Abstract
Objective:
Lactobacillus rhamnosus GG (LGG), a probiotic, given by gavage is radioprotective of the mouse intestine. LGG-induced radioprotection is TLR2 and COX-2 dependent and is associated with the migration of COX-2+ mesenchymal stem cells (MSCs) from the lamina propria of the villus to the lamina propria near the crypt epithelial stem cells. Our goals was to define the mechanism of LGG radioprotection including identification of the TLR2 agonist, and the mechanism of the MSC migration, and to determine the safety and efficacy of this approach in models relevant to clinical radiation therapy.
Design:
Intestinal radioprotection was modeled in vitro with cell lines and enteroids as well as in vivo by assaying clinical outcomes and crypt survival. Fractionated abdominal and single dose radiation were used along with syngeneic CT26 colon tumor grafts to assess tumor radioprotection.
Results:
LGG with a mutation in the processing of lipoteichoic acid (LTA), a TLR2 agonist, was not radioprotective, while LTA agonist and native LGG were. An agonist of CXCR4 blocked LGG-induced MSC migration and LGG-induced radioprotection. LGG given by gavage induced expression of CXCL12, a CXCR4 agonist, in pericryptal macrophages and depletion of macrophages by clodronate liposomes blocked LGG-induced MSC migration and radioprotection. LTA effectively protected the normal intestinal crypt, but not tumors in fractionated radiation regimens.
Conclusions:
LGG acts as a “time-release capsule” releasing radioprotective LTA. LTA then primes the epithelial stem cell niche to protect epithelial stem cells by triggering a multicellular, adaptive immune signaling cascade involving macrophages and PGE2 secreting MSCs.
Keywords: Radiation Therapy, Probiotics, LGG, Prostaglandins, Stem Cells, Intestinal Epithelium
INTRODUCTION
The normal small intestinal epithelium is highly sensitive to radiation and is a major site of injury during abdominal and pelvic radiation therapy.[1, 2] Radiation of the normal epithelium results in radiation-induced gastrointestinal syndrome (RIGS).[3, 4] In humans, the major symptomassociated with RIGS is diarrhea.[3] Diarrhea induced by radiation injury in the small intestine is a limiting factor in the dosing of radiation therapy for rectal cancer and other abdominal malignancies. To date, there is no effective therapeutic intestinal radioprotectant in routine clinical use.However, preclinical models and some clinical trials suggest that administration of probiotic bacteria have the potential to address this unmet need.[5, 6, 7]
Wepreviously described the intestinal radioprotective effects of the probiotic Lactobacillus rhamnosus GG(LGG)using a single dose (12Gy) of total body irradiation (TBI) in mice.[8]LGG or LGG conditioned media (LGG-CM) given by gavageprior to radiation decreased weight loss and increased survival in mice. Moreover, gavage with LGG improved intestinal crypt survival and diminished radiation-induced apoptosis in the intestinal epithelial stem cell zone. Administration of LGG resulted in the migration of COX-2 expressing mesenchymal stem cells (MSCs) to an area adjacent to crypt epithelial cells. LGG was not radioprotective in mice deficient in TLR2 or cyclooxygenase-2 (COX-2) suggesting that the mechanism of LGG radioprotection involves signaling through TLR2 and COX-2 dependent prostaglandin production.[8]
Notably, others studies have found that LGG and other probiotics also have potential for use as adjunctive therapy in cancer.[9, 10] LGG interferes with tumor promotion in dimethyl hydrazine (DMH)-induced colon cancer in rats.[11] LGG was also found to induce apoptosis and reduce expression of angiogenic and inflammatory proteins in DMH-induced colon cancer.[12]
These promising studies prompted the initiation of an FDA-approved clinical trial of LGG as a radioprotectant in patients receiving abdominal or pelvic radiation (NCT01790035). However, several important mechanistic and clinically relevant aspects of LGG-mediated radioprotection remained to be defined. These included a need to identify the ligand and relevant cell type ofTLR2 signaling, to establish the mechanisms mediating MSC migration and to determine safety and efficacy of this approach in models relevant to clinical radiation therapy.
In the current study we identify lipoteichoic acid (LTA)as aradioprotective TLR2 agonist released by LGG and macrophages as the relevant TLR2 sensing cell type. Furthermore, we define a mechanism by which a macrophage-derived chemokine prompts COX-2 expressing MSCs migration from the lamina propria of the small intestinal villi to an area adjacent to the crypt epithelial stem cells to provide radioprotection. Finally, we demonstrate that the effects of LGG mediated small intestinal radioprotection extend to fractionated dosing regimens, but do not extend to radiation-targeted colon cancer cells.
METHODS:
Animals and General Procedures
Mice on a C57BL/6J or Balb-C background purchased from Jackson Laboratories (Bar Harbor, ME) were maintained as described.[8]Animal procedures were carried out in accordance with the Washington University School of Medicine Animal Studies Committee, which approved the protocols. Radiation was given using Gammacell 40 137Cs irradiator (Atomic Energy of Canada Ltd, Chalk River, Ontario, Canada) at 78.8 cGy/min, or using RS-2000 irradiator (Rad Source Technologies, Inc., Suwanee, GA 30024, USA) at 100 cGy/min with 160kVp X-rays using a 0.3mm copper filter.
Materials
An LGG with a mutation in the processing of LTA was obtained from Sigrid D.J. De Keersmaecker at the Catholic University of Leuven, Belgium.[13, 14]LTA from LGG is D-alanylated. Alanylation of LTA affects its biologic properties. The D-alanylation of lipoteichoic acid (dltD) operon includes proteins that adenylate teichoic acids. This mutation inactivated dltD resulting in complete absence of D-alanyl esters of LTA.[14] LGG in which dltD is inactivated has impaired ability to bind TLR2.[13]dmPGE2, prostaglandin E2 ELISA kits, and NS-398 were from Cayman Chemical (Ann Arbor, MI). PE conjugated rat anti-CD34 FITC conjugated, rat anti-CD4, and APC conjugated rat anti-CD8 were from BD Biosciences (San Jose, CA). Anionic liposomal clodronate was from FormuMax (Sunnyvale, CA). Staphylococcal aureus LTA and PAM3-CSK4 are from Invivogen, San Diego, CA.
Immunohistochemical analyses
Immunohistochemistry (IHC) and immunofluorescence (IF) analyses followedpublished protocols.[15]Primary antibodies were mouse monoclonal anti-COX2 (1:100; BD Biosciences, San Diego, CA), rabbit polyclonal anti-CXCL12 (1:100; Abcam, Cambridge, MA), and mouse monoclonal anti-F4/80 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies from Life Technologies (Carlsbad, CA) were AF594 goat anti-mouse, AF488 goat anti-mouse, and AF594 donkey anti-rabbit (1:200).
Crypt survival
Crypt survival was measured as described.[16, 17, 18]Mice were sacrificed 84 hours after irradiation and given BrdU 90 minutes before sacrifice. The intestines were fixed and embedded in paraffin. The viability of a surviving crypt was confirmed by the incorporation of BrdU into five or more epithelial cells within each regenerative crypt.
Enteroid culture
Enteroids from C57BL/6 mice were established as described.[19, 20]Mouse intestines were minced and digested with collagenase. Cells were washed, filtered, centrifuged, and resuspended in Matrigel. Enteroids were maintained in 50% L-WRN conditioned medium (ATCC CRL-3276).[19, 20]See Supplemental Methods for experimental details.
HEK-Blue TLR2 reporter cells
HEK-Blue TLR2 reporter cells (InvivoGen; #hkb-mtlr2) and control cells were used to assess the presence of TLR2 agonists as described and according to manufacturer protocols. TLR2 stimulation is measured by the activation of NFκB and alkaline phosphatase.
LGG and gene expression
WT C57BL/6 mice were treated with LGG or vehicle by gavage on three consecutive days and then sacrificed. Laser capture microdissection (LCM) was performed on pericryptal cells as described.[21] RNA was isolated, amplified and converted to cDNA by reverse transcription PCR using Sigma whole transcriptome amplification kit (MilliporeSigma) cDNA was purified using a QiaQuick PCR purification kit (Qiagen, Germantown, MD). cDNA was amplified by PCR and analyzed using Agilent mouse gene expression 4X44K microarray.
Fractionated total abdominal radiation (TAI)
TAI was carried out in the RS-2000 irradiator. Mice were anesthetized with 2% isoflurane. The mouse head, forelimbs, hind limbs, and thorax was shielded with lead. Mice received 4Gy TAI on 7 or 8 consecutive days and intraperitoneal (i.p.)injections of vehicle or LTA at 1h before each radiation dose.
Statistical analysis
The two-sided, non-paired Student t test orANOVA were used forcomparisons between treatment and control groups. Survival data were assessedusing the MantelCox log rank test.
RESULTS:
LTA is a radioprotective agent in LGG.
LGG and LGG-CMare radioprotective through a TLR2 mediated mechanism.[8]To determine if LGG-CM containsa TLR2 agonist we incubated HEK-Bluecells expressing TLR2 and HEK-Bluenull cells with media, LGG-CM or PAM3-CSK4, a synthetic TLR2 agonist.[22]Incubation of HEK-Bluecells expressing TLR2 with LGG-CM resultedin NFκB activation indicating that LGG-CM contains a TLR2 agonist(Fig 1A).
