Abstract
Ambulatory blood pressure monitoring provides a more precise measure of blood pressure status than clinic blood pressure, and is currently recommended in the evaluation of high blood pressure in children and adolescents. However, ambulatory blood pressure monitoring may not always be available. Our aim was to determine the clinic blood pressure percentile most likely to predict ambulatory hypertension. We evaluated clinic and ambulatory blood pressure in 247 adolescents (median age 15.7 years, 63% white, 54% male). Clinic blood pressure percentile (based on the Fourth report and the 2017 AAP clinical practice guidelines) and ambulatory blood pressure status (normal vs. hypertension) were determined by age, sex and height-specific cut-points. Sensitivity and specificity of different clinic blood pressure percentiles and cut-offs to predict ambulatory hypertension were calculated. Forty (16%) and 67 (27%) patients had systolic hypertension based on the 4th report, and the 2017 guidelines, respectively, whereas 38 (15%) had wake ambulatory systolic hypertension. The prevalence of ambulatory wake systolic hypertension increased across clinic systolic blood pressure percentiles, from 3% when clinic systolic blood pressure was <50th percentile to 41% when ≥95th percentile. The 2017 guidelines’ 85th systolic percentile had similar sensitivity (86.8%) and better specificity (57.4% vs. 48.1%) than “elevated blood pressure” (≥90th percentile or ≥120 mm Hg) to diagnose ambulatory hypertension. When evaluating adolescents for hypertension, 2017 guidelines’ clinic systolic 85th percentile may be the optimal threshold at which to perform ambulatory blood pressure monitoring.
Keywords: hypertension, pediatrics, ambulatory blood pressure monitoring
Summary
Evaluating BP using the 2017 CPG may provide superior prediction of ambulatory HTN than categorizing clinic BP with Fourth report, with the 85th systolic percentile having the best sensitivity and specificity in prediction of ambulatory HTN. When evaluating adolescents referred for suspected hypertension, especially in case of limited ABPM availability, 2017 CPG SBP percentile of ≥85 may be the optimal threshold to perform an ABPM.
Introduction
A history of high blood pressure (BP) in children and adolescents is associated with adult hypertension (HTN)1, 2 and adverse cardiovascular outcomes3–5. HTN is diagnosed based on the presence of persistent high BP in the clinic setting,6, 7 but the 2017 clinical practice guideline (CPG) recommends 24-hour ambulatory BP (ABP) for confirmation of HTN. Several studies have shown relatively poor correlation between clinic and 24-hour ABP8–12, a more robust measure of BP status, which has stronger association with target organ damage (TOD) in both adult13, 14 and pediatric15–21 populations. A study by Davis et al.9 even suggested universal ABP monitoring (ABPM) as the most economic approach for the evaluation of children and adolescents with high clinic BP.
In 2017, the American Academy of Pediatrics published new clinical practice guidelines (CPG) for screening and management of high BP in children and adolescents,7 replacing the previous Fourth report6. A significant change in the 2017 CPG is the publication of new normative pediatric BP tables based on normal-weight children only (excluding children with BMI >85% percentile); these BP values are lower than those in the Fourth Report. Additionally, the 2017 CPG is aligned with the 2017 American College of Cardiology/American Heart Association guidelines of high BP in Adults,22 in its adoption of single-value BP of 120/80 mm Hg and 130/80 mm Hg for “elevated BP” and HTN, respectively, in all patients ≥13 years of age.
Consistent with the literature regarding the advantages of ABPM, the 2017 CPG also formally recommend performance of ABPM in every patient with persistent HTN (over 3 clinic visits) or elevated BP (over 1 year). However, ABPM is not readily available in all pediatric clinical practices, therefore implementation of this recommendation may be difficult.
The Study of Hypertension In Pediatrics, Adult Hypertension Onset in Youth Study (SHIP AHOY) is a cross-sectional cohort study designed to determine BP levels and phenotypes (clinic + ABPM) that predict BP related TOD in adolescents.23 Using a sample of the first 247 participants in this cohort, with both clinic BP measured by rigorous protocol and ABP data, we sought to identify the clinic BP cut-point that best predicts ambulatory HTN. As this is a transition period from the Fourth Report to the 2017 CPG, we categorized clinic BP based on both guidelines, to allow comparison between both them in predicting ambulatory HTN.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. All information on the rationale and design of the SHIP AHOY study is available elsewhere.23 Briefly, the study recruited otherwise healthy adolescents across a wide range of BP percentiles, including healthy volunteers or referred patients. Participants were eligible if they were 11–19 years of age without exclusion criteria (pregnant or breast-feeding females; symptomatic stage 2 HTN; use of antihypertensive medication within the past 6 months; receiving metformin and lipid-lowering agents; or medications known to affect BP, including glucocorticoids, calcineurin inhibitors, and oral decongestants). Patients were also excluded if they had any medical condition known to be associated with the potential for elevated BP, such as: diabetes; clinically significant proteinuria (verified first morning urine protein/creatinine ratio of ≥1.0); known history of chronic kidney disease and/or an estimated glomerular filtration rate ≤90 mL/min/1.73m2; congestive heart failure, obstructive valvular disease or cardiomyopathy; secondary causes of HTN; uncorrected coarctation of the aorta; diagnosis of obstructive sleep apnea; or any other clinically significant unstable medical condition.
