Abstract
Objective
This study focused on the efficacy and safety of thalidomide for patients with β-thalassemia in a multicenter trial.
Methods
Patients with non-transfusion-dependent thalassemia (NTDT) or transfusion-dependent thalassemia (TDT), who were unable to pursue conventional therapy with transfusion and chelation, were recruited over 3 years in three centers in southern China. We evaluated the efficacy and safety of thalidomide in the short-term (three months) and long-term follow-up (12 and 24 months). Response to thalidomide was defined as follows: Main Responder (MaR) showing an increase in hemoglobin (Hb) level of >2.0 g/dl or free from blood transfusion and Minor Responder (MiR) achieving elevated Hb level of 1.0–2.0 g/dl or ≥50% reduction in blood transfusion frequency.
Results
The overall response rate (ORR) was 93.5%, with MaR and MiR rates accounting for 62.9% and 30.6% in short-term follow-up. For patients with NTDT, the Hb level increased from a baseline mean of 6.8±1.1 g/dl to 9.7±1.9 g/dl (P<0.001). Elevated Hb was mainly attributable to increased fetal hemoglobin (HbF) levels. Among patients with TDT, while an increase in the average Hb concentration was observed, there was a significant drop in yearly transfusions from 20.7±7.7 to 5.8±6.8 blood units per year (P<0.001). The response of patients in both categories was sustained even after an average follow up of 14.6±9.6 months (3–37 months). Minimal side-effects were documented throughout, except peripheral neurotoxicity in one patient. Logistic regression analysis identified the ratio of HbF at baseline (P=0.038, OR=1.111, 95% CI: 1.006–1.226) as an independent risk factor for the primary response to thalidomide.
Conclusion
Thalidomide had significant therapeutic effects on patients with β-thalassemia with a sustained response. Peripheral neuropathy is one of the most feared complications. While these preliminary results support the potential long-term efficacy of thalidomide as a therapeutic agent for β-thalassemia, several issues need to be addressed before its application in the clinic.
Keywords: Thalidomide, β-thalassemia, Fetal hemoglobin, Efficacy, Safety
Introduction
Thalassemia incorporates a group of hereditary hematological diseases caused by disorders in α/β-globin chain synthesis. Currently, thalassemia syndromes can be classified phenotypically into non-transfusion-dependent thalassemia (NTDT) or transfusion-dependent thalassemia (TDT) based on their clinical severity and transfusion requirements.1 However, more transfusions may also be required for NTDT patients whose clinical course has evolved with age. Such patients ultimately become regularly transfused.2,3 The predominant hemoglobin (Hb) variant expressed by the fetus and newborn is fetal hemoglobin (HbF, α2γ2), is progressively replaced with adult hemoglobin (HbA, α2β2) after birth. In patients with β-thalassemia, γ-globin can combine with redundant α-globin chains and compensate for the lack of β-globin chains.1 Observational studies have suggested that inducing synthesis of HbF may be effective in alleviating clinical manifestations in β-thalassemia patients.4,5 Drugs capable of improving the synthesis of HbF and then anemia and quality of life have therefore been investigated.
Several promising fetal hemoglobin (HbF) inducers, including hydroxyurea, erythropoietin, 5-azacytidine, and sodium butyrate,4,6 have achieved limited success for the treatment of β-thalassemia to date. Hydroxyurea, approved by the FDA for the treatment of sickle cell disease (SCD), is also the most widely used HbF inducer in β-thalassemia. However, the clinical application of hydroxyurea is limited by the low number of significant responders,7,8 reduced clinical response with long follow-up,9,10 and bone marrow suppression.11 Therefore, better treatments facilitating improved outcomes are increasingly important. Thalidomide, a synthetic glutamic acid derivative, is widely used as an immunomodulator for the treatment of various hematological cancers due to its anti-inflammatory, anti-angiogenic, and anti-tumor effects.12 Moreover, thalidomide is an HbF inducer that promotes γ-globin gene expression.13,14 A few case reports and retrospective analyses have documented significant effects of thalidomide on NTDT or TDT,15–20 that our group subsequently confirmed in a clinical trial.21 However, the reliability of these studies was softened by the few patients studied and short-term follow-up. In the current study, we analyzed the efficacy and safety of thalidomide for β-thalassemia in a relatively large patient sample over a long-term follow-up period.
