Abstract
Abstract: Parasite‐driven declines in wildlife have become increasingly common and can pose significant risks to natural populations. We used the IUCN Red List of Threatened and Endangered Species and compiled data on hosts threatened by infectious disease and their parasites to better understand the role of infectious disease in contemporary host extinctions. The majority of mammal species considered threatened by parasites were either carnivores or artiodactyls, two clades that include the majority of domesticated animals. Parasites affecting host threat status were predominantly viruses and bacteria that infect a wide range of host species, including domesticated animals. Counter to our predictions, parasites transmitted by close contact were more likely to cause extinction risk than those transmitted by other routes. Mammal species threatened by parasites were not better studied for infectious diseases than other threatened mammals and did not have more parasites or differ in four key traits demonstrated to affect parasite species richness in other comparative studies. Our findings underscore the need for better information concerning the distribution and impacts of infectious diseases in populations of endangered mammals. In addition, our results suggest that evolutionary similarity to domesticated animals may be a key factor associated with parasite‐mediated declines; thus, efforts to limit contact between domesticated hosts and wildlife could reduce extinction risk.
Keywords: domesticated animal, IUCN Red List, mammal conservation, parasite, pathogen, transmission mode, virus
Keywords: animal domesticado, conservación de mamíferos, Lista Roja de IUCN, modo de transmisión, parásito, patógeno, virus
Abstract
Resumen: Las declinaciones de vida silvestre debido a parásitos se han vuelto más comunes y pueden ser riesgos significativos para las poblaciones naturales. Utilizamos la Lista Roja de IUCN de Especies Amenazadas y en Peligro y compilamos datos sobre huéspedes amenazados por enfermedades infecciosas y sus parásitos para comprender el papel de enfermedades infecciosas en las extinciones contemporáneas de huéspedes. La mayoría de las especies de mamíferos consideradas amenazadas por parásitos fueron carnívoros o artiodáctilos, dos clados que incluyen a la mayoría de los animales domesticados. Los parásitos que afectan el estatus amenazado de huéspedes son predominantemente virus y bacterias que infectan a una amplia gama de especies huésped, incluyendo animales domésticos. Contrario a nuestras predicciones, los parásitos transmitidos por contacto cercano tuvieron mayor probabilidad de causar riesgo de extinción que los transmitidos por otras rutas. Las especies de mamíferos amenazadas por parásitos no fueron mejor estudiadas para enfermedades infecciosas que otros mamíferos amenazados y no tuvieron más parásitos ni difirieron en cuatro características clave que afectan la riqueza de especies de parásitos en otros estudios comparativos. Nuestros hallazgos subrayan la necesidad de mayor información relacionada con la distribución e impacto de enfermedades infecciosas en poblaciones de mamíferos en peligro. Adicionalmente, nuestros resultados sugieren que la similitud evolutiva con animales domesticados puede ser un factor clave asociados con las declinaciones debidas a parásitos; por lo tanto, los esfuerzos para limitar contacto entre huéspedes domesticados y vida silvestre podrían reducir el riesgo de extinción.
Introduction
Parasites are ubiquitous in the lives of wild animals and represent a major component of biological diversity (Price 1980). Infectious diseases can also cause significant population declines in wildlife, as illustrated by canine distemper virus in Serengeti lions (Roelke‐Parker et al. 1996), Ebola outbreaks in African apes (Leroy et al. 2004), and multiple pathogens that affect amphibian populations (Daszak et al. 1999; Pounds et al. 2006). Despite evidence that parasites can increase extinction risk in wild animals (Woodroffe 1999; Daszak et al. 1999), few researchers have investigated the factors associated with parasite‐mediated declines or extinctions (but see Lafferty & Gerber 2002; de Castro & Bolker 2005; Smith et al. 2006). Even less is known about the phylogenetic distribution of infectious disease risks among wild host species and the ecological factors that drive these patterns.
Threatened host populations are predicted to harbor relatively few parasite species as a consequence of limited geographic ranges and small, isolated populations (Lyles & Dobson 1993; Lafferty & Gerber 2002). Mathematical models of single‐host, single‐parasite systems suggest that horizontally transmitted parasites will be lost if host populations decline below a critical threshold density (Anderson & May 1992). For several reasons, however, parasite‐induced extinction are more probable in situations involving multihost parasites and frequency‐dependent transmission and when in concert with other drivers of extinction (de Castro & Bolker 2005). First, many parasites can infect multiple species, and high prevalence in alternative hosts coupled with cross‐species transmission can lead to parasite‐induced declines in species that would normally be unable to maintain the parasite (Gog et al. 2002; Fenton & Pedersen 2005). In carnivores, for example, several near extinctions have been caused by generalist parasites transmitted to populations of threatened species (Woodroffe 1999; Lafferty & Gerber 2002). Second, the transmission of some parasites, such as sexually transmitted and vector‐borne diseases, may be decoupled from host population density (Getz & Pickering 1983); these parasites can therefore continue to spread even as host populations decline (Boots & Sasaki 2003). Finally, many threatened mammals are predisposed to extinction risk because their populations are fragmented and small and they have low genetic variability (Woodroffe 1999; Altizer et al. 2003). These factors can increase host susceptibility and exposure to infectious diseases (Lyles & Dobson 1993), or infectious diseases might represent an additional factor that increases stochastic extinction risk (McCallum & Dobson 1995).
Over 24% of all extant mammal species are threatened with extinction according to the World Conservation Union (IUCN 2006). Parasites rarely have been implicated as a direct cause of host extinction risk relative to other factors such as habitat loss, hunting, and invasive species (Wilcove et al. 1998; Purvis et al. 2000). Indeed, Smith et al. (2006) found infectious disease to be an uncommon cause of extinction and critical endangerment for most plants and animals. Only 3.7% of 833 known species extinctions have been attributed in part or directly to infectious disease. Because of uncertainty in threat assessments and a strong temporal bias in reporting infectious disease as a threatening process, however, such low estimates probably represent only a subset of the species at risk of disease‐mediated extinction (Smith et al. 2006).