Fig. 1. LTA is the radioprotective agent in LGG-CM.
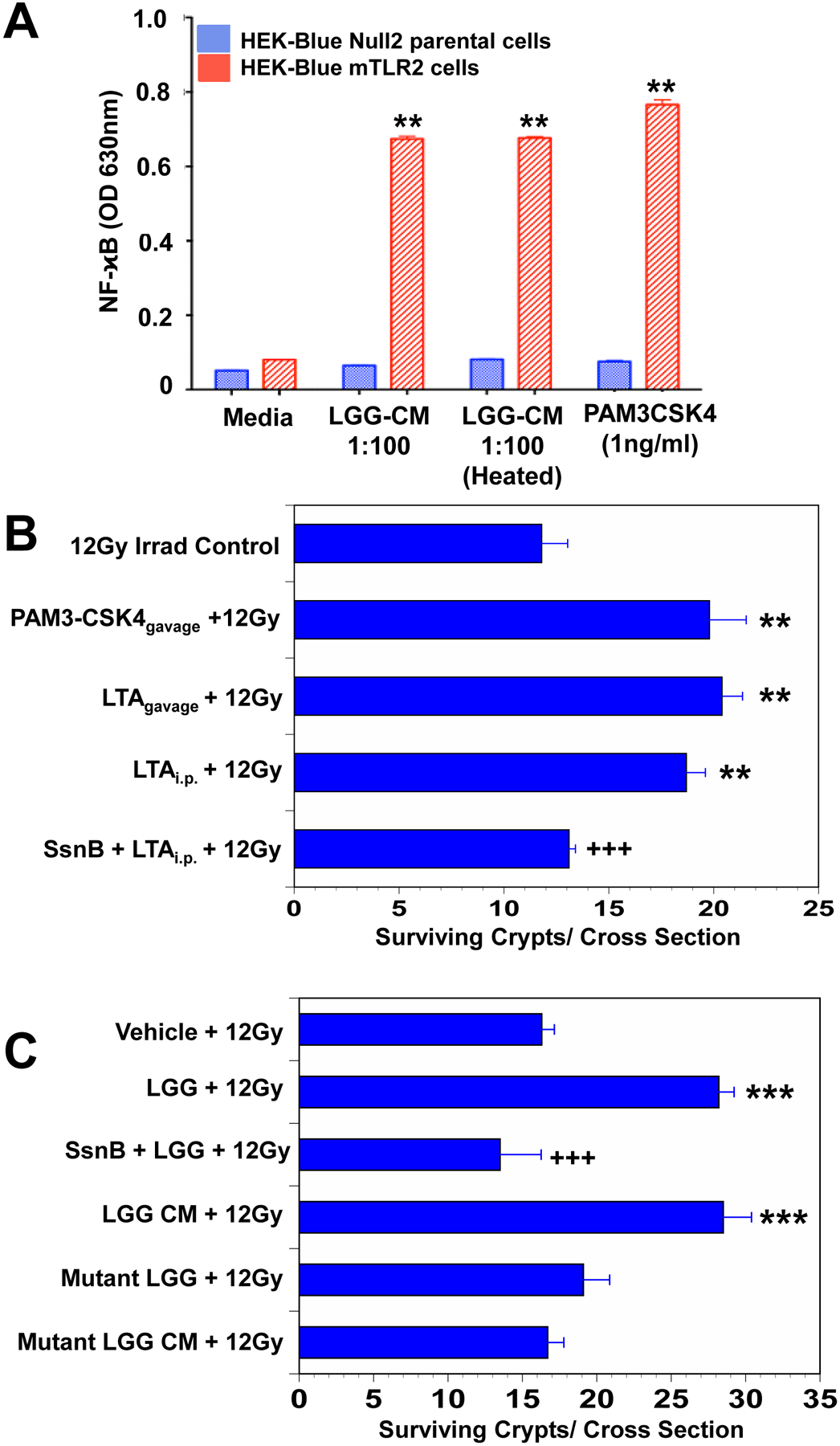
(A) LGG-CM, and the TLR2 agonist PAM3-CSK4 activated NFκB and alkaline phosphatase in HEK-Blue mTLR2 reporter cells. Alkaline phosphatase activation is assessed spectrophotometrically. Data are means ± SEM for 6 samples per treatment group. **P<.001 compared with media controls. (B) Mice pretreated for 3 consecutive days with the TLR2 agonist LTA from Staphylococcus aureus by gavage or by intraperitoneal (i.p.) injection, or with the synthetic TLR2 agonist PAM3-CSK4 by gavage prior to receiving 12Gy TBI had significantly improved crypt survival. The TLR2 antagonist Sparstolonin B (SsmB) blocked LTA-induced radioprotection. **P<.001 compared with irradiated control. Data are means ± SEM for 10 mice per treatment group. (C) Mice had significantly improved crypt survival after radiation when pretreated with WT LGG or WT LGG-CM by gavage for 3 consecutive days. SsmBblocked LGG-induced radioprotection. There was no radioprotection in the small intestines of mice pretreated with mutant LGG dltD, which produces inactive modified LTA, or mutant LGG-CM. ***P<.0006 compared with irradiated control. +++P<.005 compared with LTA ip + 12 Gy or LGG+12Gy N=10 mice per treatment group.
Radiation kills epithelial stem cells in a dose-dependent fashion. Eighty-four hours after radiation each surviving stem cell will have generated a new crypt.[8, 16] In the absence of radiation, there are about 120 crypts per cross-section. Eighty-four hours after 12Gy TBI there averaged 15 surviving crypts.LTAis a component of the lactobacillus cell wall.[23, 24]To determine if LTA is radioprotective ofthe small intestine,mice were treated with vehicle or LTA (gavage or i.p.) or PAM3-CSK4 by gavage prior to 12Gy TBI. Administration of PAM3-CSK4 or LTA resulted in almost doublingcrypt survival (Fig 1B). Sparstolonin B, a TLR2 antagonist, blocked LTA-induced radioprotection. The data suggest that LTA is radioprotective of the intestine and is consistent with LTA being the radioprotectiveTLR2 binding ligand of LGG and LGG-CM.
To determine whether LTA is aradioprotective component of LGG, we performed experiments with a mutated LGGin which LTA is incapable of binding to TLR2 (dltD).Mice were treated with vehicle or WTLGG, or LGG plus Sparstolonin B,or WTLGG-CM, or a mutant LGG or mutant LGG-CM by gavage prior to 12Gy TBI. Mice that received WTLGG or WTLGG-CM had the expected increase in crypt survival whereas the mice that received mutant-LGG or mutant-LGG-CM did not (Fig 1C). The data indicate that LTA is a radioprotective agent in LGG and LGG-CM.Sparstolonin B blocked the radioprotective effects of LGG.
Migration of COX-2+MSCs is required for the radioprotective effects of LGG.
Administration of LGG results in the TLR2-dependent migration of COX-2+MSCs from the lamina propria in the villi to the lamina propria adjacent to epithelial stem cells in the crypt.[8]MSCs express CXCR4, a chemokine receptor,and migrate in response to CXCL12 binding to CXCR4.[25, 26] To determine if CXCR4 signaling is involved in LGG-induced MSC migration,mice were given either vehicle or AMD3100,a CXCR4 antagonist,[27]prior to receiving LGG or vehicle by gavage. LGG induced the migration of COX-2+MSCs from the lamina propria of the villi to an area near the crypt epithelial cells (Fig 2A and 2B). LGG-induced migration of MSCs was not observed in animals treated with AMD3100 suggesting that the migration is dependent upon CXCR4 activation.
Fig. 2. Inhibition of CXCR4 by AMD3100 blocks LGG-induced migration of COX2 expressing MSCs and LGG-induced radioprotection in mice.