Participants were categorized by clinic systolic BP (SBP) as low-risk SBP (Clinic SBP<80th percentile), mid-risk SBP (80th - <90th percentiles), or high-risk SBP (≥90th percentile), with an intention to recruit similar number of participants in each category. The study was initiated prior to the publication of the 2017 CPG, and BP stratification at enrollment was based on the Fourth Report BP tables and percentiles.
On enrollment, demographic (age, sex, race, ethnicity) and medical history information were collected; weight and height were measured. The study protocol has undergone institutional review board (IRB) review, and written informed consent/assent was obtained from all enrolled participants according to local IRB requirements.
Clinic BP Measurements
Clinic BP status was determined based on the average of 6 BPs obtained by auscultation over two visits 1–2 weeks apart, measured according to a standardized method consistent with the Fourth Report and 2017 CPG guidelines. The mid-upper arm circumference was measured, and a cuff was selected so that the length of the cuff bladder would be equal to 80%−100% of the arm circumference. All participants had suitable cuff size for the arm circumference, with no cases of arm circumference too big (>52.0 cm) for the largest cuff. The BP was taken in the seated position in the right arm, resting at heart level, after 5 minutes of rest with an aneroid sphygmomanometer (Mabis Medic-Kit 5, Mabis Healthcare, Waukegan, IL) purchased for the sake of this study. Each site’s personnel received standardized training in BP measurement. BP was measured 4 times at 2 minute intervals on each of the two visits, discarding the first measurement on each occasion. The mean of the six remaining BP measurements was used in analysis.
Ambulatory BP Measurement
Ambulatory BP was measured with the OnTrak 90227 device (SpaceLabs™, Snoqualmie, WA), an oscillometric BP monitor that uses the same algorithm as the device that was used to generate the most commonly used pediatric normative ABPM dataset24. Using the arm circumference measurement obtained as part of the auscultatory BP measurement, a properly sized cuff was selected and the monitor placed on the participant. Three resting BP’s were obtained immediately after monitor placement to confirm correct placement and function of the monitor. For each 26-hour recording, measurements were obtained every 20 minutes through the day and night. A diary was kept by the participant to record time of sleep, time of waking, and timing of any napping. Diary data were used to divide the ABPM studies into accurate sleep-wake periods. No hours of monitoring were discarded, consistent with current AHA recommendations for pediatric ABPM.25
BP Status Classification
Clinic BP was initially classified according the Fourth report on BP in children6: 1) Normal BP: SBP and DBP <90th percentile and <120/80 mm Hg; 2) Pre-hypertension: SBP or DBP greater than or equal to the 90th or 120/80 mm Hg, but less than the 95th percentile; 3) HTN: SBP or DBP ≥95th percentile. Blood pressure was then re-classified based on the percentiles and recommendations of the 2017 CPG7:
Normal BP: BP <90th percentile for age, sex and height; or <120/<80 mmHg for adolescents ≥13 years old;
Elevated BP: BP reading ≥90th percentile and <95th percentile for age, sex and height; or 120–129/<80 mmHg for adolescents ≥13 years old;
Hypertension: BP >95th percentile for age, sex and height; or ≥130/80 mmHg for adolescents ≥13 years old.
Additional classification divided the cohort based on a universal cut-off of SBP ≥120 and/or DBP ≥80 across the entire age range.
ABPM was analyzed based on the AHA recommendations for pediatric ABPM,25 using pediatric normative ABPM data obtained with the same device24: 1) Normal ambulatory BP: mean 24 hour SBP/DBP, and both wake and sleep BP <95th percentile for sex and height; 2) Ambulatory HTN: mean 24 hour SBP/DBP, or wake/sleep BP ≥95th percentile for sex and height. Ambulatory BP index was calculated as the mean measured BP divided by the 95th percentile for sex and height, meaning that patients with normal ABP had ABP index <1, while ambulatory HTN was define as ABP index of ≥126.
Statistical analysis
For descriptive statistics, categorical variables are presented as percentages and continuous variables are presented as mean ±SD or median (IQR) depending on their distribution. McNemar’s test was used for comparison between prevalence of the various clinic BP strata and Student’s t test and Wilcoxon signed-rank test were used to compare different BP percentiles and indices of the study sample. Clinic SBP was stratified in the following groups: <50th, 50–79th, 80–89th, 90–94th, and ≥95th percentiles. Sensitivities and specificities for diagnosis of ambulatory HTN were calculated for BP percentiles ≥80 (in 5 percentile increments) as well as for, elevated BP (2017 CPG),120/80 as universal cut-offs across the entire age range, and prehypertension (Fourth Report). We also compared the sensitivities and specificities of different cut-offs to diagnose wake systolic HTN based on only 3 readings (from the visit just prior to ABPM placement). of the Tables detailing the 2017 CPG clinic SBP percentiles by age and height percentiles were generated based on the program published at https://sites.google.com/a/channing.harvard.edu/bernardrosner/pediatric-blood-press/childhood-blood-pressure. All statistical analyses were performed using SAS 9.3 statistical software (SAS Institute Inc, Cary, NC)
Results
Study Population
Participant characteristics are presented in table 1. Median age was 15.7 years, 55% were male, 63% white, and 16% Hispanic. The sample cohort was relatively overweight, with median BMI of 25.8 kg/m2, and median BMI percentile of 91.