Here we assessed how mammal clades differ in their vulnerability to infectious disease threats in an effort to better understand the role of parasites in contemporary mammal extinctions and to pinpoint conservation strategies that might reduce extinction risk. To identify factors associated with disease‐mediated threats, we independently assessed the parasites reported to cause declines in threatened mammals. We define parasites broadly to include macroparasites (i.e., helminths, arthropods, protozoa) and microbial pathogens (i.e., viruses and bacteria). We focused on hosts for which parasites have been cited previously as a major cause of threat according to the IUCN Red List, independently assessed the parasites reported to cause population declines in these mammals, and developed a comprehensive data set on host and parasite characteristics.
Because domesticated animals are maintained at high population densities and have a near‐global distribution, they can serve as reservoirs of infectious disease for wild mammals (Lafferty & Gerber 2002). We therefore expected that mammals from clades that contain domesticated animals would be more likely to be threatened by parasites shared with domesticated species. We further predicted that parasites reported to cause population declines in threatened mammals would have broad host ranges and be transmitted via biting arthropods, contaminated food or water, or other indirect routes that are decoupled from direct host contact. Finally, we investigated sampling gaps and potential biases in the degree to which parasites have been identified as risk factors for threatened mammals. Relative to other threatened hosts, we tested whether mammal species identified as threatened by parasites were better studied in terms of infectious diseases, had higher levels of parasitism overall, or possessed host traits that might facilitate parasite establishment, including higher population density, greater body mass, or larger geographic ranges.
Methods
Host Data
We used the 2006 IUCN Red List to compile a list of threatened mammal species (IUCN 2006), and standardized the mammal taxonomy according to Wilson and Reader (1993). For each mammal species for which data were available (n= 4864), we recorded threat status as nonthreatened (least concern), near threatened, and threatened (conservation‐dependent, vulnerable, endangered, critically endangered, and extinct in the wild). Independent of host threat status, we also recorded mammal species or subspecies for which parasites have been identified as a major cause of threat based on summary documentation in the IUCN Red List for “major threats.” Identification of a major threat is based on two classifications: “invasive alien species – pathogens/parasites” (threat 2.4), and “changes in native species dynamics – pathogens/parasites” (threat 8.5). For many species, key information regarding the causes of endangerment is available only in detailed reports that are not accessible via on‐line searches of the IUCN Red List (Clavero & Garcia‐Berthou 2005). This can lead to missing records and potential systematic biases if, for example, some types of species have summary documentation available, whereas others do not. This is unlikely to be a problem in our data set, however, because summary documentation on cause of threat was specified for 73% of threatened species and included species from each of the major mammal clades.
Parasite Data
For each mammal species identified as threatened by parasites, we recorded all parasites that were documented as causing population declines or having significant negative effects on host fitness. We searched for common and scientific names of host species in combination with a string of parasite‐related terms on four on‐line databases: Web of Science, BIOSIS, ZOORECORD, and PUBMED. We also obtained “species assessment accounts” for each parasite‐threatened mammal from the IUCN specialist groups (http://www.iucnredlist.org/). Each host species was assigned to one of three categories based on the availability of data on parasite‐induced population declines or documented negative effects on host fitness: (1) direct evidence from the published literature; (2) general reference to disease as a threat from the IUCN Species Assessment Accounts or Conservation Plans, but no information based on literature searches; or (3) no published evidence of parasites causing declines and no mention of disease as a threat in the IUCN Species Assessment Accounts. Only a few species were identified in this third group; as discussed later, they may represent errors in the IUCN summary documentation or species that are not currently threatened by parasites but that have a predicted future risk from infectious diseases.
For each parasite we recorded the type (virus, bacterium, protozoan, helminth, arthropod, or fungus) and degree of host specificity. Host specificity values were ranked from 1 to 5 based on reports of naturally occurring infections from Pedersen et al. (2005). Scores were assigned as follows: (1) a single host species, (2) >1 host species in a single genus, (3) >1 genus within a host family, (4) >1 family within a host order, (5) infects hosts from >1 order. We also categorized transmission modes according to whether parasites could be transmitted by (1) close contact (biting, touching, sexual contact, or vertical transmission); (2) nonclose contact (environmental contact, including water, soil, or fecal transmission); (3) arthropod vectors; or (4) intermediate hosts. These categories are not mutually exclusive, and approximately 48% of parasites were transmitted by multiple routes. Finally, we noted whether each parasite had been reported to infect domesticated livestock (cattle, pigs, goats, sheep, or horses) or cats and dogs based on searches of the primary literature.
Initial analyses indicated that the majority of mammal species reported as threatened by parasites were carnivores and artiodactyls. To compare characteristics of parasites that cause host declines against a comprehensive database of other mammalian parasites, we used infectious disease records from natural populations of carnivores and artiodactyls (Global Mammal Parasite Database [GMPD], http://www.mammalparasites.org; Nunn & Altizer 2005). This database provides records of 601 parasite species from artiodactyls and 836 parasites from carnivores, including information on host specificity and transmission mode.
Sampling Gaps
We obtained information on sampling effort and host traits for threatened carnivores and artiodactyls to test whether species identified as threatened by parasites were listed as such due to a set of unique biological characteristics that predisposed them to disease risk. First, we examined host traits demonstrated to covary with both extinction risk and parasite species richness in previous studies (e.g., Cardillo et al. 2004; Purvis et al. 2005). For this purpose, data were obtained from a mammalian trait database (Purvis et al. 2000; PanTHERIA: http://www.biodiversitydata.group.cam.ac.uk/pantheria/) on the following variables: adult body mass (grams), geographic range size (square kilometers), longevity (maximum recorded life span in years), and population density (average number of individuals per square kilometer).
