Abstract
Background
The myelodysplastic syndrome (MDS) comprises a diverse group of haematopoietic stem cell disorders. Due to symptomatic anaemia, most people with MDS require supportive therapy including repeated red blood cell (RBC) transfusions. In combination with increased iron absorption, this contributes to the accumulation of iron resulting in secondary iron overload and the risk of organ dysfunction and reduced life expectancy. Since the human body has no natural means of removing excess iron, iron chelation therapy, i.e. the pharmacological treatment of iron overload, is usually recommended. However, it is unclear whether or not the newer oral chelator deferasirox leads to relevant benefit.
Objectives
To evaluate the effectiveness and safety of oral deferasirox for managing iron overload in people with myelodysplastic syndrome (MDS).
Search methods
We searched the following databases up to 03 April 2014: MEDLINE, EMBASE, The Cochrane Library, Biosis Previews, Web of Science, Derwent Drug File and four trial registries: Current Controlled Trials (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov), ICTRP (www.who.int./ictrp/en/), and German Clinical Trial Register (www.drks.de).
Selection criteria
Randomised controlled trials (RCTs) comparing deferasirox with no therapy, placebo or with another iron‐chelating treatment schedule.
Data collection and analysis
We did not identify any trials eligible for inclusion in this review.
Main results
No trials met our inclusion criteria. However, we identified three ongoing and one completed trial (published as an abstract only and in insufficient detail to permit us to decide on inclusion) comparing deferasirox with deferoxamine, placebo or no treatment.
Authors' conclusions
We planned to report evidence from RCTs that evaluated the effectiveness of deferasirox compared to either placebo, no treatment or other chelating regimens, such as deferoxamine, in people with MDS. However, we did not identify any completed RCTs addressing this question.
We found three ongoing and one completed RCT (published as an abstract only and in insufficient detail) comparing deferasirox with deferoxamine, placebo or no treatment and data will hopefully be available soon. These results will be important to inform physicians and patients on the advantages and disadvantages of this treatment option.
Plain language summary
Deferasirox for managing iron overload in people with myelodysplastic syndrome
Review question
We aimed to review the evidence about the effects of deferasirox (an oral therapeutic option) on reducing iron overload in people with myelodysplastic syndrome (MDS), which is a diverse group of haematopoietic stem cell disorders.
Background
Repeated red blood cell transfusions can lead to clinically relevant secondary (i.e. due to the transfusions) iron overload in some people with MDS, particularly of lower risk groups over the course of their disease. Since the human body has no natural means of removing excess iron, drugs to remove the excess iron (iron chelation therapy) might be indicated to prevent organ complications. Since the newer oral iron chelator deferasirox has become available, iron chelation therapy is offered more widely to people with MDS.
We wanted to assess whether administering deferasirox is beneficial in people with MDS.
Key results
We searched the available literature up to 03 April 2014. We could not include any data in this review that answered our question. However, we found three ongoing trials and one completed trial investigating deferasirox in people with MDS of lower risk groups (low and intermediate‐1 risk MDS). As the completed trial has only been reported in insufficient detail (in abstract format), we were unable to definitively decide on inclusion of this study or draw any conclusions from this. Once available, these results will be important to inform physicians and patients on the comparative advantages and disadvantages of this treatment option.
Background
Description of the condition
The myelodysplastic syndrome (MDS) comprises a diverse group of haematopoietic stem cell disorders which are usually classified according to the World Health Organization (WHO) MDS classification (Harris 1999; Vardiman 2002). They are characterised by abnormal differentiation and maturation of blood cells, bone marrow failure and a genetic instability with an enhanced risk of transformation to leukaemia. The annual incidence reported in the literature is between 2.1 to 12.6 cases per 100,000 people per year (Aul 2001). Men aged over 70 years are mainly affected, with incidence rates reaching 50 cases per 100,000 people per year (Aul 2001).
People with MDS can be subdivided in prognostic groups according to the International Prognostic Scoring System (IPSS) taking into account bone marrow blast percentage, cytogenetic profile and the number of cytopenias (Greenberg 1997). For higher risk groups, drug treatment (e.g. with azacitidine), intensive chemotherapy or even haematopoetic stem cell transplantation (depending on disease‐ and patient‐related factors) is usually required. Recently, a new WHO classification‐based prognostic scoring system (WPSS) has been proposed (Malcovati 2007), classifying patients into five risk groups according to WHO subgroups, karyotype abnormalities according to IPSS and transfusion requirements.
Mainly for the risk groups designated low and intermediate‐1 (according to IPSS) only supportive therapy including red blood cell (RBC) transfusions for symptomatic anaemia might be indicated (NCCN Myelodysplastic Panel Members 2010). Regular RBC transfusions in combination with prolonged dyserythropoiesis and increased iron absorption contribute to the accumulation of iron resulting in secondary iron overload. This can ultimately lead to organ dysfunction affecting the liver, endocrine glands and the heart, resulting in reduced life expectancy (Malcovati 2005; Takatoku 2007). Since the human body has no natural means of getting rid of excess iron, iron chelation therapy, i.e. pharmacological treatment of iron overload, is usually recommended (List 2006; Valent 2008).
Description of the intervention
Deferoxamine (DFO, Desferal®), reviewed in detail in a Cochrane Review (Fisher 2013), has been the treatment of choice for iron overload for the last 40 years. Due to its long standing availability it is the only chelating agent for which profound effects on the long‐term survival of a large cohort of patients with thalassaemia have been shown (Zurlo 1989; Brittenham 1994; Gabutti 1996; Borgna‐Pignatti 2004). To be clinically effective DFO has to be administered as a subcutaneous infusion over eight to 12 hours, five to seven days per week. This regimen has been demonstrated to reduce the body iron load, prevent the onset of iron‐induced complications and even reverse some of the organ‐damage due to iron (Olivieri 1994). However, the arduous schedule of overnight subcutaneous infusions often leads to reduced compliance (Olivieri 1997; Cappellini 2005). Another problem concerns the toxicity of DFO, particularly at higher doses. Toxicities beside local skin reactions also include ophthalmologic (optic neuropathy, retinal pigmentation) and hearing problems (high frequency sensorineural hearing loss). Rare adverse effects like growth retardation, renal impairment (Koren 1991), anaphylactic reactions and pulmonary fibrosis (Freedman 1990) have been reported.
Oral preparations have been highly sought after for many years. In 1987, two studies showed that the orally active iron chelator deferiprone (1,2 dimethyl‐3‐hydroxypyrid‐4‐1, also known as L1, CP20, Ferriprox® or Kelfer) could achieve effective short‐term iron chelation (Kontoghiorghes 1987a; Kontoghiorghes 1987b). Doubts on the efficacy to reduce liver iron and prevent liver damage arose due to individuals with progression to overt liver fibrosis (Olivieri 1998), but the hypothesis of direct liver toxicity of deferiprone could not be confirmed (Wanless 2002; Wu 2006). In the meantime several studies have shown the efficacy of deferiprone for iron chelation (Ceci 2002; Maggio 2002) and in particular its benefit on cardiac iron and cardiac morbidity (Peng 2008). However, its use has remained limited due to its range of adverse effects (Hoffbrand 2003). These include gastrointestinal disturbances, arthropathy, neutropenia and agranulocytosis (Hoffbrand 1989). Recently, studies on combination therapy of deferoxamine and deferiprone have been performed (Kattamis 2003; Origa 2005; Farmaki 2006; Galanello 2006; Tanner 2007; Kolnagou 2008). A Cochrane Review on the effectiveness of deferiprone in people with thalassaemia has recently been published (Roberts 2007).
How the intervention might work
Deferasirox (4‐[3,5‐bis(2‐hydroxyphenyl)‐1H‐1,2,4‐triazol‐1‐yl]‐benzoic acid, also known as CGP 72670, ICL670 or Exjade®) is a new oral chelator available for routine use. The US Food and Drug Administration (FDA) (Food and Drug Administration (FDA) 2010) and the European Medicines Agency (EMA) (European Medicines Agency 2010) have approved it for the treatment of secondary iron overload. It is rapidly absorbed after administration and has a bioavailability of about 70%. Safety and tolerability was shown in a randomised dose escalation trial in 24 people with β‐thalassaemia (Nisbet‐Brown 2003). The elimination half‐life of eight to 16 hours allows a once daily administration after the tablets have been added to water or juice. Being a tridentate chelator, two molecules of deferasirox are needed to bind one molecule of iron. The excretion of the bound iron is mainly via faeces.
Adverse effects, known from the phase III study in people with thalassemia by Cappellini 2006 (n= 584 patients) and from experiences in people with thalassaemia, include gastrointestinal disturbances (nausea, stomach pain or diarrhoea) that are generally mild and a diffuse rash being more common at higher doses (Cappellini 2006). More rarely, fever, headache and cough have been encountered. The main adverse effect with the use of deferasirox seems to be a mild to moderate elevation of the creatinine level in about a third of patients. Elevations of liver enzyme levels have also been described with a lower incidence (5.6%) (Cappellini 2006). As with standard therapy (DFO), hearing loss and ocular disturbances including cataracts and retinal disorders have been reported with a lower incidence (< 1%).
Why it is important to do this review
Deferoxamine necessitates serious commitment on the user's side and deferiprone is only approved as second line therapy in some countries due to its adverse effects. Thus, much hope is being placed in the new oral chelator deferasirox, which apparently offers a promising line of treatment due to its iron chelation properties and safety and tolerability profile. Therefore, a systematic review of the effectiveness and safety of deferasirox according to Cochrane standards is urgently needed.
Objectives
To evaluate the effectiveness and safety of oral deferasirox for managing iron overload in people with MDS.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), of parallel group or cross‐over design, published or unpublished. To be eligible, either 80% of trial participants should have had MDS or data should have been available for the subgroup of participants with MDS.
Types of participants
People with diagnosis of MDS regardless of age, type of MDS and setting. To be eligible people were required to receive either more than two RBC concentrates per month or to have elevated ferritin levels of > 1000 ng/mL on at least two occasions.
Types of interventions
For oral deferasirox (all schedules and doses), we considered the following comparisons:
deferasirox compared with no therapy or placebo
deferasirox compared with another iron chelating treatment schedule (e.g. deferoxamine or deferiprone or any combination thereof).
These comparisons constituted two separate groups and we planned to analyse them separately.
Types of outcome measures
We did not exclude studies on the basis of reported outcomes.
Primary outcomes
Overall survival (measured at any point in time).
Secondary outcomes
-
Reduced end‐organ damage due to iron deposition:
cardiac failure (necessitating medical treatment)
endocrine disease (necessitating substitution therapy of hormones or treatment of diabetes)
histological evidence of hepatic fibrosis
pathological surrogate markers of end‐organ damage (i.e. elevated liver enzymes, elevated fasting glucose or pathological oral glucose tolerance test (OGTT), pathological measures (e.g. ejection fraction) in echocardiography).
-
Measures of iron overload:
serum ferritin (ng/mL)
iron levels in biopsies of liver and other tissue (mg/g liver dry weight)
tissue iron assessment by SQUID (superconducting quantum interference device) (mg/g liver wet weight)
tissue iron assessment by MRI (magnetic resonance imaging) (ms).
Measures of iron excretion (urine and faeces) over 24 hours (mg/kg/day).
-
Any adverse events:
raised levels of creatinine or kidney failure (above upper normal limit or rise of more than 20% above baseline level)
skin rash
gastrointestinal disturbances
neutropenia / agranulocytosis (ANC less than 1000/µL)
raised levels of liver enzymes (above upper normal limit or raise of more than 20% above baseline level) or progression to liver fibrosis
hearing loss
eye problems (e.g. retinal toxicity)
unanticipated adverse events as reported in the primary studies.
Participant satisfaction (measured e.g. by questionnaire) and compliance with chelation treatment (measured by the number of people in each arm that show adequate level of adherence to treatment (intake or application of iron chelator on five or more days per week).
Cost of intervention per year.
