Abstract
Objective:
Previous research examining telomeres in individuals with neuropsychiatric disorders show that greater illness, symptoms, or cognitive impairment are linked with shorter telomeres. However, the relationships of telomere length and neuropsychological processes or psychiatric symptoms are not understood in individuals with Attention-Deficit Hyperactivity Disorder (ADHD).
Methods:
390 young adults with and without ADHD completed a multi-informant diagnostic assessment and neuropsychological testing battery. Participant DNA was isolated from saliva samples and telomere length was determined using qPCR.
Results:
Linear regression models demonstrated the only significant association to survive correction for multiple testing was for childhood hyperactivity-impulsivity symptoms and longer telomere length.
Conclusions:
Contrary to expectations, longer telomere length in young adults was associated only with childhood ADHD symptoms, particularly hyperactivity-impulsivity, in this sample. These findings are an important demonstration that the neuropsychological deficits and symptoms experienced by individuals diagnosed with ADHD during adulthood may not be negatively associated with telomere length.
Telomeres, the “endcaps” of chromosomes, and their related biology are critical components of faithfully maintaining cellular genetic information (Chan & Blackburn, 2004). Telomere length is an increasingly used biomarker of health and aging, as it may indicate the extent to which cells have aged and undergone damage (Blasco, 2005; Chan & Blackburn, 2004). Specifically, telomere length shortens as cells progress from more proliferative to more differentiated states. Telomere length is also a marker of cellular senescence, when cells are no longer capable of division and indicative of cellular adversity. Telomeres are shorter after exposure to oxidative stress, ionizing damage, and chemical stress (Astuti, Wardhana, Watkins, Wulaningsih, & Network, 2017; Hewitt et al., 2012; Lustig et al., 2016; Opresko, Fan, Danzy, Wilson, & Bohr, 2005). Telomere changes may be mechanistically important to cellular function due to telomeres protecting genetic material in the chromosome from degradation and the transcriptional role of telomere-associated molecules (O’Sullivan & Karlseder, 2010; Ye, Renault, Jamet, & Gilson, 2014).
Given the relationship between cellular age and telomere length, efforts have been made to better understand factors that may accelerate or slow telomere shortening. Along these lines, some evidence suggests a relationship between decreased telomere length and neuropsychiatric disorders. These studies have been motivated, among other things, by the notion that psychological stress, which contributes to the occurrence of neuropsychiatric disorders, may result in physiological stress and telomere changes. Prior research has reported shorter peripheral telomeres among adults with schizophrenia (Kao et al., 2008), adults with depression (Ridout, Ridout, Price, Sen, & Tyrka, 2016; Simon et al., 2006; Verhoeven et al., 2014; Wikgren et al., 2012; Wolkowitz, Epel, Reus, & Mellon, 2010) and children with disruptive behavior disorders (i.e., ADHD, ODD; Costa et al., 2015; Wojcicki et al., 2015) compared to healthy controls.
Some prior work has also reported no association between telomere length and extent of depressive symptoms (Shaffer et al., 2012; Simon et al., 2015). Additionally, gender may moderate these associations with symptom level, with some studies only finding effects in women with schizophrenia (Shalev et al., 2014) or men with internalizing disorders (Whisman & Richardson, 2017; Wolkowitz et al., 2017). Further, the chronicity and severity of having psychiatric symptoms may contribute to physiological stress and influence telomeres. Impairment in individual functioning associated with high levels of psychiatric symptoms may be a source of chronic psychological stress. Therefore, shortened telomere length in individuals with psychiatric disorders may be a mechanism underlying the increases in physical morbidity and mortality common among individuals with psychiatric disorders (Atlantis, Vogelzangs, Cashman, & Penninx, 2012; Chida, Hamer, Wardle, & Steptoe, 2008; Costa et al., 2015; Denollet, Maas, Knottnerus, Keyzer, & Pop, 2009; Lima et al., 2015; Luppino et al., 2010; Polho, De-Paula, Cardillo, dos Santos, & Kerr, 2015).
Conceptually, there are many reasons to expect that telomere length may be altered among individuals with ADHD (especially in adults), particularly given the observed relationship between experiences of psychological stress and shorter telomere length (Mathur et al., 2016). ADHD is a neurodevelopmental disorder characterized by impairment in multiple domains (e.g., social and educational functioning). ADHD symptoms and impairment have been found to persist into adulthood in over 50% of cases (Faraone, Biederman, & Mick, 2006). Symptoms of ADHD in childhood are associated with multiple psychological stressors, including peer rejection, academic difficulties, and parental and familial discord (Faraone & Antshel, 2008; Strine et al., 2006), which appear to be cumulative and result in significant increases in psychological stress among adults with the disorder (Hirvikoski, Lindholm, Nordenstrom, Nordstrom, & Lajic, 2009). The continuation of ADHD symptoms into adulthood combined with the presence of persistent and accumulating stress may activate the mechanisms that cause accelerated shortening of telomeres.
Along these lines, there is also emerging evidence that telomere length may also be associated with cognitive deficits. Cohen-Manheim and colleagues (2016) examined associations between telomere attrition and cognitive functioning over a period of 13 years (e.g., from early adulthood to mid-life). Telomere length was robustly and negatively associated with global cognitive performance as well as performance in perceptual reasoning, processing speed, and memory. ADHD has long been associated with numerous (yet heterogeneous) deficits in cognitive functioning, particularly in the domains of inhibition, working memory, and processing speed (Gansler et al., 1998; Nigg et al., 2005; Sandson, Bachna, & Morin, 2000; van Lieshout, Luman, Buitelaar, Rommelse, & Oosterlaan, 2013). Thus, persistent deficits in cognitive performance in these domains observed among individuals with ADHD may increase risk for more rapid cellular aging, indexed via telomere length. Additionally, deficits in these processes may contribute to increased stress because of the ways in which they interfere with occupational and social functioning (Friedrichs, Igl, Larsson, & Larsson, 2012; Miklosi, Mate, Somogyi, & Szabo, 2016). Thus, impairments in social, academic, and/or occupational domains and neuropsychological deficits among adults with ADHD all may contribute to increased psychological stress, which in turn, may impact telomere attrition.
Indeed, several studies of psychopathology in adults (e.g., schizophrenia, post-traumatic stress disorder, major depression, anxiety) have found this exact negative association between increased symptoms and shortened telomere lengths. However, only one prior study has previously examined telomere length in the context of Attention-Deficit Hyperactivity Disorder (ADHD; Costa et al., 2015). In the previous study, hyperactive-impulsive but not inattentive symptoms were negatively correlated with telomere length in children under 16 years of age. Notably, associations between ADHD diagnosis, neuropsychological processes, psychosocial impairments, or psychological stress were not evaluated. Moreover, telomere length has not been examined in relation to adult ADHD symptoms. The effect of ADHD symptom chronicity, or persistence, from childhood to adulthood, and the extent to which ADHD symptoms in adulthood may be related to telomere length is not known.
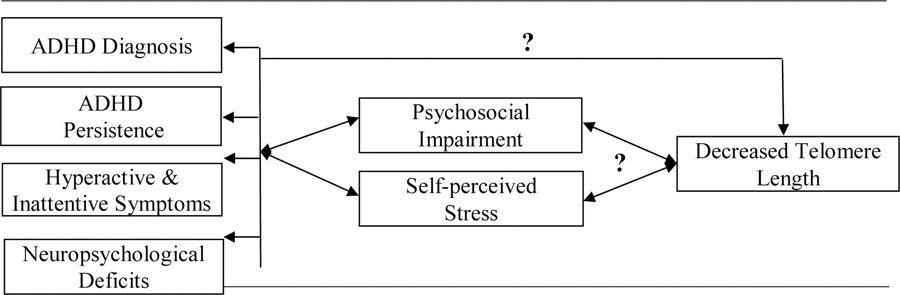
The current study aimed to fill this knowledge gap by examining the association between telomere length and ADHD diagnosis, ADHD symptoms, symptom persistence, neuropsychological processes, psychosocial impairment, and psychological stress (Figure 1). Specifically, our primary hypotheses are that there will be negative associations between telomere length and ADHD diagnosis, ADHD symptoms, symptom persistence, and neuropsychological processes. Our secondary hypothesis is that there will be a negative association between telomere length and psychosocial impairments after accounting for significantly associated ADHD symptoms or neuropsychological deficits.
Figure 1.