To examine whether uneven sampling effort among hosts could bias the species recognized as threatened by parasites, we compiled measures of study effort for artiodactyls and carnivores. First, we recorded the total number of published references for each species by searching for scientific names on the Web of Science (1975–2004). Second, we repeated each search with a series of key words related to infectious disease. Finally, we obtained total counts of the parasite species reported to infect each host species (parasite species richness) from the GMPD.
Data Analyses
We used chi‐square tests to examine the taxonomic distribution of mammals threatened by parasites relative to all mammals and to other threatened mammals, and to compare the characteristics of threatening parasites to traits of other parasites represented in the GMPD. We also examined whether mammals classified as threatened by parasites were better studied or differed in biological traits from other threatened mammals. For this we used an analysis of variance (ANOVA) to test cause of threat (parasites vs. other factors) against host citation counts and biological traits (model: dependent variable = host order + threat + host order × threat). Finally, we used analysis of covariance (ANCOVA) to compare parasite species richness (PSR) between host species threatened by parasites relative to other threatened hosts (model: PSR = host order + threat + citations counts + host order × threat + threat × citation counts). We included log‐transformed citation counts as a measure of sampling effort because more parasites are likely to be found in well‐studied species (Nunn et al. 2003). Statistical tests were performed in SPSS version 13.0 (SPSS 2004) and R (version 2.2.0; http://www.r-project.org/).
Results
Host Characteristics
We identified 54 mammal species for which infectious diseases were cited as a threatening process (see Supplementary Material). Of these, 43 species were classified as threatened, 9 were considered of lower conservation concern, and 2 were listed as data‐deficient. The majority (88%) of mammals threatened by parasites were from two orders: Artiodactyla (13% of 218 species) and Carnivora (5.3% of 281 species). This nonrandom distribution across taxonomic orders was highly significant when parasite‐threatened hosts were compared against all other mammal species (Fig. 1a; restricted to orders > 15 spp.; χ2= 416.3, df = 15, p < 0.0001), and with just the subset of threatened species in each order (χ2= 164.5, df = 15, p < 0.0001).
Figure 1.
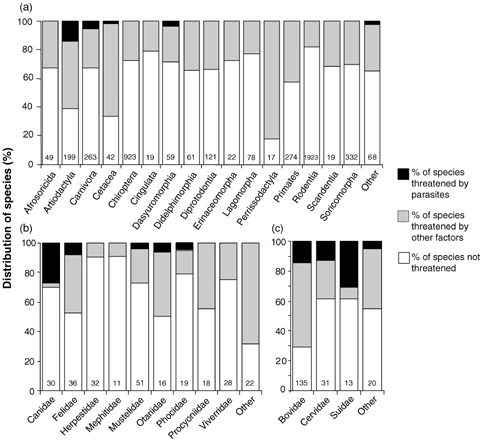
Distribution of threat status among (a) mammalian orders and host families within the (b) Carnivora and (c) Artiodactyla, based on 2006 IUCN Red List. Only host orders with >15 species and host families with >10 species are shown separately; the rest are combined in “other” and excluded from statistical tests. Numbers represent the total number of species, excluding those listed as data deficient.
Within the carnivores, species in the families Canidae (26.7% of 30 species) and Felidae (8.3% of 36 species) were more commonly recognized as threatened by parasites than members of other families (Fig. 1b). This among‐family effect was significant when parasite‐threatened hosts were compared with all other species (families > 10 spp.; χ2= 22.1, df = 8, p= 0.005) and with the subset of threatened species (χ2= 30.8, df = 8, p= 0.0002). Within the Artiodactyla, species in the families Bovidae (14% of 135 species) and Suidae (30.7% of 13 species) were more commonly identified as threatened by parasites than species in other families (Fig. 1c). This effect was not significant when parasite‐threatened hosts were compared with all other species (families > 10 spp.; χ2= 2.72, df = 2, p= 0.26), but was highly significant when compared with the subset of threatened hosts (χ2= 10.1, df = 2, p= 0.006).
Parasite Characteristics
We identified 31 parasites that caused population declines or had strong negative effects on the fitness of parasite‐threatened mammals (Table 1). Over half of these parasites were viruses (41%) and bacteria (28%); the remainder included helminths (16%), arthropods (9%), protozoa (3%), and fungi (3%). The distribution of parasite types significantly differed between carnivores and artiodactyls (Fig. 2, χ2= 11.3, df = 5, p= 0.045). Most parasites reported as causing threat in artiodacytls (n= 17) were bacteria (47%) or viruses (29%); we found no records for protozoa or fungi (Fig. 2a). Among parasites reported as threatening carnivores (n= 13), the majority were viruses (56%; Fig. 2b).
Table 1.
