubMed] [Google Scholar]
- 40.Zheng B, et al. , Nonredundant roles of the mPer1 and mPer2 genes in the mammalian circadian clock. Cell, 2001. 105(5): p. 683–94. [DOI] [PubMed] [Google Scholar]
- 41.Miyamoto Y and Sancar A, Vitamin B2-based blue-light photoreceptors in the retinohypothalamic tract as the photoactive pigments for setting the circadian clock in mammals. Proc Natl Acad Sci U S A, 1998. 95(11): p. 6097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thresher RJ, et al. , Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science, 1998. 282(5393): p. 1490–1494. [DOI] [PubMed] [Google Scholar]
- 43.Vitaterna MH, et al. , Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci U S A, 1999. 96(21): p. 12114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Horst GT, et al. , Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature, 1999. 398(6728): p. 627–30. [DOI] [PubMed] [Google Scholar]
- 45.Okamura H, et al. , Photic induction of mPer1 and mPer2 in cry-deficient mice lacking a biological clock. Science, 1999. 286(5449): p. 2531–4. [DOI] [PubMed] [Google Scholar]
- 46.Kume K, et al. , mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell, 1999. 98(2): p. 193–205. [DOI] [PubMed] [Google Scholar]
- 47.Ralph MR and Menaker M, A mutation of the circadian system in golden hamsters. Science, 1988. 241(4870): p. 1225–1227. [DOI] [PubMed] [Google Scholar]
- 48.Shimomura K and Menaker M, Light-induced phase shifts in tau mutant hamsters. Journal of Biological Rhythms, 1994. 9(2): p. 97–110. [DOI] [PubMed] [Google Scholar]
- 49.Lowrey PL, et al. , Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science, 2000. 288(5465): p. 483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Etchegaray JP, et al. , Casein kinase 1 delta (CK1delta) regulates period length of the mouse suprachiasmatic circadian clock in vitro. PLoS One, 2010. 5(4): p. e10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu Y, et al. , Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature, 2005. 434(7033): p. 640–4. [DOI] [PubMed] [Google Scholar]
- 52.Isojima Y, et al. , CKIepsilon/delta-dependent phosphorylation is a temperature- insensitive, period-determining process in the mammalian circadian clock. Proc Natl Acad Sci U S A, 2009. 106(37): p. 15744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee H, et al. , Essential roles of CKIdelta and CKIepsilon in the mammalian circadian clock. Proc Natl Acad Sci U S A, 2009. 106(50): p. 21359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Preitner N, et al. , The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell, 2002. 110(2): p. 251–60. [DOI] [PubMed] [Google Scholar]
- 55.Asher G, et al. , SIRT1 Regulates Circadian Clock Gene Expression through PER2 Deacetylation. Cell, 2008. 134(2): p. 317–328. [DOI] [PubMed] [Google Scholar]
- 56.Nakahata Y, et al. , The NAD+-Dependent Deacetylase SIRT1 Modulates CLOCK- Mediated Chromatin Remodeling and Circadian Control. Cell, 2008. 134(2): p. 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakahata Y, et al. , Circadian Control of the NAD+ Salvage Pathway by CLOCK- SIRT1. Science, 2009. 324(5927): p. 654–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramsey KM, et al. , Circadian Clock Feedback Cycle Through NAMPT-Mediated NAD+ Biosynthesis. Science, 2009. 324(5927): p. 651–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Etchegaray JP, et al. , Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature, 2003. 421(6919): p. 177–82. [DOI] [PubMed] [Google Scholar]
- 60.Liu AC, et al. , Redundant Function of REV-ERBα and β and Non-Essential Role for Bmal1 Cycling in Transcriptional Regulation of Intracellular Circadian Rhythms. PLoS Genet, 2008. 4(2): p. e1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siepka SM, et al. , Circadian Mutant Overtime Reveals F-box Protein FBXL3 Regulation of Cryptochrome and Period Gene Expression. Cell 129(5): p. 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dardente H, et al. , Implication of the F-Box Protein FBXL21 in circadian pacemaker function in mammals. PLoS One, 2008. 3(10): p. e3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hirano A, et al. , FBXL21 regulates oscillation of the circadian clock through ubiquitination and stabilization of cryptochromes. Cell, 2013. 152(5): p. 1106–18. [DOI] [PubMed] [Google Scholar]
