Abstract
The global pandemic of SARS-CoV-2, the causative viral pathogen of COVID-19, has driven the biomedical community to action—to uncover and develop antiviral interventions. One potential therapeutic approach currently being evaluated in numerous clinical trials is the agent remdesivir, which has endured a long and winding developmental path. Remdesivir is a nucleotide analogue prodrug that perturbs viral replication, originally evaluated in clinical trials to thwart the Ebola outbreak in 2014. Subsequent evaluation by numerous virology laboratories demonstrated the ability of remdesivir to inhibit coronavirus replication, including SARS-CoV-2. Here, we provide an overview of remdesivir’s discovery, mechanism of action, and the current studies exploring its clinical effectiveness.
Short abstract
We review clinical development of remdesivir, a prodrug with a demonstrated ability to inhibit SARS-CoV-2 replication, which supports its clinical evaluation for COVID-19 treatment.
Introduction
Coronaviruses are a family of enveloped viruses with a positive-sense, single-stranded RNA genome that infects animal species and humans. Among coronavirus members are those responsible for the common cold, severe acute respiratory syndrome coronavirus (SARS), Middle East respiratory syndrome-related coronavirus (MERS), and the recently emerged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, the causative pathogen of the disease COVID-19).1
Coronaviruses primarily cause respiratory and intestinal infections in animals and humans.2 Discovered in the 1960s, they were originally thought to be only responsible for mild disease, with strains such as HCoV 229E and HCoV OC43 responsible for the common cold.3 That changed in 2003 with the SARS pandemic and in 2012 with the outbreak of MERS, both zoonotic infections that resulted in mortality rates greater than 10% and 35%, respectively.4 Both coronaviruses likely emerged from native bat populations, which maintain a broad diversity of coronaviruses, and were transmitted through an intermediate host to humans. Loss of natural habitat and increased exposure to new hosts are likely responsible for the increased frequency of zoonotic infections originating from bats.5,6 Evidence also supports that the novel coronavirus which emerged in the Wuhan region of China in late 2019 also originated from bats.7 This novel coronavirus, SARS-CoV-2, resulted in an outbreak of pathogenic viral pneumonia in Wuhan, Hubei Province, China, as reported to the World Health Organization (WHO) in December 2019. Subsequent spread has led to a global pandemic (officially declared by the WHO on March 11, 20208).
COVID-19 disease appears to be a spectrum of clinical presentations ranging from asymptomatic to severe respiratory failure. Common symptomology at the onset of illness are fever, cough, and general myalgia, with less common symptoms including sputum production, headache, and diarrhea.9−11 An initial case analysis from China through mid-February 2020 found 14% of cases were associated with severe disease (dyspnea, respiratory frequency ≥ 30/min, blood oxygen saturation ≤ 93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio < 300, and/or lung infiltrates > 50% within 24–48 h), and 5% of cases were critical (i.e., respiratory failure, septic shock, and/or multiple organ dysfunction or failure).12 A more extensive meta-analysis found a slightly higher severe disease percentage (20.3%).13 The disease case fatality rate (CFR) varies depending on region, population demographics, and heath care capabilities; for instance, in Italy an overall CFR of 7.2% is estimated, in part driven by the higher proportion of individuals of advanced age compared to China.14 On the basis of global data, the CFR from COVID-19 based on confirmed cases is estimated to be ∼6.9%.15 Disease progression to acute respiratory distress syndrome typically occurs in older patients (over 63), often with underlying medical conditions such as hypertension or diabetes;16 elevated risk of mortality was associated with advanced age, sepsis, blood clotting deficiencies.17,18 In individuals less than 60 years of age, an increased body to mass index (over 30) was associated with increased disease severity and progression to acute respiratory distress syndrome.19 Other symptoms, including neurologic symptoms and coagulopathies, have also been reported in a portion of infected individuals.20−24
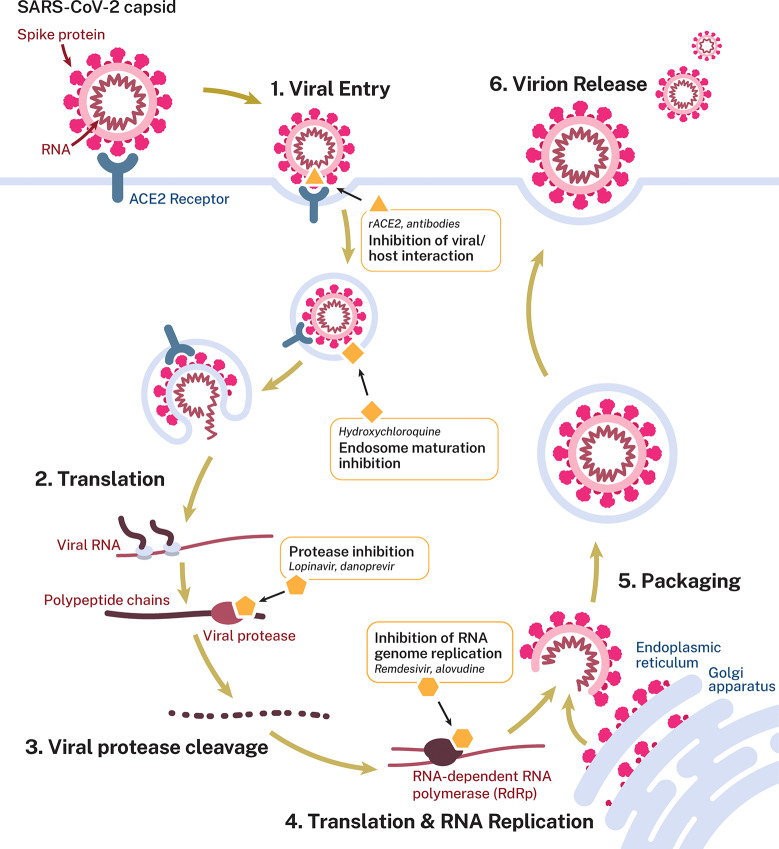
Similar to other coronaviruses, SARS-CoV-2 primarily infects the respiratory and gastrointestinal tract, with a cell tropism of nasal epithelial cells, pneumocytes, and alveolar macrophages in the lung and enterocytes in the bowel.25−27 Although not limited to only these specific cell types, evidence does support that cell binding via the viral S protein to the host receptor angiotensin-converting enzyme 2 (ACE2) is required for infection (Figure 1).28,29 Following entry of the virus into the host cell, the virus complex is then translocated to the endosome, where endosomal acid proteases cleave the S protein mediating membrane fusion.28 The viral genome is released and translated into the viral replicase polyproteins PP1a and PP1ab, which are cleaved into functional proteins by viral proteases. Subgenomic templates for mRNA synthesis and translation of the viral structural proteins occur through discontinuous transcription.2 Viral genome replication is mediated by the viral replication complex, which includes an RNA-dependent RNA polymerase (RdRp), helicase, exonucleaseN, and other accessory proteins. Subsequent assembly of viral nucleocapsids from the packaged viral genomes and translated viral structural proteins occurs at the endoplasmic reticulum-Golgi intermediate compartment,30 with infectious virions then released from the cell through exocytosis.
Figure 1.