Hypothetical associations examined in the current study
METHODS
Participants
Participants were 390 adults aged 18–38 years who were recruited from the local community vie advertisements in local newspapers, email list-serves, and from local clinics. Participants with a confirmed or suspected diagnosis of ADHD as well as those without ADHD were invited to participate in a study of ADHD in young adults, in order to capture the full dimension of symptoms and impairment, given the dimensional nature of ADHD (Marcus & Barry, 2011). Participants were excluded for the following reasons: not fluent in English or a native English speaker, impaired vision or hearing that was not corrected, or history of Tourette’s disorder, schizophrenia, psychosis, or autism spectrum disorder.
ADHD Assessment
Participants completed a three-hour laboratory visit that included a diagnostic interview to assess current and lifetime ADHD symptoms (based on Kessler et al., 2005), a battery of neuropsychological tests, and a series of self-report questionnaires assessing their current and childhood symptoms history as well as a variety of comorbid problems and experiences of stress. All tasks were administered to participants in a fixed order. Participants taking stimulant medication were required to have completed a 24- to 48- hour washout prior to neuropsychological testing (M washout = 46.2 hours, SD = 18.3). Participants provided contact information for at least one informant who provided ratings of current and childhood symptoms, as well as ratings of participant psychosocial impairment. Mean composite scores of self and informant ratings of current and childhood ADHD symptoms and of psychosocial impairment were used for analyses. In cases where informant report was not available, only self-report was used.
ADHD Diagnosis
ADHD diagnostic status was determined by a clinical psychologist (M.A.N.), using self and informant report data from questionnaires and responses from the diagnostic interview to determine final DSM-5 diagnoses. An overall symptom count of current and childhood ADHD symptoms was calculated by applying an “or” algorithm to self and informant data (i.e., a symptom was counted as present if the participant or an informant rated it as present, with each informant maximally endorsing 3 unique symptoms). Additionally, scores of current and childhood impairment, symptom presence in more than one context (e.g., school and home), and onset of symptoms before age 12 were also determined for each participant. Thus, each participant was assigned a childhood and current diagnostic presentation type based on DSM-5 criteria.
ADHD Persistence
Both categorical and dimensional approaches were utilized to characterize persistence of ADHD. A categorical approach was utilized based on current and childhood diagnostic status. Participants who met DSM-5 diagnostic criteria during childhood (prior to age 12) and currently (based on symptoms reports during the past 6 months) were classified as “persisters;” those who met full diagnostic criteria during childhood but no longer met diagnostic criteria in adulthood were classified as “remitters.”
A dimensional rating of persistence was calculated at the symptom level by examining self and informant childhood and current symptom ratings on the Barkley Adult ADHD Rating Scale (BAARS) Current and Childhood Symptoms (Barkley, 2011). Each symptom was given a dimensional persistence score (0–2) based on both childhood and current ratings (2= persistent and occurring often or very often, 1=decline from often/very often to occasionally present, 0=not persistent, either not present at all, or declined from often/very often to not present). Mean persistence scores were calculated for each of the 18 DSM ADHD symptoms using self-reports and informant reports. Mean self- and informant symptom sum scores were then calculated for overall ADHD symptoms as well as for inattention and hyperactivity-impulsivity symptoms. Internal consistencies for the BAARS self and informant reports in the current sample were excellent (α = 0.94 and 0.93 respectively).
Neuropsychological Battery
Several neuropsychological tests were selected to measure components of participant executive function, as well as non-executive neuropsychological processes that are thought to be impaired in individuals with ADHD. The following tests were administered to participants: Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), Delis-Kaplan Executive Function System (D-KEFS) Color-Word Interference Test (Delis, Kaplan, & Kramer, 2001), D-KEFS Trail Making Test (Delis et al., 2001), the Stop Task (Logan, 1994), and Conners Continuous Performance Test (Conners & MHS Staff, 2002).
A latent variable model approach was employed to identify sources of common variance across test scores from each cognitive domain assessed. A confirmatory factor analytic model based on prior work (Nikolas & Nigg, 2013) was fitted to the data in order to best capture the common, systematic variance among the different tasks. A six-factor model provided a good fit to the data (χ2=192.39, df=73, p<.001, Confirmatory Fit Index [CFI]=.970, Root Mean Square Error of Approximation [RMSEA]=.065). The resultant six factors were labeled processing speed, working memory, interference control, inhibition, arousal, and reaction time variability. Standardized factor scores for each of the six factors were calculated using a regression-based approach and were retained for analyses.
IQ.
The two sub-test version of the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999) was administered to estimate full-scale IQ.
Processing speed.
Five measures loaded onto the processing speed factor. These included completion times for the Color Naming and Word Reading subtests of the D-KEFS Color-Word Interference task and the Number Sequencing, Letter Sequencing, and Motor Speed subtests of the D-KEFS Trailmaking task. The D-KEFS Color-Word Interference Color Naming subtest requires participants to name colors that are presented as a series of color patches on a page as quickly as possible without making mistakes. Alternatively, the Word Reading subtest requires participants to read color names (i.e., blue, red, green) presented as a series on a page as quickly as possible without making mistakes. For the D-KEFS Trailmaking Number Sequencing subtest, participants are presented with two pages of numbers and told to connect the numbers in serial order (1–16) as quickly and accurately as possible. The Letter Sequencing subtest is similar to the Number Sequencing subtest, except participants connect the numbers in alphabetic order (A-P). On the Motor Speed subtest, participants just must connect lines between empty circles as quickly as possible.
Working memory.
Two measures loaded onto the working memory factor; completion time of the Number-Letter switching Subtest of the D-KEFS Trailmaking task (Delis et al., 2001) and the raw score of the WAIS-IV Digit Span Backward subtest (Wechsler, 2008). One the Number-Letter Switching subtest, participants are presented with two pages with numbers (1–16) and letters (A-P) on the pages and are asked to alternate sequencing between numbers and letters (e.g., connecting 1-A-2-B, etc.). For the WAIS-IV Digit Span Backward subtest, participants recalled a series of orally presented digits in reverse order, with the span increasing from 2 digits up to 9 digits. Participants completed each span section until they failed to correctly complete two trials of the same span of digits.
Interference control.
Two measures also loaded onto the interference control factor. These included completion time of the Color-Word Inhibition/Switching subtest of the D-KEFS Color-Word Interference task (Delis et al., 2001) and total omission errors from the Conners Continuous Performance Task (CPT). For the Color-Word Inhibition/Switching task, participants are presented with color names printed in different-colored ink, and some of the color names were inside boxes. Participants were told to name the color of the ink for color names not in boxes, but to read the color name for words inside boxes. The CPT is a twenty-minute computerized task in which a series of letters appear rapidly on the screen. Participants are instructed to press the space bar for every letter except “X”. Omission errors on the CPT reflects the number of times participants failed to make an accurate key press.
Inhibition.
Two measures loaded onto the inhibition factor. First, was the stop signal reaction time calculated from performance on the Stop Task (Logan 1994). The Stop Task measures the ability to suppress a prepotent motor response. During the Stop Task, participants are presented with either a circle or a square on a computer screen and told to respond with one of two keys to indicate which shape they had seen (Go Response trials). For a portion of the trials (25%), a tone sounded shortly after the presentation of the shape, and this indicated the participant should withhold responding with the keys. The average stop signal reaction time was computed as a measure of how much warning each participant needed to interrupt a response and was calculated by subtracting the average stop signal delay from the average Go Response time, with greater values indexing greater inhibition difficulty. The commission errors index from the CPT also loaded onto the inhibition factor. This measure indicated how many times during the course of the task participants made an inaccurate key press (e.g., with the letter “X”).
Arousal.
The d-prime (d’) score from Conners Continuous Performance Task (CPT), Version II (Conners & MHS Staff, 2002) is a sensitivity index of participant responding based on both omission and commission errors and was used to measure participant arousal. A higher d’ score indicated greater sensitivity in differentiating targets (Xs) from non-targets (non-Xs); however, this was reverse-scored for data analysis such that higher scores indicated worse performance.
Reaction time variability.
Two indices loaded onto the reaction time variability factor; these included the standard deviation for reaction time to the Go Response trials on the Stop Task (Logan, 1994) and the reaction time variability score on the Conners CPT (Conners & MHS Staff, 2002).
Questionnaire Battery
Several questionnaires were used to collect demographic and family history information and to assess participant perceived stress, daily hassles, and psychosocial impairment. Questionnaires assessing perceived stress and daily hassles were completed by participants only, whereas the questionnaire assessing psychosocial impairment was completed by participants and informants. All questionnaires were completed via an online data collection instrument (Qualtrics).
