Abstract
Background
Acute bronchitis is one of the most common diagnoses made by primary care physicians. It is traditionally treated with antibiotics (although the evidence for their effectiveness is weak, and modest at best) and other even less effective treatments. Chinese medicinal herbs have also been used as a treatment.
Objectives
This review aimed to summarise the existing evidence on the comparative effectiveness and safety of Chinese medicinal herbs for treating uncomplicated acute bronchitis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 4) which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to 19 September 19, 2011), EMBASE (1988 to 19 September 2011) and CNKI and the Chinese Biomedical Database (CBM) (1980 to 19 September, 2011).
Selection criteria
Randomised controlled trials (RCTs) comparing Chinese medicinal herbs with placebo, antibiotics or other Western medicines for the treatment of uncomplicated acute bronchitis.
Data collection and analysis
At least two review authors independently extracted data and assessed trial quality.
Main results
In this updated review, 74 studies involving 6877 participants were reported as RCTs by the study authors. None of them met the inclusion criteria for this review. Out of the 74 trials, we identified 39 as non‐RCTs and 35 compared different Chinese herbal medicines in the intervention and control groups.
Authors' conclusions
There is insufficient quality data to recommend the routine use of Chinese herbs for acute bronchitis. Trial design limitations of the individual studies meant that we could not draw any conclusions about the benefits of Chinese herbs for acute bronchitis. In addition, the safety of Chinese herbs is unknown due to the lack of toxicological evidence for these herbs, although adverse events were reported in some case reports.
Keywords: Humans; Acute Disease; Bronchitis; Bronchitis/drug therapy; Clinical Trials as Topic; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Phytotherapy; Phytotherapy/methods
Plain language summary
Chinese medicinal herbs for acute bronchitis
We assessed the therapeutic effect of traditional Chinese herbal medicines commonly used in China for acute bronchitis. There is no evidence from randomised controlled trials (RCTs) to demonstrate that Chinese medicinal herbs are efficacious in treating acute bronchitis.
We identified 74 studies involving 6877 participants which reported to randomly allocate participants to their treatment groups. However, we did not identify any true RCTs for inclusion. The common reasons for exclusion were a potential high risk of selection bias and conflict of interest. These design limitations resulted in us being unable to draw conclusions on the effects of Chinese herbal medicines for acute bronchitis. In addition, the safety of Chinese medicinal herbs is unknown due to the lack of toxicological evidence, although some adverse events, for example, slight gastrointestinal reactions, skin rash, etc., were reported in some case reports but not in the so called 'random' studies which we excluded. High quality RCTs are needed in the future.
Background
Description of the condition
Acute bronchitis is one of the most common diagnoses made by primary‐care physicians (Kirkwood 1982; Marsland 1976; Meza 1994). This condition accounted for approximately 2.5 million visits to physicians in the United States in 1998 (Slusarcick 2000). It consistently ranks as one of the top 10 diagnoses for which patients seek medical care, with cough being the most frequently mentioned symptom (Slusarcick 2000). During each episode, patients receive an average of two prescriptions and miss two to three days of work (Oeffinger 1997).
Viruses are the most common cause of the bronchial inflammation associated with acute bronchitis in otherwise healthy adults. Only a small proportion of acute bronchitis infections are caused by non‐viral agents, with the most common organism being Mycoplasma pneumoniae (M. pneumoniae) (Evans 1961; Evans 1967; Mogabgab 1968). Study findings suggest that Chlamydia pneumoniae (C. pneumoniae) may also cause acute bronchitis (Falck 1994; Hahn 1991).
Description of the intervention
Antibiotics are the common therapy offered to patients with acute bronchitis (William 1998). However, evidence for the effectiveness of antibiotics over placebo is of modest benefit only (Fahey 2004). Symptomatic treatments have even less evidence, and include antitussives for cough; expectorants for sputum too viscous to expectorate; bronchodilators for cough associated with any associated asthma; and antipyretic analgesics for fever.
Traditional Chinese medicine (TCM) follows a particular theoretical and methodological pathway to look at the cause concept, diagnosis and treatment of an illness. Chinese medicinal herbs are made from natural plants and the typical TCM method is based on using several herbs to treat the disease. Depending on the symptoms or causes, specific herbs are selected using a particular method, to create a formulation of multiple, active ingredients. In recent decades more and more clinical doctors are following TCM practices to integrate traditional medicine with Western medicine, particularly in terms of diagnostic, aetiological and treatment theories.
How the intervention might work
Depending on the symptoms or causes, various medicinal herbs are used for treating acute bronchitis. In China, Chinese herbs are generally considered to be effective and are commonly prescribed by physicians for patients with acute bronchitis. Some Chinese herbs are considered to have antiviral, antiasthmatic, antitussive and fever‐relieving properties. In pharmacological experiments, radix scutellariae has been shown to have antiphlogistic properties (Huang 1990); radix glycyrrhizae has expectorant properties (Zhu 1976); and folium perillae has antitussive properties (Yu 1986).
Why it is important to do this review
Natural medicinal herbs are a potential drug resource. The effects of any medicinal herb need to be studied systematically. Every year in China hundreds of millions of dollars are spent on treating acute bronchitis. In the United States the evaluation and treatment of this illness is estimated to cost between USD 200 million and USD 300 million annually (Dunlay 1984). Therefore, a systematic review of the evidence as to whether or not these medicinal herbs are effective, can be a helpful tool when making health policy decisions.
This review aimed to summarise the existing evidence on the comparative effectiveness and safety of Chinese medicinal herbs for treating uncomplicated acute bronchitis.
Objectives
To assess whether Chinese medicinal herbs are an effective treatment for acute bronchitis.
To compare the effectiveness of Chinese medicinal herbs and routine treatments for acute bronchitis.
To estimate adverse effects of Chinese medicinal herbs used to treat acute bronchitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Trials which included participants of either gender or any age with a clinical syndrome of cough and productive sputum, or with a physician's diagnosis of acute bronchitis were to be included. Trials which included participants with pre‐existing chronic bronchitis (that is, acute exacerbation of chronic bronchitis) or other infectious diseases and fever‐causing diseases were not to be included.
Types of interventions
Studies comparing any Chinese herbal combination to placebo, antibiotics, or other routine care, were acceptable.
Types of outcome measures
We planned to include outcomes of clinical importance such as:
time to resolution of cough, sputum production and return to normal activity;
proportion of participants with cough, night cough, productive cough, activity limitations, or abnormal lung examination at a designated follow‐up visit;
global assessment (symptoms, signs and laboratory examination) of improvement by clinicians at follow‐up; and
adverse effects.
Primary outcomes
Recovery, defined as resolution of all symptoms of acute bronchitis due to the treatment.
No change in symptoms.