Life cycle of SARS-CoV-2 in host cells. SARS-CoV-2 primarily infects the respiratory tract (nasal epithelial cells, pneumocytes, and alveolar macrophages) and the gastrointestinal tract (enterocytes). The virus enters though direct interaction between the viral S protein and the cellular receptor angiotensin-converting enzyme 2 (ACE2). Following entry, the viral genome is released and translated into the viral replicase polyproteins PP1a and PP1ab, which are cleaved into functional proteins by viral proteases.2 Viral genome replication is mediated by the viral replication complex, including the RNA-dependent RNA polymerase (RdRp). Viral nucleocapsids are assembled from the packaged viral genomes and translated viral structural proteins and released through exocytosis. Potential targets and postulated mechanism of action for antiviral interventions are shown: blocking virus/host cell interaction through the use of antibodies/nanobodies (and convalescent plasma therapy) or recombinant ACE2 protein; use of hydroxychloroquine (based on in vitro data) to inhibit endosome maturation; use of protease inhibitors to inhibit viral/endosome membrane fusion or viral polypeptide maturation; nucleoside/nucleotide analogues to inhibit viral genome replication.
As a new disease, SARS-CoV-2 does not have any clinically proven therapeutics. Furthermore, a significant amount of preclinical research was reported in the search for therapeutic treatments for the related viruses SARS and MERS. As the SARS and MERS coronavirus outbreaks did not persist, no therapeutic or vaccine development programs were completed. The consequence is that drug repositioning and repurposing has received a significant amount of attention,31 and approved agents including hydroxychloroquine, azithromycin, ritonavir, ruxolitinib, and camostat have entered clinical trials to address the current SARS-CoV-2 pandemic (Figure 1).32−34 Although some candidates do have pre-existing data to support activity against coronaviruses, other repurposing candidates for potential use against SARS-CoV-2 are based on their ability to inhibit SARS-CoV-2 viral replication in vitro.29,35−37 These include hydroxychloroquine, a known autophagy inhibitor that suppresses lysosomal function,38 and the serine protease inhibitor camostat.29 The ability of these compounds to act as prophylactic agents, treat disease, or even modulate viral replication in vivo has not been demonstrated, although clinical evaluation of several of these potential therapeutics is ongoing.
One of the first clinical candidates that has received attention is remdesivir, a pre-existing drug candidate developed by Gilead Sciences as part of an antiviral development effort, with initial results against Ebola virus (EBOV) reported in 2015.39 It was recently authorized for compassionate use and has now entered controlled clinical trials. Like all other therapeutic approaches for patients with COVID-19, remdesivir was not developed specifically to treat COVID-19, and here we review its discovery and mode of action.
Development of Remdesivir
Remdesivir (GS-5734) was developed by Gilead Sciences and emerged from a collaboration between Gilead, the U.S. Centers for Disease Control and Prevention (CDC) and the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID). They sought to identify therapeutic agents for treating RNA-based viruses that maintained global pandemic potential, such as those that indeed emerged following the initiation of the program, including EBOV and the Coronaviridae family viruses exemplified by Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS).
As a starting point for discovery, a library of ∼1000 small molecules focused around nucleoside analogues was compiled, based on prior knowledge of effective antiviral compounds targeting RNA viruses. Nucleosides are poorly cell-permeable (and therefore can have a low hit rate in cell-based screens such as antiviral screens), so modified nucleosides such as monophosphate, ester, and phosphoramidate prodrugs composed a significant portion of the library. Such prodrugs are typically more permeable and metabolized to liberate the nucleoside or phosphorylated nucleoside within cells.40−42 While the data from the original full screen does not appear to have been disclosed, a 1′-CN modified adenosine C-nucleoside hit (GS-441524), along with a prodrug form of the monophosphate of GS-441524 (GS-5734, later renamed as remdesivir), was found to be highly potent.43 GS-441524 and its S-acyl-2-thioethyl monophosphate prodrug had previously been reported in 2012 as potent leads from a series of 10-substituted 4-aza-7,9-dideazaadenosine C-nucleosides, with broad activity against a panel of RNA viruses: yellow fever virus (YFV), Dengue virus type 2 (DENV-2), influenza A, parainfluenza 3, and SARS.44 The primary assay used was the cytoprotection effect (CPE) assay, in which live virus is incubated with a target cell line and the antiviral activity is inferred by the ability of a test agent to rescue cell death, measured using a standard cell viability reagent.45 In a 2012 study, GS-5734 showed CPE activity against SARS strain Toronto 2 (IC50 = 2.2 μM) without causing cytotoxicity toward the host Vero African green monkey kidney epithelial cells used in the CPE assay (note that different target cells were utilized in viral CPE assays).
When the Ebola outbreak occurred in 2014, the assembled library was utilized to identify and prioritize compounds with efficacy against EBOV. The study by Madelain et al. found that GS-5734 reduced EBOV replication in HeLa cells with an IC50 ≈ 100 nM, and it retained potency in in vivo nonhuman primate EBOV infection models, while GS-441524 was inactive.46,47 In addition to demonstrating activity against EBOV, Warren et al. showed that remdesivir also had antiviral activity against several other viruses, including the coronavirus MERS, with an IC50 of 340 nM in vitro.
With the demonstration that GS-5734 (remdesivir) possessed broad activity against RNA viruses, multiple groups assessed antiviral activity both in vitro and in vivo,45,48,49 validating its activity against coronaviruses. Antiviral activity was confirmed against SARS, MERS zoonotic coronaviruses,49 as well as the circulating human coronaviruses HCoV-OC43 and HCoV-229E, causative agents of the common cold.50 Furthermore, de Wit et al. demonstrated that remdesivir had both prophylactic and therapeutic activity against MERS in a nonhuman primate in vivo model.51
The pharmacokinetics of remdesivir have been summarized in compassionate use documentation published by the European Medicines Agency (EMA, 2020). Remdesivir is administered via an intravenous injection (IV) with a loading dose on day 1 (200 mg in adults, adjusted for body weight in pediatric patients) followed by a daily maintenance dose (100 mg in adults) for up to 10 days. In nonhuman primates, daily administration of 10 mg/kg of remdesivir yielded a short plasma half-life of the prodrug (t1/2= 0.39 h), but sustained intracellular levels of the triphosphate form.45
In vitro and preclinical in vivo animal models supported the effectiveness of remdesivir against SARS-CoV-2 and related coronaviruses. These include a recent in vitro study of remdesivir assessing antiviral activity against SARS-CoV-2 (previously known as 2019-nCov, strain nCoV-2019BetaCoV/Wuhan/WIV04/2019) using qRT-PCR quantification of viral copy number in infected Vero E6 cells. This study demonstrated an IC50 of 770 nM and an IC90 equal to 1,760 nM (with cytotoxic concentration >100 mM).52 In addition, works by Sheahan et al. and de Wit et al. demonstrated in vivo efficacy of remdesivir at inhibiting viral replication and reducing viral related pathology against related coronaviruses.51,53 These findings, along with the safety profile of remdesivir in the clinical trial assessment against EBOV,54 support the evaluation of remdesivir as a potential therapeutic drug for repurposing against the SARS-CoV-2 pandemic.
Driven by the EBOV outbreak in 2014 and based on in vitro and animal model in vivo efficacy against EBOV,45 Gilead Sciences initiated clinical evaluation of remdesivir for EBOV. Gilead pursued FDA evaluation under the FDA’s Animal Rule, permitting the reliance on efficacy findings from animal studies for drugs in which it is not feasible or ethical to conduct human trials. As such, remdesivir was included in a randomized, controlled trial of Ebola virus therapeutics in patients within the Democratic Republic of the Congo (NCT02818582); however, midstudy primary analyses found remdesivir inferior to the antibody based therapeutics MAb114 and REGN-EB3, with respect to mortality, and the remdesivir intervention arm was terminated.54 Mulangu et al. reported one serious adverse event related to remdesivir, an instance of hypotension, along with elevated creatinine and aspartate aminotransferase plasma levels (a suggestive marker for impaired kidney or liver function, respectively) in remdesivir-treated patients compared to either antibody based therapeutic arms. Although remdesivir was inferior against EBOV based on efficacy compared to antibody therapy, the study arm did provide an initial insight into the safety profile in patients.