Perceived Stress.
The total score from the Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1983; Taylor, 2015) was used as a measure of participant stress. The Perceived Stress Scale is the most widely used measure of perceived stress, or the degree to which an individual appraises situations in one’s life as stressful. This measure consists of 10 items assessing participants’ perceptions of stress in the past month. Participants rate their experiences using a 5-point scale (0 = never, 1 = almost never, 2 = sometimes, 3 = fairly often, 4 = very often). Internal consistency for the PSS in the current sample was good (α = 0.88).
Daily Hassles.
The total score from the Daily Hassles Scale (Kohn & Macdonald, 1992) was used as a measure of participant daily hassles. This measure consists of 51 items assessing the extent to which a variety of daily hassles have affected the participant’s life in the past month. Participants rate each hassle using a 4-point Likert scale (1 = not at all a part of my life, 2 = only slightly part of my life, 3 = distinctly part of my life, 4 = very much part of my life). Internal consistency for the perceived Daily Hassles Scale in the current sample was acceptable (α = 0.77).
Impairment.
The Barkley Functional Impairment Scale (BFIS; Barkley, 2011) was used to assess participant impairment in 15 areas of psychosocial functioning (e.g., at home, with friends, at school). Impairment in each of the 15 areas was rated using a Likert scale from 0 to 9 (0 = not impaired at all; 9 = extremely impaired). Previous work has found the 15 areas can be reduced to three impairment factors: relationship impairment, professional impairment, and daily living impairment (Kamradt, Ullsperger, & Nikolas, 2014). Ratings for the psychosocial areas that load on each factor were summed for the impairment factor scores. Internal consistencies for the BFIS self and informant reports in the current sample were excellent (α = 0.96 and 0.90, respectively).
Telomere Length Measurement
DNA was isolated from saliva samples from 389 participants following the protocol from Oragene OG500 collection kit (DNA Genotek, Ottawa, Ontario, Canada). DNA concentration was determined by Qubit fluorometry (ThermoFisher Scientific, Waltham, MA, USA). Average telomere length was measured using an adaptation of qPCR methods (Drury et al., 2014). 33 ng of DNA was used for each assay containing 1X Power SYBR green (Applied Biosystems, Foster City, CA, USA) and 900 nM forward and reverse telomere (T) or 600 nM forward and reverse single copy albumin gene (S) primers (Table 1). Within a single experimental plate, all samples were run in triplicate for telomere and albumin reactions and cycle threshold (CT) values for each sample for each gene were averaged. All experimental plates were also run in duplicate with sample positions reversed. Individual samples were required to have an inter-plate coefficient of variation (CV) below 0.6. The geometric mean of all CV values for sample reactions on duplicate plates was required to be below 0.1 (Drury et al., 2014) . T/S Ratio was calculated using the ΔCT method as .
Table 1.
Telomere assay primer sequences
| Primer | Sequence (5’ -> 3’) |
|---|---|
| Telc | TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA |
| Telg | ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT |
| Albu | CGGCGGCGGGCGGCGCGGGCTGGGCGGAAATGCTGCACAGAATCCTTG |
| Albd | GCCCGGCCCGCCGCGCCCGTCCCGCCGGAAAAGCATGGTCGCCTGTT |
Analytic Strategy
Participants with a history of cancer were excluded from analyses given the known association between telomere length and cancer. Participants whose samples passed telomere quality control were compared to participants whose samples failed quality control to determine if participants included in subsequent analyses differed from those not included. Then, telomere length was tested for normality with the Shapiro-Wilk test of normality. Given the non-normal distribution of the telomere length data, a natural logarithmic transformation was used. Telomere length outliers (participant telomere length differed from mean telomere length by more than two standard deviations) were identified and winsorized to two standard deviations.
Next, correlations were computed between telomere length and variables identified as potential covariates. A variable was evaluated as a covariate if previous research has found an association between the potential covariate variable and telomere length (e.g., cigarette smoke exposure). Potential covariates with p ≤ 0.1 were retained as covariates in subsequent analyses. The potential covariates analyzed were: sex; age; race; current stimulant medication use; childhood stimulant medication use; maternal education; paternal education; current income; childhood income; prenatal tobacco exposure; prenatal alcohol exposure; preterm birth; childhood second-hand smoke exposure; and Family Environment Scale conflict score (Adler et al., 2013; Carroll, Diez-Roux, Adler, & Seeman, 2013; Drury et al., 2014; Geronimus et al., 2015; Hadchouel et al., 2015; Ip et al., 2017; Kajantie et al., 2012; Lu et al., 2017; Mayer et al., 2006; Mitchell et al., 2014; Needham et al., 2013; Salihu et al., 2015; Smeets, Codd, Samani, & Hokken-Koelega, 2015; Zhu et al., 2011).
Linear regression was used for the main analyses examining telomere length and ADHD symptoms, diagnosis, persistence, and neuropsychological processes. Each independent variable was tested separately. Initial analyses examined associations between telomere length and the independent variables without controlling for covariates. Then, analyses were conducted with the covariates identified via the correlational analyses detailed above entered first followed by the independent variable. Thirteen hypothesized predicting independent variables were tested: IQ; processing speed factor score; working memory factor score; interference control factor score; inhibition factor score; arousal factor score; response variability factor score; ADHD diagnosis; ADHD persistence; childhood inattention symptoms; childhood hyperactivity-impulsivity symptoms; current inattention symptoms; and current hyperactivity-impulsivity symptoms. For the linear regression analysis examining ADHD diagnosis, participants who met diagnostic criteria were compared to controls and participants who experienced subthreshold symptoms were excluded from this analysis. For the linear regression analysis examining ADHD persistence, participants who experienced symptom remittance (remitters) were compared to participants who experienced symptoms persistence (persisters). Additionally, Analysis of Variance (ANOVA) was utilized to compare telomere length across remitters, persisters, and controls.
Following the main analyses, linear regression was used to determine if there was an association between telomere length and stress or psychosocial impairment. The independent variables related to stress and psychosocial impairment used in the supplementary analyses were Perceived Stress Scale score; Daily Life Hassles score; professional impairment factor score; daily responsibility impairment factor score; and relational impairment factor score. Each independent variable was analyzed separately. The covariates that were used in the main analyses were entered first in the supplementary analyses. Then, since stress and psychosocial impairments were conceptualized to be a result of the experience of ADHD symptoms and deficits in associated neuropsychological processes, significant independent variables identified in the main analyses were entered second. The variables for stress and psychosocial impairments were entered third.
Finally, the Hochberg method of correction was used to correct for multiple testing. Briefly, this method uses stepwise adjustments that adjust p values sequentially (step-up approach) and maintain the observed p-value order. This approach is recommended for neuropsychological outcomes that are mildly correlated (Blakesley et al., 2009). Given the exploratory nature of the current analyses, a False Detection Rate of .20 was used.
RESULTS
Telomere Data
Fourteen individuals were excluded from analyses because of a positive cancer history. 325 samples out of 375 (87%) passed the quality control procedures for the telomere length assay. Participant data for samples that passed quality control versus samples that did not pass quality control are presented in Table 2. Participants with telomere length data were more likely to be control participants. Otherwise, participants with telomere length data were not different from participants whose sample did not pass quality control on the numerous covariate and predictor variables used in analyses. After excluding the participants with samples that did not pass quality control, there were 214 participants who met diagnostic criteria for ADHD, 19 participants who had subthreshold symptoms of ADHD, and 92 participants who were deemed controls. With regard to persistence of ADHD symptoms from childhood to adulthood, there were 74 participants who experienced symptom remittance, 151 participants who experienced symptom persistence, and 85 participants who did not experience threshold or subthreshold symptoms during childhood or adulthood. Data from 15 individuals who did not have symptoms reported in childhood but reported subthreshold symptoms (n=11) or threshold symptoms (n=4) only in adulthood were not included in the analysis of symptom persistence.
Table 2.