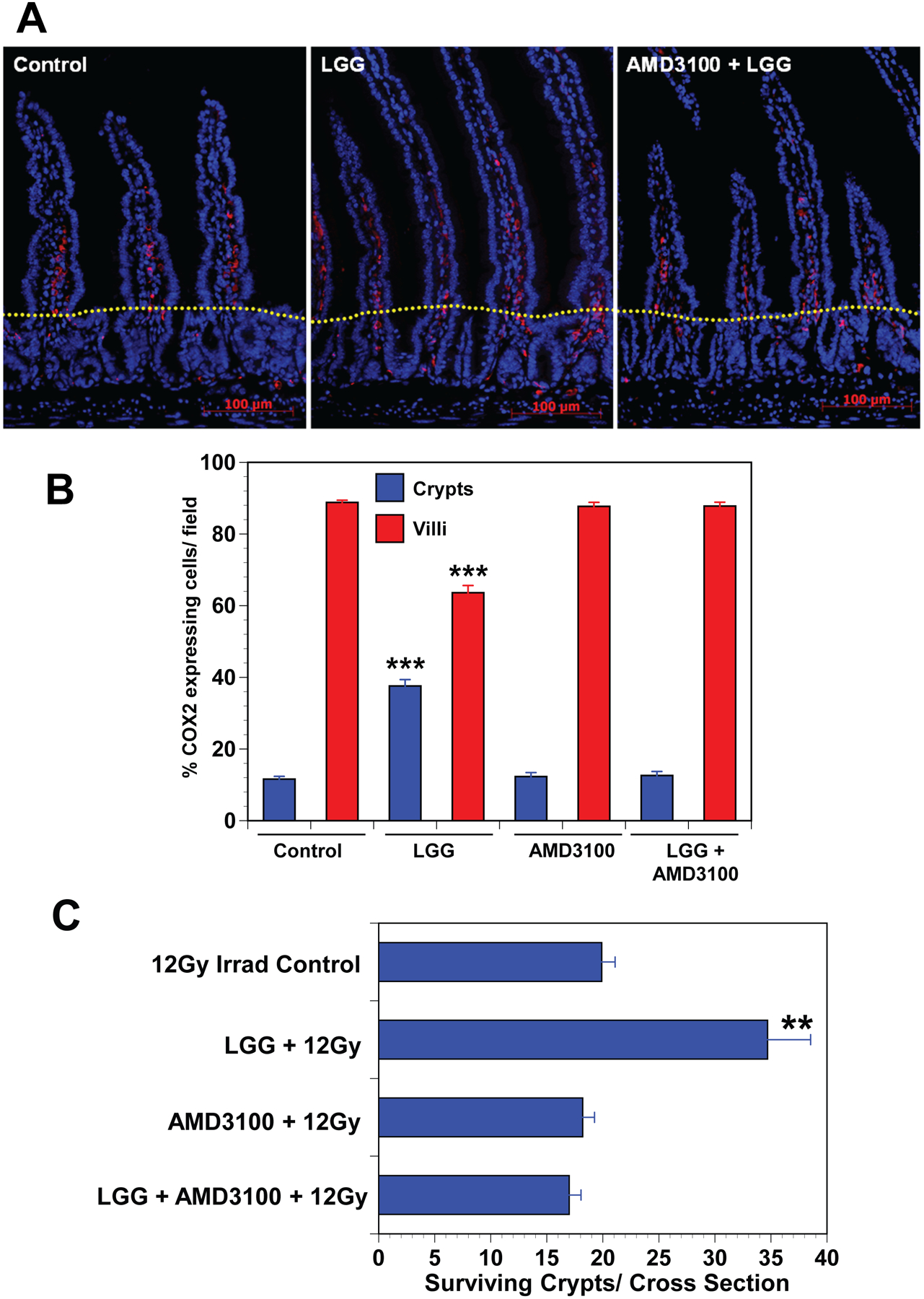
(A) Immunofluorescence for COX2 in the small intestine shows an increased percentage of MSCs in the crypt zone of LGG-treated mice compared with controls or mice treated with LGG and AMD3100 treated mice. Dotted yellow line separates crypt and villus zones. (B) Cell counts provide quantitative data indicating that LGG-induced migration of COX2 expressing MSCs from the villus to the crypt zone, and that AMD3100 blocks LGG induced migration. Data are means ± SEM for 5 to 7 mice per treatment group. ***P<.0001 compared with controls. (C) Crypt survival is significantly improved in LGG treated mice, and AMD3100 blocks LGG induced radioprotection. Data are means ± SEM for 5 to 7 mice per treatment group. **P<.005 compared with irradiated controls.
To determine if LGG-induced migration of MSCs is required for the radioprotective effects of LGG, mice were pre-treated with vehicle or AMD3100 i.p. prior to receiving either vehicle or LGG by gavage. Mice were irradiated with 12Gy TBIand sacrificed84 hours later. AMD3100 blocked the LGG-associated increase in crypt survival suggesting that the radioprotective effects of LGG are mediated by MSC migration induced by CXCR4 activation (Fig 2C). Together these data illustrate that LGG-induced MSC migration is required for radioprotection and involves CXCR4 activation.
LGG induces CXCL12 in pericryptal macrophagesfavouring LGG-induced MSC migration
Having demonstrated that MSC migration in response to LGG is mediated by CXCR4 we next sought to determine if LGG induced the expression of CXCL12, the chemotactic ligand for CXCR4. To address this question we performed laser capture microdissection (LCM) on pericryptal cells of the lamina propria from intestines of mice treated with vehicle or LGG. Gene expression was assessed by gene chip analysis. In response to LGG there was decreased expression of several chemokine receptors (CCR10, CXCR1 and CXCR6) and increased expression of CX3CR1 (Fig 3A, 3B). There was not a statistically significant change in CXCR4. Administration of LGG was associated with downregulation of the chemokineCCL25 and upregulation of several others (CCL4, CCL12, CXCL2, CXCL3, CXCL10 and CXCL12) but only CXCL12 binds to CXCR4. Treatment with LGG was also associated with increased expression of COX-2 (Ptgs2) and cPLA2 (Pla2g4c), which releases arachidonic acid from phospholipids.[28]Having found that LGG induces the migration of COX-2+ MSCs in a CXCR4-dependent fashion andincreasesexpression of the CXCR4 ligand, CXCL12, in the pericryptal region, we next sought to determine the cellular source of CXCL12. Immunofluorescence for CXCL12 in the mouse intestine demonstrates expression in some epithelial cells and in lamina propria cells including macrophages (Fig 3C). However, the only pericryptal cells expressing CXCL12 are macrophages.
Fig. 3. LGG induces CXCL12 in pericryptal macrophages.
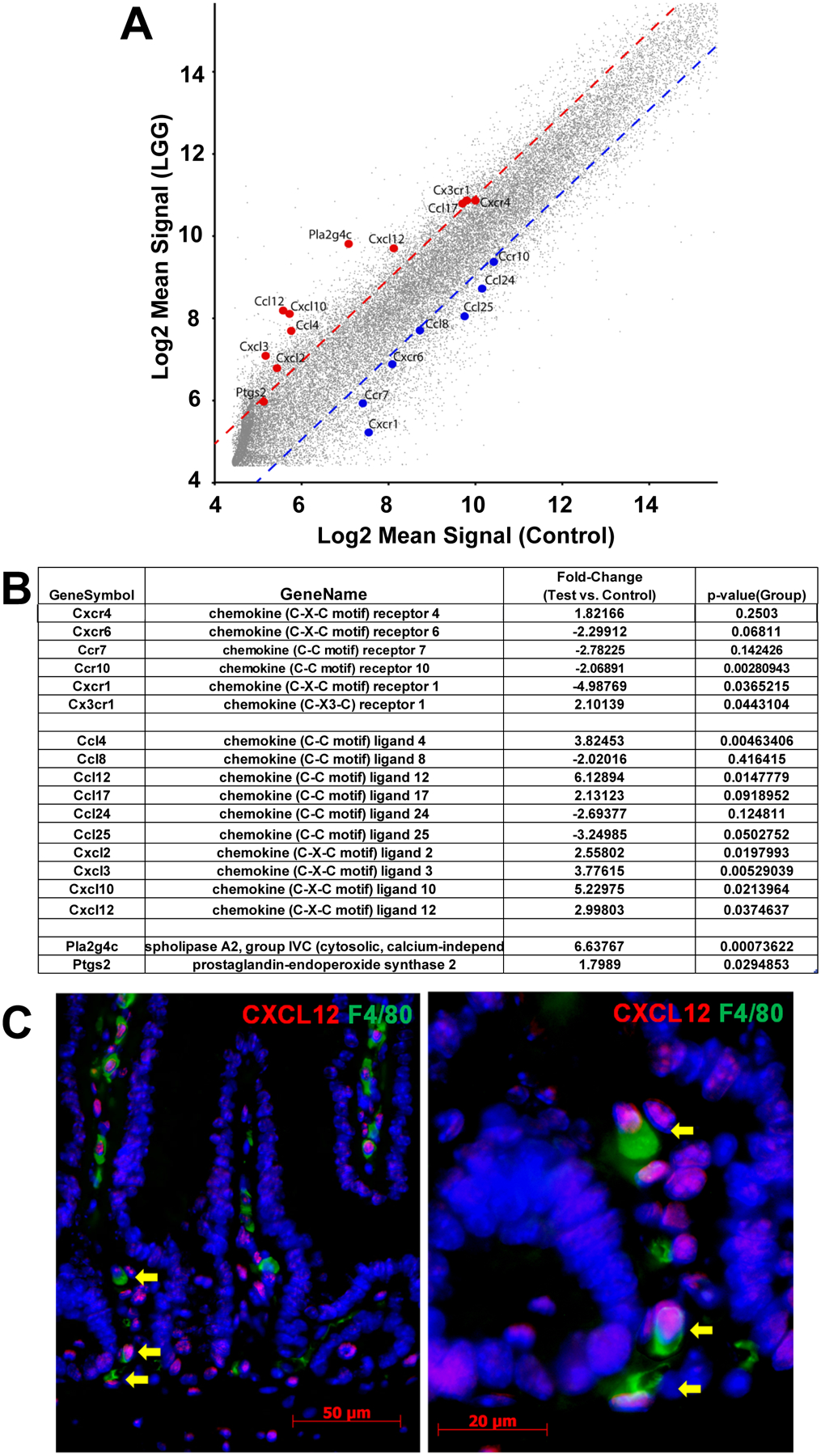
(A,B) Mice were given LGG or vehicle by gavage and sacrificed 24 hours later. Laser Capture Microdissection (LCM) was performed and analyzed by gene chip. (A) Figure illustrates the relative expression of gene expression in the pericryptal mRNA from LGG treated and vehicle treated mice. Dotted lines represent 2 standard deviations from the means. Genes for chemokines, chemokine receptors and enzymes related to arachidonic acid metabolism are shown. (B) Table presents data for chemokine receptors, chemokines and arachidonic acid metabolism genes, comparing gene expression in pericryptal cells from vehicle treated and LGG treated mice. (C) Immunofluorescence for CXCL12(red) and F4/80(green) in mouse small intestines shows that some macrophages, including pericryptal macrophages (arrows), express CXCL12.