Table 1.
Study Population Characteristics (n=247)
| Characteristic | Median (IQR)/n (%) |
|---|---|
| Age, y | 15.7 (14.3–16.9) |
| Male Sex | 133 (54) |
| Race | |
| White | 156 (63) |
| Black | 65 (26) |
| Asian | 11 (5) |
| Other | 15 (6) |
| Hispanic | 39 (16) |
| Height, m | 1.67 (1.61–1.75) |
| Weight, kg | 74.2 (60.7–92.4) |
| BMI, kg/m2 | 25.7 (22.0–32.0) |
| BMI Percentile | 91.0 (66.6–98.3) |
Clinic BP status
Our study population was relatively hypertensive, with a median BP of 121/81, reflecting the intension to recruit similar number of participants to each risk group. Fifty-one percent of the study population were stratified in the low risk BP group, 20% in the mid-risk group and 29% in the high-risk BP group. Clinic BP status is summarized in table 2. Median BP percentiles and the prevalence of both systolic and diastolic HTN were significantly higher when BP was categorized based on the 2017 CPG. For example, 27% of participants were classified as having systolic HTN using the 2017 CPG, while only 16% had systolic HTN by the Fourth Report (p<0.001). The median DBP percentiles were significantly higher than the median SBP percentiles, regardless of which guideline was used. Accordingly, the prevalence of diastolic HTN was significantly higher than for systolic HTN. The prevalence of HTN defined as either SBP and/or DBP HTN was 43% based on the Fourth Report compared with 61% based on the 2017 CPG (p<0.001).
Table 2.
Clinic BP Status of the Study population (n=247)
| BP status | Fourth Report | 2017 CPG |
|---|---|---|
| SBP %ile, median (IQR) | 80 (51–92) | 84 (59–93)* |
| <90, n (%) | 172 (70) | 154 (62) |
| 90–95, n (%) | 35 (14) | 42 (17) |
| ≥95, n (%) | 40 (16) | 51 (21)* |
| SBP Category, n (%) | ||
| Normal | 107 (43) | 105 (43) |
| Pre-HTN/Elevated BP | 100 (40) | 75 (30) |
| HTN | 40 (16) | 67 (27)* |
| ≥120 | 139 (56%) | |
| DBP %ile, median (IQR) | 90 (72–97)† | 93 (75–98)*,† |
| <90, n (%) | 126 (51) | 108 (44) |
| 90–95, n (%) | 25 (10) | 26 (11) |
| ≥95, n (%) | 96 (39)† | 111 (45)*,† |
| DBP Status, n (%) | ||
| Normal | 112 (45) | 110 (45) |
| Pre-HTN/Elevated BP | 39 (16) | 4 (2) |
| HTN | 96 (39)† | 132 (54)*,† |
| ≥80 | 132 (53%) | |
p<0.001 compared with Fourth Report
p<0.001 compared with SBP
Ambulatory HTN Status
ABP status is presented in table 3. The ABPM studies obtained were 26 hours (IQR 25.6–26.6) in duration, contained 72 readings (IQR 65–77) per study with a success rate of 86% (IQR 76%−92%). Median wake BP was 122/71 mmHg, and median sleep BP was 107/56 mmHg. As opposed to the Clinic BP, ambulatory SBP index was significantly higher than ambulatory DBP index (p<0.001 for both wake and sleep hours). Fifteen percent had wake systolic HTN, and 13% had wake diastolic HTN. During sleep time, 17% had systolic HTN and 12% had diastolic HTN. Taking into account SBP/DBP and wake/sleep ABP, 71 (29%) participants had ambulatory HTN.
Table 3.
Ambulatory BP status of the study population (n=247)
| BP Parameter | Systolic | Diastolic |
|---|---|---|
| Mean awake, mmHg | 122 (114–130) | 71 (66–77)* |
| Wake Index | 0.90 (0.86–0.96) | 0.85 (0.80–0.93)† |
| Mean Sleep, mmHg | 107 (100–114) | 56 (53–62) |
| Sleep Index | 0.90 (0.85–0.96) | 0.85 (0.80–0.92)† |
| Prevalence of wake HTN (%) | 38 (15) | 31 (13)† |
| Prevalence of sleep HTN (%) | 40 (17) | 30 (12)‡ |
| Prevalence of Wake and/or Sleep HTN (%) | 56 (23) | 48 (20) |
p<0.001 compared with clinic DBP
p<0.001 compared with SBP
p<0.05 compared with SBP
Systolic Ambulatory BP Status Based on Clinic BP
Prevalence of wake, sleep and overall (wake and/or sleep) systolic ambulatory HTN by different clinic SBP percentile categories is presented in Figure 1A, 1B, and 1C respectively. Prevalence of ambulatory HTN increased across Clinic SBP percentiles, from 3% when SBP percentile was <50 to 41% when SBP percentile was ≥95 in the case of wake systolic HTN, and from 4% when SBP percentile was <50 to 53% when SBP percentile was ≥95, for overall (wake and/or sleep) systolic HTN. When using the 2017 CPG percentiles, fewer patients with clinic SBP percentiles <95 had ambulatory HTN. Even though the 2017 was less likely to miss ambulatory HTN with a clinic SBP<95th percentile, there was still a high prevalence of white-coat HTN (high in clinic, normal on ABP), such that whatever guidelines were used, about 60% of subjects with clinic BP >95th percentile, had normal ambulatory BP.