Remdesivir Mode of Action
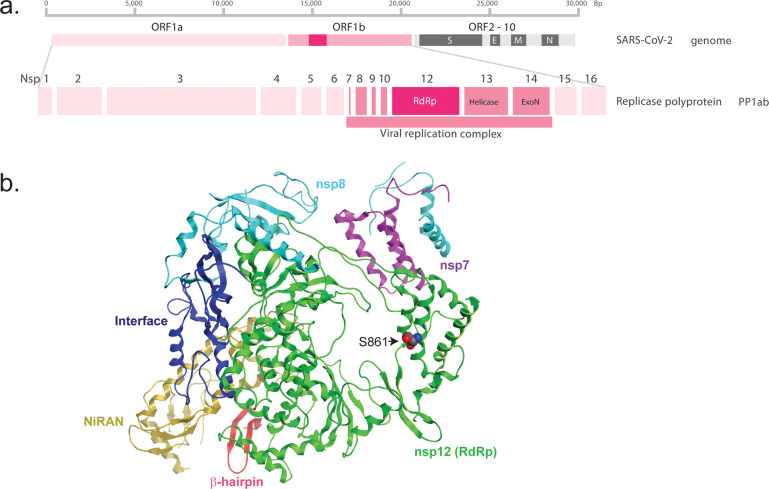
Antiviral chemotherapeutic interventions often target specific viral enzymes or attack a weak point of viral replication within the host, such as targeting the divergent RNA-dependent RNA polymerase (RdRp; Figure 2). Nucleoside analogues represent a class of antiviral agents that has proven efficacious against several viruses, including hepatitis B and C as well as HIV. Generally, these fall into three general classes: mutagenic nucleosides, obligate chain terminators, or delayed chain terminators.55 Ribavirin, a mutagenic nucleoside, targets the viral reliance on an RdRp to catalyze the replication of the RNA genome from the original RNA template.56,57 In a seminal paper, Crotty et al. demonstrated that the RNA virus poliovirus exists on the edge of viability, due to the proportion of virus particles with deleterious mutations. Furthermore, treatment with concentrations of ribavirin that caused a 9.7-fold increase in mutations was sufficient to induce “error catastrophe,” in effect lethally mutating the poliovirus, reducing infectivity by 99.3%.58 Obligate chain terminators, such as azidothymidine (AZT), lack the reactive 3′-hydroxyl group, which directly prevents additional DNA synthesis after incorporation.59 Lastly, delayed chain terminators, which include remdesivir, block transcription despite still possessing the 3′-hydroxyl and thus can still form a phosphodiester bond with the next incorporated nucleotide. However, evidence suggests that the 1′CN substituent of remdesivir sterically clashes with RdRp (residue S861) upon further chain elongation (remdesivir + three additional nucleotides), distorting the positioning of the RNA and hampering translocation to the remdesivir + fourth position (Figure 2).60
Figure 2.
SARS-CoV-2 genome and RNA-dependent RNA polymerase structure. (a) Representation of the SARS-CoV-2 RNA genome. As SARS-CoV-2 is a positive-sense RNA virus, the genome serves as a direct template for protein translation. Replication of the viral genome requires a functional viral replication complex, including an RNA-dependent RNA polymerase (RdRp). (b) Domain organization of the SARS-CoV-2 RdRp (encoded by nsp12) domains bound to cofactors nsp7 and dimers of nsp8, that serve as essential cofactors that increase polymerase activity. The rendering was based on the cryo-EM structure at a resolution of 2.9-Å, published by Gao et al, 2020 (PDB: 6M71). The nsp12 RdRp domain is shown in green, nsp7 in purple, nsp8 in cyan, nidovirus RdRp-associated nucleotidyltransferase (NiRAN) domain in yellow, interface in blue, and a newly identified β-hairpin domain is shown in red.61 Highlighted is RdRp residue S861, which is predicted to sterically interact with the 1′CN substituent of remdesivir inducing delayed chain termination.60
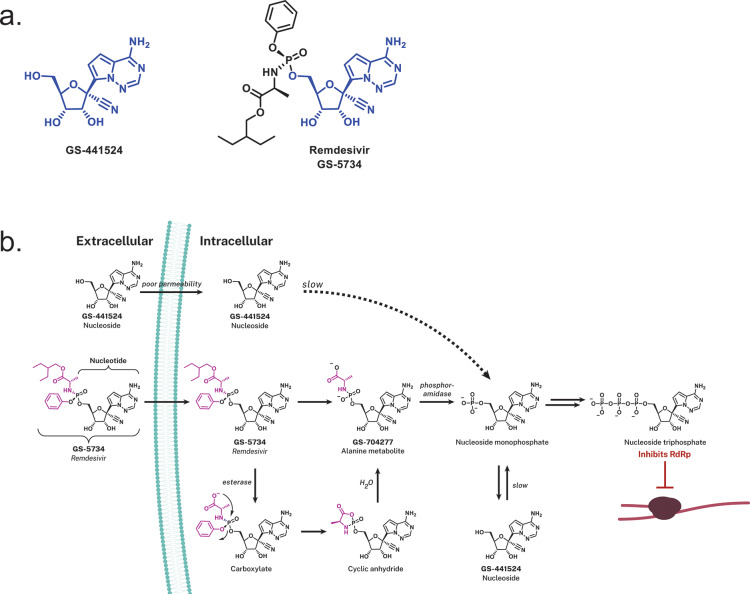
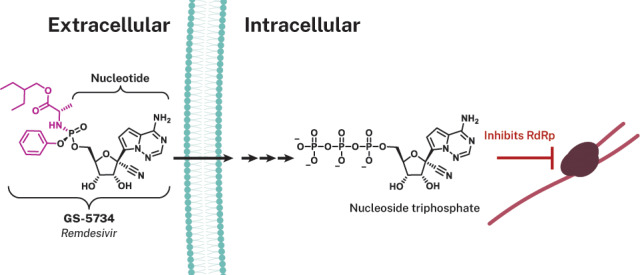
Remdesivir (GS-5734), a prodrug, is metabolized within cells into an alanine metabolite (GS-704277), further processed into the monophosphate derivative and ultimately into the active nucleoside triphosphate derivative (Figure 3). Nucleotide analogues are not highly cell permeable, and once in the cell they require di- and then triphosphorylation to produce the nucleoside triphosphate (NTP) that can be utilized by the viral RNA-dependent polymerases for genome replication. As such, NTPs can then be misintegrated into viral RNA by the viral RNA-dependent RNA polymerase (RdRP; Figure 2). To address this, an approach to antiviral drug design can employ the utilization of phosphoramidate prodrugs (ProTides, inferred as prodrugs of nucleotides).62−65 Protides are composed of a nucleoside monophosphate capped with an aryl group and an amino acid ester (a phosphoramidate). Following diffusion into the cell, the prodrug is presumed to metabolize in a sequence of hydrolytic steps that starts with esterase-mediated ester hydrolysis to a carboxylate that cyclizes internally to the phosphonate ejecting the phenoxide; the resultant unstable cyclic anhydride is hydrolyzed open by water to the alanine metabolite GS-704277 whose P–N bond is hydrolyzed by a phosphoramidase-type enzyme (Figure 3).66−69 This final step liberates the nucleoside monophosphate, which is highly polar, and does not diffuse back across the cell membrane (essentially trapping it within the cell). Subsequent phosphorylation by host cell kinases convert the compound into the NTP analogue that can be used as a substrate by the viral RdRp enzyme.70 While the nucleoside analogue core of remdesivir, GS-441524, can diffuse into cells, the initial phosphorylation step for nucleosides is rate-limiting (slow), which is believed to account for the reduced antiviral activity of GS-441524 compared to remdesivir.49,71 This approach has been successfully applied to a number of FDA-approved antiviral drugs including the Gilead products sofosbuvir (for treating HCV) and tenofovir alafenamide (first approved for treating HIV).64
Figure 3.
