Recently we reported that close-proximity metallic silver islands or colloids can alter the radiative decay rate, Gm, and/or excitation rate, Em, of fluorophores [1–5]. We have shown that the quantum yield of low quantum yield fluorophores can be increased, with a maximum predicted increase of 1/Q0, (Q0-quantum yield in absence of metal), whereas significant increases in emission intensity from high quantum yield species, in the absence of any non-radiative rate modifications, can only be observed by substantial increases in Em. Complimentary to our previous results and interpretations, we can now report that enhanced and localized multiphoton excitation of rhodamine B (RhB) fluorescence occurs near metallic silver islands.
The increase in fluorescence emission intensity for RhB molecules adjacent to metallic silver islands (Fig. 1) is accompanied by a reduction in lifetime, compared to that observed using 1-photon excitation. Given the high quantum yield of RhB (Q0 = 0.48), these results can be explained by the metallic particles significantly increasing the Em of the RhB molecules. Moreover, given the sample geometry (Fig. 2) and the absence of any notable increase in emission intensity using 1-photon excitation, as well the fact that the 1-photon mean lifetime remained essentially unchanged both in the presence and absence of silver, suggests that enhanced 2-photon excitation is localized to regions in close proximity to the silver islands.
Fig. 1.
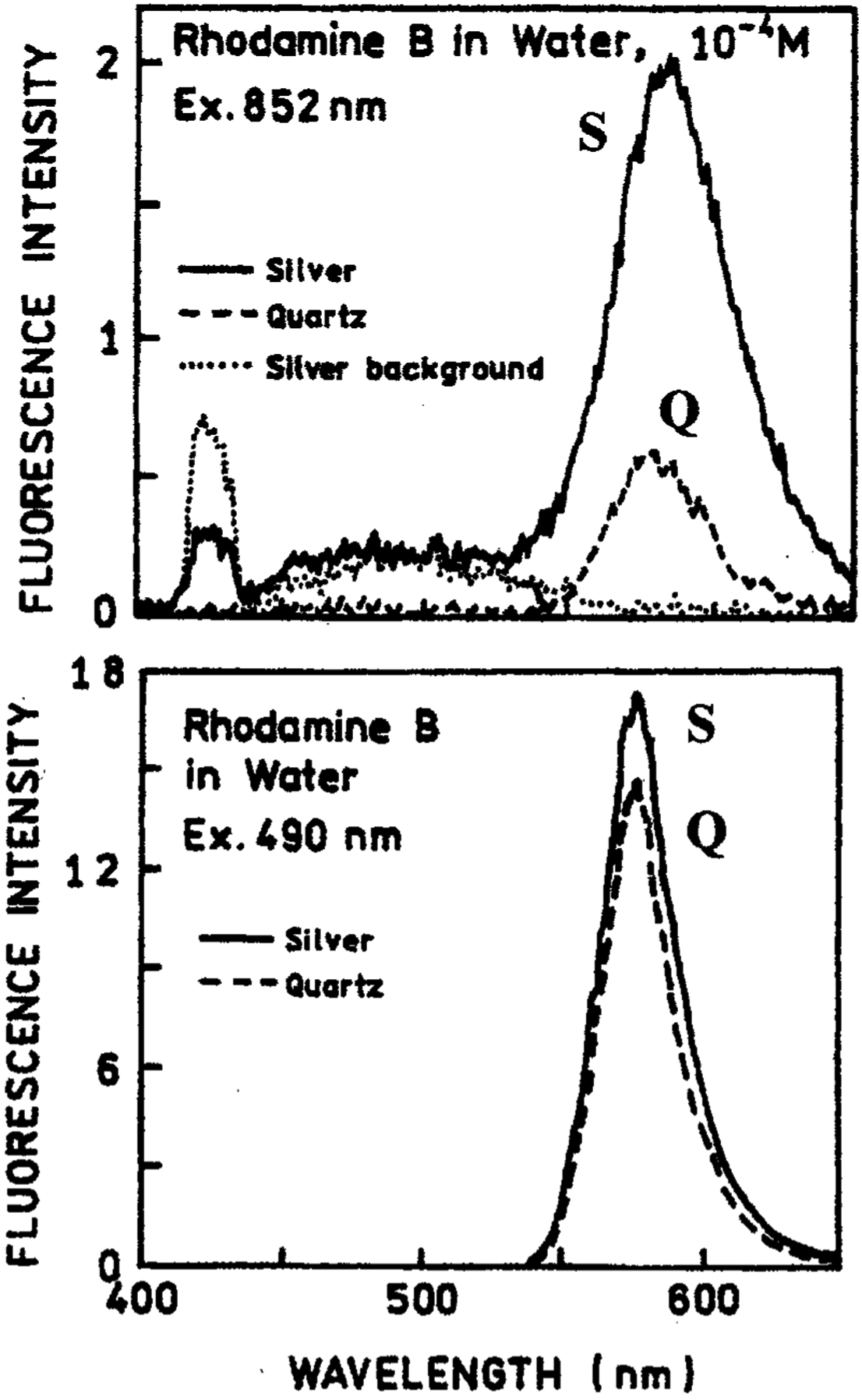
(Top) Emission spectra of 10−4 M RhB between silver island films (S) with 2-photon excitation at 852 nm (−) from a Tsunami modelocked Ti:sapphire laser, 80 MHz repetition rate, 90 fs pulse and about 0.5 W average power. Also shown are the emission spectra observed from uncoated quartz slides (Q), and silver islands alone without RhB. (Bottom) RhB between silver islands, S(−), or quartz plates, Q(…), with 1-photon excitation at 490 nm.
Fig. 2.
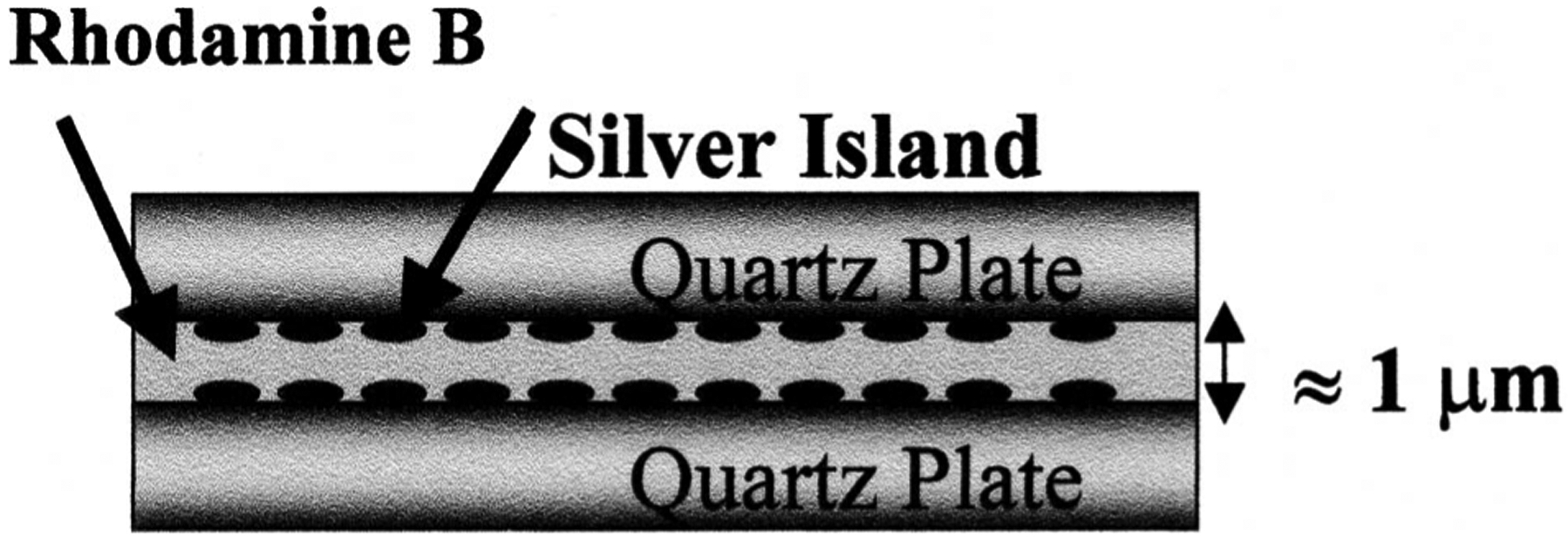
Sample geometry. Two silver islanded quartz plates sandwich ≈1 mm RhB solution. Given that metal-fluorophore interactions arethought to extend some 200Å into the solution, in this sample geometry only ≈4% of RhB molecules are within this active region. This suggests that the true enhancement effect in Fig. 1 is at least 25 times higher.
