Abstract
Aim:
Phenylethanolamine N-methyltransferase (PNMT) catalyzes the conversion of sympathetic neurotransmitter norepinephrine to epinephrine. We examined the association of PNMT polymorphisms with acute and chronic pain in sickle cell disease (SCD).
Methods:
Utilization of emergency care owing to painful crisis was used as a marker for acute pain in 131 patients with SCD.
Results:
rs876493 A allele, rs2934965 T allele and rs2941523 G allele were significantly associated with decreased utilization (p ≤ 0.05). rs876493 A allele showed association with utilization in females (p = 0.003), not males (p = 0.803). rs2934965 T allele and rs2941523 G allele were predicted to cause loss of putative transcription factor binding sites. This is the first report of the association of PNMT polymorphisms with acute crisis pain in SCD. Together with our previous findings in catechol-o-methyltransferase, polymorphisms in catecholamine metabolizing enzymes appear to primarily influence acute pain in SCD.
Keywords: : acute crisis pain, chronic pain, PNMT, sickle cell disease, SNP
Sickle cell disease (SCD) is a fatal blood disorder caused by the inheritance of mutations in the gene coding for the hemoglobin β-chain subunit [1]. The more severe mutation (HbS) favors the formation of extended polymeric tendrils of hemoglobin in the deoxygenated state precipitating the characteristic sickle shape red blood cells (RBCs). Short-lived, with decreased oxygen carrying capacity, the sickled RBCs often undergo intravascular hemolysis due to their rigidity and instability [2,3].
Consequently, sickled RBCs cluster thereby restricting blood flow and imparting endothelial dysfunction [1,4,5]. Poor gas exchange provokes an ischemic-like condition, commonly referred to as vaso-occlusive crisis (VOC), characterized by severe acute pain and target organ damage [1,6,7]. Acute pain presents itself during VOC and its etiology has been extensively studied; however, chronic pain in SCD is little understood. One study estimated that a third of all adult SCD patients experience chronic noncrisis pain almost daily [8]. While another study found that 80% of adult outpatients with SCD reported to be experiencing a constant pain pattern and >90% of them selected neuropathic pain descriptors to describe their pain quality [9]. A more recent study reported that on an average >50% of SCD patients aged 20 or older experience chronic pain [10]. Although the exact mechanisms are unknown, a number of working models have been proposed, including a state of heightened sensitivity secondary to exhaustive pain signaling, elevations in reactive oxygen species [6,7], peripheral nerve damage and central nerve sensitization attributed to the manifestation of allodynia and hyperalgesia [11,12]. While both opioid and non-opioid-based drugs are employed to manage acute and chronic sickle cell pain, the wide variability in the severity, frequency and pattern of the pain phenotypes makes pain management a challenge [13,14].
Phenylethanolamine N-methyltransferase (PNMT) is the enzyme responsible for the methylation of norepinephrine into epinephrine in the adrenal medulla [15–19]. Norepinephrine is a known endogenous pain modulator with predominant inhibitory effects [6]. On the other hand, epinephrine can sensitize nociceptive neurons resulting in hyperalgesia [20–22]. Moreover, studies in murine models have shown that epinephrine can mediate vaso-occlusion by increasing sickle RBC adhesion to the endothelium [23]. Thus, we hypothesized that genetic variants of the PNMT gene that alter its expression or activity may contribute to the heterogeneity of sickle cell pain by modulating levels of norepinephrine and epinephrine in the body.
The PNMT gene is composed of three exons interspaced by two intronic regions, and preceded by a promoter region [24,25]. Studies have shown that PNMT expression is regulated by a number of transcription factors located at the promoter region [26–28] and polymorphisms of PNMT have been implicated in altered transcriptional activity [16,25]. To our knowledge, polymorphisms of the PNMT gene have not been previously studied in the context of pain in SCD. In this study, we studied polymorphisms of the PNMT gene and their associations with acute and chronic pain in SCD.
Methods
Subject recruitment
Subjects were recruited during their scheduled visits at the University of Illinois Hospital and Health Sciences System in Chicago, Illinois. During these visits, blood or buccal swabs were collected as samples for subsequent DNA extraction and genotyping. Written informed consent was obtained from each subject and the study was approved by the University of Illinois at Chicago’s Institutional Review Board.
DNA extraction & genotyping
DNA extraction technique varied depending on the sample type. For buccal swabs, a modified phenol/chloroform protocol was used to isolate DNA [29]. Blood samples were either subjected to a modified salting-out procedure [30] or processed using the QuickGene-mini80 isolation device (AutoGen, MA, USA). Post DNA extraction, purified samples were genotyped utilizing the MassARRAY iPLEX Platform (Sequenom, CA, USA). Single-nucleotide polymorphisms (SNPs) included in the study had >95% genotyping call rate and were in Hardy–Weinberg equilibrium as determined by a χ2 goodness-of-fit test (p > 0.05).