We planned to present data for the following time points: six months, 12 months, 24 months, 36 months, etc. Otherwise, we planned to present data for the latest time points available.
We did not anticipate that there would be any additional outcome measures. However, we planned to collect data from outcomes not defined a priori but which will arise from updated versions from the review, if we considered the outcome of clinical relevance.
Search methods for identification of studies
We did not apply any language restrictions.
Electronic searches
For this update, we searched the following databases for relevant trials:
Via Wiley Interscience: The Cochrane Library (Issue 4, 2014 for Cochrane Database of Systematic Reviews, Issue 3, 2014 for Cochrane Central Register of Controlled Trials, Issue 1, 2014 for Other Reviews (DARE), Technology Assessments and Economic Evaluations, Issue 3, 2012 for Methods Studies); via OvidSP: MEDLINE (1946 to March Week 4 2014), MEDLINE in Process and Other Non‐Indexed Citations (to April 2, 2014); via PubMed: MEDLINE subset "supplied by publisher" (to April 2, 2014); via DIMDI: EMBASE and EMBASE Alert (2010 to April 1, 2014); via Thomson Reuters: Web of Science (2010 to April 1, 2014), Biosis Previews (2010 to April 1, 2014); via DIMDI: Derwent Drug File (2010 to April 1, 2014). We performed the searches on 2nd and 3rd April 2014. We used an RCT filter MEDLINE, EMBASE, Biosis Previews, ISI Web of Science and Derwent Drug File searches; also, we limited the search to reports published between 2010 and 2014. For details of the search strategies see Appendix 1.
Since research into deferasirox treatment is ongoing, we searched the following four trial registries up to 16 April 2014 for all years available in all possible fields using the basic search function (using separately the following keyword terms: "deferasirox", "ICL670", "ICL 670" and "exjade"):
Current Controlled Trials: www.controlled‐trials.com (all available registers were searched)
ClinicalTrials.gov: www.clinicaltrials.gov
ICTRP: www.who.int/ictrp/en/
German Clinical Trial Register: www.drks.de
For the previous version of this review, we searched several databases and ongoing trials registers from 24 June 2010 up to 01 July 2010. Please see Appendix 2 for full details.
Searching other resources
In addition we searched the abstract books of two major haematology conferences from 2000 to 2013: the European Haematology Association conference and the American Society of Hematology conference.
We intended to screen reference lists of all identified papers to identify other potentially relevant citations.
We contacted selected experts in the field and the manufacturer of deferasirox (Novartis) to request information on unpublished studies that involved deferasirox.
Data collection and analysis
Selection of studies
One review author (JM and LS (update search)) screened all titles and abstracts of papers identified by the trial search strategy for relevance. We only excluded citations that were clearly irrelevant at this stage. We obtained full text copies of all potentially relevant papers and two review authors (JM, DB for previous version of review; LS, JM for current update) independently screened the full papers, identified relevant studies and assessed eligibility of studies for inclusion. We resolved any disagreement on eligibility through discussion and consensus or if necessary through referral to a third party (GA or CN). We excluded all irrelevant records and recorded details of the studies and the reasons for their exclusion. We planned to categorise studies that lacked important information (including foreign language studies awaiting translation) and report them as studies pending inclusion/exclusion decision for future updates.
Data extraction and management
This was not applicable as no trials met our inclusion criteria. For details on planned methods please see section "Differences between protocol and review".
Assessment of risk of bias in included studies
This was not applicable as no trials met our inclusion criteria.
Measures of treatment effect
This was not applicable as no trials met our inclusion criteria.
Unit of analysis issues
This was not applicable as no trials met our inclusion criteria.
Dealing with missing data
We contacted the original investigators of Castellano 2011 to request more detailed data regarding patient eligibility criteria. However, we were informed that data are not to be shared prior to publication.
Assessment of heterogeneity
This was not applicable as no trials met our inclusion criteria.
Assessment of reporting biases
We tried to minimise the likelihood of publication bias by using a comprehensive search strategy including the search of abstracts and contacting the manufacturer of deferasirox. However, we did not identify any trials for inclusion.
Data synthesis
This was not applicable as no trials met our inclusion criteria.
Subgroup analysis and investigation of heterogeneity
This was not applicable as no trials met our inclusion criteria.
Sensitivity analysis
This was not applicable as no trials met our inclusion criteria.
Results
Description of studies
Results of the search
Based on the searches for this review update (run in April 2014), we identified 546 unique citations (Figure 1). We included two additional abstracts after we contacted the authors of Castellano 2011. After we screened titles and abstracts of these citations we identified seven as potentially eligible. We excluded five citations after full text screening for the following reasons:
1.
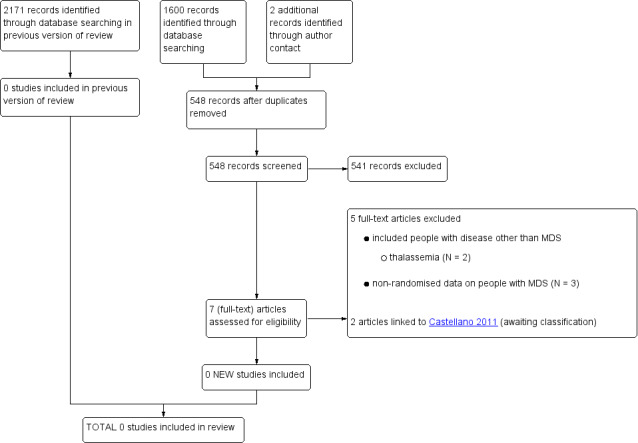
Study flow diagram (update search performed in April 2014),
-
included people with disease other than MDS
thalassaemia (N = 2)
non‐randomised data on people with MDS (N = 3) (see Characteristics of excluded studies).
We identified 110 unique references to trials after searching the four trial registers (run on 02/03 April 2014). We found two ongoing (Novartis 2013; NCT02038816) and one completed RCTs (published as abstract only) (Castellano 2011) by this search, in addition to the ongoing trial already identified in the previous version of this review (TELESTO 2009). We categorised the Castellano 2011 trial as awaiting classification (since the two published abstracts do not provide enough detail to decide on definitive inclusion). A fifth study, which we identified through searching the registers (Pennell 2014, CORDELIA), was planned to also include people with MDS. However, as reported in Pennell 2014, CORDELIA, none of the four screened MDS patients fulfilled the other inclusion criteria of the trial.
Previous search
For the previous version of this review, we performed the literature search in August 2008, June 2009 and lastly between 24 and 30 June 2010. Altogether, we identified 2171 citations, including 1191 duplicates. After we screened titles and abstracts of the 980 unique citations, we excluded 686 citations. In total, we screened 294 full texts but we no RCTs met our inclusion criteria. Our reasons for exclusion were as follows:
-
included people with disease other than MDS
thalassaemia (N = 140)
sickle cell disease (N = 38)
other condition (N = 11)
review article or editorial/comment (N = 46)
intervention other than deferasirox (N = 1)
cost‐effectiveness analysis (N = 5)
non‐randomised data on people with MDS (N = 52) (see Characteristics of excluded studies)
ongoing study (N = 1) (see Characteristics of ongoing studies).
After we searched the three trial registers for the previous version of this review (last run on 30 June 2010) we identified 49 unique references to trials. However, we identified only one ongoing RCT including MDS patients (TELESTO 2009).
Included studies
In this version of the review, we could not include any data from completed trials.
Excluded studies
We excluded 25 studies, most of which were non‐randomised studies. For details please see Characteristics of excluded studies.
Risk of bias in included studies
We did not find any trials that were eligible for inclusion.
Effects of interventions
We did not find any eligible trials for inclusion in this review.
Discussion
Summary of main results
Through our comprehensive searches, we identified three ongoing and one completed trial. However, no data from any of these RCTs are currently available for inclusion in this review.
Overall completeness and applicability of evidence
This was not applicable as we did not include any trials in this review.
Quality of the evidence
This was not applicable.
Potential biases in the review process
We conducted a very comprehensive search including searches of several study registers. This led to the identification of three ongoing and one completed RCTs. However, despite correspondence with trial authors, we were unable to decide on definite inclusion of the completed RCTs, nor include any data in this current review version.
Agreements and disagreements with other studies or reviews
People with MDS potentially constitute the largest group of patients at risk of iron overload. However, the impact of iron chelation therapy in people with MDS has not been investigated as extensively as in other transfusion‐dependent anaemias such as thalassaemia (Roberts 2007; Cianciulli 2008; Porter 2009; Fisher 2013). In theory, the biological rationale behind treating iron overload in patients with other anaemias should also apply to people with MDS. Thus, clinical practice guidelines do suggest consideration of iron chelation therapy for certain subgroups of MDS patients (Bennett 2008; Gattermann 2008; Greenberg 2011; Malcovati 2013). With the emergence of deferasirox, which avoids the cumbersome application mode of deferoxamine, iron chelation therapy has been offered more widely to MDS patients. However, data from thalassaemia patients is not likely to be directly transferred to people with MDS considering that people with MDS are older and suffer from a different disease entity. For example, people with MDS are potentially more prone to iron‐related organ dysfunction and certain adverse effects. Furthermore, reduced life‐expectancy in people with MDS compared to people with other anaemias could render the beneficial effects of iron chelation therapy clinically less relevant since such patients may not survive long enough to accumulate iron to toxic levels.
The negative impacts of iron overload and the benefits of iron chelation therapy for people with MDS are nevertheless suggested by recent retrospective survey data (Takatoku 2007) and observational studies (Rose 2007; Leitch 2008), although not all studies could confirm beneficial effects of iron chelation therapy (Chee 2008). More specifically, several retrospective analyses and observational studies suggest a benefit with regard to certain outcomes in patients with MDS from deferasirox (see the Excluded studies section; also Porter 2004; List 2006, US3 study; Gattermann 2007, EPIC; List 2012; Cermak 2013). Additionally, several narrative reviews of deferasirox have been published over recent years (Shah 2012; Adams 2013; Breccia 2013). However, the impact of iron chelation therapy (either with deferasirox or any other iron chelator) on long‐term outcomes or patient‐relevant outcomes such as organ dysfunction or mortality has not been evaluated in rigorous RCTs (Leitch 2009b). Indeed, we found no RCTs published to date that studied the impact of iron chelation with deferasirox for patients with MDS.
Since iron chelation therapy has such a positive impact on survival in thalassaemia patients and is increasingly being offered to people with MDS, further evaluation of the effects of iron overload and chelation therapy in patients with MDS is urgently required in order for clinical practice guidelines based on high quality evidence to be formulated. In particular, the profile of adverse effects urgently needs to be established to allow adequate balancing of the benefits and potential harms of iron chelation therapy for people with MDS. Adverse events may differ compared to people with thalassaemia; an RCT conducted with acute myeloid leukaemia patients points to poor tolerability and excess gastrointestinal and infectious toxicity (Kennedy 2013). Furthermore, the generation of further data supporting the application of this intervention instead of no intervention or other iron chelating regimens seems to be even more important considering the costs implied by a continuous therapy with deferasirox (Delea 2005; Karnon 2007a; Karnon 2007b; Bozkaya 2008).
To address some of the uncertainties in the evidence base, three prospective RCTs are currently enrolling patients (TELESTO 2009; Novartis 2013; NCT02038816), while one has already completed patient recruitment and is awaiting publication (Castellano 2011). Castellano 2011 has already presented findings as an abstract at two conferences. Castellano et al. reported statistically significant decreases in serum ferritin for both groups over time, but comparative analyses between groups are not provided. Also, these abstracts did not provide sufficient information on patient eligibility criteria in order to be included in the current version of this review.
These trials should improve the evidence base and possibly lead to clearer indications being established for iron chelation therapy in MDS patients. If the effectiveness of deferasirox in MDS is confirmed in the future, further comparisons with other iron chelation regimens, such as deferiprone, will be worthwhile since the profile of adverse effects, in particular, varies between different chelating agents.
Authors' conclusions
Implications for practice.