Participant data for samples that passed quality control (n = 325) vs. samples that did not pass quality control (n = 50) and p-values for test statistic comparing the two groups
| Variable | Did Not Pass | Passed | p |
|---|---|---|---|
| Sex (% male) | 66.0 | 56.6 | .223 |
| Age (mean years) | 22.9 | 23.3 | .480 |
| Ethnicity | |||
| White (%) | 90.0 | 86.1 | .654 |
| African American (%) | 2.0 | 2.8 | 1.000 |
| Latinx (%) | 2.0 | 3.4 | 1.000 |
| Other (%) | 6.0 | 7.7 | 1.000 |
| Current income z-score (mean) | −.09 | −.02 | .518 |
| Childhood income z-score (mean) | .10 | −.02 | .489 |
| Current stimulant medication use (% yes) | 26.0 | 32.6 | .416 |
| Childhood stimulant medication use (% yes) | 23.2 | 21.4 | .842 |
| Prenatal tobacco exposure (% yes) | 2.6 | 4.6 | 1.000 |
| Prenatal alcohol exposure (% yes) | 2.6 | 2.3 | 1.000 |
| Preterm birth (% preterm) | 2.3 | 7.8 | .335 |
| Childhood secondhand smoke exposure (% yes) | 31.8 | 24.8 | .356 |
| Family Environment Scale – Conflict score (mean) | 52.7 | 49.4 | .076 |
| Highest level maternal education | |||
| High School (%) | 20.4 | 14.8 | .371 |
| Some college or college degree (%) | 47.7 | 60.0 | .141 |
| Graduate degree (%) | 31.8 | 23.1 | .256 |
| Highest level paternal education | |||
| High School (%) | 13.6 | 20.4 | .413 |
| Some college or college degree (%) | 45.5 | 50.9 | .522 |
| Graduate degree (%) | 38.6 | 26.6 | .108 |
| ADHD diagnosis (% yes) | 51.0 | 69.9 | .013 |
| ADHD persistence (% yes) | 80.8 | 67.1 | .185 |
| Current inattention symptoms (mean) | 2.9 | 3.6 | .099 |
| Current hyperactivity-impulsivity symptoms (mean) | 2.5 | 2.9 | .270 |
| Childhood inattention symptoms (mean) | 3.6 | 4.4 | .084 |
| Childhood hyperactivity-impulsivity symptoms (mean) | 3.6 | 3.9 | .369 |
| IQ (mean) | 113.1 | 113.2 | .779 |
| Processing speed factor z-score (mean) | .04 | −.02 | .676 |
| Working memory factor z-score (mean) | −.01 | −.01 | .974 |
| Interference control factor z-score (mean) | .06 | −.02 | .786 |
| Inhibition factor z-score (mean) | .26 | −.03 | .146 |
| Arousal factor z-score (mean) | −.28 | .02 | .092 |
| Response variability factor z-score (mean) | .12 | −.02 | .838 |
| Professional impairment factor (mean) | 17.0 | 18.5 | .396 |
| Daily responsibility impairment factor (mean) | 9.0 | 9.2 | .614 |
| Relational impairment factor (mean) | 13.6 | 12.7 | .600 |
| Perceived Stress Scale score (mean) | 20.0 | 19.3 | .456 |
| Daily Life Hassles Scale score (mean) | 6.1 | 5.1 | .091 |
Note: Normality of scale variables was assessed with the Shapiro-Wilk test of normality. The independent samples t-test was used to compare groups on scale variables that were normally distributed, and the Mann-Whitney test was used to compare groups on scale variables that were non-normally distributed. For dichotomous variables, group differences were tested using a Chi-square Independence test (n => 5 for each group) or a Fisher Exact Test (n < 5 in a group).
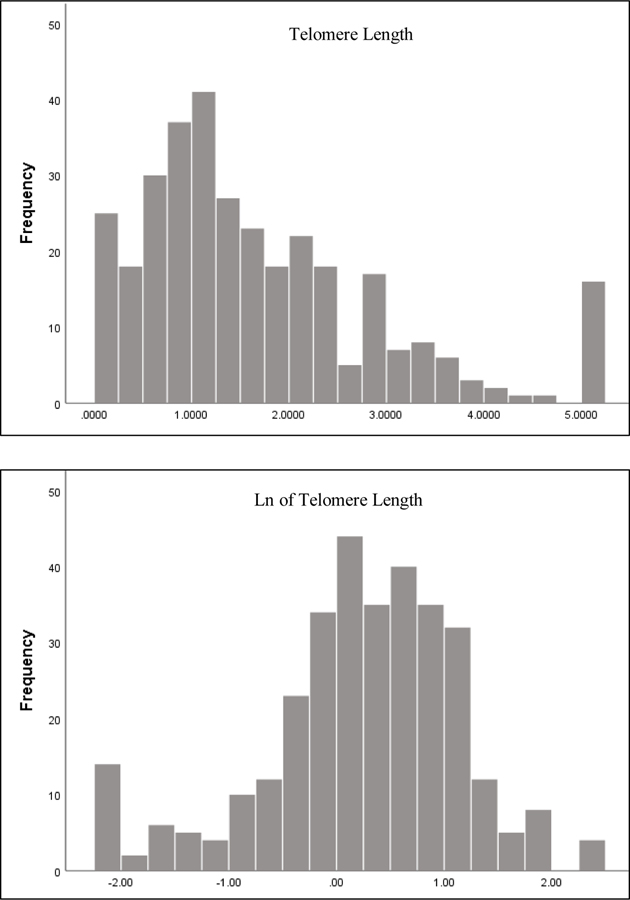
The Shapiro-Wilk test of normality was significant, indicating telomere length was not normally distributed (Figure 2). As a result, a log transformation was performed on the telomere length data (Figure 2).
Figure 2. Histogram of telomere length and natural logarithmic transformed telomere length, respectively.
Note: Outliers have been winsorized to two standard deviation in both histograms.
Thirteen participant samples were identified as outliers (more than 2 SDs from the mean telomere length). Telomere length values for these participants were winsorized to 2 SDs in an effort to maximize the number of participants included in analyses whilst simultaneously limiting the effect of the outlier values on results.
Covariates
Correlations for all of the potential covariates examined are presented in Table 3. Four variables met criteria to be included as a covariate (p < 0.1). Mother’s highest level of education is a graduate degree (r = −.109, p = 0.065) and use of stimulants in childhood (r = −.102, p = 0.091) was associated with shorter telomeres whereas preterm birth (r = .112, p = 0.057) and mother’s highest level of education is some high school (r = .132, p = 0.025) were associated with longer telomeres.
Table 3.
Correlations between potential covariates and telomere length
| Sex | r = .005, p = .935 |
| Age | r = −.005, p = .934 |
| Current income | r = .028, p = .636 |
| Current stimulant medication use | r = −.082, p = .138 |
| Race/ethnicity | |
| Caucasian race | r = .091, p = .100 |
| African American | r = −.030, p = .590 |
| Hispanic/Latino | r = −.023, p = .677 |
| Other | r = −.084, p = .130 |
| Prenatal tobacco exposure | r = .092, p = .137 |
| Prenatal alcohol exposure | r = .096, p = .126 |
| Preterm birth (gestational age < 37 weeks) | r = .112, p = .057 |
| Childhood secondhand smoke exposure | r = .028, p = .639 |
| Childhood stimulant medication use | r = −.102, p = .091 |
| Childhood income | r = −.010, p = .871 |
| Family Environment Scale – Conflict score | r = −.035, p = .564 |
| Highest level maternal education | |
| High School | r = .132, p = .025 |
| Some college or college degree | r = −.017, p = .768 |
| Graduate degree | r = −.109, p = .065 |
| Highest level paternal education | |
| High School | r = .085, p = .150 |
| Some college or college degree | r = −.086, p = .144 |
| Graduate degree | r = .001, p = .983 |
Note: Sex was coded as male = 0, female = 1; Age, current income, childhood income, and Family Environment Scale – conflict score are continuous variables; All other variables are dichotomous and were coded no = 0, yes = 1
Linear Regression
Regression parameters for the main analyses examining ADHD diagnosis, symptoms, and associated cognitive functions are presented in Table 4 (without covariates) and Table 5 (with covariates). When analyzed without covariates, none of the independent variables were significantly associated with telomere length. When analyzed with covariates, two independent variables were significantly associated with telomere length. Current ADHD diagnostic status was associated with longer telomere length (β = 0.13, 95% CI = 0.00, 0.26, p = 0.046,) such that individuals who currently met diagnostic criteria for ADHD had longer telomere length. Similarly, childhood hyperactive-impulsive symptoms were associated with longer telomere length (β = 0.17, 95% CI = 0.04, 0.29, p = 0.009) such that individuals who displayed more hyperactive-impulsive symptoms during childhood had longer telomere length. Childhood hyperactive-impulsive symptoms was the only significant predictor after applying the Hochberg correction for multiple comparisons. Surprisingly, none of the neuropsychological factors were significantly associated with telomere length. Similar to the linear regression results for persistence (persisters versus remitters), the ANOVA of telomere length across persisters, remitters, and controls showed no group differences (F(2, 307) = 1.73, p = 0.18), indicating that telomere length did not significantly vary across these groups.