Pain assessment
The marker for baseline chronic pain was the composite pain index (CPI) score. Subjects rated their noncrisis chronic pain symptoms on a computerized reporting tool that employs the McGill Pain Questionnaire, as described in previous studies [31,32]. These inputs were weighted and aggregated to obtain CPI scores.
The marker for acute crisis pain was acute care utilization, that is, number of visits to the emergency department or acute care center prompted by crisis pain. A history of each subject’s utilization was enumerated over a period of 12 months from their baseline chronic pain assessment. Data were collected via medical records at the emergency department, University of Illinois Health System and phone calls to enrolled subjects.
While the McGill Pain Questionnaire is a well-established method of pain scoring and has been validated in sickle cell cohorts [32], it is important to note that all pain-assessment methods and tools are limited by the inherent subjective quality of pain as a symptom.
Statistics & bioinformatics
Statistical analysis was performed using R v3.4.0 (R Foundation for Statistical Computing, Vienna, Austria). For association analysis, additive, dominant, and recessive genetic models were utilized [33,34]. The major allele served as the reference allele and the minor allele as the risk allele in all models. In the additive model, effect of each minor allele is determined. The dominant model estimates the effect of carrying one or both minor alleles, and the recessive model estimates the effect of being homozygous with the minor alleles. CPI scores were modeled using multiple linear regression, and utilization scores were modeled using negative binomial regression. Models were adjusted for covariates: age, sex and sickle cell type. Sex-specific analysis was performed in only the dominant model owing to limited sample size in each sub-category. Linkage disequilibrium plots were generated using the software, Haploview v4.2 (Broad Institute, MA, USA). Identified haplotypes were analyzed for association with CPI and utilization groups. The analysis of utilization groups involved comparison of subjects without utilization versus those with utilization and also between subjects with low (zero to three events) utilization versus those with high (four or more events) utilization. Bioinformatics tools SNPinfo:FuncPred (https://snpinfo.niehs.nih.gov/snpinfo/snpfunc.html) and PROMO (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3) were used to predict if SNPs were in or near putative transcription factor binding sites (TFBS) and potential functional roles [35–37]. Input sequences for PROMO were obtained from the dbSNP database.
Results
Blood samples collected from a cohort of African-American subjects were used to genotype four SNPs on the PNMT gene. A summary of the baseline characteristics of the subjects is shown in Table 1. In total, 131 subjects were included of whom 77.9% were homozygous with sickle hemoglobin. The average subject age was 34 years (34.3 ± 11.8) with females accounting for 66% of the study population. As a group, subjects of this cohort had an average utilization number of 4.5 ± 5.3 with the numbers ranging from 0 to 38; and an average CPI of 40.6 with a range of 14.8–86.5 on a scale of 0–100, highlighting the wide variability in pain phenotypes, both acute and chronic pain.
Table 1. . Demographic characteristics (n = 131).
| Demographics | Values | Phenotype | Values |
|---|---|---|---|
| Age (years) | Mean ± SD: 34.3 ± 11.8 | Composite Pain Index (0–100) | Mean ± SD: 40.6 ± 13.4 |
| Range: 15–70 | Range: 14.8–86.5 | ||
| Sex, n (%) | Male: 45 (34.4) | Utilization score | Mean ± SD: 4.5 ± 5.3 |
| Female: 86 (65.6) | Range: 0–38 | ||
| Sickle cell type† n (%) | SCD-SS: 102 (77.9) | Utilization groups n (%) | Zero (0): 19 (14.5) |
| SCD-SC: 15 (11.5) | Low (1–3): 56 (42.7) | ||
| Other: 14 (10.7) | High (≥4): 56 (42.7) |
Sickle cell types: SCD-SS, SCD-SC, others include SCD-Sβ+ and SCD-Sβ°.
SCD: Sickle cell disease; SCD-Sβ°: Sickle cell disease-sickle β° thalassemia; SCD-Sβ+: Sickle cell disease-sickle β+ thalassemia; SCD-SC: Sickle cell disease-sickle hemoglobin C; SCD-SS: Sickle cell disease-homozygous hemoglobin S; SD: Standard deviation.
The major and minor allele relationships for the PNMT polymorphisms as well as their genotype frequencies within the recruited population are shown in Table 2. None of the four SNPs showed significant deviation from Hardy–Weinberg equilibrium. Additive, dominant and recessive genotype models were applied to discern the minor allele’s pattern of penetrance, if any, for each of the four PNMT polymorphisms. Analyses with CPI scores did not yield any significant associations (Table 3). In the analysis of utilization scores, three of the four SNPs (rs2934965, rs876493, rs2941523) achieved a statistically significant reduction in the utilization rate in at least one genetic model (Table 4). The T allele of rs2934965[G>T] exhibited a significant reduction in utilization rate in the additive model (incidence risk ratio = 0.61; p = 0.044). The allele A of rs876493[G>A] was found to be significantly associated with a reduction in the utilization rate in both the additive and dominant models (additive: B = 0.68; p = 0.012; dominant: B = 0.67; p = 0.030). The allele G of rs2941523[C>G] achieved statistically significant reduction in utilization rate in both the additive and recessive models (additive: B = 0.68; p = 0.033; recessive: B = 0.12; p = 0.017). In contrast, the synonymous variant rs5638 was not found to be associated with a significant change in utilization rate.
