Abstract
Pulmonary sarcomatoid carcinoma (PSC) is a group of five rare non-small cell lung cancer subtypes. In the present study, the clinical characteristics and outcomes of patients with PSC registered in the Surveillance, Epidemiology and End Results (SEER) database were investigated. For this purpose, data for patients with PSC (n=1,723) who received their initial diagnosis between 1988 and 2016 were collected from the SEER database. Survival analysis was performed using the Kaplan-Meier curves and the log-rank test. Subsequently, multivariate analyses with the Cox proportional hazards model were used to identify significant independent predictors. A nomogram model was established to predict survival performance using the concordance index (C-index). From the total cohort, patients with pulmonary blastoma demonstrated improved 1-year overall survival (OS) rate compared with other pathological types (P<0.001). The 2-year overall survival rates of the ‘only radiotherapy’ cohort and the ‘no specific treatment’ cohort were 9.1 and 5.4% (P<0.001), respectively. Radiotherapy significantly improved the OS rate in stage I–III patients with PSC (P<0.001) when stratified by stage. After matching the propensity scores, the ‘surgery combined with radiotherapy’ group comprised 156 patients and the ‘surgery-only’ group had 247 patients (1:1.6). However, no significant differences in prognosis were found between the 2 subgroups (P=0.052). The multivariate Cox analysis demonstrated that older age (≥76 years old), male, unmarried, pathological type, larger tumor size (≥56 mm), later tumor node metastasis stages and treatment modalities were independent prognostic factors. A nomogram model was established to predict the survival of patients with PSC. This model incorporated the seven aforementioned independent prognostic factors (C-index for survival, 0.75; 95% confidence interval, 0.74–0.76). Radiotherapy needs to considered for stage I–III patients with PSC who undergo radiation therapy without surgical resection.
Keywords: radiotherapy, pulmonary sarcomatoid carcinoma, surveillance, epidemiology and end results database, treatment, surgery
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a rare type of non-small cell lung cancer (NSCLC) composed of poorly differentiated cells and sarcoma or sarcomatoid components (spindle and/or giant cells) (1). PSC accounts for 0.3–1.0% of all lung cancer cases (2,3). Systematic reports of PSC are uncommon due to the inherent rarity of the disease. Therefore, decisions regarding treatment strategies are based on clinicopathological descriptions of small case series or single case reports (4,5).
Previously, Yendamuri et al (2) identified 1,921 patients with PSC from the Surveillance, Epidemiology and End Results (SEER) database (1973–2008). Another study conducted by Steuer et al (6) identified 7,965 patients with PSC from the National Cancer Database (1998–2011). The aforementioned studies confirmed that PSC is a relatively rare histological type of lung cancer with lower overall survival (OS) rates compared with other NSCLC types. Complete resection is an essential treatment in the absence of metastasis in patients with PSC. However, the role of adjuvant chemotherapy remains unclear (7,8). To the best of our knowledge, few studies (6,9) have focused on the effect of radiotherapy on the survival of patients with PSC. An unfavorable prognosis is anticipated due to the pathological features of poorly differentiated tumors. The different pathological types of PSC are also poorly studied.
To date, the PSC treatment strategy that is selected depends on the disease stage (10). Thus, similar to other types of NSCLC, clinicians need to pay attention to early-stage resectable PSC. Clinicians must achieve a rapid preoperative diagnosis and minimize the delay before surgery to avoid tumor progression. Radical surgical resection (11) is recommended for operable patients and also facilitates accurate diagnosis. Patients with PSC significantly benefit from cancer-directed surgery (12,13).
In the present study, five specific types of sarcomatoid carcinoma were used to address the aforementioned unresolved questions using the World Health Organization (WHO) classification system (2015). The five specific types of sarcomatoid carcinoma studied were pure spindle cell, pure giant cell, carcinosarcoma, pulmonary blastoma and pleomorphic, which includes tumors with spindle and giant cells (14). The Surveillance, Epidemiology, and End Results (SEER) program is supported by the National Cancer Institute. The program collects a variety of details, including cancer incidence and survival, from 18 population-based cancer registries throughout the USA, covering 27.8% of the USA population (15). A retrospective analysis of patients with PSC registered in the SEER database between 1988 and 2016 was performed to determine the prognostic impact of clinicopathological characteristics and treatment modalities.
Materials and methods
Patients
All patients with histologically confirmed PSC between 1988 and 2016 were extracted from the SEER database (https://seer.cancer.gov/) using SEER*Stat 8.3.5 software (https://seer.cancer.gov/seerstat/). The age range of the patients was 18–94 years (mean age, 65.9 years), and there was a slightly higher percentage of males (59.9%; n=1,032) compared with females (40.1%; n=691). Patients with primary PSC (n=1,723) as their only primary cancer were included. The inclusion criteria were: i) Pathologically confirmed PSC between 1988 and 2016; and ii) the site recode ICD-O-3/WHO 2008 definition (International Classification of Diseases for Oncology, Third Edition) (16) was ‘Lung and Bronchus’. The exclusion criteria included: i) Multiple primary tumors; ii) no prognostic data; and iii) lack of clinicopathological data.