Based on the limited evidence from retrospective analyses and observational studies, some current clinical practice guidelines do recommend consideration of iron chelation therapy for low risk MDS. However, these recommendations can not be supported by high quality data from RCTs. Therefore, data from the ongoing or completed but not fully published trial are urgently needed to inform doctors whether widespread use of deferasirox outside clinical studies is warranted. The decision to use deferasirox for individual patients with MDS, while based on personal preferences, should be informed by the potential benefits and harms and the resource use incurred.
Implications for research.
RCTs investigating the effectiveness of iron chelation therapy, in general and of deferasirox in particular, in people with MDS are urgently needed. Future RCTs should include (1) patient‐relevant outcomes and (2) investigate also long‐term benefits and adverse effects. Furthermore, the value of iron chelation therapy should be differentially investigated (3) for the various subtypes of MDS. If the value of iron chelation therapy is unambiguously established, (4) comparative trials defining the advantages and disadvantages of the various iron chelating regimens should follow.
What's new
| Date | Event | Description |
|---|---|---|
| 23 October 2014 | New search has been performed | Updated |
| 23 October 2014 | New citation required but conclusions have not changed | We performed a search update and identified two new ongoing studies and one completed study (published as an abstract in insufficient detail). In total, we included three ongoing and one completed study identified through searching trial registries. We did not include any new studies in this update. |
Acknowledgements
We thank the peer reviewers for their valuable comments which helped us to improve the protocol and review. We also thank the editorial team, especially Nicole Skoetz, for their support in preparing the protocol and this review. Christina Reese helped with the literature searches and retrieval of full articles. Claire McLeod gave valuable input at the protocol stage.
Appendices
Appendix 1. Search strategies April 2014
| MEDLINE (OvidSP) | |
| #1 | deferasirox*.mp. |
| #2 | (ICL670* or ICL 670*).mp. |
| #3 | (CGP72670* or CGP 72670*).mp. |
| #4 | exjade*.mp. |
| #5 | 2‐hydroxyphenyl.mp. |
| #6 | triazol‐1‐yl.mp. |
| #7 | benzoic acid.mp. |
| #8 | 5 and 6 and 7 |
| #9 | 1 or 2 or 3 or 4 or 8 |
| #10 | (2010* or 2011* or 2012* or 2013* or 2014*).ed,ep,dc. or ("2010" or "2011" or "2012" or "2013" or "2014").yr. |
| #11 | 9 and 10 |
| #12 | randomized controlled trial.pt. |
| #13 | controlled clinical trial.pt. |
| #14 | randomi#ed.ab. |
| #15 | placebo.ab. |
| #16 | drug therapy.fs. |
| #17 | randomly.ab. |
| #18 | trial.ab. |
| #19 | groups.ab. |
| #20 | 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 |
| #21 | exp animals/ not humans.sh. |
| #22 | 20 not 21 |
| #23 | 11 and 22 |
|
Notes .mp. = title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier. .sh. or / = Medical Subject Heading exp = explode .ti. title .ab. = abstract .pt. = publication type .yr. = year .ed. = entry date .ep. = electronic date of publication .dc. = date created | |
| MEDLINE Daily Update (OvidSP) | |
| #1 | deferasirox*.mp. |
| #2 | (ICL670* or ICL 670*).mp. |
| #3 | (CGP72670* or CGP 72670*).mp. |
| #4 | exjade*.mp. |
| #5 | 2‐hydroxyphenyl.mp. |
| #6 | triazol‐1‐yl.mp. |
| #7 | benzoic acid.mp. |
| #8 | 5 and 6 and 7 |
| #9 | 1 or 2 or 3 or 4 or 8 |
|
Notes .mp. = title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier. | |
| MEDLINE In‐Process & Other Non‐Indexed Citations (OvidSP) | |
| #1 | deferasirox*.mp. |
| #2 | (ICL670* or ICL 670*).mp. |
| #3 | (CGP72670* or CGP 72670*).mp. |
| #4 | exjade*.mp. |
| #5 | 2‐hydroxyphenyl.mp. |
| #6 | triazol‐1‐yl.mp. |
| #7 | benzoic acid.mp. |
| #8 | 5 and 6 and 7 |
| #9 | 1 or 2 or 3 or 4 or 8 |
|
Notes .mp. = title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier. | |
| PubMed – subset "supplied by publisher" (www.pubmed.gov) | |
| #1 | Search deferasirox*[tw] |
| #2 | Search ICL670*[tw] OR ICL 670*[tw] |
| #3 | Search CGP72670*[tw] OR CGP 72670*[tw] |
| #4 | Search exjade*[tw] |
| #5 | Search 2‐hydroxyphenyl[tw] |
| #6 | Search triazol‐1‐yl[tw] |
| #7 | Search benzoic acid[tw] |
| #8 | Search #5 AND #6 AND #7 |
| #9 | Search #1 OR #2 OR #3 OR #4 OR #8 |
| #10 | Search #9 AND publisher[sb] |
|
Notes [tw] = textword: title, abstract, other abstract, MeSH terms, MeSH Subheadings, Publication Types, Substance Names, Personal Name as Subject, Corporate Author, Secondary Source, and Other Terms. [sb] = subset | |
| EMBASE and EMBASE Alert (DIMDI www.dimdi.de) | |
| #1 | EM05; EA08 |
| #2 | FT=DEFERASIROX* |
| #3 | FT=ICL670* OR FT=ICL 670* |
| #4 | FT=CGP72670* OR FT=CGP 72670* |
| #5 | FT=EXJADE* |
| #6 | FT=2‐HYDROXYPHENYL |
| #7 | FT=TRIAZOL‐1‐YL |
| #8 | FT=BENZOIC ACID |
| #9 | 6 AND 7 AND 8 |
| #10 | 2 OR 3 OR 4 OR 5 OR 9 |
| #11 | 10 AND PY>=2010 |
| #12 | (FT=RANDOM* OR FT=PLACEBO* ) OR FT=DOUBLE‐BLIND* |
| #13 | 11 AND 12 |
| #14 | 13 NOT SU=MEDLINE |
|
Notes EM05 = EMBASE ab 2005; EA08 = EMBASE Alert PY = Publication year SU = subunit | |
| The Cochrane Library (Wiley: www.thecochranelibrary.com) | |
| #1 | deferasirox* |
| #2 | ICL670* OR (ICL next 670*) |
| #3 | CGP72670* OR (CGP next 72670*) |
| #4 | exjade* |
| #5 | 2 next hydroxyphenyl |
| #6 | triazol next 1 next yl |
| #7 | benzoic next acid |
| #8 | (#5 AND #6 AND #7) |
| #9 | (#1 OR #2 OR #3 OR #4 OR #8) |
| #10 | #9 Publication Date from 2010 to 2014 (Word variations have been searched) |
| Biosis Previews (Thomson Reuters) | |
| #1 | ts=deferasirox* |
| #2 | ts=(ICL670* or "ICL 670*") |
| #3 | ts=(CGP72670* or "CGP 72670*") |
| #4 | ts=exjade* |
| #5 | ts="2‐hydroxyphenyl" |
| #6 | ts="triazol‐1‐yl" |
| #7 | ts="benzoic acid" |
| #8 | #7 AND #6 AND #5 |
| #9 | #8 OR #4 OR #3 OR #2 OR #1 |
| #10 | ts=(rando* or placebo or trial or "single‐blind*" or "double‐blind*" or groups) |
| #11 | #10 AND #9 |
|
Notes Ts = topic:
Indexes = BIOSIS Previews Timespan = 2010 to 2014 | |
| Web of Science via Web of Knowledge (Thomson Reuters) | |
| #1 | ts=deferasirox* |
| #2 | ts=(ICL670* or "ICL 670*") |
| #3 | ts=(CGP72670* or "CGP 72670*") |
| #4 | ts=exjade* |
| #5 | ts="2‐hydroxyphenyl" |
| #6 | ts="triazol‐1‐yl" |
| #7 | ts="benzoic acid" |
| #8 | #7 AND #6 AND #5 |
| #9 | #8 OR #4 OR #3 OR #2 OR #1 |
| #10 | ts=(rando* or placebo or trial or "single‐blind*" or "double‐blind*" or groups) |
| #11 | #10 AND #9 |
|
Notes TS = topic: Title, Abstract, Author Keywords, Keywords Plus® Indexes = SCI‐EXPANDED, SSCI, A&HCI, CPCI‐S, CPCI‐SSH Timespan = 2010 to 2014 | |
| Derwent Drug file (DIMDI www.dimdi.de) | |
| #1 | DD83 |
| #2 | DEFERASIROX* |
| #3 | FT=ICL670* OR FT=ICL 670* |
| #4 | FT=CGP72670* OR FT=CGP 72670* |
| #5 | FT=EXJADE* |
| #6 | FT=2‐HYDROXYPHENYL |
| #7 | FT=TRIAZOL‐1‐YL |
| #8 | FT=BENZOIC ACID |
| #9 | 6 AND 7 AND 8 |
| #10 | 2 OR 3 OR 4 OR 5 OR 9 |
| #11 | 10 AND PY>=2010 |
| #12 | ((((FT=RANDO* OR FT=PLACEBO ) OR FT=TRIAL ) OR (CT D "SINGLE‐BLIND"* OR UT="SINGLE‐BLIND"* OR IT="SINGLE‐BLIND"* OR SH="SINGLE‐BLIND"*)) OR FT=DOUBLE‐BLIND* ) OR FT=GROUPS |
| #13 | 11 AND 12 |
|
Notes DD83 = Derwent Drug File PY = Publication year | |
Appendix 2. Search strategies June 2010
| MEDLINE & MEDLINE In‐Process (Ovid) | |
| #1 | deferasirox*.mp |
| #2 | (ICL670* or ICL 670*).mp |
| #3 | (CGP72670* or CGP 72670*).mp |
| #4 | exjade*.mp |
| #5 | 2‐hydroxyphenyl.mp |
| #6 | triazol‐1‐yl.mp |
| #7 | benzoic acid.mp |
| #8 | and/5‐7 |
| #9 | or/1‐4,8 |
| #10 | remove duplicates from 9 |
|
Notes .mp = title, original title, abstract, name of substance word, subject heading word, unique identifier The chemical substance name "4‐(3,5‐bis(2‐hydroxyphenyl)‐(1,2,4)‐triazol‐1‐yl) benzoic acid" was searched by splitting it up in searchable terms (2‐hydroxyphenyl, triazol‐1‐yl, benzoic acid) and combining those by AND (lines #5 ‐ #8). | |
| EMBASE (Ovid) | |
| #1 | deferasirox*.mp |
| #2 | (ICL670* or ICL 670*).mp |
| #3 | (CGP72670* or CGP 72670*).mp |
| #4 | exjade*.mp |
| #5 | 2‐hydroxyphenyl.mp |
| #6 | triazol‐1‐yl.mp |
| #7 | benzoic acid.mp |
| #8 | and/5‐7 |
| #9 | or/1‐4,8 |
| #10 | remove duplicates from 9 |
|
Notes .mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name The chemical substance name "4‐(3,5‐bis(2‐hydroxyphenyl)‐(1,2,4)‐triazol‐1‐yl) benzoic acid" was searched by splitting it up in searchable terms (2‐hydroxyphenyl, triazol‐1‐yl, benzoic acid) and combining those by AND (lines #5 ‐ #8). | |
| The Cochrane Library (Wiley Interscience) | |
| #1 | deferasirox* |
| #2 | ICL670* or ICL next 670* |
| #3 | CGP72670* or CGP next 72670* |
| #4 | exjade* |
| #5 | 2‐hydroxyphenyl |
| #6 | triazol‐1‐yl |
| #7 | benzoic acid |
| #8 | (#5 AND #6 AND #7) |
| #9 | (#1 OR #2 OR #3 OR #4 OR #8) |
|
Notes The chemical substance name "4‐(3,5‐bis(2‐hydroxyphenyl)‐(1,2,4)‐triazol‐1‐yl) benzoic acid" was searched by splitting it up in searchable terms (2‐hydroxyphenyl, triazol‐1‐yl, benzoic acid) and combining those by AND (lines #5 ‐ #8). Issues searched: Issue 6, 2010 for Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials and Issue 2, 2010 for Other Reviews (DARE), Methods Studies, Technology Assessments, Economic Evaluations | |
| BIOSIS Previews (Ovid) | |
| #1 | deferasirox*.mp |
| #2 | (ICL670* or ICL 670*).mp |
| #3 | (CGP72670* or CGP 72670*).mp |
| #4 | exjade*.mp |
| #5 | 2‐hydroxyphenyl.mp |
| #6 | triazol‐1‐yl.mp |
| #7 | benzoic acid.mp |
| #8 | and/5‐7 |
| #9 | or/1‐4,8 |
| #10 | remove duplicates from 9 |
|
Notes .mp = abstract, biosystematic codes, original language book title (non‐english), book title (english), chemicals & biochemicals, concept codes, diseases, geopolitical locations, gene name, major concepts, miscellaneous descriptors, methods & equipment, organisms, parts, structures & systems of organisms, sequence data, super taxa, title, time, taxa notes The chemical substance name "4‐(3,5‐bis(2‐hydroxyphenyl)‐(1,2,4)‐triazol‐1‐yl) benzoic acid" was searched by splitting it up in searchable terms (2‐hydroxyphenyl, triazol‐1‐yl, benzoic acid) and combining those by AND (lines #5 ‐ #8). | |