LGG-induced migration of COX-2+ MSCsisTLR2-dependent and macrophages express TLR2.[29]To determine if macrophages are required for LGG-induced MSC migration, we treated mice with clodronate liposomes,whicheffectively depletedintestinal macrophages (Fig 4A). Mice received LGG or vehicleand MSC migration was assessed. LGG induced COX-2+ MSC migration in vehicle-treated mice but not in clodronate-treated mice (Fig 4B).[30] To determine if LGG-induced radioprotection is macrophage-dependent mice were treated with clodronate liposomes and then givenvehicle or LGG by gavage prior to 12Gy TBI. Clodronate depletion of macrophages resulted in a reduction in the number of surviving crypts suggesting that macrophages directly or indirectly provide a factor that supports epithelial stem cell survival after radiation (Fig 4C). Mice given clodronate followed by LGG had no increase in crypt survival compared to mice receiving clodronate alone suggesting that the radioprotection provided by LGG requires the presence of macrophages.
Fig. 4. Depletion of macrophages with anionic liposomal clodronate blocks LGG-induced MSC migration and LGG radioprotection.
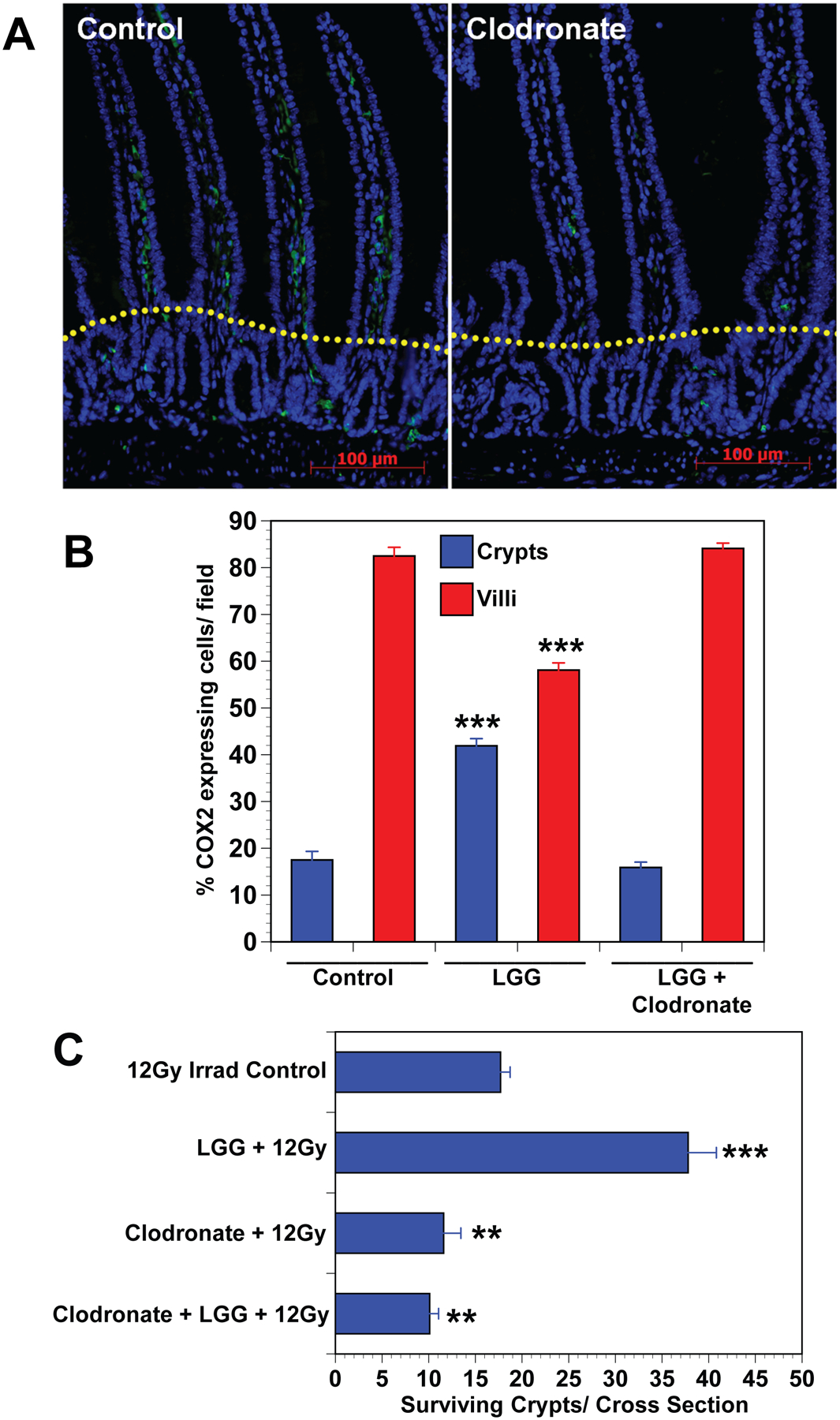
(A) Immunofluorescence for F4/80 expressing macrophages compares placebo treated mice having normal macrophage population with anionic clodronate liposome treated mice showing macrophage depletion. Dotted yellow line separates crypt and villus zones. (B) Cell counts provide quantitative data showing that treatment of mice with clodronate blocks LGG induced migration of COX2 expressing MSCs from the villus to the crypt zone in the small intestine. Data are means ± SEM for 5 to 7 mice per treatment group. ***P<.0001 compared with controls. (C) Crypt survival is significantly improved in LGG treated mice, and depletion of macrophages by treatment with clodronate blocks LGG induced radioprotection. Clodronate liposome (7mg/ml) were given i.p. at 4 days before (200μl/20g mouse), and again at 2 days before (100μl/20g mouse), and finally at eight hours before receiving 12Gy TBI. Data are means ± SEM for 5 to 7 mice per treatment group. **P<.001, ***P<.0001 compared with controls.
LTA induced PGE2 in MSCs protects epithelial stem cells from radiation.
PGE2 is radioprotective of the intestinal epithelium.[31] The radioprotective effects of LGG are mediated by COX-2.[8] Immunofluorescence for COX-2 in the intestine demonstrates staining only in lamina propria MSCs.[32, 33] To determine the effects of LTA on PGE2 production and the cellular source of PGE2 we incubated mouse enteroidsor MSCs with LTA and measured PGE2 levels in the supernatant. MSCs were isolated and cultured as previously described.[33]MSCs made PGE2 at baseline and there was an increase in PGE2 synthesis after incubation with LTA (Fig 5A). The LTA-induced increase in PGE2 synthesis was blocked by NS398, a selective COX-2 inhibitor, and by indomethacin, which inhibits both COX-1 and COX-2. Enteroids did not produce measurable PGE2 atbaseline or after incubation with LTA.
Fig. 5. LTA induced PGE2 in MSCs protects epithelial stem cells from radiation.