Parasites identified as causing population declines or reduced host fitness in mammals listed on the IUCN Red List as threatened by pathogens.a
| Parasite nameb | Carnivores | Artiodactyls | Other |
|---|---|---|---|
| Virus | |||
| Morbillivirus, canine distemper virus | 10 | 0 | 0 |
| Parvovirus, canine parvovirus | 4 | 0 | 0 |
| Vesivirus, feline calicivirus | 1 | 0 | 0 |
| Coronavirus, feline infectious peritonitis virus | 1 | 0 | 0 |
| Parvovirus, feline panleukopenia virus | 1 | 0 | 0 |
| Gammaretrovirus, feline leukemia virus | 0 | 0 | 0 |
| Apthovirus, foot‐and‐mouth disease virus | 0 | 7 | 0 |
| Lentivirus, jembrana disease virus | 0 | 1 | 0 |
| Morbillivirus, monk seal morbillivirus | 1 | 0 | 0 |
| Rhadinovirus, ovine herpesvirus 2 | 0 | 1 | 0 |
| Varicellovirus, pseudorabies virus | 1 | 0 | 0 |
| Lyssavirus, rabies virus | 9 | 2 | 0 |
| Morbillivirus, rinderpest virus | 0 | 7 | 0 |
| Bacteria | |||
| Bacillus anthracis (anthrax) | 0 | 5 | 0 |
| Chlamydia sp. (infectious keratoconjuctivitis) | 0 | 1 | 0 |
| Fusobacterium necrophorum (hoof rot) | 0 | 2 | 0 |
| Mannheimia haemolytica (pasteurellosis) | 0 | 1 | 0 |
| Mycoplasma conjunctivae (infectious keratoconjuctivitis) | 0 | 1 | 0 |
| Mycobacterium bovis (bovine tuberculosis) | 0 | 2 | 0 |
| Pasteurella spp. (pasteurellosis) | 0 | 2 | 0 |
| Yersinia pestis (plague) | 0 | 0 | 1 |
| Helminths | |||
| Angiocaulus gubernaculatus (nematode) | 1 | 0 | 0 |
| Dioctophyma renale, giant kidney worm | 1 | 0 | 0 |
| Dirofilaria immitis, heartworm | 1 | 0 | 0 |
| Protostrongylus spp., lungworm | 0 | 1 | 0 |
| Taenia hydatigena, thin‐necked bladderworm | 0 | 1 | 0 |
| Arthropods | |||
| Otodectes cynotis, ear canker mite | 1 | 0 | 0 |
| Psoroptes sp. (psoroptic mange) | 0 | 1 | 0 |
| Sarcoptes scabei (sarcoptic mange) | 3 | 1 | 0 |
| Protozoa | |||
| Toxoplasma gondii (toxoplasmosis) | 2 | 0 | 2 |
| Fungi | |||
| Enchepalitozoon cuniculi (encephalitozoonosis) | 1 | 0 | 0 |
aNumbers reflect the number of mammal species threatened by each pathogen.
bParasite names follow the International Committee on the Taxonomy of Viruses (ICTV) Database ( http://www.ncbi.nlm.nih.gov/ICTVdb/Ictv/ ), guidelines published by the National Center for Biotechnology Information (NCBI), and taxonomic authorities for other parasite groups. Names of diseases are in parentheses.
Figure 2.
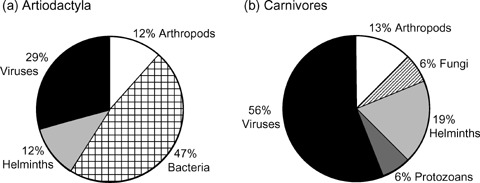
Taxonomic distribution of six major groups of parasites reported as threatening (a) artiodactyls and (b) carnivores.
Parasites reported as causing declines in threatened mammals significantly differed in taxonomy and transmission mode from an independent database of parasites reported from wild mammals (GMPD). For both artiodactyls and carnivores, the taxonomic distributions of threatening parasites were significantly different from the database of parasites reported for each clade (Fig. 3; Artiodactyla: χ2= 20.2, df = 5, p < 0.001; Carnivora: χ2= 26.3, df = 6, p < 0.001). These differences were due to a higher proportion of viruses (and for artiodactyls, bacteria) among the threatening parasites and a much lower proportion of helminths in both clades (Fig. 3a). Over 70% of the threatening viruses were ssRNA viruses; nevertheless, the distribution of virus types (ssRNA, dsRNA, ssDNA, and dsDNA) did not differ significantly from viruses in the GMPD database (artiodactyl and carnivore parasites combined: χ2= 5.67, df = 3, p= 0.12).
Figure 3.
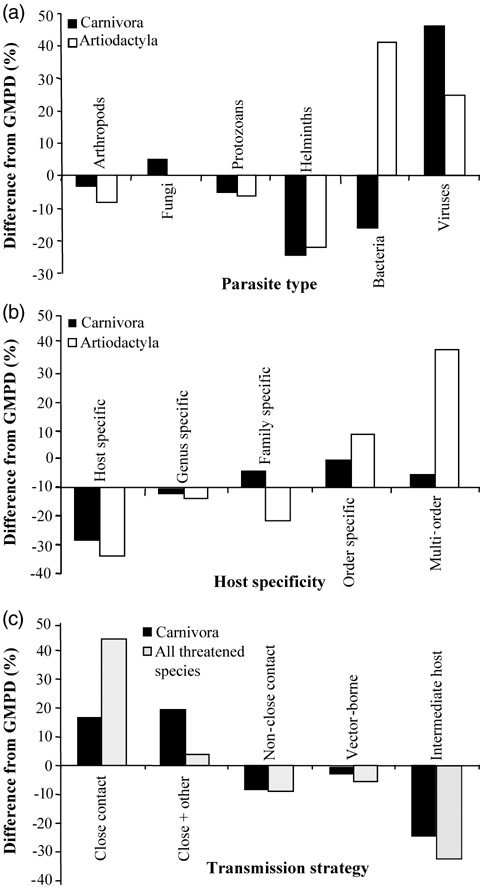
(a) Changes in the distribution of parasites reported to threaten artiodactyls (white) and carnivores (black) relative to parasites from the Global Mammal Parasite Database (GMPD). (b) Differences in the level of host specificity between parasites in the GMPD and parasites that threaten wild mammals. (c) Differences in the distribution of transmission strategies across parasites from the GMPD (data available for carnivores only) relative to those that threatened all wild mammals (gray). Positive values represent a higher proportion of threatening parasites than would be expected from the GMPD database; negative proportions show the opposite trend.
All parasites identified as causing declines or reduced fitness in threatened mammals could infect multiple host species. The most host‐specific of these parasites were restricted to a single host family (9% of 31), whereas the majority (66%) could infect species from multiple host orders, including nonmammalian hosts in some cases. Compared with parasites listed in the GMPD, we found a significantly higher proportion of generalist parasites and many fewer host‐specific parasites among those that threaten wildlife (Fig. 3b). These differences in host specificity were significant for parasites from artiodactyls (χ2= 17.9, df = 4, p= 0.0012), but not for carnivores (χ2= 4.3, df = 4, p= 0.365). Nearly all of the parasites listed as threatening wild mammals (96%) were also reported to infect domesticated carnivores or livestock.