Table 2. . Phenylethanolamine N-methyltransferase polymorphism genotypes and allele frequencies.
| dbSNP ID | Location | Allele | N (%) | Genotype | N (%) |
|---|---|---|---|---|---|
| rs5638 | GRCh38 chr17: 39669996 | A | 217 (84.1) | AA | 89 (69.0) |
| G | 41 (15.9) | AG | 39 (30.2) | ||
| GG | 1 (0.8) | ||||
| rs2934965 | GRCh38 chr17: 39667402 | G | 241 (92.0) | GG | 111 (84.7) |
| T | 21 (8.0) | GT | 19 (14.5) | ||
| TT | 1 (0.8) | ||||
| rs876493 | GRCh38 chr17: 39668292 | G | 173 (69.2) | GG | 57 (45.6) |
| A | 77 (30.8) | GA | 59 (47.2) | ||
| AA | 9 (7.2) | ||||
| rs2941523 | GRCh38 chr17: 39667508 | C | 222 (84.7) | CC | 94 (71.8) |
| G | 40 (15.3) | CG | 34 (26.0) | ||
| GG | 3 (2.3) |
Table 3. . Association analyses of Composite Pain Index with phenylethanolamine N-methyltransferase polymorphisms.
| Single-nucleotide polymorphism | Model | B† (97.5% CI) | p-value |
|---|---|---|---|
| Additive (AA vs AG vs GG) | 1.51 (-3.42–6.43) | 0.546 | |
| rs5638 | Dominant (AA vs AG + GG) | 2.12 (-3.00–7.24) | 0.413 |
| Recessive (AA + AG vs GG) | -13.76 (-40.75–13.23) | 0.315 | |
| Additive (GG vs GT vs TT) | 0.36 (-5.73–6.44) | 0.908 | |
| rs2934965 | Dominant (GG vs GT + TT) | 0.21 (-6.33–6.76) | 0.949 |
| Recessive (GG + GT vs TT) | 3.44 (-23.72–30.59) | 0.803 | |
| Additive (GG vs GA vs AA) | -1.75 (-5.65–2.15) | 0.376 | |
| rs876493 | Dominant (GG vs GA + AA) | -2.64 (-7.44–2.15) | 0.278 |
| Recessive (GG + GA vs AA) | -0.06 (-9.45–9.34) | 0.991 | |
| Additive (CC vs CG vs GG) | -0.14 (-4.76–4.48) | 0.952 | |
| rs2941523 | Dominant (CC vs CG + GG) | 0.95 (-4.26–6.16) | 0.719 |
| Recessive (CC + CG vs GG) | -10.37 (-26.07–5.33) | 0.194 |
Unstandardized regression coefficient.
Regression models are adjusted for covariates (age, sex and sickle cell type).
Table 4. . Association analyses of utilization scores with phenylethanolamine N-methyltransferase polymorphisms.
| Single-nucleotide polymorphism | Model | Incidence risk ratio (97.5% CI) | p-value |
|---|---|---|---|
| Additive (AA vs AG vs GG) | 0.96 (0.66–1.41) | 0.839 | |
| rs5638 | Dominant (AA vs AG + GG) | 0.98 (0.67–1.46) | 0.930 |
| Recessive (AA + AG vs GG) | 0.33 (0.01–5.97) | 0.412 | |
| Additive (GG vs GT vs TT) | 0.61 (0.38–1.01) | 0.044* | |
| rs2934965 | Dominant (GG vs GT + TT) | 0.63 (0.38–1.06) | 0.073 |
| Recessive (GG + GT vs TT) | 0.15 (0.01–2.72) | 0.164 | |
| Additive (GG vs GA vs AA) | 0.68 (0.51–0.93) | 0.012* | |
| rs876493 | Dominant (GG vs GA + AA) | 0.67 (0.47–0.97) | 0.030* |
| Recessive (GG + GA vs AA) | 0.51 (0.24–1.15) | 0.088 | |
| Additive (CC vs CG vs GG) | 0.68 (0.47–0.99) | 0.033* | |
| rs2941523 | Dominant (CC vs CG + GG) | 0.73 (0.49–1.09) | 0.115 |
| Recessive (CC + CG vs GG) | 0.12 (0.02–0.65) | 0.017* |
Regression models are adjusted for covariates (age, sex and sickle cell type).
*p-values < 0.05.
Strikingly, sex-specific analyses of utilization showed statistically significant associations in females with the rs876493 allele but not in males. Females had a 44% reduction in utilization when carrying either one or two risk alleles – GA or AA genotypes (p = 0.003, Table 5). Sex-specific analysis did not yield any significant associations for the other three SNPs in the dominant model, in agreement with our findings from the overall analysis.