Figure 1 -.
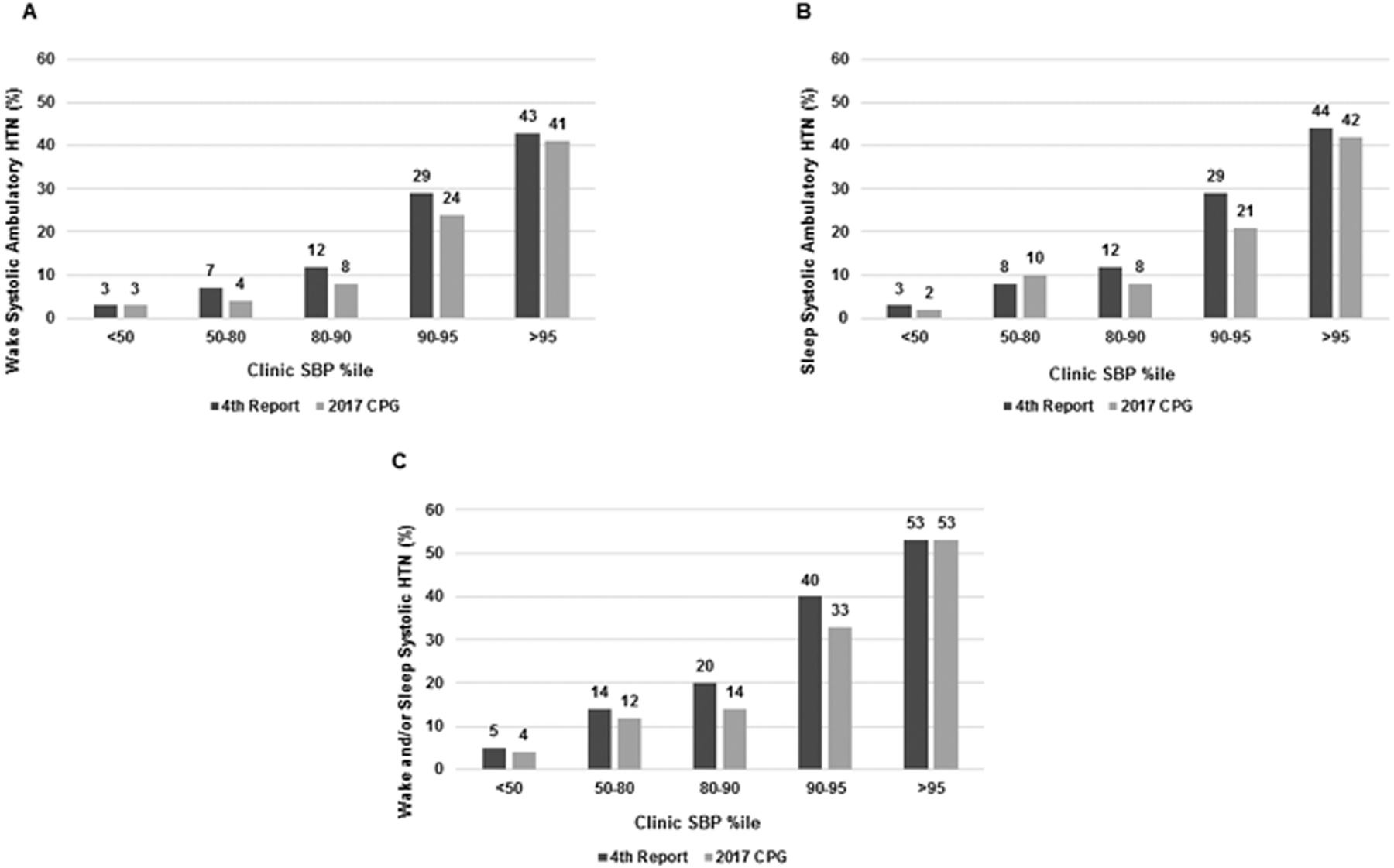
Prevalence of systolic ambulatory HTN based on different clinic systolic BP percentile categories: (A) wake systolic HTN; (B) sleep systolic HTN; (C) wake and/or sleep systolic HTN
Sensitivities and specificities of different Clinic BP cut-points to predict ambulatory HTN are presented in table 4 and supplementary table S1. Both elevated SBP (based on the 2017 CPG) and a universal cut-off of SBP ≥120 had sensitivity 86.8%, but specificity of only 47.9 and 49.2%, respectively, to predict wake ambulatory HTN. Sensitivity decreased and specificity increased with higher BP percentiles. The 2017 CPG 85th percentile cut-off was the only cut-off with both similar sensitivity (86.8%) and higher specificity (57.4%) than “elevated SBP” level or SBP≥120. Overall, the Fourth Report’s percentiles as cut-offs had lower sensitivities and higher specificities than their equivalent CPG percentiles (table S1, please see http://hyper.ahajournals.org).