| Web of Science (Thomson Reuters) | |
| #1 | ts=deferasirox* |
| #2 | ts=(ICL670* or "ICL 670*") |
| #3 | ts=(CGP72670* or "CGP 72670*") |
| #4 | ts=exjade* |
| #5 | ts="2‐hydroxyphenyl" |
| #6 | ts="triazol‐1‐yl" |
| #7 | ts="benzoic acid" |
| #8 | #5 AND #6 AND #7 |
| #9 | #1 OR #2 OR #3 OR #4 OR #8 |
|
Notes ts = topic The chemical substance name "4‐(3,5‐bis(2‐hydroxyphenyl)‐(1,2,4)‐triazol‐1‐yl) benzoic acid" was searched by splitting it up in searchable terms (2‐hydroxyphenyl, triazol‐1‐yl, benzoic acid) and combining those by AND (lines #5 ‐ #8). Date searched: 27 June 2010 | |
| Derwent Drug File and XTOXLINE (DIMDI) | |
| #1 | deferasirox* (text field) |
| #2 | ICL670* or ICL 670* (text field) |
| #3 | CGP72670* or CGP 72670* (text field) |
| #4 | exjade* (text field) |
| #5 | 2‐hydroxyphenyl (text field) |
| #6 | triazol‐1‐yl (text field) |
| #7 | benzoic acid (text field) |
| #8 | #5 and #6 and #7 |
| #9 | #1 or #2 or #3 or #4 or #8 |
|
Notes The chemical substance name "4‐(3,5‐bis(2‐hydroxyphenyl)‐(1,2,4)‐triazol‐1‐yl) benzoic acid" was searched by splitting it up in searchable terms (2‐hydroxyphenyl, triazol‐1‐yl, benzoic acid) and combining those by AND (lines #5 ‐ #8). | |
| Current Controlled Trials | |
| #1 | "deferasirox" OR "ICL670" OR "ICL 670" OR "exjade" |
|
Notes Date searched: 28 June 2010 | |
| ClinicalTrials.gov | |
| #1 | "deferasirox" OR "ICL670" OR "ICL 670" OR "exjade" |
|
Notes Date searched: 28 June 2010 | |
| ICTRP | |
| #1 | "deferasirox" OR "ICL670" OR "ICL 670" OR "exjade" |
|
Notes Date searched: 28 June 2010 | |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Angelucci 2012 | Study not randomised, not controlled. Prospective, 1‐year, multi‐centre, single‐arm trial including 152 lower risk and transfusion dependent MDS patients. |
| Brosnahan 2008 | Case report of MDS patient with acute interstitial nephritis. |
| Cermak 2013 | Study not randomised. Comparative study on 48 patients receiving deferiprone (January 2006 to July 2007) and 65 patients receiving deferasirox (January 2008 to August 2010). |
| Di Tucci 2007 | Case report of patient with primary myelofibrosis (PMF). |
| Fox 2009 | Study not randomised. Matched‐pair analysis on 186 MDS patients. |
| García‐Delgado 2009 | Study not randomised, not controlled. Single‐arm study evaluating the effect of deferasirox on oxidative stress and vascular dysfunction in MDS patients with iron overload (4 people with MDS). |
| Gattermann 2007, EPIC | Study not randomised, not controlled. Prospective, 1‐year, multi‐centre, single‐arm, open‐label phase IIIB/IV trial (1744 people with various transfusion‐dependent anaemias including 341 people with MDS and 116 with AA). |
| Gattermann 2009 | Study not randomised, not controlled. Prospective, multi‐centre, post‐marketing, single‐arm study on 123 chelation‐naive (Extend) and 44 pre‐chelated (Exjange) MDS patients. |
| Ghoti 2009 | Study not randomised, not controlled. Single‐arm study evaluating decrease in intra‐ and extra‐cellular free iron species and oxidative stress parameters during treatment with deferasirox in iron‐loaded patients with MDS. |
| Greenberg 2006, US2 study | Study not randomised, not controlled. Prospective, phase II, multi‐centre, single‐arm study on 30 people with MDS. |
| Leitch 2007 | Study not randomised, not controlled. Retrospective analysis of 41 people with PMF. |
| Leitch 2009 | Case report. |
| List 2006, US3 study | Study not randomised, not controlled. Prospective, phase II, open‐label, single‐arm study on 176 people with MDS. |
| Messa 2008 | Retrospective case series of 7 people with MDS and 3 people with PMF. |
| Metzgeroth 2009 | Study not randomised, not controlled. Prospective, phase II, open‐label, single‐arm, single‐centre study of 12 people with MDS. |
| Min 2008 | Study not randomised, not controlled. Prospective, multi‐centre, open‐label, single‐arm study of 29 people with MDS and 50 people with AA. |
| Miyazawa 2006 | Study not randomised, not controlled. Prospective, phase I, open‐label, multi‐centre, dose‐escalation study on 26 people with MDS or AA. |
| Nolte 2013 | Study not randomised, not controlled. Open label, 1‐year, single‐arm, multi‐centre trial on 50 patients with low and intermediate‐1 risk MDS and transfusional iron overload. |
| Peng 2013 | Study on patients with thalassemia (Chinese full text not available; assessment based on English abstract and references). |
| Pennell 2014, CORDELIA | Study planned to include MDS patients; however, of the four screened patients with MDS, none fulfilled the inclusion criteria. |
| Porter 2004 | Study not randomised, not controlled. Prospective, phase II, single‐arm study of 184 people with transfusion‐dependent anaemia including 47 people with MDS. |
| Rachmilewitz 2008 | Study not randomised, not controlled. Prospective, single‐arm study of 15 people with MDS. |
| Remacha 2010 | Study not randomised, not controlled. Cross‐sectional study on 549 MDS patients. |
| Rose 2007 | Study not randomised, not controlled. Survey of 170 people with MDS from 18 French GFM centres. |
| Wimazal 2009 | Case series of 14 MDS patients treated with deferasirox (500 to 1500 mg/day) for up to 24 months. |
Only reports on observational studies or case series are mentioned in this table.
AA ‐ aplastic anaemia;
GFM ‐ Groupe Francophone des Myelodysplasies;
MDS ‐ myelodysplastic syndrome;
PMF ‐ primary myelofibrosis.
Characteristics of studies awaiting assessment [ordered by study ID]
Castellano 2011.
| Methods | A single‐centre, prospective, randomised, controlled phase III clinical study to evaluate the efficacy and safety of deferasirox and desferoxamine in patients with iron overload |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Group A: Deferasirox 20 mg/kg/day Group B: Desferoxamine 30 mg/kg/day |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Notes |
Contact information: Pilar Giraldo Castellano Servicio de Hematología Hospital Universitario Miguel Servet Paseo Isabel La Católica 1‐3 50009 Zaragoza Spain Telephone: +34 670285339 Email: giraldo.p@gmail.com |
We have given the Information in tables according to entries in the ISRCTN registry and www.clinicaltrials.gov. We reviewed the registry entries on 16 April 2014.
Characteristics of ongoing studies [ordered by study ID]
NCT02038816.
| Trial name or title | Azacitidine Plus Deferasirox (ICL670) in Higher Risk MDS |
| Methods | An open‐label, phase II, randomised efficacy study in patients with higher risk MDS |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: Deferasirox plus azacitidine Active comparator: Azacitidine |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | Study start date: March 2014; Estimated primary completion date: October 2016 (Final data collection date for primary measure) |
| Contact information | Odette Cancer Centre, Sunnybrook Health Sciences Centre Toronto, Ontario, Canada, M4N 3M5 Contact: Claudia Li (claudia.li@sunnybrook.ca) |
| Notes |
|
Novartis 2013.
| Trial name or title | Combination Study of Deferasirox and Erythropoietin in Patients With Low‐ and Int‐1‐risk Myelodysplastic Syndrome |
| Methods | An open‐label, phase II, randomised, pilot study to assess the effect in term of erythroid improvement of deferasirox combined with erythropoietin compared to erythropoietin alone in patients with low‐ and int‐1‐risk MDS |
| Participants | Inclusion criteria:
Exclusion criteria:
Other protocol‐defined inclusion/exclusion criteria may apply. |
| Interventions | Group A: Erythropoietin alpha Group B: Deferasirox + erythropoietin alpha |
| Outcomes | Primary outcome measure:
Secondary outcome measures:
|
| Starting date | Study start date: January 2014 Estimated primary completion date: June 2015 (Final data collection date for primary measure) |
| Contact information | Novartis Pharmaceuticals |
| Notes |
|
TELESTO 2009.
| Trial name or title | MDS event free survival with iron chelation therapy study (TELESTO) |
| Methods | A multi‐centre, randomised, double‐blind, placebo‐controlled clinical trial of deferasirox in patients with MDS (low/int‐1 risk) and transfusional iron overload |
| Participants | Inclusion criteria:
Exclusion criteria:
Other protocol‐defined inclusion/exclusion criteria may apply. |
| Interventions | Experimental: Deferasirox Placebo: Placebo |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | Study start date: March 2010 Estimated primary completion date: February 2018 (final data collection date for primary measure) |
| Contact information | Novartis Pharmaceuticals |
| Notes |
|
Information in tables given according to www.clinicaltrials.gov. We searched ClinicalTrials.gov on 16 April 2014.
Differences between protocol and review
There are no deviations from the protocol first published in The Cochrane Library (Meerpohl 2008).
Methods not implemented
Since we did not find any trials that met our inclusion criteria, we did not implement the following aspects of the protocol.
Data extraction and management
Aside from details relating to the quality of the included studies, we planned to extract two groups of data:
Study characteristics ‐ place of publication, date of publication, population characteristics, setting, detailed nature of intervention, detailed nature of comparator and detailed nature of outcomes. A key purpose of these data is to define unexpected clinical heterogeneity in included studies independently from analysis of results.
Results of included studies with respect to each of the main outcomes indicated in the review question. We intended to carefully record reasons why an included study does not contribute data on a particular outcome and to consider the possibility of selective reporting of results on particular outcomes.
We planned to undertake data extraction by authors independently (JM, LS), using a data extraction form developed by the review authors. We planned to resolve disagreements by consensus. Missing data was requested from the original investigators. Either JM or LS were to transcribe data from the data extraction form into Review Manager 2014 and DB would verify all data entry for discrepancies.