Secondary outcomes
Improvement, defined as improvement in symptoms by the end of the treatment.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 4; www.thecochranelibrary.com (accessed 19 September 2011)), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to 30 April 2011), EMBASE (1988 to 30 April 2011), The Chinese Cochrane Centre's Controlled Trials Register (up to 30 April 2011) and CBM (1980 to 30 April 2011). Details of the MEDLINE and CBM search strategy are in Appendix 1.
We also searched databases of ongoing trials: Current Controlled Trials (www.controlled‐trials.com); the National Research Register (www.update‐software.com/National/nrr‐frame.html); Chinese Clinical Trial Registry (www.chictr.org); and the World Health Organization (WHO) International Clinical Trials Registry (ICTR) Search Portal (http://apps.who.int/trialsearch/).
Searching other resources
We attempted to identify additional studies by searching the reference lists of relevant trials, reviews, conference proceedings and journals. In particular, with respect to journals, we searched those not indexed in the electronic databases. We contacted trial authors to obtain full details.
We contacted organisations (including the WHO, individual researchers working in the field and manufacturers of Chinese medicinal herbs) to obtain additional references, unpublished, or ongoing trials, confidential reports and raw data of published trials.
Data collection and analysis
Two review authors (LJ, KL) independently searched for trials.
Selection of studies
We reviewed titles and abstracts from articles found in the searches. We retrieved those that appeared eligible as full text articles. Two review authors (LJ, KL) independently applied the inclusion criteria. We resolved disagreements by consensus and we contacted the trial authors for more details when needed.
Data extraction and management
Two review authors (LJ, TW) independently extracted data, giving details of study population, intervention and outcomes using a standard data extraction form. We specifically designed a data extraction form for this review. We extracted data on participants, interventions and outcomes. The data extraction form included the following items.
General information: published or unpublished; title; authors; reference or source; contact address; country; urban or rural etc.; language of publication; year of publication; duplicate publications; sponsor and setting.
Trial characteristics: design; duration of follow‐up; method of randomisation; allocation concealment and blinding (participants, people administering treatment and outcome assessors).
Intervention(s): use of placebo; intervention(s) (dose, route and timing); comparison intervention(s) (dose, route and timing); and co‐medication(s) (dose, route and timing).
Patients: exclusion criteria; total number and number in comparison groups; age (children/adults); baseline characteristics; diagnostic criteria; similarity of groups at baseline (including any co‐morbidity); assessment of compliance; withdrawals and losses to follow‐up (reasons/description); and subgroups.
Outcomes: outcomes specified above; any other outcomes assessed; other events; length of follow‐up; and quality of reporting of outcomes.
Results: for outcomes (including a measure of variation) with times of assessment, if necessary, converted to measures of effect specified below; and intention‐to‐treat (ITT) analysis.
We resolved differences in the extracted data by consensus, referring back to the original article. When necessary, we sought information from the trial authors.
Two review authors (LJ, TW) independently extracted original reports of trial results. We resolved disagreement by discussion and, where necessary, in consultation with a third review author (JN). We extracted number of events and total number in each group for binary outcomes. We extracted or imputed mean standard deviations (SDs) and sample sizes of each group for continuous outcomes.
Assessment of risk of bias in included studies
We had planned for two review authors (LJ, TW) to independently assess the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
1. Sequence generation (checking for possible selection bias)
We planned to assess the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
2. Allocation concealment (checking for possible selection bias)
We planned to assess the method used to conceal the allocation sequence in sufficient detail and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We planned to assess the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth); or
unclear risk of bias.
3. Blinding (checking for possible performance bias)
We planned to assess the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We judged studies at low risk of bias if they were blinded, or if we judged that the lack of blinding could not have affected the results. We planned to assess blinding separately for different outcomes or classes of outcomes. We planned to assess the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel; and
low, high or unclear risk of bias for outcome assessors.
4. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We planned to assess each study and each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We planned to state whether attrition and exclusions were reported; the numbers included in the analysis at each stage (compared with the total randomised participants); reasons for attrition or exclusion (where reported); and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook. We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. the rate of exclusion was at least 20%; missing data imbalanced across groups; 'as treated analysis' done with substantial departure from intervention received compared to that assigned at randomisation); and
unclear risk of bias.
5. Selective reporting bias
We planned to assess each included study for the possibility of selective outcome reporting bias. We planned to assess the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest for the review have been reported);
high risk of bias (where not all of the study’s pre‐specified outcomes have been reported; one or more reported primary outcome was not pre‐specified; outcomes of interest were reported incompletely and so cannot be used; and the study fails to include results of a key outcome that would have been expected to have been reported); and
unclear risk of bias.
6. Other sources of bias
We planned to assess whether each study was free of other problems that could put it at low, high or unclear risk of bias.
7. Overall risk of bias
We planned to make explicit judgements about whether studies were at high risk of bias, according to the criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis section.
Measures of treatment effect
Dichotomous data
We had planned to present results as summary risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous data.
Continuous data
We had planned to use the mean difference (MD) if outcomes were measured in the same way between trials for continuous data. We had planned to use the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
We had planned for individual participants to be the unit of analysis.
Dealing with missing data
We had planned on noting levels of attrition for included studies. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by conducting a sensitivity analysis.
We had planned to carry out analyses, as far as possible, on an intention‐to‐treat (ITT) basis, i.e. we would include all participants randomised to each group in the analyses for all outcomes. The denominator for each outcome in each trial was to be the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We had planned to use the I² statistic to measure heterogeneity among the trials in each analysis. We had planned to explore substantial heterogeneity, in which the I² is more than 50%, by a prespecified subgroup analysis.
Assessment of reporting biases
We had planned to contact study authors for missing outcome data where we suspected reporting bias (see 'Selective reporting bias' above). Where this was not possible and the missing data were thought to introduce serious bias, we will explore the impact of including such studies in the overall assessment of results by a sensitivity analysis when we update the review in the future.
Data synthesis
We had planned to carry out a statistical analysis using Review Manager software (RevMan 2011). We planned to use the fixed‐effect inverse variance meta‐analysis for combining data where trials are examining the same intervention, and the trials' populations and methods are judged sufficiently similar. We planned to use a random‐effects meta‐analysis where we suspected clinical or methodological heterogeneity between studies sufficient to suggest that treatment effects may differ between trials. We planned to note if substantial heterogeneity was identified in a fixed‐effect meta‐analysis and repeat the analysis using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
We planned on carrying out the following subgroup analyses.
Different formulations of traditional Chinese medicine (TCM).
Different TCM modalities and different types of conventional treatments.
Different age groups, children and adults.
We had planned to conduct subgroup analyses, classifying whole trials by interaction tests as described by Deeks 2001 for fixed‐effect meta‐analyses. We had planned to assess differences between subgroups by inspection of the subgroups' CIs for random‐effects meta‐analyses. Non‐overlapping CIs would have indicated a statistically significant difference in the treatment effect between the subgroups.