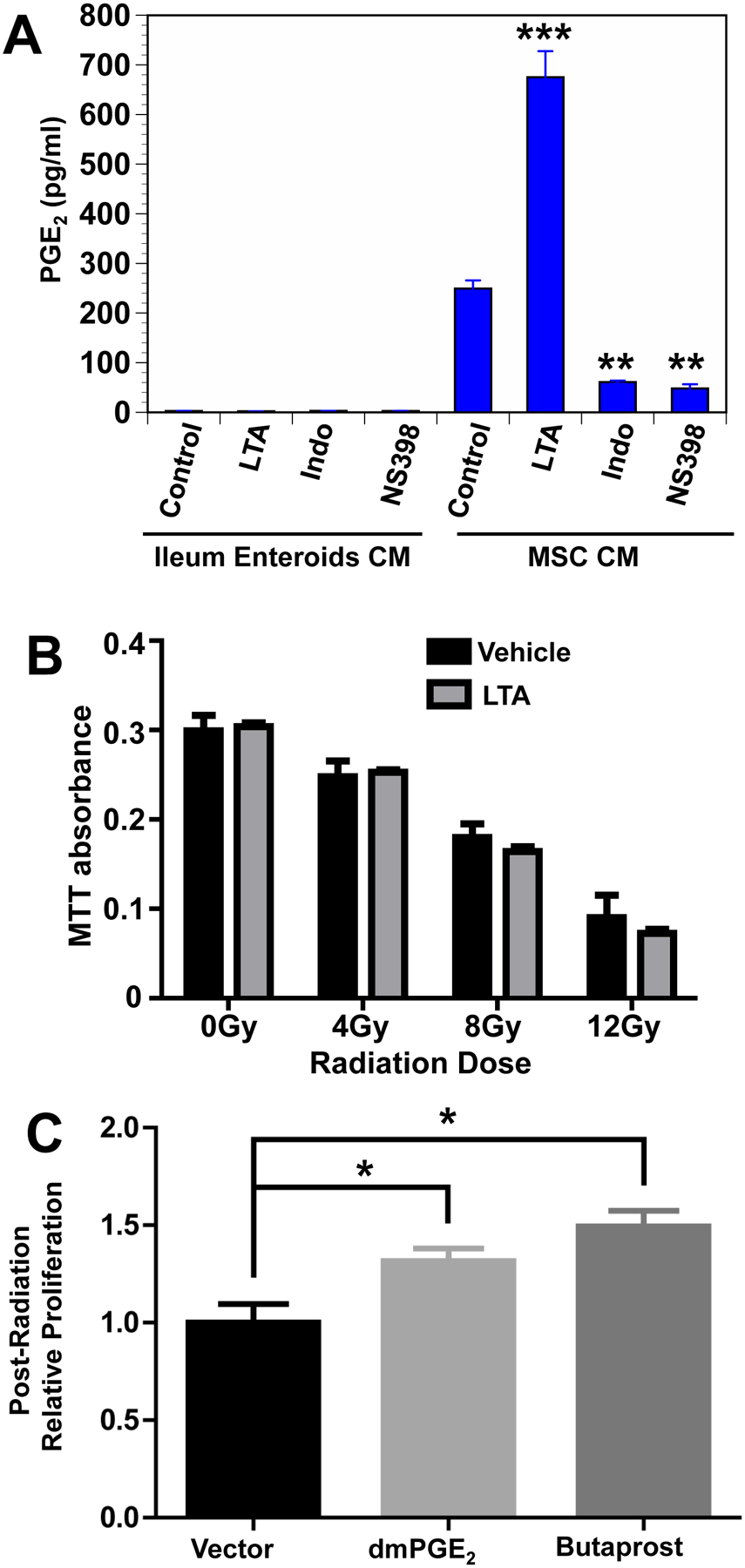
(A) LTA induces PGE production in MSCs but not in enteroids. Ileum enteroids and small intestine MSCs were incubated in media alone, or in media containing LTA (10μ/mL) or indomethacin (50μM), or NS398 (5μM). After 24-hour incubation, PGE2 concentration was determined by ELISA. Data are means ± SEM for 4 replicates in each treatment group. **P<.0002, ***P<.0004. (B) Gamma radiation reduces primary enteroid cell proliferation in a dose dependent manner. Cells were pretreated with LTA (10μg/mL) or vehicle in medium for 2 hours, then irradiated with zero, 4, 8, or 12 Gy. LTA or vehicle was removed 2 hours post irradiation and cells were cultured for 72 hours. Proliferation was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) incorporation. Using this pretreatment strategy, LTA did not protect against radiation injury. N.S., not significant. (C) dmPGE2 and the EP2 agonist butaprost are radioprotective of enteroids. WT enteroids were plated in 50% stem cell media (SCM)(11) for 18h, then grown in 5% SCM for 24h. Enteroids were then treated with dmPGE2 (10μM) or the EP2 agonist butaprost (10μM) for 1h in 5% SCM, followed by 6 Gy irradiation. Culture medium was immediately replaced with 50% SCM without drugs and enteroids were grown for 48h. Proliferation was measured by CCK-8 proliferation assay.
LGG-induced radioprotection is mediated through TLR2 and COX-2.[8] Under homeostatic conditions, intestinal epithelial cells express TLR2 but not COX-2.[32, 34]Immunofluorescence indicates that only MSCs express COX-2 at high levels in the intestine under homeostatic conditions. MSCs produce PGE2 through COX-2.[32, 33] PGE2 is radioprotective of the intestinal epithelium through binding to EP2.[35] Taken together, these data suggest that the radioprotective effects of LTA on the intestinal epithelium are not mediated by direct effects of LTA on epithelial cells, butinsteadare mediated by PGE2 produced through MSCs. Based on these data one would predict that PGE2 but not LTA would be radioprotective of enteroids.
To test this hypothesis we performed two experiments. In the first experiment mouse small intestinal enteroids were pretreated with LTA or vehicle and then irradiated with 0, 4, 8 or 12 Gy. The media was replaced and the enteroids were allowed to grow for 48 hours at which time proliferation was assessed with MTT. Increasing doses of radiation were associated with decreasing proliferation (Fig 5B). LTA had no effect on proliferation, suggesting that it is not radioprotective of enteroids. In the second experiment, enteroids were pretreated with vehicle, PGE2, or butaprost, a selective EP2 agonist. The enteroids were then irradiated (6 Gy), the media was replaced and the enteroids were allowed to grow for 48 hours after which time proliferation was assessed. Both PGE2 and butaprost enhanced proliferation suggesting that they are radioprotective of enteroids (Fig 5C).
LTA is radioprotective in mice receiving fractionated TAI.
Earlier we demonstrated the radioprotective effects of LGG and LGG-CM with a single 12Gy dose of TBI.[8]However, patients with abdominal malignancies are treated with fractionated TAI rather than a single dose TBI.[3, 36] To determine if LTA is radioprotective withfractionated abdominal radiotherapy, mice were treated with 4Gy TAIfor seven or eight consecutive days with i.p. injections of either vehicle or LTA one hour before each radiation dose. To administer radiation to the abdomen, mice were anesthetized with isoflurane, shielded with lead, and irradiated in an X-ray irradiator. Mice receiving either seven or eight fractions TAIdemonstrated weight loss and mortality (Fig 6A and 6B). Mice in all treatment groups lost weight rapidly during the course of radiation; however, the animals receiving LTA regained their weight more rapidly after radiation was completed (Fig 6A). There was a 60% survival in mice receiving seven fractions 4Gy radiationand 10% survival in mice receiving eightfractions of 4Gy radiation (Fig 6B). Administration of LTA improved survival to 100% in mice receiving seven fractions and 60% in mice receiving eight fractions of radiation. This experiment demonstrates that LTA is radioprotective not only in TBI but also in fractionated TAI.
Fig. 6. LTA improves post-irradiation weight recovery and mortality in mice receiving fractionated abdominal irradiation.
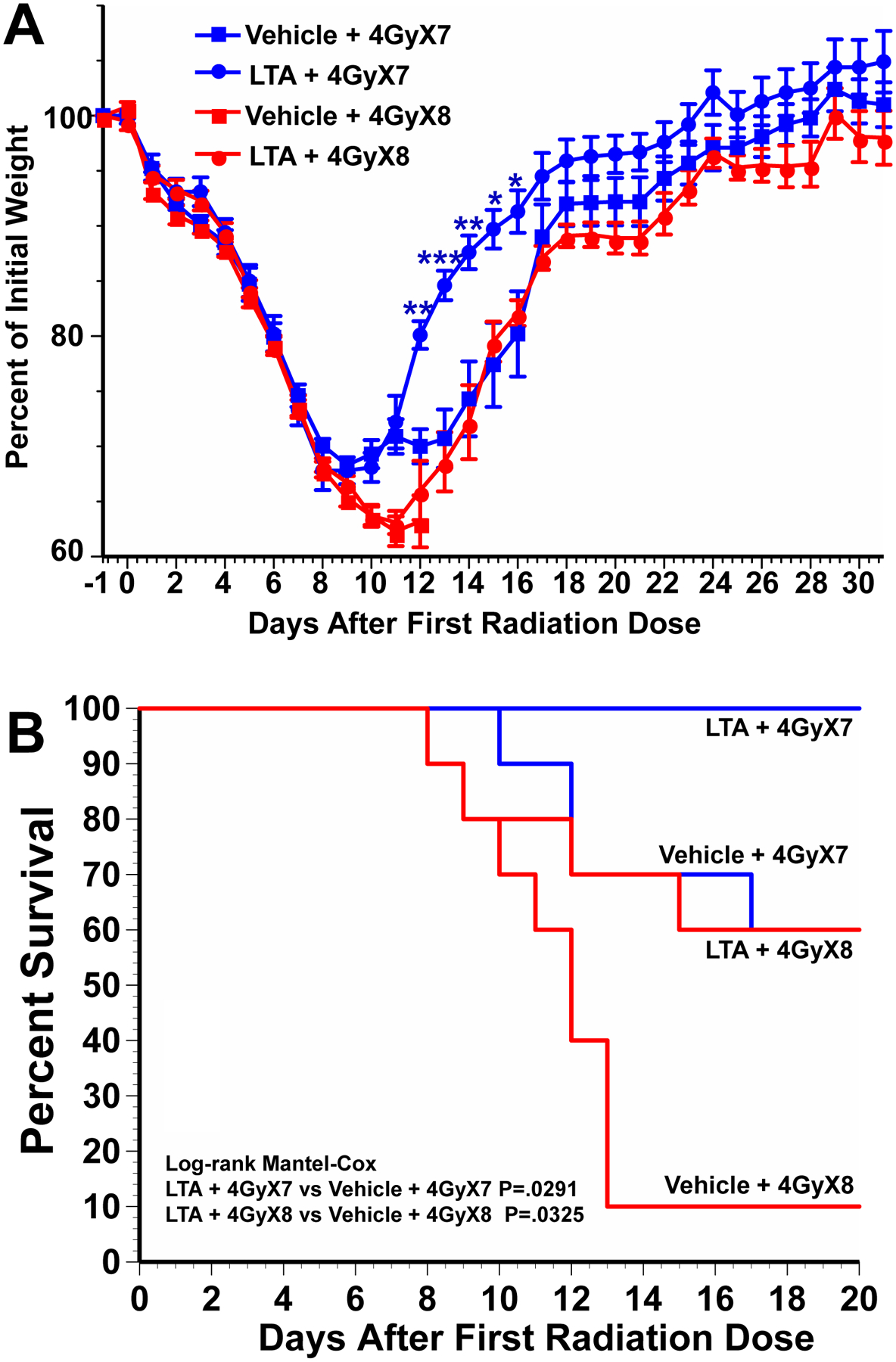
Mice received 4Gy total abdominal irradiation (TAI) on 7 or 8 consecutive days and intraperitoneal injections of LTA (5mg/kg) or vehicle at 1 hour before each radiation dose. Mice were followed for (A) weight change and (B) survival. N=10 mice per treatment group. For weight change time course, ***P<.0001, **P<.001, *P<.01 comparing LTA treated with vehicle treated controls. For survival Log-rank Mantel-COX: P=.0291 for LTA + 4GyX7 vs Vehicle + 4GyX7, P=.0325 for LTA + 4GyX8 vs Vehicle + 4GyX8.