The demographic features and clinicopathological characteristics of these patients were collected and are presented in Table I. Of the 1,723 patients, 776 underwent surgery. Of the 776 patients, 264 had stage I, 145 had stage II, 211 had stage III, 44 had stage IV PSC and 112 had no data regarding stage. The OS time was the measured primary outcome and was defined as the interval from diagnosis to death from any cause or the last follow-up in the SEER database. Tumor-Node-Metastasis (TNM) staging was performed according to the American Joint Committee on Cancer (AJCC) 3rd Edition (1988–2003) (https://seer.cancer.gov/seerstat/variables/seer/ajcc-stage/3rd.html), 6th Edition (2004–2009) and 7th Edition (2010–2016) (https://cancerstaging.org/references-tools/deskreferences/Pages/default.aspx). Several variables, such as age at diagnosis, sex, laterality, primary site, pathological type, differentiation, TNM and the use of surgery and radiotherapy were collected.
Table I.
Demographic and clinical characteristics patients with pulmonary sarcomatoid carcinoma.
| Demographic/clinical characteristic | Cases, n (%) |
|---|---|
| Age at diagnosis, years | |
| <76 | 1,309 (75.97) |
| ≥76 | 414 (24.03) |
| Sex | |
| Male | 1,032 (59.90) |
| Female | 691 (40.10) |
| Ethnicity | |
| White | 1,375 (79.80) |
| Black | 239 (13.87) |
| Others | 109 (6.33) |
| Marital status | |
| Married | 777 (45.10) |
| Unmarried | 946 (54.90) |
| Laterality | |
| Left-origin of primary | 739 (42.89) |
| Right-origin of primary | 964 (55.95) |
| Bilateral, single primary | 20 (1.16) |
| Primary site | |
| Main bronchus | 53 (3.08) |
| Upper lobe | 972 (56.41) |
| Middle lobe | 89 (5.17) |
| Lower lobe | 469 (27.22) |
| Overlapping lesion | 42 (2.44) |
| Lung, NOS | 98 (5.69) |
| Pathological Type | |
| Giant cell carcinoma | 535 (31.05) |
| Spindle cell carcinoma | 445 (25.83) |
| Pleomorphic carcinoma | 403 (23.39) |
| Carcinosarcoma | 288 (16.72) |
| Pulmonary blastoma | 52 (3.02) |
| Differentiation, grade | |
| I | 20 (1.16) |
| II | 14 (0.81) |
| III | 627 (36.39) |
| IV | 332 (19.27) |
| ND | 730 (42.37) |
| Tumor size, mm | |
| <56 | 923 (53.57) |
| ≥56 | 800 (46.43) |
| TNM | |
| I | 318 (18.46) |
| II | 175 (10.16) |
| III | 427 (24.78) |
| IV | 665 (38.60) |
| ND | 138 (8.01) |
| Surgery | |
| No | 940 (54.56) |
| Yes | 776 (45.04) |
| ND | 7 (0.41) |
| Radiation | |
| No | 1,038 (60.24) |
| Yes | 685 (39.76) |
| Chemotherapy | |
| No | 1,080 (62.68) |
| Yes | 643 (37.32) |
ND, not determined; TNM, Tumor-Node-Metastasis; NOS, not otherwise specified.
Statistical analysis
The optimal cut-off value of age and tumor size for OS rate was determined using X-tile (Yale School of Medicine) software (version 3.6.1). The Kaplan-Meier method was used to generate survival curves. Differences in OS rate stratified by each covariate were analyzed by the log-rank test. Univariate and multivariate cox proportional hazard models were used to assess the associations between each covariate and OS rate. The results were presented as hazard ratios and 95% confidence intervals (CIs). Propensity matching was performed in R v.3.5.2 (https://www.r-project.org/) using the nearest neighbor matching and a caliper width of 0.02. A nomogram was developed and modeled using the rms package of R software (version 5.1–4; http://cran.r-project.org/web/packages/rms/index.html). Statistical analysis was conducted using SPSS 20 (IBM Corp.). P<0.05 (two sided) was considered to indicate a statistically significant difference.
Results
Baseline characteristics of patients with PSC
A total of 3,897 patients initially diagnosed with PSC between 1975 and 2016 were identified in the SEER database. Of these, 1,723 eligible patients were included in the study. The study workflow and selection process are presented in Fig. 1. Patient demographic and clinicopathological characteristics are presented in Table I.
Figure 1.
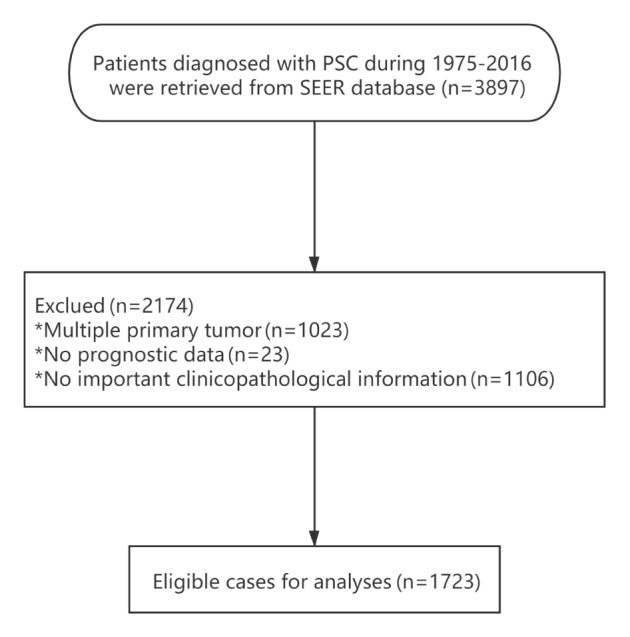
Flow diagram of the selection process of patients with PSC included in the study. PSC, pulmonary sarcomatoid carcinoma; SEER, Surveillance, Epidemiology and End Results.