Table 5. . Gender-based association analyses of utilization scores in the dominant model.
| Single-nucleotide polymorphism | Sex | Total subjects | IRR (97.5% CI) | p-value |
|---|---|---|---|---|
| rs5638 | M | 45 | 1.1 (0.49–2.47) | 0.819 |
| F | 84 | 0.74 (0.48–1.15) | 0.171 | |
| rs2934965 | M | 45 | 0.51 (0.19–1.51) | 0.187 |
| F | 86 | 0.58 (0.33–1.04) | 0.060 | |
| rs876493 | M | 43 | 1.1 (0.53–2.28) | 0.803 |
| F | 82 | 0.56 (0.38–0.82) | 0.003* | |
| rs2941523 | M | 45 | 0.62 (0.24–1.76) | 0.285 |
| F | 86 | 0.76 (0.50–1.17) | 0.203 |
Regression models are adjusted for covariates (age, sex, and sickle cell type).
*p-values < 0.05.
F: Female; IRR: Incidence risk ratio; M: Male.
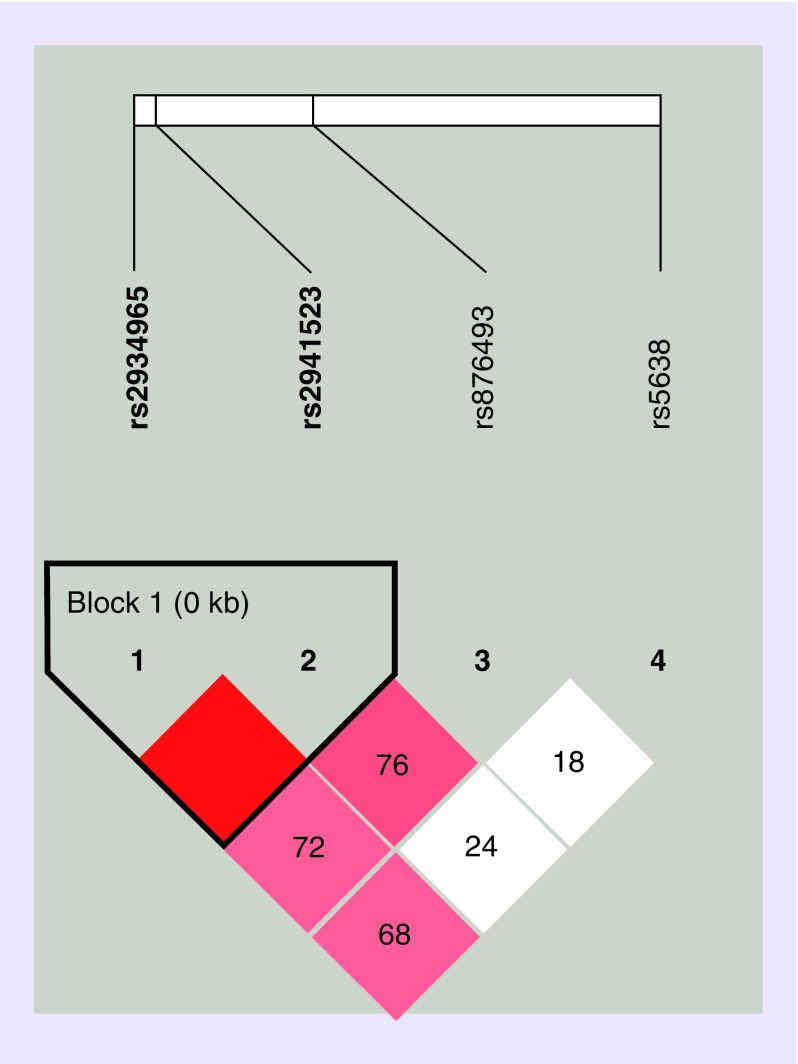
In the assessment of linkage disequilibrium, we found rs2934965 and rs2941523 exhibit strong linkage, forming a haploblock (Figure 1). Since the two SNPs individually exhibited associations with utilization, the haplotype of the two SNPs was further analyzed with respect to CPI as well as utilization groups, including 1) subjects with utilization versus those without utilization, and 2) low (up to three events) versus high (four or more events) utilization groups. Upon haplotype analysis, no association was found to reach statistical significance for either CPI or the utilization categories (Table 6).
Figure 1. . Linkage disequilibrium plot of four phenylethanolamine N-methyltransferase single-nucleotide polymorphisms.
Haplotype block organization of four PNMT single-nucleotide polymorphisms generated from Haploview 4.2 using the standard color scheme with D′ values showing the linkage disequilibrium coefficient. Red = high D′ and high LOD, white = low D′ and low LOD; shades of pink/red = low D′ and high LOD.
LOD: Logarithm of the odds.