- 64.Yoo SH, et al. , Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell, 2013. 152(5): p. 1091–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reick M, et al. , NPAS2: An Analog of Clock Operative in the Mammalian Forebrain. Science, 2001. 293(5529): p. 506–509. [DOI] [PubMed] [Google Scholar]
- 66.DeBruyne JP, et al. , A Clock Shock: Mouse CLOCK Is Not Required for Circadian Oscillator Function. Neuron, 2006. 50(3): p. 465–477. [DOI] [PubMed] [Google Scholar]
- 67.DeBruyne JP, Weaver DR, and Reppert SM, CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci, 2007. 10(5): p. 543–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Honma S, et al. , Dec1 and Dec2 are regulators of the mammalian molecular clock. Nature, 2002. 419(6909): p. 841–844. [DOI] [PubMed] [Google Scholar]
- 69.Sato F, et al. , Functional analysis of the basic helix-loop-helix transcription factor DEC1 in circadian regulation. European Journal of Biochemistry, 2004. 271(22): p. 4409–4419. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, et al. , DNA binding, but not interaction with Bmal1, is responsible for DEC1- mediated transcription regulation of the circadian gene mPer1. Biochem. J, 2004. 382(3): p. 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He Y, et al. , The Transcriptional Repressor DEC2 Regulates Sleep Length in Mammals. Science, 2009. 325(5942): p. 866–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patke A, Murphy PJ, Onat OE, Krieger AC, Özçelik T, Campbell SS, Young MW. Mutation of the Human Circadian Clock Gene CRY1 in Familial Delayed Sleep Phase Disorder. Cell 2017. April 6;169(2):203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. American Academy of Sleep Medicine 2014.
- 74.Toh KL1, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptácek LJ, Fu YH. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 2001. February 9;291(5506):1040–3. [DOI] [PubMed] [Google Scholar]
- 75.Hirano A, Shi G, Jones CR, Lipzen A, Pennacchio LA, Xu Y, Hallows WC, McMahon T, Yamazaki M, Ptáček LJ, Fu YH. A Cryptochrome 2 mutation yields advanced sleep phase in humans. Elife 2016. August 16;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shanware NP, Hutchinson JA, Kim SH, Zhan L, Bowler MJ, Tibbetts RS. Casein kinase 1-dependent phosphorylation of familial advanced sleep phase syndrome-associated residues controls PERIOD 2 stability. J Biol Chem 2011. April 8;286(14):12766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shimomura K, Low-Zeddies SS, King DP, Steeves TD, Whiteley A, Kushla J, Zemenides PD, Lin A, Vitaterna MH, Churchill GA, Takahashi JS. Genome-wide epistatic interaction analysis reveals complex genetic determinants of circadian behavior in mice. Genome Res 2001;11(6):959–80. 10.1101/gr.171601. [DOI] [PubMed] [Google Scholar]
- 78.Shimomura K, Lowrey PL, Vitaterna MH, Buhr ED, Kumar V, Hanna P, Omura C, Izumo M, Low SS, Barrett RK, LaRue SI, Green CB, Takahashi JS. Genetic suppression of the circadian Clock mutation by the melatonin biosynthesis pathway. Proc Natl Acad Sci U S A 2010;107(18):8399–403. 10.1073/pnas.1004368107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roseboom PH, Namboodiri MA, Zimonjic DB, Popescu NC, Rodriguez IR, Gastel JA, Klein DC. Natural melatonin ‘knockdown’ in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res 1998;63(1):189–97. [DOI] [PubMed] [Google Scholar]