Survival analyses
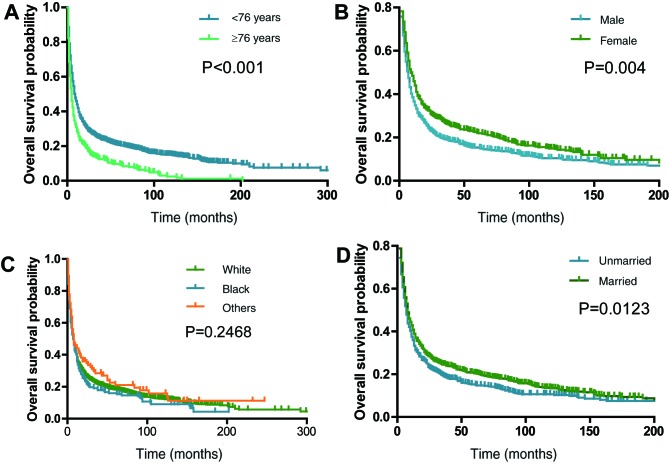
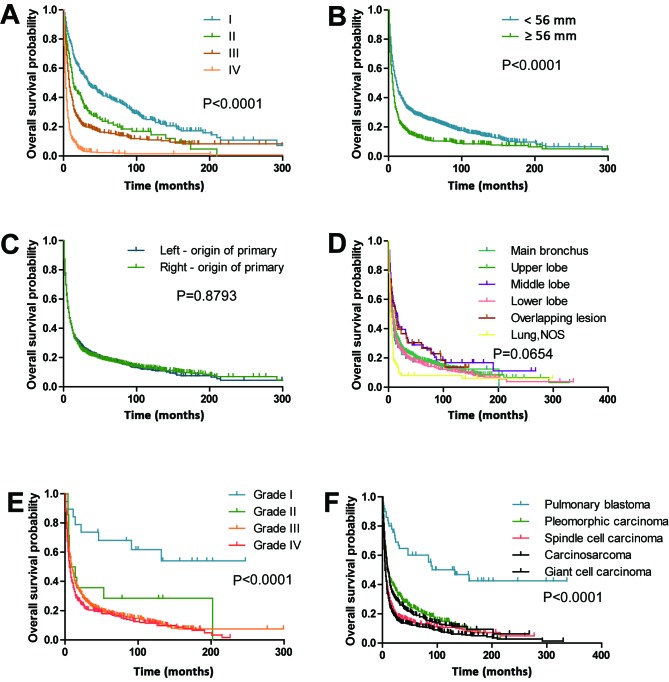
The median survival time was 8 months (range, 0–337 months). The 1-, 2-, 3- and 5-year survival rates were 38.6, 26.3, 22.1 and 18.1%, respectively. The optimal cut-off values of age and tumor size at diagnosis were 76.0 years and 56 mm, respectively, as set by X-tile analysis. The OS rates of patients with clinicopathological and demographic characteristics, such as male (P=0.004; Fig. 2B), aged ≥76 years (P<0.0001; Fig. 2A), unmarried (P=0.0123; Fig. 2D), poor tumor-differentiated grade (P<0.0001; Fig. 3E) and large tumor size ≥56 mm (P<0.0001; Fig. 3B), were significantly worse compared with other patients. The analyses stratified by stage demonstrated that stage I patients with PSC demonstrated improved OS outcomes compared with stage II–IV patients (P<0.0001; Fig. 3A). The 5-year OS rates for patients with PSC were as follows: Stage I, 40.3; stage II, 23.8; stage III, 16.0 and stage IV, 2.3%. Patients with pulmonary blastoma demonstrated improved OS rates compared with other pathological types (P<0.0001; Fig. 3F). Other factors, including laterality (Fig. 3C), ethnicity (Fig. 2C) and primary site (Fig. 3D), did not significantly affect OS rate.
Figure 2.
Kaplan-Meier survival curves demonstrating the effects of demographic covariates on overall survival rate of patients with pulmonary sarcomatoid carcinoma. Overall survival rate of patients based on (A) age at diagnosis, (B) sex, (C) ethnicity and (D) marital status.
Figure 3.
Effects of clinicopathological covariates on the overall survival rate of patients with pulmonary sarcomatoid carcinoma. Kaplan-Meier curves of (A) stage, (B) tumor size, (C) laterality, (D) primary site, (E) differentiation and (F) pathological type. NOS, not otherwise specified.