Patients and Methods
In the period from May 2016 to June 2019, 71 patients with duration of therapy over 3 months, and follow-up data were recruited. The following inclusion criteria were adopted in the trial: 1) patients with a clinical and genetic diagnosis of β-thalassemia requiring blood transfusion, but unable to afford regular transfusions or iron chelation due to economic or other reasons; 2) patients between 14 and 65 years of age; 3) gender not limited; and 4) an ECOG physical score between 0 and 2 points. Patients with liver, renal, cardiac, pulmonary, or neurological deficits were excluded, as were patients with a history of thrombotic episodes. All females were checked for pregnancy, and pregnant patients were ruled out. All women enrolled were informed that they should absolutely avoid pregnancy during treatment and until 6 months after the withdrawal of medicine. Patients were informed of the side effects and possible benefits of thalidomide. Full informed consent was required before treatment was initiated. All patients were followed up by the hematology department of each research center during the observation period and received thalidomide treatment for at least three months. Except for supportive care with transfusions and iron chelation therapy, patients were required not to have received any therapy that affects Hbs for at least 3 months before starting the thalidomide treatment. The thalidomide protocol for patients with β-thalassemia was approved by the Medical Ethics Committee of the 923rd Hospital of the Joint Logistics Support Force of the Peoples Liberation Army, People’s Hospital of Guiping and People’s Hospital of Hezhou. The clinical trial was registered at ClinicalTrials.gov, registration number: NCT02995707.
The initial dose of thalidomide used was 50 mg/d, and a daily dose of 100 mg/d was given to patients needing blood transfusions at least twice a month. Aspirin (100 mg/d) was prescribed to patients post-splenectomy or those with platelet counts >500 × 109/L to prevent thrombosis. Patients were regularly followed up monthly during the first three months of treatment and every 2–3 months afterward. Baseline and follow-up records were reviewed for demographic data, transfusion history, splenic size, adverse reaction, and duration of therapy. A halving of the dosage was prescribed in cases where side effects were graded III or above. For patients who failed to respond, 50% of the current dose was increased every month after 3 months of treatment. If no response was observed within 6 months, thalidomide was discontinued, and the patient advised to resume conventional management. Within the four research centers, complete blood counts were analyzed using an XE 5000 automatic blood cell analyzer (Sysmex Corporation, Kobe, Japan). Different Hb levels were quantified using Bio-Rad Variant II high-pressure liquid chromatography (HPLC) (Bio-Rad, Hercules, CA, USA). Biochemical parameters and serum ferritin levels (SF) were assessed using a multichannel analyzer (Abbot Aeroset, Abbott Diagnostics, Bohemia, NY, USA) and chemiluminescence (Beckman Coulter, Inc., CA, USA).
Standard for determination of efficacy
For patients with NTDT, response to thalidomide was defined as follows: Main Responder (MaR) showing an increase in Hb level >2.0g/dl, Minor Responder (MiR) achieving elevation in Hb level of 1.0–2.0g/dl, and No Responder (NR) showing a <1g/dl increase in Hb level. For patients with TDT, the groups were defined by the following parameters: MaR, removal from the blood transfusion, MiR, ≥50% reduction in transfusion requirement, and NR, <50% reduction in transfusion requirement.4,7
Statistical analysis
SPSS Statistics 21.0 (SPSS Inc., Chicago, IL, USA) was applied for data analysis. Numerical data were presented as means ± SD or median and interquartile range (IQR). A paired t-test or Mann-Wilcoxon rank-sum test was applied to compare the changes in continual variables before and after treatment. Comparisons in numerical variables between two groups were performed with Student’s t-test or Mann-Whitney rank-sum test. Hb levels were assessed for comparability at each time-point using repeated measures analysis of variance (ANOVA). Chi-square or Fisher exact test was used to compare categorical variables for small sample size, followed by logistic regression using significant results from univariate analysis to confirm the association. P values <0.05 were considered statistically significant.
Results
Of the enrolled 71 patients, six were treated for less than 3 months, and three were without any follow-up data after treatment, leading to the final inclusion of 62 patients. The patient group comprised 27 males and 35 females, 39 NTDT and 23 TDT patients, and 29 splenectomized and 33 nonsplenectomized patients. The average age of patients was 27.2±7.9 years (range, 15–45 years). During the treatment period, the initial dose of 50 mg and 100 mg were 58 patients and 4 patients. Moreover, the median dose of thalidomide at the last follow-up was 50 mg/d (range 12.5 mg/d–150 mg/d). Fourteen of the patients discontinued treatment, four of whom were NRs and stopped within 3–6 months, and seven patients experienced dose modifications. The average duration of thalidomide treatment was 14.6±9.6 months (range, 3–37 months), with 34 patients treated over 12 months and 11 over 24 months.
Clinical features
In our cohort, alleviation of fatigue, and an increase in the energy state, well-being, and physical activity was detected in 88.7% (55/62) of the patients. Facial changes were observed in 69.4% (43/62) of the patients at the end of the study. In nonsplenectomized responders, average spleen size (length × width) was not significantly altered (110.1±20.3 cm2 vs 114.7±29.6 cm2, n=14, P=0.493).