- 80.Kasahara T, Abe K, Mekada K, Yoshiki A, Kato T. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc Natl Acad Sci U S A 2010;107(14):6412–7. 10.1073/pnas.0914399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee- Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005;308(5724):1043–5. 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, Delplanque J, Lobbens S, Rocheleau G, Durand E, De Graeve F, Chevre JC, Borch-Johnsen K, Hartikainen AL, Ruokonen A, Tichet J, Marre M, Weill J, Heude B, Tauber M, Lemaire K, Schuit F, Elliott P, Jorgensen T, Charpentier G, Hadjadj S, Cauchi S, Vaxillaire M, Sladek R, Visvikis-Siest S, Balkau B, Levy-Marchal C, Pattou F, Meyre D, Blakemore AI, Jarvelin MR, Walley AJ, Hansen T, Dina C, Pedersen O, Froguel P. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet 2009;41(1):89–94. 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 83.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, Bugliani M, Saxena R, Fex M, Pulizzi N, Isomaa B, Tuomi T, Nilsson P, Kuusisto J, Tuomilehto J, Boehnke M, Altshuler D, Sundler F, Eriksson JG, Jackson AU, Laakso M, Marchetti P, Watanabe RM, Mulder H, Groop L. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet 2009;41(1):82–8. 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, Loos RJ, Manning AK, Jackson AU, Aulchenko Y, Potter SC, Erdos MR, Sanna S, Hottenga JJ, Wheeler E, Kaakinen M, Lyssenko V, Chen WM, Ahmadi K, Beckmann JS, Bergman RN, Bochud M, Bonnycastle LL, Buchanan TA, Cao A, Cervino A, Coin L, Collins FS, Crisponi L, de Geus EJ, Dehghan A, Deloukas P, Doney AS, Elliott P, Freimer N, Gateva V, Herder C, Hofman A, Hughes TE, Hunt S, Illig T, Inouye M, Isomaa B, Johnson T, Kong A, Krestyaninova M, Kuusisto J, Laakso M, Lim N, Lindblad U, Lindgren CM, McCann OT, Mohlke KL, Morris AD, Naitza S, Orru M, Palmer CN, Pouta A, Randall J, Rathmann W, Saramies J, Scheet P, Scott LJ, Scuteri A, Sharp S, Sijbrands E, Smit JH, Song K, Steinthorsdottir V, Stringham HM, Tuomi T, Tuomilehto J, Uitterlinden AG, Voight BF, Waterworth D, Wichmann HE, Willemsen G, Witteman JC, Yuan X, Zhao JH, Zeggini E, Schlessinger D, Sandhu M, Boomsma DI, Uda M, Spector TD, Penninx BW, Altshuler D, Vollenweider P, Jarvelin MR, Lakatta E, Waeber G, Fox CS, Peltonen L, Groop LC, Mooser V, Cupples LA, Thorsteinsdottir U, Boehnke M, Barroso I, Van Duijn C, Dupuis J, Watanabe RM, Stefansson K, McCarthy MI, Wareham NJ, Meigs JB, Abecasis GR. Variants in MTNR1B influence fasting glucose levels. Nat Genet 2009;41(1):77–81. 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimomura K, Kumar V, Koike N, Kim TK, Chong J, Buhr ED, Whiteley AR, Low SS, Omura C, Fenner D, Owens JR, Richards M, Yoo SH, Hong HK, Vitaterna MH, Bass J, Pletcher MT, Wiltshire T, Hogenesch J, Lowrey PL, Takahashi JS. Usf1, a suppressor of the circadian Clock mutant, reveals the nature of the DNA-binding of the CLOCK:BMAL1 complex in mice. Elife 2013;2:e00426 10.7554/eLife.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ferguson A, Lyall LM, Ward J, Strawbridge RJ, Cullen B, Graham N, Niedzwiedz CL, Johnston KJA, MacKay D, Biello SM, Pell JP, Cavanagh J, McIntosh AM, Doherty A, Bailey MES, Lyall DM, Wyse CA, Smith DJ. Genome-Wide Association Study of Circadian Rhythmicity in 71,500 UK Biobank Participants and Polygenic Association with Mood Instability. EBioMedicine 2018;35:279–87. 10.1016/j.ebiom.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dashti HS, Jones SE, Wood AR, Lane JM, van Hees VT, Wang H, Rhodes JA, Song Y, Patel K, Anderson SG, Beaumont RN, Bechtold DA, Bowden J, Cade BE, Garaulet M, Kyle SD, Little MA, Loudon AS, Luik AI, Scheer F, Spiegelhalder K, Tyrrell J, Gottlieb DJ, Tiemeier H, Ray DW, Purcell SM, Frayling TM, Redline S, Lawlor DA, Rutter MK, Weedon MN, Saxena R. Genome-wide association study identifies genetic loci for self- reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun 2019;10(1):1100 10.1038/s41467-019-08917-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones SE, Lane JM, Wood AR, van Hees VT, Tyrrell J, Beaumont RN, Jeffries AR, Dashti HS, Hillsdon M, Ruth KS, Tuke MA, Yaghootkar H, Sharp SA, Jie Y, Thompson WD, Harrison JW, Dawes A, Byrne EM, Tiemeier H, Allebrandt KV, Bowden J, Ray DW, Freathy RM, Murray A, Mazzotti DR, Gehrman PR, Lawlor DA, Frayling TM, Rutter MK, Hinds DA, Saxena R, Weedon MN. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun 2019;10(1):343 10.1038/s41467-018-08259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Akhtar RA, Reddy AB, Maywood ES, et al. Circadian Cycling of the Mouse Liver Transcriptome, as Revealed by cDNA Microarray, Is Driven by the Suprachiasmatic Nucleus. Current Biology 2002;12(7):540–550. [DOI] [PubMed] [Google Scholar]