LGG is not radioprotective of colon cancer in vivo or in vitro.
LGG would be of little use as adjuvant therapy in patients receiving abdominal radiation if it were radioprotective of the tumor as well as the intestinal epithelium. To address this question, we performed in vitro and in vivo studies using CT26 cells, a colon cancer cell line developed in Balb C mice.[37]To determine the effect of LGG-CM treatment on viability and survival of CT26 cells, we performed clonogenic assays. Cells were treated with 1:50 and 1:100 of LGG-CM or media control and irradiated with 0,2,4,6 or 8 Gy. There was no significant difference in the numbers of colonies from CT26 cells treated with LGG-CM 1:50 or LGG-CM 1:100 compared to mediatreated cells.
BalbC mice were injected heterotopically with CT26 cells to allow localized radiation and to generate tumor growth delay curves. CT26 cells were injected subcutaneously in the right hind limb of BalbC mice. To determine if LGG is radioprotective of the tumors, tumor-bearing mice were treated with PBS alone, irradiation alone, LGG alone for 6 days, or LGG for 6 days followed by irradiation (five daily fractions of 2 Gy). Tumor volumes were measured. LGG did not protect the CT26 heterotopic tumors fromradiation; in fact, it delayed the growth of the tumor (Fig 7B). The tumor growth was evaluated by monitoring the time taken to reach a tumor volume of 2500 mm3. The CT26 tumors in irradiated mice took 40% longer to reach 2500mm3when compared to tumors in unirradiated micecontrol (10.5 days vs 7.5 days in un-irradiated mice). Treatment with LGG did not affect the time to reach 2500 mm3(Fig 7C).
Fig. 7. LGG-CM is not radioprotective of CT26 colon cancer cells in vitro or in vivo.
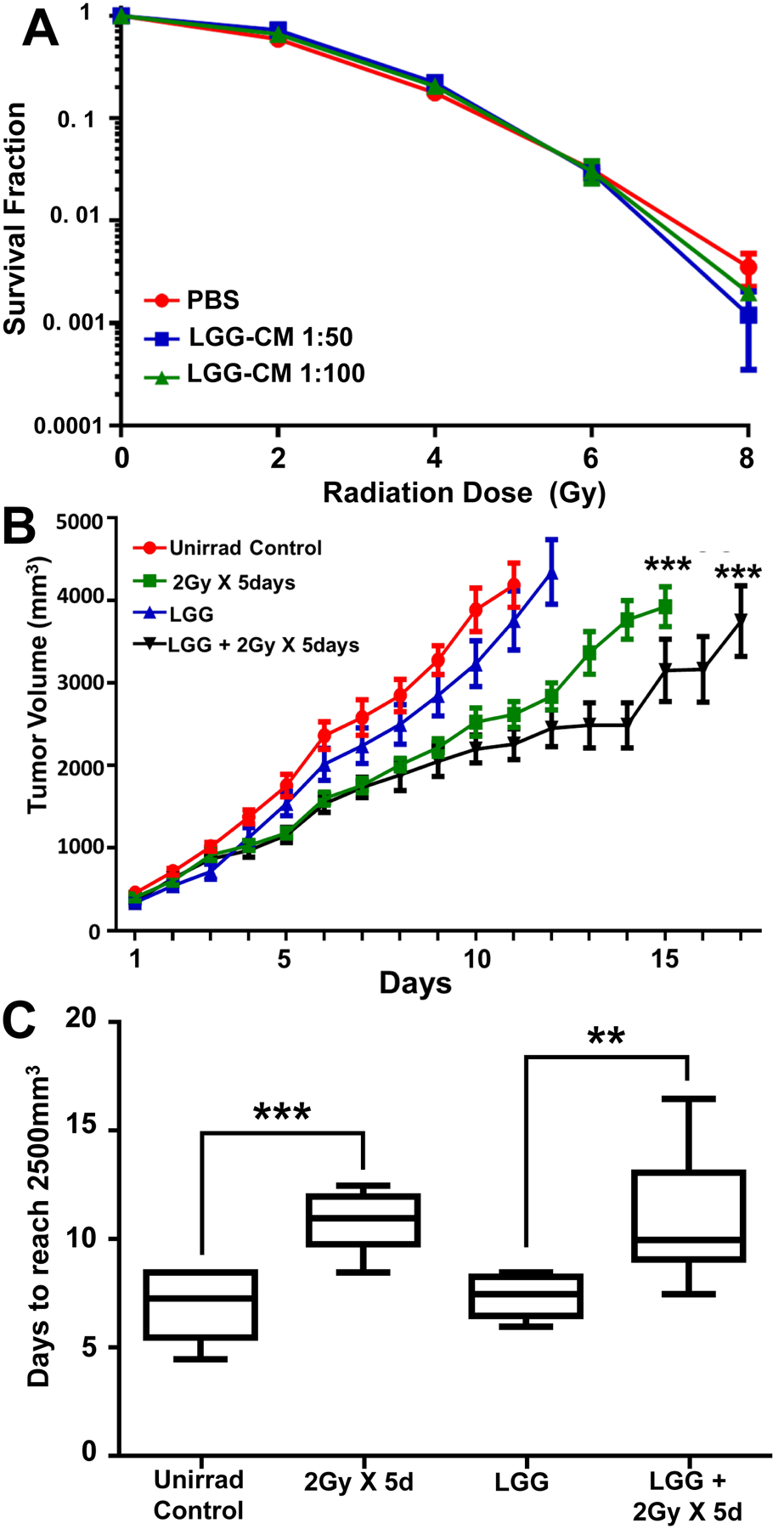
(A) Cells were plated in 10% FBS RPMI media and left to attach overnight, Media was changed to LGG-CM at 1 hour before irradiation. Media was again changed at 1 hour after irradiation back to 10% FBS RPMI and cells allowed to grow for 5 days. Cells were fixed with 70% ethanol and stained with 1% methylene blue. (B) LGG does not provide radioprotection to CT26 cell tumors grown in mice. CT26 cells (7.50 × 105) were injected into the flanks of mice and allowed to grow for 7 days, at which time mice were stratified into four treatment groups, each representing similar distributions of tumor size. Mice were treated once a day for 6 consecutive days with vehicle (groups 1 and 2) or LGG (groups 3 and 4) by gavage. Mice in groups 2 and 4 also received 2Gy of radiation to the tumor alone on days 2 through 6. Tumor volumes were measured with a digital caliper. ***P<.0001 compared with unirradiated controls using Tukey’s multiple comparisons test. (C) Data from B showing that tumors in irradiated mice take 40% longer (10.5 days) to reach 2500mm3 compared with tumors in unirradiated mice (7.5 days).Data are means ± SEM for 10 mice per treatment group. **P<.003, ***P<.0001 compared with unirradiated controls.
LTA is not radioprotective of the bone marrow.
We assumedthat the radioprotective effects of LGG would be confined to the epithelial cells of the intestine and colon since LGG would be confined to the intestinal lumen and only the cells immediately adjacent to the lumen would be exposed to LGG and thus to LTA. To assess the specificity of the radioprotective effects of LGG for the GI tract we performed experiments to determine if LGG was radioprotective of the bone marrow. The radioprotective effects of LGG on the circulating lymphocytes were assessed by counting the number of CD4+ and CD8+ cells in the peripheral blood after radiation.[38] Irradiation resulted in a 90% decrease in both CD4+ and CD8+ cells in the peripheral blood (Supp Fig 1A and 1B). Treatment with LGG did not affect the number of CD4+ or CD8+ cells in the peripheral blood after radiation. Bone marrow from the femurs was analyzed for CD34+ stem cells (Supp Fig 1C).[38] Irradiation led to a 45% reduction in the stem cell number. Pretreatment with LGG or LGA did not affect the number of stem cells after irradiation.