Sensitivity analysis
We planned to test the robust nature of the evidence by sensitivity analyses by comparing the results of a fixed‐effect model with a random‐effects model; robust evidence should not be reversed by changing the models.
Results
Description of studies
The process of study selection is presented in Figure 1.
1.
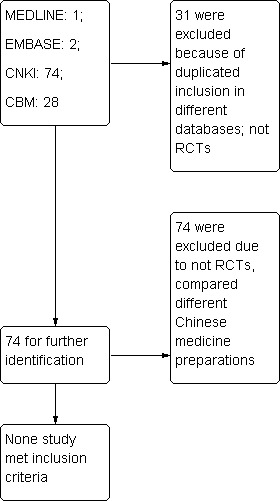
Flow diagram ‐ study selection
Results of the search
Our new searches yielded 39 trials which had been classified as RCTs. In all, we found a total of 74 trials involving 6877 participants in our review. We telephoned the original trial authors of the 39 trials for the purpose of identifying the method of the randomisation they used. Unfortunatly, none of the trials met our inclusion criteria.
Excluded studies
As a result of telephone interviews, we found that no study met our inclusion criteria. Thirty‐nine studies stated "randomly allocated the participants" but were not RCTs. They were either retrospective case record analyses, studies that had not developed a protocol before recruiting participants, or incorrect randomisation methods had been used in the trials (An 2003; Bian 2011; Chai 2011; Chu 2011; Hang 2011; Liu 2011; Luo 2005; Qing 2002; Shi 2010; Song 2011; Tian 2005; Wang 2001a; Wang 2001b; Wang 2005; Wang 2011a; Wu 2010; Xu 2011; Yang 2002; Yang 2005; Yang 2011c; Yang 2011b; Ye 2011; Yu 1998; Yu 2003; Yuan 2001; Yuan 2002; Zeng 2005; Zhai 2000; Zhang 1989; Zhang 2001; Zhang 2002; Zhang 2005; Zhang 2010; Zhang 2011e; Zhang 2011a; Zhang 2011b; Zhang 2011c; Zhang 2011d; Zhou 2003). In 35 studies (Chen 2000; Chen 2002; Chen 2003; Chen 2011b; Chen 2011a; Cui 2011; Dong 2011; Gao 2011; Han 2001; Han 2001; He 2011; Li 2010; Li 2011b; Li 2011a; Liang 2001a; Liang 2011b; Lin 2000; Lin 2011; Liu 1998; Liu 2010; Meng 1999; Ren 2010; Sun 2008; Sun 2010; Tan 2002; Wang 2002; Wang 2011b; Wu 2000; Xu 2001; Yang 2003; Yang 2010; Yang 2011a; Yin 2001; Zeng 2002; Zhou 2011) Chinese medicinal herbs were administered in both the intervention and control groups, which did not meet the inclusion criteria. The reasons for exclusion can be seen in the Characteristics of excluded studies table.
Risk of bias in included studies
We could not carry out a risk of bias assessment as none of the studies were eligible for inclusion in the review.
Effects of interventions
While none of the identified studies met the inclusion criteria, we extracted data from Wang 2005 and Sun 2008 in order to allow readers of our review to understand what problems were presented in those studies. We identified the following results.
Time to resolution of symptoms and signs in study Wang 2005
1. Cough resolution
In Wang 2005, 13 children (13/200) in the Huoke granules group and 19 in the control group (19/100) did not show any improvement in cough resolution. The difference is statistically significant (risk ratio (RR) 0.34; 95% confidence interval (CI) 0.18 to 0.66). When comparing the duration of cough in the Huoke granules group with that in the antibiotics group, the beneficial effect of Huoke granules was statistically significance (mean difference (MD) ‐0.37 days; 95% CI ‐0.57 to ‐0.05). This showed that coughs resolved over a much shorter time in children with acute bronchitis when treated with Huoke granules than penicillin.
2. Fever clearance
In Wang 2005, there were 89 children with fever in the Huoke granules group; after treatment there was no improvement in three children. There were 44 children with fever in the control group; after treatment there was no improvement in seven children. The difference is statistically significant (RR 0.21; 95% CI 0.06 to 0.78). When comparing the duration of fever between the Huoke granules group with the antibiotics and Xiaoer Shangfeng Zhike tangjiang syrup group, the beneficial effect of Huoke granules was statistically significance (MD ‐1.07 days; 95% CI ‐1.31 to ‐0.83).
3. Sputum resolution
In Wang 2005, there were 157 children with sputum in the Huoke granule group; after treatment there was no improvement in 11 children. There were 78 children with sputum in the antibiotics group; after treatment there was no improvement in 14 children. The difference was statistically significant (RR 0.39; 95% CI 0.19 to 0.82). Comparing the duration of sputum in the Huoke granules group with the antibiotics and Xiaoer Shangfeng Zhike tangjiang syrup group, the beneficial effect of antibiotics and Xiaoer Shangfeng Zhike tangjiang syrup showed statistical significance (MD 0.67 days; 95% CI 0.43 to 0.91).
4. Number of participants with no improvement in rales
In Wang 2005, comparing Huoke granules with antibiotics on the rate of no improvement in rales, there was no statistical significance (RR 0.39; 95% CI 0.11 to 1.33). This shows that the effect of Huoke granules on the resolution for rales was similar to the penicillin and Xiaoer Shangfeng Zhike tangjiang syrup.
5. Number of patients with no improvement in chest X‐ray
In Wang 2005, Huoke granules with antibiotics showed statistical significance on the rate of no improvement in chest X‐ray than Xiaoer Shangfeng Zhike tangjiang syrup (RR 0.42; 95% CI 0.20 to 0.91).
4. Adverse events
No adverse effects during treatment were reported.
Rate of effect in study Sun 2008
1. Recovery
In Sun 2008, 29 participants (29/97) in the experimental group and 14 in the control group (14/45) recovered. There was no difference in the recovery (RR 0.96; 95% CI 0.57 to 1.63).
2. No improvement
In Sun 2008, there were six children in the experimental group and two in the control group who appeared not to improve following treatment. There was no difference between the groups (RR 0.72; 95% CI 0.15 to 3.42).
3. Change in traditional Chinese medicine (TCM) signs
In Sun 2008, 91 participants in the experimental group (91/97) and 43 (43/45) in the control group appeared to have an improvement in their TCM signs. There was no statistical significantly difference (RR 1.02; 95% CI 0.94 to 1.10).
4. Adverse events
In Sun 2008, two participants in the experimental group and one in the control group reported slight discomfort.