Table 4.
Regression parameters for the main analyses examining ADHD diagnosis, symptoms, and associated cognitive functions. Each independent variable was examined separately. Parameters (βs and 95% confidence interval for βs) the independent variables.
| IQ | −0.09 (−0.20, 0.01) |
| Processing speed factor | −0.01 (−0.11, 0.09) |
| Working memory factor | −0.03 (−0.14, 0.08) |
| Interference control factor | −0.01 (−0.12, 0.09) |
| Inhibition factor | −0.02 (−0.13, 0.09) |
| Arousal factor | 0.03 (−0.08, 0.14) |
| Response variability factor | 0.00 (−0.17, 0.17) |
| ADHD diagnosis | 0.06 (−0.06, 0.17) |
| ADHD persistence | 0.11 (−0.02, 0.24) |
| Child inattention | 0.02 (−0.10, 0.13) |
| Child hyperactivity-impulsivity | 0.09 (−0.02, 0.20) |
| Current inattention | 0.06 (−0.06, 0.17) |
| Current hyperactivity-impulsivity | 0.09 (−0.02, 0.21) |
Note: None of the independent variables were significant
Table 5.
Regression parameters for the main analyses examining ADHD diagnosis, symptoms, and associated cognitive functions. Each independent variable was examined separately. Parameters (βs and 95% confidence interval for βs) are presented for the 4 covariates and the independent variables.
| Childhood Stimulants | Preterm Birth | Maternal High School | Maternal Graduate | Independent Variable | |
|---|---|---|---|---|---|
| IQ | −0.07 (−0.19, 0.05) | 0.13 (0.01, 0.25) | 0.12 (0.00, 0.24) | 0.09 (−0.21, 0.03) | −0.07 (−0.21, 0.06) |
| Processing speed factor | −0.07 (−0.19, 0.05) | 0.12 (0.00, 0.24) | 0.12 (0.00, 0.24) | 0.10 (−0.22, 0.03) | 0.01 (−0.11, 0.13) |
| Working memory factor | −0.07 (−0.19, 0.05) | 0.12 (0.00, 0.24) | 0.13 (0.00, 0.25) | 0.10 (−0.22, 0.02) | −0.02 (−0.14, 0.09) |
| Interference control factor | −0.07 (−0.19, 0.05) | 0.12 (0.00, 0.24) | 0.12 (0.00, 0.25 | 0.10 (−0.21, 0.03) | 0.00 (−0.12, 0.12) |
| Inhibition factor | −0.07 (−0.19, 0.05) | 0.12 (0.00, 0.24) | 0.12 (0.00, 0.25) | 0.09 (−0.21, 0.03) | −0.02 (−0.14, 0.09) |
| Arousal factor | −0.07 (−0.19, 0.05) | 0.12 (0.00, 0.24) | 0.12 (0.00, 0.25) | 0.10 (−0.22, 0.03) | 0.01 (−0.11, 0.12) |
| Response variability factor | −0.07 (−0.19, 0.05) | 0.12 (0.00, 0.24) | 0.12 (0.00, 0.24) | 0.10 (−0.22, 0.03) | 0.01 (−0.12, 0.13) |
| ADHD diagnosis | −0.12 (−0.25, 0.01) | 0.10 (−0.02, 0.22) | 0.13 (0.01, 0.36) | 0.09 (−0.21, 0.04) | 0.13 (0.00, 0.26) |
| ADHD persistence | −0.10 (−0.24, 0.05) | 0.10 (−0.05, 0.24) | 0.17 (0.03, 0.32) | 0.03 (−0.18, 0.12) | 0.13 (−0.02, 0.27) |
| Child inattention | −0.10 (−0.23, 0.03) | 0.12 (0.00, 0.24) | 0.13 (0.01, 0.25) | 0.10 (−0.22, 0.02) | 0.09 (−0.04, 0.22) |
| Child hyperactivity-impulsivity | −0.14 (−0.27, −0.01) | 0.12 (0.00, 0.23) | 0.12 (0.00, 0.24) | 0.10 (−0.22, 0.02) | 0.17 (0.04, 0.29)* |
| Current inattention | −0.08 (−0.20, 0.04) | 0.12 (0.00, 0.24) | 0.13 (0.00, 0.25) | 0.09 (−0.21, 0.03) | 0.06 (−0.06, 0.17) |
| Current hyperactivity-impulsivity | −0.09 (−0.21, 0.03) | 0.11 (−0.01, 0.23) | 0.13 (0.01, 0.25) | 0.09 (−0.21, 0.03) | 0.09 (−0.03, 0.21) |
Note: Bold font indicates p < 0.05;
child hyperactivity-impulsivity was the only significant predictor after applying the Hochberg correction.
Regression parameters for the analyses examining impairment and stress are presented in Table 6. Childhood hyperactive-impulsive symptoms was included as a covariate in addition to the four covariates used in the previous linear regression analyses. None of the impairment domains or stress indices were significant predictors of telomere length.
Table 6.
Regression parameters for secondary analyses examining psychosocial impairment and self-perceived stress. Each independent variable was examined separately. Parameters (βs and 95% confidence interval for βs) are presented for the four original covariates, childhood hyperactive-impulsive symptoms, and the independent variables.
| Childhood Stimulants | Preterm Birth | Maternal High School | |
|---|---|---|---|
| Relational impairment | −0.15 (−0.28, −0.02) | 0.11 (−0.01, 0.23) | 0.11 (−0.02, 0.23) |
| Professional impairment | −0.16 (−0.29, −0.02) | 0.12 (0.00, 0.24) | 0.11 (−0.02, 0.23) |
| Daily responsibility impairment | −0.15 (−0.28, −0.02) | 0.12 (0.00, 0.23) | 0.13, (0.00, 0.25) |
| PSS total score | −0.14 (−0.27, −0.01) | 0.12 (0.00, 0.24) | 0.13 (0.00, 0.25) |
| DLH total score | −0.14 (−0.28, −0.01) | 0.11 (−0.02, 0.23) | 0.12 (0.00, 0.24) |
| Maternal Graduate | Childhood H-I Symptoms | Independent Variable | |
| Relational impairment | 0.10 (−0.22, 0.02) | 0.18 (0.04, 0.31) | 0.02 (−0.09, 0.14) |
| Professional impairment | 0.10 (−0.22, 0.02) | 0.18 (0.05, 0.32) | 0.02 (−0.11, 0.17) |
| Daily responsibility impairment | 0.10 (−0.22, 0.02) | 0.17 (0.03, 0.30) | 0.03 (−0.09, 0.14) |
| PSS total score | 0.09 (−0.21, 0.04) | 0.16 (0.03, 0.30) | −0.02 (−0.16, 0.11) |
| DLH total score | 0.08 (−0.21, 0.04) | 0.20 (0.06, 0.33) | −0.08 (−0.21, 0.04 |
Note: Bold font indicates p < 0.05
DISCUSSION
The current study examined whether there was an association between telomere length and a variety of factors associated with ADHD. Specifically, ADHD diagnosis, symptoms, persistence of symptoms, neuropsychological processes, psychosocial impairments, and self-perceived stress were evaluated as predictive measures of telomere length. Childhood hyperactive-impulsive symptoms was the only predictor associated with telomere length. Moreover, this association was in the opposite direction of what was predicted, with significantly longer telomere length associated with more hyperactive-impulsive symptoms in childhood. Contrary to the hypotheses, all other predictors were not associated with telomere length after correcting for multiple testing.