DISCUSSION:
LGG was demonstrated to beradioprotective in themouse intestine.[8] LGG is now undergoing clinical trials as a radioprotectant for patients receiving radiation therapy (NCT01790035). Here, we have extended these studies to define the mechanism of LGG intestinal radioprotection and illustrate its application in clinically relevant models. Mechanistically, we show that LGG (through LTA) primes the epithelial stem cell niche to protect epithelial stem cells from radiation toxicity by triggering a multicellular, adaptive immune signaling cascade that imitates a process normally initiated only after injury occurs. Furthermore, in radiation regimens that reflect those used in clinical practice we demonstrate that the radioprotective effects of LGG extend to the normal small intestine, but not the tumor.
We define LTA as key to probiotic LGG mediated radioprotection. Wildtype LGG is radioprotective whereasLGG deficient in LTA alanylation is not; thissuggests that LTA is a radioprotective agent in LGG.LTA,found in the protoplast fraction of lactobacilli, is normally shed continuously from Gram-positive bacteria during replication and released after bacteriolysis by lysozyme, bactericidal peptides, cathepsin and elastase peptide.[23]Not only LGG but also LGG-CM is radioprotective whereas the conditioned media from mutant LGG is not; this is consistent with the release of LTA from LGG. Thus, LGG acts as a “time-release capsule” for its biologically active component, LTA. It is possible that other probiotics may also have therapeutic activity as time release capsules for LTA or mediate radioprotection by other mechanisms.[39]TLR2 signaling mediates intestinal homeostasis and tumorigenesis through both epithelial cell autonomous and epithelial cell eccentric mechanisms.[40, 41]To determine whether the radioprotective effects of LTA are epithelial cell autonomous,we performed experiments using mouse enteroids. LTA was not radioprotective of enteroids suggesting that the radioprotective effects of LTA in the intestine are mediated through binding of LTA to TLR2 on some non-epithelial cell. Macrophage depletion blocksLGG-induced MSC migration and LGG-induced radioprotection suggesting that macrophages are the relevant TLR2 expressing cells mediating LGG-induced radioprotection.
The radioprotective effects of LGG require the migration of COX-2 expressing MSCs from the lamina propria of villus to the pericryptal lamina propria. MSCs are widely distributed in the body butonly the MSCs of the GI tract constitutively express COX-2.[33] The expression of COX-2 in MSCs of the GI tract is promoted by COX-2 mRNA stabilization downstream of Fgf9, a growth factor constitutively expressed by the intestinal epithelium.[33] The migration of COX-2 expressing MSCsis driven by the release of CXCL12 by pericryptal macrophages in response to LTA binding to macrophage TLR2. CXCL12 binds to CXCR4 on MSCs inducing their migration to the pericryptal region. PGE2 released by the COX-2 expressing MSCs has an antiapoptotic effect on the adjacent epithelial stem cells.[42] PGE2 has a short half-life and only affects cells in the immediate vicinity of the PGE2 secreting cells.[43] Thus, MSC migration to a place near the epithelial stem cells allows PGE2 released by MSCs to block radiation-induced epithelial stem cell apoptosis.
Migration of COX-2 expressing MSCs is also seen in hyaluronic acid induced intestinal radioprotection.[44] Administration of exogenous hyaluronic acid induces the migration of MSCs from the villus to the pericryptal region in a TLR4-dependent fashion. The role of pericryptal macrophages and CXCL12 in hyaluronic acid induced migration was not addressed but it is reasonable to assume that a similar mechanism is involved.
Migration of COX-2 expressing MSCs is seen in wound repair. In DSS colitis epithelial repair is mediated by TLR4 activation and the migration of COX-2-expressing MSCs.[32] CXCL12 expression is increased in DSS colitis.[45]In a second wound repair model the effect of exogenous colonic MSCs was assessed in biopsy forceps injury to the colon.[46]Exogenous MSCs injected into the colonic wall migrate to sites of injury induced by biopsy forceps and accelerate wound repair. Administration of AMD3100 blocked both MSC migration and the acceleration of wound repair suggesting that CXCL12 binding to CXCR4 drives MSC migration in wound repair. Here we find that the same migration of COX-2 expressing MSCs is the mechanism for LGG-induced radioprotection. Thus, the radioprotective effects of LGG are mediated by jump-starting the wound repair process by inducing the first step in wound repair, the migration of COX-2 expressing MSCs, prior to radiation injury.
Although LGG protects the normal small intestine, it is not radioprotective of tumor cells either in vitro or in vivo even though the tumor cells express TLR2.[47] This is consistent with the finding that the in vivo radioprotective effects of LTA, a TLR2 agonist,are not epithelial cell autonomous but rather are mediated through pericryptal macrophages and PGE2 released by COX-2 expressing MSCs.LGG was also not radioprotective of CT26 syngeneic colon cancer isografts grown in mice. Two explanations for this are postulated. One is that LGG is confined to the intestinal lumen and the radioprotective effects of LTA shed by LGG is confined to the cells that line the intestinal lumen. The CT26 tumors growing in the mouse flank would not be exposed to LTA when LGG is given by gavage. The second possible explanation is that in CT26 syngeneic isograftsthere are no COX-2 expressing MSCs that could release PGE2 in response to TLR2 activation.
LGG was also not radioprotective of the normal bone marrow. Here again the likely explanation is that LGG is confined to the intestinal lumen and the bone marrow would not be exposed to LGG or LTA. TLR2 activation can induce radioprotection in the bone marrow as previously demonstrated by parenteral administration of the synthetic TLR2 agonist PAM3-CSK4;[22] however, in this study it was not clear which cells were responding to the PAM3-CSK4.
Here we found that LTA is a radioprotective agent in LGG. The radioprotective effects of LGG in the intestine are mediated by the release of LTA, the activation of macrophage TLR2 and the migration of COX-2 expressing MSCs to an area adjacent to the epithelial stem cells. PGE2 released from MSCs protects epithelial stem cells from radiation-induced apoptosis. The radioprotective effects of LGG are mediated by jump-starting the wound repair process by inducing the migration of COX-2 expressing MSCs prior to irradiation. This raises the possibility that other agents that lead to the prepositioning of COX-2 expressing MSCs near the epithelial stem cells may also protect the intestinal epithelium from injury induced by radiation and chemotherapy.
Supplementary Material
SIGNIFICANCE.
What is already known about this subject?
The lactobacillus probiotic LGG is radioprotective of the intestinal epithelium.
The radioprotective effects of LGG are mediated through TLR2 and COX-2.
An FDA-approved clinical trial of LGG as a radioprotectant in patients receiving radiation therapy for pelvic malignancies is in progress.
What are the new findings?
LGG acts as a “time release capsule” for lipoteichoic acid, a TLR2 agonist and the radioprotective agent in LGG.
Lipoteichoic acid binding to TLR2 on pericryptal macrophages induces the production of the chemokine CXCL12 which binds to CXCR4 on COX-2 expressing MSCs inducing their migration to an area adjacent to the epithelial stem cells.
PGE2 released by COX-2 expressing mesenchymal stem cells protects epithelial stem cells from radiation induced apoptosis.
In the case of LGG, radioprotection is induced by jumpstarting the first step in wound repair, the migration of COX-2 expressing MSCs.
LGG is radioprotective of the intestine in mice receiving fractionated abdominal radiation.
LGG is not radioprotective of CT26 syngeneic tumor isografts.
How might it impact practice in the foreseeable future?
These findings define the mechanism of action for a probiotic undergoing clinical trials as a radioprotectant.
These findings suggest that pharmacologic manipulation of the migration of COX-2 expressing MSCs may result in new drugs for radioprotection.
Demonstration that probiotics can act as “time release capsules” for biologically active agents.
These findings support the practicality of LGG as a radioprotective agent in man by demonstrating radioprotection in mice receiving fractionated abdominal radiation and by demonstrating that its radioprotective effects do not extend to radiation-targeted colon cancer cells.
Funding:
NIH-DK33165, DK077653, DK109384, CA206039, Washington University DDRCC, P30-DK052574, and the Department of Radiation Oncology at Washington University.
Abbreviations:
- RIGS
Radiation-induced gastrointestinal syndrome
- LGG
Lactobacullus rhamnosus GG
- i.p.