Discussion
Summary of main results
Since there is no gold standard test, the diagnosis for acute bronchitis must be based on clinical assessment. Both Wang 2005 and Sun 2008 included participants with a recent onset of a respiratory illness with productive cough and fever and excluded participants with chronic pulmonary disease. Clinical characteristics of the enrolled participants varied but were consistent with the variety of similar definitions generally used by primary physicians. Therefore, these results would appear to be generalised to the management of acute bronchitis in community practices.
Although 74 studies were reported as RCTs by the trial authors, none of them met the inclusion criteria of this review. Out of the 74 trials, 39 were identified as non‐RCTs and 35 compared different Chinese herbal medicines in the intervention and control groups.
Overall completeness and applicability of evidence
There was no evidence from RCTs of Chinese herbal medicines for acute bronchitis. However, the majority of studies considered for inclusion did not give adequate methodological information, which is a common phenomenon in Chinese journal articles (Wu 2007). In this updated review, we clarified the reliability of the randomisation allocation procedure used in each trial. We achieved this by interviewing the original authors of the studies which mentioned 'randomly allocated participants' in the text. As a result, we found that all of the trials did not use true randomisation methods.
In addition to the limitations of the lack of description of the randomisation and allocation concealment, a number of studies showed the following typical methodological limitations presented in some TCM studies published in Chinese journals.
Use of a 'positive drug' as the control: aimed to clarify that the experimental intervention had the same effect as the control, but most of these control drugs lack evidence of effect. For example, in the excluded study Sun 2008 we searched the databases and found that there was no evidence about the Chinese medicine Piba Zhike granules. Therefore we were unable to provide evidence for the Chinese medicine Ji Zhi granules. Similarly, the evidence of penicillin combined with the Chinese medicine Xiaoer Shangfeng Zhike syrup used in the control group of study Wang 2005 has not been found. The effect of the control drug should be tested a priori comparing it with the placebo. However, we were unable to find any such evidence of this.
In Wang 2005, the effect of the Chinese medicine Huoke granule was much better than the penicillin plus Chinese medicine Xiaoer Shangfeng Zhike syrup (RR 2.07, 95% CI 1.19 to 3.60). However, the time point of detection of the outcomes was unclear. Therefore, the effect was questionable.
Conflict of interest: most drugs tested for effectiveness were prepared, or suggested, or made by the trial authors themselves. For example, Huoke granule was made by the authors' hospital (Wang 2005). Therefore there was a high risk of bias in terms of conflict of interest.
Blinding for avoiding detection bias in assessment of some outcomes is very important, particularly for the objective outcomes, for example, change of symptoms and subject reports of how the participants felt. All of the outcomes in the study by Sun 2008 were objective outcomes, but as blinding was not used to detect the results, there was a high risk of detection bias.
The sample size problem: some studies, for example Sun 2008 included 433 participants, but included three different diseases (acute bronchitis, acute onset of chronic bronchitis and cough). Only 142 participants suffered from acute bronchitis. Whether the power is sufficient or not is unclear due to the changed sample size.
The effect of most TCMs was tested in one study only.
Most of the studies were conducted in lower level hospitals or smaller clinics. This means that most of the trials did not receive ethical approval and therefore may not be true trials, but rather analysis reports from case notes.
Potential biases in the review process
We contacted the trial authors to avoid including false RCTs. Potential biases may have taken place in the searching process while we were unable to contact industries for unpublished studies.
Agreements and disagreements with other studies or reviews
We were unable to find other evidence from systematic reviews or meta‐analyses of TCM for acute bronchitis.
Authors' conclusions
Implications for practice.
There is no evidence from RCTs of Chinese herbal medicines for acute bronchitis.
Implications for research.
There is a widespread belief among Chinese clinicians and patients that Chinese medicinal herbs are effective in improving signs and symptoms of acute bronchitis. Due to the absence of RCTs in this field, we were unable to draw a conclusion for recommendation. We suggest that additional, well‐designed RCTs with adequate power to provide a definitive answer, need to be conducted. For many Chinese researchers, allocation concealment should be emphasised and the approaches should be reported clearly. The randomisation procedure should also be clearly described. Blinding should be conducted in trials on Chinese medicinal herbs, though this may be difficult. Studies should emphasise adverse effects, and more toxicological research on Chinese medicinal herbs should be conducted, which would provide important information for clinicians. The consolidated standards for reporting trials (CONSORT) for TCM is recommended as the standard for reporting TCM trials (Wu 2007).
What's new
| Date | Event | Description |
|---|---|---|
| 19 September 2011 | New search has been performed | We updated our searches and excluded 39 new trials as none of them met our inclusion criteria for this review. |
| 19 September 2011 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 8 May 2009 | Amended | Contact details updated. |
| 10 June 2008 | Amended | Converted to new review format. |
| 6 July 2007 | New citation required and conclusions have changed | Conclusions changed. |
| 1 March 2007 | New search has been performed | Searches conducted. |
| 2 March 2005 | New search has been performed | Searches conducted. |
Notes
This updated review did not include any new trials. We investigated the methodological quality of all identified RCTs by interviewing the original trial authors by telephone: we excluded all trials included in the original review for failing to use a random allocation method.
Acknowledgements
The authors would like to thank Liz Dooley for assistance in the editorial process; and Cheryl Flynn, Durhane Wong‐Rieger, George Lewith, Edzard Ernst and Nelcy Rodriguez for comments on the first draft of this review. We would also like to thank the following people for commenting on the updated draft review: Ann Fonfa, George Lewith, George Lenon, Sree Nair and Geoff Spurling.
Appendices
Appendix 1. Search strategy
The search terms were adapted to search the other electronic databases.
MEDLINE (OVID) 1 exp BRONCHITIS/ 2 bronchitis.mp. 3 or/1‐2 4 exp Drugs, Chinese Herbal/ 5 exp Medicine, Chinese Traditional/ 6 exp Medicine, Oriental Traditional/ 7 (medicinal herb$ or Chinese herb$ or Chinese medic$).mp. 8 or/4‐7 9 3 and 8
CNKI and VIP
1. 急性支气管炎 ti.
2. 中医
3. 中药
4. 中医药
5. 随机 ti. ab. tx.