Table 6. . Haplotype analysis of haploblock rs2934965[G>T]- rs2941523[C>G].
| Haplotype | Frequency (%) | 0 versus 1 or more utilization | 0–3 versus 4 or more utilization | CPI | |||
|---|---|---|---|---|---|---|---|
| Odds ratio | p-value | Odds ratio | p-value | B† | p-value | ||
| GG | 7 | 0.79 | 0.748 | 0.71 | 0.528 | -0.73 | 0.827 |
| TG | 8 | 0.45 | 0.170 | 0.46 | 0.126 | -0.30 | 0.922 |
Unstandardized regression coefficient.
Reference haplotype: GC (frequency = 85%).
Regression models are adjusted for covariates (age, sex and sickle cell type).
CPI: Composite pain index.
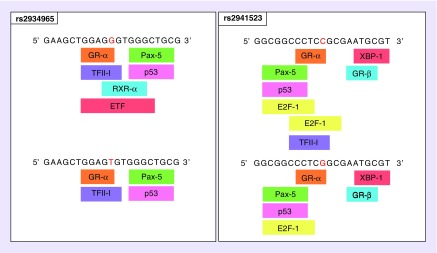
Using bioinformatics tool SNPinfo:FuncPred we found that rs2934965, rs876493 and rs2941523 are located at potential transcription factor binding sites (TFBS). We further explored the effect of nucleotide change on TFBS using PROMO (Figure 2). We found that for rs2934965 a change from G to T (variant minor allele) led to loss of putative binding sites retinoid X receptor α (RXR-α) and TEA domain family member 2 (ETF). For rs2941523 it was predicted that a change from C to G (variant minor allele) led to loss of putative binding site TFII–I (transcription factor II–I). The specific input sequence of rs876493, which was investigated, did not yield any change in TFBS upon nucleotide variation.
Figure 2. . Functional predictions for rs2934965 and rs2941523.
Changes in the transcription factor binding sites of rs2934965[G>T] and rs2941523[C>G] as predicted by PROMO. Nucleotide variations in the input sequence are indicated by red letter.
Discussion
In this study we studied the association of SNPs of PNMT with acute and chronic pain phenotypes in SCD. Production of epinephrine functions in part to maintain hemodynamic stability, though maladaptation and dysregulation secondary to polymorphisms are found in the literature [38–41].
Enhanced PNMT activity has been proposed to aggravate ischemic penumbras, thus precipitating painful episodes of VOC [6]. While epinephrine contributes to hyperalgesia [22], norepinephrine suppresses pain centrally [42]. Moreover, epinephrine can induce endothelial adhesion of sickle RBCs, consequently triggering VOC [23]. Since decreased transcription of PNMT would translate to reduced levels of epinephrine, it may be expected that polymorphisms reducing PNMT activity or expression would result in fewer or less severe incidences of VOC. As a consequence, such polymorphisms might favor lower acute pain or utilizations due to painful crisis. Correspondingly, our findings show that SCD subjects bearing minor alleles of rs876493, rs2934965 and rs2941523 in their genome experience acute pain crises less frequently. The lack of association of all four SNPs with chronic pain in SCD, despite their significant association with acute crisis pain, is not surprising given the different pathophysiology of the pain phenotypes. We have previously reported similar findings for the Val158Met polymorphism in COMT, another catecholamine-metabolizing enzyme [43]. Here it was found that while the Met allele showed significant association with acute crisis pain, chronic pain scores were not significantly different between the two alleles.
Of the three PNMT SNPs that showed significant associations with acute crisis pain, rs2934965 and rs2941523 lie within 2000 bases upstream of the 5′ end and rs876493 is located in the 5′UTR [16,44]. PNMT variants have also been studied in neurological disorders among non-Hispanic Caucasians. In Alzheimer’s disease (AD) the G allele of rs876493 significantly associated with early onset of AD [45]. In contrast to most reports of the A allele being protective, Mann et al. found that the AA genotype of the variant was in fact more prevalent in subjects with multiple sclerosis [46]. Our study found that the A variant of this SNP (rs876493) associated with lesser episodes of acute pain.
PNMT expression is highly regulated by transcription factors (TFs) and response elements in the promoter region [47,48]. Some of the TFs known to proximally regulate PNMT transcription include egr-1, Sp1, AP-2, glucocorticoid receptor and hypoxia-inducible factor-α [38,47–49]. Rodriguez-Flores et al. explored transcription factors binding at rs876493 SNP site and reported that the variant allele A of the SNP allowed for SOX17 binding and had an inhibitory effect on transcription [25]. Our finding that the A allele of rs876493 associated with decreased acute pain or utilization due to painful crisis is in agreement with this functional effect of the SNP.
It has been demonstrated that different haplotypes of SNPs in the gene’s 5′ flanking region and promoter region have significantly different transcriptional activity [16,25]. Our analyses of the SNP regions showed that not only does rs876493 lie in putative TFBS but rs2934965 and rs2941523 are also in or near a number of TFBS. In fact, our findings indicate that the minor allele of the rs2934965 (T allele) and rs2941523 (G allele) can cause potential loss of TFBS.