Short-term follow-up
After a 3-month treatment period, 62.9% (39/62) and 30.6% (19/62) of the patients showed MaR and MiR status, respectively, while 6.5% (4/62) were classified as NR. As shown in Table 1, for patients with NTDT, the Hb level increased from a baseline mean of 6.8±1.1 g/dl to 9.7±1.9 g/dl (P<0.001), with an average increase of 2.9±1.6g/dl. Elevated Hb was mainly attributable to increased HbF levels. The average HbF percentage increased from a pretreatment level of 41.9±23.4% to 54.3±23.0% (P<0.001) after treatment. Among patients with TDT, transfusions were terminated in 43.5% (10/23) of the patients and decreased by more than 50% in 52.2% (12/23) of the patients, an increase in the average hemoglobin concentration was contemporarily observed. There was a significant drop in yearly transfusions from 20.7±7.7 to 5.8±6.8 blood units per year. After treatment, the red blood cell (RBC) count was markedly increased, nucleated red blood cells (NRBC) were significantly decreased, and reticulocyte counts were not significantly changed. As mean cell volume (MCV) decreased, mean corpuscular Hb concentration (MCHC) significantly increased. Parameters reflecting hemolysis, including bilirubin and lactate dehydrogenase (LDH), showed a significant decrease. However, after the treatment, the overall average SF was not decreased but increased to a significant extent.
Table 1.
Laboratory data of the patients with β-thalassemia and treatment with thalidomide.
| Parameters | NTDT | TDT | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Before | After | P value | Before | After | P value | |||
|
|
|
|||||||
| Hb (g/dl) | 3M (n=39) | 6.8±1.1 | 9.7±1.9 | <0.001 | 3M (n=23) | 6.1±1.7 | 8.5±1.4 | <0.001 |
| 12M (n=20) | 7.0±1.0 | 10.2±1.8 | <0.001 | 12M (n=14) | 5.6±1.8 | 9.1±1.6 | <0.001 | |
| 24M (n=7) | 7.1±0.7 | 10.0±1.5 | <0.001 | 24M (n=4) | 4.8±2.0 | 9.9±1.1 | <0.001 | |
| RBC(×1012/L) | 3M (n=39) | 3.3±0.7 | 4.7±0.9 | <0.001 | 3M (n=23) | 2.7±0.7 | 4.0±0.7 | <0.001 |
| 12M (n=20) | 3.4±0.5 | 4.9±1.0 | <0.001 | 12M (n=14) | 2.4±0.7 | 4.3±0.8 | <0.001 | |
| 24M (n=7) | 3.4±0.4 | 4.5±1.0 | 0.002 | 24M (n=4) | 2.2±0.7 | 4.7±0.3 | 0.007 | |
| HbF(%) | 3M (n=32) | 41.9±23.4 | 54.3±23.0 | <0.001 | 3M (n=14) | 34.7±21.3 | 52.2±22.7 | <0.001 |
| 12M (n=11) | 38.9±22.3 | 46.4±20.3 | 0.047 | 12M (n=5) | 35.6±22.6 | 60.8±18.8 | 0.015 | |
| 24M (n=6) | 39.1±19.4 | 44.1±20.1 | 0.023 | - | - | - | - | |
| NRBC (%) | 3M (n=29) | 176.6±215.1 | 63.8±90.9 | <0.001 | 3M (n=14) | 283.4±645.0 | 194.3±267.4 | 0.553 |
| 12M (n=14) | 178.4±208.6 | 86.7±102.5 | 0.015 | 12M (n=5) | 38.8±45.2 | 7.3±8.9 | 0.138 | |
| 24M (n=3) | 393.0±205.7 | 251.3±182.1 | 0.068 | - | - | - | - | |
| Ret (%) | 3M (n=27) | 7.7±5.0 | 7.7±5.7 | 0.957 | 3M (n=12) | 6.9±7.0 | 6.6±6.4 | 0.824 |
| 12M (n=10) | 6.6±6.0 | 8.7±7.6 | 0.468 | 12M (n=4) | 2.3±1.6 | 8.0±7.4 | 0.249 | |
| 24M (n=5) | 10.3±7.0 | 11.4±9.5 | 0.784 | 24M (n=3) | 3.1±1.4 | 3.8±2.6 | 0.597 | |
| MCV (fL) | 3M (n=39) | 71.3±9.1 | 66.6±7.8 | <0.001 | 3M (n=23) | 72.6±8.1 | 69.1±7.9 | 0.005 |