6. #1 AND (#2 OR #3 OR #4) AND #5
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| An 2003 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Bian 2011 | Non‐RCT; did not meet outcome inclusion criteria |
| Chai 2011 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Chen 2000 | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Chen 2002 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Chen 2003 | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Chen 2011a | Did not meet outcome inclusion criteria, comparing 2 Chinese herbal medicines |
| Chen 2011b | No data on outcome measure, compared 2 Chinese herbal medicines |
| Chu 2011 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Cui 2011 | Did not meet participants inclusion criteria, comparing 2 Chinese herbal medicines |
| Dong 2011 | Did not meet the medicine comparison inclusion criteria, comparing 2 Chinese herbal medicines |
| Gao 2011 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Guan 2010 | Did not meet medicine comparison inclusion criteria, comparing 2 Chinese herbal medicines |
| Han 2001 | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Hang 2011 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| He 2011 | Did not meet participants inclusion criteria, comparing 2 Chinese herbal medicines |
| Li 2010 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Li 2011a | Did not meet medicine comparison inclusion criteria, comparing 2 Chinese herbal medicines |
| Li 2011b | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Liang 2001a | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Liang 2011b | Did not meet participants inclusion criteria, comparing 2 Chinese herbal medicines |
| Lin 2000 | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Lin 2011 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Liu 1998 | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Liu 2010 | Did not meet outcome inclusion criteria, comparing 2 Chinese herbal medicines |
| Liu 2011 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Luo 2005 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Meng 1999 | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Qing 2002 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Ren 2010 | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Shi 2010 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Song 2011 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Sun 2008 | Did not meet outcome inclusion criteria, comparing 2 Chinese herbal medicines |
| Sun 2010 | Did not meet outcome inclusion criteria, comparing 2 Chinese herbal medicines |
| Tan 2002 | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Tian 2005 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Wang 2001a | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Wang 2001b | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Wang 2002 | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Wang 2005 | The authors mentioned that "the 200 children were allocated to the treatment group and 100 to the control group according to the random, balance principle" but the method was not mentioned in detail and the numbers of children in 2 groups were actually not balanced Treatment group: 109 males, 91 females, age 1 to 14 years old (average 6.53) Control group: 52 males, 48 females, age 1 to 14 years old (average 6.37) Treatment group: Huoke granule Control group: penicillin and Xiaoer Zhike syrup Caprin was given for the children who suffering fever in both groups Outcomes: recovery, improvement, no improvement |
| Wang 2011a | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Wang 2011b | Did not meet outcome inclusion criteria, comparing 2 Chinese herbal medicines |
| Wu 2000 | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Wu 2010 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Xu 2001 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Xu 2011 | Did not meet outcome inclusion criteria, comparing 2 Chinese herbal medicines |
| Yang 2002 | The methods of randomisation allocation were incorrect |
| Yang 2003 | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Yang 2005 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Yang 2010 | Did not meet outcome inclusion criteria, comparing 2 Chinese herbal medicines |
| Yang 2011a | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Yang 2011b | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Yang 2011c | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Ye 2011 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Yin 2001 | Chinese medicinal herbs were administered in both intervention and control groups, which does not meet the intervention criteria |
| Yu 1998 | The method of randomisation allocation was incorrect |
| Yu 2003 | The method of randomisation allocation was incorrect |
| Yuan 2001 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Yuan 2002 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Zeng 2002 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Zeng 2005 | "Allocated the patients according to random number table method" was mentioned. However, we had the following doubts regarding allocation
Taixiang Wu telephoned Dr. Zeng, the trial author, to ask about the three questions above. Eventually, Dr. Zeng acknowledged that this was a case analysis, not an RCT. He did not develop a protocol before recruiting the participants |
| Zhai 2000 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Zhang 1989 | The methods of randomisation allocation were incorrect |
| Zhang 2001 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Zhang 2002 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Zhang 2005 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Zhang 2010 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Zhang 2011a | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Zhang 2011b | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Zhang 2011c | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Zhang 2011d | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Zhang 2011e | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Zhou 2003 | The trial was described as an RCT but was found not to be an RCT following a telephone interview with the trial authors |
| Zhou 2011 | Did not meet outcome inclusion criteria, comparing 2 Chinese herbal medicines |
Differences between protocol and review
The GRADE tool was used to assess the quality of the evidence.
Contributions of authors