Surgery and radiotherapy effects on OS of patients with PSC
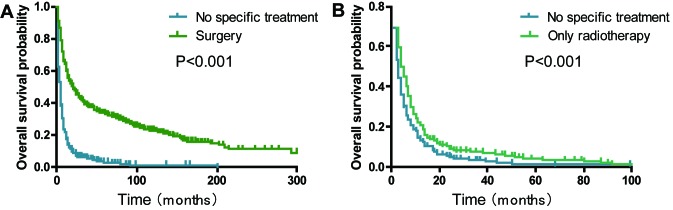
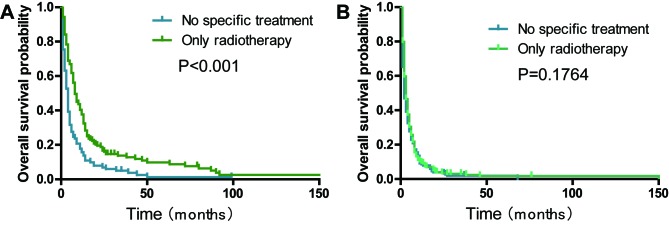
Patients with a history of surgical treatment at the primary site exhibited a significantly improved OS compared with those that received no specific treatment (median survival, 21.0 vs. 4.0 months; P<0.001; Fig. 4A). The 2-year OS rate of the ‘only radiotherapy’ group was significantly higher compared with the ‘no specific treatment’ group (9.1 vs. 5.4%; P<0.001; Fig. 4B). The analyses stratified by stage demonstrated that radiotherapy significantly improved the OS rate in stage I–III patients with PSC without surgical resection (P<0.001; Fig. 5A) and stage IV patients with PSC without surgical resection (P=0.1764, Fig. 5B). Subgroup analyses for AJCC 3rd, 6th and 7th groups demonstrated that similar trends still existed (all P<0.05; Fig. S1).
Figure 4.
Kaplan-Meier survival curves comparing the effects of surgery and radiotherapy on the overall survival rate of patients with pulmonary sarcomatoid carcinoma. (A) Surgery and (B) only radiotherapy.
Figure 5.
Effects of only radiotherapy stratified by stage on the overall survival rate of patients with pulmonary sarcomatoid carcinoma. Kaplan-Meier curves of patients with (A) stage I–III and (B) stage IV pulmonary sarcomatoid carcinoma.
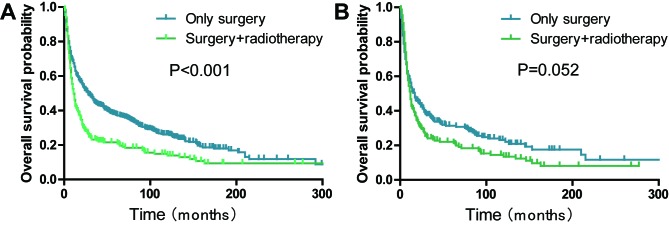
Furthermore, the patients that received only surgery demonstrated improved progress compared with those who received surgery combined with radiotherapy (P<0.001; Fig. 6A). After matching the propensity scores, the ‘surgery plus radiotherapy group’ consisted of 156 and ‘only surgery group’ of 247 patients with PSC (1:2). However, no significant differences in the prognosis were found between the two subgroups (P=0.052; Fig. 6B).
Figure 6.
Kaplan-Meier survival curves demonstrating the effects of surgery and radiotherapy on the overall survival rate of patients with pulmonary sarcomatoid carcinoma. (A) Before PSM and (B) after PSM. PSM, propensity score matching.
Independent prognostic risk factors that affect OS of patients with PSC
In the present study, univariate and multivariate Cox proportional hazard analyses of the variables potentially influencing OS of patients with PSC were investigated. Univariate analysis revealed that older age, male, unmarried, giant cell carcinoma, worse pathological grade, larger tumor size, later TNM stages and no special treatments were associated with a worse OS rate (all P<0.05; Table II). Using multivariate analysis, all factors except for differentiation (P=0.190, Table II) were still identified as independent risk factors of prognosis for patients with PSC.
Table II.
Univariate and multivariate Cox proportional hazard analyses of the clinical characteristics for overall survival of patients with pulmonary sarcomatoid carcinoma.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age at diagnosis, years | ||||||
| <76 | 1 (Ref.) | – | 1 (Ref.) | – | ||
| ≥76 | 1.531 | 1.351–1.735 | <0.001 | 1.440 | 1.265–1.639 | <0.001 |
| Sex | ||||||
| Male | 1 (Ref.) | – | 1 (Ref.) | – | ||
| Female | 0.821 | 0.734–0.919 | 0.001 | 0.863 | 0.768–0.968 | 0.012 |
| Marital status | ||||||
| Unmarried | 1 (Ref.) | – | 1 (Ref.) | – | ||
| Married | 0.850 | 0.762–0.949 | 0.004 | 0.869 | 0.776–0.974 | 0.016 |
| Pathological Type | ||||||
| Pulmonary blastoma | 1 (Ref.) | – | 1 (Ref.) | – | ||
| Pleomorphic carcinoma | 2.317 | 1.451–3.699 | <0.001 | 2.043 | 1.269–3.291 | 0.003 |
| Spindle cell carcinoma | 3.315 | 2.083–5.275 | <0.001 | 2.210 | 1.373–3.557 | 0.001 |
| Carcinosarcoma | 2.823 | 1.752–4.547 | <0.001 | 2.634 | 1.623–4.276 | <0.001 |
| Giant cell carcinoma | 3.638 | 2.291–5.777 | <0.001 | 2.704 | 1.684–4.341 | <0.001 |
| Differentiation | 0.190 | |||||
| I | 1 (Ref.) | – | ||||
| II | 2.776 | 1.127–6.836 | 0.026 | |||
| III | 3.038 | 1.569–5.884 | 0.001 | |||
| IV | 3.621 | 1.861–7.046 | <0.001 | |||
| Unknown | 3.785 | 1.956–7.324 | <0.001 | |||
| Tumor size, mm | ||||||
| <56 | 1 (Ref.) | – | 1 (Ref) | – | ||
| ≥56 | 1.574 | 1.409–1.757 | <0.001 | 1.292 | 1.152–1.449 | <0.001 |
| TNM | ||||||
| I | 1 (Ref.) | – | 1 (Ref.) | – | ||
| II | 1.416 | 1.137–1.764 | 0.002 | 1.335 | 1.067–1.670 | 0.011 |
| III | 2.044 | 1.729–2.417 | <0.001 | 1.593 | 1.320–1.923 | <0.001 |
| IV | 4.874 | 4.140–5.739 | <0.001 | 2.947 | 2.413–3.600 | <0.001 |
| Treatment modality | ||||||
| Only Surgery | 0.203 | 0.174–0.237 | <0.001 | 0.407 | 0.336–0.494 | <0.001 |
| Surgery plus RT | 0.292 | 0.242–0.353 | <0.001 | 0.406 | 0.332–0.497 | <0.001 |
| Only RT | 0.644 | 0.558–0.743 | <0.001 | 0.672 | 0.580–0.778 | <0.001 |
| No special treatment | 1 (Ref.) | – | 1 (Ref.) | – | ||
CI, confidence interval; HR, hazard ratio; TNM, Tumor-Node-Metastasis; RT, radiotherapy; Ref., reference.