Interestingly, when we analyzed male and female subjects separately, the A allele of rs876493 conferred pain protection only in females. Sex is known to influence baseline PNMT activity by the cyclic and cascading effects of sex hormones [50,51]. But while this relationship is solidified among the literature, we find that studies have not been conducted for evaluating the role of sex in polymorphisms of the PNMT gene.
In our assessment of linkage disequilibrium for the four SNPs involved, we identified strong pair-wise linkage between rs2934965 and rs2941523. Analysis of this novel haploblock, however, did not reveal any significantly associated haplotypes. While most published studies evaluating PNMT polymorphisms have been performed in Caucasian or Asian populations, our study examined these SNPs in African-American subjects. One study looking at the role of PNMT SNPs in essential hypertension compared allelic frequencies between African-Americans and European-Americans, and found that the A allele of the variant rs876493 was more prevalent among whites [39]. Furthermore, they observed that rs876493 was in linkage disequilibrium with rs3764351 in both the African-American and European-American cohort. However, there was no significant association of the haplotypes with hypertension. On the contrary, this 2-SNP haplotype A-A was found to significantly associate with decreased risk of essential hypertension in a Han Chinese population [40]. A couple of other studies addressing Alzheimer’s disease and reward dependence have also reported the rs876493–rs3764351 linkage but failed to find significant associations with the haplotypes [45,52].
Conclusion
In summary, this is the first study reporting the association of PNMT polymorphisms with pain in SCD. We identified that allele A of SNP rs876493 in the 5′UTR of PNMT, previously implicated to alter transcriptional activity of the PNMT gene, significantly associated with reduced incidence of acute pain crises in SCD subjects. Similarly, 2kb upstream variants rs2934965 T allele and rs2941523 G allele also associated with decreased incidence of acute pain crises. Associations with chronic pain were, however, not significant. Bioinformatic analyses revealed that three (rs2934965, rs876493 and rs2941523) of the four SNPs were in or near putative TFBS. It was predicted that the variant minor alleles T of rs2934965 and G of rs2941523 may lead to loss of some of these TFBS. This warrants the need to perform further functional evaluation of these two understudied SNPs. Moreover, we found that while female patients carrying GA or AA genotype of rs876493 associated with significantly lower utilization scores compared with those who carried the GG genotype, the same was not true for males. We also found strong linkage disequilibrium between SNPs rs2934965 and rs2941523, though haplotypic associations with acute or chronic pain did not reach significance.
Additional studies with larger patient cohorts are needed to confirm these findings. Future studies should also account for the effect of medications such as hydroxyurea or glutamine or arginine supplementation on crisis frequency. While a number of subjects at our center participated in the hydroxyurea study, we currently do not have comprehensive medication prescription/use information for all of our study participants. Nonetheless, our findings indicate a possible role of genetic variants of PNMT on acute pain variability in SCD, thereby contributing to the growing evidence on the function of PNMT and epinephrine in the pathophysiology of this complex disease. Along with our previous findings in COMT, this study also indicates that polymorphisms in catecholamine-metabolizing enzymes may primarily influence acute pain in SCD.
Summary points.
Phenylethanolamine N-methyltransferase polymorphisms rs876493 A allele, rs2934965 T allele and rs2941523 G allele significantly associated with decreased utilization.
rs876493 A allele associated with significantly lower utilization only in female patients, not in males.
rs2934965, rs876493 and rs2941523 lie in or near putative transcription factor binding sites.
Variant alleles T of rs2934965 and G of rs2941523 may lead to loss of putative transcription factor binding sites.
Associations with chronic pain phenotype of sickle cell disease were not significant.
rs2934965 and rs2941523 were in strong linkage disequilibrium but haplotypic associations were not significant with either phenotype.
This is the first evidence of association of phenylethanolamine N-methyltransferase polymorphisms with acute crisis pain in sickle cell disease.
Footnotes
Financial & competing interests disclosure
This study was supported in part by grants from the Illinois Department of Public Health (IDPH) and the National Heart, Lung, and Blood Institute (NHLBI) (R01 HL124945 and R35 HL140031). EH Jhun was supported by a predoctoral fellowship (T32 DE018381) from National Institute of Dental and Craniofacial Research (NIDCR). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the IDPH, NIH, NHLBI, NIDCR or Veteran’s Administration. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study was conducted as approved by the University of Illinois at Chicago institutional review board and informed consent was obtained from all participants involved.