These results raise questions about whether child and adult hyperactivity-impulsivity symptoms are distinctly different from other psychiatric symptoms in their link with telomere length. Our findings would suggest that telomere length is related to childhood symptoms and not to symptoms or stress as a result of symptoms in adulthood at the time of telomere assessment. Moreover, the direction of association suggests that more symptoms of hyperactivity-impulsivity in childhood is protective against telomere shortening. Telomere length is highly dependent on parental or inherited factors (paternal age, genes for telomerase and other telomere maintaining factors), physiologic and environmental context, somatic growth rate, and age (Corbett & Alda, 2015; Monaghan & Ozanne, 2018). We were not able to assess for inheritance in this study but did covary for age. From these other sources that contribute to telomere length, an intriguing possibility for our findings is decreased rate of somatic growth in individuals with childhood hyperactive-impulsive symptoms (Spencer, Biederman, & Wilens, 1998). This was apparent even when covarying for childhood stimulant use, a possible source of slowed growth (Faraone, Lecendreux, & Konofal, 2012). Alternatively, our findings may indicate a particular physiology or environment which leads to telomere maintenance and reduced cellular stress that affected individuals who had more childhood hyperactive-impulsive symptoms. If the effects of medication were not fully controlled in our analysis, their role as potential antioxidants could contribute to these effects (Guney et al., 2015; Schmidt et al., 2010).
To date, only one other study has examined telomere length in relation to ADHD symptoms (Costa et al., 2015). Differing from the current results, Costa et al. (2015) found telomere length to be negatively associated with hyperactive-impulsive symptoms in children under age 16. It is important to note that the previous study used a population of 61 children and adolescents whose families had sought psychiatric services for attention and/or behavior problems. There were also other differences in the current and previous approaches. Thus, it may be that the differences in methods regarding participant age (young adult versus child/adolescent), source of DNA (blood versus saliva), qPCR parameters, covariates, population background (Brazilian versus US), and sample population (community versus clinic) account for the discrepancy in the hyperactive-impulsive symptom association with shorter telomere length. Moreover, discrepant findings are not out of the ordinary in research on telomere length and psychiatric diagnoses. For instance, research on schizophrenia and telomere length has also found mixed results (Cui, Prabhu, Nguyen, Devi, & Chung, 2017; Kao et al., 2008; Maurya et al., 2018; Nieratschker et al., 2013).
One interpretation of the absence of a negative association between telomere length and our hypothesized predictors - ADHD diagnosis, persistence, neurocognitive deficits, and psychosocial impairments – is the neurodevelopmental nature of ADHD as opposed to stress-related disorders, such as depression or anxiety. Another possible interpretation, invoking theories of telomere erosion in the setting of physiological stress is that individuals with ADHD do not experience the same magnitude of physiological stress as individuals with other psychiatric disorders, for which telomere shortening has been repeatedly found. While multiple studies show that the impairments of ADHD can contribute to psychological stress, individuals with ADHD may not experience as much stress from equally impairing symptoms compared to individuals with other psychiatric disorders perhaps because they adapt to functioning with such symptoms at an early age.
One finding of interest in this study not addressed by a priori hypotheses was the relationship between childhood stimulant use and shorter telomere length. We analyzed our data with this as a covariate to eliminate potential confounding. In addition to its impact on neuronal signaling, psychostimulant medication may have an influence on non-neural cellular processes—for example, cultured bone progenitors proliferate less in response to methylphenidate (Gumustas et al., 2017). A limitation of our covariate approach is the potential elimination of real diagnosis and symptom related variance in telomere length. This may also contribute to a difference between our results and those previously reported (Costa et al., 2015).
There are several limitations of the current study that are important to consider. First, the sample was recruited from the community, as opposed to a clinic-recruited sample. The rate of treatment of ADHD symptoms, the level of impairments, and patterns of comorbidity in the current sample do not differ from typical clinical populations, but the sample does differ in the exposure to psychosocial adversity compared to most clinical populations. The individuals included in the current study largely came from middle class households and had, on average, 14 years of education. Thus, it may be that the individuals in the current sample did not experience a similar magnitude of psychosocial adversity, and therefore psychological stress, as individuals seen in psychiatric clinics. Finally, many of the measures, including the items used to derive the psychosocial impairments, were self-report.
Future research on telomere length and ADHD should assess the association in different populations. Clinic-referred populations may have individuals who have endured higher levels of psychosocial adversity and stress. Thus, it is important to understand whether the current findings extend to a larger population of individuals experiencing more extreme adversity. Additionally, future work should collect information on Adverse Childhood Experiences as individuals with ADHD have an increased risk for experiencing abuse during childhood (Hunt, Slack, & Berger, 2017).
The current study provides an important contribution to the field of research on ADHD and psychiatric diagnoses more broadly. Although ADHD is a disorder that may cause impairment and psychological stress, the degree of impairments and stress may not cause physiological stress to the extent that telomere length in peripheral cells are impacted. However, the current findings should be interpreted cautiously given the limitations of the study and the dearth of research on ADHD and telomere length. Future efforts should continue to examine the association of telomere length and ADHD in clinic-referred populations, across different ages, and with additional measurement of childhood adversity.
Acknowledgments
Funding: Allison Momany was supported by NIHGMS grant T32 GM108540.
Funding The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Allison Momany was supported by NIHGMS grant T32 GM108540. The research was supported by a University of Iowa DeLTA Center Interdisciplinary Research Grant.
Biographical Statements:
Allison Momany is a Clinical Psychology PhD candidate in the Department of Psychological and Brain Sciences at the University of Iowa and is mentored by Dr. Nikolas. Her research interests include prenatal, neonatal, and genetic risk factors for neurodevelopmental disorders.
Stephanie Lussier completed a Master of Science in biostatistics at the University of Iowa College of Public Health. While completing her degree, Stephanie worked in Dr. Stevens’ laboratory. She is currently employed by Rho Federal Systems Division.
Molly Nikolas, PhD, is an associate professor in the Department of Psychological and Brain Sciences at the University of Iowa. Her research examines gene-environment interplay in developmental psychopathology.
Hanna Stevens, MD, PhD is an associate professor of psychiatry and the division director of child psychiatry in the Carver College of Medicine at the University of Iowa. Her research seeks to understand molecular and cellular aspects of early brain development and their relevance to psychiatric disorders.
Footnotes
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Adler N, Pantell MS, O’Donovan A, Blackburn E, Cawthon R, Koster A, … Epel E. (2013). Educational attainment and late life telomere length in the Health, Aging and Body Composition Study. Brain Behav Immun, 27(1), 15–21. doi: 10.1016/j.bbi.2012.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti Y, Wardhana A, Watkins J, Wulaningsih W, & Network PR. (2017). Cigarette smoking and telomere length: A systematic review of 84 studies and meta-analysis. Environ Res, 158, 480–489. doi: 10.1016/j.envres.2017.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlantis E, Vogelzangs N, Cashman K, & Penninx BJ. (2012). Common mental disorders associated with 2-year diabetes incidence: the Netherlands Study of Depression and Anxiety (NESDA). J Affect Disord, 142 Suppl, S30–35. doi: 10.1016/S0165-0327(12)70006-X [DOI] [PubMed] [Google Scholar]