Predicting OS of patients with PSC using a nonogram model
Age and tumor size were stratified using X-tile software. This revealed that the optimal cut-off points for age for predicting progression were 63 and 79 years old. The optimal cut-off points for tumor size for predicting progression were 50 and 75 mm. Subsequently, patients with PSC were divided into three groups according to these optimal cut-off values. These groups included the following: i) Young group (<63 years); ii) middle aged group (63-79 years); and iii) old group (>79 years). In addition patients were grouped according to tumor size: i) <50 mm; ii) 50–75 mm; and iii) >75 mm. Significant differences were observed between the Kaplan-Meier curves for OS among the three age and tumor size groups (all P<0.05; data not shown).
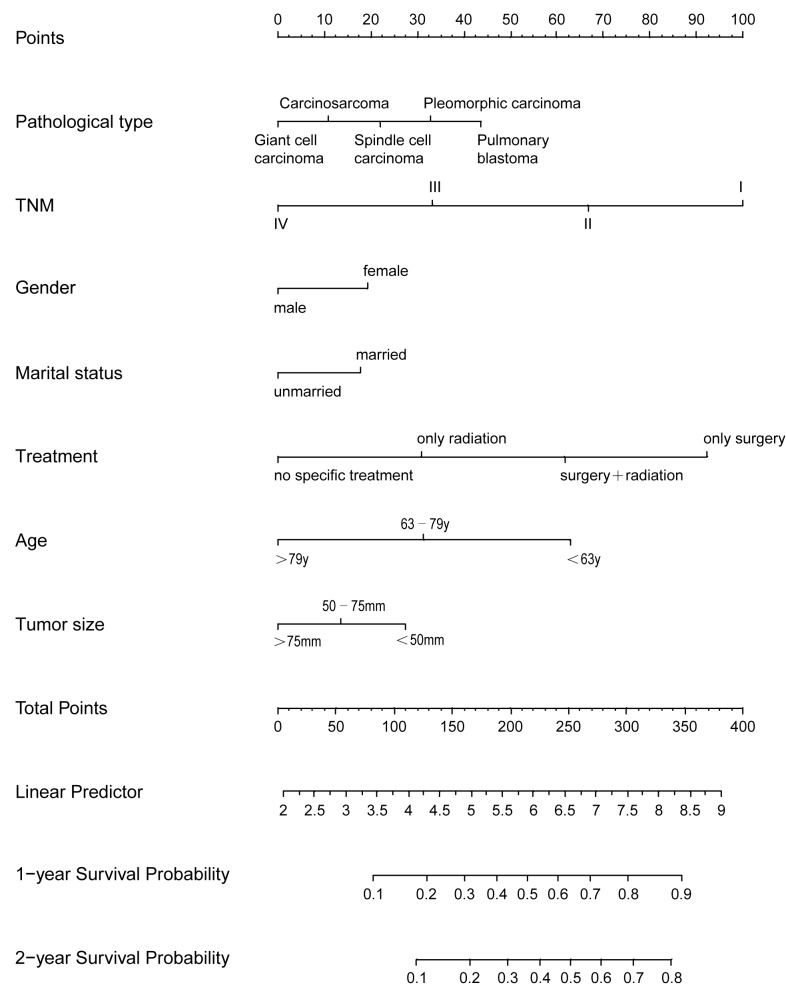
Next, a nomogram model was established to predict the survival of patients with PSC. This model incorporated all the independent prognostic factors found using multivariate analysis (Fig. 7). In the model, a high score was associated with good prognosis. TNM, treatment modality and age contributed the most to prognosis, followed by pathological type and tumor size. The C-index of the nomogram model for OS prediction was 0.75 (95% CI, 0.74–0.76).
Figure 7.
Nomogram model for predicting 1- and 2-year overall survival rates of patients with pulmonary sarcomatoid carcinoma. y, years; TNM, tumor-node-metastasis.