Counter to our expectations, a high proportion of threatening parasites were transmitted by close contact only (30% of 31 species) or a combination of close contact and other transmission modes (45%; Fig. 3c). Relatively few threatening parasites were transmitted by arthropod vectors, intermediate hosts, or through nonclose contact. Compared with the transmission modes of parasites in the GMPD (available for carnivores only; n= 836 parasites), a higher proportion of threatening parasites were transmitted by close contact only than expected by chance, and a lower proportion were transmitted by other modes. This difference was significant for all threatening parasites (Fig. 3c; χ2= 50.7, df = 5, p < 0.0001) and remained significant when restricted to the subset of threatening parasites from carnivores only (Fig. 3c; n= 17; χ2= 9.5, df = 4, p= 0.05).
Sampling Gaps
In searching for infectious diseases reported to infect threatened mammal species, our efforts revealed several information gaps. First, of the 54 mammals for which parasites were noted as a cause of threat, we identified specific threatening parasites for only 34 species based on searches of the published literature. For another 14 mammals, the IUCN Species Assessment Accounts discussed disease as a general cause of concern, but no specific parasites were identified or the assessment was based on predicted disease risk. For six mammal species (11%), the published literature provided no evidence that the species had been studied for infectious diseases, and IUCN Species Assessment Accounts provided no specific details on the parasite‐related threats.
To better understand the degree to which highly threatened mammals have been sampled for infectious diseases, we searched all critically endangered and endangered artiodactyls (n= 40), carnivores (n= 20), and primates (n= 28) from the 2006 IUCN Red List for parasite records in the GMPD. Among the most threatened artiodactyls, only 12% had reports of parasites found in wild populations. The proportions of carnivores (54%) and primates (67%) with parasite records were higher, but still indicated that natural populations of endangered species have been poorly studied for infectious diseases.
Within artiodactyls and carnivores, we found no evidence that species identified as threatened by parasites were listed as such because they were better studied than other threatened species (total citation counts: F 1,115= 0.51, p= 0.48; parasite‐related citation counts: F 1,114= 0.67, p= 0.42). Relative to other threatened mammals, species threatened by infectious disease also did not have significantly greater parasite species richness (based on parasites reported from wild populations; Table 2). In addition, we found no evidence that host species threatened by parasites differed in four key traits relative to other threatened mammal species (Table 2).
Table 2.
Analysis of parasite species richness and host life‐history traits in relation to whether parasites were reported as a cause of threat based on the IUCN Red List (abbreviated as “threat”).*
| Parasite species richness (F1,96, p) | Mass (F1,101, p) | Geographic range (F1,97, p) | Longevity (F1,85, p) | Density (F171, p) | |
|---|---|---|---|---|---|
| Threat | 0.19, 0.66 | 0.47, 0.50 | 0.70, 0.41 | 0.81, 0.37 | 0.75, 0.39 |
| Host order | 0.03. 0.86 | 26.9, 0.001 | 1.08, 0.30 | 3.12, 0.08 | 12.0, 0.001 |
| Threat*order | 0.99, 0.32 | 0.59, 0.45 | 0.22, 0.64 | 3.31, 0.07 | 0.01, 0.94 |
| WOS citations | 25.2, 0.001 | — | — | — | — |
| Threat*citations | 0.00, 0.99 | — | — | — | — |
| Total n | 102 | 105 | 101 | 88 | 75 |
*Analyses included data for up to 105 species of threatened artiodactyls and carnivores, depending on the availability of host trait data. Dependent variables are shown as column headings, and independent variables appear as row headings. A continuous covariate (Web of Science [WOS] citation counts) was included in analyses of parasite species richness (PSR) only. All variables were log‐transformed prior to analysis.
Discussion
Wild mammals are a well‐studied taxonomic group that is currently experiencing a wave of extinctions (Purvis et al. 2000; Cardillo et al. 2005). It has become increasingly clear that infectious diseases represent an emerging cause of population declines and extinctions in threatened mammal species (Daszak et al. 2000; Lafferty & Gerber 2002; Smith et al. 2006). Our results demonstrate that several host and parasite characteristics are important for predicting infectious disease risks for threatened mammal. First, the majority (88%) of mammals identified as threatened by parasites were from just two taxonomic orders: artiodactyls and carnivores. Within these clades, host families with the greatest frequency of species threatened by parasites also included all major groups of domesticated livestock and companion animals (including dogs, cats, goats, sheep, cattle, and pigs). By comparison, no bats (0.0% of 1024) and only one rodent species (<0.01% of 2041) were identified as being threatened by parasites. One explanation for this pattern is that close phylogenetic relationships with domesticated mammals, which are globally distributed and maintained at high densities, can intensify the risk of cross‐species transmission to related wildlife species. Given that parasites are more likely to be shared or to jump between closely related host species (Brooks & McLennan 1991) and that over 80% of livestock and domesticated carnivore parasites can also infect wildlife (Cleaveland et al. 2001), it is not surprising that species in these lineages suffer more heavily from infectious diseases.
Parasites reported as threatening wild mammals were principally viruses and bacteria with broad host ranges that nearly always included domesticated animals. These parasites included well‐known agents such as foot‐and‐mouth disease virus, morbilliviruses that cause canine distemper and rinderpest, and bacteria that cause bovine tuberculosis and anthrax (Table 1). Counter to expectations, 75% of parasites identified as threatening were transmitted by close contact, rather than by arthropod vectors or contact with contaminated soil or water. Although previous studies have highlighted the characteristics of parasites that pose significant health risk to humans and animals (Taylor et al. 2001; Gog et al. 2002; Pedersen et al. 2005), our study represents the first to evaluate these factors in the context of extinction risk. Consistent with these studies (Taylor et al. 2001; Woolhouse et al. 2001), we found that viruses, due to their typically wide host ranges and high mutation rates (Domingo & Holland 1994), are most likely to threaten mammals. This is further supported by our finding that ssRNA viruses, which have higher mutation rates than DNA viruses (Drake et al. 1998), comprised over 70% of the viruses identified as causing declines in threatened mammals.