In the previous version, Wei J (JW), Wu T (TW) and Ni J (JN) were responsible for the development of the review, searching for trials, quality assessment of the trials, telephone interviewing of the initial authors, data extraction, data analysis and review development and updating. Liu G (GL) was responsible for the quality assessment of trials, data extraction, and data analysis. Qiao J (JQ), Wei J (JW), Duan X (XD), Zhou L (LZ), Wang Q (QW), and Zheng J (JZ) searched for trials. Chen X (XC) contributed to the original search for trials. JL, KL and TW are responsible for updating this version of the review.
Sources of support
Internal sources
Chinese Cochrane Centre, West China Hospital of Sichuan University, China.
External sources
Chinese Medical Board of New York (CMB), USA.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
An 2003 {published data only}
- An J, Wang J, Zhang H, Wang Y, Wang Z. Clinical observation of 'Tan Re Qing' Injection for 68 cases of acute bronchitis. Zhong Guo Zhong Yi Ji Zheng 2003;12(4):320‐1. [Google Scholar]
Bian 2011 {published data only}
- Bian FP, Jia SQ. The treatment of acute bronchitis in 74 cases on children by ReduNing. China's Naturopathy 2011;19(8):22. [Google Scholar]
Chai 2011 {published data only}
- Chai LM. Clinical observation on treatment of bronchitis by Jia Jian Chinqindan soup in 30 cases. Hunan Journal of Traditional Chinese Medicine 2011;27(3):20‐1. [Google Scholar]
Chen 2000 {published data only}
- Chen S. 'Zhi Sou Tang' for 58 cases of acute bronchitis. Guang Xi Zhong Yi Xue Yuan Xue Bao 2000;17(2):28‐9. [Google Scholar]
Chen 2002 {published data only}
- Chen R, Pan C. Synergic effect of 'Shi Wei Long Dan Hua Ke Li' for child acute bronchitis. Zhong Guo Zhong Yi Yao Xing Xi Za Zhi 2002;9(9):92‐3. [Google Scholar]
Chen 2003 {published data only}
- Chen K. Clinical observation of 'Jia Wei San Ao Tang' for child acute bronchitis. Zhong Guo Zhong Yi Ji Zheng 2003;12(5):422. [Google Scholar]
Chen 2011a {published data only}
- Chen X. Clinical observation on the treatment of acute bronchitis by ReduNing. Chinese Journal of Misdiagnoses 2011;11(12):2867. [Google Scholar]
Chen 2011b {published data only}
- Chen DH, Lin CW, Yuan BC. Clinical efficiency observation on the treatment of acute bronchitis by Lu Hua An Gan Cao. Rural Medicine Platform 2011;15(4):332‐3. [Google Scholar]
Chu 2011 {published data only}
- Chu MZ, Jiang LZ. Ke Lin Mei Su combined with Rong Min Capsules in the treatment of acute bronchitis. Pharmacy and Clinic 2011;49(15):55‐6. [Google Scholar]
Cui 2011 {published data only}
- Cui JM, Hai GF, Fei BF. The treatment of acute bronchitis by Tanre Qing injection. Journal of Xinxiang Medical College 2011;28(4):516‐7. [Google Scholar]
Dong 2011 {published data only}
- Dong WW, Kong JF. Treatment of acute bronchitis by An Ning Pai. Journal of Qiqihar University of Medicine 2011;32(5):744. [Google Scholar]
Gao 2011 {published data only}
- Gao QF, Chen J, Long Y. Treatment of acute bronchitis by Fei Li Ke in children. Chinese Pediatrate Integrate Traditional Western Medicine 2011;3(1):65‐6. [Google Scholar]
Guan 2010 {published data only}
- Guan ZD. Clinical observation the treatment on child's acute bronchitis by Tan Re Qing. Aerospace Medicine 2010;21(1):68. [Google Scholar]
Han 2001 {published data only}
- Han W, Xu L, Yang C, Zhang J. Clinical research of 'Qin Dan Ke Chuai Kang' for acute bronchitis. Zhong Guo Zhong Yi Ji Zheng 2001;10(3):132‐3. [Google Scholar]
Hang 2011 {published data only}
- Huang Y, Wang XL. The treatment report of acute bronchitis by Western and Chinese medicine in 88 cases. Chinese Community Doctors 2011;13(10):195. [Google Scholar]
He 2011 {published data only}
- He PR, Liu YC. Clinical analysis on the treatment of child's acute bronchitis treated by Western and Chinese medicine. Medical Information 2011;7(1):3432‐3. [Google Scholar]
Li 2010 {published data only}
- Li J, Li M. The treatment of child's acute bronchitis by Xiao Yan Zhi Ke Jian. China's Naturopathy 2010;18(2):25. [Google Scholar]
Li 2011a {published data only}
- Li SS, Nie XJ, Chen HC. The treatment of acute bronchitis in 110 cases by Reduning injection. Guide of China Medicine 2011;9(17):292‐3. [Google Scholar]
Li 2011b {published data only}
- Li WJ, Lei Y, Mi HT, Li B, Hang ZW. Effective observation on the treatment of acute bronchitis by Qing Fei Tie Xue Wei Tie in children. Journal of New Chinese Medicine 2011;43(8):119‐20. [Google Scholar]
Liang 2001a {published data only}
- Liang W. Synergic effect of 'Huo Xue Hua Yu Fa' for childhood acute bronchitis. Guang Xi Zhong Yi Xue Yuan Xue Bao 2001;4(4):65‐6. [Google Scholar]
Liang 2011b {published data only}
- Liang M. The treatment of acute bronchitis in 41 cases by Yan Hu Ning. Chinese Community Doctors 2011;13(13):216‐7. [Google Scholar]
Lin 2000 {published data only}
- Lin W, Chai M, Song L. Chinese Medicinal herbs for 120 cases of child acute bronchitis. Zhong Yi Yao Xin Xi 2000;6:42. [Google Scholar]
Lin 2011 {published data only}
- Lin GQ, Yan PK, Luo QF, XIE JK. Efficiency of Kuoyu Dongganfen in the treatment of acute bronchitis. Guide to Chinese Medicine 2011;9(21):248‐50. [Google Scholar]
Liu 1998 {published data only}
- Liu M, Qi C, Kou H. Chinese Medicine compounded with Western medicine for 62 cases of acute bronchitis. Shan Xi Zhong Yi 1998;19(4):145‐6. [Google Scholar]
Liu 2010 {published data only}
- Liu CJ. Observation on the clinical curative effect of the Qin lian wen dan decoction for tracheobronchitis. Journal of Practical Traditional Chinese Medicine 2010;26(5):298‐9. [Google Scholar]
Liu 2011 {published data only}
- Liu LM. Clinical observation on the treatment of acute bronchitis by Tan Re Qing. Jilin Medicine 2011;32(2):375. [Google Scholar]
Luo 2005 {published data only}
- Luo FK, Wu YQ, Li YE. 'Yan Hu Ning' Injection for 128 cases of acute bronchitis. Guangxi Medical Journal 2005;27(6):922‐3. [Google Scholar]
Meng 1999 {published data only}
- Meng D, Lin S, Chen S. Clinical research of 'Shuang San Kou Fu Ye' treating acute bronchitis. Liao Ning Zhong Yi Za Zhi 1999;26(1):18. [Google Scholar]
Qing 2002 {published data only}
- Qing Z. Clinical observation of 'Dai Zhe Zhi Sou San Jia Wei' for acute bronchitis. Liao Ning Zhong Yi Za Zhi 2002;29(6):329. [Google Scholar]
Ren 2010 {published data only}
- Ren WM, Nie H, Wang ZX. Clinical observation on acute bronchitis by Xiao Yan Zhi Ke soup. Acta Chinese Medicine and Pharmacology 2010;38(3):123. [Google Scholar]
