Abstract
Background
Age‐related macular degeneration (AMD) is one of the leading causes of permanent blindness worldwide. The current mainstay of treatment for neovascular AMD (nAMD) is intravitreal injection of anti‐vascular endothelial growth factor (anti‐VEGF) agents: aflibercept, ranibizumab, and off‐label bevacizumab. Injections can be given monthly, every two or three months ('extended‐fixed'), or as needed (pro re nata (PRN)). A variant of PRN is 'treat‐and‐extend' whereby injections are resumed if recurrence is detected and then delivered with increasing intervals. Currently, injection frequency varies among practitioners, which underscores the need to characterize an optimized approach to nAMD management.
Objectives
To investigate the effects of monthly versus non‐monthly intravitreous injection of an anti‐VEGF agent in people with newly diagnosed nAMD.
Search methods
We searched CENTRAL, MEDLINE, Embase, LILACS, and three trials registers from 2004 to October 2019; checked references; handsearched conference abstracts; and contacted pharmaceutical companies to identify additional studies.
Selection criteria
We included randomized controlled trials (RCTs) that compared different treatment regimens for anti‐VEGF agents in people with newly diagnosed nAMD. We considered standard doses only (ranibizumab 0.5 mg, bevacizumab 1.25 mg, aflibercept 2.0 mg, or a combination of these).
Data collection and analysis
We used standard Cochrane methods for trial selection, data extraction, and analysis.
Main results
We included 15 RCTs. The total number of participants was 7732, ranging from 37 to 2457 in each trial. The trials were conducted worldwide. Of these, six trials exclusively took place in the US, and three included centers from more than one country. Eight trials were at high risk of bias for at least one domain and all trials had at least one domain at unclear risk of bias.
Seven trials (3525 participants) compared a PRN regimen with a monthly injection regimen, of which five trials delivered four to eight injections using standard PRN and three delivered nine or 10 injections using a treat‐and‐extend regimen in the first year. The overall mean change in best‐corrected visual acuity (BCVA) at one year was +8.8 letters in the monthly injection group. Compared to the monthly injection, there was moderate‐certainty evidence that the mean difference (MD) in BCVA change at one year for the standard PRN subgroup was –1.7 letters (95% confidence interval (CI) –2.8 to –0.6; 4 trials, 2299 participants), favoring monthly injections. There was low‐certainty evidence of a similar BCVA change with the treat‐and‐extend subgroup (0.5 letters, 95% CI –3.1 to 4.2; 3 trials, 1226 participants).
Compared to monthly injection, there was low‐certainty evidence that fewer participants gained 15 or more lines of vision with standard PRN treatment at one year (risk ratio (RR) 0.87, 95% CI 0.76 to 0.99; 4 trials, 2299 participants) and low‐certainty evidence of a similar gain with treat‐and‐extend versus monthly regimens (RR 1.11, 95% CI 0.91 to 1.36; 3 trials, 1169 participants).
The mean change in central retinal thickness was a decrease of –166 μm in the monthly injection group; the MD compared with standard PRN was 21 μm (95% CI 6 to 32; 4 trials, 2215 participants; moderate‐certainty evidence) and with treat‐and extend was 22 μm (95% CI 37 to –81 μm; 2 trials, 635 participants; low‐certainty evidence), in favor of monthly injection. Only one trial (498 participants) measured quality of life and reported no evidence of a difference between regimens, but data could not be extracted (low‐certainty evidence).
Both PRN regimens (standard and 'treat‐and‐extend') used fewer injections than monthly regimens (standard PRN: MD –4.6 injections, 95% CI –5.4 to –3.8; 4 trials, 2336 participants; treat‐and‐extend: –2.4 injections, 95% CI –2.7 to –2.1 injections; moderate‐certainty evidence for both comparisons). Two trials provided cost data (1105 participants, trials conducted in the US and the UK). They found that cost differences between regimens were reduced if bevacizumab rather than aflibercept or ranibizumab were used, since bevacizumab was less costly (low‐certainty evidence).
PRN regimens were associated with a reduced risk of endophthalmitis compared with monthly injections (Peto odds ratio (OR) 0.13, 95% CI 0.04 to 0.46; 6 RCTs, 3175 participants; moderate‐certainty evidence). Using data from all trials included in this review, we estimated the risk of endophthalmitis with monthly injections to be 8 in every 1000 people per year. The corresponding risk for people receiving PRN regimens was 1 in every 1000 people per year (95% CI 0 to 4).
Three trials (1439 participants) compared an extended‐fixed regimen (number of injections reported in only one large trial: 7.5 in one year) with monthly injections. There was moderate‐certainty evidence that BCVA at one year was similar for extended‐fixed and monthly injections (MD in BCVA change compared to extended‐fixed group: –1.3 letters, 95% CI –3.9 to 1.3; RR of gaining 15 letters or more: 0.94, 95% CI 0.80 to 1.10). The change in central retinal thickness was a decrease of 137 μm in the monthly group; the MD with the extended‐fixed group was 8 μm (95% CI –11 to 27; low‐certainty evidence). The frequency of endophthalmitis was lower in the extended‐fixed regimen compared to the monthly group, but this estimate was imprecise (RR 0.19, 95% CI 0.03 to 1.11; low‐certainty evidence). If we assumed a risk of 8 cases of endophthalmitis in 1000 people receiving monthly injections over one year, then the corresponding risk with extended‐fixed regimen was 2 in 1000 people (95% CI 0 to 9).
Other evidence comparing different extended‐fixed or PRN regimens yielded inconclusive results.
Authors' conclusions
We found that, at one year, monthly regimens are probably more effective than PRN regimens using seven or eight injections in the first year, but the difference is small and clinically insignificant. Endophthalmitis is probably more common with monthly injections and differences in costs between regimens are higher if aflibercept or ranibizumab are used compared to bevacizumab.
This evidence only applies to settings in which regimens are implemented as described in the trials, whereas undertreatment is likely to be common in real‐world settings. There are no data from RCTs on long‐term effects of different treatment regimens.
Plain language summary
Comparing different injection frequencies for neovascular age‐related macular degeneration
What was the aim of this review? The aim of this Cochrane Review was to find out if anti‐vascular endothelial grown factor (anti‐VEGF) injections for neovascular age‐related macular degeneration (nAMD) can be given less frequently than every month.
Key messages This review found that people receiving monthly injections had slightly better vision (one or two letters more on a vision test chart, less than half‐line of vision) at one year compared with people receiving injections 'as needed' (average: seven injections), but there was no difference with a modified 'as needed' regimen called treat‐and‐extend (average: nine injections). People receiving monthly injections had more injections and this increased the risk of rare, but severe side effects such as infections.
What was studied in this review? Neovascular age‐related macular degeneration occurs in older people and affects the central part of vision. In nAMD, new blood vessels grow at the back of the eye.
People with nAMD can benefit from injections of medicines into the eye. These ‘anti‐VEGF’ medicines block the growth of new blood vessels. Currently, there is variation in how often these injections are given. A greater number of injections may result in better vision but also increase harm, such as endophthalmitis, a sight‐threatening infection of the eye. More injections are also more costly for the health service.
What were the main results of the review? Cochrane researchers identified 15 studies (7732 participants) comparing non‐monthly and monthly injections. Six out of 15 studies were funded by drug manufacturers.
The review found:
People who had less frequent anti‐VEGF injections may have had slightly worse vision at one year compared with people having monthly injections when injections (seven on average) are delivered 'as needed'. This was a difference of 1 or 2 more letters read on a vision test chart and an approximate 10% increased chance of gaining 15 or more letters of vision with monthly injections. There was no evidence of difference between monthly injections and treat‐and‐extend (nine injections on average).
There was an increased chance of endophthalmitis with monthly injections. Endophthalmitis is rare, occurring in approximately 8 in 1000 people having monthly injections for one year, and in approximately 1 per 1000 people (range 0 to 4) having less than monthly injections 'as needed'.
How up‐to‐date was this review? The search was updated on 18 October 2019.
Summary of findings
Summary of findings 1. As needed compared to monthly injections for administration of anti‐vascular endothelial growth factor agents for neovascular age‐related macular degeneration.
| PRN compared to monthly injections for administration of anti‐vascular endothelial growth factor agents for neovascular age‐related macular degeneration | ||||||
|
Patient or population: people with neovascular age‐related macular degeneration Setting: eye services delivering intravitreal injections of anti‐vascular endothelial growth factor agents Intervention: PRN injections Comparison: monthly injections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with monthly injections | Risk with PRN | |||||
| Change in BCVA at 1 year (ETDRS letters score, the higher the score the better) | The mean change in BCVA at 1 year was +8.8 letters |
Standard PRN MD 1.68 letters lower (–2.81 to –0.55) |
— | 2299 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | Test for subgroup differences P = 0.26 |
|
Treat‐and‐extend MD 0.51 higher (–3.14 to 4.16) |
— | 1226 (3 RCTs) |
⊕⊕⊝⊝ Lowa,b | |||
| Gain of ≥ 15 letters visual acuity at 1 year | 294 per 1000 |
Standard PRN 256 per 1000 (223 to 291) |
RR 0.87 (0.76 to 0.99) |
2299 (4 RCTs) | ⊕⊕⊝⊝ Lowa,c | Test for subgroup differences P = 0.04 |
|
Treat‐and‐extend 326 per 1000 (268 to 400) |
RR 1.11 (0.91 to 1.36) |
1169 (3 RCTs) |
⊕⊕⊝⊝ Lowa,c | |||
| Change in central retinal thickness at 1 year (μm, the thinner the better on average) | The mean change in central retinal thickness at 1 year was –165.5 μm |
Standard PRN MD 20.8 μm higher (5.8 to 35.9) |
— | 2215 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | Test for subgroup differences P = 0.97 |
|
Treat‐and‐extend MD 22.0 μm higher (–37.2 to –81.1) |
— | 635 (2) |
⊕⊕⊝⊝ Lowa,c | |||
| Change in QoL scores at 1 year (higher was better) | Data could not be extracted. Author reported that measures of QoL (median EuroQoL EQ‐5D) did not differ to a significant degree between monthly and PRN at 1 year. | — | 498 (1 RCT) | ⊕⊕⊝⊝ Lowa,c | — | |
| Number of injections at 1 year | The mean number of injections at 1 year was 11.3 |
Standard PRN MD 4.57 lower (–5.38 to –3.76 lower) |
— | 2336 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | Test for subgroup differences P < 0.01 |
|
Treat‐and extend MD 2.42 lower (–2.71 to –2.14) |
— | 1232 (3 RCTs) |
⊕⊕⊕⊝ Moderatea | |||
| Cost of treatment per person at 1 year | We could not estimate the difference in mean cost of treatment per person at 1 year for different regimens. Differences between regimens were reduced if bevacizumab was used. | — | 1105 (2 RCTs) | ⊕⊕⊝⊝ Lowd | — | |
| Endophthalmitis (ocular adverse event) | 8 per 1000 | 1 per 1000 (0 to 4 per 1000) |
Peto OR 0.13 (0.04 to 0.46) |
3175 (6 RCTs) |
⊕⊕⊕⊝ Moderatea | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCVA: best‐corrected visual acuity; CI: confidence interval; ETDRS: Early Treatment Diabetic Retinopathy Study; MD: mean difference; OR: odds ratio; PRN: as needed; QoL: quality of life; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for potential risk of bias as more than half were 'unclear' or 'high.' bDowngraded one level for inconsistency due to heterogeneity in the treat‐and‐extend subgroup cDowngraded one level for imprecision as no quantitative data could be extracted: "no difference" reported but the precision of this statement was unclear. dDowngraded two levels for indirectness as drug cost data available from two studies (CATT 2011; IVAN 2012b), and a full economic evaluation available from one study (IVAN 2012b): data available from two countries (US and UK), not including measures of variation in total cost per regimen, with unknown applicability to other settings.
Summary of findings 2. Extended‐fixed compared to monthly injections for administration of anti‐vascular endothelial growth factor agents for neovascular age‐related macular degeneration.
| Extended‐fixed compared to monthly injections for administration of anti‐vascular endothelial growth factor agents for neovascular age‐related macular degeneration | ||||||
|
Patient or population: people with neovascular age‐related macular degeneration Setting: eye services delivering intravitreal injections of anti‐vascular endothelial growth factor agents Intervention: extended‐fixed injections, such as injections every 2 or 3 months Comparison: monthly injections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with monthly injections | Risk with extended‐fixed | |||||
| Change in BCVA at 1 year (ETDRS letters score, the higher the score the better) | The mean change in BCVA at 1 year was 9 letters' improvement | MD 1.32 letters lower (–3.93 to 1.29) | — | 1439 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
| Gain of ≥ 15 letters visual acuity at 1 year | 300 per 1000 | 280 per 1000 (240 to 330) | RR 0.94 (0.80 to 1.10) | 1441 (3 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
| Change in central retinal thickness at 1 year (μm, the thinner the better on average) | The mean change central retinal thickness at 1 year was –137 μm | MD 8.16 higher (–11.07 to 27.40) | — | 1439 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | — |
| Change in QoL score at 1 year (measured with NEI VFQ‐25 questionnaire; the higher score the better) | The mean change in QoL score at 1 year was an improvement of about 5 | MD 0.59 lower (–2.22 to 1.04) | — | 1220 (1 RCT) | ⊕⊕⊕⊝ Moderatea | — |
| Number of injections at 1 year | Only 1 trial (1220 participants) comparing an extended (bimonthly) with a monthly regimen: 7.5 injections with the extended‐fixed regimen (scheduled for 8 injections), 12.3 in the monthly regimen (scheduled for 13 injections); no measures of variation reported and limited variation in the number of injections within each arm expected. | Not applicable | — | |||
| Cost of treatment per person at 1 year | None of the trials in this comparison category measured treatment cost. | Not applicable | — | |||
| Endophthalmitis (ocular adverse event) | 8 per 1000 | 2 per 1000 (0 to 9) |
RR 0.19 (0.03 to 1.12) | 1132 (3 RCTs) |
⊕⊕⊝⊝ Lowa,b | — |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCVA: best‐corrected visual acuity; CI: confidence interval; ETDRS: Early Treatment Diabetic Retinopathy Study; MD: mean difference; NEI VFQ‐25: National Eye Institute 25‐Item Visual Functioning Questionnaire; QoL: quality of life; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for potential risk of bias as more than half were 'unclear' or 'high.' bDowngraded one level for imprecision due to wide confidence intervals around estimate.
Background
Description of the condition
Age‐related macular degeneration (AMD) is a progressive, degenerative disease of the central retina, known as the macula, that can result in central vision loss. It is the leading cause of irreversible vision loss in industrialized countries and the third major cause of blindness globally (Bourne 2014; WHO 2016). The main risk factor for AMD is age (Klein 1992; Leibowitz 1980); other risk factors include cigarette smoking, white race, and genetic variation (Christen 1996; Evans 2005; Friedman 1999; Friedman 2004; Miller 2013; Seddon 1996; Swaroop 2007). There are two main forms of AMD: non‐neovascular, known as 'dry' or 'non‐exudative,' and neovascular, known as 'wet' or 'exudative,' types.
This review focused on neovascular AMD (nAMD). Approximately 20% of dry AMD cases transform to exudative disease through development of choroidal neovascularization (CNV), the abnormal proliferation of blood vessels in the inner choroid layer (Harper 2010). Defects in Bruch's membrane and the retinal pigment epithelium (RPE) enable extension of choroidal blood vessels into the subpigment epithelial space and eventually the subretinal space. Leakage or bleeding from these vessels causes exudative or hemorrhagic retinal detachments, triggering fibrosis. The resulting scarred retina has significantly decreased visual capacity (Harper 2010; Solomon 2014).
Fluorescein angiography (FA) findings are the gold standard for diagnosing CNV. Fluorescein dye is injected into a vein and travels into the eye; characteristic patterns of hyperfluorescence and hypofluorescence outline pathology. CNV diagnosis is supported by hyperfluorescent lesions in the macula that increase in intensity and size within a few minutes. Over the years, spectrum‐domain optical coherence tomography (SD‐OCT) has emerged as the main tool for monitoring disease and evaluating treatment response. OCT provides cross‐sectional views of the layers of the retina (AAO 2015), and it can be obtained quickly and non‐invasively. Since 2014, OCT angiography has become available to evaluate AMD lesions non‐invasively, although its use is limited by additional cost and some challenges to properly obtain and interpret the images.
CNV represents pathologic angiogenesis, the development of new capillaries, in the choroid. In nAMD, chronic exposure to hypoxia, ischemia, inflammation, or a combination of these tips the balance between angioinhibitors and angioactivators toward the formation of new blood vessels (Bressler 2009; Gunda 2013). The natural progression of nAMD without effective treatment eventually results in an end‐stage subretinal disciform scar and loss of vision.
Description of the intervention
The current mainstay treatment for nAMD is intravitreous injections of anti‐vascular endothelial growth factor (anti‐VEGF) agents. VEGF is an endothelial cell‐specific mitogen that promotes the proliferation of new vessels and increased vascular permeability (Ferrara 2004). It is upregulated in nAMD and is a key factor in the pathogenesis of CNV. Anti‐VEGF agents, including ranibizumab, bevacizumab, and aflibercept, target this angioactivator in their treatment of nAMD (Bressler 2009; Ferrara 2004; Gunda 2013).
Ranibizumab, a monoclonal antibody fragment against VEGF‐A, was approved by the US Food and Drug Administration (FDA) for the treatment of nAMD in 2006. Two pivotal trials demonstrated its efficacy and safety, ANCHOR and MARINA (ANCHOR 2009; MARINA 2006). Bevacizumab, a monoclonal antibody against VEGF‐A, has been used alongside ranibizumab as a cheaper anti‐VEGF alternative. Although it is FDA approved only for the treatment of colorectal cancer, non‐small cell lung cancer, cervical cancer, glioblastoma, and renal cell carcinoma, it is used off‐label to treat nAMD. Several trials have demonstrated comparable efficacies and safeties between these two anti‐VEGF agents (CATT 2012; GEFAL 2013; IVAN 2012a; MANTA 2013; Moja 2014; Solomon 2014). However, the marketed dosage of bevacizumab is too large for use in the eye. The appropriate dose of bevacizumab for intravitreous injection has to be compounded by pharmacies, which introduces contamination risk. Its use in the eye is not regulated by the FDA. The FDA approved a third anti‐VEGF intravitreous agent, aflibercept, in 2011 for the treatment of nAMD. It is a decoy receptor that blocks VEGF‐A, VEGF‐B, and placental growth factor. VIEW 1 and VIEW 2 trials (VIEW 2012) demonstrated the non‐inferiority of aflibercept efficacy when compared to ranibizumab (Sarwar 2016).
The first FDA approved anti‐VEGF drug, pegaptanib (VISION 2006), is no longer in use because of the better visual acuity results from ranibizumab, bevacizumab, and aflibercept (Sarwar 2016; Solomon 2014). Photodynamic therapy (PDT) decreases rates of visual loss from subfoveal nAMD and still has clinical application in rare cases (TAP 2001; VIP 2001; Wormald 2007; Yonekawa 2015). Our review focused on treatment regimens using ranibizumab, bevacizumab, and aflibercept intravitreous injections.
There is currently no standard regimen for injection frequency after the initial three monthly loading doses. Ophthalmologists administer anti‐VEGF injections at frequencies that vary based on physician practice and individual cases after the first three injections of anti‐VEGF agents. Intravitreous injections of ranibizumab were administered monthly in the MARINA and ANCHOR trials (ANCHOR 2009; MARINA 2006). With a higher binding affinity and thus longer therapy window than ranibizumab, aflibercept's non‐inferior effects were demonstrated with injections every two months after three initial monthly loading doses (VIEW 2012).
Additional studies have investigated ranibizumab, bevacizumab, and aflibercept efficacy using a variety of monthly and non‐monthly injection regimens. Non‐monthly dosing has included: loading doses (monthly for the first three months) followed by as needed (or pro re nata [PRN]), every eight weeks, quarterly, crossover from monthly to PRN, or formula‐based (such as treat‐and‐extend protocol) (Abedi 2014; CATT 2012; CLEAR‐IT 2 2011a; HARBOR 2014; IVAN 2012a; PIER 2010; PrONTO 2009; Schmidt‐Erfurth 2011; SECURE 2013; SUSTAIN 2011; VIEW 2012). PRN is a reactive scheme in which injections are administered whenever disease activity is detected with OCT, commonly as intraretinal or subretinal fluid, or when visual loss is associated with clinical signs of CNV recurrence, such as subretinal hemorrhage or exudation. The treat‐and‐extend regimen is also reactive, since an injection is given when recurrence is observed, but further injections are delivered extending their interval (generally by two weeks) even if no recurrence is observed; if recurrence is observed, then further treatment is administered shortening the following interval (e.g. by two weeks). In this review, we included the treat‐and‐extend regimen in the PRN category given its reactive characteristics, but we acknowledge that a larger number of injections are expected. Although all investigations have supported the use of anti‐VEGF agents, it is unclear which regimen is superior with respect to efficacy and safety to 'standard' PRN, the terms we will use here in subgroup analyses of PRN regimens.
The ideal treatment protocol would minimize the number of injections to decrease adverse effects and maximize therapeutic outcomes. The potential adverse effects are rare but may have serious consequences for vision from the procedure and the drug itself. Serious risks from the injection process include endophthalmitis, retinal hemorrhage, retinal detachment, RPE detachment, retinal edema, and vitreous detachment (CATT 2012; CLEAR‐IT 2 2011a). Potential adverse drug events include systemic arterial thromboembolic events such as myocardial infarction and cerebral vascular accident (CATT 2012). Although Solomon and colleagues found the occurrence of systemic adverse events to be comparable across anti‐VEGF and control groups and between ranibizumab and bevacizumab when given the same injections regimens, the number of participants in the trials included in their review was insufficient to detect meaningful differences in rare adverse events (Solomon 2014). Furthermore, their review did not compare dosing regimens. Inclusion of more trials in our review may reveal other adverse systemic effects of individual anti‐VEGF agents in addition to those risks posed by the injection procedure.
Delivering injections more frequently than therapeutically required also imposes an unnecessary cost burden on individuals and national healthcare systems.
How the intervention might work
Pivotal anti‐VEGF trials followed monthly injection regimens to investigate drug efficacy. Initial trials of ranibizumab, bevacizumab, and aflibercept used monthly administration of the drugs (ANCHOR 2009; CATT 2012; CLEAR‐IT 2 2011a; IVAN 2012a; MARINA 2006). Mean change of best‐corrected visual acuity (BCVA) after two years was +8.1 for monthly ranibizumab 0.5 mg, +7.8 for monthly bevacizumab 1.25 mg, and +9 for monthly aflibercept 2.0 mg (ANCHOR 2009; CATT 2012; CLEAR‐IT 2 2011a).
Subsequent trials have investigated ranibizumab, bevacizumab, and aflibercept efficacy using a variety of monthly and non‐monthly injection regimens. The VIEW trials compared monthly injections with bimonthly injection of aflibercept 2.0 mg after three initial monthly doses. Results demonstrated comparable effects on BCVA due to aflibercept's longer therapy window than ranibizumab (CLEAR‐IT 2 2011a; VIEW 2012; Yonekawa 2015). Trials also have investigated PRN, quarterly, crossover from monthly to PRN, and formula‐based (i.e. treat‐and‐extend protocol) regimens. Effects on BCVA from these trials have been mixed (Abedi 2014; CATT 2012; CLEAR‐IT 2 2011a; HARBOR 2014; IVAN 2012a; PIER 2010; PrONTO 2009; Schmidt‐Erfurth 2011; SECURE 2013; SUSTAIN 2011; VIEW 2012). Schmucker and colleagues performed a systematic review and meta‐analysis of PRN injections versus monthly injections of anti‐VEGF in 2015; the review and meta‐analysis, which included reports from three trials of more than 2000 participants (CATT 2012; HARBOR 2014; IVAN 2012a), found that those on PRN treatment had slightly but statistically significantly worse BCVA and an increased risk of systemic adverse events compared to those given monthly injections (Schmucker 2015). As their findings were based on only three trials, it was not known which injection regimen satisfied therapeutic standards while minimizing injection frequency to eliminate unnecessary risk of adverse events and to control cost.
Why it is important to do this review
Although nAMD is less prevalent than non‐exudative disease, it accounts for 80% of severe vision loss due to AMD (worse than 20/200 Snellen acuity) (Leibowitz 1980). Risk factors for conversion from non‐exudative AMD to nAMD include a decrease in visual acuity to 75 or fewer Early Treatment Diabetic Retinopathy Study (ETDRS) letters from a baseline of more than 85 letters and older age (Friberg 2012).
As global populations age, the number of people affected by AMD is expected to rise. Approximately 1.25 million people with nAMD were reported in the USA in 2004. By 2020, the prevalence of nAMD in the USA is expected to increase to an estimated 1.875 million cases (Friedman 2004). AMD imposes a significant decrement in people's quality of life, with the impact from severe AMD likened to that of end‐stage cancer or a stroke requiring constant nursing care (Brown 2006). Several studies have suggested AMD as a risk factor for depression, a major cause of disability (Casten 2004). Thirty percent of people with AMD have depression, compared with 15% of adults aged 65 years and older who have clinically significant depressive symptoms in the USA and internationally (Casten 2004; Fiske 2009). nAMD not only has negative effects on individual patients, but also has negative social and economic consequences. Using utility analysis, researchers have estimated a gross domestic product cost of USD 5.396 billion per year due to lost productivity (Brown 2005).
Previous Cochrane Reviews have investigated and demonstrated the efficacy and safety of intravitreous anti‐VEGF agents for the treatment of nAMD (Solomon 2014). However, ever‐growing burdens on the patient and healthcare systems necessitate cost‐effective therapies for nAMD and consideration of the lower cost of bevacizumab versus ranibizumab and aflibercept (CATT 2011; IVAN 2012b). It remains unknown which treatment regimen is optimal when balancing efficacy, safety, and cost.
Objectives
To investigate the effects of monthly versus non‐monthly intravitreous injection of an anti‐VEGF agent in people with newly diagnosed nAMD.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs).
Types of participants
We included trials in which participants had a diagnosis of nAMD as defined by study investigators.
Types of interventions
Intervention (main): non‐monthly intravitreous injection of an anti‐VEGF agent, including loading doses (monthly for first three months followed by PRN, every eight weeks, quarterly, crossover from monthly to PRN, or treat‐and‐extend protocol).
Comparison: monthly intravitreous injection of an anti‐VEGF agent.
We only included trials that utilized a standard anti‐VEGF dose (ranibizumab 0.5 mg, bevacizumab 1.25 mg, and aflibercept 2.0 mg).
As explained in the Description of the intervention section, we grouped the treat‐and‐extend regimen with PRN regimens, in which injections are prescribed when CNV recurrence is detected clinically, typically because of exudation with OCT or hemorrhage detection. However, in the treat‐and‐extend regimen, further injections are prescribed at increasing intervals even if the macula is dry. Thus, this regimen is more intensive than 'standard' PRN (no treatment algorithm, such as in treat‐and‐extend protocols) and is favored by many clinicians. Therefore, we decided post hoc to conduct subgroup analyses to compare standard PRN and treat‐and‐extend with monthly regimens. We also decided post hoc to include trials directly comparing different non‐monthly regimens because this is also very relevant to clinicians.
Types of outcome measures
Primary outcomes
BCVA measured in ETDRS letters on a logMAR chart and analyzed as mean change of BCVA from baseline to one year of follow‐up.
Secondary outcomes
Mean change of BCVA measured in ETDRS letters on a logMAR chart from baseline to two years of follow‐up.
Proportion of participants with an improvement of BCVA by 15 ETDRS letters (0.3 logMAR or 3 Snellen lines) or more at one and two years of follow‐up.
Mean change in optical coherence tomography (OCT) central subfoveal retinal thickness (CRT) in micrometers from baseline to one and two years of follow‐up.
Mean change in quality of life from baseline to one and two years of follow‐up using any validated questionnaire.
Use of resources: number of injections in the first year and within two years and their cost estimates.
Adverse events
We compared systemic adverse events (e.g. all‐cause death, serious systemic adverse events) within the first year of treatment and follow‐up. For ocular adverse events, we focused on endophthalmitis because it is the most devastating ocular complication and may be related to the number of injections.
We considered outcomes at '12 months' to be any observation between nine and 15 months. If change in outcome measures between baseline and one‐ and two‐year follow‐up was not reported or calculable, we collected data at the last follow‐up.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for RCTs. The search excluded trials initiated prior to 2004 because intravitreous anti‐VEGF agents were introduced after 2004. There were no language restrictions in this search. The date of the search was 18 October 2019.
Cochrane Central Register of Controlled Trials (CENTRAL), which contains the Cochrane Eyes and Vision Trials Register (2019, Issue 10; Appendix 1).
MEDLINE Ovid (from January 1946 to 18 October 2019; Appendix 2).
Embase Ovid (from January 1980 to 18 October 2019; Appendix 3).
LILACS (Latin American and Caribbean Health Sciences Literature Database; from 1982 to 18 October 2019; Appendix 4).
ISRCTN registry (www.isrctn.com/editAdvancedSearch: searched 18 October 2019; Appendix 5).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov: searched 18 October 2019; Appendix 6).
World Health Organization International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp; searched 18 October 2019; Appendix 7).
Searching other resources