| 12M (n=20) | 70.0±8.0 | 66.2±7.7 | 0.039 | 12M (n=14) | 71.0±9.3 | 66.6±9.0 | 0.031 | |
| 24M (n=7) | 71.0±7.5 | 69.7±3.9 | 0.452 | 24M (n=4) | 66.0±7.4 | 65.3±5.6 | 0.624 | |
| MCH (pg) | 3M (n=39) | 21.3±2.8 | 20.7±2.9 | 0.168 | 3M (n=23) | 22.6±2.8 | 21.5±2.4 | 0.014 |
| 12M (n=20) | 20.7±2.6 | 20.5±2.2 | 0.610 | 12M (n=14) | 22.0±3.0 | 21.5±2.5 | 0.421 | |
| 24M (n=7) | 20.8±2.2 | 21.5±1.5 | 0.166 | 24M (n=4) | 21.0±2.8 | 21.1±2.2 | 0.818 | |
| MCHC (g/L) | 3M (n=39) | 299.2±19.1 | 314.3±15.0 | <0.001 | 3M (n=23) | 311.0±14.0 | 312.0±14.3 | 0.793 |
| 12M (n=20) | 297.2±18.4 | 310.5±18.1 | 0.001 | 12M (n=14) | 310.2±13.9 | 322.5±19.9 | 0.020 | |
| 24M (n=7) | 294.6±15.2 | 309.1±12.1 | 0.006 | 24M (n=4) | 316.3±10.5 | 323.5±21.7 | 0.342 | |
| TBIL (μmol/L) | 3M (n=36) | 67.7±28.6 | 54.9±22.8 | 0.001 | 3M (n=22) | 55.3±27.7 | 51.9±24.2 | 0.205 |
| 12M (n=19) | 69.8±30.8 | 55.5±20.8 | 0.008 | 12M (n=10) | 47.6±22.6 | 44.2±30.0 | 0.395 | |
| 24M (n=7) | 78.7±40.5 | 62.7±30.4 | 0.125 | 24M (n=3) | 47.4±12.3 | 48.9±19.3 | 0.851 | |
| IBIL (μmol/L) | 3M (n=36) | 50.0±26.9 | 41.4±23.8 | 0.012 | 3M (n=22) | 38.7±21.0 | 35.9±20.6 | 0.228 |
| 12M (n=19) | 50.5±27.0 | 39.6±20.2 | 0.052 | 12M (n=10) | 34.0±23.3 | 31.7±26.6 | 0.278 | |
| 24M (n=7) | 63.1±39.7 | 45.0±28.8 | 0.110 | 24M (n=3) | 34.7±13.2 | 31.5±14.8 | 0.581 | |
| LDH (IU/L) | 3M (n=20) | 412.2±207.6 | 263.4±186.3 | <0.001 | 3M (n=10) | 329.3±127.2 | 238.3±115.1 | 0.004 |
| SF (ng/ml) | 3M (n=30) | 1821.6±1488.3 | 2158.5±1529.5 | 0.039 | 3M (n=18) | 3035.3±2280.4 | 3848.5±2827.7 | 0.043 |
| 12M (n=18) | 1928.7±1483.0 | 2693.3±2032.6 | 0.009 | 12M (n=8) | 2303.1±1850.3 | 3442.4±2099.7 | 0.026 | |
| 24M (n=7) | 1595.3±1021.2 | 2654.0±1882.6 | 0.025 | 24M (n=3) | 1449.7±772.9 | 1924.1±342.6 | 0.402 | |
Abbreviations: NTDT: non-transfusion-dependent thalassemia; TDT: transfusion-dependent thalassemia; Hb: hemoglobin; HbF: fetal hemoglobin; RBC: red blood cell count; NRBC: nucleated red blood cells; Ret: reticulocyte; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; TBIL: total bilirubin; IBIL: indirect bilirubin; LDH: lactate dehydrogenase; SF: serum ferritin.
Long-term follow-up
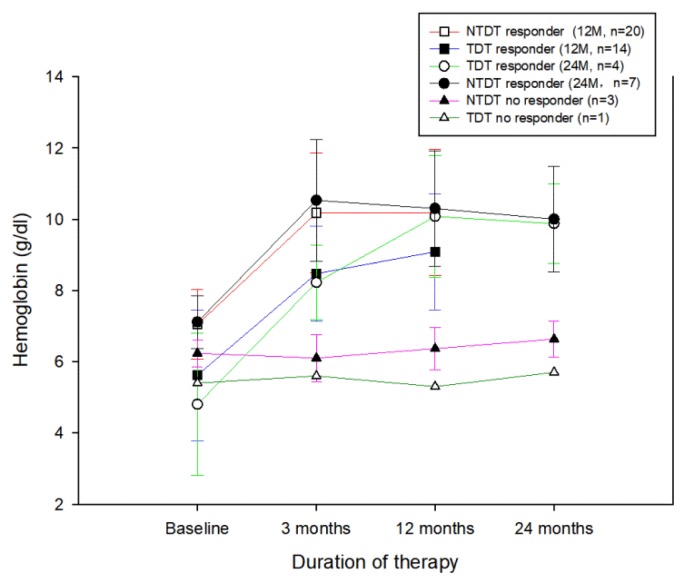
Four NRs among the 62 patients discontinued therapy, respectively, after 3–6 months. Table 1 depicts the changes in clinical and laboratory efficacy indicators of patients with NTDT or TDT after long-term follow-up. In total, 34 of the 58 responders were treated with thalidomide for >12 months, averaging 21.9±6.7 months (12–37 months). In 28 responders, the long-term follow-up response was not significantly different from the 3-month response. Among the remaining six patients, two showed a decreased therapeutic effect, changing from MaR to MiR status, and four improved from MiR to MaR. Repeated measures ANOVA showed that Hb levels at different follow-up time points of 3 and 12 months after treatment increased significantly, compared with baseline values in NTDT (F=58.682, P<0.001) and TDT (F=22.259, P<0.001), with no significant differences between the 3 and 12-month period (P>0.05) (Figure 1).