Discussion
The present study summarized the clinical characteristics of patients with PSC and identified several survival factors using data from the SEER database from 1975–2016. In the present study, the median survival of patients with PSC was 8 months, and the 1-year survival rate was 38.6%. The 2-, 3-, and 5-year survival rates were 26.3, 22.1 and 18.1%, respectively. Compared with previous studies (6,17,18), the present study demonstrated that the survival rate was slightly low with a median survival range of 11–19 months, a 1-year survival rate of 32–71% and a 5-year survival rate of 17–29%. This outcome may be attributable to patients enrolled in previous studies that had surgical resection (8,12,13,17). One of these studies (13) also had a high proportion of patients with early-stage PSC.
Morphologically, PSC is generally characterized by a high degree of variability and heterogeneity of the tumor (19,20). The present study also revealed that the most common histological subtype was giant cell carcinoma, followed by spindle cell carcinoma, pleomorphic carcinoma and carcinosarcoma. Previous reports demonstrated that pleomorphic carcinoma was a common histological subtype (1,3,21,22). The WHO 2015 lung cancer classification (14) reported that the least common subtype of PSC is pulmonary blastoma (23), which is consistent with the findings of the present study. Previous studies have identified that pulmonary blastoma counts accounts for 3.02% of all PSC cases (3,6). Most types of sarcomatoid carcinoma are poorly differentiated, pleomorphic-like carcinoma (7,24), spindle cell carcinoma (25), giant cell carcinoma (26) and carcinosarcoma (21,25). Pulmonary blastoma is a subtype of a well-differentiated biphasic tumor and mainly composed of a primitive epithelial component (21). The present results demonstrated that 51.8% of pulmonary blastoma cases were well differentiated or moderately differentiated. Poorly differentiated or undifferentiated occurred in a majority of other types of PSC (93.6–99.7%). The present study also demonstrated that patients with pulmonary blastoma exhibited improved survival, with a 60.2% 5-year OS rate compared with 12.2–23.7% for the other types of sarcamatoid carcinoma. Therefore, pulmonary blastoma is a well-differentiated malignancy and has a favorable survival outcome.
In the present study, 776 of the 1,723 patients underwent surgery. Of the 776 patients who underwent surgery, 67.2% had stage I–II PSC. Meanwhile, of the 776 patients who underwent surgery, 255 (32.8%) had stage III/IV PSC. PSC has been reported to be associated with high rates of recurrence and low OS rates even after complete surgical resection (6,11,17). Aforementioned studies (13,27) also investigated the effects of neoadjuvant chemotherapy and radiotherapy on the OS rate of patients with PSC. In patients with high-risk stages IB, II and IIIA NSCLC, platinum-based combination regimens are the mainstream treatment strategies (28). A beneficial effect of chemotherapy on the OS rate of patients with PSC can be difficult to detect due to the low incidence of this disease (3). Furthermore, to the best of our knowledge, studies investigating the effects of chemotherapy on the OS rate of patients with PSC are uncommon. The present study considered multimodal treatment; however, could not assess the potential survival impact of chemotherapy among patients with PSC due to the lack of specific information regarding chemotherapy in the SEER database.
The role of radiotherapy in patients with PSC is poorly defined (29). Radiotherapy has been used alone and in combination with chemotherapy in patients with unresectable disease or in an adjuvant setting for those with high rates of recurrence (30). Clinically, radiotherapy is always reserved for patients with rare types of lung cancer (31,32). To date, few studies have focused on the value of radiotherapy in PSC. Several prospective randomized studies have failed to demonstrate advantages in delivering radiotherapy in patients with PSC (24). In the present study, radiotherapy has an important impact on the survival of patients with PSC stratified by stage, particularly stages I–III. However, patients with PSC who had undergone surgery combined with radiotherapy had a worse OS compared with those who only received surgery. Propensity score matching was also used to reduce bias in the present study. After matching the propensity scores, the prognostic analysis did not significantly differ between the two groups. Chaft et al (3) conducted a prospective study on patients with PSC and demonstrated patients that underwent surgery plus adjuvant therapy did not have a statistically significant survival benefit compared with those who received surgery alone. This finding is similar to the results obtained by the current study.
The present study had several limitations. First, the study was conducted in a retrospective manner, which always leads to a selection bias. Second, patients with pulmonary blastoma were few in number in the SEER database, which may be a strong confounding factor affecting the result of the survival analysis. Third, specific information, such as chemotherapy sequence with surgery, dose, and agent of chemotherapy are lacking in the SEER database. Therefore, the present study could not account for the effect of chemotherapy on PSC prognosis. Fourth, a number of other factors may influence the results of the present study, such as radiation regimen and the technological infrastructure of the equipment.
In conclusion, PSC is a type of poorly differentiated NSCLC composed of spindle or giant cells. Surgery is often the first choice of treatment for patients with PSC. Furthermore, radiotherapy can influence the long-term outcome in stage I–III patients with PSC who only underwent radiation therapy without surgical resection. The present population cohort study was conducted to stratify prognoses of patients with PSC despite incidence being low. Age, tumor size, TNM and pathological type were independent risk factors for the prognosis of patients with PSC. Female, married and treatment modality were independent protective factors for patients with PSC.
In summary, for patients with stage I–III who are unsuitable for surgery, radiotherapy has been demonstrated to provide a better prognosis compared with no specific treatment. In order to provide an individualized prediction method, the present study developed a novel nomogram. Taken together, the present results can aid clinical decision-making to improve prognosis.