We reviewed the reference lists of included trial reports and related systematic reviews to identify additional relevant trials. We contacted pharmaceutical companies that sponsored studies on anti‐VEGF drugs for information about any ongoing or completed clinical trials for which findings have not been published. We searched abstracts from the annual meetings of the Association for Research in Vision and Ophthalmology (ARVO), the European VitreoRetinal Society, the Macula Society, the Retina Society, subspecialty meetings from the American Academy of Ophthalmology, and the American Society of Retinal Surgeons for ongoing trials from 2004 to 29 October 2019.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts resulting from the searches using web‐based software (Covidence). We resolved disagreements by discussion. Citations considered irrelevant at this stage were not documented in the review other than to note the number of these in a flow chart. We obtained full‐text copies of reports from potentially relevant records.
Two review authors independently assessed the full‐text reports for inclusion according to the Criteria for considering studies for this review. We resolved disagreements by discussion with a third review author. We corresponded with investigators to clarify trial eligibility, as appropriate. We were not masked to the names of the authors, institutions, or journal publication when we reviewed full‐text reports.
We listed all studies excluded after review of full‐text reports and provided a brief justification for exclusion in the Characteristics of excluded studies table.
For potentially eligible studies identified from trials registers, we proceeded as follows.
If the study had a completion date more than two years earlier than our search date, we looked for publications from the study and contacted the investigators as necessary to obtain published or unpublished data from the trial. If eligible, the trial was included in the review irrespective of whether we could identify a publication.
If the study had a completion date within two years later than the date of our search or in the future, we documented the study in the Characteristics of ongoing studies table.
Data extraction and management
Two review authors independently extracted study characteristics including study methods, participants, interventions, outcomes, and funding sources. We contacted the authors of trial reports for data on primary and secondary outcomes in the individual trials when the information was not clearly presented or not available from the full‐text reports. We extracted data on BCVA, adverse events, and other relevant outcomes. We extracted data from figures published in the trial reports when applicable and communicated with the authors to verify extracted data. One review author entered data into Review Manager 5 (Review Manager 2014), and a second review author verified the data entry.
Assessment of risk of bias in included studies
We specifically considered and reported on the following sources of bias.
Selection bias (random sequence generation, allocation concealment before randomization): was the sequence of allocation generated using a random procedure and was the allocation concealed to people recruiting/enrolling participants and to participants before randomization?
Performance bias (masking of participants and researchers): were the recipients of care unaware of their assigned intervention? Were people providing care unaware of the assigned intervention? This judgment concerned all outcomes.
Detection bias (masking of outcome assessors): were people evaluating outcomes unaware of the assigned intervention? This judgment concerned all outcomes.
Attrition bias: were the rates of follow‐up and compliance similar in the trial treatment groups? Was the analysis by intention‐to‐treat (ITT)? Were there any postrandomization exclusions?
Selective outcome reporting bias: was there any evidence that outcomes that were measured had not been reported?
We graded each trial for each domain at low risk of bias, high risk of bias, or unclear risk of bias (lack of information or uncertainty of potential for bias), as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We contacted trial investigators for clarification of parameters graded as 'unclear' and proceeded with available information when they did not respond.
Measures of treatment effect
We calculated the mean difference (MD) and 95% confidence interval (CI) for the following continuous outcomes: mean change in BCVA, mean change in CRT, and number of injections. We calculated the risk ratio (RR) and 95% CI for the following dichotomous outcomes: proportion of participants with an improvement of BCVA, and incidence of adverse events.
Where possible, we checked for the skewness of continuous data by considering the ratio of the mean to the standard deviation for continuous variables with a natural ceiling, such as BCVA or retinal thickness.
We planned to use the standardized mean difference (SMD) with 95% CI whenever trials measured a continuous outcome on different scales, such as quality of life scores from different questionnaires. The SMD expresses the size of the intervention effect in each trial relative to the variability observed in that trial. If one scale increased with severity while another decreased with severity, we ensured that all the scales measured improvement in the same direction, either by multiplying the mean values of trials using one type of scale by –1 or by subtracting the mean from the maximum possible value for the scale.
Unit of analysis issues
We anticipated unit of analysis issues with respect to eyes in few trials because most trials of treatment of nAMD designated one eye of a participant as the study eye. Therefore, participants were randomized to treatment of one eye per participant and outcomes reported for study eyes. Nonetheless, we planned that, if trials included more than 10% of participants with both eyes in the analysis, regardless of whether the two eyes of a participant were assigned to the same or a different injection regimen, we would conduct a sensitivity analysis which excluded these trials. In the current version of this review, one eye per participant was the unit of analysis in all trials.
When studies had more than two treatment arms, we only used those with treatment regimens meeting our inclusion criteria for each comparison. If similar regimens were used (e.g. with both bevacizumab and ranibizumab), we pooled these arms using standard Cochrane formulas.
Dealing with missing data
Whenever possible, we conducted an ITT analysis. We used outcome data imputed by the trial investigators whenever appropriate, but we did not impute missing data ourselves.
When ITT outcome data were not available, we did an available‐case analysis. This approach assumes that data are missing at random. We assessed whether this assumption was reasonable by collecting data from each included trial on the number of participants excluded or lost to follow‐up and reasons for loss to follow‐up by treatment group, when reported.
Assessment of heterogeneity
We examined the overall characteristics of the trials, in particular the type of participants and types of interventions, to assess the extent to which the trials were similar enough to make pooling outcome data sensible.
We looked at the forest plots of outcome estimates to see how consistent the results of the trials were, with particular attention to the size and direction of effects and overlap of CIs.
We calculated I2 statistics, which is the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (i.e. chance; Higgins 2002). We considered I2 values over 50% to indicate substantial statistical heterogeneity and considered the Chi2 test. As the Chi2 test has low power to identify heterogeneity when the number of trials is small, we considered P less than 0.1 to indicate statistical significance.
Assessment of reporting biases
We assessed selective outcome reporting for each trial by comparing the outcomes specified in a protocol, research plan, or clinical trial registry with the outcomes reported. When there was no prepublication document available, we compared the outcomes specified in the design and methods sections of trial reports to the outcomes reported. When there were 10 or more trials included in a meta‐analysis, we planned to use a funnel plot to assess potential publication bias.
Data synthesis
We combined data using a random‐effects model in Review Manager 5 (Review Manager 2014). Whenever there were fewer than three trials in a comparison, we used a fixed‐effect model.
If there was inconsistency between study results (e.g. the effects were in different directions or the I2 value was more than 50% and the Chi2 P value was less than 0.1), we did not combine the data but described the pattern of the individual study estimates. If there was statistical heterogeneity but all the effect estimates were in the same direction such that a pooled estimate provided a good summary of the individual trial results, we elected to combined the data.
Subgroup analysis and investigation of heterogeneity
Our primary analysis compared the monthly injection arm of all trials with all the reduced frequency regimens simultaneously. If there were sufficient trials and outcome data, we compared the effect of treatment regimens in the following subgroups:
different anti‐VEGF agents (this analysis was not possible);
different decision‐making criteria, for example visual acuity based versus OCT based (this analysis was not possible);
'standard' PRN versus treat‐and‐extend regimens.
We had enough data only to investigate the comparison of 'standard' PRN versus treat‐and‐extend regimens.
Sensitivity analysis
We planned to perform the following sensitivity analyses on the primary outcome:
excluding trials at high risk of bias in one or more domains;
excluding trials with more than 10% of participants with both eyes in primary analyses;
comparing fixed‐effect and random‐effect models (if three or more trials).
'Summary of findings' tables
We prepared 'Summary of findings' tables to present estimated relative and absolute risks. Two review authors independently graded the overall certainty of the evidence for each outcome using the GRADE classification (GRADEpro GDT). We included the following outcomes in the 'Summary of findings' tables.
Mean change in BCVA measured on a logMAR chart from baseline to one year of follow‐up.
Proportion of participants with an improvement of BCVA by 15 ETDRS letters (0.3 logMAR or 3 Snellen lines) or more at one and two years of follow‐up.
Mean change in OCT CRT in micrometers from baseline to one year of follow‐up.
Mean change in quality of life from baseline to one and two years of follow‐up using any validated questionnaire.
Use of resources: number of injections in the first year and within two years and their cost estimates.
Ocular and systemic adverse effects.
Results
Description of studies
Results of the search
The electronic database searches of this review, last conducted on 29 October 2019, yielded 4227 records (Figure 1). We removed 507 duplicates and screened the title and abstracts of 3720 records. We selected 99 records for full‐text review. We classified four records as awaiting classification because only conference abstracts were available, and one record as an ongoing trial. We excluded 26 reports of 21 studies, two studies that were not RCTs and 19 studies in which the intervention or comparator did not meet our eligibility criteria. Overall, we included 15 trials (reported in 68 records) for qualitative analysis and 14 trials for meta‐analysis of our primary outcome. Fewer RCTs were included in the meta‐analyses of secondary outcomes and the number of studies included in each varied.
1.
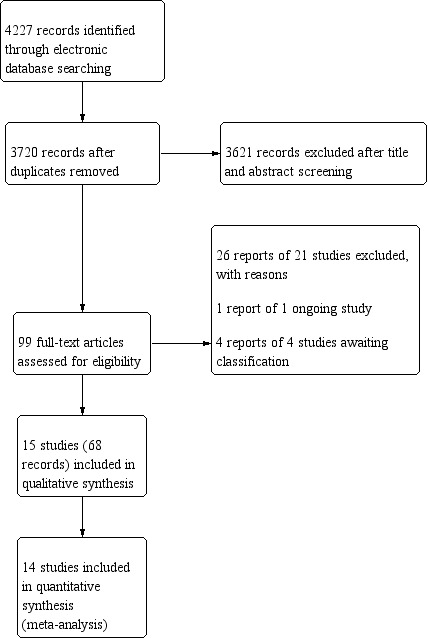
Study flow diagram.
Acronyms used to refer to the trials in this review are listed under the Included studies section. Descriptions are available in the Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification, and Characteristics of ongoing studies sections.
Included studies
Types of participants
This review included 7732 participants randomized in 15 RCTs, ranging from 37 to 2457 participants within each trial, with a median age of 77.5 years (ranging from 68 to 80 years). All 15 trials randomized one eye per participant. The trials were conducted worldwide: six trials exclusively took place in the US (Barikian 2015a; CATT 2011; CLEAR‐IT 2 2011b; HARBOR 2013; Sarraf 2013; TREX‐AMD 2015), three in the UK(BeMOc 2013; GMAN 2015; IVAN 2012b), one in the Netherlands (Lushchyk 2013), one in China (NATTB 2012), one in both France and Lebanon (El‐Mollayess 2012), one in Canada (CANTREAT 2019), and two with centers across many countries (TREND 2017; VIEW 2012).
Thirteen trials used a predefined BCVA criterion to determine participation eligibility and generally excluded people with near‐normal or very low BCVA, who are still treated in clinical practice (Barikian 2015a; CANTREAT 2019; CATT 2011; CLEAR‐IT 2 2011b; El‐Mollayess 2012; GMAN 2015; HARBOR 2013; IVAN 2012b; Lushchyk 2013; NATTB 2012; Sarraf 2013; TREX‐AMD 2015; VIEW 2012).
Ten trials included participants who had never received any treatment in the study eye at time of enrollment (Barikian 2015a; CANTREAT 2019; CATT 2011; CLEAR‐IT 2 2011b; HARBOR 2013; IVAN 2012b; Lushchyk 2013; NATTB 2012; TREX‐AMD 2015; VIEW 2012), meaning that our results were based on the first treatment year. Two trials did not describe the treatment history in their inclusion and exclusion criteria (El‐Mollayess 2012; GMAN 2015). Investigators of Sarraf 2013 excluded people who had received anti‐VEGF therapy within 30 days of enrollment, greater than three prior anti‐VEGF injections, more than one PDT session, and any prior AMD treatment aside from vitamins and minerals. Investigators of TREND 2017 excluded participants treated by anti‐VEGFs within the six months leading up to the study.
Additional details about the participants in each trial are available in the Characteristics of included studies table.
Types of interventions
The interventions evaluated in each trial are available in the Characteristics of included studies table. Overall, the trials evaluated monthly versus non‐monthly injection regimens. Most trials assessed participants every four weeks to monitor and evaluate the need for retreatment when applicable.
Seven trials started with at least three consecutive monthly loading injections in any intervention group (CANTREAT 2019; GMAN 2015; HARBOR 2013; IVAN 2012b; Sarraf 2013; TREX‐AMD 2015; VIEW 2012). This was followed by monthly versus PRN, including treat‐and‐extend injections in CANTREAT 2019, HARBOR 2013, IVAN 2012b, Sarraf 2013, and TREX‐AMD 2015. VIEW 2012 evaluated monthly versus extended‐fixed injections, and GMAN 2015 compared extended‐fixed and PRN injections. The treat‐and‐extend protocol was considered a subgroup of the PRN as explained in the Description of the intervention section (CANTREAT 2019; TREND 2017; TREX‐AMD 2015).
The trials utilized three most prescribed anti‐VEGF agents at their accepted dosages: aflibercept 2.0 mg, bevacizumab 1.25 mg, and ranibizumab 0.5 mg.
The specific criteria for injection in PRN groups varied across trials, though all the protocols required retreatment based on a specified decrease in BCVA, new macular fluid, an increase in CRT, or evidence of new CNV on FA, or a combination of these criteria. Seven trials initiated retreatment in the presence of new macular hemorrhage (Barikian 2015a; CANTREAT 2019; CATT 2011; El‐Mollayess 2012; GMAN 2015; IVAN 2012b; Sarraf 2013). Three trials initiated retreatment when there was an increase in CNV lesion size (Barikian 2015a; El‐Mollayess 2012; GMAN 2015). In two trials, new, persistent, or enlarging pigment epithelial detachment was an indication for retreatment (Barikian 2015a; Sarraf 2013). In two trials, new classic CNV was an indication for retreatment (Barikian 2015a; El‐Mollayess 2012).
Although a few studies allowed clinicians to reduce follow‐up intervals or administer additional injections when deemed appropriate, or both, the treat‐and‐extend protocol in CANTREAT 2019, TREND 2017, and TREX‐AMD 2015 encompassed predesignated algorithms that customized follow‐up and dosing frequency. In CANTREAT 2019, on achievement of disease stability, the interval between each subsequent injection was extended by two weeks (intervals of 6 weeks, 8 weeks, 10 weeks, and a maximum of 12 weeks) until clinical or diagnostic evidence of disease instability was observed based on OCT findings, ETDRS BCVA, or both. In TREND 2017, participants received monthly injections until resolution of fluid on OCT. At that point, the follow‐up visit was extended to six weeks. Treatment intervals were extended by two weeks at each evaluation where there was no disease activity, as defined by the absence of fluid on OCT. If there was return of fluid, the follow‐up interval was decreased by two weeks until the participant was back to a four‐week interval. There was room in the trial design to allow for modifications based on the investigators' judgment. In TREX‐AMD 2015, follow‐up intervals were no more frequent than four weeks or less frequent than 12 weeks. Upon resolution of macular fluid, the interval between visits was lengthened by two‐week increments. When there was recurrent fluid, the interval between visits was reduced by two‐week increments until the eye cycled back to no fluid. If this happened, the interval between visits was extended by only one‐week increments until fluid recurred, at which time the interval between visits was reduced by only one‐week increments until dry. At this point, the same follow‐up interval was maintained for one more visit before extending the interval by one‐week increments so long as the macula was dry. In participants who developed three recurrences of macular fluid, the interval was continued for three consecutive visits no matter the fluid status, followed by re‐initiation of the treat‐and‐extend protocol. Participants received an injection at every visit.
Types of outcome measures
Sarraf 2013 was a small trial aiming to investigate factors that predicted RPE tears and did not provide data to compare regimens, despite the fact that 37 participants were randomized to four groups, of which two were ranibizumab 0.5 mg receiving PRN or monthly injections; thus, 14 trials were included in the analysis.
Visual acuity
Thirteen of the 15 trials based their primary outcome on BCVA (BeMOc 2013; CANTREAT 2019; CATT 2011; El‐Mollayess 2012; GMAN 2015; HARBOR 2013; IVAN 2012b; Lushchyk 2013; NATTB 2012; Sarraf 2013; TREND 2017; TREX‐AMD 2015; VIEW 2012). The primary outcome of our review, mean change in BCVA from baseline to one year of follow‐up, was the main outcome measure for eight of the included trials (CANTREAT 2019; CATT 2011; El‐Mollayess 2012; HARBOR 2013; NATTB 2012; Sarraf 2013; TREND 2017; TREX‐AMD 2015). It was a secondary outcome in four trials (Barikian 2015a; CLEAR‐IT 2 2011b; GMAN 2015; VIEW 2012). Two trials used proportion of participants maintaining vision at week 52 (defined as loss of fewer than 15 letters on the ETDRS chart) as their primary outcomes (BeMOc 2013; VIEW 2012).
Central subfoveal retinal thickness
One of the secondary outcomes of our review was change in OCT CRT in micrometersfrom baseline to one or two years of follow‐up. This was the primary outcome measured by investigators in CLEAR‐IT 2 2011b and the basis for a secondary outcome measured in 13 of the remaining 14 trials (Barikian 2015a; BeMOc 2013; CANTREAT 2019; CATT 2011; El‐Mollayess 2012; GMAN 2015; HARBOR 2013; IVAN 2012b; Lushchyk 2013; NATTB 2012; TREND 2017; TREX‐AMD 2015; VIEW 2012). The primary outcome measured in Barikian 2015a was the mean initial central fluid‐free interval after induction period.
Other functional measures and quality of life
In IVAN 2012b, authors utilized additional clinical quantifiers of visual function: the Pelli‐Robson chart for measuring contrast sensitivity, the Bailey‐Lovie near reading card for evaluation of near visual acuity, and the Belfast reading chart to measure reading speed. They also investigated health‐related quality of life and participant treatment satisfaction using EQ‐5D, the published EuroQol Group quality of life assessment tool (EuroQol 1990). Investigators in BeMOc 2013 and VIEW 2012 used the National Eye Institute 25‐Item Visual Functioning Questionnaire to assess vision‐related quality of life (NEI VFQ‐25), but these results have not been published yet.
Adverse events
All 15 trials reported ocular adverse events up to at least one year of follow‐up. All but one trial (Sarraf 2013), also disclosed systemic events. Sarraf 2013 reported the incidences of postinjection RPE tears and postinjection retinal epithelial detachments.
Economic considerations
Several trials provided data and analysis of cost considerations in nAMD treatment using anti‐VEGF injections. Seven trials reported the number of injections utilized in each group (CANTREAT 2019; CATT 2011; El‐Mollayess 2012; HARBOR 2013; IVAN 2012b; TREX‐AMD 2015; VIEW 2012); two trials discussed annual drug cost (CATT 2011; NATTB 2012); and one trial provided evaluation of cumulative resource use, cost, and cost effectiveness (IVAN 2012b).
Excluded studies
We excluded 21 studies after full‐text assessment for reasons provided in the Characteristics of excluded studies table. Nineteen studies were excluded because intervention and/or comparator did not meet our eligibility criteria and two studies were not RCTs.
Four studies were ongoing, of which three were conference abstract and one was unpublished (Characteristics of studies awaiting classification). One trial is awaiting classification since the trial has been completed but is unpublished (Characteristics of ongoing studies).
Risk of bias in included studies
The risk of bias assessment for each trial appears in the Characteristics of included studies table and in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Five of the 15 studies provided adequate description of both random sequence generation and allocation concealment to indicate low risk of selection bias (CANTREAT 2019; CATT 2011; GMAN 2015; IVAN 2012b; HARBOR 2013). Three trials were unclear for either sequence generation or allocation concealment (El‐Mollayess 2012; TREX‐AMD 2015; VIEW 2012), and seven trials were unclear for both (Barikian 2015a; BeMOc 2013; CLEAR‐IT 2 2011b; Lushchyk 2013; NATTB 2012; Sarraf 2013; TREND 2017).
Blinding
Two trials had a low risk of performance bias as they masked participants and personnel (IVAN 2012b; VIEW 2012); seven trials were at high risk of bias due to lack of masking (CANTREAT 2019; CATT 2011; El‐Mollayess 2012; GMAN 2015; Lushchyk 2013; NATTB 2012; Sarraf 2013), and the others did not give enough details (unclear risk of bias). Eight trials masked outcome assessors at least regarding BCVA, our primary outcome (CATT 2011; CLEAR‐IT 2 2011b; El‐Mollayess 2012; GMAN 2015; IVAN 2012b; NATTB 2012; TREND 2017; VIEW 2012), two trials were at high risk of bias (Lushchyk 2013; Sarraf 2013), and the other trials were at unclear risk of bias.
Incomplete outcome data
Overall, the risk of attrition bias was low across the trials included in our review. In the 10 trials at low risk, six reported missing data less than 5% (Barikian 2015a; BeMOc 2013; CLEAR‐IT 2 2011b; El‐Mollayess 2012; Sarraf 2013; TREX‐AMD 2015), six reported losses to follow‐up that were balanced with similar reasons in the comparison groups (CATT 2011; GMAN 2015; HARBOR 2013; IVAN 2012b; TREND 2017; VIEW 2012). NATTB 2012 lost about 13% of participants in each arm but did not report reasons for missing data and was at unclear risk of bias. Lushchyk 2013 reported an imbalance of losses in the two arms and was at high risk of bias (28.1% in the bevacizumab every four weeks group, 9.5% in the bevacizumab every six weeks group, and 15.6% in the bevacizumab every eight weeks group). CANTREAT 2019 also reported an imbalance in losses to follow‐up (6.3% in the treat‐and‐extend arm and 12.3% in the monthly arm) with no reasons given (unclear risk).
Selective reporting
There was low risk of selective reporting in eight of the 15 trials, six had unclear risk, and one had high risk (TREND 2017). The seven trials with low reporting bias presented outcomes that were consistent with those found in their registered clinical trial protocols (CATT 2011; CLEAR‐IT 2 2011b; GMAN 2015; HARBOR 2013; Lushchyk 2013; NATTB 2012; VIEW 2012). Five trials did not have a protocol for comparison (Barikian 2015a; BeMOc 2013; El‐Mollayess 2012; Sarraf 2013). TREND 2017 did not report some outcomes that had been prespecified in the protocol. TREX‐AMD 2015 was still ongoing at the time of our review, with only one‐year results reported and the intention of carrying out the trial for two years. IVAN 2012b reported treatment satisfaction, survival‐free from treatment failure, and serum analysis at one year but not at two years.
Other potential sources of bias
There were no other sources of bias identified.
Conflict of interest
Seven studies were free from conflict of interest (Barikian 2015a; BeMOc 2013; CATT 2011; El‐Mollayess 2012; HARBOR 2013; IVAN 2012b; NATTB 2012). There was unclear risk in Lushchyk 2013, whose authors did not disclose funding sources and declarations of interest. Six other trials were industry‐funded (CANTREAT 2019; CLEAR‐IT 2 2011b; Sarraf 2013; TREND 2017; TREX‐AMD 2015; VIEW 2012).
Effects of interventions
A summary of the treatments and regimens of trials included in this review is presented in Table 3 and Table 4.
1. Treatment groups in included trials.
|
Study Treatment period |
Intervention 1 | Intervention 2 | Intervention 3 | Intervention 4 |
| Aflibercept 2.0 mg | ||||
|
CLEAR‐IT 2 2011b* 1 year |
Every 12 weeks | Every 4 weeks | — | — |
|
VIEW 2012* 1 year; PRN for all groups at end of first year |
Every 8 weeks after 3 initial monthly doses | Every 4 weeks | — | — |
| Bevacizumab 1.25 mg | ||||
|
Barikian 2015a 1 year |
PRN after first injection | Every 2 weeks for first 3 injections, then PRN | Every 4 weeks for first 3 injections, then PRN | — |
|
BeMOc 2013 1 year |
No loading: PRN with no initial mandatory loading injections | Loading: 3 initial monthly loading injections, then PRN | — | — |
|
El‐Mollayess 2012 1 year |
Variable dosing | Every 4–6 weeks | — | — |
|
GMAN 2015 2 years |
3 initial monthly loading injections, then PRN | 3 initial monthly loading injections, then every 12 weeks | — | — |
|
Lushchyk 2013 1 year |
Every 8 weeks | Every 6 weeks | Every 4 weeks | — |
|
NATTB 2012 1 year |
Every 6 weeks for first 3 injections, then every 12 weeks for last 2 injections | Every 6 weeks | — | — |
| Ranibizumab 0.5 mg | ||||
|
CANTREAT 2019 1 year |
PRN (treat‐and‐extend) | Every 4 weeks | — | — |
|
HARBOR 2013b 1 year |
PRN | Every 4 weeks | — | — |
|
Sarraf 2013c 1 year |
Every 4 weeks for 4 injections, then PRN | Every 4 weeks | — | — |
|
TREND 2017 1 year |
2 loading doses (day 1, month 1) followed by PRN | Every 4 weeks | — | — |
|
TREX‐AMD 2015 1 year |
Every 4 weeks for 3 injections, then PRN | Every 4 weeks | — | — |
| Bevacizumab and ranibizumab | ||||
|
Study Treatment period |
Bevacizumab 1.25 mg | Ranibizumab 0.5 mg | ||
|
CATT 2011 1 year; rerandomized at end of first year |
PRN after first injection for 2 years | Every 4 weeks for 1 year, then rerandomized to monthly or variable dosing | PRN after first injection for 2 years | Every 4 weeks for 1 year, then rerandomized to monthly or variable dosing |
|
IVAN 2012b 2 years |
Every 4 weeks for 3 injections, then PRN in 3 month cycles | Every 4 weeks | Every 4 weeks for 3 injections, then PRN in 3 month cycles | Every 4 weeks |
PRN: as needed. aThree intervention groups using other doses not analyzed. bTwo intervention groups using other doses not analyzed. cTwo intervention groups using other doses not analyzed.
2. Treatment regimens evaluated by included trials.
| Study name Treatment period | Drug interventions | Treatment schedule interventions | |
| Intervention 1 | Intervention 2 | ||
| PRN vs monthly dosing | |||
|
CANTREAT 2019 1 year |
Ranibizumab 0.5 mg | PRN (treat‐and‐extend) | Every 4 weeks |
| CATT 2011 1 year; rerandomized at end of first year | Bevacizumab 1.25 mg or ranibizumab 0.5 mg | PRN after first injection for 2 years | Every 4 weeks for 1 year, then rerandomized to monthly or variable dosing |
| El‐Mollayess 2012 1 year | Bevacizumab 1.25 mg | PRN | Every 4–6 weeks |
| HARBOR 2013 1 year | Ranibizumab 0.5 mg | PRN | Every 4 weeks |
| IVAN 2012b 2 years | Bevacizumab 1.25 mg or ranibizumab 0.5 mg | Every 4 weeks for 3 injections, then PRN in 3 month cycles | Every 4 weeks |
| Sarraf 2013 1 year | Ranibizumab 0.5 mg | Every 4 weeks for 4 injections, then PRN | Every 4 weeks |
| TREND 2017 | Ranibizumab 0.5 mg | Every 4 weeks for 2 injections, then PRN | Every 4 weeks |
| TREX‐AMD 2015 1 year | Ranibizumab 0.5 mg | Every 4 weeks for 3 injections, then PRN | Every 4 weeks |
| Extended‐fixed vs monthly dosing | |||
| CLEAR‐IT 2 2011b 1 year | Aflibercept 2.0 mg | Every 12 weeks | Every 4 weeks |
| Lushchyk 2013 1 year | Bevacizumab 1.25 mg | Every 6–8 weeks | Every 4 weeks |
| VIEW 2012 1 year; PRN for all groups at end of first year | Aflibercept 2.0 mg | Every 8 weeks after 3 initial monthly doses | Every 4 weeks |
| Other extended‐fixed dosing comparisons | |||
| GMAN 2015 2 years | Bevacizumab 1.25 mg | 3 initial monthly loading injections, then PRN | 3 initial monthly loading injections, then every 12 weeks |
| NATTB 2012 1 year | Bevacizumab 1.25 mg | Every 6 weeks for first 3 injections, then every 12 weeks for last 2 injections | Every 6 weeks |
| No loading injections vs loading injections | |||
| Barikian 2015a 1 year | Bevacizumab 1.25 mg | No loading: PRN after first injection | Loading: every 2–4 weeks for first 3 injections, then PRN |
| BeMOc 2013 1 year | Bevacizumab 1.25 mg | No loading: PRN with no initial mandatory loading injections | Loading: 3 initial monthly loading injections, then PRN |
PRN: as needed.
As needed (PRN) versus monthly injections
Seven trials investigated the effects of PRN versus fixed monthly injections, of which four adopted standard PRN (CATT 2011; El‐Mollayess 2012; HARBOR 2013; IVAN 2012b) and three used a treat‐and‐extend regimen (CANTREAT 2019; TREND 2017; TREX‐AMD 2015). The drugs used were: ranibizumab only (CANTREAT 2019; HARBOR 2013; Sarraf 2013; TREND 2017); bevacizumab only (El‐Mollayess 2012); and both ranibizumab and bevacizumab in the other trials. There were no data on comparisons of regimens available in Sarraf 2013, a small trial which investigated the occurrence of RPE tears. Participants in TREND 2017 received two loading doses (day one, month one).
As explained in the Subgroup analysis and investigation of heterogeneity section, we present 'standard' PRN and treat‐and‐extend as subgroups in analyses, since this comparison has been of clinical interest in recent years, which is supported by significant differences in some subgroup analyses.
Visual acuity
All seven trials (3525 participants) with a 'PRN versus monthly' protocol evaluated the mean change in BCVA and gain of 15 letters or more in visual acuity at one year (Table 1). Pooled estimates are presented separately for the 'standard' PRN and the treat‐and‐extend groups and test for subgroup differences are reported.
Standard PRN treatment delivered a median of 7.5 injections (3.8 to 7.7) and yielded a clinically small difference that favored monthly injections (MD –1.68 letters, 95% CI –2.81 to –0.55; 4 studies, 2299 participants; I2 = 0%; moderate‐certainty evidence due to risk of bias [–1]). The treat‐and‐extend regimen delivered a median of 9.4 injections with no evidence of a difference in visual acuity change compared to monthly injections (0.51 letters, 95% CI –3.14 to 4.16; 3 studies, 1226 participants; I2 = 78%; low‐certainty evidence due to risk of bias [–1] and inconsistency [–1]); the estimates from these studies were heterogeneous, and in CANTREAT 2019 a reduced‐intensity treat‐and‐extend regimen gained more visual acuity than the monthly regimen. The test for subgroup differences between standard PRN and treat‐and‐extend regimen was not significant (P = 0.26) (Figure 4; Analysis 1.1).
4.