Intraperitoneal
- TBI
Total body irradiation
- CM
Conditioned media
- COX-2
Cyclooxygenase-2
- LTA
Lipoteichoic acid
- LCM
Laser capture microdissection
- TAI
Total abdominal radiation
- MSCs
Mesenchymal stem cells
Footnotes
Competing Interest:
NONE declared
Data Sharing agreement: Not applicable
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, et al. Animal models for medical countermeasures to radiation exposure. Radiat Res 2010;173:557–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potten CS. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol 1990;58:925–73. [DOI] [PubMed] [Google Scholar]
- 3.Bismar MM, Sinicrope FA. Radiation enteritis. Current Gastroenterology Reports 2002;4:361–5. [DOI] [PubMed] [Google Scholar]
- 4.Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy--pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol 2014;11:470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardill HR, Van Sebille YZA, Ciorba MA, Bowen JM. Prophylactic probiotics for cancer therapy-induced diarrhoea: a meta-analysis. Current opinion in supportive and palliative care 2018;12:187–97. [DOI] [PubMed] [Google Scholar]
- 6.Packey CD, Ciorba MA. Microbial influences on the small intestinal response to radiation injury. Curr Opin Gastroenterol 2010;26:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciorba MA, Hallemeier CL, Stenson WF, Parikh PJ. Probiotics to prevent gastrointestinal toxicity from cancer therapy: an interpretive review and call to action. Current opinion in supportive and palliative care 2015;9:157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciorba MA, Riehl TE, Rao MS, Moon C, Ee X, Nava GM, et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 2012;61:829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pace F, Pace M, Quartarone G. Probiotics in digestive diseases: focus on Lactobacillus GG. Minerva gastroenterologica e dietologica 2015;61:273–92. [PubMed] [Google Scholar]
- 10.Banna GL, Torino F, Marletta F, Santagati M, Salemi R, Cannarozzo E, et al. Lactobacillus rhamnosus GG: An Overview to Explore the Rationale of Its Use in Cancer. Frontiers in pharmacology 2017;8:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldin BR, Gualtieri LJ, Moore RP. The effect of Lactobacillus GG on the initiation and promotion of DMH-induced intestinal tumors in the rat. Nutrition and cancer 1996;25:197–204. [DOI] [PubMed] [Google Scholar]
- 12.Gamallat Y, Meyiah A, Kuugbee ED, Hago AM, Chiwala G, Awadasseid A, et al. Lactobacillus rhamnosus induced epithelial cell apoptosis, ameliorates inflammation and prevents colon cancer development in an animal model. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 2016;83:536–41. [DOI] [PubMed] [Google Scholar]
- 13.Claes IJJ, Lebeer S, Shen C, Verhoeven TLA, Dilissen E, De Hertogh G, et al. Impact of lipoteichoic acid modification on the performance of the probiotic Lactobacillus rhamnosus GG in experimental colitis. Clin Exp Immunol 2010;162:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velez MP, Verhoeven TLA, Draing C, Von Aulock S, Pfitzenmaier M, Geyer A, et al. Functional analysis of D-alanylation of lipoteichoic acid in the probiotic strain Lactobacillus rhamnosus GG. Appl Environ Microb 2007;73:3595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riehl TE, Santhanam S, Foster L, Ciorba M, Stenson WF. CD44 and TLR4 mediate hyaluronic acid regulation of Lgr5+ stem cell proliferation, crypt fission, and intestinal growth in postnatal and adult mice. American journal of physiology Gastrointestinal and liver physiology 2015;309:G874–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potten CS, Merritt A, Hickman J, Hall P, Faranda A. Characterization of radiation-induced apoptosis in the small intestine and its biological implications. Int J Radiat Biol 1994;65:71–8. [DOI] [PubMed] [Google Scholar]
- 17.Riehl T, Cohn S, Tessner T, Schloemann S, Stenson WF. Lipopolysaccharide is radioprotective in the mouse intestine through a prostaglandin-mediated mechanism. Gastroenterology 2000;118:1106–16. [DOI] [PubMed] [Google Scholar]
- 18.Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. International journal of radiation biology and related studies in physics, chemistry, and medicine 1970;17:261–7. [DOI] [PubMed] [Google Scholar]
- 19.Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nature protocols 2013;8:2471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, et al. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 2015;64:911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stappenbeck TS, Hooper LV, Manchester JK, Wong MH, Gordon JI. Laser capture microdissection of mouse intestine: characterizing mRNA and protein expression, and profiling intermediary metabolism in specified cell populations. Methods Enzymol 2002;356:167–96. [DOI] [PubMed] [Google Scholar]
- 22.Shakhov AN, Singh VK, Bone F, Cheney A, Kononov Y, Krasnov P, et al. Prevention and Mitigation of Acute Radiation Syndrome in Mice by Synthetic Lipopeptide Agonists of Toll-Like Receptor 2 (TLR2). Plos One 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginsburg I Role of lipoteichoic acid in infection and inflammation. The Lancet Infectious diseases 2002;2:171–9. [DOI] [PubMed] [Google Scholar]
- 24.Matsuguchi T, Takagi A, Matsuzaki T, Nagaoka M, Ishikawa K, Yokokura T, et al. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin Diagn Lab Immunol 2003;10:259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marquez-Curtis LA, Janowska-Wieczorek A. Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. BioMed research international 2013;2013:561098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu YJ, Zhao RCH. The Role of Chemokines in Mesenchymal Stem Cell Homing to Myocardium. Stem Cell Rev Rep 2012;8:243–50. [DOI] [PubMed] [Google Scholar]
- 27.Hummel S, Van Aken H, Zarbock A. Inhibitors of CXC chemokine receptor type 4: putative therapeutic approaches in inflammatory diseases. Current opinion in hematology 2014;21:29–36. [DOI] [PubMed] [Google Scholar]
- 28.Clark JD, Lin LL, Kriz RW, Ramesha CS, Sultzman LA, Lin AY, et al. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell 1991;65:1043–51. [DOI] [PubMed] [Google Scholar]
- 29.Dobrovolskaia MA, Medvedev AE, Thomas KE, Cuesta N, Toshchakov V, Ren T, et al. Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: effects of TLR “homotolerance” versus “heterotolerance” on NF-kappa B signaling pathway components. Journal of immunology 2003;170:508–19. [DOI] [PubMed] [Google Scholar]
- 30.van Rooijen N, Sanders A, van den Berg TK. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. Journal of immunological methods 1996;193:93–9. [DOI] [PubMed] [Google Scholar]
- 31.Hanson WR, Ainsworth EJ. 16,16-Dimethyl prostaglandin E2 induces radioprotection in murine intestinal and hematopoietic stem cells. Radiat Res 1985;103:196–203. [PubMed] [Google Scholar]
- 32.Newberry RD, McDonough JS, Stenson WF, Lorenz RG. Spontaneous and continuous cyclooxygenase-2-dependent prostaglandin E2 production by stromal cells in the murine small intestine lamina propria: directing the tone of the intestinal immune response. Journal of immunology 2001;166:4465–72. [DOI] [PubMed] [Google Scholar]
- 33.Walker MR, Brown SL, Riehl TE, Stenson WF, Stappenbeck TS. Growth factor regulation of prostaglandin-endoperoxide synthase 2 (Ptgs2) expression in colonic mesenchymal stem cells. J Biol Chem 2010;285:5026–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 2004;126:1054–70. [DOI] [PubMed] [Google Scholar]
- 35.Houchen CW, Sturmoski MA, Anant S, Breyer RM, Stenson WF. Prosurvival and antiapoptotic effects of PGE2 in radiation injury are mediated by EP2 receptor in intestine. American journal of physiology Gastrointestinal and liver physiology 2003;284:G490–8. [DOI] [PubMed] [Google Scholar]
- 36.Stansborough RL, Bateman EH, Al-Dasooqi N, Bowen JM, Keefe DMK, Yeoh ASJ, et al. Fractionated abdominal irradiation induces intestinal microvascular changes in an in vivo model of radiotherapy-induced gut toxicity. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2017;25:1973–83. [DOI] [PubMed] [Google Scholar]
- 37.Son TG, Gong EJ, Bae MJ, Kim SD, Heo K, Moon C, et al. Protective effect of genistein on radiation-induced intestinal injury in tumor bearing mice. BMC complementary and alternative medicine 2013;13:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CL, Lento WE, Castle KD, Chao NJ, Kirsch DG. Inhibiting glycogen synthase kinase-3 mitigates the hematopoietic acute radiation syndrome in mice. Radiat Res 2014;181:445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones RM, Desai C, Darby TM, Luo L, Wolfarth AA, Scharer CD, et al. Lactobacilli Modulate Epithelial Cytoprotection through the Nrf2 Pathway. Cell Rep 2015;12:1217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004;118:229–41. [DOI] [PubMed] [Google Scholar]
- 41.Vaishnava S, Behrendt CL, Ismail AS, Eckmann L, Hooper LV. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proceedings of the National Academy of Sciences of the United States of America 2008;105:20858–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tessner TG, Muhale F, Riehl TE, Anant S, Stenson WF. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. The Journal of clinical investigation 2004;114:1676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamberg M, Samuelsson B. On the metabolism of prostaglandins E 1 and E 2 in man. The Journal of biological chemistry 1971;246:6713–21. [PubMed] [Google Scholar]
- 44.Riehl TE, Foster L, Stenson WF. Hyaluronic acid is radioprotective in the intestine through a TLR4 and COX-2-mediated mechanism. American journal of physiology Gastrointestinal and liver physiology 2012;302:G309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikami S, Nakase H, Yamamoto S, Takeda Y, Yoshino T, Kasahara K, et al. Blockade of CXCL12/CXCR4 Axis Ameliorates Murine Experimental Colitis. J Pharmacol Exp Ther 2008;327:383–92. [DOI] [PubMed] [Google Scholar]
- 46.Manieri NA, Mack MR, Himmelrich MD, Worthley DL, Hanson EM, Eckmann L, et al. Mucosally transplanted mesenchymal stem cells stimulate intestinal healing by promoting angiogenesis. Journal of Clinical Investigation 2015;125:3606–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou XT, Hong T, Yu Q, Nie SP, Gong DM, Xiong T, et al. Exopolysaccharides from Lactobacillus plantarum NCU116 induce c-Jun dependent Fas/Fasl-mediated apoptosis via TLR2 in mouse intestinal epithelial cancer cells. Sci Rep-Uk 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