Remdesivir and its intracellular conversion. (a) Chemical structures of GS-441524 that compose the nucleoside analogue core (blue) of remdesivir (GS-5734). (b) Intracellular processing of the prodrug remdesivir (GS-5734), the aryloxy phosphoramidate (purple) prodrug of GS-441524 monophosphate. Upon diffusion of remdesivir into the cell, it is metabolized into the nucleoside monophosphate form via a sequence of steps that are presumably initiated by esterase-mediated hydrolysis of the amino acid ester that liberates a carboxylate that cyclizes on to the phosphorus displacing the phenoxide. The unstable cyclic anhydride is hydrolyzed by water to the alanine metabolite GS-704277 whose P–N bond is hydrolyzed by phosphoramidase-type enzymes to liberate the nucleoside monophosphate or nucleotide analog. The artificial nucleoside monophosphate is routed to further phosphorylation events (hijacking the endogenous phosphorylation pathway) yielding the active nucleoside triphosphate analogue form that is utilized by the viral RNA-dependent RNA polymerase (RdRp). Utilization of the GS-441524 nucleoside triphosphate analogue by RdRp inhibits viral replication through inducing delayed chain termination.
Remdesivir’s antiviral activity, sterically interacting with the viral RdRp to induce delayed chain termination, has been demonstrated in vitro against multiple coronaviruses (SARS, MERS, contemporary human CoV and bat-CoVs).72 Remdesivir was also shown to perturb pan-CoV RdRp function by inhibiting viral replication of SARS, MERS, and the model β-coronavirus murine hepatitis virus (MHV), even in settings with intact exonuclease proofreading activity.45 Biochemical data from recombinant respiratory syncytial virus (RSV) RdRp suggested the primary mechanism of action was through delayed chain termination.73−75 Importantly, remdesivir inhibits viral replication (demonstrated with both Ebola and RSV) in cell-based assays with IC50 values of approximately 100 nM, whereas human RNA Polymerase (RNAP) II and human mitochondrial RNAP are not inhibited in the presence of compound,75 providing approximately 500-fold selectivity. This selectivity is achieved, at least in part, due to the nucleoside analogues being poor substrates for the human polymerases.76 Interestingly, in vitro assays demonstrate that the triphosphate form of the inhibitor was incorporated at increased rates compared to natural nucleotide pools,77 likely adding to strong antiviral potency of remdesivir through premature RNA synthesis termination.
Clinical Studies for COVID-19
With the COVID-19 outbreak increasing in size and a lack of alternative therapeutics, two clinical trials using remdesivir were designed and initiated in China. On February 5, 2020, a phase 3 randomized, quadruple-blind, placebo-controlled clinical trial was registered at Capital Medical University, with the goal to determine safety and efficacy of remdesivir in patients with mild to moderate SARS-CoV-2 infection (NCT04252664, since suspended).78 A day later, a second trial (NCT04257656, since terminated) was registered at the same location, focused on patients with advanced COVID-19 respiratory disease.79 Both trials had planned to track the primary outcome as time to clinical improvement, up to 28 days: normalization of fever, oxygen saturation, and respiratory rate, and alleviation of cough which is sustained for 72 h. Both trials delivered remdesivir as a 200 mg loading dose on the first day, with 9 subsequent days of maintenance dosing at 100 mg; this regime is identical to that utilized in the previous NCT03719586 Ebola trial, which appears to be the model for all subsequent trials involving remdesivir (discussed below; Figure 4 and Table 1, registered trials of remdesivir).
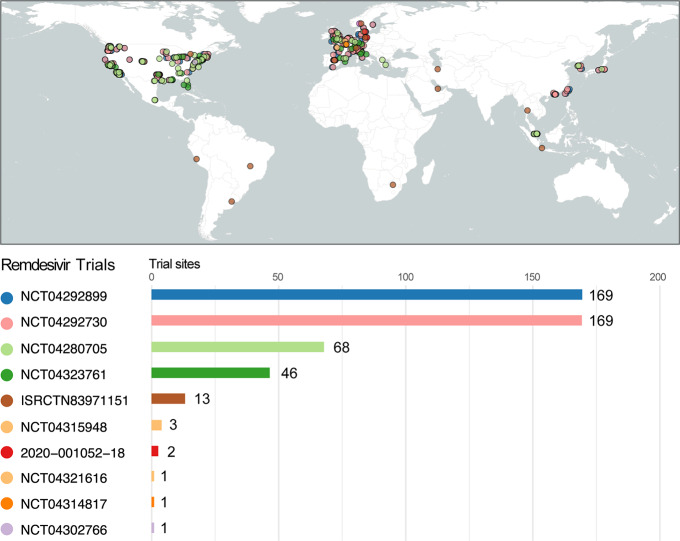
Figure 4.
Remdesivir global clinical trials. Shown are the locations of the clinical study sites for the ongoing clinical studies of remdesivir for SARS-CoV-2/COVID-19. Number of sites participating for each respective study, if no specific information was given, shown are the countries participating (e.g., ISRCTN83971151). Listed are the number of sites participating for each respective study, if no detailed information was provided; shown are the number of countries participating. NCT04302766 is an expanded access trial with no specific sites listed in the registration. Figure created with R,91 utilizing the packages rnaturalearth,92 sf,93 and ggplot2.94
Table 1. Registered Remdesivir Trials for SARS-CoV-2/COVID-19a.