Forest plot of comparison: 1 As needed (PRN) versus monthly injections, outcome: 1.1 Mean change in best‐corrected visual acuity at 1 year.
1.1. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 1: Mean change in best‐corrected visual acuity at 1 year
Regarding the RR of gaining 15 letters or more at one year, compared to monthly injection, standard PRN regimen slightly reduced the chances of improving vision (RR 0.87, 95% CI 0.76 to 0.99; 4 studies, 2299 participants; I2 = 0%; low‐certainty evidence due to risk of bias [–1] and imprecision [–1]), whereas the treat‐and‐extend was similar to the monthly regimen (RR 1.11, 95% CI 0.91 to 1.36; 3 studies, 1169 participants; I2 = 0%; low‐certainty evidence due to risk of bias [–1] and imprecision [–1]). The test for subgroup differences suggested less chances of 3‐line gain with 'standard' PRN compared to treat‐and‐extend regimens (P = 0.04) (Analysis 1.2).
1.2. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 2: Gain ≥ 15 letters visual acuity at 1 year
Two trials provided results at two years and also favored the monthly regimen compared to standard PRN (CATT 2011; IVAN 2012b). The MD in mean BCVA change between PRN and fixed monthly injections at two years was –2.23 letters (95% CI –3.93 to –0.53; 2 trials, 1295 participants; I2 = 0%; moderate‐certainty evidence due to risk of bias [–1]; Analysis 1.3). The RR of gaining 15 letters or more at two years was 0.80 but the CI approached no evidence of a difference (95% CI 0.66 to 0.96; 2 trials, 1295 participants; I2 = 74%; low‐certainty evidence due to risk of bias [–1] and imprecision [–1]; the two included trials showed heterogeneous results, but both were in the direction of benefit and we did not downgrade the certainty of evidence for inconsistency; Analysis 1.4). A further trial (CANTREAT 2019) provided results at two years and compared a treat‐and‐extend regimen with monthly injections, finding no difference in the mean change of visual acuity (MD 0.80 letters, 95% CI ‐1.38 to 2.98 letters; participants = 580) and RR of gaining 15 letters or more (RR 1.10, 95% CI 0.82 to 1.46; participants = 580; low certainty of evidence due to risk of bias and imprecision). Estimates of functional benefit were heterogeneous between standard PRN and treat‐and‐extend regimens, which could be due to a higher number of injection with a treat‐and‐extend regimen (MD in number of injections versus monthly: standard PRN: ‐9.78, 95% CI ‐10.29 to ‐9.27; participants = 1303; studies = 2; I2 = 0%: treat‐and‐extend:. ‐6.20, 95% CI ‐6.99 to ‐5.41; participants = 576).
1.3. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 3: Mean change in best‐corrected visual acuity at 2 years
1.4. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 4: Gain ≥ 15 letters visual acuity at 2 years
Central retinal thickness
The MD in mean change of CRT at one year between standard PRN and monthly regimens was 20.8 μm in favor of monthly regimen (95% CI 5.8 to 35.9 μm; 4 trials, 2215 participants; I2 = 0%; moderate‐certainty evidence due to risk of bias [–1]; Analysis 1.5). The MD for the treat‐and‐extend subgroup versus monthly was 22.0 μm (95% CI 37.2 to –81.1 μm; moderate‐certainty evidence due to risk of bias [–1] and imprecision [–1]). The CIs of subgroups of 'standard' PRN and treat‐and‐extend regimen subgroups overlapped (test for subgroup differences: P = 0.96).
1.5. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 5: Mean change central retinal thickness at 1 year
Results at two years were available from 1273 participants in two trials (CATT 2011; IVAN 2012b). The MD in mean change in CRT at two years between standard PRN and monthly was 24.5 μm (95% CI 6.1 to 42.9; 2 trials, 1273 participants; moderate‐certainty evidence due to risk of bias [–1]; Analysis 1.6), which was approximately the same as one‐year results.
1.6. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 6: Mean change central retinal thickness at 2 years
Quality of life
Only one trial assessed visual function as a quality of life measure (IVAN 2012b). The IVAN investigators found that EQ‐5D, Macular Disease Dependent Quality of Life, and Macular Disease Treatment Satisfaction Questionnaire scores did not differ between monthly and PRN dosing regimens at one year. We could not extract quality of life data since the authors reported they were skewed.
Economic considerations
Compared to monthly regimen, the number of injections was significantly lower (P < 0.001) both in the 'standard' PRN (–4.57 injections, 95% CI –5.38 to –3.76; 4 trials, 2336 participants; I2 = 0%; moderate‐certainty evidence due to risk of bias [–1]) and in the treat‐and‐extend subgroup (–2.42, 95% CI –2.71 to –2.14; 3 trials, 1232 participants; I2 = 0%; moderate‐certainty evidence due to risk of bias [–1]). The test for subgroup differences was statistically significant (P < 0.001). Results at two years were available from two trials (CATT 2011; IVAN 2012b). The mean number of injections was much lower in the 'standard' PRN group (MD –9.78, 95% CI –10.29 to –9.27; 2 trials, 1303 participants; I2 = 0%; moderate‐certainty evidence due to risk of bias [–1]; Analysis 1.7).
1.7. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 7: Mean number of injections during 2 years
We could not estimate the MD in mean cost of treatment per person at one year. Investigators from IVAN 2012b reported monthly ranibizumab was the most expensive treatment regimen when taking into account the costs of monitoring, adverse events, and drugs. In both monthly and PRN arms, bevacizumab was less costly than ranibizumab (two‐year cost for continuous treatment GBP 651 for bevacizumab and GBP 16,286 for ranibizumab). The authors determined the main source of cost in ranibizumab treatment was drug pricing (85% of total costs), whereas fees from treatment administration and monitoring comprised 65% of the total cost of bevacizumab therapy. In CATT 2011, the mean total drug cost at one year was USD 23,400 in the monthly ranibizumab group, USD 13,800 in the PRN ranibizumab group, USD 595 in the monthly bevacizumab group, and USD 385 in the PRN bevacizumab group.
Adverse events
Because adverse events were rare, we did not conduct subgroup analyses by 'standard' PRN versus treat‐and‐extend regimen.
The risk of endophthalmitis was lower with any PRN compared to monthly injections (Peto odds ratio (OR) 0.13, 95% CI 0.04 to 0.46; 6 trials, 3175 participants; I2 = 0%; moderate‐certainty evidence for risk of bias [–1]; Analysis 1.8). In this analysis, we used the Peto OR since data were sparse and sample size per arm was similar across all trials, making this effect measure appropriate.
1.8. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 8: Endophthalmitis
Serious systemic adverse events were more common with PRN compared to monthly treatment (RR 1.23, 95% CI 1.05 to 1.44; 6 trials, 3175 participants; I2 = 0%; low‐certainty evidence due to risk of bias [–1] and imprecision [–1]; Analysis 1.9).
1.9. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 9: Serious systemic adverse events
There were no differences between the regimens regarding death (RR 1.11, 95% CI 0.55 to 2.23; 7 trials, 3701 participants; I2 = 47%; Analysis 1.10) and arterial thromboembolic events (RR 0.97, 95% CI 0.44 to 2.13; 6 trials, 3175 participants; I2 = 56%; Analysis 1.11), but estimates were imprecise (low‐certainty evidence due to risk of bias [–1] and imprecision [–1]).
1.10. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 10: Death
1.11. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 11: Arterial thromboembolic events
Sensitivity analyses
The sensitivity analysis excluding trials with at least one high‐risk domain and including three of six trials still favored monthly versus PRN regimen but was less precise and included no difference (Analysis 1.13). The sensitivity analysis using fixed, rather than random, effects was similar to the primary analysis (Analysis 1.14).
1.13. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 13: Sensitivity analysis excluding high‐risk of bias: mean change in best‐corrected visual acuity at 1 year
1.14. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 14: Sensitivity analysis using fixed effects: mean change in best‐corrected visual acuity at 1 year
Extended‐fixed versus monthly injections
Three trials investigated extended‐fixed versus monthly injections (CLEAR‐IT 2 2011b; Lushchyk 2013; VIEW 2012). CLEAR‐IT 2 2011b compared aflibercept monthly with every three months. In Lushchyk 2013, the treatment groups were: bevacizumab monthly, bevacizumab every six weeks, and bevacizumab every eight weeks. In VIEW 2012, the comparisons of interest for our review were between aflibercept or ranibizumab monthly and aflibercept every two months. The non‐monthly participants in CLEAR‐IT 2 2011b and Lushchyk 2013 did not receive loading doses whereas those in VIEW 2012 did. Results were not available for two‐year follow‐up.
Visual acuity
The MD in mean change in BCVA at one year was –1.32 letters but the CIs included null (95% CI –3.93 to 1.29; 3 trials, 1439 participants; I2 = 66%; moderate‐certainty evidence due to risk of bias [–1]; Analysis 2.1; Figure 5). The RR of gaining 15 letters or more at one year was 0.94, and the CIs suggested no evidence of a difference (95% CI 0.80 to 1.10; 3 trials, 1441 participants; I2 = 0%; moderate‐certainty evidence due to risk of bias [–1]; Analysis 2.2). There were no data at two years.
2.1. Analysis.