Assessment of risk of bias in included studies
We did not perform an assessment of risk of bias because of insufficient data. We planned that JM and LS would assess every trial independently using a simple form following the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
We would have assessed the following domains as either 'Yes' (i.e. low risk of bias), 'Unclear' (i.e. uncertain risk of bias) or 'No' (i.e. high risk of bias):
Sequence generation
Allocation concealment
Blinding (of participants, personnel and outcome assessors)
Incomplete outcome data
Selective outcome reporting
Other sources of bias
We planned to discuss any inconsistencies between the review authors in the interpretation of risk of bias and their significance to the selected trials and to resolve any differences with a third author (DB). No study was to be automatically excluded as a result of a rating of 'Unclear' or 'No'. We intended that we would present the evaluation of the risk of bias in included studies in tabular form in the 'Results' section of the review.
Measures of treatment effect
We planned to analyse extracted data using the most up‐to‐date version of RevMan available at the time of analysis (Review Manager 2014).
We planned to extract hazard ratios with their 95% confidence intervals (CIs) for the time‐to‐event data mortality and end‐organ damage (see below). If hazard ratios were not given, we would have used indirect estimation methods described by Parmar 1998 and Williamson 2002 to calculate them.
If we were neither able to extract these data from the study reports nor able to receive the necessary information from the primary investigators, we would, as an alternative, have used the proportions of participants with the respective outcomes measured at three months, six months and then at six‐monthly intervals (i.e. 12 months, 18 months and so on) to be able to calculate relative risks. If outcome data were recorded at other time periods, then we would have given consideration to examining these as well.
We planned to express results for binary outcomes as relative risk (RR) with 95% CIs as measure of uncertainty. We planned to express continuous outcomes as mean difference (MD) with 95% CIs as measure of uncertainty.
Unit of analysis issues
When conducting a meta‐analysis combining results from cross‐over studies we planned to use the methods recommended by Elbourne 2002. For combining parallel and cross‐over trials, we would use methods described by Curtin (Curtin 2002a; Curtin 2002b; Curtin 2002c).
For some outcomes, a possible perception of the comparison might be whether deferasirox is equivalent to standard treatment with deferoxamine. Therefore, as secondary analysis, we planned to consider per‐protocol analysis, as is often used for equivalence studies, for our primary outcome as well as for the groups one to five of our secondary outcomes.
For time‐to‐event data, we would have considered a relative difference in hazard ratios of less than 10% equivalent. For relative risks, we would have defined non‐inferiority as a relative risk difference of less than 10% in treatment failures compared to standard therapy. For the continuous outcomes of "measures of iron overload and iron excretion" as well as "costs" we would also have considered a relative difference of 10% as equivalent. Resulting CIs would have been discussed with respect to methods suggested by Witte 2004.
In principle it is conceivable that the evidence found might be suitable for indirect comparisons or multiple‐treatment meta‐analysis. If so, we planned to apply these concepts according to methods discussed by Glenny 2005 and Salanti 2008, respectively, as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), once they are supported by Review Manager 2014.
Dealing with missing data
We planned to follow the general recommendations for dealing with missing data in Cochrane Reviews (Higgins 2011b):
Whenever possible we planned to contact the original investigators to request missing data.
We would have clearly stated the assumptions of any methods used to cope with missing data (e.g. imputation of missing data and accounting for the fact that these were imputed with uncertainty).
We would have performed sensitivity analyses to assess how sensitive results are to reasonable changes in the assumptions that are made.
We would have addressed the potential impact of missing data on the findings of the review in the Discussion section.
Assessment of heterogeneity
We planned to assess statistical heterogeneity using the I2 statistic (Higgins 2002; Higgins 2003). This measure describes the percentage of total variation across studies that is caused by heterogeneity rather than by chance (Higgins 2003). The values of I2 lie between 0% and 100%. We planned to use a simplified categorisation of heterogeneity with the following categories: low (I2 less than 30%), moderate (I2 between 30% and 60%) and high (I2 more than 60%) (Deeks 2011).
In future review updates, if we detect moderate or high heterogeneity we intend to explore clinical heterogeneity by examining differences between groups as detailed below.
Assessment of reporting biases
If we include more than 10 trials, we will use funnel plots to graphically assess the likelihood of publication bias. We will take care in translating the results of included studies into recommendations for action by involving all review authors in drawing conclusions.
Data synthesis
We planned to conduct meta‐analyses of pooled data from all contributing trials using a fixed‐effect model for the primary analysis. Since no trials met our inclusion criteria, we did not perform meta‐analyses.
For future updates of the review we will use both a fixed‐effect and a random‐effects model and report results from both models.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses for the different prognostic subtypes of MDS according to the IPSS (Greenberg 1997), as well as to the morphological MDS subtypes according to the WHO classification (Harris 1999; Vardiman 2002). We planned to assess clinical heterogeneity by examining differences due to baseline measures of iron overload, dose of intervention, concomitant use of growth factors (erythropoietin, G‐CSF) and age. In addition, we planned to assess heterogeneity regarding study characteristics as described in the paragraph on Data collection and analysis and Assessment of risk of bias in included studies.
Sensitivity analysis
We did not perform sensitivity analyses based on assessment of risk of bias and publication status (unpublished and published studies) as we could not include any trials in this review. However, for future updates we plan to investigate the robustness of our results through a sensitivity analysis on the basis of the methodological quality of the included trials by defining the following groups: low risk of bias (successful blinding of all patients, people involved in treatment and care and outcome assessors; adequate allocation concealment; and loss to follow‐up of less than 20%); high risk of bias (no blinding; inadequate allocation concealment; and loss to follow‐up of more than 20%); unclear risk of bias (rating of unclear risk of bias in at least one of these three categories).
'Summary of findings' table
We will develop a 'Summary of findings' table according to the GRADE methodology (Balshem 2011; Guyatt 2011). We plan to present information on the following outcomes: mortality; cardiac failure; endocrine disease; iron status (assessed by magnetic resonance imaging (MRI) or superconducting quantum interference device (SQUID)), if not available, ferritin; two most relevant or severe AEs; discontinuations.
Contributions of authors
Joerg Meerpohl: conceived, designed and coordinated the review. He performed data collection and data management, as well as analysis and interpretation of the data. He wrote the review and approved the final version.
Lisa Schell: performed data collection and data management (search update). She conducted the literature searches in 2014, was involved with writing the review update and approval of the final version.
Gerta Ruecker: provided statistical advice and methodological support. She gave general advice on the review and approved the final version.
Nigel Fleeman: was co‐author of the HTA report by McLeod (McLeod 2009), gave general advice on the review and approved the final version.
Edith Motschall: gave advice on the search strategy and conducted the literature searches in 2010.
Charlotte Niemeyer: interpreted the data, provided clinical expertise and general advice on the review and approved the final version.
Dirk Bassler: performed data collection and data management. He analysed and interpreted the data, was involved with writing the review and approved the final version.
Sources of support
Internal sources
German Cochrane Centre, Freiburg, Germany.
External sources
No sources of support supplied
Declarations of interest
Joerg Meerpohl enrolled two adolescents with thalassaemia and one with Diamond‐Blackfan anaemia in a post‐marketing surveillance study on deferasirox and participated once in a Novartis advisory board meeting on paediatric iron overload over five years ago. The other review authors have no known conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Angelucci 2012 {published data only}
- 2006‐006147‐31. An open multicenter clinical trial to evaluate the safety, tolerability and efficacy of Deferasirox (ICL670) in patients affected by Myelodysplastic syndrome and transfusional chronic hemosiderosis. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=2006‐006147‐31 (accessed 16 April 2014).
- Angelucci E, Santini V, Tucci AA, Finelli C, Cantore N, Quarta G, et al. Deferasirox chelation therapy in transfusion dependent MDS patients. Final report from the Gimema MDS0306 prospective trial. Blood 2012;120(21):425. [Google Scholar]
Brosnahan 2008 {published data only}
- Brosnahan G, Gokden N, Swaminathan S. Acute interstitial nephritis due to deferasirox: a case report. Nephrology, Dialysis, Transplantation 2008;23(10):3356‐8. [DOI] [PubMed] [Google Scholar]
Cermak 2013 {published data only}
- Cermak J, Jonasova A, Vondrakova J, Cervinek L, Belohlavkova P, Neuwirtova R. A comparative study of deferasirox and deferiprone in the treatment of iron overload in patients with myelodysplastic syndromes. Leukemia Research 2013;37(12):1612‐5. [DOI] [PubMed] [Google Scholar]
Di Tucci 2007 {published data only}
- Tucci AA, Murru R, Alberti D, Rabault B, Deplano S, Angelucci E. Correction of anemia in a transfusion‐dependent patient with primary myelofibrosis receiving iron chelation therapy with deferasirox (Exjade ®, ICL670). European Journal of Haematology 2007;78(6):540‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2009 {published data only}
- Fox F, Kuendgen A, Nachtkamp K, Strupp C, Haas R, Germing U, et al. Matched‐pair analysis of 186 MDS patients receiving iron chelation therapy or transfusion therapy only. Blood 2009;114(22):694. [DOI] [PubMed] [Google Scholar]
García‐Delgado 2009 {published data only}
- García‐Delgado R, Fernández‐Ramos A, Rosell A, Gallardo AI, Martín M, Campos A, et al. Effect of deferasirox on oxidative stress and vascular dysfunction in MDS patients with iron overload. Haematologica 2009;94(Suppl. 2):536. [Google Scholar]
- García‐Delgado R, Rosell A, Fernández‐Ramos A, Gallardo A, Campos A, Castillo S. Effect of deferasirox on oxidative stress and vascular dysfunction in MDS patients with iron overload. Cytometry Part B‐Clinical Cytometry 2009;76B(6):436. [Google Scholar]