Shi 2010 {published data only}
- Shi JH, Xu HQ, Fang CY. The treatment of child's acute bronchitis by Yan Hu Ning. Journal of Emergency Traditional Chinese Medicine 2010;19(2):206‐7. [Google Scholar]
Song 2011 {published data only}
- Song ZB, Wang Y. Clinical research on acute bronchitis treated by Kang Dujun Xiao capsules. Chinese Traditional Medicine and Pharmacy 2011;18(9):104‐5. [Google Scholar]
Sun 2008 {published data only}
- Sun KH, Yang WW, Cheng JG, Jiang H, Liao Z, Zhang Y. A clinical study of Ji Zhi granule in the treatment of acute bronchitis, cough after cold, chronic bronchitis acute onset. Journal of Emergency Traditional Chinese Medicine 2008;17(1):26‐7. [Google Scholar]
Sun 2010 {published data only}
- Sun Y, Chu F, Cheng S, Guo L, Huang Z. The efficacy and safety of Fufangyuanzhi mixture for acute bronchitis. Chinese Medical Herald 2010;7(5):16‐7. [Google Scholar]
Tan 2002 {published data only}
- Tan Y, Tan Y. Clinical observation of 'Xian Bai Ju Hua' for 27 cases of child acute bronchitis. Zhong Guo Min Zu Min Jian Yi Yao Za Zhi 2002;55:82‐4. [Google Scholar]
Tian 2005 {published data only}
- Tian F, Shen H. Integrated traditional Chinese and Western medicine for 60 cases of acute bronchitis. Modern Journal of Integrated Traditional Chinese and Western Medicine 2005;14(13):1713‐4. [Google Scholar]
Wang 2001a {published data only}
- Wang K, Li G, Gao L, Tian L. Shi Wei Long Dan Hua Ke Li' for 55 cases of child acute bronchitis. Zhong Guo Zhong Yi Yao Xin Xi Za Zhi 2001;8(11):81. [Google Scholar]
Wang 2001b {published data only}
- Wang G, Liu C. Zhi Sou San Jia Jian' for 228 cases of acute bronchitis. Zhong Guo Shi Yan Fang Ji Xue Za Zhi 2001;7(5):56. [Google Scholar]
Wang 2002 {published data only}
- Wang X, Li J, Lin Z, Fan Y, Yang Z. Clinical research of 'Chuan Fu Ning' injection for acute bronchitis. Zhong Guo Zhong Yi Ji Zheng 2002;11(5):363‐4. [Google Scholar]
Wang 2005 {published data only}
- Wang LY, Li AY. Treatment of acute bronchitis by Huoke Granules in 200 children. Shanghai Zhong Yi Yao Za Zhi (Shanghai Journal of Traditional Chinese Medicine) 2005;39(1):30‐1. [Google Scholar]
Wang 2011a {published data only}
- Wang JF. Efficiency observation on the treatment of acute bronchitis by self‐made soup. Contemporary Medicine 2011;17(15):157‐8. [Google Scholar]
Wang 2011b {published data only}
- Wang J, Wang M. The treatment report of acute bronchitis by Western and Chinese medicine in 90 cases. Traditional Chinese Medicine 2011;8(8):90‐1. [Google Scholar]
Wu 2000 {published data only}
- Wu T, Wang J, Fan H. Clinical observation of 'Qing Re Xie Fei He Ji' for acute bronchitis. He Bei Zhong Yi 2000;22(3):167‐8. [Google Scholar]
Wu 2010 {published data only}
- Wu XF, Gao ZT. The treatment of acute bronchitis by Tanre Qing plus Toubao Paitong. Modern Journal of Integrated Traditional Chinese and Western Medicine 2010;19(4):448. [Google Scholar]
Xu 2001 {published data only}
- Xu B, Ying Y. 200 cases of 'Zi Ni San Ye Shuang Hua Tang' for treating childhood acute bronchitis. Zhe Jiang Zhong Yi Za Zhi 2001;36(8):347. [Google Scholar]
Xu 2011 {published data only}
- Xu ZY, Li JY, Zhang Y, Xun LQ. The treatment of acute bronchitis on children in 40 cases by Tan Re Qing. China Journal Modern Drug Application 2011;5(10):93‐4. [Google Scholar]
Yang 2002 {published data only}
- Yang L. Clinical observation of 'Yu Xing Cao' injection with penicillin for 200 cases of child acute bronchitis. An Hui Yi Yao 2002;6(4):28. [Google Scholar]
Yang 2003 {published data only}
- Yang W, Zhou S. Clinical analysis of 'Zeng Wei Zhi Sou Ke Li' for 32 cases of 'Feng Tan Nian Fei Zheng' in acute bronchitis. Zheng Guo Zhong Yi Yao Ke Ji 2003;10(1):53‐4. [Google Scholar]
Yang 2005 {published data only}
- Yang C, Lv Y. 'Ban Bian Lian' Compound for acute bronchitis in children. Journal of Practical Traditional Chinese Medicine 2005;21(3):136. [Google Scholar]
Yang 2010 {published data only}
- Yang MX. The treatment of acute bronchitis by Tanre Qing injection in 27 cases. Asia‐Pacific Traditional Medicine 2010;6(3):51‐2. [Google Scholar]
Yang 2011a {published data only}
- Yang QH. Clinical observation on childhood acute bronchitis by Tan Re Qing in 54 cases. Liaoning Medicine Journal 2011;25(1):14. [Google Scholar]
Yang 2011b {published data only}
- Yang GX, Yang JB, Wang HD. The treatment of acute bronchitis in 37 cases by Yan Hu Ning. Chinese Medicine Modern Distance Education of China 2011;9(2):49‐50. [Google Scholar]
Yang 2011c {published data only}
- Yang CG, Lu MY, Chen HX. Efficiency comparison of two kinds of medicine in the treatment of acute bronchitis in children. Guide to Chinese Medicine 2011;9(1):127‐8. [Google Scholar]
Ye 2011 {published data only}
- Ye ZY, Pan JH, Huang SY. Efficiency observation on child's acute bronchitis by Tianlong Cha He Qianjin Weijing soup. Journal of Emergency Traditional Chinese Medicine 2011;20(1):27‐8. [Google Scholar]
Yin 2001 {published data only}
- Yin X, Chang C, Xu G. Clinical research of Chinese Medicine compounded with Western medicine for acute bronchitis. Bin Zhou Yi Xue Yuan Xue Bao 2001;24(4):404. [Google Scholar]
Yu 1998 {published data only}
- Yu D, Huang W. Effective observation of integrated Chinese and Western medicine for acute bronchitis. Jiang Xi Zhong Yi Yao 1998;29(2):39. [Google Scholar]
Yu 2003 {published data only}
- Yu X, Qiao S, Wang K. Synergic effect of 'Fu Fang Gua Zi Jin Ke Li' for acute bronchitis. Shi Yong Zhong Xi Yi Jie He Lin Chuang 2003;3(5):32. [Google Scholar]
Yuan 2001 {published data only}
- Yuan B. Curative effect of 118 cases of 'Xiao'er Xiao Ji Zhi Ke Kou Fu Ye' treating child acute bronchitis. Jiang Su Zhong Yi 2001;22(12):16. [Google Scholar]
Yuan 2002 {published data only}
- Yuan Z. 'E Shu You' glucose injection for 42 cases of acute bronchitis. Xian Dai Yi Yao Wei Sheng 2002;18(11):1014. [Google Scholar]
Zeng 2002 {published data only}
- Zeng Y, Deng L. Clinical observation of 'Yu Xing Cao' atomization for child acute bronchitis. Jiang Xi Zhong Yi Yao 2002;33(6):20. [Google Scholar]
Zeng 2005 {published data only}
- Zeng YH. Treatment on 100 cases of acute tracheobronchitis by Weijing decoction combined with Sangxing decoction. Zhong Guo Shi Yong Zhong Xi Yi Za Zhi (Chinese Journal 0f the Practical Chinese with Modern Medicine) 2005;18(2):204‐5. [Google Scholar]
Zhai 2000 {published data only}
- Zhai Y. 'Zeng Xiao Zhi Ke He Ji' for 46 cases of acute bronchitis. Tian Jin Yao Xue 2000;12(1):37. [Google Scholar]