Comparison 2: Extended‐fixed versus monthly injections, Outcome 1: Mean change in best‐corrected visual acuity at 1 year
5.

Forest plot of comparison: 2 Extended‐fixed versus monthly injections, outcome: 2.1 Mean change in best‐corrected visual acuity at 1 year.
2.2. Analysis.

Comparison 2: Extended‐fixed versus monthly injections, Outcome 2: Gain ≥ 15 letters visual acuity at 1 year
Central retinal thickness
The MD in mean change in CRT at one year between extended‐fixed and monthly regimens was 8.16 μm (95% CI –11.07 to 27.40; 3 trials, 1439 participants; I2 = 36%; low‐certainty evidence due to risk of bias [–1] and imprecision [–1]; Analysis 2.3). There were no data at two years.
2.3. Analysis.

Comparison 2: Extended‐fixed versus monthly injections, Outcome 3: Mean change of central retinal thickness at 1 year
Quality of life
One trial in this comparison category assessed quality of life using the NEI VFQ‐25 tool (VIEW 2012). Among 1220 participants, the overall MD in quality of life scores at one year was –0.59, which suggested no evidence of a difference between regimens (95% CI –2.22 to 1.04; a score difference of 5 was considered clinically significant; moderate‐certainty evidence due to risk of bias [–1]; Analysis 2.4)
2.4. Analysis.

Comparison 2: Extended‐fixed versus monthly injections, Outcome 4: Mean change in quality of life scores at 1 year
Economic considerations
We could not estimate the MD in mean number of injections across the three trials. Two trials did not report the number of injections per treatment arm (CLEAR‐IT 2 2011b; Lushchyk 2013). In VIEW 2012, participants in the aflibercept every two months group received a mean of 7.5 injections (scheduled for eight injections), whereas the mean number of injections in the monthly groups was 12.3 (scheduled for 13 injections).
None of the trials in this comparison category measured treatment cost.
Adverse events
All three trials reported endophthalmitis, which was less common for the extended‐fixed regimen (RR 0.19, 95% CI 0.03 to 1.12; 3 trials, 1132 participants; I2 = 0%; low‐certainty evidence due to risk of bias [–1] and imprecision [–1]; Analysis 2.5). Two trials reported serious systemic adverse events and were similar for the two regimens (RR 0.98, 95% CI 0.74 to 1.30; 2 trials, 1068 participants; I2 = 43%; low‐certainty evidence due to risk of bias [–1] and imprecision [–1]; Analysis 2.6). Data were too sparse to be used regarding death and arterial thromboembolic events.
2.5. Analysis.

Comparison 2: Extended‐fixed versus monthly injections, Outcome 5: Endophthalmitis
2.6. Analysis.

Comparison 2: Extended‐fixed versus monthly injections, Outcome 6: Serious systemic adverse events
Sensitivity analyses
The sensitivity analyses excluding one trial with high‐risk domains, out of three included studies, favored monthly versus PRN regimen but still included no difference (Analysis 2.7). The sensitivity analyses using fixed rather than random effects was more precise than the primary analysis and significantly favored monthly injections (MD –1.36, 95% CI –2.64 to –0.08; 3 trials, 1439 participants; I2 = 66%; Analysis 2.8).
2.7. Analysis.

Comparison 2: Extended‐fixed versus monthly injections, Outcome 7: Sensitivity analysis excluding high risk of bias: mean change in best‐corrected visual acuity at 1 year
2.8. Analysis.

Comparison 2: Extended‐fixed versus monthly injections, Outcome 8: Sensitivity analysis using fixed effects: mean change in best‐corrected visual acuity at 1 year
Other extended‐fixed dosing or as needed comparisons
Two trials compared treatment regimens that did not include monthly dosing (GMAN 2015; NATTB 2012), aside from a loading series of three monthly injections in one trial (GMAN 2015). After initial loading doses, participants in GMAN 2015 received bevacizumab PRN or extended‐fixed dosing every three months. In NATTB 2012, participants were randomized to bevacizumab every six weeks (regimen A) or bevacizumab every six weeks for three injections followed by extended‐fixed dosing every three months for the last two injections (regimen B). The final results of NATTB 2012 were at 48 weeks of follow‐up. We were unable to perform meta‐analyses across the two trials given the divergent comparison regimens.
Visual acuity
The authors of GMAN 2015 reported results from their trial at 92 weeks; the mean gain in BCVA was 5.5 letters (95% CI 2.9 to 8.0) in the extended‐fixed dosing group versus 0.6 letters (95% CI –2.0 to 3.1) in the PRN group. Mean BCVA was significantly better in the extended‐fixed dosing group than in the PRN group (MD 4.8 letters, 95% CI 1.2 to 8.3; Analysis 3.1), which was consistent with the results regarding gain of 15 or more letters (RR 0.54, 95% CI 0.33 to 0.87; Analysis 3.2). This evidence was of low certainty due to risk of bias (–1) and imprecision (–1).
3.1. Analysis.

Comparison 3: As needed (PRN) or extended‐fixed versus other extended‐fixed injections, Outcome 1: Mean change in best‐corrected visual acuity
3.2. Analysis.