Figure 1.
Hemoglobin levels of the patients receiving thalidomide therapy.
The duration of therapy for 11 patients exceeded 24 months, with an average treatment period of 30.1±3.8 months (range 24–37 months). Ten patients maintained clinical response during the observation period, and only one changed status from MaR to MiR. Similarly, Hb levels of the groups at different follow-up time-points (3, 12, and 24 months) were significantly increased compared with baseline levels (NTDT: F=29.411, P<0.001; TDT: F=15.835, P=0.001). Average Hb was comparable at 3, 12, and 24 months (P>0.05) (Figure 1).
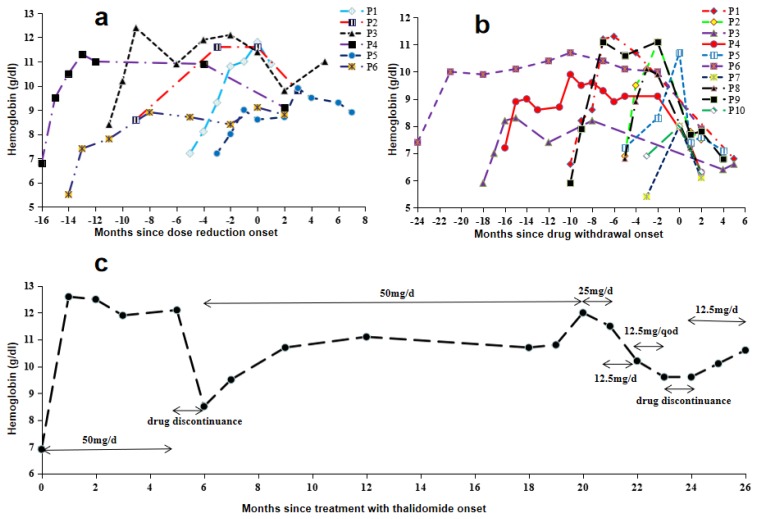
Effect of dose adjustment on Hb
In total, seven patients experienced dose reduction during treatment. The levels of Hb before and after reduction are shown in Figure 2a. Two patients with doses reduced from 100 to 50 mg/d maintained constant levels of Hb. The Hb level decreased in the remaining four patients for whom the dose was reduced from 50 to 25 mg/d, but three retained MaR status, and only one changed to MiR.
Figure 2.
Hemoglobin levels of patients with drug discontinuation and adjustment of dosages. (a) Hemoglobin levels of patients with dose reduction. P1, P2, P3 and P4 indicate patients with a dose reduction from 50 mg/d to 25 mg/d. P5 and P6 signify patients with a dose reduction from 100 mg/d to 50 mg/d. (b) Hemoglobin levels of the patient subjected to multiple dose adjustments. (c) Hemoglobin levels of patients with drug discontinuation.
Interestingly, one patient required multiple dose adjustments from the initial 50 to 25 mg/d, which was subsequently reduced to 12.5 mg/d, and finally, 12.5 mg/q.d., with the maintenance of Hb level at >9 g/dl, and while the Hb levels decreased after drug withdrawal, it was restored after resuming treatment (Figure 2c). Only two patients were administered increased doses during treatment. In order to no deviate from blood transfusion, one patient increased the dose from 50 to 100 mg/d and reached MiR; no improvement was observed in another patient, even upon increasing the dose from 100 to 150 mg/d.
Effect of drug withdrawal on Hb
Treatment was discontinued in 14 patients (seven MaR, three MiR, and four NR). Within the responders, two patients discontinued the drug due to constipation and menstruation disorders while the others discontinued treatment because they could not insist on drug intake or were preparing for conception. The changes in Hb in 10 responders before and after drug withdrawal are presented in Figure 2b. Regardless of the length of treatment, the Hb level was significantly decreased after drug discontinuation for one month and further declined to baseline levels with the extension of withdrawal time.
Predictors of response
To identify potential predictors of the thalidomide response, we divided all patients into two groups (MaR and MiR+NR). The primary response to thalidomide was significantly correlated with the HbF ratio before treatment (P=0.003) and splenic status (P=0.025), but not related to age, sex, phenotype, duration of treatment, thalidomide dose or baseline Hb level (Table 2). To view the effect of blood transfusion on hemoglobin, the authors analyzed patients with NTDT, separately. Logistic regression analysis identified the ratio of HbF at baseline (P=0.038, OR=1.111, 95% CI: 1.006–1.226) as an independent risk factor for the main response to thalidomide.