| study identifier | study title | start date | status | sponsor | interventions | phase | study design | expected completion date | location (s) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT04280705b | Adaptive COVID-19 Treatment Trial (ACTT) | February 21, 2020 | recruiting | National Institute of Allergy and Infectious Diseases (NIAID) | remdesivir; remdesivir placebo | 3 | clinical trial, randomized parallel assignment | double (participant, investigator) | treatment | April 1, 2023 | multiple US; multiple Korea; Tokyo, Japan; Singapore |
| ISRCTN83971151c, NCT04330690 | Public Health Emergency SOLIDARITY Trial of Treatments for COVID- 19 Infection in Hospitalized Patients | March 1, 2020 | Available | World Health Organization | remdesivir; lopinavir/ritonavir; lopinavir/ritonavir, interferon β-1a; hydroxychloroquine; standard of care | 3 | clinical trial, randomized | none (open label) | treatment | March 25, 2021 | multiple sites - countries of recruitment: Argentina, Brazil, Canada, Germany, Indonesia, Iran, Norway, Peru, Qatar, South Africa, Spain, Switzerland, Thailand |
| NCT04292899b | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Severe Coronavirus Disease (COVID-19) | March 6, 2020 | recruiting | Gilead Sciences | remdesivir; Standard of Care | 3 | clinical trial, randomized parallel assignment | none (open label) | treatment | May 1, 2020 | multiple US; multiple Hong Kong; Multiple Italy; multiple Korea; multiple Singapore; multiple Spain; multiple Taiwan |
| NCT04292730b study | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment | March 15, 2020 | recruiting | Gilead Sciences | remdesivir; standard of care | 3 | clinical trial, randomized parallel assignment | none (open label) | treatment | May 1, 2020 | multiple US; multiple Hong Kong; multiple Italy; multiple Korea; multiple Singapore; multiple Spain; multiple Taiwan |
| NCT04314817b | Adverse Events Related to Treatments Used Against Coronavirus Disease 2019 | March 17, 2020 | recruiting | Groupe Hospitalier Pitie-Salpetriere, CMC Ambroise Paré | any drug used to treat COVID-19 | observational model, case-only | January 1, 2023 | AP-HP Assistance Publique Hopitaux de Paris, Paris, France | |||
| NCT04315948b | Trial of Treatments for COVID-19 in Hospitalized Adults | March 22, 2020 | recruiting | Institut National de la Santé Et de la Recherche Médicale, France | remdesivir; lopinavir/ritonavir; interferon β-1a; hydroxychloroquine; standard of care | 3 | clinical trial, randomized parallel assignment | none (open label) | treatment | March 1, 2023 | multiple France |
| NCT04321616b, 2020-000982-18d | The Efficacy of Different Antiviral Drugs in (Severe Acute Respiratory Syndrome-Corona Virus-2) SARS-CoV-2 | March 26, 2020 | not yet recruiting | Oslo University Hospital | remdesivir; hydroxychloroquine; standard of care | 2, 3 | clinical trial, randomized parallel assignment | none (open label) | treatment | November 1, 2020 | |
| 2020-001052-18d | A Multicenter, Adaptive, Randomized Blinded Controlled Trial of the Safety and Efficacy of Investigational Therapeutics for the Treatment of COVID-19 in Hospitalised Adults | available | Regents of the University of Minnesota | remdesivir | clinical trial, randomized parallel assignment | double (participant, investigator) | treatment | multiple sites - countries of recruitment: Denmark, Germany, Italy, Portugal, Spain, United Kingdom | |||
| NCT04302766b | Expanded Access Remdesivir (RDV; GS-5734) | available | U.S. Army Medical Research and Development Command | remdesivir | expanded access | ||||||
| NCT04323761b | Expanded Access Treatment Protocol: Remdesivir (RDV; GS-5734) for the Treatment of SARS-CoV-2 (CoV) Infection | available | Gilead Sciences | remdesivir | expanded access | ||||||
Registered remdesivir clinical studies (as of 4/15/2020) for SARS-CoV-2/COVID-19.
Clinicaltrials.gov registered.
ISRCTN registered (www.isrctn.com).
Clinicaltrialsregister.eu registered.
Contemporaneous to the development of the Chinese trials, the first cases of COVID-19 were emerging in the USA. On January 20, 2020, a patient reported to urgent care in Snohomish County, Washington with subjective fever and a 4-day history of cough, later to be confirmed as the first positive case of COVID-19 in the USA.80 On the seventh day of hospitalization and after worsening clinical status, the patient was given IV remdesivir under compassionate use access (Gilead Sciences), with no adverse events observed on infusion.80 The patient’s clinical condition improved the next day, though concurrent treatment with acetaminophen, ibuprofen, guaifenesin, vancomycin, cefepime, and supplemental oxygen confound the direct interpretation of remdesivir’s impact.
Subsequently, 12 patients were confirmed to be infected with SARS-CoV-2 between January 20, 2020, and February 5, 2020.81 Of these 12 patients, seven were hospitalized and three received remdesivir (compassionate use access; Gilead Sciences) upon worsening clinical disease. Treatment was continued for 4–10 days with 200 mg IV on the first day and 100 mg each following day. Following the initial dose, all patients experienced “transient gastrointestinal symptoms, including nausea, vomiting, gastroparesis, or rectal bleeding,” although treatment was continued until improvement in respiratory symptoms, with all 12 patients reporting symptom resolution by February 22, 2020.81 The small sample size and lack of controlled randomization preclude analysis of clinical efficacy or safety.
The National Institute of Allergies and Infectious Diseases (NIAID), NIH initiated the Adaptive COVID-19 Treatment Trial (ACTT), a double-blind, randomized, placebo-controlled phase 3 trial to evaluate the safety and efficacy of remdesivir compared with a remdesivir placebo-control (NCT04280705).82 NIAID developed this study in part based on the existing Chinese clinical trials in addition to consulting with the WHO.83 This study is currently recruiting patients, tracking the primary outcome of patient status severity on an eight-point ordinal scale, with multiple secondary outcomes of interest. A total of 75 clinical sites are anticipated to participate in the study, with distribution across the United States, and an estimated primary completion date of April 2023.
Subsequently, Gilead Sciences initiated two clinical trials that began in mid-March, comparing remdesivir to standard of care in patients with moderate or severe coronavirus disease (COVID-19) in an open-label, randomized trial, NCT04292899.84 This trial will explore the safety and efficacy of remdesivir in combination with standard of care to compare study arms of 5- or 10-day remdesivir dosing on the primary outcome of fever and oxygen saturation. NCT04292730 maintains three study arms to compare remdesivir provided over 5 or 10 days, to standard of care alone, with the primary outcome being the proportion of patients discharged by the 14th day.85
To determine the most effective treatments for COVID-19 and ensure sufficient power to observe definitive results, the WHO announced the SOLIDARITY clinical trial, a four-arm trial comparing remdesivir, lopinavir/ritonavir, lopinavir/ritonavir with interferon-β1a, and chloroquine or hydroxychloroquine (ISRCTN83971151). With the goal of reducing trial design time and start-up, the WHO seeks to rapidly facilitate comparison of treatments on a worldwide scale. Data will be analyzed on an interim basis by an independent group of experts, the Global Data and Safety Monitoring Committee,86 enabling the modification of study design if particular treatments show early promise. As of March 27, 2020, over 70 countries had committed to participating.
In a trial sponsored by the Oslo University Hospital, the WHO NOR (Norwegian)-COVID 19 study is a multicenter, adaptive, randomized, open label study to evaluate the safety and efficacy of hydroxychloroquine, remdesivir, and current standard of care (NCT04321616, 2020-001052-18).87 The comparative arms of the study are daily remdesivir, hydroxychloroquine loading dose of 800 mg × 2 followed by 400 mg × 2 daily for a total of 10 days, or the standard of care. Primary outcome is all cause in-hospital mortality, with secondary measures of duration of mechanical ventilation, ICU duration, 28-day mortality, viral clearance, readmittance, occurrence of coinfections, and organ dysfunction. Inclusion criteria include confirmed SARS-CoV-2 infection by PCR, 18 years of age, and admittance to the hospital ward or ICU. Importantly, exclusion criteria include prolonged QT interval (>450 ms) due to the known toxicity issues associated with hydroxychloroquine.
An observational study sponsored by the Groupe Hospitalier Pitie-Salpetriere, with collaborator CMC Ambroise Paré, was initiated to investigate adverse events in COVID-19 treatment (NCT04314817).88 The study will consider events as classified by the international classification of disease ICD-10, and track lopinavir/ritonavir, chloroquine, azithromycin, remdesivir, and interferon-β1a, potentially expanding the scope in the future prior to the primary completion date in January 2021.
The DisCoVeRy trial is an adaptive, open-label, randomized interventional trial that includes five treatment modalities (NCT04315948): standard of care alone or standard of care plus the following: remdesivir, hydroxychloroquine, lopinavir and ritonavir, or lopinavir, ritonavir, and interferon-β1a.89 The remdesivir dose regime is identical to existing trials, with maintenance dosing continuing up to 10 days. Lopinavir and ritonavir tablets are to be administered every 12 h for 14 days (400 mg of lopinavir /100 mg of ritonavir). In combination with the lopinavir/ritonavir schedule, interferon-β1a will be administered subcutaneously at a dose of 44 μg, for three doses in 6 days (day 1, day 3, day 6). Hydroxychloroquine will be given 400 mg, twice on the first day, followed by 400 mg once daily for 9 days. Initially, the study will include five French hospitals (Paris – Hôpital Bichat-AP-HP, Lille, Nantes, Strasbourg, Lyon) with potential expansion to other participating sites.90 The primary outcome is the reported disease severity on a seven-point ordinal scale, assessed on the 15th day, with secondary outcomes tracking various physiological and clinical metrics.