Gattermann 2007, EPIC {published data only}
- Cappellini DM, El‐Beshlawy A, Porter JB, Kattamis A, Taylor K, Rose C, et al. Concomitant medications and gastrointestinal events in thalassemia and MDS patients receiving deferasirox for transfusional iron overload: data from the EPIC study. Blood 2012;120(21):5182. [Google Scholar]
- Cappellini MD, Porter J, El‐Beshlawy A, Li C‐K, Seymour JF, Elalfy M, et al. Tailoring iron chelation by iron intake and serum ferritin: the prospective EPIC study of deferasirox in 1744 patients with transfusion‐dependent anemias. Haematologica 2010;95(4):557‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattermann N, Schmid M, Della Porta M, Taylor K, Seymour JF, Habr D, et al. Efficacy and safety of deferasirox (Exjade ®) during 1 year of treatment in transfusion‐dependent patients with myelodysplastic syndromes: results from EPIC trial. Blood 2008;112(11):235‐6. [Google Scholar]
- Gattermann N, Schmid M, Guerci‐Bresler A, Della Porta M, Taylor K, Habr D, et al. Correlation between decreased serum ferritin and improved liver transaminases during deferasirox (Exjade ®) treatment in iron‐overloaded patients with myelodysplastic syndromes. Blood 2009;114(22):1462‐3. [Google Scholar]
- Gattermann N, Schmid M, Guerci‐Bresler A, Della Porta M, Taylor K, Habr D, et al. Reduction in serum ferritin (SF) is associated with improvement in liver transaminase levels during treatment with deferasirox (Exjade ®) in iron‐overloaded patients with myelodysplastic syndromes (MDS). Leukemia Research 2009;33(Suppl 1):S140‐1. [Google Scholar]
- Gattermann N, Schmid M, Vassilieff D, Rose C, Della Porta M, Finelli C, et al. Severe iron overload in patients with myelodysplastic syndromes (MDS) enrolled in a large study of deferasirox (Exjade ®, ICL670). Leukemia Research 2007;31(Suppl 1):109‐10. [Google Scholar]
- Kattamis A, El‐Beshlawy A, Seymour JF, Li CK, Nick H, Habr D, et al. Labile plasma iron levels in heavily iron‐overloaded patients with transfusion‐dependent anaemias in response to deferasirox therapy: results from the large‐scale, prospective 1‐year EPIC trial. Haematologica 2009;94(Suppl 2):75. [Google Scholar]
- Lee JW, Yoon S‐S, Shen ZX, Hsu H‐C, Ganser A, Habr D, et al. Iron chelation in regularly transfused patients with aplastic anemia: efficacy and safety results from the large deferasirox EPIC trial. Blood 2008;112(11):167‐8. [Google Scholar]
- Porter J, Bowden DK, Economou M, Troncy J, Ganser A, Habr D, et al. Health‐related quality of life, treatment satisfaction, adherence and persistence in β‐thalassemia and myelodysplastic syndrome patients with iron overload receiving deferasirox: results from the EPIC clinical trial. Anemia 2012;2012:297641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JB, Elalfy M, Viprakasit V, Giraudier S, Chan LL, Lai Y, et al. Serum ferritin, labile plasma iron and transferrin saturation: comparison between underlying anemias with transfusional iron overload before and after treatment with deferasirox. Blood 2012;120(21):2126. [Google Scholar]
- Porter JB, Yoon SS, Shen ZX, Thein SL, Lin KH, Habr D, et al. Iron chelation therapy with deferasirox for transfusional iron‐overloaded patients with aplastic anaemia and other rare anaemias. Haematologica 2009;94(Suppl 2):80. [Google Scholar]
- Schmid M, Cappellini MD, Porter JB, Greenberg PL, Lawniczek T, Glaser S, et al. Safety of deferasirox (Exjade ®) in myelodysplastic syndromes (MDS) and non‐MDS patients with transfusional iron overload: a pooled analysis focusing on renal function. Blood 2009;114(22):702‐3. [Google Scholar]
- Schmid M, Guerci‐Bresler A, Della Porta M, Taylor K, Habr D, Domokos G, et al. Deferasirox (Exjade ®) is effective and well tolerated in chelation‐naive and previously chelated patients with transfusion‐dependent myelodysplastic syndromes (MDS). Blood 2009;114(22):1464. [Google Scholar]
- Schmid M, Guerci‐Bresler A, Della Porta M, Taylor K, Habr D, Domokos G, et al. Efficacy and safety of deferasirox (Exjade ®) in chelation‐naive and previously chelated patients with transfusion‐dependent myelodysplastic syndromes (MDS). Leukemia Research 2009;33(Suppl 1):S141‐2. [Google Scholar]
Gattermann 2009 {published data only}
- Gattermann N, Leismann O, Schlag R, Blumenstengel K, Goebeler M, Groschek M, et al. Deferasirox (Exjade ®) treatment of chelation‐naive and pre‐chelated MDS patients with transfusional iron‐overload in the medical practice: results from the observational studies Extend and Exjange. Blood 2009;114(22):1463‐4. [Google Scholar]
Ghoti 2009 {published data only}
- Ghoti H, Fibach E, Merkel D, Amer J, Nagler A, Levi I, et al. Decrease in intra‐ and extra‐cellular free iron species and oxidative stress parameters and increase in serum and urinary hepcidin during treatment with deferasirox in iron‐loaded patients with MDS. Haematologica 2009;94(Suppl 2):322. [Google Scholar]
- Ghoti H, Fibach E, Merkel D, Amer J, Nagler A, Perez‐Avraham G, et al. Decrease in intra‐ and extra‐cellular free iron species and oxidative stress parameters and increase in serum and urinary hepcidin during treatment with deferasirox in iron‐loaded patients with MDS. Leukemia Research 2009;33(Suppl 1):S120‐1. [Google Scholar]
Greenberg 2006, US2 study {published data only}
- Baer R, Esposito J, Kang B, Virkus J, Paley C. Iron overload in patients with MDS: Baseline data from studies of the once‐daily oral iron chelator, deferasirox. Haematologica 2007;92(2):379‐80. [Google Scholar]
- Greenberg PL, Koller CA, Glynos T, Paley C, Schiffer C. Change in liver iron concentration (LIC), serum ferritin (SF) and labile plasma iron (LPI) over 1 year of deferasirox (Exjade ®) therapy in a cohort of patients with MDS. Leukemia Research 2009;33(Suppl 1):S120. [Google Scholar]
- Greenberg PL, Schiffer CA, Koller CA, Kang B, Decker J, Paley C. Liver iron concentration measurements using MRI R2 in MDS patients in a deferasirox (Exjade ®, ICL670) phase II study. Blood 2006;108(11, Part 2):297B. [Google Scholar]
- Schmid M, Cappellini MD, Porter JB, Greenberg PL, Lawniczek T, Glaser S, et al. Safety of deferasirox (Exjade ®) in myelodysplastic syndromes (MDS) and non‐MDS patients with transfusional iron overload: a pooled analysis focusing on renal function. Blood 2009;114(22):702‐3. [Google Scholar]
Leitch 2007 {published data only}
- Leitch H, Ezzat H, Rollins MD, Goodman TA, Leger CS, Wong DHC, et al. Improved survival in red blood cell transfusion dependent patients with primary myelofibrosis (PMF) receiving iron chelation therapy. Blood 2008;112(11):617. [DOI] [PubMed] [Google Scholar]
- Leitch HA, Chase JM, Goodman TA, Ezzat H, Rollins MD, Wong DHC, et al. Improved survival in red blood cell transfusion dependent patients with primary myelofibrosis (PMF) receiving iron chelation therapy. Hematological Oncology 2010;28(1):40‐8. [DOI] [PubMed] [Google Scholar]
- Leitch HA, Ezzat H, Rollins MD, Wong DHC, Leger CS, Ramadan KM, et al. Transfusion dependence in patients with primary myelofibrosis has a negative impact on survival independent of decreased myelopoiesis. Blood 2007;110(11, Part 2):237B. [Google Scholar]
Leitch 2009 {published data only}
- Badawi MA, Vickars LM, Chase JM, Leitch HA. Red blood cell transfusion independence following the initiation of iron chelation therapy in myelodysplastic syndrome. Advances in Hematology 2010;2010:164045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch HA, Chase J, Vickars L. Red blood cell transfusion independence following the initiation of iron chelation therapy in myelodysplastic syndrome: a case report and review of the literature. Haematologica 2009;94(Suppl 2):539. [DOI] [PMC free article] [PubMed] [Google Scholar]
List 2006, US3 study {published data only}
- Baer R, Esposito J, Kang B, Virkus J, Paley C. Iron overload in patients with MDS: baseline data from studies of the once‐daily oral iron chelator, deferasirox. Haematologica 2007;92:379‐80. [Google Scholar]
- List AF, Baer MR, Steensma D, Raza A, Esposito J, Virkus J, et al. Deferasirox (ICL670; Exjade ®) reduces serum ferritin (SF) and labile plasma iron (LPI) in patients with myelodysplastic syndromes (MDS). Blood 2007;110(11, Part 1):440A. [Google Scholar]
- List AF, Baer MR, Steensma D, Raza A, Esposito J, Virkus J, et al. Iron chelation therapy with deferasirox (ICL670) reduces serum ferritin (SF) and labile plasma iron (LPI) in patients with myelodysplastic syndromes (MDS). Haematologica 2008;93(S1):0228. [Google Scholar]
- List AF, Baer MR, Steensma D, Raza A, Esposito J, Virkus J, et al. Iron chelation with deferasirox (Exjade ®) improves iron burden in patients with myelodysplastic syndromes (MDS). Blood 2008;112(11):236. [Google Scholar]
- List AF, Baer MR, Steensma DP, Raza A, Esposito J, Martinez‐Lopez N, et al. Two‐year analysis of efficacy and safety of deferasirox (Exjade®) treatment in myelodysplastic syndrome patients enrolled in the US03 study. Blood 2009;114(22):1473. [Google Scholar]
- List AF, Esposito J, Decker J, Baer MR, Powell B, Steensma D, et al. Iron parameters in 84 MDS patients enrolled in a deferasirox (Exjade®, ICL670) multicenter trial. Blood 2006;108(11, Part 2):297B. [Google Scholar]
- Schmid M, Cappellini MD, Porter JB, Greenberg PL, Lawniczek T, Glaser S, et al. Safety of deferasirox (Exjade®) in myelodysplastic syndromes (MDS) and non‐MDS patients with transfusional iron overload: a pooled analysis focusing on renal function. Blood 2009;114(22):702‐3. [Google Scholar]
Messa 2008 {published data only}
- Messa E, Catalano R, Messa F, Arruga E, Defilippi I, Carturan S, et al. Improvement of hemoglobin level and reduction of transfusion requirement in four patients affected by myelodysplastic syndromes and primary myelofibrosis receiving deferasirox treatment. Haematologica 2008;93(S1):0063. [Google Scholar]
Metzgeroth 2009 {published data only}
- Metzgeroth G, Dinter D, Schultheis B, Dorn‐Beineke A, Lutz K, Leismann O, et al. Deferasirox in MDS patients with transfusion‐caused iron overload ‐ a phase‐II study. Annals of Hematology 2009;88(4):301‐10. [DOI] [PubMed] [Google Scholar]
Min 2008 {published data only}
- Cheong J‐W, Kim H‐J, Lee K‐H, Yoon S‐S, Lee JH, Park H‐S, et al. Efficacy of ICT with Deferasirox in transfusional iron overloaded patients with MDS or AA. Blood 2009;114(22):1466. [Google Scholar]
- Min Y, Cheong J, Kim H, Lee K, Yoon S, Lee J, et al. A multi‐center, open label study evaluating the efficacy of iron chelation therapy with deferasirox in transfusional iron overload patients with myelodysplastic syndromes or aplastic anemia using quantitative R2 MRI. Leukemia Research 2009;33(Suppl 1):S118‐9. [Google Scholar]
- Min Y‐H, Kim HJ, Lee KH, Yoon S‐S, Lee JH, Park H‐S, et al. A multi‐center, open label study evaluating the efficacy of iron chelation therapy with deferasirox in transfusional iron overload patients with myelodysplastic syndromes or aplastic anemia using quantitative R2 MRI. Blood 2008;112(11):1249. [Google Scholar]
Miyazawa 2006 {published data only}
- Iki S, Urabe A, Hata T, Ohyashiki K, Nakao S, Takatoku M, et al. Comparative pharmacokinetic/pharmacodynamic profiles in Japanese and Caucasian patients with transfusional hemosiderosis receiving treatment with deferasirox (Exjade®, ICL670). Blood 2006;108(11, Part 2):296B‐7B. [Google Scholar]
- Iki S, Urabe A, Hata T, Ohyashiki K, Nakao S, Takatoku M, et al. PK/PD profiles during deferasirox (Exjade (R), ICL670) treatment are similar in Japanese and Caucasian patients with transfusional iron overload. American Journal of Hematology 2007;82(6):514. [Google Scholar]