Relative to viruses and bacteria, fewer helminths, protozoa, and arthropods were reported to cause population declines in threatened mammals. This pattern was particularly striking for helminths, which represent the greatest diversity of parasites of wild mammals (Nunn et al. 2003; Ezenwa et al. 2006; Lindenfors et al. 2007). One explanation is that many helminth infections cause sublethal fitness effects or cyclical changes in host abundance, both of which are difficult to quantify in wild mammal populations (Hudson et al. 1998). When combined with other parasites or environmental stressors, helminths could pose significant conservation threats. For example, population declines in bighorn sheep (Ovis candadensis) may have been caused by interactions between stress, lungworm infection (Protostrongylus spp.), and bacteria (Pastuerella spp.), which lead to pneumonia outbreaks that resulted in high mortality and low reproductive success (Forrester 1971; Jones & Worley 1994). Further investigation of the effects of helminths and other macroparasites is needed to understand their role in mammalian extinction risk.
Mammals threatened by infectious diseases could represent formerly common species brought to rarity by pathogens or already rare species that experience further declines from disease (Lafferty & Gerber 2002). We did not have historical data to distinguish between these cases. Nevertheless, within the subset of threatened species, we predicted that vulnerability to pathogen‐mediated declines would be correlated with traits known to increase parasite transmission or species richness (e.g., large body size, high population density; Nunn et al. 2003; Cardillo et al. 2005). Similarly, species threatened by parasites may have been better studied for parasites than other threatened mammals. Nevertheless, we found no evidence that mammals identified as threatened by parasites were better studied for infectious disease, had higher parasites species richness, or differed in four key traits relative to other threatened mammals. Instead, phylogenetic relatedness to domesticated animals posed the greatest risk in terms of parasite‐mediated declines.
Relative to habitat loss and hunting, much less attention has focused on the role of infectious disease as a driver of contemporary mammalian extinctions. Our study revealed that few mammal species have been identified as threatened by parasites (IUCN 2006), which is consistent with a recent review of parasite‐mediated extinction risk across plants and animals (Smith et al. 2006). One contributing factor could be a lack of accessible information on infectious diseases in threatened mammal populations. Published studies of parasite infections in natural populations were available for <40% of the world's most threatened primates, carnivores, and artiodactyls. Furthermore, over 25% of the mammals classified as threatened by parasites by the IUCN had little or no published information concerning the effects of disease on wild populations. For example, an Australian marsupial (the Dibbler [Parantechinus apicalis]) was identified as threatened by parasites, but literature searches revealed instead that a plant pathogen (Phytopthora cinnamomi) was negatively affecting native plants in its preferred habitat (Friend 2004). For West Indian manatees (Trichechus manatus) and marine otters (Lontra felina), infectious disease was perceived to be a future risk even though no current parasite‐mediated declines have been reported.
In addition, several widely publicized parasite‐driven population declines are not captured in the IUCN list, including canine distemper virus outbreaks in black‐footed ferrets (Mustela nigripes) (Thorne & Williams 1988) and African lions (Panthera leo) (Roelke‐Parker et al. 1996), Chlamydia infections in koalas (Phascolarctos cinereus) (Melzer et al. 2000), phocine distemper virus infections in harbor seals (Phoca vitulina) (Jensen et al. 2002), and several pathogens affecting gorillas (Gorilla gorilla) and chimpanzees (Pan troglodytes) (Hastings et al. 1991; Walsh et al. 2003; Leroy et al. 2004). Collectively, these examples suggest that extinction risks due to parasites may be substantially underestimated and demonstrate the need to better assess the presence and impacts of infectious disease in wild mammal populations (Leendertz et al. 2006).
Our results provide optimism that strategies can be employed to reduce the risk of parasite‐mediated wildlife declines. Vaccination of domesticated animal reservoirs could be an effective control strategy for minimizing the transmission of pathogens to wildlife hosts (Lafferty & Gerber 2002). Outbreaks caused by many of the reported parasites resulted from direct contact with domesticated animals, in some cases from infections that would not have spread or persisted in wildlife in the absence of domesticated animals. As one example, rinderpest virus declined dramatically in wild populations of African bovids following the widespread vaccination of domesticated cattle (Plowright 1982). A campaign is currently underway in the Serengeti ecosystem to immunize domesticated dogs against rabies, canine distemper, and parvovirus, with the goal to reduce disease outbreaks in lions, African wild dogs, and bat‐eared foxes (Otocyon megalotis) (Cleaveland et al. 2000, 2003). Alternative strategies could focus on minimizing contacts between domestic animal reservoir hosts and endangered wildlife species (Woodroffe 1999), particularly because our results suggest that close contact is a major route of transmission for these harmful parasites. Minimizing cross‐species contact could be accomplished through physical barriers that reduce the potential for close contact or by limiting temporal overlap in the use of water resources or grazing habitats. These strategies have been effective in reducing bighorn sheep epidemics, specifically through the construction of buffer zones between agricultural areas and ranges of bighorn sheep (Jessup et al. 1991, 1995).
Mammals from clades that are closely related to domesticated animals are at the greatest risk of parasite‐mediated declines, most likely due to cross‐species transmission of generalist viruses and bacteria. Efforts to provide a more comprehensive view of the role of parasites in wildlife extinction risk will require increased collaboration among wildlife ecologists, veterinary workers, and conservation organizations. Characteristics of threatening parasites highlight the possibility that future control strategies targeted at reducing cross‐species transmission of high‐risk parasites, either by vaccination or by limiting contact with domestic animals, may significantly reduce the risk of parasite‐mediated wildlife declines.