Comparison 3: As needed (PRN) or extended‐fixed versus other extended‐fixed injections, Outcome 2: Gain ≥ 15 letters visual acuity
In NATTB 2012, there was no evidence of a difference between BCVA improvements in the two regimens at 48 weeks; the estimates were imprecise (MD –2.52 letters, 95% CI –7.14 to 2.10; Analysis 3.1). The proportion of participants who gained at least 15 letters of visual acuity was also similar in both groups (Analysis 3.2). This evidence was of low certainty due to risk of bias (–1) and imprecision (–1).
Central retinal thickness
Two trials provided data on the mean change in CRT between groups at two years (GMAN 2015) and one year (NATTB 2012). Estimates were imprecise and included no difference (Analysis 3.3; in GMAN 2015, we imputed standard deviations from non‐parametric P values for presentation purposes).
3.3. Analysis.

Comparison 3: As needed (PRN) or extended‐fixed versus other extended‐fixed injections, Outcome 3: Mean change in central retinal thickness
Quality of life
Neither of the two trials measured quality of life.
Economic considerations
Although the investigators in GMAN 2015 did not directly assess treatment cost, they reported mean number of visits and mean number of injections by arm. In the extended‐fixed dosing arm, participants had a mean of 11.9 visits and 10.8 injections (165 participants). A total of 166 participants who completed the PRN arm of the trial had a mean of 12.4 visits and 9.1 injections. In other words, participants in the extended‐fixed dosing group had slightly fewer visits but more injections on average when compared with participants in the PRN group.
Investigators in NATTB 2012 reported a mean of 7.9 injections in the extended‐fixed regimen (79 participants) and 4.9 in the PRN regimen (82 participants). This translated to mean total drug costs of USD 675.70 for the extended‐fixed regimen and USD 420.90 for the PRN regimen.
No meta‐analysis was possible because standard deviations were not available.
Adverse events
The total number of serious systemic adverse events was 19 events in the extended‐fixed dosing group and 26 in the PRN arm in GMAN 2015. There were no reports of endophthalmitis in GMAN 2015. The authors of NATTB 2012 reported no serious adverse events. The authors also reported no case of endophthalmitis over the 48‐week trial period. We did not meta‐analyze any adverse events data due to their sparseness.
Sensitivity analyses
No sensitivity analysis was possible.
No loading dose versus loading injections
Two trials compared the effects of starting treatment with loading doses (Barikian 2015a; BeMOc 2013). Barikian 2015a randomized participants to one of three groups: one initial injection followed by PRN (regimen one), one injection every two weeks for three injections followed by PRN (regimen two), and one injection every four weeks for the first three injections followed by PRN (regimen three). Participants in BeMOc 2013 either underwent PRN without a loading dose or received three monthly injections followed by PRN. Results were not available for two years of follow‐up.
Visual acuity
The MD in mean change in BCVA at one year between no loading and loading groups was –0.65 letters, with no evidence of a difference (95% CI –3.36 to 2.07; 2 trials, 159 participants; I2 = 0%; Analysis 4.1). The RR of gaining 15 letters or more at one year was 0.95 and was imprecisely estimated (95% CI 0.50 to 1.80; 1 trial, 99 participants; Analysis 4.2). The evidence was of low certainty due to risk of bias (–1) and imprecision (–1).
4.1. Analysis.

Comparison 4: No loading versus loading injections, Outcome 1: Mean change in best‐corrected visual acuity at 1 year
4.2. Analysis.

Comparison 4: No loading versus loading injections, Outcome 2: Gain ≥ 15 letters visual acuity at 1 year
Central retinal thickness
The MD in mean change in CRT at one year between no loading and loading groups was 9.42 μm, with no evidence of a difference (95% CI –11.28 to 30.12 μm; 2 trials, 159 participants; I2 = 68%), but the point estimates of the two included studies were in opposite directions (low‐certainty evidence due to risk of bias [–1] and inconsistency [–1]; Analysis 4.3).
4.3. Analysis.

Comparison 4: No loading versus loading injections, Outcome 3: Mean change in central retinal thickness
Quality of life
BeMOc 2013 found no statistically significant difference between the change in NEI VFQ‐25 scores from baseline to 54 weeks in all 12 survey domains. However, these analyses could not be replicated since data on standard deviations were not available. The mean scores were 3.05 at baseline and 3.01 at week 54 in the group without loading injections; and the mean scores were 3.02 at baseline and 3.08 at week 54 in the group that underwent loading injections.
Barikian 2015a did not assess quality of life.
Economic considerations
The authors reported that the mean number of injections over 12 months in Barikian 2015a was similar in the three study arms (6.1 in regimen 1, 6.5 in regimen 2, and 6.3 in regimen 3).
In BeMOc 2013, participants who completed the no loading dose arm of the trial received a mean of 4.7 injections (range one to nine) while those who were in the loading dose arm received a mean of 5.8 injections (range three to nine). In the no loading dose group, 18 (36.7%) participants required two injections in the first three months and four (8.2%) required only one injection in the first three months, which was fewer than the loading dose regimen.
The two trials in this comparison category did not evaluate treatment cost.
Adverse events
The incidences of serious systemic adverse events were low in both trials. BeMOc 2013 reported only one serious event, which was myocardial ischemia occurring in one participant from the loading dose arm. No serious adverse events occurred in Barikian 2015a. BeMOc 2013 had no serious ocular adverse events.
Sensitivity analyses
No sensitivity analysis was possible.
Discussion
Summary of main results
We found moderate‐certainty evidence of a statistically significant, but not clinically meaningful difference in BCVA with monthly injection compared with 'standard' PRN regimens, in which injections were prescribed when there were signs of CNV recurrence, after one year. We found low‐certainty evidence that a modified PRN regimen, the treat‐and‐extend regimen in which additional injections were delivered with increasing intervals after a recurrence were detected regardless of exudation persistence, yields similar results to monthly injections. We observed that the number of PRN injections was generally greater in trials compared to clinical practice (Kim 2016), where PRN treatment intensity is suboptimal (fewer than five injections in the first year in many settings), and worse visual outcomes are achieved in many settings.
We conducted subgroup analyses for 'standard' PRN and treated‐and‐extend subgroups (versus monthly regimen) and found they overall favored treat‐and‐extend regimens regarding vision gain, but differences in effects were small. Both the 'standard' PRN and the treat‐and‐extend regimens were more intense than common clinical practice (Kim 2016).
Regarding safety, there were more cases of endophthalmitis with monthly versus PRN regimens, which is expected since this event is procedure‐related and injections were more frequent with monthly regimens. We found more serious systemic adverse events with PRN versus monthly treatments, but this finding must be interpreted with caution since it lacks a rationale and was not supported by evidence on all‐cause mortality and arterial thromboembolic events.
Although we could not perform a meta‐analysis of treatment cost, both IVAN 2012b and CATT 2011 found the ranibizumab groups to be more expensive than the bevacizumab groups because of drug cost, regardless of regimens.
Comparison of monthly injections with extended‐fixed injection regimen showed relative effects that were consistent with those of PRN regimens, but estimates were less precise since we included only three trials.
We could not pool data from extended‐fixed versus PRN comparisons because of the lack of treatment interval uniformity (GMAN 2015; NATTB 2012).
In this review, we also found two small, single‐center trials that evaluated the efficacy and safety of loading doses prior to PRN scheduling (Barikian 2015a; BeMOc 2013). We could not combine data from the two trials given incompatible intervals. Both trials reported imprecise estimates between the group that started treatment with loading doses and the group that initiated PRN dosing at trial onset.
Overall completeness and applicability of evidence
Many of the trials in this review were multi‐centered and international, thereby supporting applicability across different practice settings and patient populations. Nonetheless, published reviews of 'real‐world' data found that the mean number of injections in the first year varied and was often very low in observational studies (Kim 2016). The same review found a direct association between the mean number of injections in a trial and mean visual outcomes.
Although we had considerable data for one‐year outcome comparisons, many of the trials did not provide data at two years of follow‐up. We were also limited in our ability to assess quality of life and economic outcomes because they were less often studied or reported. Moreover, economic outcomes are often specific to each setting and clinical pathway.
No long‐term data were available from the three trials that adopted a treat‐and‐extend regimen. One observational study reported that a mean of five annual injections were delivered until the eighth year, which suggests that a treat‐and‐extend regimen is intensive if it is properly implemented (Berg 2017).
Quality of the evidence
Overall, risk of bias present in the evidence was high or unclear in most trials, Particularly, only two trials reported adequate masking of participant and personnel (IVAN 2012b; VIEW 2012). and this item was unclear or high‐risk for all other trials. Accordingly, the certainty of evidence was low or moderate for most comparisons and outcomes.
Moreover, given the high cost of on‐label drugs, if more studies are available in the updates of this review, it will be interesting to conduct sensitivity analyses to investigate if the results change when industry‐funded RCTs are excluded.
Potential biases in the review process
We implemented several measures to minimize potential biases in the execution of this review. We conducted broad electronic searches for studies without restrictions on language. Our review excluded trials initiated prior to 2004 to reflect the timing of the introduction of anti‐VEGF injections for the treatment of CNV in AMD. We do not believe this imposed any bias on the review process. We followed the standard Cochrane Review methodology.
Agreements and disagreements with other studies or reviews
One systematic review published in 2015 compared the efficacy and safety of monthly versus PRN regimens (Schmucker 2015). It included three trials also included in our review (CATT 2011; HARBOR 2013; IVAN 2012b). Results showed that there was a small statistically significant decrease in mean BCVA and a small increase in risk of systemic adverse events among participants in the PRN treatment group. Nevertheless, the authors concluded that PRN treatment guided by visual acuity and OCT findings may be a reasonable approach to managing the majority of people with nAMD.
One systematic review that evaluated the relative efficacy of PRN versus treat‐and‐extend regimens for the treatment of nAMD conducted a meta‐analysis comprised of 1046 peer‐reviewed articles not restricted to RCTs (Chin‐Yee 2016). The review had a primary outcome of change in BCVA from baseline at one year of treatment; secondary outcomes included number of injections over the 12‐month study period and change in CRT from baseline at one year. The authors also conducted subgroup analysis of PRN groups from RCTs. The review found a significantly larger improvement in BCVA and greater number of injections in the treat‐and‐extend cohort when compared to the PRN group (both across all types of studies and within just RCTs). There was no statistically significant difference in CRT change between the two regimens. The authors concluded there may have been superior visual outcomes from treat‐and‐extend treatment versus PRN dosing. This review was difficult to compare with our review since it included non‐randomized studies.
Authors' conclusions
Implications for practice.
The current standard of care for neovascular age‐related macular degeneration (nAMD) requires intravitreous injection of anti‐vascular endothelial growth factor (anti‐VEGF) medication. Our review found that, at one year, monthly regimens are slightly more effective than standard as needed (PRN) regimens using a median of seven injections in the first year, but the difference is not clinically important. Treat‐and‐extend regimens delivered a median of nine injections and obtained similar results as monthly injections. Endophthalmitis is more common with monthly injections and differences in costs are higher if aflibercept or ranibizumab are used compared to bevacizumab. A small difference in visual benefit between PRN and monthly regimens persisted at two years in two large trials.
This evidence only applies to settings in which intensive PRN regimens are implemented, whereas undertreatment is common in real‐world settings. nAMD tends to become a chronic disease under anti‐VEGF treatment, with continuous deterioration of visual acuity. There are no data from randomized controlled trials on long‐term effects of different treatment regimens.
Implications for research.
Further long‐term, pragmatic randomized controlled trials comparing 'standard' PRN regimens with treat‐and‐extend regimens are needed to establish which treatment and monitoring intensity is to be preferred in clinical practice. In fact, PRN regimens often lead to substantial undertreatment in practice compared to trials and the adoption of a treat‐and‐extend regimen may improve treatment intensity. Such trials should collect patient‐reported outcomes and cost data regarding the use of drugs, tests, and other resources.
History
Protocol first published: Issue 5, 2016 Review first published: Issue 5, 2020
Acknowledgements
The Methods section used some text from a standard review template prepared by Cochrane Eyes and Vision (CEV). We thank Iris Gordon, Information Specialist for CEV, for devising the search strategy. We thank the CEV group for data extraction. We also thank Daniela Bacherini, Mahsa Salehi, and Barbara Hawkins for providing peer review comments to the protocol and final review.
This review was managed by CEV@US and was signed off for publication by Tianjing Li and Richard Wormald.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Macular Degeneration] explode all trees #2 MeSH descriptor: [Retinal Degeneration] explode all trees #3 MeSH descriptor: [Retinal Neovascularization] explode all trees #4 MeSH descriptor: [Choroidal Neovascularization] explode all trees #5 MeSH descriptor: [Macula Lutea] explode all trees #6 maculopath* #7 (macula* or retina* or choroid*) near/3 degenerat* #8 (macula* or retina* or choroid*) near/3 neovascul* #9 macula* near/2 lutea #10 AMD or AMRD or CNV #11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #12 MeSH descriptor: [Angiogenesis Inhibitors] explode all trees #13 MeSH descriptor: [Angiogenesis Inducing Agents] explode all trees #14 MeSH descriptor: [Endothelial Growth Factors] explode all trees #15 MeSH descriptor: [Vascular Endothelial Growth Factors] explode all trees #16 anti near/2 VEGF* #17 anti near/1 angiogen* #18 endothelial near/2 growth near/2 factor* #19 (macugen* or pegaptanib* or lucentis* or rhufab* or ranibizumab* or bevacizumab* or avastin* or aflibercept* or conbercept* or OPT 302 or Opthea* or RTH258 or Brolucizumab* or abicipar pegol) #20 VEGF TRAP* #21 #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 #22 #11 and #21
Appendix 2. MEDLINE (Ovid) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp macular degeneration/ 14. exp retinal degeneration/ 15. exp retinal neovascularization/ 16. exp choroidal neovascularization/ 17. exp macula lutea/ 18. maculopath$.tw. 19. ((macul$ or retina$ or choroid$) adj3 degener$).tw. 20. ((macul$ or retina$ or choroid$) adj3 neovasc$).tw. 21. (macula$ adj2 lutea).tw. 22. (AMD or ARMD or CNV).tw. 23. or/13‐22 24. exp angiogenesis inhibitors/ 25. angiogenesis inducing agents/ 26. endothelial growth factors/ 27. exp vascular endothelial growth factors/ 28. (anti adj2 VEGF$).tw. 29. (endothelial adj2 growth adj2 factor$).tw. 30. (anti adj1 angiogen$).tw. 31. (macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibizumab$ or bevacizumab$ or avastin or aflibercept$ or conbercept$ or OPT 302 or Opthea$ or RTH258 or Brolucizumab$ or abicipar pegol).tw. 32. VEGF TRAP$.tw. 33. or/24‐32 34. 23 and 33 35. 12 and 34
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase (Ovid) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp retina macula degeneration/ 34. exp retinal degeneration/ 35. exp subretinal neovascularization/ 36. maculopath$.tw. 37. ((macul$ or retina$ or choroid$) adj3 degener$).tw. 38. ((macul$ or retina$ or choroid$) adj3 neovasc$).tw. 39. (macula$ adj2 lutea).tw. 40. (AMD or ARMD or CNV).tw. 41. or/33‐40 42. angiogenesis/ 43. exp angiogenesis inhibitors/ 44. angiogenic factor/ 45. endothelial cell growth factor/ 46. monoclonal antibody/ 47. vasculotropin/ 48. (anti adj2 VEGF$).tw. 49. (endothelial adj2 growth adj2 factor$).tw. 50. (anti adj1 angiogen$).tw. 51. (macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibizumab$ or bevacizumab$ or avastin or aflibercept$ or conbercept$ or OPT 302 or Opthea$ or RTH258 or Brolucizumab$ or abicipar pegol).tw. 52. VEGF TRAP$.tw. 53. or/42‐52 54. 41 and 53 55. 32 and 54
Appendix 4. LILACS search strategy
(tw:(Macular Degeneration OR AMD OR ARMD )) AND (tw:(Macugen OR Pegaptanib OR Lucentis OR rhufab OR ranibizumab OR bevacizumab OR avastin OR aflibercept OR conbercept OR OPT 302 OR Opthea OR RTH258 OR Brolucizumab OR abicipar pegol))
Appendix 5. ISRCTN search strategy
(Macular Degeneration or change to or AMD OR nAMD OR ARMD) AND (Macugen OR Pegaptanib OR Lucentis OR rhufab OR ranibizumab OR bevacizumab OR avastin OR aflibercept OR conbercept OR Brolucizumab OR abicipar)
Appendix 6. ClinicalTrials.gov search strategy
(Macular Degeneration OR AMD OR nAMD OR ARMD) AND (Macugen OR Pegaptanib OR Lucentis OR rhufab OR ranibizumab OR bevacizumab OR avastin OR aflibercept OR conbercept OR OPT 302 OR Opthea OR RTH258 OR Brolucizumab OR abicipar pegol)
Appendix 7. ICTRP search strategy
Macular Degeneration OR AMD OR nAMD OR ARMD = Condition AND Macugen OR Pegaptanib OR Lucentis OR rhufab OR ranibizumab OR bevacizumab OR avastin OR aflibercept OR Conbercept = Intervention
Data and analyses
Comparison 1. As needed (PRN) versus monthly injections.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Mean change in best‐corrected visual acuity at 1 year | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 PRN | 4 | 2299 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐2.81, ‐0.55] |
| 1.1.2 Treat and extend | 3 | 1226 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐3.14, 4.16] |
| 1.2 Gain ≥ 15 letters visual acuity at 1 year | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 PRN | 4 | 2299 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.76, 0.99] |
| 1.2.2 Treat and extend | 3 | 1169 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.91, 1.36] |
| 1.3 Mean change in best‐corrected visual acuity at 2 years | 3 | 1875 | Mean Difference (IV, Fixed, 95% CI) | ‐1.08 [‐2.42, 0.26] |
| 1.3.1 PRN | 2 | 1295 | Mean Difference (IV, Fixed, 95% CI) | ‐2.23 [‐3.93, ‐0.53] |
| 1.3.2 Treat and extend | 1 | 580 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐1.38, 2.98] |
| 1.4 Gain ≥ 15 letters visual acuity at 2 years | 3 | 1875 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.75, 1.03] |
| 1.4.1 PRN | 2 | 1295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.66, 0.96] |
| 1.4.2 Treat and extend | 1 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.82, 1.46] |
| 1.5 Mean change central retinal thickness at 1 year | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.5.1 PRN | 4 | 2215 | Mean Difference (IV, Random, 95% CI) | 20.84 [5.78, 35.89] |
| 1.5.2 Treat and extend | 2 | 635 | Mean Difference (IV, Random, 95% CI) | 21.96 [‐37.22, 81.14] |
| 1.6 Mean change central retinal thickness at 2 years | 2 | 1273 | Mean Difference (IV, Fixed, 95% CI) | 24.53 [6.12, 42.93] |
| 1.7 Mean number of injections during 2 years | 3 | 1879 | Mean Difference (IV, Fixed, 95% CI) | ‐8.73 [‐9.16, ‐8.30] |
| 1.7.1 PRN | 2 | 1303 | Mean Difference (IV, Fixed, 95% CI) | ‐9.78 [‐10.29, ‐9.27] |
| 1.7.2 Treat and extend | 1 | 576 | Mean Difference (IV, Fixed, 95% CI) | ‐6.20 [‐6.99, ‐5.41] |
| 1.8 Endophthalmitis | 6 | 3175 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.04, 0.46] |
| 1.9 Serious systemic adverse events | 6 | 3175 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.05, 1.44] |
| 1.10 Death | 7 | 3701 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.55, 2.23] |
| 1.11 Arterial thromboembolic events | 6 | 3175 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.44, 2.13] |
| 1.12 Mean number of injections during 1 year | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.12.1 PRN | 4 | 2336 | Mean Difference (IV, Random, 95% CI) | ‐4.57 [‐5.38, ‐3.76] |
| 1.12.2 Treat and extend | 3 | 1232 | Mean Difference (IV, Random, 95% CI) | ‐2.42 [‐2.71, ‐2.14] |
| 1.13 Sensitivity analysis excluding high‐risk of bias: mean change in best‐corrected visual acuity at 1 year | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.13.1 PRN | 2 | 1074 | Mean Difference (IV, Random, 95% CI) | ‐1.49 [‐3.05, 0.06] |
| 1.13.2 Treat and extend | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 1.30 [‐6.15, 8.75] |
| 1.14 Sensitivity analysis using fixed effects: mean change in best‐corrected visual acuity at 1 year | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.14.1 PRN | 4 | 2299 | Mean Difference (IV, Fixed, 95% CI) | ‐1.68 [‐2.81, ‐0.55] |
| 1.14.2 Treat and extend | 3 | 1226 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐0.97, 1.91] |
1.12. Analysis.