Expanded Access
With the overwhelming influx of compassionate use requests, on March 23, 2020, Gilead Sciences suspended compassionate use access to remdesivir for all cases save children and pregnant women, shifting their focus to support mounting clinical trials and establish a system of expanded access, wherein hospitals or physicians can request emergency use of remdesivir for multiple patients at one time.95 In an open letter to the public on March 28, 2020, Gilead CEO reported that they had provided over 1000 doses of remdesivir through compassionate use requests.96 To date, the FDA has granted expanded access treatment protocols for remdesivir, sponsored by the U.S. Army Medical Research and Development Command (NCT04302766)95 and Gilead Sciences (NCT04323761).97 The primary objective of these studies is the provision of expanded access to remdesivir for the treatment of SARS-CoV2 infections. Gilead Sciences has acknowledged that production of remdesivir is an involved process, and this is being scaled up to meet demand.
Other Nucleoside Candidates
Remdesivir is certainly not the only nucleoside analogue that is being investigated for use against SARS-CoV-2, but it is the most clinically advanced. A recent publication by Sheahan et al. describes the ribonucleoside analogue, β-d-N4-hydroxycytidine (NHC, EIDD-1931), that has in vitro activity against SARS-CoV-2 and in vivo against the related SARS virus.98 Although in preclinical development, EIDD-1931 is orally bioavailable, a significant advantage compared to remdesivir, and has increased potency against viruses containing mutations in RdRp that conferred increased resistance to remdesivir, supporting the potential for a combination therapy to address the risk of SARS-CoV-2 becoming clinically drug resistant. Other clinically approved nucleoside/nucleotide analogues, such as the hepatitis C drug sofosbuvir and HIV drugs alovudine and zidovudine, have also been shown to be active against the SARS RdRp in in vitro biochemical assays and might have the potential to be repurposed against COVID-19.99 For a general review of nucleoside and nucleotide analogues for cancer and viral diseases, including approved drugs and clinical candidates, please reference Jordheim et al.100
Conclusions
As the COVID-19 pandemic races across the globe, the scientific community, from academic and government laboratories to small biotechnology companies and multinational pharmaceutical corporations, has mobilized to develop and evaluate potential therapeutics and vaccines.101−104 Repurposing or repositioning an effective small-molecule therapeutic promises to be the fastest therapeutic means to stem the tide of the pandemic.105,106 Among the candidate therapies, remdesivir has demonstrated efficacy in both in vitro and in vivo models against coronaviruses. Recently, through a compassionate use indication, remdesivir has supportive evidence for yielding some clinical improvement in COVID-19 patients.107 In addition, an interim analysis of the Adaptive COVID-19 Treatment Trial (NCT04280705) supports improvement in the primary endpoint for patients receiving remdesivir, compared to control, with a 31% faster time to recovery.108 Based on these initial findings, the U.S. Food and Drug Administration has issued an Emergency Use Authorization for the emergency use of remdesivir for the treatment of hospitalized COVID-19 patients. With no drug having FDA approval for marketing as a treatment for SARS-CoV-2, this is the first FDA authorization of an investigational therapeutic for use in treating SARS-CoV-2.109 While remdesivir represents one compound whose recent use authorization may, in part, mitigate the morbidity, mortality, and strain on global healthcare systems caused by COVID-19, additional ongoing clinical trials will provide much-needed clarity surrounding the repurposing of approved drugs and experimental agents against SARS-CoV-2.
Acknowledgments
This work was supported by the National Center for Advancing Translational Sciences, Division of Preclinical innovation. We would like to thank Philip Sanderson for critical reading and suggestions in the editing of the manuscript.
The authors declare no competing financial interest.
References
- Andersen K. G.; Rambaut A.; Lipkin W. I.; Holmes E. C.; Garry R. F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields B. N.; Knipe D. M.; Howley P. M.. Coronavirus. In Fields Virology, 6th ed.; Wolters Kluwer Health/Lippincott Williams and Wilkins, 2013; pp 825–858. [Google Scholar]
- Geller C.; Varbanov M.; Duval R. E. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses 2012, 4, 3044–3068. 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z. From SARS to MERS, Thrusting Coronaviruses into the Spotlight. Viruses 2019, 11 (1), 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menachery V. D.; Graham R. L.; Baric R. S. Jumping species-a mechanism for coronavirus persistence and survival. Curr. Opin. Virol. 2017, 23, 1–7. 10.1016/j.coviro.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani A. S.; Al-Tawfiq J. A.; Memish Z. A. Middle East respiratory syndrome coronavirus (MERS-CoV): animal to human interaction. Pathog. Global Health 2015, 109 (8), 354–62. 10.1080/20477724.2015.1122852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579 (7798), 270–273. 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Director-General’s opening remarks at the media briefing on COVID-19. In WHO Newsletter; https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19--13-april-2020. Accessed April 13, 2020.
- Chen N.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020, 395, 507–513. 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.; McGoogan J. M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323 (13), 1239–1242. 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Morales A. J.; et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med. Infect Dis 2020, 101623. 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder G.; Rezza G.; Brusaferro S.. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- Dong E.; Du H.; Gardner L.. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533, 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein S. R.; Dalan R.; Hopkins D.; Mingrone G.; Boehm B. O.. Endocrine and metabolic link to coronavirus infection. Nat. Rev. Endocrinol. 2020, 10.1038/s41574-020-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020, 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020, 395, 1054–1062. 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighter J.et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin. Infect. Dis. 2020, ciaa415, 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A. M.; Khaleeq A.; Ali U.; Syeda H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host–Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998. 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- Li Y.-C.; Bai W.-Z.; Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020, 1–4. 10.1002/jmv.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L.et al. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a retrospective case series study. MedRxiv 2020, DOI: 10.1101/2020.02.22.20026500. [Google Scholar]
- Wu Y.et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain, Behav., Immun. 2020, 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro C.et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020, S1931-5244(20)30070-0. 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K. F.; et al. Tissue and cellular tropism of the coronavirus associated with severe acute respiratory syndrome: an in-situ hybridization study of fatal cases. J. Pathol. 2004, 202 (2), 157–63. 10.1002/path.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H.et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis. 2020, ciaa410, 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W.et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M.; Marzi A.; Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol 2020, 5 (4), 562–569. 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181 (2), 271–280. e8. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch T. R.; Machamer C. E. The coronavirus E protein: assembly and beyond. Viruses 2012, 4 (3), 363–82. 10.3390/v4030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse J. S.; Lalonde T.; Xu S.; Liu W. R. Learning from the Past: Possible Urgent Prevention and Treatment Options for Severe Acute Respiratory Infections Caused by 2019-nCoV. ChemBioChem 2020, 21, 730–738. 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C. Coronavirus puts drug repurposing on the fast track. Nat. Biotechnol. 2020, 38 (4), 379–381. 10.1038/d41587-020-00003-1. [DOI] [PubMed] [Google Scholar]
- Koch S.; Pong W.. First up for COVID-19: nearly 30 clinical readouts before end of April. https://www.biocentury.com/article/304658/nearly-30-trials-for-covid-19-could-start-to-yield-data-in-the-next-couple-of-months. Accessed April 1, 2020.