Table 4.
Prediction of Wake Systolic Ambulatory HTN Based on Different Clinic SBP Parameters
| SBP parameter | Sensitivity | Specificity | |
|---|---|---|---|
| 2017 CPG | 80th %ile | 92.1% | 48.8% |
| 85th %ile | 86.8% | 57.4% | |
| 90th %ile | 81.6% | 70.3% | |
| Elevated SBP | 86.8% | 47.9% | |
| 120 mmHg | 86.8% | 49.3% | |
When clinic BP was defined based on the 3 readings obtained on the visit just prior to ABPM placement, sensitivities to diagnose wake systolic HTN were 81.6% for “elevated SBP” and 120 mmHg, and 84.2% for the CPG 85th percentile - lower than when all 6 study readings were used, but with less effect on the CPG 85th percentile. Specificities, however, of all 3 cut-offs were slightly higher – 50.2%, 50.7%, and 59.8%, for “elevated SBP”, 120 mmHg, and the CPG 85th percentile, respectively.
Data for nocturnal and overall (wake and/or sleep) systolic HTN showed that the 2017 CPG BP level at the 85th percentile had slightly lower sensitivity (82.5% vs. 87.5% in the case of nocturnal HTN, 80.4% vs. 83.9% in the case of overall HTN) but higher specificity (56.7% vs. 47.8% in the case of nocturnal HTN, 59.0% vs. 49.5% in the case of overall HTN) to diagnose ambulatory hypertension than “elevated BP”.
Diastolic BP Status Based on Clinic BP
Prevalence of wake, sleep and overall (wake and/or sleep) diastolic ambulatory HTN in different DBP percentile categories is presented in Figure 2A, 2B, and 2C, respectively. No ambulatory HTN was observed in patients with clinic DBP percentile <50; only 23% and 32% of participants with clinic DBP percentile >95th (2017 CPG) had wake and overall diastolic ambulatory HTN, respectively. Sensitivity and specificity of different DBP percentiles and prehypertension/elevated BP and ambulatory diastolic HTN are presented in table S2 (please see http://hyper.ahajournals.org). Both prehypertension (Fourth Report) and “elevated BP” (2017 CPG) had sensitivity of 87% and specificity about 50% to diagnose wake ambulatory diastolic HTN. There was no specific Clinic DBP percentile with clear advantage than prehypertension/elevated BP to predict ambulatory diastolic HTN. As in the case of SBP, using DBP of 80 mmHg as a universal cut-off across the entire age range yielded similar sensitivity as the elevated DBP cut-off, and slightly better specificity.
Figure 2 -.
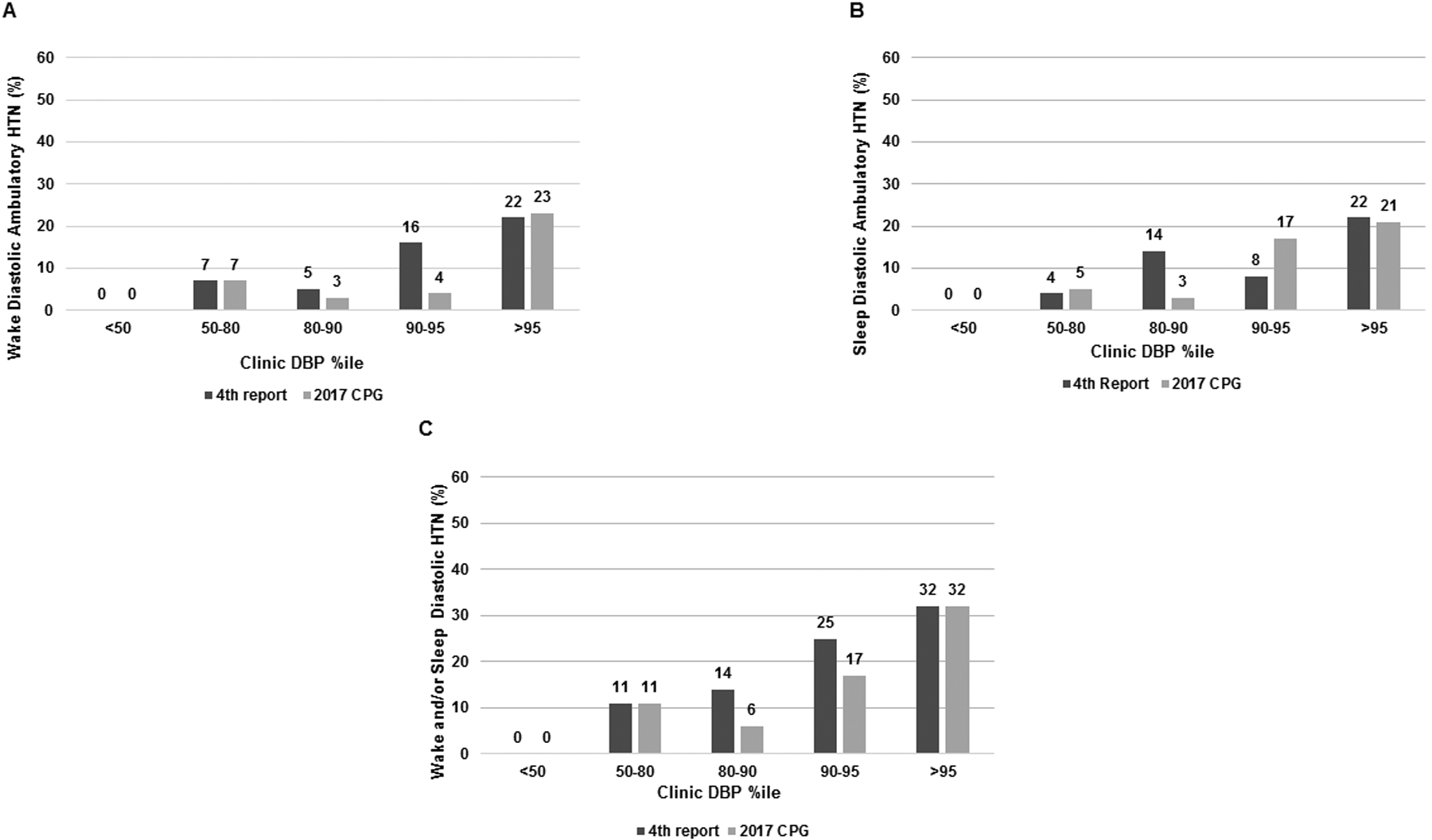
Prevalence of diastolic ambulatory HTN based on different clinic diastolic BP percentile categories: (A) wake diastolic HTN; (B) sleep diastolic HTN; (C) wake and/or sleep diastolic HTN
Discussion
In this is study of a large adolescent cohort we show that the 2017 CPG BP percentiles have superior sensitivity compared to those of the Fourth Report in predicting ambulatory HTN, and that the 2017 CPG 85th SBP percentile may serve as the best threshold to perform ABPM.