Comparison 1: As needed (PRN) versus monthly injections, Outcome 12: Mean number of injections during 1 year
Comparison 2. Extended‐fixed versus monthly injections.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Mean change in best‐corrected visual acuity at 1 year | 3 | 1439 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐3.93, 1.29] |
| 2.2 Gain ≥ 15 letters visual acuity at 1 year | 3 | 1441 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.10] |
| 2.3 Mean change of central retinal thickness at 1 year | 3 | 1439 | Mean Difference (IV, Random, 95% CI) | 8.16 [‐11.07, 27.40] |
| 2.4 Mean change in quality of life scores at 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.5 Endophthalmitis | 3 | 1132 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.03, 1.12] |
| 2.6 Serious systemic adverse events | 2 | 1068 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.30] |
| 2.7 Sensitivity analysis excluding high risk of bias: mean change in best‐corrected visual acuity at 1 year | 2 | 1282 | Mean Difference (IV, Random, 95% CI) | ‐2.14 [‐5.03, 0.75] |
| 2.8 Sensitivity analysis using fixed effects: mean change in best‐corrected visual acuity at 1 year | 3 | 1439 | Mean Difference (IV, Fixed, 95% CI) | ‐1.36 [‐2.64, ‐0.08] |
Comparison 3. As needed (PRN) or extended‐fixed versus other extended‐fixed injections.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Mean change in best‐corrected visual acuity | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 PRN (1) vs every 12 weeks (2); follow‐up at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1.2 Every 12 weeks (1) vs every 6 weeks (2); follow‐up at 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.2 Gain ≥ 15 letters visual acuity | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.1 PRN vs every 12 weeks; follow‐up at 2 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2.2 Every 12 weeks vs every 6 weeks; follow‐up at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Mean change in central retinal thickness | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.1 PRN vs every 12 weeks; follow‐up at 2 years | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.3.2 Every 12 weeks vs every 6 weeks; follow‐up at 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. No loading versus loading injections.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Mean change in best‐corrected visual acuity at 1 year | 2 | 159 | Mean Difference (IV, Fixed, 95% CI) | ‐0.65 [‐3.36, 2.07] |
| 4.2 Gain ≥ 15 letters visual acuity at 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.3 Mean change in central retinal thickness | 2 | 159 | Mean Difference (IV, Fixed, 95% CI) | 9.42 [‐11.28, 30.12] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barikian 2015a.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized (total and per group): 90 total participants; 30 participants in each of 3 groups Exclusions after randomization: none reported Number analyzed (total and per group): 90 participants; 30 participants in each of 3 groups Unit of analysis: participant (1 study eye per participant) Losses to follow‐up: none reported Intention‐to‐treat analysis: all participants randomized were analyzed Power calculation: none reported Study design comment: none |
|
| Participants |
Country: Lebanon Mean age: 77 years Gender (%): 41 (46%) women and 49 (54%) men Inclusion criteria: ages > 50 years with subfoveal CNV attributable to AMD diagnosed by FA. BCVA ≥ 50 letters (≥ 20/100 Snellen equivalent) using the ETDRS chart. Presence of subretinal fluid, cystic maculopathy, or CRT > 250 mm had to be documented on OCT with CNV < 5400 mm in greatest linear dimension. To understand and sign the study consent form. Exclusion criteria: prior treatment for CNV; submacular hemorrhage or scarring involving the fovea; corneal, lenticular, or vitreous opacification that prevented good‐quality angiograms or OCT; history of uveitis, vitrectomy, proliferative diabetic retinopathy, and other ocular conditions that affected vision. Cardiovascular, cerebrovascular, or peripheral vascular event < 6 months prior to enrollment. All CNV lesion types were included except for retinal angiomatous proliferation and polypoidal choroidal vasculopathy, since they may have responded differently to treatment. Equivalence of baseline characteristics: significantly more women recruited to the monthly induction arm compared to the biweekly induction arm. |
|
| Interventions |
Intervention: intravitreous bevacizumab 1.25 mg injection (Avastin; Roche, Basel, Switzerland) Intervention 1: first injection, then PRN Intervention2: every 2 weeks for first 3 injections, then PRN Intervention3: q4 wks for first 3 injections, then PRN Follow‐up: 12 months Frequency of assessments for retreatment: monthly |
|
| Outcomes |
Primary outcome, as defined: mean initial fluid‐free interval after induction period Secondary outcomes, as defined: mean improvement in BCVA (ETDRS charts at 4 m) and CRT Adverse events: ocular and systemic adverse events Review outcomes not reported: gain of 15 letters VA, quality of life, number of injections, cost Intervals at which outcome assessed: every month for 12 months |
|
| Notes |
Full study name: not reported Trial registration: not reported Funding sources: American University of Beirut Medical Center, Beirut, Lebanon Declarations of interest: quote: "The authors indicate no financial interest in any product discussed in this study. Z.F.B. has participated on advisory boards for Novartis and Bayer; has received honoraria from Bayer (Leverkusen, Germany) and Novartis (Basel, Switzerland) as invited speaker; and has received research grants from Novartis and Allergan (Center Valley, Pennsylvania, USA)." Study period: September 2010 throughout 2012 Subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. Quote: "Patients were randomized in a 1:1:1 ratio to 1 of 3 groups based on the induction sequence." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data reported. |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol and trial registry not reported. |
BeMOc 2013.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized (total and per group): 100 total participants; 49 participants in no loading group, 50 participants in loading group (unclear which group 1 participant was in) Exclusions after randomization: 1 participant (unclear which group) Number analyzed (total and per group): 99 participants; 49 participants in no loading group; 50 participants in loading group Unit of analysis: participant (1 study eye per participant) Losses to follow‐up: none reported Intention‐to‐treat analysis: participants analyzed as they were randomized, 1 participant excluded from analysis. Power calculation: none reported; quote: "a reasonable and pragmatic sample size of 100 patients was selected to enable the study to be carried out as a monocentric study." Study design comment: none |
|
| Participants |
Country: UK Mean age: not reported; 13 participants ages 61–70 years; 35 participants ages 71–80 years; 51 participants ages ≥ 81 years Gender (%): 72 (73%) women and 27 (27%) men Inclusion criteria: treatment‐naïve people with active subfoveal CNV of minimally classic or occult type, secondary to AMD, confirmed on FA, and no other visually significant ocular pathology Exclusion criteria: medical conditions uncontrolled hypertension; taking > 3 antihypertensive medications; change in antihypertensive drug initiated within 3 months preceding baseline visit; previous thromboembolic phenomenon; taking warfarin or anticoagulants; recent MI; recent major surgery (within 28 days); ocular conditions (glaucoma [IOP] > 25 mmHg, on antiglaucoma treatment, glaucoma surgery; active intraocular or extraocular inflammation; retinal vascular disease; other sources of CNV membrane; previous PDT; predominantly classic membranes; previous cataract surgery (within 6 months); aphakia; other retinal conditions that may affect visual outcome); other (allergy to fluorescein; inability to obtain color photographs, FA, OCT images; allergy to anti‐VEGF medications; allergy to humanized monoclonal antibody; inability to comply with follow‐up procedures (from trial registry) Equivalence of baseline characteristics: groups balanced at baseline in terms of mean VAs and mean CMT. |
|
| Interventions |
Intervention: intravitreous bevacizumab 1.25 mg injection (Avastin; Roche, Basel, Switzerland) Intervention1: PRN (no loading) Intervention2: q4 wks for first 3 injections, then PRN (loading) Follow‐up: 54 weeks Frequency of assessments for retreatment: q6 wks |
|
| Outcomes |
Primary outcome, as defined: proportion with visual stability, defined as loss of ≤ 15 letters from baseline Secondary outcomes, as defined: CMT on OCT Adverse events: ocular and systemic adverse events Review outcomes not reported: number of injections, cost Intervals at which outcome assessed: q6 wks for 54 weeks |
|
| Notes |
Full study name: not reported Trial registration: EUDRACT No: 2006‐003033‐33; ISRCTN number: 12980412 Funding sources: Frimley Park Hospital NHS Trust (UK) Declarations of interest: quote: "The authors declare no conflict of interest." Study period: November 2006 to November 2008 Subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/100 (1%) participants excluded. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol could not be retrieved from EUDRACT. Primary and secondary outcomes not reported in trial registry. |
CANTREAT 2019.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized (total and per group): Regimen randomization: 526 total participants treated with ranibizumab 0.5 mg; 268 to treat‐and‐extend group and 258 to monthly group Exclusions after randomization: 18 participants did not receive treatment and were excluded after randomization to drug treatment (9 in bevacizumab group and 9 in ranibizumab group). Number analyzed (total and per group): At 1 year' follow‐up: 526 participants (268 in treat‐and‐extend group; 258 in monthly group). Unit of analysis: participant (1 study eye per participant) Losses to follow‐up: 18/287 (6.3%) in treat‐and‐extend group; 36/293 (12.3%) in monthly group At 1 year' follow‐up: consent withdrawal was most common in the monthly (4.4%) and treat‐and‐extend (2.1%) arm. Compliance: no data Intention‐to‐treat analysis: no information on how missing data were used. Reported power calculation: yes, sample of 580 participants per group for power of 80% to detect non‐inferiority Study design comment: non‐inferiority design |
|
| Participants |
Country: Canada (27 study centers) Age: mean age for 590 participants receiving treatment was 78.8 years and similar in study arms Gender (%): 60.3% women and similar in study arms Inclusion criteria: ages > 50 years; diagnosis of treatment‐naïve CNV secondary to AMD in the study eye; BCVA score in the study eye 19–78 letters using ETDRS VA charts at a testing distance of 4 m (approximate Snellen equivalent of 20/32–20/400 at screening). Exclusion criteria: structural foveal damage; confounding severe ocular disease in the study eye; clinical suspicion of polypoidal choroidal vasculopathy in the study eye; active or suspected ocular or periocular infections Equivalence of baseline characteristics: yes Diagnoses in participants: 2.8% treated for AMD in fellow eye |
|
| Interventions |
Intervention 1: intravitreous ranibizumab 0.5 mg monthly Intervention 2: intravitreous ranibizumab 0.5 mg injected monthly until achievement of disease stability, then the interval between each subsequent injection was extended by 2 weeks (intervals of 6 weeks, 8 weeks, 10 weeks, and a maximum of 12 weeks) until clinical or diagnostic evidence of disease instability was observed based on OCT findings, ETDRS BCVA, or both. Follow‐up: 1 year Frequency of follow‐up assessments: at each visit or injection |
|
| Outcomes |
Primary outcome, as defined: mean BCVA change measured as ETDRS letters at 1 year Secondary outcomes, as defined in protocol: duration of treatment‐free intervals in the treat‐and‐extend dosing regimen arm; proportion of participants with gains of ≥ 5 letters, ≥ 10 letters, and ≥ 15 letters; proportion of participants with losses of < 5 letters, < 10 letters, and < 15 letters; mean change in ETDRS BCVA between the 2 treatment arms |
|
| Notes |
Full study name: Canadian Treat‐and‐Extend Analysis Trial with Ranibizumab (CANTREAT) Trial registration: NCT02103738 Type of study: published Funding sources: funded by Novartis Pharmaceuticals Canada. Declarations of interest: various authors reported attending and being remunerated for attendance at advisory boards for Novartis, Bayer, Allergan, AbbVie, or a combination; being employed by Novartis, Study period: study start on May 2013 Reported subgroup analyses: not reported Contacting study investigators: trial authors not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive Web‐based Response System at baseline visit, prior to injection. Randomization schedule was generated by the study biostatistician using a permuted‐block design. |
| Allocation concealment (selection bias) | Low risk | Interactive Web‐based Response System at baseline visit, prior to injection. Randomization schedule was generated by the study biostatistician using a permuted‐block design. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Masking of outcome assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Discontinuations were 6.3% in the treat‐and‐extend arm and 12.3% in the monthly arm. |
| Selective reporting (reporting bias) | Low risk | Primary outcome matched protocol in ClinicalTrial.Gov |
CATT 2011.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized (total and per group): 1208 total participants; number of participants randomized per group not reported Exclusions after randomization: 1 study center (23 participants) excluded due to protocol violations Number analyzed (total and per group): 1105 participants; 265 in bevacizumab monthly group, 284 in ranibizumab monthly group, 271 in bevacizumab PNR group, and 285 in ranibizumab PNR group Unit of analysis: participant (1 study eye per participant) Losses to follow‐up: 80 total participants: 21 in bevacizumab monthly group (4 died and 17 with missing data), 17 in ranibizumab monthly group (4 died and 13 with missing data), 29 in bevacizumab PNR group (11 died and 18 with missing data), 13 in ranibizumab PNR group (5 died and 8 with missing data) Intention‐to‐treat analysis: no, 103 participants enrolled and randomized were not included in analyses. Power calculation: yes, sample of 277 participants per group for power of 90% Study design comment: non‐inferiority design, 4 arms, 6 pair‐wise comparisons planned; at 1 year, participants in the monthly dose treatment groups were rerandomized to either continue with monthly injections or switch to PNR injections of the same treatment drug. |
|
| Participants |
Country: USA Mean age: 79 years Gender (%): 732/1185 (61.8%) women and 453/1185 (38.2%) men Inclusion criteria: ages ≥ 50 years; 1 study eye per participant with untreated active CNV due to AMD (based on presence of leakage as seen by FA and of fluid as seen by OCT); VA of 20/25–20/320 on electronic VA testing Exclusion criteria: fibrosis or atrophy in center of fovea in study eye; CNV in either eye due to other causes; RPE tear involving the macula; any concurrent intraocular condition in the study eye (e.g. cataract or diabetic retinopathy) that, in the opinion of the investigator, could either require medical or surgical intervention or contribute to VA loss during the 3‐year follow‐up; active or recent (within 4 weeks) intraocular inflammation; current vitreous hemorrhage in the study eye; history of rhegmatogenous retinal detachment or macular hole; active infectious conjunctivitis, keratitis, scleritis, or endophthalmitis; spherical equivalent > 8 diopters; intraocular surgery (including cataract surgery) in the study eye within 2 months; uncontrolled glaucoma; participants unable to be photographed to document CNV due to known allergy to fluorescein dye, lack of venous access, or cataract obscuring the CNV; premenopausal women not using adequate contraception; pregnancy or lactation; history of other disease, metabolic dysfunction, physical exam finding, or clinical laboratory finding giving reasonable suspicion of a disease or condition that contraindicated the use an investigational drug or that might have affected interpretation of the results of the study or render the person at high risk for treatment complications; current treatment for active systemic infection; uncontrolled concomitant diseases such as cardiovascular disease, nervous system, pulmonary, renal, hepatic, endocrine, or gastrointestinal disorders; history of recurrent significant infections or bacterial infections; inability to comply with study or follow‐up procedures Equivalence of baseline characteristics: slightly higher percentage of participants in bevacizumab monthly group had history of transient ischemic attack (8.7% compared with 4% in ranibizumab monthly group, 4% in ranibizumab PNR group, and 6.3% in bevacizumab PNR group) |
|
| Interventions |
Intervention 1: intravitreous bevacizumab 1.25 mg injection (Avastin; Roche, Basel, Switzerland) Intervention 2: intravitreous ranibizumab 0.5 mg injection Treatment regimen 1: PRN Treatment regimen 2: q4 wks for first year, then rerandomization to injections PRN or q4 wks |
|
| Outcomes |
Primary outcome, as defined: change in VA from baseline at 12 months with a non‐inferiority margin of 5 letters Secondary outcomes: proportion of eyes with 15‐letter change, number of injections, OCT measured change in foveal thickness, change in lesion size on OCT and FA, and annual drug cost Adverse events: ocular and systemic adverse events Review outcomes not reported: quality of life Intervals at which outcomes were assessed: weeks 4, 12, 24, 36, 52 during first year for VA; weeks 4, 8, 12, 24, 52 for changes on OCT |
|
| Notes |
Full study name: Comparison of Age‐related macular degeneration Treatment Trials Trial registration: NCT00593450 Funding: National Eye Institute, National Institutes of Health, US Declarations of interest: 1 investigator reported receiving consulting fees from GlaxoSmithKline and another consulting fees from Neurotech and SurModics Study period: accrual February 2008 through December 2009; follow‐up through December 2011 Subgroup analyses: none, but risk factors for 2‐year VA outcomes were reported (Ying 2015 under CATT 2011) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to 1 of 4 study groups. Randomization schedules were stratified according to clinical center with the use of a permuted‐block method with randomly chosen block sizes." |
| Allocation concealment (selection bias) | Low risk | Web‐based data entry system used to allocate participants to treatment groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Initially, participants were masked to which drug they received, but not to the treatment regimen. Study investigators noted, "insurance and billing documents specified ranibizumab but not study‐supplied bevacizumab. Therefore, patients may have learned or deduced their assigned drug from these financial documents." Physicians were masked to drug but not to injection regimen. Physicians were uninvolved in VA testing and in secondary outcome assessments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Electronic VA system (computerized testing) was used for primary outcome. Retinal center personnel were masked. Adverse event reporting was unmasked, but medical monitor who evaluated serious adverse events was masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 103/1208 (8.5%) participants randomized were not included in 2‐year analysis. At 2 years, outcomes were not available for all participants by their originally assigned treatment groups. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes, specified a priori, for 1‐year follow‐up reported. |
CLEAR‐IT 2 2011b.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized (total and per group): 159 total participants; 32 participants in 0.5 mg q4 wks group; 32 participants in 2 mg q4 wks group; 32 participants in 0.5 mg q12 wks group; 32 participants in 2 mg q12 wks group; 31 participants in 4 mg q12 wks group Exclusions after randomization: none reported Number analyzed (total and per group): 159 participants in total; 32 participants in 0.5 mg q4 wks group; 32 participants in 2 mg q4 wks group; 32 participants in 0.5 mg q12 wks group; 32 participants in 2 mg q12 wks group; 31 participants in 4 mg q12 wks group Unit of analysis: participant (1 study eye per participant) Losses to follow‐up: none reported Compliance: not reported Intention‐to‐treat analysis: all participants analyzed as randomized Reported power calculation: not reported Study design comment: none |
|
| Participants |
Country: USA Mean age (SD): 78.2 (not reported) years in total; by group not reported Gender (%): 38 men and 62 women in total; by group not reported Inclusion criteria: ages ≥ 50 years; diagnosis of subfoveal CNV secondary to wet AMD, and met the following inclusion criteria: CR/LT ≥ 300 μm, ETDRS BCVA letter score 73–34 letters (20/40–20/200), loss of ≥ 5 ETDRS letters in BCVA over preceding 6 months for previously treated people with minimally classic or occult lesions, linear diameter of lesion 5400 μm by FA, subretinal hemorrhage (if present) sparing the fovea and comprising ≤ 50% of total lesion, area of scar ≤ 25% of total lesion, and sufficient clarity of ocular media to allow retinal photography. Exclusion criteria: vitreous hemorrhage in preceding 4 weeks; aphakia or pseudophakia with absence of a posterior capsule (unless as a result of a YAG capsulotomy); significant subfoveal atrophy or scarring; active ocular inflammation; corneal transplant; previous uveitis in either eye; or history of macular hole of grade ≥ 3; previously received any of the following treatments in study eye: subfoveal thermal laser therapy, any operative intervention for AMD, extrafoveal laser coagulation treatment or PDT in preceding 12 weeks, pegaptanib sodium in preceding 8 weeks, systemic or intravitreous treatment with VEGF Trap‐Eye, ranibizumab, or bevacizumab at any time, juxtascleral steroids, anecortave acetate, or intravitreous triamcinolone acetonide or other steroids in preceding 24 weeks; other causes of CNV in either eye; active ocular infection; congenital lid anomalies that might interfere with intravitreous administration; any retinal disease other than CNV in either eye; previous trabeculectomy or pars plana vitrectomy; cup‐to‐disk ratio ≥ 0.8, IOP ≥ 25 mmHg, or receipt of > 2 agents for treatment of glaucoma; allergy to povidone iodine, fluorescein, or recombinant proteins; absolute neutrophil count 1000 cells/mm3; HIV positivity, active systemic infection requiring antibiotics; proteinuria > 1+ or urine protein:creatinine ratio ≥ 1 on 2 repeated determinations within 1 week; New York Heart Association class III or IV; symptomatic cardiovascular or peripheral vascular disease, malignancy other than basal cell carcinoma in preceding 2 years; and any other conditions or laboratory abnormalities that could interfere with disease assessment or patient participation in the study; use of standard agents or other anti‐VEGF agents before week 16. Equivalence of baseline characteristics: could not determine; baseline by group not reported Diagnoses in participants: subfoveal CNV secondary to wet AMD |
|
| Interventions |
Intervention 1: intravitreous injection of VEGF Trap‐Eye 0.5 mg q4 wks Intervention 2: intravitreous injection of VEGF Trap‐Eye 2 mg q4 wks Intervention 3: intravitreous injection of VEGF Trap‐Eye 0.5 mg q12 wks Intervention 4: intravitreous injection of VEGF Trap‐Eye 2 mg q12 wks Intervention 5: intravitreous injection of VEGF Trap‐Eye 4 mg q12 wks Follow‐up: 20 weeks and 1 year Frequency criteria of assessments for retreatment: an increase in CR/LT ≥ 100 μm as measured by OCT; loss of ≥ 5 ETDRS letters in conjunction with recurrent fluid as indicated by OCT; persistent fluid as indicated by OCT; new‐onset classic neovascularization; new or persistent leak on FA; or new macular hemorrhage. |
|
| Outcomes |
Primary outcome, as defined: change from baseline in CR/LT at week 12 Secondary outcomes, as defined: change in BCVA, proportion of participants with gain ≥ 15 letters, proportion of participants with loss ≥ 15 letters, and safety Adverse events (Y/N): yes Intervals at which outcome assessed: q4 wks for 20 weeks |
|
| Notes |
Full study name: CLinical Evaluation of Anti‐angiogenesis in the Retina intravitreous Trial (CLEAR‐IT 2) Type of study: published or unpublished Trial registration: NCT00320788 Funding sources: Regeneron Pharmaceuticals, Inc. and Bayer HealthCare AG Declarations of interest: quote: "David M. Brown – Alcon Laboratories – Consultant, Grant/Financial Support; Alimera – Grant/Financial Support; Allergan – Consultant, Grant/ Financial Support; Carl Zeiss Meditec – Consultant; CoMentis – Grant/ Financial Support; Eyemaginations – Consultant; Genentech – Consultant, Grant/Financial Support, Lecturer; Heidelberg Engineering – Consultant, Lecturer; Jerini Ophthalmics – Consultant, Grant/Financial Support, Lecturer; NeoVista – Consultant, Grant/Financial Support, Lecturer; Neuro‐ tech – Grant/Financial Support; Novartis Pharmaceuticals – Consultant, Grant/Financial Support; Oraya Therapeutics – Consultant; Othera – Grant/ Financial Support; Oxigene – Grant/Financial Support; Pfizer Ophthalmics – Consultant, Grant/Financial Support; Regeneron – Consultant, Grant/ Financial Support, Lecturer; Steba – Consultant. Jeffrey S. Heier: Acucela – Consultant; Alcon Laboratories – Consultant, Grant/Financial Support; Allergan – Consultant, Grant/Financial Support; Bausch & Lomb – Consultant; CoMentis – Grant/Financial Support; Eyemaginations – Consultant; Fovea – Consultant; Genentech – Consultant, Grant/Financial Support, Lecturer; Genzyme – Consultant; Heidelberg Engineering – Consultant, Lecturer; iScience – Consultant, Grant/ Financial Support; Ista Pharmaceuticals – Consultant, Grant/Financial Support; Jerini Ophthalmics – Consultant, Grant/Financial Support, Lecturer; LPath – Consultant; NeoVista – Consultant, Grant/Financial Support, Lecturer; Neurotech – Grant/Financial Support; Notal Vision – Consultant; Novartis Pharmaceuticals – Consultant, Grant/Financial Support; Optherion – Consultant; Optimedica – Royalties; Oraya Therapeutics – Consultant; Oxigene – Grant/Financial Support; Paloma – Consultant, Grant/ Financial Support; Pfizer Ophthalmics – Consultant, Grant/Financial Support; Regeneron – Consultant, Grant/Financial Support, Lecturer; Resolvyx Pharmaceuticals – Consultant; Schering Plough Research Institute – Consultant; Scyfix – Consultant; Steba – Consultant; VisionCare Ophthalmic Technologies – Consultant, Grant/Financial Support. Thomas Ciulla: Neovista – Consultant; Regeneron – Consultant; Pfizer – Consultant; Genentech – Grant/Financial Support; Regeneron – Grant/ Financial Support; Allergan – Grant/Financial Support; Alimera – Grant/ Financial Support; Othera – Grant/Financial Support; Glaxo‐Smith‐Kline – Grant/Financial Support; Optko – Grant/Financial Support; National Eye Institute/National Institutes of Health – Grant/Financial Support. Prema Abraham: Genentech – Consultant, Grant/Financial Support; Alcon – Consultant, Grant/Financial Support; Novartis – Consultant, Grant/Financial Support; Regeneron – Grant/Financial Support; Allergan – Grant/ Financial Support; Opko Health – Grant/Financial Support; Jerini Ophthalmic – Grant/Financial Support; Pfizer – Grant/Financial Support; Eli Lilly – Grant/Financial Support; Alimera – Grant/Financial Support; VRT – Grant/Financial Support; Schering‐Plough – Grant/Financial Support. George Yancopoulous, Neil Stahl, Avner Ingerman, Robert Vitti, Alyson J. Berliner, Ke Yang: Regeneron – Employee at the time the study was conducted. Quan Dong Nguyen: Bausch & Lomb – Consultant; Genentech – Grant/ Financial Support; Regeneron – Grant/Financial Support. Supported by Regeneron Pharmaceuticals, Inc. and Bayer HealthCare AG. The sponsors participated in the design of the study, conducting the study, data collection, data management, data analysis, interpretation of the data, and the preparation, review, and approval of the manuscript." Study period: May 2006 and April 2007 Reported subgroup analyses: none reported CLEAR‐IT 2 2011b: 3 intervention groups using other doses not analyzed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. Quote: "The CLEAR‐IT 2 was a prospective, double‐masked, randomized study conducted at 33 sites in the United States." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Examiners were masked to treatment assignment and performed no other study assessments." Quote: "Stratus (software version 4.0 or higher) OCT scans (Carl Zeiss Meditec, Inc., Dublin, CA) read at a masked independent central reading center (Digital Optical Coherence Tomography Reading Center [DOCTR], Cleveland, OH)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/159 (3.2%) participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes in trial registry reported in full text. |
El‐Mollayess 2012.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized (total and per group): 120 total participants; 60 participants in each group Exclusions after randomization: none reported Number analyzed (total and per group): 120 participants; 60 participants in each group Unit of analysis: participant (1 study eye per participant) Losses to follow‐up: none reported Intention‐to‐treat analysis: all participants randomized were analyzed Power calculation: quote: "detect a difference of at least 5 letters in mean visual acuity using the independent t test with 80% power and an alpha level of 5%, assuming a standard deviation of 10 letters, 60 eyes were needed in each group." Study design comment: quote: "If both eyes of the same patient were eligible, then the eye with the worse visual acuity was enrolled." |
|
| Participants |
Country: France and Lebanon Mean age: 77 years Gender (%): 78 women and 42 men Inclusion criteria: ages ≥ 50 years; subfoveal CNV attributable to AMD diagnosed by FA; presence of subretinal fluid, cystic maculopathy, or CRT > 250 μm on OCT; BCVA, using ETDRS charts, between 20/40 and 20/400 (Snellen equivalent); CNV < 5400 μm in greatest linear dimension; and ability to understand and sign a consent form. Exclusion criteria: presence of subfoveal scarring or hemorrhage; media opacity that would prevent good‐quality retinal imaging; history of uveitis, vitrectomy, diabetic retinopathy, or other condition that may have affected vision; and thromboembolic event < 6 months prior to enrollment. Equivalence of baseline characteristics: baseline characteristics by group not reported |
|
| Interventions |
Intervention: intravitreous bevacizumab 1.25 mg injection (Avastin; Roche, Basel, Switzerland) Intervention1: PRN (variable dosing) Intervention2: every 4–6 weeks (fixed‐interval dosing) Follow‐up: 12 months Frequency of assessments for retreatment: every 4–6 weeks |
|
| Outcomes |
Primary outcome, as defined: improvement in BCVA and CRT at 12 months Secondary outcomes, as defined: none reported Adverse events: ocular and systemic adverse events Review outcomes not reported: mean change in CRT, quality of life, cost Intervals at which outcome assessed: every 4–6 weeks |
|
| Notes |
Full study name: not reported Trial registration: not reported Funding sources: Department of Ophthalmology and University Research Board of American University of Beirut Medical Center, Beirut, Lebanon Declarations of interest: quote: "The authors indicate no financial interest in any product discussed in this study." Study period: May 2009 to October 2009 Subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization program (GraphPad StatMate, version 1.01i; GraphPad Software Inc, San Diego, California, USA)." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "visual acuity examiners were masked to treatment regimen and patients were instructed not to share this information with the examiner." Quote: "Treating physicians were not masked to the treatment regimen of patients under their care and no sham injections were employed." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "visual acuity examiners were masked to treatment regimen and patients were instructed not to share this information with the examiner." Quote: "The physician reviewing OCT images or other material to be recorded in the study was masked to that particular patient's identity and treatment regimen and in no way could be involved in the treatment of that patient." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients completed the 12 months of the study and were able to make scheduled visits with no greater than a 7‐day delay." |
| Selective reporting (reporting bias) | Unclear risk | Trial registry and citation to protocol not reported. |
GMAN 2015.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized (total and per group): 331 total participants; 166 participants in PRN group, 50 participants in routine group Exclusions after randomization: 48 withdrew in PRN group, 22 withdrew in routine group Number analyzed (total and per group): 166 in PRN group, 165 in routine group Unit of analysis: participant (1 study eye per participant) Losses to follow‐up: 26 in PRN group, 22 in routine group Compliance: 140 completed trial in PRN group, 143 completed trial in routine group Intention‐to‐treat analysis: 166 in PRN group, 165 in routine group Power calculation: yes, a non‐inferiority margin of 4–5 letters at 90% power for the sample size planned for the study Study design comment: none |
|
| Participants |
Country: UK Median age: 80 years Gender (%): 61% women and 39% men Inclusion criteria: ages > 50 years with a diagnosis of nAMD and BCVA of logarithm of the minimum angle of resolution 0.3–1.2 Exclusion criteria: lesion showed signs of > 50% fibrosis, hemorrhage, or serous PED. People with medical history of MI, cardiovascular accident, or gastrointestinal perforation when the trial commenced. However, as more evidence emerged suggesting a low systemic risk from the intravitreous use of anti‐VEGF drugs, the protocol was amended so that MI and gastrointestinal perforation were not exclusion criteria, and only people with history of cerebrovascular accident within 6 months were excluded. Equivalence of baseline characteristics: yes, no substantial imbalances in the ocular or demographic characteristics between the 2 groups |
|
| Interventions |
Intervention: intravitreous bevacizumab 1.25 mg injection (Avastin; Roche, Basel, Switzerland) Intervention1: 3 monthly loading doses, then PRN (PRN treatment) Intervention2: 3 monthly loading doses, then q12 wks (routine treatment) Follow‐up: 92 weeks Frequency of assessments for retreatment: q12 wks |
|
| Outcomes |
Primary outcome, as defined: mean BCVA at 92 weeks Secondary outcomes, as defined: change in mean VA from baseline to 92 weeks and % of participants who had a change in VA from baseline of ≥ 5, ≥ 10, or ≥ 15 letters, comparing contrast sensitivity, reading speed, and CMT between the 2 arms at 92 weeks Adverse events: yes Intervals at which outcome assessed: q12 wks for 92 weeks |
|
| Notes |
Full study name: The Greater Manchester Avastin for Neovascularisation Study Trial registration: ISRCTN 34221234 and EudraCT number 2007‐003853‐97 Funding sources: quote: "Supported by Greater Manchester Primary Care Trusts, National Health Service, England, and Manchester Biomedical Research Centre." Declarations of interest: quote: "The author(s) have made the following disclosure(s): S.M.: Advisory boards of and financial support _ Novartis and Bayer. T.M.A: Advisory boards of and financial support _ Novartis and Bayer." Study period: February 2008 to May 2013 Subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated allocation lists were drawn up by the trial statistician using block randomization with a variable block size." |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer‐generated allocation lists were drawn up by the trial statistician using block randomization with a variable block size." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "patients, treating clinicians, and other staff involved in the study were not masked." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The optometrists who measured BCVA, reading speed, and contrast sensitivity were masked to the study arm." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up reported and balanced in the 2 comparison groups. |
| Selective reporting (reporting bias) | Low risk | Compared with the trial registries, there did not appear to be selective outcome reporting. |
HARBOR 2013.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized (total and per group): 1098 total participants; 276 in 0.5 mg monthly group; 275 in 0.5 mg PRN group; 274 in 2.0 mg monthly group; 273 in 2.0 mg PRN group Exclusions after randomization: 1 participant was randomized before screen failure, and no baseline or postbaseline data were reported for this participant; therefore, the participant was excluded from analysis. Number analyzed (total and per group): 1098 total participants; 275 in 0.5 mg monthly group; 275 in 0.5 mg PRN group; 274 in 2.0 mg monthly group; 273 in 2.0 mg PRN group Unit of analysis: participant (1 study eye per participant) Losses to follow‐up: discontinued study: 2 in 0.5 mg monthly group; 2 in 0.5 mg PRN group; 2 in 2.0 mg monthly group; 2 in 2.0 mg PRN group. Discontinued treatment: 2 in 0.5 mg monthly group; 2 in 0.5 mg PRN group; 3 in 2.0 mg monthly group; 3 in 2.0 mg PRN group Compliance: not reported Intention‐to‐treat analysis: yes Reported power calculation: yes, 80% power in the intention‐to‐treat analysis for the 3 primary comparisons Study design comment: none |