- Zhai P.et al. The epidemiology, diagnosis and treatment of COVID-19. Int. J. Antimicrob. Agents 2020, 105955, 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva L.et al. A Large-scale Drug Repositioning Survey for SARS-CoV-2 Antivirals. bioRxiv, 2020, 2020.04.16.044016. [Google Scholar]
- Touret F.et al. In vitro screening of a FDA approved chemical library reveals potential inhibitors of SARS-CoV-2 replication. bioRxiv, 2020, 2020.04.03.023846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger B.Identification of inhibitors of SARS-CoV-2 in-vitro cellular toxicity in human (Caco-2) cells using a large scale drug repurposing collection. Research Square, 2020, 10.21203/rs.3.rs-23951/v1. [DOI]
- Zhan L.; Li J.; Wei B. Autophagy therapeutics: preclinical basis and initial clinical studies. Cancer Chemother. Pharmacol. 2018, 82 (6), 923–934. 10.1007/s00280-018-3688-3. [DOI] [PubMed] [Google Scholar]
- Warren T.et al. Nucleotide Prodrug GS-5734 Is a Broad-Spectrum Filovirus Inhibitor That Provides Complete Therapeutic Protection Against the Development of Ebola Virus Disease (EVD) in Infected Non-human Primates. Open Forum Infectious Diseases 2015, 2, 10.1093/ofid/ofv130.02. [DOI] [Google Scholar]
- De Clercq E. Strategies in the design of antiviral drugs. Nat. Rev. Drug Discovery 2002, 1, 13–25. 10.1038/nrd703. [DOI] [PubMed] [Google Scholar]
- Mehellou Y.; Balzarini J.; McGuigan C. Aryloxy phosphoramidate triesters: a technology for delivering monophosphorylated nucleosides and sugars into cells. ChemMedChem 2009, 4, 1779–1791. 10.1002/cmdc.200900289. [DOI] [PubMed] [Google Scholar]
- Seley-Radtke K. L.; Yates M. K. The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antiviral Res. 2018, 154, 66–86. 10.1016/j.antiviral.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D.; et al. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J. Med. Chem. 2017, 60, 1648–1661. 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
- Cho A.; et al. Synthesis and antiviral activity of a series of 1′-substituted 4-aza-7,9-dideazaadenosine C-nucleosides. Bioorg. Med. Chem. Lett. 2012, 22, 2705–2707. 10.1016/j.bmcl.2012.02.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N.; Ott R. D.; Isaacs R. J.; Fang H. Cell-based Assays to Identify Inhibitors of Viral Disease. Expert Opin. Drug Discovery 2008, 3, 671–676. 10.1517/17460441.3.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini M. L.; Andres E. L.; Sims A. C.; Graham R. L.; Sheahan T. P.; Lu X.; Smith E. C.; Case J. B.; Feng J. Y.; Jordan R.; Ray A. S.; Cihlar T.; Siegel D.; Mackman R. L.; Clarke M. O.; Baric R. S.; Denison M. R. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 2018, 9 (2), e00221–18. 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madelain V.; Baize S.; Jacquot F.; Reynard S.; Fizet A.; Barron S.; Solas C.; Lacarelle B.; Carbonnelle C.; Mentre F.; Raoul H.; de Lamballerie X.; Guedj J. Ebola viral dynamics in nonhuman primates provides insights into virus immuno-pathogenesis and antiviral strategies. Nat. Commun. 2018, 9, 4013. 10.1038/s41467-018-06215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan R.; et al. Broad-spectrum Investigational Agent GS-5734 for the Treatment of Ebola, MERS Coronavirus and Other Pathogenic Viral Infections with High Outbreak Potential. Open Forum Infect Dis 2017, 4, S737. 10.1093/ofid/ofx180.008. [DOI] [Google Scholar]
- Varga A.; Lionne C.; Roy B. Intracellular Metabolism of Nucleoside/Nucleotide Analogues: a Bottleneck to Reach Active Drugs on HIV Reverse Transcriptase. Curr. Drug Metab. 2016, 17, 237–252. 10.2174/1389200217666151210141903. [DOI] [PubMed] [Google Scholar]
- Brown A. J.; et al. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019, 169, 104541. 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit E.; et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc. Natl. Acad. Sci. U. S. A. 2020, 117, 6771–6776. 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T. P.; Sims A. C.; Leist S. R.; Schafer A.; Won J.; Brown A. J.; Montgomery S. A.; Hogg A.; Babusis D.; Clarke M. O.; Spahn J. E.; Bauer L.; Sellers S.; Porter D.; Feng J. Y.; Cihlar T.; Jordan R.; Denison M. R.; Baric R. S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020, 11 (1), 222. 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulangu S.; et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval J. Antimicrobial strategies: inhibition of viral polymerases by 3′-hydroxyl nucleosides. Drugs 2009, 69 (2), 151–66. 10.2165/00003495-200969020-00002. [DOI] [PubMed] [Google Scholar]
- Snell N. J. Ribavirin--current status of a broad spectrum antiviral agent. Expert Opin. Pharmacother. 2001, 2 (8), 1317–24. 10.1517/14656566.2.8.1317. [DOI] [PubMed] [Google Scholar]
- Witkowski J. T.; et al. Design, synthesis, and broad spectrum antiviral activity of 1- -D-ribofuranosyl-1,2,4-triazole-3-carboxamide and related nucleosides. J. Med. Chem. 1972, 15 (11), 1150–4. 10.1021/jm00281a014. [DOI] [PubMed] [Google Scholar]
- Crotty S.; Cameron C. E.; Andino R. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 6895–6900. 10.1073/pnas.111085598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya H.; Yarchoan R.; Broder S. Molecular targets for AIDS therapy. Science 1990, 249 (4976), 1533–44. 10.1126/science.1699273. [DOI] [PubMed] [Google Scholar]
- Gordon C. J.; Tchesnokov E. P.; Woolner E.; Perry J. K; Feng J. Y.; Porter D. P; Gotte M.. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020, jbc.RA120.013679, 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.et al. Structure of RNA-dependent RNA polymerase from 2019-nCoV, a major antiviral drug target. bioRxiv, 2020, 10.1101/2020.03.16.993386. [DOI] [Google Scholar]
- Mehellou Y.; Rattan H. S.; Balzarini J. The ProTide Prodrug Technology: From the Concept to the Clinic. J. Med. Chem. 2018, 61, 2211–2226. 10.1021/acs.jmedchem.7b00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarczyk M.; Serpi M.; Pertusati F. Phosphoramidates and phosphonamidates (ProTides) with antiviral activity. Antivir Chem. Chemother Jan-Dec 2018, 26, 2040206618775243. 10.1177/2040206618775243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley D.; et al. Synthesis and anti-HIV evaluation of some phosphoramidate derivatives of AZT: studies on the effect of chain elongation on biological activity. Antiviral Res. 1990, 14 (6), 345–56. 10.1016/0166-3542(90)90053-A. [DOI] [PubMed] [Google Scholar]
- McGuigan C.; et al. Intracellular delivery of bioactive AZT nucleotides by aryl phosphate derivatives of AZT. J. Med. Chem. 1993, 36 (8), 1048–52. 10.1021/jm00060a013. [DOI] [PubMed] [Google Scholar]
- Murakami E.; et al. Mechanism of activation of PSI-7851 and its diastereoisomer PSI-7977. J. Biol. Chem. 2010, 285 (45), 34337–47. 10.1074/jbc.M110.161802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboulard D.; et al. Characterization of the activation pathway of phosphoramidate triester prodrugs of stavudine and zidovudine. Mol. Pharmacol. 1999, 56 (4), 693–704. [PubMed] [Google Scholar]
- Tobias S. C.; Borch R. F. Synthesis and biological studies of novel nucleoside phosphoramidate prodrugs. J. Med. Chem. 2001, 44 (25), 4475–80. 10.1021/jm010337r. [DOI] [PubMed] [Google Scholar]
- Venkatachalam T. K.; et al. Protease-mediated enzymatic hydrolysis and activation of aryl phosphoramidate derivatives of stavudine. Eur. J. Med. Chem. 2005, 40 (5), 452–66. 10.1016/j.ejmech.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Schneider B.; et al. Pre-steady state of reaction of nucleoside diphosphate kinase with anti-HIV nucleotides. J. Biol. Chem. 1998, 273 (19), 11491–7. 10.1074/jbc.273.19.11491. [DOI] [PubMed] [Google Scholar]
- Munch-Petersen B.; et al. Diverging substrate specificity of pure human thymidine kinases 1 and 2 against antiviral dideoxynucleosides. J. Biol. Chem. 1991, 266 (14), 9032–8. [PubMed] [Google Scholar]
- Sheahan T. P.; et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017, 9 (396), eaal3653. 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. C.; et al. Initiation, extension, and termination of RNA synthesis by a paramyxovirus polymerase. PLoS Pathog. 2018, 14, e1006889. 10.1371/journal.ppat.1006889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchesnokov E. P.; Feng J. Y.; Porter D. P.; Gotte M. Mechanism of Inhibition of Ebola Virus RNA-Dependent RNA Polymerase by Remdesivir. Viruses 2019, 11 (4), 326. 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren T. K.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J. Y.; et al. Role of Mitochondrial RNA Polymerase in the Toxicity of Nucleotide Inhibitors of Hepatitis C Virus. Antimicrob. Agents Chemother. 2016, 60 (2), 806–17. 10.1128/AAC.01922-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon C. J.; et al. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020, 295 (15), 4773–4779. 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov . Mild/Moderate 2019-nCoV Remdesivir RCT. https://clinicaltrials.gov/ct2/show/NCT04252664. Accessed April 10, 2020.