Acknowledgments
We thank V. Ezenwa, J. Gittleman, A. Dobson, A. Cunningham, S. Price, P. Rohani, H. Wearing, J. de Roode, K. Smith, P. Daszak, and two anonymous referees for discussion and comments on the manuscript. We thank B. Ledbetter for technical assistance in extracting data from the GMPD. This research was funded in part by National Science Foundation grants DEB‐0211908 and DEB‐0129009, Conservation International, The Max Planck Society, and through the “Disease and Host Behavior” Working Group supported by the National Center for Ecological Analysis and Synthesis (NCEAS). A.B.P. was funded by an International Incoming Fellowship from the Royal Society.
Literature Cited
- Altizer, S. , Harvell D., and Friedle E.. 2003. Rapid evolutionary dynamics and disease threats to biodiversity. Trends in Ecology & Evolution 18:589–596. [Google Scholar]
- Anderson, R. M. , and May R. M.. 1992. Infectious diseases of humans: dynamics and control. Oxford University Press, New York . [Google Scholar]
- Boots, M. , and Sasaki S.. 2003. Parasite evolution and extinctions. Ecology Letters 6:176–182. [Google Scholar]
- Brooks, D. R. , and McLennan D. A.. 1991. Phylogeny, ecology, and behavior: a research program in comparative biology. University of Chicago Press, Chicago . [Google Scholar]
- Cardillo, M. , Purvis A., Sechrest W., Gittleman J. L., Bielby J., and Mace G. 2004. Human population density and extinction risk in the world's carnivores. PLoS Biology 2:909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardillo, M. , Mace G. M., Jones K. E., Bielby J., Bininda‐Edmonds O. R. P., Secrest W., Orme C. D. L., and Purvis A.. 2005. Multiple causes of high extinction risk in large mammal species. Science 309:1239–1241. [DOI] [PubMed] [Google Scholar]
- Clavero, M. , and Garcia‐Berthou E.. 2005. Invasive species are a leading cause of animal extinctions. Trends in Ecology & Evolution 20:110. [DOI] [PubMed] [Google Scholar]
- Cleaveland, S. , Appel M. G. J., Chalmers W. S. K., Chillingworth C., Kaare M., and Dye C.. 2000. Serological and demographic evidence for domestic dogs as a source of canine distemper virus infection for Serengeti wildlife. Veterinary Microbiology 72:3–4. [DOI] [PubMed] [Google Scholar]
- Cleaveland, S. , Laurenson M. L., and Taylor L. H.. 2001. Disease of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philosophical Transactions of the Royal Society B: Biological Sciences 356:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaveland, S. , Kaare M., Tiringa P., Mlegngeya T., and Barrat J.. 2003. A dog rabies vaccination campaign in rural Africa: impact on the incidence of dog rabies and human dog‐bite injuries. Vaccine 21:17–18. [DOI] [PubMed] [Google Scholar]
- Daszak, P. , Berger L., Cunningham A. A., Hyatt A. D., Green D. E., and Speare R.. 1999. Emerging infectious diseases and amphibian population declines. Emerging Infectious Disease 5:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak, P. , Cunningham A. A., and Hyatt A. D.. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287:443–449. [DOI] [PubMed] [Google Scholar]
- De Castro, F. , and Bolker B.. 2005. Mechanisms of disease‐induced extinction. Ecology Letters 8:117–126. [Google Scholar]
- Domingo, E. , and Holland J. J.. 1994. Mutation rates and rapid evolution of RNA viruses Pages 161–184 in Morse S. S., editor. The evolutionary biology of viruses. Raven Press, New York . [Google Scholar]
- Drake, J. W. , Charlesworth B., Charlesworth D., and Crow J. F.. 1998. Rates of spontaneous mutation. Genetics 148:1667–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezenwa, V. O. , Price S. A., Altizer S., Vitone N. S., and Cook K. C.. 2006. Host traits and parasite species richness: patterns and processes in even and odd‐toed hoofed mammals (Artiodactyla and Perissodactyla). Oikos 115:526–536. [Google Scholar]
- Fenton, A. , and Pedersen A. B.. 2005. Community epidemiology in theory and practice: a conceptual framework for classifying disease threats in human and wild populations. Emerging Infectious Disease 11:1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester, D.J. 1971. Bighorn sheep lungworm‐pneumonia complex Pages 158–173 in Davis J. W. and Anderson R. editors. Parasitic diseases of wild mammals. Iowa State University Press, Ames . [Google Scholar]
- Friend, T. 2004. Dibbler (Parantechnus apicalis) recovery plan. Wildlife Management Program Report 38. Department of Conservation and Land Management, Canberra , Australia . [Google Scholar]
- Getz, W. M. , and Pickering J.. 1983. Epidemic models: thresholds and population regulation. The American Naturalist 121:892–898. [Google Scholar]
- Gog, J. R. , Woodroffe R., and Swinton J.. 2002. Disease in endangered metapopulations: the importance of alternative hosts. Philosophical Transactions of the Royal Society B: Biological Sciences 269:671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings, B. E. , Kenny D., Lowenstine L. J., and Foster J. W.. 1991. Mountain gorillas and measles: ontogeny of a wildlife vaccination program. Pages 198–205 in Junge R. E., editor. Proceedings of AAZV Conference . American Association of Zoo Veterinarians Media, Philadelphia , Pennsylvania . [Google Scholar]
- Hudson, P. J. , Dobson A. P., and Newborn D.. 1998. Prevention of population cycles by parasite removal. Science 282:2256–2258. [DOI] [PubMed] [Google Scholar]
- IUCN (World Conservation Union ). 2006. IUCN Red List of threatened species. IUCN, Gland , Switzerland . Available from http://www.redlist.org (accessed November 2006). [Google Scholar]
- Jensen, T. , Van De Bildt M., Dietz H. H., Andersen T. H., Hammer A. S., Kuiken T., and Osterhaus A.. 2002. Another phocine distemper outbreak in Europe. Science 297:209. [DOI] [PubMed] [Google Scholar]