We also found a discordance between clinic and ambulatory BP, which confirms the findings of previous retrospective pediatric studies.9–12 In two of these studies, conducted on otherwise healthy patients referred for evaluation of HTN, 12%−17% of those with normal clinic BP/pre-hypertension had ambulatory HTN (masked HTN), while 46–55% of those with clinic HTN had normal ABP (white-coat HTN)9, 10. In another study, conducted on a mixed population (including patients already treated for HTN and CKD and diabetes patients), 36% patients of non-hypertensive per clinic BP had masked HTN, while 36% of hypertensives actually had white-coat HTN11.
Consistent with these studies, our findings underscore the importance of ABPM, both in confirming the diagnosis and in minimizing under-diagnosis of HTN. However, since applying ABPM on all patients with suspected HTN may be unrealistic, we tried to identify a clinic BP cut-point that would optimize the utility of ABPM. We therefore looked at different BP percentiles, as well the recently defined “elevated BP” cut-off and a universal cut-off of 120/80 as predictors of ambulatory HTN.
We demonstrated that the 2017 CPG 85th SBP percentile provided the best sensitivity/specificity combination to predict ambulatory HTN, with potentially higher specificity and similar or slightly lower sensitivity than the clinic BP category “elevated BP” (called pre-hypertension in the Fourth Report) and 120 mmHg. Interestingly, when clinic BP was defined based on readings from one visit only, the sensitivity of the CPG 85th percentile was higher than that of the other two cut-offs, further supporting the use of this cut-off if decision regarding ABPM is based on the findings of one clinic visit.
Our data extend the observations of previous studies to examine at what level of clinic BP is ambulatory HTN (and thus risk of TOD) likely to occur. In our cohort, 16/38 (42%) patients of participants with wake systolic ambulatory HTN did not have clinic HTN (according to the 2017 CPG), and 11 of those (29% of wake systolic hypertensives) had SBP ≥85th percentile, therefore, using the CPG 85th percentile as the cut-point to obtain an ABPM, similarly to using “elevated BP”, would identify ~70% of patients with masked HTN.
On the other hand, the systolic BP definition of “elevated BP” over the age of 13 (≥120 mm Hg) is in many cases, especially in older male teenagers, lower than the 85th SBP percentile. In our study, 49.4% of participants had SBP ≥85th percentile, compared to 57.5% who were above 120 mm Hg (p<0.001). Hence, fewer patients would require an ABPM, if the 85th percentile would be used to suspect ambulatory HTN. Therefore, in cases of limited availability of ABPM, the CPG 85th percentile could be a more appropriate SBP level in an adolescent for ABPM referral. For practical purposes, since the 2017 CPG guidelines detail only the 50th, 90th, and 95th percentiles, we include two tables (supplementary tables S3–S4, please see http://hyper.ahajournals.org) detailing the SBP 85th percentile by age and height percentile for both boys and girls aged 5–17.