- Barkley RA. (2011). Barkley Functional Impairment Scale (BFIS). New York, NY: Guilford Press. [Google Scholar]
- Blakesley RE, Mazumdar S, Dew MA, Houck PR, Tang G, Reynolds CF 3rd, & Butters MA. (2009). Comparisons of methods for multiple hypothesis testing in neuropsychological research. Neuropsychology, 23(2), 255–264. doi: 10.1037/a0012850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco MA. (2005). Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet, 6(8), 611–622. doi: 10.1038/nrg1656 [DOI] [PubMed] [Google Scholar]
- Carroll JE, Diez-Roux AV, Adler NE, & Seeman TE. (2013). Socioeconomic factors and leukocyte telomere length in a multi-ethnic sample: findings from the multi-ethnic study of atherosclerosis (MESA). Brain Behav Immun, 28, 108–114. doi: 10.1016/j.bbi.2012.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SRWL, & Blackburn E. (2004). Telomeres and telomerase. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 359(1441), 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Hamer M, Wardle J, & Steptoe A. (2008). Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol, 5(8), 466–475. doi: 10.1038/ncponc1134 [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R. (1983). A global measure of perceived stress. J Health Soc Behav, 24(4), 385–396. [PubMed] [Google Scholar]
- Conners CK, & MHS Staff. (2002). Conners’ Continuous Performance Test II: Computer program for windows technical guide and software manual. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Corbett N, & Alda M. (2015). On telomeres long and short. J Psychiatry Neurosci, 40(1), 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa D, Rosa DV, Barros AG, Romano-Silva MA, Malloy-Diniz LF, Mattos P, & de Miranda DM. (2015). Telomere length is highly inherited and associated with hyperactivity-impulsivity in children with attention deficit/hyperactivity disorder. Front Mol Neurosci, 8, 28. doi: 10.3389/fnmol.2015.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Prabhu VV, Nguyen TB, Devi SM, & Chung YC. (2017). Longer Telomere Length of T lymphocytes in Patients with Early and Chronic Psychosis. Clin Psychopharmacol Neurosci, 15(2), 146–152. doi: 10.9758/cpn.2017.15.2.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, & Kramer JH. (2001). Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Denollet J, Maas K, Knottnerus A, Keyzer JJ, & Pop VJ. (2009). Anxiety predicted premature all-cause and cardiovascular death in a 10-year follow-up of middle-aged women. J Clin Epidemiol, 62(4), 452–456. doi: 10.1016/j.jclinepi.2008.08.006 [DOI] [PubMed] [Google Scholar]
- Drury SS, Mabile E, Brett ZH, Esteves K, Jones E, Shirtcliff EA, & Theall KP. (2014). The association of telomere length with family violence and disruption. Pediatrics, 134(1), e128–137. doi: 10.1542/peds.2013-3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, & Antshel KM. (2008). Diagnosing and treating attention-deficit/hyperactivity disorder in adults. World Psychiatry, 7(3), 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, & Mick E. (2006). The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med, 36(2), 159–165. doi: 10.1017/S003329170500471X [DOI] [PubMed] [Google Scholar]
- Faraone SV, Lecendreux M, & Konofal E. (2012). Growth dysregulation and ADHD: an epidemiologic study of children in France. J Atten Disord, 16(7), 572–578. doi: 10.1177/1087054711413083 [DOI] [PubMed] [Google Scholar]
- Friedrichs B, Igl W, Larsson H, & Larsson JO. (2012). Coexisting psychiatric problems and stressful life events in adults with symptoms of ADHD--a large Swedish population-based study of twins. J Atten Disord, 16(1), 13–22. doi: 10.1177/1087054710376909 [DOI] [PubMed] [Google Scholar]
- Gansler DA, Fucetola R, Krengel M, Stetson S, Zimering R, & Makary C. (1998). Are there cognitive subtypes in adult attention deficit/hyperactivity disorder? J Nerv Ment Dis, 186(12), 776–781. [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Pearson JA, Linnenbringer E, Schulz AJ, Reyes AG, Epel ES, … Blackburn EH. (2015). Race-Ethnicity, Poverty, Urban Stressors, and Telomere Length in a Detroit Community-based Sample. J Health Soc Behav, 56(2), 199–224. doi: 10.1177/0022146515582100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumustas F, Yilmaz I, Sirin DY, Gumustas SA, Batmaz AG, Isyar M, … Mahirogullari M. (2017). Chondrocyte proliferation, viability and differentiation is declined following administration of methylphenidate utilized for the treatment of attention-deficit/hyperactivity disorder. Hum Exp Toxicol, 36(9), 981–992. doi: 10.1177/0960327116678294 [DOI] [PubMed] [Google Scholar]
- Guney E, Cetin FH, Alisik M, Tunca H, Tas Torun Y, Iseri E, … Erel O. (2015). Attention Deficit Hyperactivity Disorder and oxidative stress: A short term follow up study. Psychiatry Res, 229(1–2), 310–317. doi: 10.1016/j.psychres.2015.07.003 [DOI] [PubMed] [Google Scholar]
- Hadchouel A, Marchand-Martin L, Franco-Montoya ML, Peaudecerf L, Ancel PY, Delacourt C, & group E. s. (2015). Salivary Telomere Length and Lung Function in Adolescents Born Very Preterm: A Prospective Multicenter Study. PLoS One, 10(9), e0136123. doi: 10.1371/journal.pone.0136123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt G, Jurk D, Marques FD, Correia-Melo C, Hardy T, Gackowska A, … Passos JF. (2012). Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun, 3, 708. doi: 10.1038/ncomms1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvikoski T, Lindholm T, Nordenstrom A, Nordstrom AL, & Lajic S. (2009). High self-perceived stress and many stressors, but normal diurnal cortisol rhythm, in adults with ADHD (attention-deficit/hyperactivity disorder). Horm Behav, 55(3), 418–424. doi: 10.1016/j.yhbeh.2008.12.004 [DOI] [PubMed] [Google Scholar]
- Hunt TKA, Slack KS, & Berger LM. (2017). Adverse childhood experiences and behavioral problems in middle childhood. Child Abuse Negl, 67, 391–402. doi: 10.1016/j.chiabu.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip P, Chung BH, Ho FK, Chan GC, Deng W, Wong WH, … Lau YL. (2017). Prenatal Tobacco Exposure Shortens Telomere Length in Children. Nicotine Tob Res, 19(1), 111–118. doi: 10.1093/ntr/ntw139 [DOI] [PubMed] [Google Scholar]
- Kajantie E, Pietilainen KH, Wehkalampi K, Kananen L, Raikkonen K, Rissanen A, … Hovatta I. (2012). No association between body size at birth and leucocyte telomere length in adult life--evidence from three cohort studies. Int J Epidemiol, 41(5), 1400–1408. doi: 10.1093/ije/dys127 [DOI] [PubMed] [Google Scholar]
- Kamradt JM, Ullsperger JM, & Nikolas MA. (2014). Executive Function Assessment and Adult Attention-Deficit/Hyperactivity Disorder: Tasks Versus Ratings on the Barkley Deficits in Executive Functioning Scale. Psychological Assessment, 26(4), 1095–1105. doi: 10.1037/pas0000006 [DOI] [PubMed] [Google Scholar]
- Kao HT, Cawthon RM, Delisi LE, Bertisch HC, Ji F, Gordon D, … Porton B. (2008). Rapid telomere erosion in schizophrenia. Mol Psychiatry, 13(2), 118–119. doi: 10.1038/sj.mp.4002105 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Ames M, Demler O, Faraone S, Hiripi E, … Walters EE. (2005). The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med, 35(2), 245–256. [DOI] [PubMed] [Google Scholar]
- Kohn PM, & Macdonald JE. (1992). The Survey of Recent Life Experiences: a decontaminated Hassles Scale for adults. J Behav Med, 15(2), 221–236. [DOI] [PubMed] [Google Scholar]
- Lima IMM, Barros A, Rosa DV, Albuquerque M, Malloy-Diniz L, Neves FS, … de Miranda DM. (2015). Analysis of telomere attrition in bipolar disorder. Journal of Affective Disorders, 172, 43–47. doi: 10.1016/j.jad.2014.09.043 [DOI] [PubMed] [Google Scholar]
- Logan GD. (1994). On the ability to inhibit thought and action: A users’ guide to the stop signal paradigm. In Dagenbach D & Carr TH. (Eds.), Inhibitory processes in attention, memory, and language (pp. 189–239). San Diego, CA: Academic Press. [Google Scholar]
- Lu L, Johnman C, McGlynn L, Mackay DF, Shiels PG, & Pell JP. (2017). Association between exposure to second-hand smoke and telomere length: cross-sectional study of 1303 non-smokers. Int J Epidemiol, 46(6), 1978–1984. doi: 10.1093/ije/dyx212 [DOI] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, & Zitman FG. (2010). Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry, 67(3), 220–229. doi: 10.1001/archgenpsychiatry.2010.2 [DOI] [PubMed] [Google Scholar]