It is informative to discuss the nature of this enhanced and localized excitation. In addition to metallic particles and/or colloids modifying a fluorophore’s radiative decay rate, they are also known to increase excitation rates by concentrating the incident light [6,7]. The maximum enhancement in the incident electric field has been calculated to be a factor of 140 near appropriately sized metallic ellipsoids [8]. Because the incident intensity is the square of the incident field strength for a 1-photon process, enhancements in excitation rates by a factor of up to 2*104 are possible. It is this phenomenon that one can typically attribute to increases in observed apparent quantum yields near metallic particles to greater than unity. However, a much more dramatic enhancement is possible for multiphoton excitation. For a 2-photon absorption process the rate of excitation is proportional to the square of the incident intensity. This suggests that 2-photon excitation could be enhanced by a factor of 3.8* 108. Such an enhancement in the excitation rate is thought to provide selective excitation of fluorophores near tometal islands or colloids, even if the solution contains a considerable concentration of other fluorophores that could undergo 2-photon excitation at the same wavelength, but are more distant from the metals surface (Fig. 3). This interpretation is borne out by the fact that given the overwhelming excess of high quantum yield RhB in this sample geometry (‘96% of solution is too distant for any fluorophore–metal effect), the fluorescence lifetime is still shorter than that typically observed for bulk solution RhB in the absence of metal (Fig. 4).
Fig. 3.
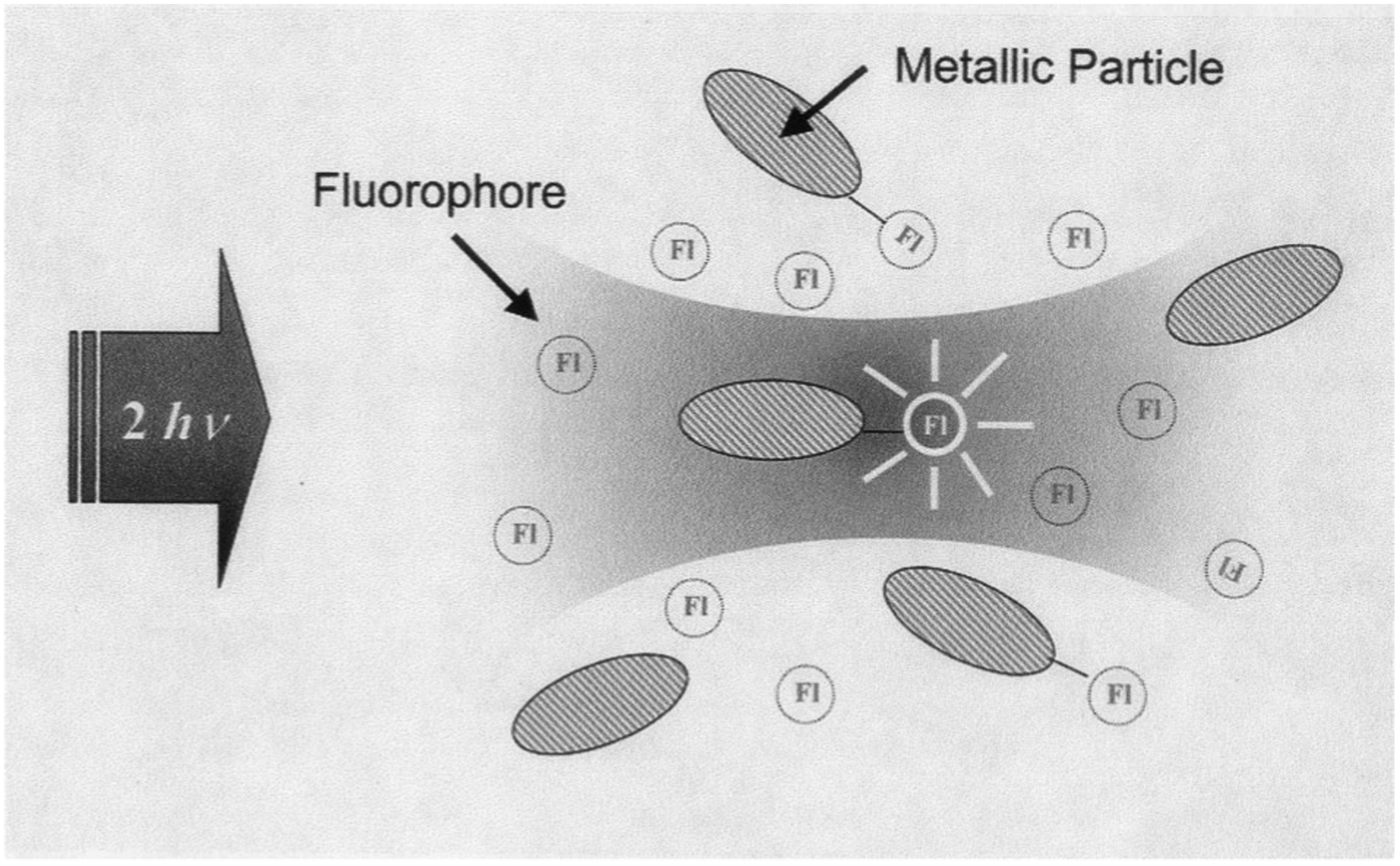
Preferential multiphoton excitation of fluorophores in close proximity to metal, in the presence of free fluorophore, Fl.
Fig. 4.
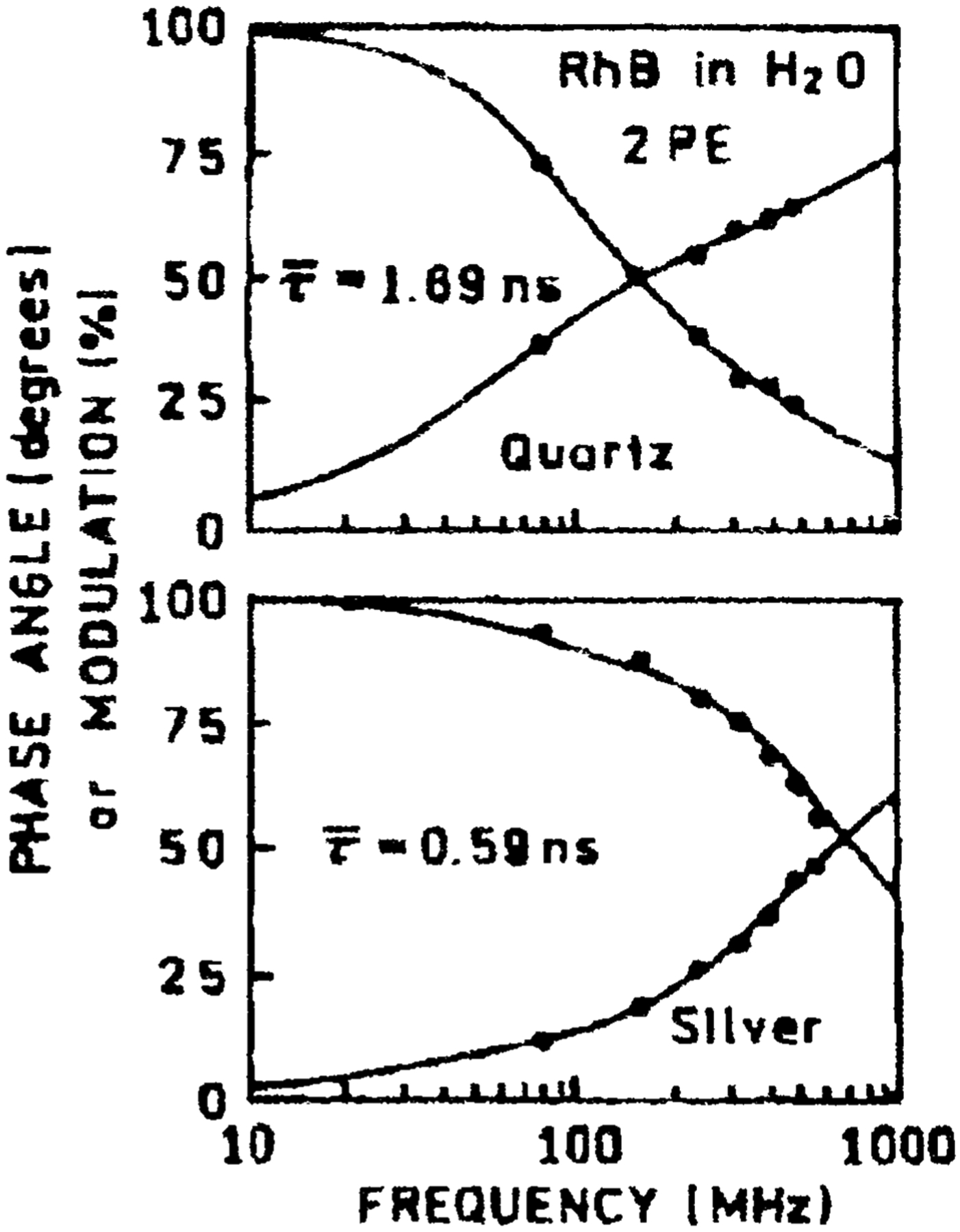
Frequency domain intensity decay of RhB between quartz slides (top) and silver island films (bottom) with 2-photon excitation at 852 nm observed at 580 nm.
In our opinion, enhanced and localized multiphoton excitation by metal particles may have numerous applications in the biochemical and biological applications of fluorescence. For example:
More Generally: The preferential enhanced excitation of fluorophores in close proximity to metallic islands or colloids even in the presence of high concentrations of other emitting species (see Fig. 3). Even further enhancements may be possible by using low Q0 species.
Specifically: By directing metallic colloids to a cell membrane by the presence of a covalently bound antibody, one may be able to specifically excite cellular autofluorescence. If the enhancement by the metallic particles is significantly large, one could imagine their use in tissues where scattering reduces the peak intensity of excitation pulses.
In the many applications of fluorescence, the photostability of fluorophores is an important consideration. This is particularly true in single molecule detection, where it has been estimated that approximately 1000 photons can be observed from a highly stable fluorophore such as a Rhodamine prior to photodecomposition [9]. Because photochemical destruction usually occurs in the excited state, a reduction in fluorescence lifetime resulting from the close proximity of metallic particles is expected to result in increased photostability. For our samples we found that for 1-photon excitation, the photostability was unaffected by the presence or absence of silver islands (Fig. 5, bottom). However for 2-photon excitation, an increased photostability was observed for RhB in the presence of silver islands (Fig. 5, top). These results are consistent with the shorter lifetime observed for RhB between the silver islands and with our interpretation that multiphoton excitation occurs preferentially near the silver islands.
Fig. 5.