Table 2.
Predictors of thalidomide response among patients with β-thalassemia.
| Variable | MaR(n=41) | MiR+NR(n=21) | P value |
|---|---|---|---|
| Sex (n,%) | |||
| Male | 20 (48.8%) | 7 (33.3%) | |
| Female | 21 (51.2%) | 14 (66.7%) | 0.110 |
| Age (years) | 27.00±7.97 | 27.67±7.90 | 0.485 |
| Phenotype (n,%) | |||
| NTDT | 28 (68.3%) | 11 (52.4%) | 0.220 |
| TDT | 13 (31.7%) | 10 (47.6%) | |
| Splenic status (n,%) | |||
| Splenectomized | 15 (36.6%) | 14 (66.7%) | 0.025 |
| Nonsplenectomized | 26 (63.4%) | 7 (33.3%) | |
| Duration of treatment (month) | 15.24±9.79 | 13.42±9.29 | 0.703 |
| Thalidomide dose(mg/day) | |||
| Median (IQR) | 50 (50–50) | 50 (50–50) | 0.545 |
| Hemoglobin before Thalidomide (g/dl) | 63.76±13.49 | 68.29±13.61 | 0.217 |
| Fetal Hemoglobin before Thalidomide (%) | 46.14±22.09 | 28.49±20.54 | 0.003 |
Abbreviations: MaR: main responder; MiR: minor responder; NR: no responder; NTDT: non-transfusion-dependent thalassemia; TDT: transfusion-dependent thalassemia; IQR: interquartile range.
Toxicity
The mild adverse effects of thalidomide were recorded in 10 patients. The most common toxicity was at the gastrointestinal level (5/62) followed by a rash (2/62) and menstruation disorders (2/62), but the symptoms were transient and recovered after symptomatic treatment or temporary drug discontinuance. During long-term follow-up, one patient developed peripheral neurotoxicity with intermittent numbness of both lower limbs. Potential underlying conditions or diseases responsible for peripheral neurotoxicity were ruled out. The initial dose of thalidomide administered was 100 mg/d for 23 months, and the cumulative dose was ~ 55 g. Distal numbness of both lower limbs occurred about 18 months after therapy, and these symptoms were incompletely reversed after drug withdrawal over a subsequent 4-month period. During the treatment period, no hematological toxicity or bone marrow suppression was detected in patients. Furthermore, thalidomide had no unfavorable effects on liver or kidney function and induced no significant changes in alanine aminotransferase (ALT) or creatinine (Cr) levels.
Discussion
In this study, we analyzed the efficacy and safety of thalidomide for patients with β-thalassemia using a relatively large cohort and long-term follow-up, with encouraging results. Primary data showed significant efficacy of thalidomide for β-thalassemia, with the rate of response for thalidomide being much better than that reported for hydroxyurea treatment.22 Guangxi Zhuang Autonomous Region, Southern China, is an area with a high prevalence of thalassemia and is economically underdeveloped.23 Many patients with thalassemia are not sufficiently transfused due to a shortage of blood products.24 Moreover, compared to transfusions, thalidomide is more convenient and economically more feasible in China. Actually, transfusions induce iron overload, which can be prevented by expensive drug chelation,25 mostly in the heart but only partially in endocrine organs.26 In addition, we observed a long-lasting effect of thalidomide for β-thalassemia in long-term follow-up relative to earlier short-term case reports.15,16 Compared with the decline in hematological response after hydroxyurea treatment for 12 months,9,10 the efficacy of thalidomide was stable over time, and no reduction of the hematological reaction was observed during long-term follow-up, which would be of considerable benefit to patients requiring continued treatment. Unfortunately, we have not observed a decline in SF levels after treatment.
Although the use of thalidomide to treat β-thalassemia has achieved good results, there is still no consensus on the optimal and maintenance dose for clinical application. At present, the therapeutic dose of thalidomide for β-thalassemia is ~50–100 mg/d,15–17,19,20 and the dose-response relationship is yet to be established. Compared with a daily dose of 50 mg/d, we observed that the dose increment did not give significant added benefit. For responders who received maintenance therapy at a relatively low dose, the response was evident despite a decrease in Hb levels. Interestingly, Hb decreased rapidly to the baseline level after drug withdrawal and was restored after the re-introduction of the drug, consistent with the results of Fozza at el.15 Therefore, thalidomide seems to have a “switching effect” on β-thalassemia, and the maintenance dose could be reduced in the future. Since patients with thalassemia require lifelong medication, it may be valuable to compare the effects of thalidomide with a different maintenance dose.