|
| Participants |
Country: 100 study centers across the US Age: 0.5 mg monthly: mean 78.8 years (SD 8.4; range 53.0–97.0); 0.5 mg PRN: mean 78.5 years (SD 8.3; range 53.0–97.0); 2.0 mg monthly: mean 79.3 years (SD 8.3; range 50.0–96.0); 2.0 mg PRN: mean 78.3 years (range 54.0–98.0) Gender (%): 0.5 mg monthly: 113 (41.1%) men and 162 (58.9%) women; 0.5 mg PRN: 112 (40.7%) men and 163 (59.3%) women; 2.0 mg monthly: 104 (38.0%) men and 170 (62.0%) women; 2.0 mg PRN: 117 (42.9%) men and 156 (57.1%) women Inclusion criteria: ages ≥ 50 years; BCVA 20/40–20/320 (Snellen equivalent), using ETDRS charts (at 4 m); active subfoveal lesions with classic CNV, some classic CNV component, or purely occult CNV; total area of lesion 12 disk areas or 30.48 mm2; and total CNV area constituted 50% of total lesion area based on FA. For the inclusion of purely occult or occult with some classic CNV, activity of the lesion had to be demonstrated by 1 of several criteria including 10% increase in CNV lesion size on interval visits, documented visual loss of 1 line of Snellen vision, or presence of hemorrhage at presentation Exclusion criteria: history of vitrectomy surgery; prior treatment with PDT with verteporfin, external beam radiation therapy, or transpupillary thermotherapy; previous intravitreous drug delivery; previous subfoveal laser photocoagulation; uncontrolled blood pressure; atrial fibrillation not managed by the participant's primary care physician or cardiologist within 3 months of the screening visit; or history of stroke within 3 months of the screening visit. Equivalence of baseline characteristics: yes, quote: "All variables were well balanced among the 4 treatment groups." Diagnoses in participants: approximately 46% of participants had minimally classic CNV lesions, 16% had predominantly classic lesions, and 38% had purely occult CNV. |
|
| Interventions |
Intervention 1: ranibizumab 0.5 mg monthly Intervention 2: ranibizumab 0.5 mg PRN Intervention 3: ranibizumab 2.0 mg monthly Intervention 4: ranibizumab 2.0 mg PRN Follow‐up: 12 months Frequency of assessments for retreatment: at month 3 visit and thereafter |
|
| Outcomes |
Primary outcome, as defined: mean change from baseline in BCVA at month 12 Secondary outcomes, as defined: mean number of ranibizumab injections up to, but not including, month 12; mean change from baseline in CFT based on SD‐OCT over time to month 12; proportion of participants who gained 15 letters from baseline in BCVA at month 12 Adverse events (Y/N): yes Intervals at which outcome assessed: safety and ocular parameters assessed on day 7; subsequently, all participants had scheduled monthly visits for evaluation of safety and efficacy. FA and fundus photography were performed at screening and at months 3, 6, and 12. |
|
| Notes |
Full study name: not reported Type of study: published Trial registration: NCT00891735 Funding sources: Genentech, Inc. (South San Francisco, CA) provided support for the study and participated in the study design; conducting the study; and data collection, management, and interpretation. Declarations of interest: quote: "B.G.B. has served as a consultant for Alimera, Elan, Genentech, Synergetics, and Thrombogenics; has received research funding from Genentech; is a member of the speakers bureau for Genentech and Regeneron; and has received royalties from AKORN. A.C.H. has served as a consultant for Alcon, Allergan, Centocor/Johnson & Johnson, Genentech, Merck, NeoVista, Ophthotech, Oraya, Paloma, PRN, QLT, Regeneron, and Thrombogenics; has received research funding from Alcon, Allergan, Genentech, National Eye Institute/ National Institutes of Health, NeoVista, Ophthotech, Oraya, PRN, QLT, Regeneron, and Second Sight; and is a member of the speakers bureau for Alcon, Genentech, and Regeneron. D.M.B. has served as a consultant for Alcon, Alimera, Allergan, Genentech, Novartis, Regeneron, and Thrombogenics; has received research funding from Abbott, Alcon, Alimera, Allergan, Eli Lilly, Genentech, GlaxoSmithKline, Ophthotech, Novartis, Regeneron, and Thrombogenics; and is a member of the speakers bureau for Genentech and Regeneron. J.S.H. has served as a consultant for Acucela, Allergan, Bayer, Forsight, Fovea, Genentech, Genzyme, GlaxoSmithKline, LPath, Neovista, Oraya, Paloma, QLT, Quark, and Regeneron; and has received research funding from Alcon, Alimera, Allergan, Fovea, Genentech, Genzyme, GlaxoSmithKline, Neovista, Neurotech, Novartis, Ophthalmic Consultants of Boston, Ophthotech, Paloma, and Regeneron. I.J.S. has served as a consultant for Genentech, Eyetech, Regeneron, and Thrombogenics; has received research funding from Genentech; is a member of the speakers bureau for Genentech, Optos, and Regeneron; and is a board member of Optos. Z.L., R.G.R., and P.L. are employees of Genentech. Support for third‐party writing assistance for this manuscript provided by Linda Merkel, PhD, and Michelle Kelly, PhD, of UBC‐Envision Group, and was provided by Genentech, Inc." Study period: recruitment from July 2009 and August 2010 Reported subgroup analyses: no HARBOR 2013: 2 intervention groups using other doses not analyzed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "each patient received a computer‐generated subject number on day 0, which randomly assigned patients in a 1:1:1:1 ratio to 1 of 4 ranibizumab treatment groups: 0.5 mg monthly, 0.5 mg PRN, 2.0 mg monthly, and 2.0 mg PRN." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was stratified by VA at day 0 (≤54 letters [approximate Snellen equivalent <20/80] vs. ≥55 letters [approximate Snellen equivalent ≥20/80]), CNV classification at baseline (predominantly classic, minimally classic, or purely occult), and study center." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "All study site personnel, the designated physician(s), central reading center personnel, patients, and the sponsor and its agents were masked to treatment drug dose assignment (0.5 mg vs. 2.0 mg). Treatment frequency (ie, monthly vs. PRN dosing) was not masked to patient and site personnel." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All study site personnel, the designated physician(s), central reading center personnel, patients, and the sponsor and its agents were masked to treatment drug dose assignment (0.5 mg vs. 2.0 mg). Treatment frequency (ie, monthly vs. PRN dosing) was not masked to patient and site personnel." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were balanced and similar in the 2 groups. |
| Selective reporting (reporting bias) | Low risk | Compared with the trial registry, there did not appear to be selective outcome reporting. |
IVAN 2012b.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized (total and per group): Drug randomization: 628 total participants; 305 in bevacizumab group and 323 in ranibizumab group Regimen randomization: 294/305 in bevacizumab group and 312/323 in ranibizumab group completed first 3 injections and were randomized to continue or discontinue treatment: 149 continued bevacizumab; 145 discontinued bevacizumab; 157 continued ranibizumab; and 155 discontinued ranibizumab Exclusions after randomization: 18 participants did not receive treatment and were excluded after randomization to drug treatment (9 in bevacizumab group and 9 in ranibizumab group). Number analyzed (total and per group): At 1 year' follow‐up: 561 total participants; 136 in continued bevacizumab group; 138 in discontinued bevacizumab group; 141 in continued ranibizumab group; and 146 in discontinued ranibizumab group At 2 years' follow‐up: 525 total participants; 127 in continued bevacizumab group; 127 in discontinued bevacizumab group; 134 in continued ranibizumab group; and 137 in discontinued ranibizumab group Unit of analysis: participant (1 study eye per participant) Losses to follow‐up: At 1 year' follow‐up: 49 total participants: 4 participants receiving treatment withdrew prior to completing 3rd injection (2 in bevacizumab group and 2 in ranibizumab group); 45 participants randomized to regimen groups exited trial before 1 year (13 in continued bevacizumab group; 7 in discontinued bevacizumab group; 16 in continued ranibizumab group; and 9 in discontinued ranibizumab group) At 2 years' follow‐up: 85 total participants: 5 participants receiving treatment withdrew prior to completing 3rd injection (3 in bevacizumab group and 2 in ranibizumab group); 80 participants randomized to regimen groups exited trial before 2 years (21 in continued bevacizumab group; 18 in discontinued bevacizumab group; 23 in continued ranibizumab group; and 18 in discontinued ranibizumab group) Compliance: the wrong study drug was administered twice during the first year At 1 year' follow‐up: adherence was 6576/6699 (98%) scheduled injections received At 2 years' follow‐up: adherence was 12761/14640 (87%) scheduled injections received Intention‐to‐treat analysis: no, 67 participants enrolled and randomized were not included in the analyses at 1 year and 103 at 2 years. Reported power calculation: yes, sample of 600 participants per group for power of 90% to detect non‐inferiority Study design comment: non‐inferiority design; 2 × 2 factorial design – randomization in 2 stages: first randomized to drug treatment (bevacizumab or ranibizumab), then to treatment regimen (continue monthly injections or discontinue monthly injections and switch to PNR injections given in 3 month cycles); results reported only as bevacizumab vs ranibizumab and continuous vs discontinuous |
|
| Participants |
Country: UK (23 study centers) Age: mean age for 610 participants receiving treatment was 78 years Gender (%): 366/610 (60%) women and 244/610 (40%) men Inclusion criteria: ages ≥ 50 years; previously untreated nAMD in study eye with any component of the neovascular lesion (CNV, blood, serous PED, elevated blocked fluorescence) involving the center of fovea, confirmed by FA; BCVA ≥ 25 letters on ETDRS chart (measured at 1 m) Exclusion criteria: neovascular lesion ≥ 50% fibrosis or blood; > 12 disk diameters; argon laser treatment in study eye within 6 months; presence of thick blood involving the center of fovea; presence of other active ocular disease causing concurrent vision loss; myopia ≥ 8 diopters; previous treatment with PDT or a VEGF inhibitor in study eye; women pregnant, lactating, or of child‐bearing potential; men with a spouse or partner of child‐bearing potential Equivalence of baseline characteristics: yes Diagnoses in participants: 301/610 (58%) had nAMD with CNV in foveal center; 308/610 (54%) had fluid in foveal center; 90/610 (16%) had hemorrhage in foveal center; 75/610 (13%) had other foveal center involvement; and 15/610 (3%) had no CNV or not possible to grade |
|
| Interventions |
Intervention 1: intravitreous bevacizumab 1.25 mg in 0.05 mL injected monthly for 2 years Intervention 2: intravitreous ranibizumab 0.5 mg injected monthly for 2 years Intervention 3: after first 3 monthly intravitreous bevacizumab 1.25 mg injections, monthly treatment was discontinued, and treatment was given PNR in cycles of 3 monthly doses Intervention 4: after first 3 monthly intravitreous ranibizumab 0.5 mg injections, monthly treatment was discontinued, and treatment was given PNR in cycles of 3 monthly doses Follow‐up: 2 years Frequency of follow‐up assessments: monthly |
|
| Outcomes |
Primary outcome, as defined: BCVA measured as ETDRS letters at 2 years Secondary outcomes, as defined in protocol: at 1 year' and 2 years' follow‐up: frequencies of adverse effects of treatment; generic and vision‐specific health‐related quality of life; treatment satisfaction; cumulative resource use/cost and cost‐effectiveness; clinical measures of vision (contrast sensitivity measured with Pelli‐Robson charts, near VA measured by Bailey‐Love near reading cards, and reading speed measured with Belfast reading charts); lesion morphology (FA and OCT); distance VA at 1 year; survival free from treatment failure Exploratory analysis: association between serum markers and cardiovascular serious adverse events Intervals at which outcomes were assessed: monthly through 24 months; various data were collected at every visit depending on assessment schedule and regimen group |
|
| Notes |
Full study name: alternative treatments to Inhibit VEGF in age‐related choroidal neovascularisation Trial registration: ISRCTN92166560. Type of study: published Funding sources: National Institute for Health Research Health Technology Assessment programme, UK Declarations of interest: various authors reported being principal investigators of trials sponsored by Novartis; attending and being remunerated for attendance at advisory boards for Novartis, Bayer, Neovista, Oraya, Allergan, Bausch and Lomb, or a combination; being employed by institution that has received payments from Novartis, Bayer, Neovista, Oraya, Alcon, Pfizer, or a combination; receiving honoraria from Novartis for lecture or teaching fees from Janssen‐Cilag, or both Study period: random enrollment 27 March 2008 to 15 October 2010 Reported subgroup analyses: 3 genetic polymorphisms (Lotery 2013 under IVAN 2012b) Contacting study investigators: trial authors not contacted as data were available in published reports. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized allocations were computer generated by a third party in blocks and stratified by center." Quote: "Randomisation was stratified by centre and was blocked to ensure roughly equal numbers of participants per group within a centre." |
| Allocation concealment (selection bias) | Low risk | Quote: "Research teams at sites recruited participants, and accessed a password‐protected website to randomize participants. Allocations were concealed until participants' eligibility and identities were confirmed." Quote: "Allocations were computer generated and concealed with an internet‐based system (Sealed Envelope, London, UK). Staff in participating centres accessed the website and, on entering information to confirm a participant's identity and eligibility, were provided with the unique study number." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | From study protocol: Quote: "Participants, clinicians and trial personnel will be masked to the VEGF inhibitor to which a participant is assigned." Quote: "We have chosen not to mask participants, clinicians and trial personnel to whether patients are allocated to continue or stop treatment at 3 months." Quote: "We intended that drug allocation should be concealed by having separate masked assessment and unmasked treating teams. This system was achieved by 14 sites. At the other 9 sites, staffing levels could not support this system and an unmasked staff member prepared ranibizumab in a syringe identical to those containing bevacizumab and did not perform assessments." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "We intended that drug allocation should be concealed by having separate masked assessment and unmasked treating teams. This system was achieved by 14 sites. At the other 9 sites, staffing levels could not support this system and an unmasked staff member prepared ranibizumab in a syringe identical to those containing bevacizumab and did not perform assessments." Quote: "Lesion morphology was assessed by independent graders masked to drug and treatment regimen." From study protocol: Quote: "We have chosen not to mask participants, clinicians and trial personnel to whether patients are allocated to continue or stop treatment at 3 months." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 67/628 (11%) participants randomized were not included in the 1‐year analysis; 111/628 (18%) participants randomized were not included in the 2‐year analysis. |
| Selective reporting (reporting bias) | Unclear risk | Differences between the protocol and published 1‐year and 2‐year results papers included: 2 secondary outcomes in the protocol were not listed in paper: treatment satisfaction and survival free from treatment failure; and exploratory (serum) analysis in protocol upgraded to a secondary outcome in paper. |
Lushchyk 2013.
| Study characteristics | ||
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized (total and per group): 191 total participants; 64 in the q8 wks group; 63 in the q6 wks group; 64 in the q4 wks group Exclusions after randomization: 2 participants due to lack of evidence of CNV Number analyzed (total and per group): 54 in the q8 wks group; 57 in the q6 wks group; 46 in the q4 wks group for efficacy analysis Unit of analysis: participant (1 study eye per participant) Losses to follow‐up: 18 (28.1%) in the intravitreous bevacizumab q4 wks group; 6 (9.5%) in the intravitreous bevacizumab q6 wks group; 10 (15.6%) in the intravitreous bevacizumab q8 wks group Intention‐to‐treat analysis: no, participants with missing data excluded from analyses Power calculation: yes; 80% Study design comment: single‐center trial |
|
| Participants |
Country: Netherlands Mean age: 77 years Gender (%): men 18 (28.1%) and women 46 (71.9%) in the intravitreous bevacizumab q4 wks group; men 25 (39.7%) and women 38 (60.3%) in the intravitreous bevacizumab q6 wks group; men 21 (32.8%) and women 43 (67.2%) in the intravitreous bevacizumab q8 wks group Inclusion criteria: ages ≥ 65 years; VA 20/200 to 20/20 (Snellen equivalent) assessed using the ETDRS VA charts; previously untreated active CNV due to AMD; presence of active leakage to establish active CNV defined as a leakage observed using FA and indocyanine green angiography, and the presence of fluid, observed using SD‐OCT, located either below the retina or below the RPE Exclusion criteria: other significant ocular disorders affecting visual; allergy to either FA and indocyanine green dye injections was known; immunocompromised people or people with an ocular surgery planned during the 1‐year follow‐up period; people who used coumarin derivatives at the time of inclusion and people who experienced clinically significant cerebrovascular accident or MI in the 6 months prior to planned inclusion Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: intravitreous bevacizumab (1.25 mg bevacizumab in a 0.05 mL solution) q4 wks Intervention 2: intravitreous bevacizumab (1.25 mg bevacizumab in a 0.05 mL solution) q6 wks Intervention 3: intravitreous bevacizumab (1.25 mg bevacizumab in a 0.05 mL solution) q8 wks Follow‐up: 1 year Frequency of assessments for retreatment: q12 wks in addition to regular injection visits |
|
| Outcomes |
Primary outcome, as defined: BCVA Secondary outcomes, as defined: fluid and foveal thickness on SD‐OCT Adverse events: yes Intervals at which outcome assessed: q12 wks |
|
| Notes |
Full study name: not reported Trial registration: NTR117 Funding sources: not reported Declarations of interest: not reported Study period: June 2008 to March 2011 Subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 'Open‐label' study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 'Open‐label' study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imbalance in losses to follow‐up across groups: 34 (17.8%) participants [18 (28.1%) in the intravitreous bevacizumab q4 wks group; 6 (9.5%) in the intravitreous bevacizumab q6 wks group; 10 (15.6%) in the intravitreous bevacizumab q8 wks group] were not included in the final efficacy analysis. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported in the final report. |
NATTB 2012.
| Study characteristics | ||
| Methods |
Study design: randomized controlled trial Number randomized (total and per group): 13 centers, 185 participants in total; 91 in the intervention 1; 94 in the intervention 2 Exclusions after randomization: none reported Number analyzed (total and per group): 79 eyes (86.8%) in the intervention 1; 82 eyes (87.2%) in the intervention 2 Unit of analysis: participant (1 study eye per participant) Losses to follow‐up: not reported Intention‐to‐treat analysis: no Power calculation: none reported Study design comment: none reported |
|
| Participants |
Country: China Age: median 67 years in the intervention 1; median 70 years in the intervention 2 Gender (%): men 60 (65.9%) and women 31 (34.4%) in the intervention 1; men 62 (66.0%) and women 32 (34.0%) in the intervention 2 Inclusion criteria: ages ≥ 50 years; previously untreated active CNV (determined by the presence of leakage, as seen on FA, and by the presence of fluid, as seen on OCT, located either within or under the neurosensory retina or under the RPE) resulting from AMD; lesion area ≤ 12 disk areas, and BCVA 5–73 letters using ETDRS charts Exclusion criteria: presence of a macular scar, CNV not resulting from AMD, and polypoidal choroidal vasculopathy Equivalence of baseline characteristics: yes |
|
| Interventions |
Intervention 1: intravitreous bevacizumab (1.25 mg bevacizumab in 0.05 mL solution) q6 wks for 8 injections Intervention 2: intravitreous bevacizumab (1.25 mg bevacizumab in 0.05 mL solution) q6 wks for the first 3 injections, followed by injections q12 wks for the last 2 injections Follow‐up: 48 weeks Frequency of assessments for retreatment: not reported |
|
| Outcomes |
Primary outcome, as defined: mean change in VA Secondary outcomes, as defined: proportion of participants with a change in VA ≥ 15 letters; number of injections; change in CRT on OCT; incidence of ocular and systemic adverse events; and annual drug cost Adverse events: yes Intervals at which outcome assessed: q6 wks |
|
| Notes |
Full study name: Bevacizumab for Neovascular Age‐related Macular Degeneration in China Trial registration: NCT01306591 Funding sources: quote: "Supported by the National Key Technology Research and Development Program in the 11th Five‐Year Plan of China (no. 2006BAI02B05)." Declarations of interest: quote: "The author(s) have no proprietary or commercial interest in any materials discussed in this article." Study period: January 2008 to January 2010 Subgroup analyses: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 'Open‐label' study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Visual acuity examiners and imaging technicians were unaware of study group assignment." Quote: "A medical monitor who was unaware of study group assignments reviewed all adverse event data;" masking of other outcome assessors not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Balanced losses to follow‐up but causes not reported: 24 (13.0%) participants (12 [13.2%] in the intravitreous bevacizumab q6 wks group; 12 [12.8%] in the intravitreous bevacizumab q6 wks followed by q12 wks group) were not included in the final efficacy analysis. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in the final report. |
Sarraf 2013.
| Study characteristics | ||
| Methods | In this study, the results were reported for subgroup, not based on intervention groups. It was unclear if this study was eligible for the review Study design: parallel‐group randomized controlled trial Number randomized (total and per group): 37 eyes of 37 participants in total; number per group not reported Exclusions after randomization: none reported Number analyzed (total and per group): 37 eyes of 37 participants in total; number per group not reported Unit of analysis: participant (1 study eye per participant) Losses to follow‐up: none reported Intention‐to‐treat analysis: not reported Reported power calculation: no Study design comment: multicenter (3 centers) trial; the results were reported for subgroup analysis (tear vs non‐tear group), not based on the randomized intervention groups |
|
| Participants |
Country: USA Age: not reported Gender: not reported Inclusion criteria: ages ≥ 50 years; vascularized PED secondary to exudative AMD, PED ≤ 12‐disk area in size; BCVA with ETDRS ≥ 19 and ≤ 69 letters (20/400 to 20/40), and surface area of any associated submacular hemorrhage or fibrosis occupying < 50% of entire PED Exclusion criteria: anti‐VEGF therapy within the past 30 days; > 3 previous anti‐VEGF injections; > 1 previous PDT session; previous AMD treatment (excluding minerals and vitamins) in the past 30 days; YAG laser in past 30 days; previous intravitreous triamcinolone therapy in the past 30 days; previous intravitreous dexamethasone therapy in the past 6 months; history of pars plana vitrectomy Equivalence of baseline characteristics: not reported by intervention groups |
|
| Interventions |
Intervention 1: intravitreous ranibizumab 0.5 mg monthly for 12 months Intervention 2: intravitreous ranibizumab 0.5 mg monthly for 4 months (months 0, 1, 2, and 3) followed by PRN treatment according to predefined criteria Intervention 3: intravitreous ranibizumab 2.0 mg monthly for 12 months Intervention 4: intravitreous ranibizumab 2.0 mg monthly for 4 months (months 0, 1, 2, and 3) followed by PRN treatment according to predefined criteria Follow‐up: 12 months Frequency of assessments for retreatment: monthly |
|
| Outcomes |
Primary outcome, as defined: mean change in BCVA ETDRS; incidence in postinjection RPE tears; PED Secondary outcomes, as defined: not distinguished Adverse events: no Intervals at which outcome assessed: BCVA and OCT measurements monthly; fundus photography and FA months 0, 1, 2, 3, 6, 9, and 12 |
|
| Notes |
Full study name: not reported Trial registration: not reported Funding sources: quote: "Supported by an Investigator‐Supported Trial grant from Genentech and by a grant (D.S.) from the Karl Kirchgessner Foundation at the Jules Stein Eye Institute." Declarations of interest: not reported Study period: not reported Subgroup analyses: tear vs non‐tear for development of RPE tear Sarraf 2013: 2 intervention groups using other doses not analyzed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 'Open‐label' study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 'Open‐label' study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. |
TREND 2017.
| Study characteristics | ||
| Methods |
Number randomized (total and per group): treatment: 323 participants (323 eyes) with ranibizumab 0.5 mg, treat and extend; control: 327 participants (327 eyes) with ranibizumab 0.5 mg, monthly; total: 650 Exclusions after randomization: adverse effects (9 in treatment, 2 in control), consent withdrawal (14 in treatment, 17 in control), death (3 in treatment, 4 in control), protocol deviation (1 in treatment, 2 in control), physician's decision (1 in treatment, 3 in control) Number analyzed (total and per group): treatment: 290, control: 295, total: 585 Unit of analysis: participant (1 eye per participant) Losses to follow‐up: 5 in treatment, 4 in control Compliance: not explicitly reported, but participants were administered treatment on visits Intention‐to‐treat analysis: no; participants who were excluded after randomization and lost to follow‐up were not included in analysis. Reported power calculation: quote: "Assuming a standard deviation (SD) of 15 ETDRS letters in the treat‐and‐extend and monthly groups, with a difference of 1.5 in mean change in BCVA from baseline in favor of the monthly regimen, and by applying an ANCOVA [analysis of covariance] model, a sample size of 322 patients per treatment group was considered (to account for loss of information resulting from missing data, the sample size was increased by 10% from 290 to 322). With this sample size, the resulting power for ANCOVA was 80% to establish noninferiority of the treat‐and‐extend regimen versus the monthly regimen at a 1‐sided 2.5% level for a noninferiority margin of 5 letters." Study design comment: not available |
|
| Participants |
Country: 18 countries: Belgium, Croatia, Denmark, Egypt, Germany, Hungary, India, Israel, Italy, Korea, Portugal, Russia, Slovakia, Slovenia, Spain, Switzerland, Turkey, UK Age: treatment: mean 75.3 years (SD 8.61); control: mean 75.2 years (SD 8.13); overall: mean 75.2 years Gender (%): treatment: 55.4% women; control: 55.4% women Inclusion criteria: treatment‐naïve participants ages ≥ 50 years with visual impairment resulting from active CNV secondary to AMD confirmed by presence of active leakage of CNV detected by FA, color fundus photography, or both; total area of fibrosis comprising < 50% of the lesion area and BCVA score 23–78 ETDRS letters at 4 m (approximately 20/32–20/320 Snellen equivalent). Exclusion criteria: any type of advanced, severe, or unstable disease, including any medical condition that could bias assessment or put the participant at special risk; history of stroke or MI within 3 months before screening or an uncontrolled systolic blood pressure > 160 mmHg or diastolic blood pressure > 100 mmHg; prior treatment of the study eye with anti‐VEGF or verteporfin PDT or corticosteroids within 6 months before screening or intraocular surgery within 3 months before screening; history of focal/grid laser photocoagulation with involvement of the macular area; or uncontrolled glaucoma or atrophy or fibrosis in study eye. Equivalence of baseline characteristics: quote: "Patient demographic and baseline ocular characteristics were well balanced between the 2 treatment groups." Diagnoses in participants: AMD, CNV |
|
| Interventions |
Intervention 1: treat‐and‐extend group received 2 initial monthly ranibizumab injections at baseline (day 1) and month 1. After 1 month, visits in the treat‐and‐extend group were scheduled based on disease activity as assessed by VA and OCT criteria. Participants were treated at monthly intervals until disease activity was resolved, as assessed by SD‐OCT according to the investigator's judgment (i.e. no intraretinal or subretinal fluid). If disease activity was not present, the next visit was scheduled in 6 weeks (i.e. the treatment interval, defined as the period between 2 ranibizumab injections, was extended by 2 weeks); however, if disease activity was present, the interval to the next visit was not extended and thus was scheduled in 4 weeks (1 month). The treatment interval could be extended by 2 weeks at each visit as long as there was no disease activity, with a maximum of a 12‐week treatment interval. During the course of the study, if disease activity was present, the treatment interval was shortened by 2 weeks, but never to fewer than 4 weeks. The participant was treated at this interval until no disease activity was present, after which an extension of 2 weeks was reactivated. The possibility to extend the interval between treatments was limited to 2 attempts. If disease activity recurred, the visit schedule was shortened by 2 weeks and fixed on this interval up to the end of the study. However, if disease activity was present along with visual impairment, the treatment interval was allowed to shorten by 4 weeks instead of 2 weeks based on the investigator's judgment. Intervention 2: monthly regimen group, treatment visits were scheduled at monthly intervals up to the end of the study. Follow‐up: planned length: not stated; actual length: 12 months Frequency of assessments for retreatment: monthly |
|
| Outcomes |
Primary outcome, as defined: change in BCVA from baseline to end of the study Secondary outcomes, as defined: change in retinal central subfield thickness from baseline to end of study, treatment exposure, and safety Adverse events (Y/N): yes: increased IOP, conjunctival hemorrhage, reduced VA, nasopharyngitis, hypertension, influenza, bronchitis, endophthalmitis Intervals at which outcome assessed: baseline, end of study |
|
| Notes |
Full study name: Treat‐and‐Extend versus Monthly Regimen in Neovascular Age‐Related Macular Degeneration Type of study: published Trial registration: NCT01948830 Funding sources: Novartis Pharma AG(Basel, Switzerland) Declarations of interest: RS: consultant – Allergan, Alimera, Alcon, Bayer, Novartis, THEA; financial support – Bayer, Alimera, Angelini, THEA, Allergan, Novartis ML: consultant, lecturer, financial support (to institution) – Novartis, Allergan, Alcon, Roche WM: employee – Novartis Pharma AG (Basel, Switzerland) CF: employee – Novartis Pharma AG (Basel, Switzerland) JM: consultant – Novartis, Allergan, Bayer, Alcon, Ophthotech, Notal Vision, Alimera, Genentech; financial support – Novartis, Bayer, Alcon, Ophthotech, Roche; lecturer – Novartis, Allergan, Ophthotech Study period: enrollment December 2013 through November 2015 Reported subgroup analyses (Y/N): if yes, specify no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Masking of participants not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "In this study, the VA assessor who assessed the parameters for the primary end point was masked to the treatment regimen and was not allowed to perform any additional study tasks." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The primary analysis was performed on the full analysis set using the last observation carried forward principle for imputing missing BCVA values at the end of the study. The full analysis set comprised all patients to whom a treatment regimen was assigned." |
| Selective reporting (reporting bias) | High risk | Some of the prespecified outcomes (e.g. NEI VFQ‐25) were not presented. |
TREX‐AMD 2015.
| Study characteristics | ||
| Methods |
Number randomized (total and per group): 60 total participants; 40 to TREX group and 20 to monthly group Exclusions after randomization: none reported Number analyzed (total and per group): 57 total participants; 37 in the TREX group and 20 in the monthly group Unit of analysis: participant (1 study eye per participant) Losses to follow‐up: 3 participants (all in the TREX group; due to temporal arteritis, lung cancer, or meningitis) Intention‐to‐treat analysis: no, 3 participants not included in analysis Power calculation: yes, quote: "we calculated an a priori power of 42% to detect noninferiority (significance 5%, 1‐sided). TREX‐AMD 1 year post‐hoc analysis demonstrated a power of 88%." Study design comment: quote: "randomized 1:2, utilizing a noninferiority limit of 5 ETDRS letters and the 12.5 ETDRS letter standard deviation reported in the LUCAS trial." |
|
| Participants |
Country: USA (2 centers) Mean age: 77 years (range 59–96 years) Gender (%): 38 (63%) women and 22 (37%) men Inclusion criteria: treatment‐naïve CNV secondary to exudative AMD with ETDRS BCVA 78–18 (Snellen equivalent, 20/32–20/500) determined by protocol trial lens refraction, and total area of subretinal hemorrhage and fibrosis comprising < 50% of the total lesion. Exclusion criteria: not reported Equivalence of baseline characteristics: could not determine; baseline by group not reported Diagnoses in participants: CNV secondary to exudative AMD |
|
| Interventions |
Intervention 1: intravitreous ranibizumab 0.5 mg in 0.05 mL, monthly for first 3 months, then treat‐and‐extend protocol (quote: "interval between treatments was tailored based on exudative disease activity: eyes were treated at each visit, no more frequently than every 4 weeks and no less frequently than every 12 weeks") Intervention 2: intravitreous ranibizumab 0.5 mg in 0.05 mL, monthly for 1 year Follow‐up: 1 year reported, 2 years planned Frequency of assessments for retreatment: every 1–4 weeks, based on exudative disease activity in the TREX group |
|
| Outcomes |
Primary outcome, as defined: ETDRS BCVA change from baseline Secondary outcomes, as defined: mean change in CRT by SD‐OCT, total number of intravitreous injections, percentage of participants with persistent exudative disease activity by SD‐OCT, percentage of participants gaining or losing 10 or 15 ETDRS letters at month 12, and the incidence and severity of ocular and systemic adverse events Adverse events (Y/N): yes Intervals at which outcome assessed: every month for 12 months |
|
| Notes |
Full study name: The Treat‐and‐Extend Protocol in Patients with Wet Age‐Related Macular Degeneration Type of study: published Trial registration: NCT01748292 Funding sources: quote: "Supported by Genentech, Inc., South San Francisco, California. The funding organization had no role in the design or conduct of this research." Declarations of interest: quote: "The author(s) have no proprietary or commercial interest in any materials discussed in this article: C.C.W.: Research support – Alcon, Allergan, Genentech, Regeneron; Consultant – Alcon, Allergan, Bayer, Genentech, Regeneron; Lecturer – Allergan, Genentech, Regeneron. D.M.B.: Research support – Alcon, Allergan, Genentech, Regeneron; Consultant – Alcon, Allergan, Bayer, Genentech, Regeneron; Lecturer – Bayer, Roche. L.C.: Research support – Genentech; Consultant – Regeneron; Lecturer – Regeneron, Genentech, Bayer; Travel – Bayer, Regeneron, Genentech. J.F.P.: Research support – Genentech. S.S.: Research support – Genentech, Carl Zeiss Meditec, Optos, Allergan; Personal fees – Genentech, Carl Zeiss Meditec, Optos, Allergan, Roche, Novartis, Alcon, Iconic." Study period: February 2013 through January 2014 Reported subgroup analyses (Y/N): none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. Quote: "The Treat‐and‐Extend Protocol in Patients with Wet Age‐Related Macular Degeneration (TREX‐AMD) is a phase IIIb, multicenter, randomized, controlled clinical trial." |
| Allocation concealment (selection bias) | Low risk | Quote: "At enrollment, patients were randomized sequentially by a blinded study coordinator to the monthly or TREX cohort." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/60 (5%) participants lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Trial planned for 2 years; results at 1 year reported (study ongoing). |
VIEW 2012.
| Study characteristics | ||
| Methods |