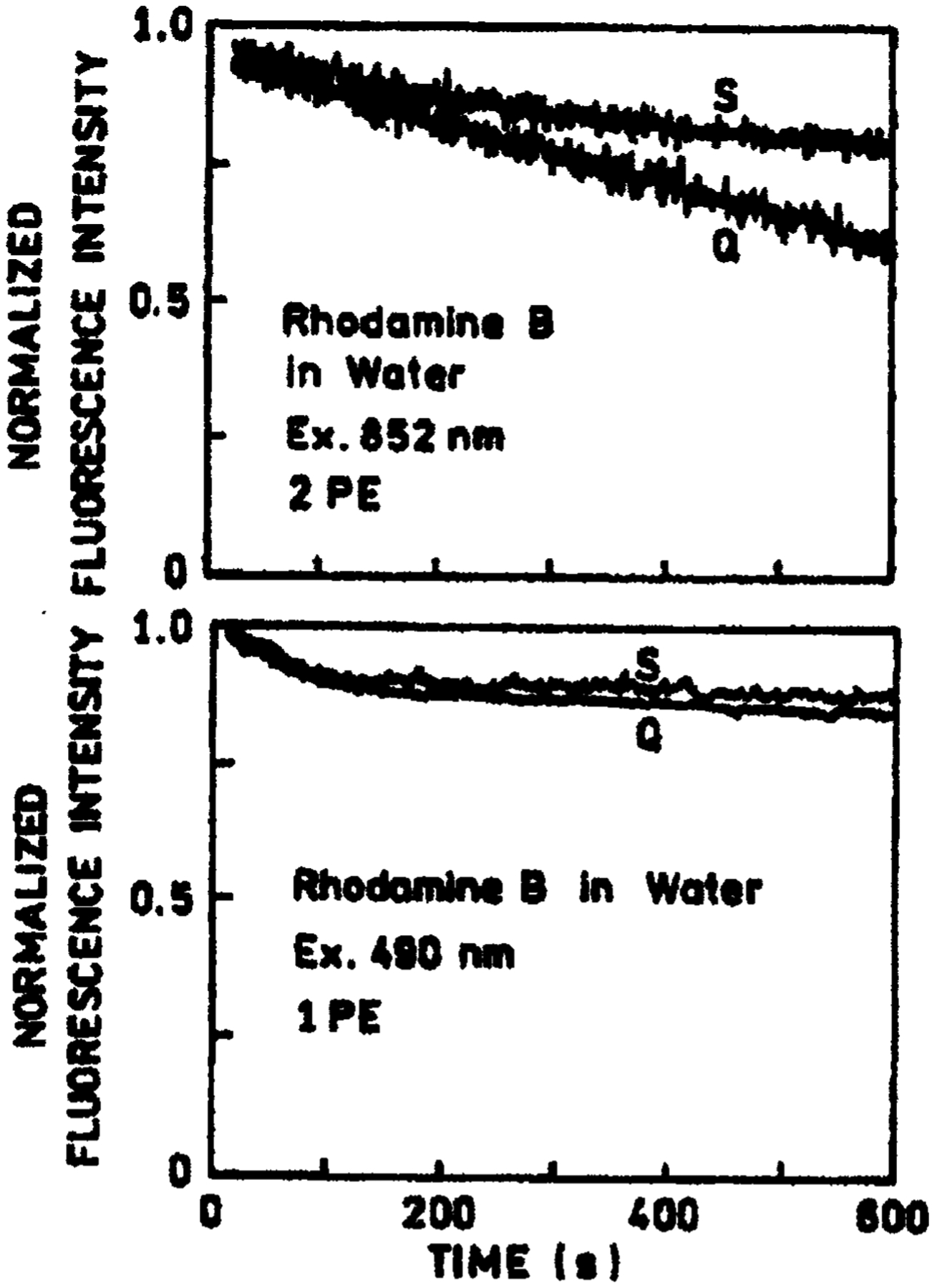
Photostability of RhB between quartz slides, (Q), and silver island films, (S), with 2-photon excitation at 852 nm (top) and with 1-photon excitation at 490 nm (bottom). The 490-nm excitation was from an argon ion laser attenuated to about 10 mW. Fluorescence was observed at 580 nm.
The use of metallic structures to enhance multiphoton excitation has already been considered in apertureless near-field scanning optical microscopy [10,11]; however, to the best of our knowledge, multiphoton excitation has not been reported near metallic colloids or islands.