- Jessup, D. A. , Boyce W. M., and Clarke R. K.. 1991. Diseases shared by wild, exotic and domestic sheep Pages 438–445 in Renecker L. A. and Hudson R. J., editors. Wildlife production: conservation and sustainable development. University of Alaska Press, Fairbanks . [Google Scholar]
- Jessup, D. A. , Boyce W. M., and Torres S. G.. 1995. Bighorn sheep health management in California: a fifteen year retrospective. Pages 55–67 in Junge R. E., editor. Proceedings of the Joint conference of the American Association of Zoo Veterinarians, the Wildlife Disease Association and the American Association of Wildlife Veterinarians. American Association of Zoo Veterinarians Media, Philadelphia , Pennsylvania . [Google Scholar]
- Jones, L. C. , and Worley D. E.. 1994. Evaluation of lungworms, nutrition, and predation as factors limiting recovery of the Stillwater bighorn sheep herd, Montana. Pages 25–34 in Pybus M., editor. Proceedings of the 9th biennial symposium of the Northern Wild Sheep and Goat Council. Northern Wild Sheep and Goat Council, Cody, Wyoming.. [Google Scholar]
- Lafferty, K. D. , and Gerber L. R.. 2002. Good medicine for conservation biology: the intersection of epidemiology and conservation theory. Conservation Biology 16:593–604. [Google Scholar]
- Leendertz, F. H. , Pauli G., Maetz‐Rensing K., Boardman W., Nunn C. L., Ellerbrok H., Jensen S. A., Junglen S., and Boesch C.. 2006. Pathogens as drivers of population declines: the importance of systematic monitoring in great apes and other threatened mammals. Biological Conservation 131:325–337. [Google Scholar]
- Leroy, E. M. , et al 2004. Multiple ebola virus transmission events and rapid decline of Central African wildlife. Science 303:387–390. [DOI] [PubMed] [Google Scholar]
- Lindenfors, P. , Nunn C. L., Jones K. E., Cunningham A. A., Sechrest W., and Gittleman J. L.. 2007. Parasite species richness in carnivores: effects of host body mass, latitude, geographic range and population density. Global Ecology and Biogeography: in press. [Google Scholar]
- Lyles, A. M. , and Dobson A. P.. 1993. Infectious disease and intensive management: population dynamics, threatened hosts and their parasites. Journal of Zoo and Wildlife Medicine 24:315–326. [Google Scholar]
- McCallum, H. , and Dobson A.. 1995. Detecting disease and parasite threats to endangered species and ecosystems. Trends in Ecology & Evolution 10:190–194. [DOI] [PubMed] [Google Scholar]
- Melzer, A. , Carrick F., Menkhorst P., Lunney D., and John B. St.. 2000. Overview, critical assessment, and conservation implications of koala distribution and abundance. Conservation Biology 14:619–628. [Google Scholar]
- Nunn, C. L. , and Altizer S.. 2005. The Global Mammal Parasite database: an online resource for infectious disease records in wild primates. Evolutionary Anthropology 14:1. [Google Scholar]
- Nunn, C. L. , Altizer S., Jones K. E., and Sechrest W.. 2003. Comparative tests of parasite species richness in primates. The American Naturalist 162:597–614. [DOI] [PubMed] [Google Scholar]
- Pedersen, A. B. , Altizer S., Poss M., Cunningham A. A., and Nunn C. L.. 2005. Patterns of host specificity and transmission among parasites of wild primates. International Journal of Parasitology 35:647–657. [DOI] [PubMed] [Google Scholar]
- Plowright, W. 1982. The effects of rinderpest and rinderpest control on wildlife in Africa. Symposium of the Zoological Society of London 50:1–28. [Google Scholar]
- Pounds, J. A. , et al 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439:161–167. [DOI] [PubMed] [Google Scholar]
- Price, P. W. 1980. Evolutionary biology of parasites. Princeton University Press, Princeton , New Jersey . [Google Scholar]
- Purvis, A. , Jones K. E., and Mace G.. 2000. Extinction. Bioessays 22:1123–1133. [DOI] [PubMed] [Google Scholar]
- Purvis, A. , Cardillo M., Grenyer R., and Collen B.. 2005. Correlates of extinction risk: phylogeny, biology, threat and scale Pages 295–316 in Purvis A., Gittleman J. L., and Brooks T. M., editors. Phylogeny and conservation. Cambridge University Press, Cambridge , United Kingdom . [Google Scholar]
- Roelke‐Parker, M. E. , et al 1996. A canine distemper virus epidemic in Serengeti lions (Panthera leo). Nature 376:441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, K. F. , Sax D. F., and Lafferty K. D.. 2006. Evidence for the role of infectious disease in species extinction and endangerment. Conservation Biology 20:1349–1357. [DOI] [PubMed] [Google Scholar]
- SPSS . 2004. SPSS. Version. 13.0. SPSS, Chicago . [Google Scholar]
- Taylor, L. H. , Latham S. M., and Woolhouse M. E. J.. 2001. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society B: Biological Sciences 356:983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne, E. T. , and Williams E. S.. 1988. Disease and endangered species: the black footed ferret as a recent example. Conservation Biology 2:66–74. [Google Scholar]
- Walsh, P. D. , et al 2003. Catastrophic ape decline in western equatorial Africa. Nature 422:611–614. [DOI] [PubMed] [Google Scholar]
- Wilcove, D. S. , Rothstein D., Dubow J., Phillips A., and Losos E.. 1998. Quantifying threats to imperiled species in the United States. BioScience 48:607–615. [Google Scholar]
- Wilson, D.E. , and Reader D. M.. 1993. Mammal species of the world. 2nd edition Smithsonian Institution Press, Washington , D.C. [Google Scholar]
- Woodroffe, R. 1999. Managing disease threats to wild mammals. Animal Conservation 2:185–193. [Google Scholar]
- Woolhouse, M. E. J. , Taylor L. H., and Haydon D. T.. 2001. Population biology of multihost pathogens. Science 292:1109–1112. [DOI] [PubMed] [Google Scholar]