Zhang 1989 {published data only}
- Zhang X, Wang M, Liu Z, Yao A. 210 cases of 'Li Qi Huo Xue Fa' treating child acute bronchitis. Shan Dong Zhong Yi Xuan Yuan Xue Bao 1989;13(2):11‐3. [Google Scholar]
Zhang 2001 {published data only}
- Zhang S. Clinical observation of 'Shuang Huang Lian' with penicillin G for acute bronchitis. Gui Lin Yi Xue 2001;2:24. [Google Scholar]
Zhang 2002 {published data only}
- Zhang H, Yang W, Sun Z. 'Shi Wei Long Dan Hua Ke Li' for 120 cases of child acute bronchitis. Zhong Guo Zhong Yi Yao Xing Xi Za Zhi 2002;9(9):93. [Google Scholar]
Zhang 2005 {published data only}
- Zhang SR. 'Tan Re Qing' for 41 cases of acute bronchitis. Zhong Guo Zhong Yi Ji Zheng 2005;14(6):513. [Google Scholar]
Zhang 2010 {published data only}
- Zhang XH. Effective observation of the treatment of bronchitis in children by Jin Zhen liquid. Modern Journal of Integrated Traditional Chinese and Western Medicine 2010;19(17):2141‐2. [Google Scholar]
Zhang 2011a {published data only}
- Zhang PY, Li BJ. The treatment of acute bronchitis by Shegan Jugeng soup. Medical Information 2011;7(1):3142‐3. [Google Scholar]
Zhang 2011b {published data only}
- Zhang L. The treatment of acute bronchitis in children by Tan Re Qing. China Medical Herald 2011;8(13):76‐7. [Google Scholar]
Zhang 2011c {published data only}
- Zhang YG, Peng CQ. Efficiency observation on the treatment of child's acute bronchitis treated by Western and Chinese medicine. Journal of Hubei University of Chinese Medicine 2011;13(3):56‐7. [Google Scholar]
Zhang 2011d {published data only}
- Zhang W, Liu XQ. The treatment of acute bronchitis of 28 cases by traditional Chinese medicine. Journal of Emergency Traditional Chinese Medicine 2011;20(4):667. [Google Scholar]
Zhang 2011e {published data only}
- Zhang XJ, Zhang XW, Wang SQ. Clinical research on the effect of Qingfei Zhike Granule on children with acute bronchitis. Journal of Pediatrics of TCM 2011;7(1):24‐7. [Google Scholar]
Zhou 2003 {published data only}
- Zhou H, Chen W, Wang L. Synergic effect of 'Yu Xing Cao' Injection for 128 cases of child acute bronchitis. Lin Chuang Hui Cui 2003;18(10):585‐6. [Google Scholar]
Zhou 2011 {published data only}
- Zhou DZ. Treatment of acute bronchitis by Bao Ke Ning capsules in 60 children. China Pharmaceuticals 2011;20(12):77. [Google Scholar]
Additional references
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic reviews in health care: meta‐analysis in context. London: BMJ Books, 2001. [Google Scholar]
Dunlay 1984
- Dunlay J, Reinhardt R. Clinical features and treatment of acute bronchitis. The Journal of Family Practice 1984;18(5):719‐22. [PubMed] [Google Scholar]
Evans 1961
- Evans AS, Brobst M. Bronchitis, pneumonitis, and pneumonia in University of Wisconsin students. The New England Journal of Medicine 1961;265:401‐9. [DOI] [PubMed] [Google Scholar]
Evans 1967
- Evans AS, Allen V, Sueltmann S. Mycoplasma pneumoniae infections in University of Wisconsin students. The American Review of Respiratory Disease 1967;96:237‐44. [DOI] [PubMed] [Google Scholar]
Fahey 2004
- Fahey T, Smucny J, Becker L, Glazier R. Antibiotics for acute bronchitis. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD000245.pub2] [DOI] [PubMed] [Google Scholar]
Falck 1994
- Falck G, Heyman L, Gnarpe J, Gnarpe H. Chlamydia pneumoniae (TWAR): a common agent in acute bronchitis. Scandinavian Journal of Infectious Diseases 1994;26:179‐87. [DOI] [PubMed] [Google Scholar]
Hahn 1991
- Hahn DL, Dodge RW, Golubjatnikov R. Association of Chlamydia pneumoniae (strain TWAR) infection with wheezing, asthmatic bronchitis, and adult‐onset asthma. JAMA 1991;266:225‐30. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Huang 1990
- Huang Li, Ye Wenhua. Primary research for pharmacology of compound baikal skullcap root and the elements of it. China Journal of Chinese Materia Medica 1990;15(2):115. [PubMed] [Google Scholar]
Kirkwood 1982
- Kirkwood CR, Clure HR, Brodsky R, Gould GH, Knaak R, Metcalf M, et al. The diagnostic content of family practice: 50 most common diagnoses recorded in the WAMI community practices. Journal of Family Practice 1982;15(3):485‐92. [PubMed] [Google Scholar]
Marsland 1976
- Marsland DW, Wood M, Mayo F. Content of family practice. Part 1. Rank order of diagnoses by frequency. The Journal of Family Practice 1976;3(1):37‐68. [PubMed] [Google Scholar]
Meza 1994
- Meza RA, Bridges‐Webb C, Sayer GP, Miles DA, Traynor V, Neary S. The management of acute bronchitis in general practice: results from the Australian Morbidity and Treatment Survey, 1990‐1991. Australian Family Physician 1994;23:1550‐3. [PubMed] [Google Scholar]
Mogabgab 1968
- Mogabgab WJ. Mycoplasma pneumoniae and adenovirus respiratory illnesses in military and university personnel. The American Review of Respiratory Disease 1968;97:345‐58. [DOI] [PubMed] [Google Scholar]
Oeffinger 1997
- Oeffinger KC, Snell LM, Foster BM, Panico KG, Archer RK. Diagnosis of acute bronchitis in adults: a national survey of family physicians. Journal of Family Practice 1997;42:402‐9. [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Slusarcick 2000
- Slusarcick AL, McCaig LF. National hospital ambulatory medical care survey: 1998 outpatient department summary. Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics 2000. [(PHS) 2000‐1250/0‐0520]
William 1998
- William J. Hueston, Arch G. Mainous III. Acute Bronchitis. http://www.aafp.org/afp/980315ap/hueston.html 1998.
Wu 2007
- Wu TX, Li YP, Bian ZX, Li TQ, Li J, Dagenais S, et al. Consolidated standards for reporting trials of traditional Chinese medicine (CONSORT for TCM). Chinese Journal of Evidence‐Base Medicine 2007;7(8):601‐5. [Google Scholar]
Yu 1986
- Yu Z, Su Z. Research of Perilla and the Recent Progress. Aboard Medicine: Volume of Pharmacology 1986;3:173. [Google Scholar]
Zhu 1976
- Zhu Y. Pharmacology and Application of Traditional Chinese Medicine. Beijing: People's Health Publishing Company, 1976. [Google Scholar]
References to other published versions of this review
Wu 2005
- Wu T, Chen X, Duan X, Juan N, Liu G, Qiao J, et al. Chinese medicinal herbs for acute bronchitis. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004560.pub3] [DOI] [PubMed] [Google Scholar]
Wu 2008
- Wei J, Ni J, Wu T, Chen X, Duan X, Liu G, et al. Chinese medicinal herbs for acute bronchitis. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004560.pub3] [DOI] [PubMed] [Google Scholar]