- 90.Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC. Circadian Programs of Transcriptional Activation, Signaling, and Protein Turnover Revealed by Microarray Analysis of Mammalian Cells. Current Biology 2002;12(7):551–557. [DOI] [PubMed] [Google Scholar]
- 91.Panda S, Antoch MP, Miller BH, et al. Coordinated Transcription of Key Pathways in the Mouse by the Circadian Clock. Cell 2002;109(3):307–320. [DOI] [PubMed] [Google Scholar]
- 92.Storch K-F, Lipan O, Leykin I, et al. Extensive and divergent circadian gene expression in liver and heart. Nature 2002;417(6884):78–83. [DOI] [PubMed] [Google Scholar]
- 93.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proceedings of the National Academy of Sciences 2014. [DOI] [PMC free article] [PubMed]
- 94.Mure LS, Le HD, Benegiamo G, et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science (New York, NY) 2018;359(6381):eaao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller BH, McDearmon EL, Panda S, et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proceedings of the National Academy of Sciences 2007;104(9):3342–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol 2007;5(2):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Le Martelot G, Canella D, Symul L, et al. Genome-Wide RNA Polymerase II Profiles and RNA Accumulation Reveal Kinetics of Transcription and Associated Epigenetic Changes During Diurnal Cycles. PLoS Biol 2012;10(11):e1001442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, Naef F. Genome-Wide and Phase-Specific DNA-Binding Rhythms of BMAL1 Control Circadian Output Functions in Mouse Liver. PLoS Biol 2011;9(2):e1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vollmers C, Schmitz Robert J, Nathanson J, Yeo G, Ecker Joseph R, Panda S. Circadian Oscillations of Protein-Coding and Regulatory RNAs in a Highly Dynamic Mammalian Liver Epigenome. Cell Metabolism 2012;16(6):833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Papazyan R, Zhang Y, Lazar MA. Genetic and epigenomic mechanisms of mammalian circadian transcription. Nature Structural &Amp; Molecular Biology 2016;23:1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Menet JS, Pescatore S, Rosbash M. CLOCK:BMAL1 is a pioneer-like transcription factor. Genes & development 2014;28(1):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Doi M, Hirayama J, Sassone-Corsi P. Circadian Regulator CLOCK Is a Histone Acetyltransferase. Cell 2006;125(3):497–508. [DOI] [PubMed] [Google Scholar]
- 103.Koike N, Yoo S-H, Huang H-C, et al. Transcriptional Architecture and Chromatin Landscape of the Core Circadian Clock in Mammals. Science (New York, NY) 2012;338(6105):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meireles-Filho Antonio CA, Bardet Anaïs F, Yáñez-Cuna JO, Stampfel G, Stark A. cis-Regulatory Requirements for Tissue-Specific Programs of the Circadian Clock. Current Biology 2014;24(1):1–10. [DOI] [PubMed] [Google Scholar]
- 105.Menet JS, Rodriguez J, Abruzzi KC, Rosbash M. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. eLife 2012;1. [DOI] [PMC free article] [PubMed]
- 106.Rodriguez J, Tang C-HA, Khodor YL, Vodala S, Menet JS, Rosbash M. Nascent- Seq analysis of Drosophila cycling gene expression. Proceedings of the National Academy of Sciences 2013;110(4):E275–E284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proceedings of the National Academy of Sciences of the United States of America 2009;106(50):21453–21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Greenwell BJ, Trott AJ, Beytebiere JR, et al. Rhythmic Food Intake Drives Rhythmic Gene Expression More Potently than the Hepatic Circadian Clock in Mice. Cell Reports 2019;27(3):649–657.e645. [DOI] [PubMed] [Google Scholar]