- Miyazawa K, Ohyashiki K, Urabe A, Hata T, Nakao S, Ozawa K, et al. A safety, pharmacokinetic and pharmacodynamic investigation of deferasirox (Exjade®, ICL670) in patients with transfusion‐dependent anemias and iron‐overload: a Phase I study in Japan. International Journal of Hematology 2008;88(1):73‐81. [DOI] [PubMed] [Google Scholar]
Nolte 2013 {published data only}
- Nolte F, Höchsmann B, Giagounidis A, Lübbert M, Platzbecker U, Haase D, et al. Results from a 1‐year, open‐label, single arm, multi‐center trial evaluating the efficacy and safety of oral Deferasirox in patients diagnosed with low and int‐1 risk myelodysplastic syndrome (MDS) and transfusion‐dependent iron overload. Annals of Hematology 2013;92(2):191‐8. [DOI] [PubMed] [Google Scholar]
Peng 2013 {published data only}
- Peng P. Long LL, Huang ZK, Zhang L, Li XH, Feng X. Comparison of deferasirox and deferoxamine treatment in iron‐overloaded patients: liver iron concentration determined by quantitative MRI‐R2* [Chinese]. Chinese Journal of Radiology 2013;47(1):55‐9. [Google Scholar]
Pennell 2014, CORDELIA {published and unpublished data}
- Pennell DJ, Porter JB, Piga A, Lai Y, El‐Beshlawy A, Belhoul KM. A 1‐year randomized controlled trial of deferasirox versus deferoxamine for myocardial iron removal in beta‐thalassemia major (CORDELIA). Blood 2014;123(10):1447‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Porter 2004 {published data only}
- Cazzola M, Gattermann N, Greenberg P, Maertens J, Soulières D, Rose M, et al. ICL670, a once‐daily oral iron chelator, is effective and well tolerated in patients with myelodysplastic syndrome (MDS) and iron overload. Haematologica 2005;90(Suppl 2):318. [Google Scholar]
- Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Cappellini MD, et al. Relative response of patients with myelodysplastic syndromes and other transfusion‐dependent anaemias to deferasirox (ICL670): a 1‐yr prospective study. European Journal of Haematology 2008;80(2):168‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter J, Vichinsky E, Rose C, Piga A, Olivieri N, Gattermann N, et al. A phase II study with ICL670 (Exjade®), a once‐daily oral iron chelator, in patients with various transfusion‐dependent anemias and iron overload. Blood 2004;104(11, Part 1):872A. [Google Scholar]
- Porter JB, Cohen A, Agaoglu L, Ganser A, Cappellini MD, Maseruka H, et al. Long‐term effects of deferasirox (Exjade®), ICL670) on serum ferritin: outcome of dose adjustments in achieving maintenance or reduction in body iron stores. Blood 2006;108(11, Part 1):502A. [Google Scholar]
- Porter JB, Piga A, Cohen A, Ford JM, Bodner J, Cappellini MD. Assessment of safety in patients receiving longer‐term iron chelation therapy with deferasirox who had achieved serum ferritin levels of < 1000 ng/ml during the study course. Haematologica 2009;94(Suppl 2):77. [Google Scholar]
- Saglio G, Gattermann N, Rabault B, Alberti D. High iron burden in patients with myelodysplastic syndromes (MDS) effectively decreased with once‐daily, oral chelation with deferasirox. Annals of Oncology 2006;17(Suppl 9):204. [Google Scholar]
- Taher A, Cappellini MD, Vichinsky E, Galanello R, Piga A, Lawniczek T, et al. Efficacy and safety of deferasirox doses of >30 mg/kg per d in patients with transfusion‐dependent anaemia and iron overload. British Journal of Haematology 2009;147(5):752‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rachmilewitz 2008 {published data only}
- Rachmilewitz E, Merkel D, Ghoti H, Amer J, Nagler A, Perez‐Avraham G, et al. Improvement of oxidative stress parameters in MDS patients with iron overload treated with deferasirox. Blood 2008;112:924‐5. [Google Scholar]
Remacha 2010 {published data only}
- Remacha AF, Arrizabalaga B, Cañizo C, Sanz G, Villegas A. Iron overload and chelation therapy in patients with low‐risk myelodysplastic syndromes with transfusion requirements. Annals of Hematology 2010;89(2):147‐54. [DOI] [PubMed] [Google Scholar]
Rose 2007 {published data only}
- Rose C, Brechignac S, Vassilief D, Beyne‐Rauzy O, Stamatoullas A, Larbaa D, et al. Positive impact of iron chelation therapy (CT) on survival in regularly transfused MDS patients. A prospective analysis by the GFM. Blood 2007;110(11, Part 1):80A‐1A. [DOI] [PubMed] [Google Scholar]
Wimazal 2009 {published data only}
- Wimazal F, Nösslinger T, Baumgartner C, Sperr WR, Pfeilstöcker M, Valent P. Deferasirox induces regression of iron overload in patients with myelodysplastic syndromes. European Journal of Clinical Investigation 2009;39(5):406‐11. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Castellano 2011 {published data only}
- Andrade‐Campos MM, Medrano‐Engay MB, Royo P, Giraldo P. Impact of chelation therapy on patients with iron overload. +12 months follow‐up after QUELAFER study. European Leukemia Net (ELN) Frontiers Meeting; 2013 Oct 11‐13; Prague. http://www.leukemia‐net.org/content/home/eln_meetings/eln_frontiers_meeting/e10287/infoboxContent10288/ELN_Abstracts_Prag_2013_low.pdf. Frankfurt: European LeukemiaNet, (accessed 03 April 2014).
- Medrano‐Engay B, Irun P, Sarria L, Andrade M, Murillo I, Montes A. Evaluation of quality of life and analysis of efficacy and safety of two iron chelators in patients with iron overload. Haematologica. 2013; Vol. 98 (supplement 1):704.
References to ongoing studies
NCT02038816 {unpublished data only}
- NCT02038816. Azacitidine plus deferasirox (ICL670) in higher risk myelodysplastic syndromes (MDS). http://clinicaltrials.gov/show/NCT02038816 (accessed 16 April 2014).
Novartis 2013 {unpublished data only}
- CICL670A2421. An open‐label, phase II, randomized, pilot study to assessthe effect in term of erythroid improvement of deferasiroxcombined with erythropoietin compared to erythropoietinalone in patients with low‐ and int‐1‐risk myelodysplasticsyndrome. https://www.clinicaltrialsregister.eu/ctr‐search/trial/2013‐000981‐12/ES.
- NCT01868477. Combination study of deferasirox and erythropoietin in patients with low‐ and Int‐1‐risk myelodysplastic syndrome. http://clinicaltrials.gov/show/NCT01868477 (accessed 16 April 2014).
TELESTO 2009 {unpublished data only}
- 2009‐012418‐38. A multi‐center, randomized, double‐blind, placebocontrolled clinical trial of deferasirox in patients with myelodysplastic syndromes (low/int‐1 risk) and transfusional iron overload. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2009‐012418‐38.
- Angelucci E, Bowen D, Magalhães SMM, Lawniczek T, Douma S, Jakobs P, et al. A multicenter, randomized, double‐blind, placebo‐controlled trial of deferasirox (Exjade ®) in patients with low/intermediate‐1 risk MDS and transfusional iron overload. Blood 2009; Vol. 114, issue 22:4854.
- NCT00940602. Myelodysplastic syndromes (MDS) event free survival with iron chelation therapy study (TELESTO). http://clinicaltrials.gov/show/NCT00940602 (accessed 16 April 2014).
Additional references
Adams 2013
- Adams RL, Bird RJ. Safety and efficacy of deferasirox in the management of transfusion‐dependent patients with myelodysplastic syndrome and aplastic anaemia: a perspective review. Therapeutic Advances in Hematology 2013;4(2):93‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aul 2001
- Aul C, Giagounidis A, Germing U. Epidemiological features of myelodysplastic syndromes: results from regional cancer surveys and hospital‐based statistics. International Journal of Hematology 2001;73(4):405‐10. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Bennett 2008
- Bennett JM, MDS Foundation's Working Group on Transfusional Iron Overload. Consensus statement on iron overload in myelodysplastic syndromes. American Journal of Hematology 2008;83(11):858‐61. [DOI] [PubMed] [Google Scholar]
Borgna‐Pignatti 2004
- Borgna‐Pignatti C, Rugolotto S, Stefano P, Zhao H, Cappellini MD, Vecchio GC, et al. Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica 2004;89(10):1187‐93. [PubMed] [Google Scholar]
Bozkaya 2008
- Bozkaya D, Migliaccio‐Walle K, Baladi J‐F. Impact of management of iron overload in patients with myelodysplastic syndromes. Blood 2008;112(11):473. [Google Scholar]
Breccia 2013
- Breccia M, Alimena G. Efficacy and safety of deferasirox in myelodysplastic syndromes. Annals of Hematology 2013;92(7):863‐70. [DOI] [PubMed] [Google Scholar]
Brittenham 1994
- Brittenham GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, et al. Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major. New England Journal of Medicine 1994;331(9):567‐73. [DOI] [PubMed] [Google Scholar]
Cappellini 2005
- Cappellini MD. Overcoming the challenge of patient compliance with iron chelation therapy. Seminars in Hematology 2005;42(2 Suppl 1):S19‐21. [DOI] [PubMed] [Google Scholar]
Cappellini 2006
- Cappellini MD, Cohen A, Piga A, Bejaoui M, Perrotta S, Agaoglu L, et al. A phase 3 study of deferasirox (ICL670), a once‐daily oral iron chelator, in patients with beta‐thalassemia. Blood 2006;107(9):3455‐62. [DOI] [PubMed] [Google Scholar]
Ceci 2002
- Ceci A, Baiardi P, Felisi M, Cappellini MD, Carnelli V, Sanctis V, et al. The safety and effectiveness of deferiprone in a large‐scale, 3‐year study in Italian patients. British Journal of Haematology 2002;118(1):330‐6. [DOI] [PubMed] [Google Scholar]
Chee 2008
- Chee CE, Steensma DP, Wu W, Hanson CA, Tefferi A. Neither serum ferritin nor the number of red blood cell transfusions affect overall survival in refractory anemia with ringed sideroblasts. American Journal of Hematology 2008;83(8):611‐3. [DOI] [PubMed] [Google Scholar]
Cianciulli 2008
- Cianciulli P. Treatment of iron overload in thalassemia. Pediatric Endocrinology Reviews 2008;6(Suppl 1):208‐13. [PubMed] [Google Scholar]
Curtin 2002a
- Curtin F, Altman DG, Elbourne D. Meta‐analysis combining parallel and cross‐over clinical trials. I: Continuous outcomes. Statistics in Medicine 2002;21(15):2131‐44. [DOI] [PubMed] [Google Scholar]
Curtin 2002b
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. II: Binary outcomes. Statistics in Medicine 2002;21(15):2145‐59. [DOI] [PubMed] [Google Scholar]
Curtin 2002c
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. III: The issue of carry‐over. Statistics in Medicine 2002;21(15):2161‐73. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Delea 2005
- Delea TE, Thomas SK, Baladi J‐F, Phatak PD. Cost‐effectiveness analysis of oral iron chelation therapy with deferasirox (Exjade®, ICL670) versus infusional chelation therapy with deferoxamine in patients with transfusion‐dependent myelodysplastic. Blood 2005;106(11, Part 2):484B‐5B. [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
European Medicines Agency 2010
- European Medicines Agency. Exjade: European Public Assessment Report: Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for_the_public/human/000670/WC500033927.pdf (accessed 13 October 2014).
Farmaki 2006
- Farmaki K, Angelopoulos N, Anagnostopoulos G, Gotsis E, Rombopoulos G, Tolis G. Effect of enhanced iron chelation therapy on glucose metabolism in patients with beta‐thalassaemia major. British Journal of Haematology 2006;134(4):438‐44. [DOI] [PubMed] [Google Scholar]
Fisher 2013
- Fisher SA, Brunskill SJ, Doree C, Gooding S, Chowdhury O, Roberts DJ. Desferrioxamine mesylate for managing transfusional iron overload in people with transfusion‐dependent thalassaemia. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD004450] [DOI] [PMC free article] [PubMed] [Google Scholar]
Food and Drug Administration (FDA) 2010
- Food, Drug Administration (FDA). Exjade. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021882s010lbl.pdf (accessed 24 September 2010).