Limited complications were reported in β-thalassemia, with peripheral neuropathy, which is one of the most feared complications, being documented in one patient. The mechanism of thalidomide-induced neurotoxicity remains to be clarified. Several studies have explored risk factors for thalidomide neuropathy, including age, daily dose, duration of drug exposure, cumulative dose, and preexisting neuropathy.27 The incidences of peripheral neurotoxicity that have been reported are relatively low and are limited at a daily dose of >50 mg.27–29 Although only one patient presented with peripheral neurotoxicity in our study, physicians contemplating its use should be vigilant to its occurrence and take preventive action. Furthermore, it is valuable to explore lower therapeutic or maintenance doses to maximize the benefit/risk rate.
The current study is the first to assess the efficacy and safety of thalidomide in a relatively large cohort over a long-term follow-up period and provide new insights on the continued efficacy and safety of thalidomide in patients with β-thalassemia.
A limitation of this study was its design, having included patients from three centers. Nevertheless, the effects of this limitation were attenuated by the fact that all centers used 1) the same inclusion criteria, 2) equal treatment and management of patients, and 3) the long duration of follow-up.
Conclusions
Thalidomide is a promising modality of treatment in patients with β-thalassemia. It can significantly improve Hb levels minimizing the need for blood transfusion. However, several issues remain to be resolved, such as establishing the optimal maintenance dose to further improving the curative effect while avoiding long-term complications.
Acknowledgments
We are grateful to our patients for participating in this study.
Footnotes
Competing interests: The authors declare no conflict of Interest.
Author contribution. All authors examined the available material, wrote the review, reviewed and revised the manuscript and provided their approval of the final version of the manuscript. All authors agree to be accountable for all aspects of the work.
References
- 1.Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia Lancet. 2018;391(10116):155–167. doi: 10.1016/S0140-6736(17)31822-6. [DOI] [PubMed] [Google Scholar]
- 2.Camaschella C, Cappellini MD. Thalassemia intermedia. Haematologica. 1995;80(1):58–68. [PubMed] [Google Scholar]
- 3.Taher AT, Radwan A, Viprakasit V. When to consider transfusion therapy for patients with non-transfusion-dependent thalassaemia. Vox Sang. 2015;108(1):1–10. doi: 10.1111/vox.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musallam KM, Taher AT, Cappellini MD, Sankaran VG. Clinical experience with fetal hemoglobin induction therapy in patients with β-thalassemia. Blood. 2013;121(12):2199–212. doi: 10.1182/blood-2012-10-408021. quiz 2372. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Zeng J, Roscoe BP, et al. Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat Med. 2019;25(5):776–783. doi: 10.1038/s41591-019-0401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ch A, Alimirzoyeva Z, Hasanova M, Mammadova T, Shirinova A. Clinical application of recombinant erythropoietin in beta-thalassaemia intermedia. Georgian Med News. 2016;(255):86–92. [PubMed] [Google Scholar]
- 7.El-Beshlawy A, El-Ghamrawy M, EL-Ela MA, et al. Response to hydroxycarbamide in pediatric β-thalassemia intermedia: 8 years’ follow-up in Egypt. Ann Hematol. 2014;93(12):2045–50. doi: 10.1007/s00277-014-2154-5. [DOI] [PubMed] [Google Scholar]
- 8.Karimi M, Haghpanah S, Farhadi A, Yavarian M. Genotype-phenotype relationship of patients with β-thalassemia taking hydroxyurea: a 13-year experience in Iran. Int J Hematol. 2012;95(1):51–6. doi: 10.1007/s12185-011-0985-6. [DOI] [PubMed] [Google Scholar]
- 9.Rigano P, Pecoraro A, Calzolari R, et al. Desensitization to hydroxycarbamide following long-term treatment of thalassaemia intermedia as observed in vivo and in primary erythroid cultures from treated patients. Br J Haematol. 2010;151(5):509–15. doi: 10.1111/j.1365-2141.2010.08397.x. [DOI] [PubMed] [Google Scholar]
- 10.Mancuso A, Maggio A, Renda D, Di Marzo R, Rigano P. Treatment with hydroxycarbamide for intermedia thalassaemia: decrease of efficacy in some patients during long-term follow up. Br J Haematol. 2006;133(1):105–6. doi: 10.1111/j.1365-2141.2006.06002.x. [DOI] [PubMed] [Google Scholar]
- 11.Ehsani MA, Hedayati-Asl AA, Bagheri A, Zeinali S, Rashidi A. Hydroxyurea-induced hematological response in transfusion-independent beta-thalassemia intermedia: case series and review of literature. Pediatr Hematol Oncol. 2009;26(8):560–5. doi: 10.3109/08880010903271671. [DOI] [PubMed] [Google Scholar]