- 109.Maret S, Dorsaz S, Gurcel L, et al. Homer1a is a core brain molecular correlate of sleep loss. Proceedings of the National Academy of Sciences of the United States of America 2007;104(50):20090–20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Möller-Levet CS, Archer SN, Bucca G, et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proceedings of the National Academy of Sciences 2013;110(12):E1132–E1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Archer SN, Laing EE, Möller-Levet CS, et al. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proceedings of the National Academy of Sciences 2014;111(6):E682–E691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science (New York, NY) 2010;330(6002):379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang P, Turek FW. The endogenous circadian clock programs animals to eat at certain times of the 24-hour day: What if we ignore the clock? Physiology & behavior 2018;193(Pt B):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Griffett K, Burris TP. The mammalian clock and chronopharmacology. Bioorganic & medicinal chemistry letters 2013;23(7):1929–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dallmann R, Okyar A, Lévi F. Dosing-Time Makes the Poison: Circadian Regulation and Pharmacotherapy. Trends in Molecular Medicine 2016;22(5):430–445. [DOI] [PubMed] [Google Scholar]
- 116.Fischer D, Lombardi DA, Marucci-Wellman H, Roenneberg T. Chronotypes in the US - Influence of age and sex. PloS one 2017;12(6):e0178782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kasukawa T, Sugimoto M, Hida A, et al. Human blood metabolite timetable indicates internal body time. Proceedings of the National Academy of Sciences of the United States of America 2012;109(37):15036–15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ueda HR, Chen W, Minami Y, et al. Molecular-timetable methods for detection of body time and rhythm disorders from single-time-point genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America 2004;101(31):11227–11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Braun R, Kath WL, Iwanaszko M, et al. Universal method for robust detection of circadian state from gene expression. Proceedings of the National Academy of Sciences 2018;115(39):E9247–E9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li JZ, Bunney BG, Meng F, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proceedings of the National Academy of Sciences of the United States of America 2013;110(24):9950–9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eckel-Mahan Kristin L, Patel Vishal R, de Mateo S, et al. Reprogramming of the Circadian Clock by Nutritional Challenge. Cell 2013;155(7):1464–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sato S, Solanas G, Peixoto FO, et al. Circadian Reprogramming in the Liver Identifies Metabolic Pathways of Aging. Cell 2017;170(4):664–677.e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dyar KA, Eckel-Mahan KL. Circadian Metabolomics in Time and Space. Frontiers in neuroscience 2017;11:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hurley JM, Jankowski MS, De Los Santos H, et al. Circadian Proteomic Analysis Uncovers Mechanisms of Post-Transcriptional Regulation in Metabolic Pathways. Cell systems 2018;7(6):613–626.e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schenk S, Bannister SC, Sedlazeck FJ, et al. Combined transcriptome and proteome profiling reveals specific molecular brain signatures for sex, maturation and circalunar clock phase. eLife 2019;8. [DOI] [PMC free article] [PubMed]
- 126.Wang Y, Song L, Liu M, et al. A proteomics landscape of circadian clock in mouse liver. Nature communications 2018;9(1):1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang J, Mauvoisin D, Martin E, et al. Nuclear Proteomics Uncovers Diurnal Regulatory Landscapes in Mouse Liver. Cell Metab 2017;25(1):102–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Robles MS, Humphrey SJ, Mann M. Phosphorylation Is a Central Mechanism for Circadian Control of Metabolism and Physiology. Cell Metab 2017;25(1):118–127. [DOI] [PubMed] [Google Scholar]