As BP levels above 120/80 are associated with adverse outcomes in adults,22, 27, 28 we performed additional analysis applying this cut-off for all participants, including 11–13 years old children. In this age range, elevated BP is defined as BP ≥90th percentile, which in contrast to older adolescents, is generally lower than 120/80. Using this threshold yielded similar sensitivity and slightly better specificity than the “elevated BP” cut-off (but still lower than that of the 85th percentile). The number of 11–13 year olds in our study was small (18 participants), but this might suggest that in this age range, BP ≥120/80 may be an appropriate cut-off to perform ABPM.
Our results also showed that the 2017 CPG BP values have a consistently higher sensitivity for ambulatory HTN detection, compared to the Fourth Report percentiles. This is not surprising, since as opposed to the Fourth Report, the CPG percentiles are based on subjects with normal weight status only, while the Fourth Report included youth with obesity, a known risk factor for BP elevation. Therefore, in most cases, a patient with a specific BP percentile according to the Fourth Report has a higher BP percentile according to the 2017 CPG, as demonstrated by the higher BP percentiles of our population based on the 2017 CPG, compared to the Fourth Report (median SBP of 84 vs. 80; median DBP of 93 vs. 90).
One limitation of our study is that we over-sampled participants with higher BP levels and participants were relatively overweight or obese. This is representative of the typical pediatric patients referred for hypertension evaluation, and do not represent a general childhood population. In addition, although the Clinic BP guidelines were revised to include a single numerical HTN definition of ≥130/80 mmHg in all patients ≥13 years old, the ambulatory BP guidelines have not been revised and still use specific height and sex percentiles cut-offs. It is therefore challenging to define phenotypes of ambulatory HTN according to the new CPG guidelines and we were limited to focusing on associations between BP percentiles and ambulatory HTN. Moreover, although ABPM is a more robust measure of BP29, it might have limited reproducibility too30. Another limitation is that clinic BP was measured by auscultation, whereas ambulatory BP is assessed by oscillometry, methods which are not perfectly correlated. It is also unclear how our determination of clinic BP (average of 6 measurements obtained over 2 visits) is applicable to clinical settings, in which readings in 3 consecutive occasions are required to diagnose hypertension.7
Perspectives
Evaluating BP using the 2017 CPG may provide superior prediction of ambulatory HTN than categorizing clinic BP with Fourth Report. Use of the CPG 85th systolic percentile generates the best sensitivity and specificity in prediction of ambulatory HTN. When evaluating adolescents referred to clinic for suspected hypertension, especially in case of limited ABPM availability, CPG SBP percentile of ≥85 may be the optimal threshold to perform an ABPM. Additional research is needed to determine if referral for ABPM at the 85th percentile leads to changes in hypertension therapy and ultimate reduction in BP-related target organ damage.
Supplementary Material
Novelty and Significance.
What Is New?
First study using clinic blood pressure cut-offs based on the 2017 Clinical Practice Guidelines (CPG) for high blood pressure in Children and Adolescents to predict ambulatory HTN.
Comparison between percentiles and cut-offs of the 2017 CPG and the Fourth Report (2004) as predictors of ambulatory HTN.
What is Relevant?
Identifying a clinic BP cut-off with an optimal sensitivity/specificity combination to diagnose HTN, thereby optimizing ABPM utilization, especially in cases of limited ABPM availability.
Sources of Funding
This study was supported by AHA grant 15SFRN23680000 and partially by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1 TR001425. The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Heart Association or the National Institutes of Health.
Footnotes
Conflicts of Interest
None
References
- 1.Sun SS, Grave GD, Siervogel RM, Pickoff AA, Arslanian SS and Daniels SR. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119:237–46. [DOI] [PubMed] [Google Scholar]
- 2.Chen X and Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tracy RE, Newman WP 3rd, Wattigney WA, Srinivasan SR, Strong JP and Berenson GS. Histologic features of atherosclerosis and hypertension from autopsies of young individuals in a defined geographic population: the Bogalusa Heart Study. Atherosclerosis. 1995;116:163–79. [DOI] [PubMed] [Google Scholar]
- 4.Daniels SR, Loggie JM, Khoury P and Kimball TR. Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation. 1998;97:1907–11. [DOI] [PubMed] [Google Scholar]
- 5.Urbina EM, Khoury PR, McCoy C, Daniels SR, Kimball TR and Dolan LM. Cardiac and vascular consequences of pre-hypertension in youth. J Clin Hypertens (Greenwich). 2011;13:332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National High Blood Pressure Education Program Working Group on High Blood Pressure in C and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 7.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV, Urbina EM, Subcommittee On S and Management Of High Blood Pressure In C. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140:e20171904. [DOI] [PubMed] [Google Scholar]