Supplementary Material
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Social Development Project of Jiangsu Province (grant no. BE2019768), and the Zhangjiagang City Science and Technology Support Plan (Social Development) Medical and Health Project (grant no. ZKS1918). The funding institutions did not have any roles in the study design, data collection or analysis.
Availability of data and materials
The datasets generated and/or analyzed during the present study are available in the SEER database (https://seer.cancer.gov/).
Authors' contributions
XL and YC have made substantial contributions to data collection, data analysis and manuscript writing. ZYa, YH and ZYu performed data analysis. QL contributed to acquisition and interpretation of data. GF designed the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sim JK, Chung SM, Choi JH, Oh JY, Lee SH, Kim JH, Min KH, Hur GY, Shim JJ, Kang KH, et al. Clinical and molecular characteristics of pulmonary sarcomatoid carcinoma. Korean J Intern Med. 2018;33:737–744. doi: 10.3904/kjim.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yendamuri S, Caty L, Pine M, Adem S, Bogner P, Miller A, Demmy TL, Groman A, Reid M. Outcomes of sarcomatoid carcinoma of the lung: A Surveillance, Epidemiology, and end results database analysis. Surgery. 2012;152:397–402. doi: 10.1016/j.surg.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Chaft JE, Sima CS, Ginsberg MS, Huang J, Kris MG, Travis WD, Azzoli CG. Clinical outcomes with perioperative chemotherapy in sarcomatoid carcinomas of the lung. J Thorac Oncol. 2012;7:1400–1405. doi: 10.1097/JTO.0b013e3182614856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaguchi D, Ichikawa M, Ito M, Okamoto S, Kimura H, Watanabe K. Dramatic response to nivolumab after local radiotherapy in pulmonary pleomorphic carcinoma with rapid progressive post-surgical recurrence. Thorac Cancer. 2019;10:1263–1266. doi: 10.1111/1759-7714.13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoine M, Vieira T, Fallet V, Hamard C, Duruisseaux M, Cadranel J, Wislez M. Pulmonary sarcomatoid carcinoma. Ann Pathol. 2016;36:44–54. doi: 10.1016/j.annpat.2015.11.007. (In French) [DOI] [PubMed] [Google Scholar]
- 6.Steuer CE, Behera M, Liu Y, Fu C, Gillespie TW, Saba NF, Shin DM, Pillai RN, Pakkala S, Owonikoko TK, et al. Pulmonary sarcomatoid carcinoma: An analysis of the national cancer data base. Clin Lung Cancer. 2017;18:286–292. doi: 10.1016/j.cllc.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 7.Hendriksen BS, Hollenbeak CS, Reed MF, Taylor MD. Perioperative chemotherapy is not associated with improved survival in stage I pleomorphic lung cancer. J Thorac Cardiovasc Surg. 2019;158:581–591.e11. doi: 10.1016/j.jtcvs.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Maneenil K, Xue Z, Liu M, Boland J, Wu F, Stoddard SM, Molina J, Yang P. Sarcomatoid carcinoma of the lung: The Mayo clinic experience in 127 patients. Clin Lung Cancer. 2018;19:e323–e333. doi: 10.1016/j.cllc.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Schrock AB, Li SD, Frampton GM, Suh J, Braun E, Mehra R, Buck SC, Bufill JA, Peled N, Karim NA, et al. Pulmonary sarcomatoid carcinomas commonly harbor either potentially targetable genomic alterations or high tumor mutational burden as observed by comprehensive genomic profiling. J Thorac Oncol. 2017;12:932–942. doi: 10.1016/j.jtho.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Rahouma M, Kamel M, Narula N, Nasar A, Harrison S, Lee B, Stiles B, Altorki NK, Port JL. Pulmonary sarcomatoid carcinoma: An analysis of a rare cancer from the Surveillance, Epidemiology, and End Results database. Eur J Cardiothorac Surg. 2018;53:828–834. doi: 10.1093/ejcts/ezx417. [DOI] [PubMed] [Google Scholar]
- 11.Lewis JA, Petty WJ, Urbanic J, Bernstein ED, Ahmed T. Cure of oligometastatic classic biphasic pulmonary blastoma using aggressive Tri-modality treatment: Case series and review of the literature. Cureus. 2018;10:e3586. doi: 10.7759/cureus.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JS, Lee Y, Han J, Kim HK, Choi YS, Kim J, Shim YM, Kim K. Clinicopathologic outcomes of curative resection for sarcomatoid carcinoma of the lung. Oncology. 2011;81:206–213. doi: 10.1159/000333095. [DOI] [PubMed] [Google Scholar]
- 13.Lococo F, Rapicetta C, Cardillo G, Stefani A, Margaritora S, Leuzzi G, Rossi G, Petracca Ciavarella L, Morandi U, Facciolo F, et al. Pathologic findings and long-term results after surgical treatment for pulmonary sarcomatoid tumors: A multicenter analysis. Ann Thorac Surg. 2017;103:1142–1150. doi: 10.1016/j.athoracsur.2016.08.114. [DOI] [PubMed] [Google Scholar]
- 14.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, et al. The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 15.NCI SEER, corp-author. Number of Persons by Race and Hispanic Ethnicity for SEER Participants (2010 Census Data 1) https://seer.cancer.gov/registries/data.html. [Mar 3;2020 ]; [Google Scholar]