References
- 1.Alexy T, Sangkatumvong S, Connes P. et al. Sickle cell disease: selected aspects of pathophysiology. Clin. Hemorheol. Microcirc. 44(3), 155–166 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fertrin KY, Costa FF. Genomic polymorphisms in sickle cell disease: implications for clinical diversity and treatment. Expert Rev. Hematol. 3(4), 443–458 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Telen MJ, Malik P, Vercellotti GM. Therapeutic strategies for sickle cell disease: towards a multi-agent approach. Nat. Rev. Drug Discov. 18(2), 139–158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trinh-Trang-Tan MM, Vilela-Lamego C, Picot J, Wautier MP, Cartron JP. Intercellular adhesion molecule-4 and CD36 are implicated in the abnormal adhesiveness of sickle cell SAD mouse erythrocytes to endothelium. Haematologica 95(5), 730–737 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 376(9757), 2018–2031 (2010). [DOI] [PubMed] [Google Scholar]
- 6.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood 120(18), 3647–3656 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Tran H, Gupta M, Gupta K. Targeting novel mechanisms of pain in sickle cell disease. Blood 130(22), 2377–2385 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith WR, Penberthy LT, Bovbjerg VE. et al. Daily assessment of pain in adults with sickle cell disease. Ann. Intern. Med. 148(2), 94–101 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Wilkie DJ, Molokie R, Boyd-Seal D. et al. Patient-reported outcomes: descriptors of nociceptive and neuropathic pain and barriers to effective pain management in adult outpatients with sickle cell disease. J. Natl Med. Assoc. 102(1), 18–27 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pope M, Albo C, Kidwell KM. et al. Evolution of chronic pain in sickle cell disease. Blood 128(22), 1297 (2016). [Google Scholar]
- 11.Campbell CM, Moscou-Jackson G, Carroll CP. et al. An evaluation of central sensitization in patients with sickle cell disease. J. Pain 17(5), 617–627 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He Y, Wilkie DJ, Nazari J. et al. PKCdelta-targeted interventionrelieves chronic pain in a murine sickle cell disease model.. J Clin Invest 126(8), 3053–3057 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballas SK. Pain management of sickle cell disease. Hematol. Oncol. Clin. North Am. 19(5), 785–802 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Brandow AM, Zappia KJ, Stucky CL. Sickle cell disease: a natural model of acute and chronic pain. Pain 158(Suppl. 1), S79–S84 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Axelrod J. Purification and properties of phenylethanolamine-N-methyl transferase. J. Biol. Chem. 237, 1657–1660 (1962). [PubMed] [Google Scholar]
- 16.Ji Y, Salavaggione OE, Wang L. et al. Human phenylethanolamine N-methyltransferase pharmacogenomics: gene re-sequencing and functional genomics. J. Neurochem. 95(6), 1766–1776 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Ji Y, Snyder EM, Fridley BL. et al. Human phenylethanolamine N-methyltransferase genetic polymorphisms and exercise-induced epinephrine release. Physiol. Genomics 33(3), 323–332 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Martin JL, Begun J, McLeish MJ, Caine JM, Grunewald GL. Getting the adrenaline going: crystal structure of the adrenaline-synthesizing enzyme PNMT. Structure 9(10), 977–985 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Toth M, Ziegler M, Sun P, Gresack J, Risbrough V. Impaired conditioned fear response and startle reactivity in epinephrine-deficient mice. Behav. Pharmacol. 24(1), 1–9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khasar SG, McCarter G, Levine JD. Epinephrine produces a β-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J. Neurophysiol. 81(3), 1104–1112 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Wang ZJ, Wilkie DJ, Molokie R. Neurobiological mechanisms of pain in sickle cell disease. Hematology Am. Soc. Hematol. Educ. Program 2010, 403–408 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen X, Levine JD. Epinephrine-induced excitation and sensitization of rat C-fiber nociceptors. J. Pain 6(7), 439–446 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Zennadi R, Moeller BJ, Whalen EJ. et al. Epinephrine-induced activation of LW-mediated sickle cell adhesion and vaso-occlusion in vivo. Blood 110(7), 2708–2717 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziegler MG, Bao X, Kennedy BP, Joyner A, Enns R. Location, development, control, and function of extraadrenal phenylethanolamine N-methyltransferase. Ann. N. Y. Acad. Sci. 971, 76–82 (2002). [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez-Flores JL, Zhang K, Kang SW. et al. Conserved regulatory motifs at phenylethanolamine N-methyltransferase (PNMT) are disrupted by common functional genetic variation: an integrated computational/experimental approach. Mamm. Genome 21(3–4), 195–204 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen P, Khurana S, Peltsch H. et al. Prenatal glucocorticoid exposure programs adrenal PNMT expression and adult hypertension. J. Endocrinol. 227(2), 117–127 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Jiang W, Uht R, Bohn MC. Regulation of phenylethanolamine N-methyltransferase (PNMT) mRNA in the rat adrenal medulla by corticosterone. Int. J. Dev. Neurosci. 7(5), 513–520 (1989). [DOI] [PubMed] [Google Scholar]