- 12.Stewart AK. Medicine. How thalidomide works against cancer. Science. 2014;343(6168):256–7. doi: 10.1126/science.1249543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalali FMA, Dehghani FA, Hajizamani S, Mossahebi-Mohammadi M, Yaghooti H, Saki N. Thalidomide is more efficient than sodium butyrate in enhancing GATA-1 and EKLF gene expression in erythroid progenitors derived from HSCs with β-globin gene mutation. Int J Hematol Oncol Stem Cell Res. 2016;10(1):37–41. [PMC free article] [PubMed] [Google Scholar]
- 14.Aerbajinai W, Zhu J, Gao Z, Chin K, Rodgers GP. Thalidomide induces gamma-globin gene expression through increased reactive oxygen species-mediated p38 MAPK signaling and histone H4 acetylation in adult erythropoiesis. Blood. 2007;110(8):2864–71. doi: 10.1182/blood-2007-01-065201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fozza C, Pardini S, Giannico DB, et al. Dramatic erythroid response to low-dose thalidomide in two patients with transfusion independent thalassemia and severe post-transfusional alloimmune hemolysis. Am J Hematol. 2015;90(7):E141. doi: 10.1002/ajh.24030. [DOI] [PubMed] [Google Scholar]
- 16.Ricchi P, Costantini S, Spasiano A, et al. The long-term and extensive efficacy of low dose thalidomide in a case of an untransfusable patient with Non-Transfusion-Dependent Thalassemia. Blood Cells Mol Dis. 2016;57:97–9. doi: 10.1016/j.bcmd.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Zhu W, Cai N, Bu S, Li J, Huang L. Thalidomide induces haematologic responses in patients with β-thalassaemia. Eur J Haematol. 2017;99(5):437–441. doi: 10.1111/ejh.12955. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Ren Q, Zhou Y, Li P, Lin W, Yin X. Thalidomide has a significant effect in patients with thalassemia intermedia. Hematology. 2018;23(1):50–54. doi: 10.1080/10245332.2017.1354427. [DOI] [PubMed] [Google Scholar]
- 19.Aguilar-Lopez LB, Delgado-Lamas JL, Rubio-Jurado B, Perea FJ, Ibarra B. Thalidomide therapy in a patient with thalassemia major. Blood Cells Mol Dis. 2008;41(1):136–7. doi: 10.1016/j.bcmd.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Masera N, Tavecchia L, Capra M, et al. Optimal response to thalidomide in a patient with thalassaemia major resistant to conventional therapy. Blood Transfus. 2010;8(1):63–5. doi: 10.7175/cmi.v4i2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren Q, Zhou YL, Wang L, et al. Clinical trial on the effects of thalidomide on hemoglobin synthesis in patients with moderate thalassemia intermedia. Ann Hematol. 2018;97(10):1933–1939. doi: 10.1007/s00277-018-3395-5. [DOI] [PubMed] [Google Scholar]
- 22.Kosaryan M, Zafari M, Alipur A, Hedayatizadeh-Omran A. The effect and side effect of hydroxyurea therapy on patients with β-thalassemia: a systematic review to December 2012. Hemoglobin. 2014;38(4):262–71. doi: 10.3109/03630269.2014.927770. [DOI] [PubMed] [Google Scholar]
- 23.Xiong F, Sun M, Zhang X, et al. Molecular epidemiological survey of haemoglobinopathies in the Guangxi Zhuang Autonomous Region of southern China. Clin Genet. 2010;78(2):139–48. doi: 10.1111/j.1399-0004.2010.01430.x. [DOI] [PubMed] [Google Scholar]
- 24.Yin XL, Wu ZK, He YY, Zhou TH, Zhou YL, Zhang XH. Treatment and complications of thalassemia major in Guangxi, Southern China. Pediatr Blood Cancer. 2011;57(7):1174–8. doi: 10.1002/pbc.23101. [DOI] [PubMed] [Google Scholar]
- 25.Jialian L, Yong L, Xue L, Jiayou Z. Economic Evaluation of Chelation Regimens for β-Thalassemia Major: a Systematic Review. Mediterr J Hematol Infect Dis. 2019;11(1) doi: 10.4084/mjhid.2019.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Sanctis V, Soliman AT, Canatan D, et al. Thyroid Disorders in Homozygous β-Thalassemia: Current Knowledge, Emerging Issues and Open Problems. Mediterr J Hematol Infect Dis. 2019;11(1):e2019029. doi: 10.4084/mjhid.2019.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baas P, Boogerd W, Dalesio O, Haringhuizen A, Custers F, van Zandwijk N. Thalidomide in patients with malignant pleural mesothelioma. Lung Cancer. 2005;48(2):291–6. doi: 10.1016/j.lungcan.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Bastuji-Garin S, Ochonisky S, Bouche P, et al. Incidence and risk factors for thalidomide neuropathy: a prospective study of 135 dermatologic patients. J Invest Dermatol. 2002;119(5):1020–6. doi: 10.1046/j.1523-1747.2002.19502.x. [DOI] [PubMed] [Google Scholar]
- 29.Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40(7):872–82. doi: 10.1016/j.ctrv.2014.04.004. [DOI] [PubMed] [Google Scholar]