Freedman 1990
- Freedman MH, Grisaru D, Olivieri N, MacLusky I, Thorner PS. Pulmonary syndrome in patients with thalassemia major receiving intravenous deferoxamine infusions. American Journal of Diseases of Children 1990;144(5):565‐9. [DOI] [PubMed] [Google Scholar]
Gabutti 1996
- Gabutti V, Piga A. Results of long‐term iron‐chelating therapy. Acta Haematologica 1996;95(1):26‐36. [DOI] [PubMed] [Google Scholar]
Galanello 2006
- Galanello R, Kattamis A, Piga A, Fischer R, Leoni G, Ladis V, et al. A prospective randomized controlled trial on the safety and efficacy of alternating deferoxamine and deferiprone in the treatment of iron overload in patients with thalassemia. Haematologica 2006;91(9):1241‐3. [PubMed] [Google Scholar]
Gattermann 2008
- Gattermann N. Overview of guidelines on iron chelation therapy in patients with myelodysplastic syndromes and transfusional iron overload. International Journal of Hematology 2008;88(1):24‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glenny 2005
- Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D'Amico R, et al. Indirect comparisons of competing interventions. Health Technology Assessment 2005;9(26):1‐134. [DOI] [PubMed] [Google Scholar]
Greenberg 1997
- Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997;89(6):2079‐88. [PubMed] [Google Scholar]
Greenberg 2011
- Greenberg PL, Attar E, Bennett JM, Bloomfield CD, Castro CM, Deeg HJ, et al. NCCN Clinical Practice Guidelines in Oncology: myelodysplastic syndromes. Journal of the National Comprehensive Cancer Network 2011;9(1):30–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Harris 1999
- Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller‐Hermelink HK, Vardiman J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting‐Airlie House, Virginia, November 1997. Journal of Clinical Oncology 1999;17(12):3835‐49. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffbrand 1989
- Hoffbrand AV, Bartlett AN, Veys PA, O'Connor NT, Kontoghiorghes GJ. Agranulocytosis and thrombocytopenia in patient with Blackfan‐Diamond anaemia during oral chelator trial. Lancet 1989;2(8660):457. [DOI] [PubMed] [Google Scholar]
Hoffbrand 2003
- Hoffbrand AV, Cohen A, Hershko C. Role of deferiprone in chelation therapy for transfusional iron overload. Blood 2003;102(1):17‐24. [DOI] [PubMed] [Google Scholar]
Karnon 2007a
- Karnon J, Baladi JF. Modelling the cost‐effectiveness of deferasirox (Exjade) in the treatment of iron overloaded myelodysplastic syndrome patients. Leukemia Research 2007;31(Suppl 1):S134. [Google Scholar]
Karnon 2007b
- Karnon J, Akehurst RL, Jewitt K, Ossa D. Cost utility analysis deferasirox versus deferoxamine (desferal) for patients requiring iron chelation therapy in the United Kingdom. Haematologica 2007;92(1):222. [Google Scholar]
Kattamis 2003
- Kattamis A, Kassou C, Berdousi H, Ladis V, Papassotiriou I, Kattamis C. Combined therapy with desferrioxamine and deferiprone in thalassemic patients: effect on urinary iron excretion. Haematologica 2003;88(12):1423‐5. [PubMed] [Google Scholar]
Kennedy 2013
- Kennedy GA, Morris KL, Subramonpillai E, Curley C, Butler J, Durrant S. A prospective phase II randomized study of deferasirox to prevent iatrogenic iron overload in patients undertaking induction/consolidation chemotherapy for acute myeloid leukemia. British Journal of Haemotology 2013;161(6):794‐801. [DOI] [PubMed] [Google Scholar]
Kolnagou 2008
- Kolnagou A, Economides C, Eracleous E, Kontoghiorghes GJ. Long term comparative studies in thalassemia patients treated with deferoxamine or a deferoxamine/deferiprone combination. Identification of effective chelation therapy protocols. Hemoglobin 2008;32(1‐2):41‐7. [DOI] [PubMed] [Google Scholar]
Kontoghiorghes 1987a
- Kontoghiorghes GJ, Aldouri MA, Sheppard L, Hoffbrand AV. 1,2‐Dimethyl‐3‐hydroxypyrid‐4‐one, an orally active chelator for treatment of iron overload. Lancet 1987;1(8545):1294‐5. [DOI] [PubMed] [Google Scholar]
Kontoghiorghes 1987b
- Kontoghiorghes GJ, Aldouri MA, Hoffbrand AV, Barr J, Wonke B, Kourouclaris T, et al. Effective chelation of iron in beta thalassaemia with the oral chelator 1,2‐dimethyl‐3‐hydroxypyrid‐4‐one. British Medical Journal (Clinical Research Edition) 1987;295(6612):1509‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koren 1991
- Koren G, Kochavi‐Atiya Y, Bentur Y, Olivieri NF. The effects of subcutaneous deferoxamine administration on renal function in thalassemia major. International Journal of Hematology 1991;54(5):371‐5. [PubMed] [Google Scholar]
Leitch 2008
- Leitch H, Leger C, Goodman T, Wong K, Wong D, Ramadan K, et al. Improved survival in patients with myelodysplastic syndrome receiving iron chelation therapy. Clinical Leukemia 2008;2(3):205‐11. [Google Scholar]
Leitch 2009b
- Leitch HA, Vickars LM. Supportive care and chelation therapy in MDS: are we saving lives or just lowering iron?. Hematology. American Society of Hematology Education Program 2009;1:664‐72. [DOI] [PubMed] [Google Scholar]
List 2006
- List AF. Treatment of iron overload in myelodysplastic syndrome. Hematology/Oncology Clinics of North America 2006;20(Suppl 1):6‐17. [Google Scholar]
List 2012
- List AF, Baer MR, Steensma DP, Raza A, Esposito J, Martinez‐Lopez N, et al. Deferasirox reduces serum ferritin and labile plasma iron in RBC transfusion‐dependent patients with myelodysplastic syndrome. Journal of Clinical Oncology 2012;30(17):2134‐9. [DOI] [PubMed] [Google Scholar]
Maggio 2002
- Maggio A, D'Amico G, Morabito A, Capra M, Ciaccio C, Cianciulli P, et al. Deferiprone versus deferoxamine in patients with thalassemia major: a randomized clinical trial. Blood Cells, Molecules and Diseases 2002;28(2):196‐208. [DOI] [PubMed] [Google Scholar]
Malcovati 2005
- Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. Journal of Clinical Oncology 2005;23(30):7594‐603. [DOI] [PubMed] [Google Scholar]
Malcovati 2007
- Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, et al. Time‐dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. Journal of Clinical Oncology 2007;25(23):3503‐10. [DOI] [PubMed] [Google Scholar]
Malcovati 2013
- Malcovati L, Hellström‐Lindberg E, Bowen D, Adès L, Cermak J, Cañizo C, et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood 2013;122(17):2943‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
McLeod 2009
- McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al. Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation. Health technology assessment (Winchester, England) 2009;13(1):iii‐iv, ix‐xi, 1‐121. [PUBMED: 19068191] [DOI] [PubMed] [Google Scholar]
Meerpohl 2008
- Meerpohl JJ, Antes G, Rücker G, McLeod C, Fleeman N, Niemeyer C, et al. Deferasirox for managing iron overload in patients with myelodysplastic syndrome. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007461.pub2] [DOI] [Google Scholar]
NCCN Myelodysplastic Panel Members 2010
- NCCN Myelodysplastic Panel Members. Myelodysplastic syndromes. http://www.nccn.org/professionals/physician_gls/PDF/mds.pdf accessed 24 September 2010.
Nisbet‐Brown 2003
- Nisbet‐Brown E, Olivieri NF, Giardina PJ, Grady RW, Neufeld EJ, Séchaud R, et al. Effectiveness and safety of ICL670 in iron‐loaded patients with thalassaemia: a randomised, double‐blind, placebo‐controlled, dose‐escalation trial. Lancet 2003;361(9369):1597‐602. [DOI] [PubMed] [Google Scholar]
Olivieri 1994
- Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A, et al. Survival in medically treated patients with homozygous beta‐thalassemia. New England Journal of Medicine 1994;331(9):574‐8. [DOI] [PubMed] [Google Scholar]
Olivieri 1997
- Olivieri NF, Brittenham GM. Iron‐chelating therapy and the treatment of thalassemia. Blood 1997;89(3):739‐61. [PubMed] [Google Scholar]
Olivieri 1998
- Olivieri NF, Brittenham GM, McLaren CE, Templeton DM, Cameron RG, McClelland RA, et al. Long‐term safety and effectiveness of iron‐chelation therapy with deferiprone for thalassemia major. New England Journal of Medicine 1998;339(7):417‐23. [DOI] [PubMed] [Google Scholar]
Origa 2005
- Origa R, Bina P, Agus A, Crobu G, Defraia E, Dessi C, et al. Combined therapy with deferiprone and desferrioxamine in thalassemia major. Haematologica 2005;90(10):1309‐14. [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Peng 2008
- Peng CT, Tsai CH, Wu KH. Effects of chelation therapy on cardiac function improvement in thalassemia patients: literature review and the Taiwanese experience. Hemoglobin 2008;32(1‐2):49‐62. [DOI] [PubMed] [Google Scholar]
Porter 2009
- Porter JB. Optimizing iron chelation strategies in beta‐thalassaemia major. Blood Reviews 2009;23(Suppl 1):S3‐7. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2007
- Roberts DJ, Brunskill S, Doree C, Williams S, Howard J, Hyde C. Oral deferiprone for iron chelation in people with thalassaemia. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004839.pub2] [DOI] [PubMed] [Google Scholar]
Salanti 2008
- Salanti G, Higgins JP, Ades A, Ioannidis JP. Evaluation of networks of randomized trials. Statistical Methods in Medical Research 2008;17(3):279‐301. [DOI] [PubMed] [Google Scholar]
Shah 2012
- Shah J, Kurtin SE, Arnold L, Lindroos‐Kolqvist P, Tinsley S. Management of transfusion‐related iron overload in patients with myelodysplastic syndromes. Clinical Journal of Oncology Nursing 2012;16(3):37‐46. [DOI] [PubMed] [Google Scholar]
Takatoku 2007
- Takatoku M, Uchiyama T, Okamoto S, Kanakura Y, Sawada K, Tomonaga M, et al. Retrospective nationwide survey of Japanese patients with transfusion‐dependent MDS and aplastic anemia highlights the negative impact of iron overload on morbidity/mortality. European Journal of Haematology 2007;78(6):487‐94. [DOI] [PubMed] [Google Scholar]
Tanner 2007
- Tanner MA, Galanello R, Dessi C, Smith GC, Westwood MA, Agus A, et al. A randomized, placebo‐controlled, double‐blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation 2007;115(14):1876‐84. [DOI] [PubMed] [Google Scholar]
Valent 2008
- Valent P, Krieger O, Stauder R, Wimazal F, Nösslinger T, Sperr WR, et al. Iron overload in myelodysplastic syndromes (MDS) ‐ diagnosis, management, and response criteria: a proposal of the Austrian MDS platform. European Journal of Clinical Investigation 2008;38(3):143‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vardiman 2002
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 2002;100(7):2292‐302. [DOI] [PubMed] [Google Scholar]
Wanless 2002
- Wanless IR, Sweeney G, Dhillon AP, Guido M, Piga A, Galanello R, et al. Lack of progressive hepatic fibrosis during long‐term therapy with deferiprone in subjects with transfusion‐dependent beta‐thalassemia. Blood 2002;100(5):1566‐9. [DOI] [PubMed] [Google Scholar]
Williamson 2002
- Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;21(22):3337‐51. [DOI] [PubMed] [Google Scholar]
Witte 2004
- Witte S, Victor N. Some problems with the investigation of noninferiority in meta‐analysis. Methods of Information in Medicine 2004;43(5):470‐4. [PubMed] [Google Scholar]
Wu 2006
- Wu SF, Peng CT, Wu KH, Tsai CH. Liver fibrosis and iron levels during long‐term deferiprone treatment of thalassemia major patients. Hemoglobin 2006;30(2):215‐8. [DOI] [PubMed] [Google Scholar]
Zurlo 1989
- Zurlo MG, Stefano P, Borgna‐Pignatti C, Palma A, Piga A, Melevendi C, et al. Survival and causes of death in thalassaemia major. Lancet 1989;2(8653):27‐30. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Meerpohl 2010
- Meerpohl JJ, Antes G, Rucker G, Fleeman N, Motschall E, Niemeyer CM, et al. Deferasirox for managing iron overload in people with myelodysplastic syndrome. The Cochrane database of systematic reviews 2010, (11):CD007461. [PUBMED: 21069694] [DOI] [PubMed] [Google Scholar]