- 16.NCI SEER, corp-author. Site Recode ICD-O-3/WHO 2008 Definition*^. https://seer.cancer.gov/siterecode/icdo3_dwhoheme/#footnotes. [Mar 3;2020 ]; [Google Scholar]
- 17.Ung M, Rouquette I, Filleron T, Taillandy K, Brouchet L, Bennouna J, Delord JP, Milia J, Mazières J. Characteristics and clinical outcomes of sarcomatoid carcinoma of the lung. Clin Lung Cancer. 2016;17:391–397. doi: 10.1016/j.cllc.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Liang X, Li Q, Xu B, Hu S, Wang Q, Li Y, Zong Y, Zhang S, Li C. Mutation landscape and tumor mutation burden analysis of Chinese patients with pulmonary sarcomatoid carcinomas. Int J Clin Oncol. 2019;24:1061–1068. doi: 10.1007/s10147-019-01454-6. [DOI] [PubMed] [Google Scholar]
- 19.Boland JM, Mansfield AS, Roden AC. Pulmonary sarcomatoid carcinoma-a new hope. Ann Oncol. 2017;28:1417–1418. doi: 10.1093/annonc/mdx276. [DOI] [PubMed] [Google Scholar]
- 20.Basu A, Moreira AL, Simms A, Brandler TC. Sarcomatoid carcinoma in cytology: Report of a rare entity presenting in pleural and pericardial fluid preparations. Diagn Cytopathol. 2019;47:813–816. doi: 10.1002/dc.24183. [DOI] [PubMed] [Google Scholar]
- 21.Franks TJ, Galvin JR. Sarcomatoid carcinoma of the lung: Histologic criteria and common lesions in the differential diagnosis. Arch Pathol Lab Med. 2010;134:49–54. doi: 10.5858/2008-0547-RAR.1. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama S, Murakami T, Tao H, Onoda H, Hara A, Miyazaki R, Furukawa M, Hayashi M, Inokawa H, Okabe K, Akagi Y. Tumor spread through air spaces identifies a distinct subgroup with poor prognosis in surgically resected lung pleomorphic carcinoma. Chest. 2018;154:838–847. doi: 10.1016/j.chest.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Gu L, Xu Y, Chen Z, Pan Y, Lu S. Clinical analysis of 95 cases of pulmonary sarcomatoid carcinoma. Biomed Pharmacother. 2015;76:134–110. doi: 10.1016/j.biopha.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Yin J, Yang Y, Ma K, Yang X, Lu T, Wang S, Shi Y, Zhan C, Zhu Y, Wang Q. Clinicopathological characteristics and prognosis of pulmonary pleomorphic carcinoma: A population--based retrospective study using SEER data. J Thorac Dis. 2018;10:4262–4273. doi: 10.21037/jtd.2018.06.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weissferdt A, Kalhor N, Rodriguez Canales J, Fujimoto J, Wistuba II, Moran CA. Spindle cell and pleomorphic (‘sarcomatoid’) carcinomas of the lung: An immunohistochemical analysis of 86 cases. Hum Pathol. 2017;59:1–9. doi: 10.1016/j.humpath.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Weng SS, Cao Y, Tang XJ, Zhu LZ, Tan YN, Dong CX, Chen JQ, Shen H, Yuan Y. Epidemiological features of lung giant cell carcinoma and therapy for patients with EGFR mutations based on case reports and the surveillance, epidemiology, and end results (SEER) database. Oncotarget. 2017;8:25323–25333. doi: 10.18632/oncotarget.15831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong JY, Choi MK, Uhm JE, Park MJ, Lee J, Park YH, Ahn JS, Park K, Han JH, Ahn MJ. The role of palliative chemotherapy for advanced pulmonary pleomorphic carcinoma. Med Oncol. 2009;26:287–291. doi: 10.1007/s12032-008-9117-4. [DOI] [PubMed] [Google Scholar]
- 28.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ, Dobelbower M, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama S, Sasaki M, Morinaga S, Minematsu N. Nonsmall cell lung carcinoma with giant cell features expressing programmed Death-ligand 1: A report of a patient successfully treated with pembrolizumab. Case Rep Oncol Med. 2018;2018:5863015. doi: 10.1155/2018/5863015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei W, Zhou J, Zhang Q, Liao DH, Liu QD, Zhong BL, Liang ZB, Zhang YC, Jiang R, Liu GY, et al. Postoperative intensity-modulated radiation therapy reduces local recurrence and improves overall survival in III-N2 non-small-cell lung cancer: A single-center, retrospective study. Cancer Med. 2020 Feb 26; doi: 10.1002/cam4.2937. doi: 10.1002/cam4.2937 (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin BD, Jiao XD, Liu K, Wu Y, He X, Liu J, Zang YS. Clinical, pathological and treatment factors associated with the survival of patients with primary pulmonary salivary gland-type tumors. Lung Cancer. 2018;126:174–181. doi: 10.1016/j.lungcan.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Yang H, Lin Y, Liang Y. Treatment of lung carcinosarcoma and other rare histologic subtypes of non-small cell lung cancer. Curr Treat Options Oncol. 2017;18:54. doi: 10.1007/s11864-017-0494-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the present study are available in the SEER database (https://seer.cancer.gov/).