- 8.Sorof JM, Poffenbarger T, Franco K and Portman R. Evaluation of white coat hypertension in children: importance of the definitions of normal ambulatory blood pressure and the severity of casual hypertension. Am J Hypertens. 2001;14:855–60. [DOI] [PubMed] [Google Scholar]
- 9.Davis ML, Ferguson MA and Zachariah JP. Clinical predictors and impact of ambulatory blood pressure monitoring in pediatric hypertension referrals. J Am Soc Hypertens. 2014;8:660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubrano R, Paoli S, Spiga S, Falsaperla R, Vitaliti G, Gentile I and Elli M. Impact of ambulatory blood pressure monitoring on the diagnosis of hypertension in children. J Am Soc Hypertens. 2015;9:780–4. [DOI] [PubMed] [Google Scholar]
- 11.Halbach SM, Hamman R, Yonekawa K and Hanevold C. Utility of ambulatory blood pressure monitoring in the evaluation of elevated clinic blood pressures in children. J Am Soc Hypertens. 2016;10:406–12. [DOI] [PubMed] [Google Scholar]
- 12.Johnson PK, Ferguson MA and Zachariah JP. In-Clinic Blood Pressure Prediction of Normal Ambulatory Blood Pressure Monitoring in Pediatric Hypertension Referrals. Congenit Heart Dis. 2016;11:309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van Der Niepen P, O’Brien E and Office versus Ambulatory Pressure Study I. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–15. [DOI] [PubMed] [Google Scholar]
- 14.Dolan E, Stanton A, Thijs L, Hinedi K, Atkins N, McClory S, Den Hond E, McCormack P, Staessen JA and O’Brien E. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156–61. [DOI] [PubMed] [Google Scholar]
- 15.Belsha CW, Wells TG, McNiece KL, Seib PM, Plummer JK and Berry PL. Influence of diurnal blood pressure variations on target organ abnormalities in adolescents with mild essential hypertension. Am J Hypertens. 1998;11:410–7. [DOI] [PubMed] [Google Scholar]
- 16.Sorof JM, Cardwell G, Franco K and Portman RJ. Ambulatory Blood Pressure and Left Ventricular Mass Index in Hypertensive Children. Hypertension. 2002;39:903–908. [DOI] [PubMed] [Google Scholar]
- 17.Lurbe E, Torro I, Alvarez V, Nawrot T, Paya R, Redon J and Staessen JA. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension. 2005;45:493–8. [DOI] [PubMed] [Google Scholar]
- 18.Stabouli S, Kotsis V, Toumanidis S, Papamichael C, Constantopoulos A and Zakopoulos N. White-coat and masked hypertension in children: association with target-organ damage. Pediatr Nephrol. 2005;20:1151–5. [DOI] [PubMed] [Google Scholar]
- 19.Lande MB, Carson NL, Roy J and Meagher CC. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006;48:40–4. [DOI] [PubMed] [Google Scholar]
- 20.Sharma AP, Mohammed J, Thomas B, Lansdell N, Norozi K and Filler G. Nighttime blood pressure, systolic blood pressure variability, and left ventricular mass index in children with hypertension. Pediatr Nephrol. 2013;28:1275–82. [DOI] [PubMed] [Google Scholar]
- 21.Conkar S, Yilmaz E, Hacikara S, Bozabali S and Mir S. Is Daytime Systolic Load an Important Risk Factor for Target Organ Damage in Pediatric Hypertension? J Clin Hypertens (Greenwich). 2015;17:760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017:HYP.0000000000000065. [Google Scholar]
- 23.Mendizabal B, Urbina EM, Becker R, Daniels SR, Falkner BE, Hamdani G, Hanevold CD, Hooper SR, Ingelfinger JR, Lande M, Martin LJ, Meyers K, Mitsnefes M, Rosner B, Samuels JA and Flynn JT. SHIP-AHOY (Study of High Blood Pressure in Pediatrics: Adult Hypertension Onset in Youth): Rationale, Design, and Methods. Hypertension. 2018;72:00-00:DOI: 10.1161/HYPERTENSIONAHA.118.11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wuhl E, Witte K, Soergel M, Mehls O, Schaefer F and German Working Group on Pediatric H. Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens. 2002;20:1995–2007. [DOI] [PubMed] [Google Scholar]
- 25.Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, Zachariah JP, Urbina EM, American Heart Association Atherosclerosis H and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Y. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63:1116–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuels J, Ng D, Flynn JT, Mitsnefes M, Poffenbarger T, Warady BA, Furth S and Chronic Kidney Disease in Children Study G. Ambulatory blood pressure patterns in children with chronic kidney disease. Hypertension. 2012;60:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leiba A, Twig G, Vivante A, Skorecki K, Golan E, Derazne E, Tzur D, Grossman E, Dichtiar R, Kark JD and Shohat T. Prehypertension among 2.19 million adolescents and future risk for end-stage renal disease. J Hypertens. 2017;35:1290–1296. [DOI] [PubMed] [Google Scholar]
- 28.Xi B, Zhang T, Li S, Harville E, Bazzano L, He J and Chen W. Can Pediatric Hypertension Criteria Be Simplified? A Prediction Analysis of Subclinical Cardiovascular Outcomes From the Bogalusa Heart Study. Hypertension. 2017;69:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stergiou GS, Alamara CV, Salgami EV, Vaindirlis IN, Dacou-Voutetakis C and Mountokalakis TD. Reproducibility of home and ambulatory blood pressure in children and adolescents. Blood Press Monit. 2005;10:143–7. [DOI] [PubMed] [Google Scholar]
- 30.Keren S, Leibowitz A, Grossman E and Sharabi Y. Limited reproducibility of 24-h ambulatory blood pressure monitoring. Clin Exp Hypertens. 2015;37:599–603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