- Lustig A, Shterev I, Geyer S, Shi A, Hu Y, Morishita Y, … Hayashi T. (2016). Long term effects of radiation exposure on telomere lengths of leukocytes and its associated biomarkers among atomic-bomb survivors. Oncotarget, 7(26), 38988–38998. doi: 10.18632/oncotarget.8801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DK, & Barry TD. (2011). Does attention-deficit/hyperactivity disorder have a dimensional latent structure? A taxometric analysis. J Abnorm Psychol, 120(2), 427–442. doi: 10.1037/a0021405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur MB, Epel E, Kind S, Desai M, Parks CG, Sandler DP, & Khazeni N. (2016). Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain Behav Immun, 54, 158–169. doi: 10.1016/j.bbi.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya PK, Rizzo LB, Xavier G, Tempaku PF, Ota VK, Santoro ML, … Belangero SI. (2018). Leukocyte telomere length variation in different stages of schizophrenia. J Psychiatr Res, 96, 218–223. doi: 10.1016/j.jpsychires.2017.10.016 [DOI] [PubMed] [Google Scholar]
- Mayer S, Bruderlein S, Perner S, Waibel I, Holdenried A, Ciloglu N, … Moller P. (2006). Sex-specific telomere length profiles and age-dependent erosion dynamics of individual chromosome arms in humans. Cytogenet Genome Res, 112(3–4), 194–201. doi: 10.1159/000089870 [DOI] [PubMed] [Google Scholar]
- Miklosi M, Mate O, Somogyi K, & Szabo M. (2016). Adult Attention Deficit Hyperactivity Disorder Symptoms, Perceived Stress, and Well-Being: The Role of Early Maladaptive Schemata. J Nerv Ment Dis, 204(5), 364–369. doi: 10.1097/NMD.0000000000000472 [DOI] [PubMed] [Google Scholar]
- Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, … Notterman D. (2014). Social disadvantage, genetic sensitivity, and children’s telomere length. Proc Natl Acad Sci U S A, 111(16), 5944–5949. doi: 10.1073/pnas.1404293111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan P, & Ozanne SE. (2018). Somatic growth and telomere dynamics in vertebrates: relationships, mechanisms and consequences. Philos Trans R Soc Lond B Biol Sci, 373(1741). doi: 10.1098/rstb.2016.0446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham BL, Adler N, Gregorich S, Rehkopf D, Lin J, Blackburn EH, & Epel ES. (2013). Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc Sci Med, 85, 1–8. doi: 10.1016/j.socscimed.2013.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieratschker V, Lahtinen J, Meier S, Strohmaier J, Frank J, Heinrich A, … Hovatta I. (2013). Longer telomere length in patients with schizophrenia. Schizophr Res, 149(1–3), 116–120. doi: 10.1016/j.schres.2013.06.043 [DOI] [PubMed] [Google Scholar]
- Nigg JT, Stavro G, Ettenhofer M, Hambrick DZ, Miller T, & Henderson JM. (2005). Executive functions and ADHD in adults: evidence for selective effects on ADHD symptom domains. J Abnorm Psychol, 114(4), 706–717. doi: 10.1037/0021-843X.114.3.706 [DOI] [PubMed] [Google Scholar]
- Nikolas MA, & Nigg JT. (2013). Neuropsychological performance and attention-deficit hyperactivity disorder subtypes and symptom dimensions. Neuropsychology, 27(1), 107–120. doi: 10.1037/a0030685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan RJ, & Karlseder J. (2010). Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol, 11(3), 171–181. doi: 10.1038/nrm2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opresko PL, Fan J, Danzy S, Wilson DM 3rd, & Bohr VA. (2005). Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res, 33(4), 1230–1239. doi: 10.1093/nar/gki273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polho GB, De-Paula VJ, Cardillo G, dos Santos B, & Kerr DS. (2015). Leukocyte telomere length in patients with schizophrenia: A meta-analysis. Schizophr Res, 165(2–3), 195–200. doi: 10.1016/j.schres.2015.04.025 [DOI] [PubMed] [Google Scholar]
- Ridout KK, Ridout SJ, Price LH, Sen S, & Tyrka AR. (2016). Depression and telomere length: A meta-analysis. J Affect Disord, 191, 237–247. doi: 10.1016/j.jad.2015.11.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihu HM, Pradhan A, King L, Paothong A, Nwoga C, Marty PJ, & Whiteman V. (2015). Impact of intrauterine tobacco exposure on fetal telomere length. Am J Obstet Gynecol, 212(2), 205 e201–208. doi: 10.1016/j.ajog.2014.08.026 [DOI] [PubMed] [Google Scholar]
- Sandson TA, Bachna KJ, & Morin MD. (2000). Right hemisphere dysfunction in ADHD: visual hemispatial inattention and clinical subtype. J Learn Disabil, 33(1), 83–90. doi: 10.1177/002221940003300111 [DOI] [PubMed] [Google Scholar]
- Schmidt AJ, Krieg JC, Clement HW, Gebhardt S, Schulz E, & Heiser P. (2010). Impact of drugs approved for treating ADHD on the cell survival and energy metabolism: an in-vitro study in human neuronal and immune cells. J Psychopharmacol, 24(12), 1829–1833. doi: 10.1177/0269881109105563 [DOI] [PubMed] [Google Scholar]
- Shaffer JA, Epel E, Kang MS, Ye S, Schwartz JE, Davidson KW, … Shimbo D. (2012). Depressive symptoms are not associated with leukocyte telomere length: findings from the Nova Scotia Health Survey (NSHS95), a population-based study. PLoS One, 7(10), e48318. doi: 10.1371/journal.pone.0048318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, Goldman-Mellor S, … Caspi A. (2014). Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol Psychiatry, 19(11), 1163–1170. doi: 10.1038/mp.2013.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, … Wong KK. (2006). Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry, 60(5), 432–435. doi: 10.1016/j.biopsych.2006.02.004 [DOI] [PubMed] [Google Scholar]
- Simon NM, Walton ZE, Bui E, Prescott J, Hoge E, Keshaviah A, … Wong KK. (2015). Telomere length and telomerase in a well-characterized sample of individuals with major depressive disorder compared to controls. Psychoneuroendocrinology, 58, 9–22. doi: 10.1016/j.psyneuen.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets CC, Codd V, Samani NJ, & Hokken-Koelega AC. (2015). Leukocyte Telomere Length in Young Adults Born Preterm: Support for Accelerated Biological Ageing. PLoS One, 10(11), e0143951. doi: 10.1371/journal.pone.0143951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T, Biederman J, & Wilens T. (1998). Growth deficits in children with attention deficit hyperactivity disorder. Pediatrics, 102(2 Pt 3), 501–506. [PubMed] [Google Scholar]
- Strine TW, Lesesne CA, Okoro CA, McGuire LC, Chapman DP, Balluz LS, & Mokdad AH. (2006). Emotional and behavioral difficulties and impairments in everyday functioning among children with a history of attention-deficit/hyperactivity disorder. Prev Chronic Dis, 3(2), A52. [PMC free article] [PubMed] [Google Scholar]
- Taylor JM. (2015). Psychometric analysis of the Ten-Item Perceived Stress Scale. Psychol Assess, 27(1), 90–101. doi: 10.1037/a0038100 [DOI] [PubMed] [Google Scholar]
- van Lieshout M, Luman M, Buitelaar J, Rommelse NN, & Oosterlaan J. (2013). Does neurocognitive functioning predict future or persistence of ADHD? A systematic review. Clin Psychol Rev, 33(4), 539–560. doi: 10.1016/j.cpr.2013.02.003 [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, Revesz D, Epel ES, Lin J, Wolkowitz OM, & Penninx BW. (2014). Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry, 19(8), 895–901. doi: 10.1038/mp.2013.151 [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1999). Manual for the Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler D. (2008). Manual for the Wechsler Adult Intelligence Scale - Fourth Edition. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Whisman MA, & Richardson ED. (2017). Depressive Symptoms and Salivary Telomere Length in a Probability Sample of Middle-Aged and Older Adults. Psychosom Med, 79(2), 234–242. doi: 10.1097/PSY.0000000000000383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikgren M, Maripuu M, Karlsson T, Nordfjall K, Bergdahl J, Hultdin J, … Norrback KF. (2012). Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry, 71(4), 294–300. doi: 10.1016/j.biopsych.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Wojcicki JM, Heyman MB, Elwan D, Shiboski S, Lin J, Blackburn E, & Epel E. (2015). Telomere length is associated with oppositional defiant behavior and maternal clinical depression in Latino preschool children. Transl Psychiatry, 5, e581. doi: 10.1038/tp.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Reus VI, & Mellon SH. (2010). Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety, 27(4), 327–338. doi: 10.1002/da.20686 [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Jeste DV, Martin AS, Lin J, Daly RE, Reuter C, & Kraemer H. (2017). Leukocyte telomere length: Effects of schizophrenia, age, and gender. J Psychiatr Res, 85, 42–48. doi: 10.1016/j.jpsychires.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Ye J, Renault VM, Jamet K, & Gilson E. (2014). Transcriptional outcome of telomere signalling. Nat Rev Genet, 15(7), 491–503. doi: 10.1038/nrg3743 [DOI] [PubMed] [Google Scholar]
- Zhu H, Wang X, Gutin B, Davis CL, Keeton D, Thomas J, … Dong Y. (2011). Leukocyte telomere length in healthy Caucasian and African-American adolescents: relationships with race, sex, adiposity, adipokines, and physical activity. J Pediatr, 158(2), 215–220. doi: 10.1016/j.jpeds.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]