- ClinicalTrials.gov . Severe 2019-nCoV Remdesivir RCT. https://clinicaltrials.gov/ct2/show/NCT04257656. Accessed April 10, 2020.
- Holshue M. L.; et al. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020, 382 (10), 929–936. 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawski S. A.et al. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. MedRxiv, 2020, 10.1101/2020.03.09.20032896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov . Adaptive COVID-19 Treatment Trial (ACTT). https://clinicaltrials.gov/ct2/show/NCT04280705. Accessed April 10, 2020.
- Routh J.https://www.nih.gov/news-events/news-releases/nih-clinical-trial-remdesivir-treat-covid-19-begins; NIH, NIAID, Accessed April 6, 2020.
- BusinessWire, Foster City, CA, 2020. https://www.gilead.com/news-and-press/press-room/press-releases/2020/2/gilead-sciences-initiates-two-phase-3-studies-of-investigational-antiviral-remdesivir-for-the-treatment-of-covid-19).
- ClinicalTrials.gov . Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment (NCT04292730). https://clinicaltrials.gov/ct2/show/NCT04292730. Accessed April 10, 2020.
- WHO . “Solidarity” clinical trial for COVID-19 treatments. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments. Accessed April 7, 2020.
- ClinicalTrials.gov . The Efficacy of Different Anti-viral Drugs in (Severe Acute Respiratory Syndrome-Corona Virus-2) SARS-CoV-2. https://clinicaltrials.gov/ct2/show/NCT04321616. Accessed April 6, 2020.
- ClinicalTrials.gov . Adverse Events Related to Treatments Used Against Coronavirus Disease 2019 (CovidTox). https://clinicaltrials.gov/ct2/show/NCT04314817. Accessed April 6, 2020.
- ClinicalTrials.gov . Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy), https://clinicaltrials.gov/ct2/show/NCT04315948. Accessed April 6, 2020.
- INSERM . Launch of a European clinical trial against COVID-19. https://presse.inserm.fr/en/launch-of-a-european-clinical-trial-against-covid-19/38737/. Accessed April 9, 2020.
- R Core Team . R Foundation for Statistical Computing. http://www.R-project.org/; Vienna, Austria, 2019.
- South A. rnaturalearth: World Map Data from Natural Earth, 2017. https://CRAN.R-project.org/package=rnaturalearth.
- Pebesma E.Simple Features for R: Standardized Support for Spatial Vector Data. R Journal 201810, 439. 10.32614/RJ-2018-009. [DOI] [Google Scholar]
- Wickham H.ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag, 2016. [Google Scholar]
- ClinicalTrials.gov . Expanded Access Remdesivir (RDV; GS-5734). https://clinicaltrials.gov/ct2/show/NCT04302766. Accessed April 6, 2020.
- O’Day D. An Open Letter from our Chairman and CEO. https://www.gilead.com/stories/articles/an-open-letter-from-our-chairman-and-ceo. Accessed April 7, 2020.
- ClinicalTrials.gov . Expanded Access Treatment Protocol: Remdesivir (RDV; GS-5734) for the Treatment of SARS-CoV2 (CoV) Infection. https://clinicaltrials.gov/ct2/show/NCT04323761. Accessed April 11, 2020.
- Sheahan T. P.; Sims A. C.; Zhou S.; Graham R. L.; Pruijssers A. J.; Agostini M. L.; Leist S. R.; Schafer A.; Dinnon K. H.; Stevens L. J.; Chappell J. D.; Lu X.; Hughes T. M.; George A. S.; Hill C. S.; Montgomery S. A.; Brown A. J.; Bluemling G. R.; Natchus M. G.; Saindane M.; Kolykhalov A. A.; Painter G.; Harcourt J.; Tamin A.; Thornburg N. J.; Swanstrom R.; Denison M. R.; Baric R. S.. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020. Published online April 6, 12, eabb5883, 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J.et al. Nucleotide analogues as inhibitors of SARS-CoV polymerase. BioRxiv, 2020, 10.1101/2020.03.12.989186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordheim L. P.; et al. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discovery 2013, 12 (6), 447–64. 10.1038/nrd4010. [DOI] [PubMed] [Google Scholar]
- Amanat F.; Krammer F. SARS-CoV-2 Vaccines: Status Report. Immunity 2020, 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. H.; Strych U.; Hotez P. J.; Bottazzi M. E. The SARS-CoV-2 Vaccine Pipeline: an Overview. Curr. Trop. Med. Rep. 2020, 10.1007/s40475-020-00201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson J.The pandemic pipeline. Nat. Biotechnol. 2020, 10.1038/d41587-020-00005-z. [DOI] [PubMed] [Google Scholar]
- Philippidis A.Catching Up to Coronavirus: Top 60 Treatments in Development. Genetic Engineering & Biotechnology News. https://www.genengnews.com/virology/coronavirus/catching-up-to-coronavirus-top-60-treatments-in-development/. Accessed April 10, 2020.
- Allison M. NCATS launches drug repurposing program. Nat. Biotechnol. 2012, 30, 571–572. 10.1038/nbt0712-571a. [DOI] [PubMed] [Google Scholar]
- Kouznetsova J.; Sun W.; Martinez-Romero C.; Tawa G.; Shinn P.; Chen C. Z; Schimmer A.; Sanderson P.; McKew J. C; Zheng W.; Garcia-Sastre A. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerging Microbes Infect. 2014, 3, e84. 10.1038/emi.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J.et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAID News Release, April 29, 2020. NIH Clinical Trial Shows Remdesivir Accelerates Recovery from Advanced COVID-19. (https://www.niaid.nih.gov/news-events/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19).
- FDA News Release, May 1, 2020. Remdesivir EUA Letter of Authorization. (https://www.fda.gov/media/137564/download).