Biography
Joseph R. Lakowicz Ph.D., is director of the Center for Fluorescence Spectroscopy at the University of Maryland School of Medicine in Baltimore. He has a B.S. in chemistry from LaSalle University and an M.S. and a Ph.D. in Biochemistry from the University of Illinois atUrbana. He is also the founding editor of the Journal of Biomedical Optics and the Journal of Fluorescence. He is a co-founder of the Society of Fluorescence.
Ignacy Gryczynski Ph.D., is a research professor at the University of Maryland School of Medicine. He has an M.S. and a Ph.D. in physics from the University of Gdansk Institute of Physics in Poland.
Joanna Malicka Ph.D., is a postdoctoral fellow at the Center for Fluorescence Spectroscopy. She has an M.S. and a Ph.D. in chemistry from the University of Gdansk.
Zygmunt Gryczynski Ph.D., is an associate professor in the Department of Biological Chemistry and Molecular Biology at the University of Maryland in Baltimore. He is also the assistant director of the Center for Fluorescence Spectroscopy. He has an M.S. and a Ph.D. in physics from the University of Gdansk Institute of Physics.
Chris D. Geddes Ph.D., is an assistant professor at the University of Maryland Biotechnology Center, Center for Fluorescence Spectroscopy in Baltimore. He has a B.Sc. from Lancaster University in England and a Ph.D. in physical chemistry (fluorescence spectroscopy) from the University of Wales, Swansea. He is the editor of the Journal of Fluorescence.
REFERENCES
- 1.Lakowicz JR (2001) Radiative Decay Engineering: Biophysical and Biomedical Applications, Anal. BioChem 298, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakowicz JR, Shen Y, D’Auria S, Malicka J, Fang J, Gryczynski Z, and Gryczynski I (2002) Radiative Decay Engineering 2. Effects of silver island films on fluorescence intensity Lifetimes and Resonance energy transfer, Anal. Biochem 301, 261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lakowicz JR, Gryczynski I, Shen YB, Malicka J, and Gryczynski Z (2001) Intensified fluorescence, Photonics Spectra 35, 96–104. [Google Scholar]
- 4.Geddes CD and Lakowicz JR (2002) Metal Enhanced Fluorescence, J. Fluoresc In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gryczynski I, Malika J, Gryczynski Z, Geddes CD, and Lakowicz JR (2002) The CFS engineers the intrinsic radiative decay rate of low quantum yield fluorophores, J. Fluoresc 12, 11–13. [Google Scholar]
- 6.Chen CJ and Osgood RM (1983) Direct observation of the local field enhanced surface photochemical reactions, Phys. Rev. Lett 50, 1705–1708. [Google Scholar]
- 7.Link S and El-Sayed MA (2000) Shape and size dependence of radiative, nonradiative and photothermal properties of gold nanocrystals, Int. Rev. Phys. Chem 19, 409–453. [Google Scholar]
- 8.Kummerlen J, Leitner A, Brunner H, Aussenegg FR, and Wokaun A (1993) Enhanced dye fluorescence over silver island films. Analysis of the distance dependence, Mol. Phys, 80, 1031–1046. [Google Scholar]
- 9.Ambrose WP, Goodwin PM, Jett JH, Van Orden A, Werner JH, and Keller RA (1999) Single molecule fluorescence spectroscopy at ambient temperature, Chem. Rev 99, 2929–2956. [DOI] [PubMed] [Google Scholar]
- 10.Kawata Y, Xu C, and Denk W (1999) Feasibility of molecularresolution fluorescence near-field microscopy using multiphoton absorption and field enhancement near a sharp tip, J. Appl. Phys 85, 1294–1301. [Google Scholar]
- 11.Sanchez EJ, Novotny L, and Xie XS (1999) Near-field fluorescence microscopy based on two-photon excitation with metal tips, Phys. Rev. Lett, 82, 4014–4017. [Google Scholar]