- 28.Wong DL, Tai TC, Wong-Faull DC, Claycomb R, Kvetnansky R. Genetic mechanisms for adrenergic control during stress. Ann. N. Y. Acad. Sci. 1018, 387–397 (2004). [DOI] [PubMed] [Google Scholar]
- 29.Vandenbergh DJ, Anthony K, Whitfield KE. Optimizing DNA yield from buccal swabs in the elderly: attempts to promote buccal cell growth in culture. Am. J. Hum. Biol. 15(5), 637–642 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16(3), 1215 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilkie DJ, Molokie RE, Suarez ML, Ezenwa MO, Wang ZJ. Composite Pain Index: reliability, validity, and sensitivity of a patient-reported outcome for research. Pain Med. 16(7), 1341–1348 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jha A, Suarez ML, Ferrans CE, Molokie R, Kim YO, Wilkie DJ. Cognitive testing of PAINReportIt in adult African Americans with sickle cell disease. Comput. Inform. Nurs. 28(3), 141–150 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadhu N, Jhun EH, Yao Y. et al. Single nucleotide polymorphisms of GCH1 associates with sickle cell disease pain in African Americans. Experimental Hematology 66, 42–49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jhun EH, Sadhu N, Hu X. et al. Beta2-adrenergic receptor polymorphisms and haplotypes associate with chronic pain in sickle cell disease. Frontiers in Pharmacology 10, 84 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Z, Taylor JA. SNPinfo: integrating GWAS and candidate gene information into functional SNP selection for genetic association studies. Nucleic Acids Res. 37, W600–W605 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farré D, Roset R, Huerta M. et al. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 31(13), 3651–3653 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Messeguer X, Escudero R, Farré D, Núñez O, Martínez J, Albà MM. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics 18(2), 333–334 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Grandbois J, Khurana S, Graff K, Nguyen P, Meltz L, Tai TC. Phenylethanolamine N-methyltransferase gene expression in adrenergic neurons of spontaneously hypertensive rats. Neurosci. Lett. 635, 103–110 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Cui J, Zhou X, Chazaro I. et al. Association of polymorphisms in the promoter region of the PNMT gene with essential hypertension in African Americans but not in whites. Am. J. Hypertens. 16(10), 859–863 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Huang C, Zhang S, Hu K, Ma Q, Yang T. Phenylethanolamine N-methyltransferase gene promoter haplotypes and risk of essential hypertension. Am. J. Hypertens. 24(11), 1222–1226 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Jacob HJ, Lindpaintner K, Lincoln SE. et al. Genetic mapping of a gene causing hypertension in the stroke-prone spontaneously hypertensive rat. Cell 67(1), 213–224 (1991). [DOI] [PubMed] [Google Scholar]
- 42.Pertovaara A. Noradrenergic pain modulation. Prog. Neurobiol. 80(2), 53–83 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Jhun E, He Y, Yao Y, Molokie RE, Wilkie DJ, Wang ZJ. Dopamine D3 receptor Ser9Gly and catechol-o-methyltransferase Val158Met polymorphisms and acute pain in sickle cell disease. Anesth. Analg. 119(5), 1201–1207 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kepp K, Juhanson P, Kozich V, Ots M, Viigimaa M, Laan M. Resequencing PNMT in European hypertensive and normotensive individuals: no common susceptibilily variants for hypertension and purifying selection on intron 1. BMC Med. Genet. 8, 47 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mann MB, Wu S, Rostamkhani M, Tourtellotte W, MacMurray J, Comings DE. Phenylethanolamine N-methyltransferase (PNMT) gene and early-onset Alzheimer disease. Am. J. Med. Genet. 105(4), 312–316 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Mann MB, Wu S, Rostamkhani M, Tourtellotte W, MacMurray JP, Comings DE. Association between the phenylethanolamine N-methyltransferase gene and multiple sclerosis. J. Neuroimmunol. 124(1-2), 101–105 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Khurana S, Peng S, McDonald E, Yates W, Venkataraman K, Tai TC. Phenylethanolamine N-methyltransferase gene expression in PC12 cells exposed to intermittent hypoxia. Neurosci. Lett. 666, 169–174 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Wong DL, Tai TC, Wong-Faull DC. et al. Stress and adrenergic function: HIF1α, a potential regulatory switch. Cell. Mol. Neurobiol. 30(8), 1451–1457 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tai TC, Wong-Faull DC, Claycomb R, Wong DL. Hypoxia and adrenergic function: molecular mechanisms related to Egr-1 and Sp1 activation. Brain Res. 1353, 14–27 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Fernandez-Ruiz JJ, Bukhari AR, Hernandez ML, Alemany J, Ramos JA. Sex- and age-related changes in catecholamine metabolism and release of rat adrenal gland. Neurobiol. Aging 10(4), 331–335 (1989). [DOI] [PubMed] [Google Scholar]
- 51.Casimiri V, Cohen WR, Parvez S, Hobel C, Parvez H. Phenylethanolamine-N-methyl transferase and catechol-O-methyl transferase activity in rat uterus. Cyclic and steroid-induced changes. Acta Obstet. Gynecol. Scand. 72(8), 606–610 (1993). [DOI] [PubMed] [Google Scholar]
- 52.Yamano E, Isowa T, Nakano Y. et al. Association study between reward dependence temperament and a polymorphism in the phenylethanolamine N-methyltransferase gene in a Japanese female population. Compr. Psychiatry 49(5), 503–507 (2008). [DOI] [PubMed] [Google Scholar]