Study design: 2 parallel‐group randomized controlled trials Number randomly assigned: 2457 total participants (2457 eyes); 615 in aflibercept 0.5 mg q4 wks group (excluded); 617 in aflibercept 2.0 mg q4 wks group; 616 in aflibercept 2.0 mg q8 wks group; 609 in ranibizumab group. Exclusions after randomization: Full analysis – 45 total participants: 4 in aflibercept 2.0 mg q4 wks group; 9 in aflibercept 2.0 mg q8 wks group; 14 in ranibizumab group. Safety analysis – 38 total participants: 4 in aflibercept 2.0 mg q4 wks group; 6 in aflibercept 2.0 mg q8 wks group; 14 in ranibizumab group. Losses to follow‐up: 251 participants discontinued treatment at 1‐year follow‐up: 53 in aflibercept 2.0 mg q4 wks group; 63 in aflibercept 2.0 mg q8 wks group; 60 in ranibizumab group. Number analyzed: Full analysis – 2412 total participants at 1‐year follow‐up: 613 in aflibercept 2.0 mg q4 wks group; 607 in aflibercept 2.0 mg q8 wks group; 595 in ranibizumab group. Safety analysis – 2419 total participants at 1‐year follow‐up: 613 in aflibercept 2.0 mg q4 wks group; 610 in aflibercept 2.0 mg q8 wks group; 595 in ranibizumab group. Unit of analysis: participant (1 study eye per participant) How were missing data handled? missing values imputed using last observation carried forward approach Power calculation: none reported |
|
| Participants |
Country: US and Canada (154 study sites) and Argentina; Australia; Austria; Brazil; Belgium; Colombia; Czech Republic; France; Germany; Hungary; India; Israel; Italy; Japan; Latvia; Mexico; Netherlands; Poland; Portugal; South Korea; Singapore; Slovakia; Spain; Sweden; Switzerland; UK (172 study sites) Mean age (range not reported): 78 years in aflibercept 2.0 mg q4 wks group, 78 years in aflibercept 2.0 mg q8 wks group, and 78 years in ranibizumab group, 74 years in aflibercept 2.0 mg q4 wks group, 74 years in aflibercept 2.0 mg q8 wks group, and 73 years in ranibizumab group Gender: 110 men (36.2%) and 194 women (63.8%) in aflibercept 2.0 mg q4 wks group, 123 men (40.9%) and 178 women (59.1%) in aflibercept 2.0 mg q8 wks group, and 132 men (43.4%) and 172 women (56.6%) in ranibizumab group, 133 men (43.0%) and 176 women (57.0%) in aflibercept 2.0 mg q4 wks group, 131 men (42.8%) and 175 women (57.2%) in aflibercept 2.0 mg q8 wks group, and 122 men (41.9%) and 169 women (58.1%) in ranibizumab group Inclusion criteria: ages ≥ 50 years; diagnosed with nAMD in the study eye; active subfoveal CNV lesions of any subtype (12 optic disk areas or smaller) constituting ≥ 50% of total lesion size; BCVA 73–25 ETDRS chart letters (20/40–20/320 Snellen equivalent); willingness and ability to return for clinic visits and complete study‐related procedures; ability to provide informed consent Exclusion criteria: prior or concomitant treatment for AMD in study eye; prior treatment with anti‐VEGF therapy; subretinal hemorrhage or scar or fibrosis constituting > 50% of total lesion size or involving the center of the fovea in study eye; RPE tears or rips involving the macula in study eye; history of other ocular conditions such as vitreous hemorrhage, retinal detachment, macular hole, corneal transplant, corneal dystrophy, diabetic retinopathy, diabetic macular edema, uveitis, scleromalacia; presence of other ocular conditions such as uncontrolled glaucoma, significant media opacities, phakia or pseudophakia with absence of posterior capsule, intraocular inflammation or infection; prior vitrectomy, trabeculectomy, or other filtration surgery or therapy in study eye Equivalence of baseline characteristics: yes; quote: "baseline demographics and disease characteristics were evenly balanced among all treatment groups." |
|
| Interventions |
Intervention 1: intravitreous aflibercept 0.5 mg q4 wks (excluded) Intervention 2: intravitreous aflibercept 2.0 mg q4 wks Intervention 3: intravitreous aflibercept 2.0 mg q8 wks after 3 initial doses at weeks 0, 4, and 8 (to maintain masking, sham injections were given at the interim 4‐week visits after week 8) Intervention 4: intravitreous ranibizumab 0.5 mg q4 wks Length of follow‐up: 1 year for primary endpoint; dosing for all groups changed to PNR after 1 year and follow‐up at 2 years from baseline |
|
| Outcomes |
Primary outcome, as defined in study reports: proportion of participants maintaining vision at week 52 (losing < 15 letters on ETDRS chart) Secondary outcomes, as defined in study reports: change in BCVA, proportion of participants gaining ≥ 15 letters, change in total NEI VFQ‐25 score, change in CNV area on FA, retinal thickness and persistent fluid as assessed by OCT, mean number of intravitreous injections, adverse events Intervals at which outcomes assessed: q4 wks through 96 weeks; week 1 after first treatment for safety assessment; weeks 12, 24, 36, and 52 for the NEI VFQ‐25 assessment |
|
| Notes |
Type of study reports: published journal articles; clinical trial registration Trial registration: NCT00509795. and NCT00637377 Funding sources: quote: "Sponsored by Regeneron Pharmaceuticals, Inc, Tarrytown, New York, and Bayer HealthCare, Berlin Germany. The sponsors participated in the design and conduct of the study, analysis of the data, and preparation of the manuscript." Disclosures of interest: quote: "J.S.H. is a consultant to and has received research funding from Alimera, Allergan, Fovea, Genentech, Genzyme, GlaxoSmithKline, Neovista, and Regeneron Pharmaceuticals. He has also received travel support from Regeneron Pharmaceuticals. D.M.B. is a consultant to Alimera, Allergan, Bayer, Genentech/Roche, Novartis, Regeneron Pharmaceuticals, and Thrombogenics and has received research funding from Alcon, Alimera, Allergan, Eli Lilly, Genentech, GlaxoSmithKline, Novartis, Regeneron Pharmaceuticals, and Thrombogenics. He has also received travel support from Regeneron Pharmaceuticals and lecture fees from Genentech. V.C. is a consultant to Alimera and Bayer and has received research funding from Alcon, Allergan, Bayer, Novartis, and Pfizer. He is an advisory board member for Allergan and Novartis and has also received travel support from Bayer. J.‐F.K. is a consultant to Alcon, Bayer, and Thea and an advisory board member for Allergan, Bayer, and Novartis. He has received travel support from Regeneron Pharmaceuticals. P.K.K. is a consultant to Bayer, Genentech, Novartis, and Regeneron Pharmaceuticals. He has received research funding from Regeneron Pharmaceuticals. Q.D.N. is a consultant to Bausch & Lomb and Santen and has received research funding from Genentech, Novartis, and Pfizer. B.K. has received travel support from Bayer. A.H. is a consultant to Alcon, Allergan, Centocor, Johnson & Johnson, Neovista, Merck, Ophthotech, Oraya, Paloma, P.R.N., Q.L.T., Regeneron Pharmaceuticals, and Thrombogenics. He has received research funding and lecture fees from Alcon, Allergan, Genentech, Neovista, Ophthotech, Oraya, P.R.N., Q.L.T., Regeneron Pharmaceuticals, and Second Sight. Y.O. is a consultant to Alcon and Bayer and has received travel support from Bayer. G.D.Y., N.S., R.V., A.J.B., and Y.S. are employees of Regeneron Pharmaceuticals. M.A., G.G., B.S., and R.S. are employees of Bayer HealthCare. C.S.'s institution has received payments from the Medical University of Vienna for data monitoring/reviewing and statistical analysis. U.S.‐E. is a consultant to Alcon, Allergan, Bayer HealthCare, and Novartis, and an advisory board member for Alcon and Novartis. She has received travel support from Bayer HealthCare and lecture fees from Bayer HealthCare and Novartis." Study period: July 2007 through September 2010 and March 2008 through September 2010 Subgroup analyses: yes; Japanese subgroup VIEW 2012: 2 intervention groups using other dose or drug not analyzed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation unclear. Quote: "Consecutively enrolled patients were assigned to treatment groups on the basis of a predetermined central randomization scheme with balanced allocation, managed by an interactive voice response system." |
| Allocation concealment (selection bias) | Low risk | Central randomization Quote: "Consecutively enrolled patients were assigned to treatment groups on the basis of a predetermined central randomization scheme with balanced allocation, managed by an interactive voice response system." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients were masked as to treatments. An unmasked investigator also was responsible for the receipt, tracking, preparation, destruction, and administration of study drug, as well as safety assessments both pre‐ and post‐dose … All other study site personnel were masked to treatment assignment by separating study records or masked packaging." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A separate masked physician assessed adverse events and supervised the masked assessment of efficacy. All other study site personnel were masked to treatment assignment by separating study records or masked packaging. OCT technicians and visual acuity examiners remained masked relative to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A full analysis set and a per protocol set were reported. Last observation carried forward approach used to impute missing values; 88.1–91.1% of participants per study treatment group completed 52 weeks of follow‐up. |
| Selective reporting (reporting bias) | Low risk | Study registered at clinicaltrials.gov; intended outcomes reported. |
AMD: age‐related macular degeneration; BCVA: best‐corrected visual acuity; CFT: central foveal thickness; CMT: central macular thickness; CNV: choroidal neovascularization; CR/LT: central retinal/lesion thickness; CRT: central subfoveal retinal thickness; ETDRS: Early Treatment Diabetic Retinopathy Study; FA: fluorescein angiography; IOP: intraocular pressure; MI: myocardial infarction; nAMD: neovascular age‐related macular degeneration; NEI VFQ‐25: National Eye Institute 25‐Item Visual Function Questionnaire; OCT: optical coherence tomography; PDT: photodynamic therapy; PED: pigment epithelial detachment; PRN: as needed; q4 wks: every four weeks; q6 wks: every six weeks; q8 wks: every eight weeks; q12 wks: every 12 weeks; RPE: retinal pigment epithelium; SD: standard deviation; SD‐OCT: spectral domain optical coherence tomography; VA: visual acuity; VEGF: vascular endothelial growth factor; YAG: yttrium‐aluminum‐garnet.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arnold 2015 | Intervention/comparator did not meet eligibility criteria. |
| Arnold 2016 | Intervention/comparator did not meet eligibility criteria. |
| Avery 2016 | Intervention/comparator did not meet eligibility criteria. |
| Barikian 2015b | Intervention/comparator did not meet eligibility criteria. |
| Berg 2016 | Intervention/comparator did not meet eligibility criteria. |
| Bishop 2014 | Intervention/comparator did not meet eligibility criteria. |
| Eldem 2015 | Intervention/comparator did not meet eligibility criteria. |
| Enseleit 2017 | Intervention/comparator did not meet eligibility criteria. |
| EXCITE 2011 | Intervention/comparator did not meet eligibility criteria. |
| Feltgen 2014 | Intervention/comparator did not meet eligibility criteria. |
| Feltgen 2017 | Intervention/comparator did not meet eligibility criteria. |
| FLUID 2016 | Intervention/comparator did not meet eligibility criteria: 2 PRN criteria compared |
| Mahmood 2015 | Intervention/comparator did not meet eligibility criteria. |
| Mori 2017 | Intervention/comparator did not meet eligibility criteria. |
| RIVAL 2014 | Drugs compared rather than regimens. |
| SAVE 2013 | Intervention/comparator did not meet eligibility criteria: ranibizumab 2.0 mg used. |
| SEVEN‐UP 2016 | Not a randomized controlled trial. |
| Takayama 2017 | Intervention/comparator did not meet eligibility criteria. |
| Tempelaar 2015 | Not a randomized controlled trial. |
| Waldstein 2016 | Intervention/comparator did not meet the eligibility criteria. |
| Wijeyakumar 2015 | Intervention/comparator did not meet the eligibility criteria. |
Characteristics of studies awaiting classification [ordered by study ID]
Kon‐Jara 2007.
| Methods | Conference abstract Purpose: to evaluate efficacy of retreatments with intravitreous bevacizumab (Avastin) to maintain or to improve VA in CNV in 2 treatment regimens, 1 according to symptomatology and 1 according to an established algorithm; and to determine which protocol was better at controlling disease activity. Methods: prospective, randomized, experimental, and descriptive open‐label study of 14 participants with CNV secondary to AMD injected with intravitreous bevacizumab (Avastin) 2.5 mg in 0.1 mL. Standardized ophthalmic evaluation performed at baseline and weeks 2, 6, and 12. |
| Participants | |
| Interventions | Intervention 1: people treated with clinical, OCT, or angiographic signs of activity Intervention 2: people treated according to an algorithm every 6 weeks. |
| Outcomes | Main outcomes: ETDRS BCVA at baseline, and after 2, 6, and 12 weeks; OCT and fluorescein angiogram at baseline, and after 6 and 12 weeks |
| Notes | Results: intervention 1 (7 eyes), participants presented a transient improvement during first 2 weeks with later worsening of BCVA. Intervention 2 (7 eyes), participants presented significant clinical improvement that was sustained until the end of study. Most common adverse events were conjunctival hyperemia and subconjunctival hemorrhage at the injection site. Mean BCVA improved from baseline throughout the study (P > 0.001) in both groups. Compared with baseline, BCVA was improved at weeks 2, 6, and 12. At week 6, most of the lesions area were stable or decreased in OCT and fluorescein angiogram. Conclusions: periodic injection of bevacizumab every 6 weeks according to an established algorithm resulted in a better control of CNV and BCVA improvement or stabilization compared to treatment according to participant symptomatology. Abstract NIH registration APEC‐0012 |
MATE 2015.
| Methods | Target number of participants Planned sample size: 40; UK sample size: 40; description: people with treatment naïve neovascular AMD patients will be recruited into the study from the Ophthalmology departments of the participating NHS hospitals of York, Hull, Leeds, Bradford, and Harrogate. The sample size is 40 and the recruitment period is expected to last approximately 6 months. Participants are randomly allocated to 1 of 2 groups. Participants in both groups receive an initial 3 doses of aflibercept 2 mg, given by intravitreal injection, spaced 4 weeks apart. Following this, for participants in the first group, the interval between treatments is extended to once every 8 weeks for the first year of treatment. In the second year of treatment, the treating physician can extend the intervals between treatments at their discretion. For participants in the second group, the treating physician is able to extend the treatment intervals at their discretion until the most appropriate dosing regimen for each individual participant is found. At the end of the study, the potential benefits of each dosing method are compared to find the best way to conduct a larger study in the future. |
| Participants | Inclusion criteria: aged ≥ 50 years; able to provide written, informed consent to the study; able and willing to attend for hospital visits at the frequency required; visual impairment predominantly due to neovascular AMD; active, treatment naïve, angiographically active choroidal neovascular membrane in the study eye secondary to neovascular AMD with any part of the lesion or its sequelae (e.g. subretinal fluid, intraretinal fluid, hemorrhage, pigment epithelial detachment, subretinal pigment epithelium fluid) in a subfoveal location; VA of 78–24 ETDRS letters at screening and baseline in the study eye; if both eyes are eligible at baseline, the worst seeing eye will be included in the study although the final decision will rest with the investigator. Any deviation from entering the worst seeing eye into the study will be explained and documented in the patient notes and the case report form. The choice of eye selected for inclusion into the study will be determined and documented before the patient is randomized. A patient who has both eyes that may be eligible may therefore undergo a different treatment regimen in each eye, however they will be treated with aflibercept in both eyes. Hospital visits will be co‐ordinated to minimize the number of attendances required and therefore the inconvenience for the patient. Exclusion criteria: inability to comply with the study or follow‐up procedures; pregnant or lactating women; women of childbearing potential unless they are using effective methods of contraception (total abstinence, female or male sterilization, barrier contraception, intrauterine device, oral or injectable hormonal methods of contraception); previous treatment for CNV in the study eye; fibrosis consisting of > 50% of the lesion or involving the center of the fovea; coexisting pathology within 0.5 disk diameters of the fovea that could prevent an improvement in VA in the opinion of the investigator (e.g. macular hole, dense epi–retinal membrane); cataract (causing significant visual impairment), aphakia, vitreous hemorrhage, retinal detachment, proliferative retinopathy, or CNV due to any cause other than AMD at screening and baseline; allergy to aflibercept or fluorescein; history of cerebrovascular accident, transient ischemic attack, or myocardial infarction within 3 months of the screening visit; any type of systemic disease or treatment that may affect or expect to affect the clinical status of the patient to a significant degree; blood pressure > 160 mmHg systolic or > 100 mmHg diastolic at screening or baseline; any active periocular infection or inflammation at screening or baseline; uncontrolled glaucoma (30 mmHg) at screening or baseline; neovascularisation of the iris at screening or baseline; treatment with any antiangiogenic drugs to either eye within 3 months of baseline; Nd‐YAG laser capsulotomy within the last 2 months or expected within 6 months of baseline in the affected eye; use of other investigational drugs within 30 days; use of systemic anti‐vascular endothelial growth factor agents within 3 months prior to baseline; use of systemic corticosteroids for ≥ 30 consecutive days within the 3 months prior to baseline; current or planned medications known to be toxic to the lens, retina, or optic nerve (e.g. hydroxychloroquine, desferoxamine, tamoxifen, or ethambutol) |
| Interventions | Participants are randomly allocated to 1 of 2 groups. Both groups will receive intravitreal injections of aflibercept 2 mg. Intervention 1: initial 3 doses of monthly aflibercept injections followed by 8 weekly treatments for the first year with an opportunity to extend the treatment intervals in the second year of treatment at the discretion of the treating physician. Intervention 2: initial 3 doses of monthly aflibercept followed by extension of treatment intervals at the discretion of the treatment physician until an interval appropriate for the individual is found. This has the potential to allow a minimum number of visits, on each of which treatment is administered, while maintaining an acceptable efficacy. |
| Outcomes | Not reported. |
| Notes | ISRCTN58955026; doi.org/10.1186/ISRCTN58955026 |
Nunes 2014.
| Methods | Conference abstract Purpose: to study the efficacy and cost‐effectiveness of therapy with intravitreous ranibizumab and bevacizumab in exudative AMD. Methods: cost‐effectiveness analysis and a prospective randomized controlled trial comparing the efficacy of ranibizumab and bevacizumab as therapy for wet AMD under the Brazilian Universal Health System with a time horizon of 1 year. QALY and the incremental cost‐effectiveness ratio calculated, according to utility values for VA changes in participants with AMD. |
| Participants | 45 participants with exudative AMD |
| Interventions | Randomized (1:1:1) in 3 groups Intervention 1: monthly intravitreous bevacizumab 1.25 mg Intervention 2: intravitreous bevacizumab 1.25 mg every 2 weeks Intervention 3: monthly intravitreous ranibizumab 0.5 mg All participants received 3 months' loading dose, followed with as‐needed regimen. Participants followed for 1 year. |
| Outcomes | |
| Notes | Results: based on the incremental cost‐effectiveness ratio, BRL 941,583.33 (about USD 410,000) would be necessary to have 1 additional QALY when comparing ranibizumab and bevacizumab. Similar results found when analyzed different treatment strategies. From the 45 participants included in the RCT, 44 concluded the first year of follow‐up. 1 participant died due to pneumonia. The mean initial VA was 52.2 ETDRS letters in intervention 1, 51.1 in intervention 2, and 54.9 in intervention 3 (P = 0.816). At month 12, the mean VA increased to 7.2 ETDRS letters in intervention 1, 13.4 in intervention 2, and 12.3 in intervention 3 (intervention 1: P = 0.054; intervention 2: P = 0.008; intervention 3: P = 0.002). This increase in VA was statistically similar among groups (P = 0.602). The mean number of injections was 10.5 in intervention 1, 16.4 in intervention 2, and 10.6 in intervention 3 (intervention 1 vs 2: P = 0.003; intervention 2 vs 3: P = 0.003; intervention 1 vs 3: P = 0.980). There was no significant IOP variation and only 1 eye developed cataract over 1 year. Low rate of ocular or systemic adverse events (or both) in interventions 1 and 2 and no adverse event in intervention 3. Important design issues for this clinical trial included use of cost‐effectiveness as outcome and an every‐2‐weeks group. Conclusions: efficacy of intravitreous bevacizumab may have been comparable to intravitreous ranibizumab in the therapy of exudative AMD. |
Ohnaka 2017.
| Methods | Conference abstract Purpose: to evaluate 2 intravitreous aflibercept (IVTAFL) treat‐and‐extend dosing regimens in Japanese people with wet AMD. |
| Participants | |
| Interventions | ALTAIR (NCT02305238) was a 96‐week, randomized, open‐label, phase 4 study conducted at 40 sites across Japan. Intervention 1: 3 monthly doses of intravitreous aflibercept before randomization (1:1) at week 16 then IVT‐AFL‐2W Intervention 2: 3 monthly doses of intravitreous aflibercept before randomization (1:1) at week 16 then IVT‐AFL‐4W |
| Outcomes | Primary endpoint: mean change in BCVA ETDRS letters from baseline to week 52. Other endpoints: proportion of participants losing < 15 ETDRS letters, mean change in CRT, and TEAEs at week 52. |
| Notes | Results: 254 participants included in safety analyses and 246 participants included in efficacy analyses. Baseline BCVA 54.8 ETCRS letters in IVT‐AFL‐2W group and 55.3 ETCRS letters in IVT‐AFL‐4W group. Mean change in BCVA from baseline to week 52: 9.0 ETDRS letters in IVT‐AFL‐ 2W group and 8.4 ETCRS letters in IVT‐AFL‐4W group. Proportion of participants losing < 15 ETDRS letters: 96.7% in IVT‐AFL‐2W group and 95.9% in IVT‐AFL‐4W group. Mean change in CRT: –134.4 in IVT‐AFL‐2W group and –126.1 in IVT‐AFL‐4W group. Mean number of injections: 7.2 in IVT‐AFL‐2W group and 6.9 in IVT‐AFL‐4W group. Mean injection interval (weeks 16–52): 10.0 in IVT‐AFL‐2W group vs 10.9 in IVT‐AFL‐4W group. Most common ocular TEAEs were conjunctival hemorrhage (2.4%) and RPE tear (2.4%) in IVT‐AFL‐2W group and conjunctival hemorrhage (5.7%) in IVT‐AFL‐4W group. Conclusions: both IVT‐AFL treat‐and‐extend regimens improved visual and anatomical outcomes at week 52 with extended dosing intervals in participants with wet AMD. Ocular TEAEs were consistent with the known safety profile of IVT‐AFL. |
AMD: age‐related macular degeneration; BCVA: best‐corrected visual acuity; CNV: choroidal neovascularization; CRT: central retinal thickness; ETDRS: Early Treatment Diabetic Retinopathy Study; IOP: intraocular pressure; IVT‐AFL‐2W: intravitreous aflibercept with a two‐week adjustment; IVT‐AFL‐4W: intravitreous aflibercept with a four‐week adjustment; OCT: optical coherence tomography; QALY: quality‐adjusted life year; RCT: randomized controlled trial; TEAE: treatment‐emergent adverse event; VA: visual acuity.
Characteristics of ongoing studies [ordered by study ID]
Foss 2015.
| Study name | Comparing different dosing regimens of bevacizumab in the treatment of neovascular macular degeneration: study protocol for a randomized controlled trial |
| Methods |
Number randomized (total and per group): N/A Exclusions after randomization: N/A Number analyzed (total and per group): N/A Unit of analysis: participant (1 or both eyes per participant; if bilateral disease develops, participant remains in the trial and treated with the same allocation as the first eye) Losses to follow‐up: N/A Compliance: N/A Intention‐to‐treat analysis: quote: "The main approach to between‐group comparisons will be to analyse all participants as randomised regardless of adherence with allocation. In addition, for the primary outcome, a per protocol analysis will be conducted that excludes participants with protocol violations (specifically, failure to collect outcome data or patients who received treatment in addition to the trial intervention, such as ranibizumab)." Reported power calculation: quote: "With a non‐inferiority hazard ratio margin of 1.4 for between‐arm main effects, 90% power and one‐sided 5% alpha, a total of 304 events are required to be observed, and the target sample size for recruitment was 2,000 participants. In January 2014 after three years of recruitment, we reviewed the assumptions underlying the original recruitment target. Based on 437 randomised participants and 374 person‐years of observation, we revised the annual event rate of the primary outcome from 10 to 20%, and additionally accounted for annual censoring (death, suspension of treatment following six months of stable disease, withdrawal of consent for study participation or no response to attempted contact) of 16%, which had not been incorporated into the original calculation. The target number of 304 events remains unchanged, but the target number of randomised participants required to achieve this has been revised to around 900 to 1,000." Study design comment: N/A |
| Participants |
Country: UK Age: N/A Gender: N/A Inclusion criteria: ages ≥ 50 years, newly referred for treatment of nAMD or reactivation of nAMD, no treatment for nAMD to either eye for the previous 6 months, eligible for anti‐VEGF treatment of nAMD in the National Health Service. Exclusion criteria: known hypersensitivity to recombinant human or humanized antibodies, woman of child‐bearing potential and not willing to use contraception, men with spouse of child‐bearing potential not willing to use condoms, pregnant or breastfeeding Equivalence of baseline characteristics: quote: "Baseline data collection (including general and ophthalmic history, examination and baseline morphology of nAMD lesion) at the initial visit." Diagnoses in participants: nAMD |
| Interventions |
Intervention 1: 3 monthly injections. Standard‐dose (1.25 mg) bevacizumab and review every 4–6 weeks Intervention 2: 3 monthly injections. Standard‐dose (1.25 mg) bevacizumab and review every 8–10 weeks Intervention 3: 3 monthly injections. Half dose (0.625 mg) bevacizumab and review every 8–10 weeks Intervention 4: 3 monthly injections. Half dose (0.625 mg) bevacizumab and review every 8–10 weeks Follow‐up: Planned length: N/A Actual length: N/A Frequency of assessments for retreatment: every 8–10 weeks for each group |
| Outcomes |
Primary outcome, as defined: time to treatment failure, defined as loss of > 5 letters (logMAR VA chart) from the baseline established as the mean of the VAs at the first 3 visits. The primary analysis will be at the margins, unless there is evidence of an interaction, in which case low dose plus bimonthly, low dose plus monthly, and standard dose plus bimonthly will each be compared with standard dose plus monthly. Secondary outcomes, as defined: not reported Adverse events (Y/N): N/A Intervals at which outcome assessed: not reported |
| Starting date | 12 September 2019: the intention to publish date has been changed from to 30 June 2020. 5 October 2017: the following changes were made to the trial record: 1. The overall trial end date was changed from 1 January 2016 to 31 March 2017. 2. Ethics approval details added. 3. Plain English summary added. 4. Publication and dissemination plan and IPD sharing statement added. 3 October 2017: the following changes were made to the trial record: 1. The recruitment end date was changed from 1 January 2016 to 31 March 2017. 2. The target number of participants was changed from 2000 to 811. 10 April 2013: the overall trial end date was changed from 1 January 2013 to 1 January 2016. |
| Contact information | |
| Notes |
Type of study: ongoing Trial registration (Y/N): International Standard Randomised Controlled Trial Number: ISRCTN95654194, registered on 22 September 2009. Funding sources: the costs for the Bristol Trials Unit were met by the East Midlands Special Commissioning Group (EMSCG). The current running costs for the Nottingham Clinical Trials Unit are currently met by NHS England. The trial was set up to be the major vehicle for delivery of NHS case and accordingly the authors are employed by their respective Hospital Trusts who pay their salaries. Alexander Foss is paid a 1 PA uplift for running the trial, which is paid from Nottingham University Hospitals who, in turn are commissioned to run the service, initially by EMSCG and now by the clinical commissioning groups. Declarations of interest: Bell, Fell, and Qualie are healthcare commissioners who fund services for nAMD and the use of bevacizumab represents a saving on their budgets. Study period: recruitment to the TANDEM trial is ongoing. First participant was randomized in November 2010 and recruitment is expected to end at the end of 2016. Reported subgroup analyses (Y/N): if yes, specify: yes, planned: quote: "Secondary analyses of the primary outcome will include additional adjustment for any variables exhibiting marked imbalance at baseline, and investigation of subgroup effects according to: baseline VA in study eye ≤44 versus >44; 2) baseline CNV size ≤4 versus >4 and nAMD lesion composition. These analyses will be conducted by fitting interaction terms to the regression models. It is recognised that the study is not powered to detect differential treatment effects among subgroups, and these analyses will be viewed as exploratory. Secondary outcomes will be analysed using a similar approach as for the primary analysis, with regression model appropriate for type of outcome. All between‐group comparisons will be described using appropriate estimates of effects (that is, hazard ratio, odds ratio or difference in means, depending on outcome type) and 95% confidence intervals." |
AMD: age‐related macular degeneration; BCVA: best‐corrected visual acuity; CNV: choroidal neovascularization; ETDRS: Early Treatment Diabetic Retinopathy Study; FA: fluorescein angiography; IRF: intraretinal fluid; IOP: intraocular pressure; OCT: optical coherence tomography; N/A: not available; nAMD: neovascular age‐related macular degeneration; PBS: Pharmaceutical Benefits Scheme; RCT: randomized controlled trial; SD: standard deviation; SRF: subretinal fluid; VA: visual acuity; VEGF: vascular endothelial growth factor; YAG: yttrium‐aluminum‐garnet.
Differences between protocol and review
Throughout the review we changed 'schedule' to 'regimen' as this is more commonly used term.
We decided post‐hoc to consider two groups of non‐monthly regimens. We grouped the treat‐and‐extend regimen with PRN regimens, in which injections were prescribed when CNV recurrence was detected clinically. In the treat‐and‐extend regimen, further injections were prescribed at increasing intervals even if the macula was dry. The objectives were amended accordingly. To further explore the issue of treatment intensity, we also conducted post‐hoc meta‐regression of the mean difference in visual acuity between monthly and non‐monthly regimens against the mean number of injections in the PRN study arm.
When data were extracted regarding ocular adverse events, we decided to report only on the number of participants with endophthalmitis, because it is the most devastating ocular complication and may be related to the number of injections. This was also adopted in the 'Summary of findings' tables.
Regarding use of resources, we reported the number of injections in one year as the major cost, but we could not report on differences in the treatment cost per person.
Contributions of authors
EL and MGK conceived, designed, and wrote the protocol. EL, SD and GV designed and wrote this review. EL, SD, and GV reviewed and classified literature. All authors contributed to the final manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
Methodological support provided by the Cochrane Eyes and Vision US Project, supported by co‐operative agreement 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA
-
National institute for Health Research (NIHR), UK
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, National Health Service, or the Department of Health.
Declarations of interest
EL: none. SD: none. KL: none. MGK: none. GV: none.
New
References
References to studies included in this review
Barikian 2015a {published data only}
- Barikian A, Mahfoud Z, Abdulaal M, Safar A, Bashshur ZF. Induction with intravitreal bevacizumab every two weeks in the management of neovascular age-related macular degeneration. American Journal of Ophthalmology 2015;159(1):121-7. [DOI] [PubMed] [Google Scholar]
BeMOc 2013 {published data only}
- Menon G, Chandran M, Sivaprasad S, Chavan R, Narendran N, Yang Y. Is it necessary to use three mandatory loading doses when commencing therapy for neovascular age-related macular degeneration using bevacizumab? (BeMOc Trial). Eye 2013;27(8):959-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
CANTREAT 2019 {published data only (unpublished sought but not used)}
- Kertes PJ, Galic IJ, Greve M, Williams RG, Rampakakis E, Scarino A, et al. Canadian treat-and-extend analysis trial with ranibizumab in patients with neovascular age-related macular disease: one-year results of the randomized Canadian treat-and-extend analysis trial with ranibizumab study. Ophthalmology 2019;126(6):841-8. [DOI] [PubMed] [Google Scholar]
CATT 2011 {published data only}
- Altaweel MM, Daniel E, Martin DF, Mittra RA, Grunwald JE, Lai MM, et al. Outcomes of eyes with lesions composed of >50% blood in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT). Ophthalmology 2015;122(2):391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong EW, Al-Qureshi SH. Anti-vascular endothelial growth factor treatment for neovascular age-related macular degeneration: Comparison of Age-related Macular Degeneration Treatments Trials 5 year outcomes and implication for clinical practice. Clinical & Experimental Ophthalmology 2017;45(4):333-5. [DOI] [PubMed] [Google Scholar]
- Ciulla TA, Ying GS, Maguire MG, Martin DF, Jaffe GJ, Grunwald JE, et al. Influence of the vitreomacular interface on treatment outcomes in the Comparison of AMD Treatments Trials (CATT). Investigative Ophthalmology & Visual Science 2015;56(7):3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciulla TA, Ying GS, Maguire MG, Martin DF, Jaffe GJ, Grunwald JE, et al. Influence of the vitreomacular interface on treatment outcomes in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2015;122(6):1203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E, Grunwald JE, Kim BJ, Maguire MG, Jaffe GJ, Toth CA, et al. Visual and morphologic outcomes in eyes with hard exudate in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology Retina 2017;1(1):25-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E, Grunwald JE, Kim BJ, Maguire MG, Shaffer JA, Jaffer GJ, et al. Hard exudates and treatment outcomes in the Comparison of Age-related macular degeneration Treatments Trials (CATT). Investigative Ophthalmology & Visual Science 2016;57(12):2653. [Google Scholar]
- Daniel E, Maguire M, Shaffer J, Ying G, Grunwald JE, Jaffe G, et al. Retinal angiomatous proliferation and treatment outcomes in the Comparison of Age-Related Macular Degeneration Treatments Trials (CATT). Investigative Ophthalmology & Visual Science 2015;56:2833. [Google Scholar]
- Daniel E, Shaffer J, Ying GS, Grunwald JE, Martin DF, Jaffer GJ, et al. Outcomes in eyes with retinal angiomatous proliferation in the Comparison of Age-related macular degeneration Treatments Trials (CATT). Ophthalmology 2016;123(3):609-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald JE, Pistilli M, Daniel E, Ying GS, Fine SL, Martin DF, et al. Size and growth of geographic atrophy in the Comparison of Age-related Macular Degeneration Treatments Trials (CATT). Investigative Ophthalmology & Visual Science 2014;55(13):1649. [Google Scholar]
- Grunwald JE, Pistilli M, Daniel E, Ying GS, Pan W, Jaffe GJ, et al. Incidence and growth of geographic atrophy during 5 years of Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2017;124(1):97-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald JE, Pistilli M, Ying GS, Maguine MG, Daniel E, Martin DF. Growth of geographic atrophy in the Comparison of Age-related macular degeneration Treatments Trials. Ophthalmology 2015;122(4):809-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Ying GS, Maguire MG, Stone RA, Martin DF. Impact of change in retinal thickness on refractive error in the Comparison of Age-related macular degeneration Treatments Trials (CATT). Investigative Ophthalmology & Visual Science 2014;55(13):657. [Google Scholar]
- Kim BJ, Ying GS, Huang J, Levy NE, Maguire MG. Sporadic visual acuity loss in the Comparison of AMD Treatments Trials (CATT). Investigative Ophthalmology & Visual Science 2014;55(13):4962. [Google Scholar]
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119(7):1388-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ, et al, CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. New England Journal of Medicine 2011;364(20):1897-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistilli M, Ying GS, Jaffe GJ, Toth CA, Martin DF. Resolution of retinal fluid on optical coherence tomography in the Comparison of AMD Treatments Trials (CATT). Investigative Ophthalmology & Visual Science 2015;56(7):1657. [Google Scholar]
- Shah N, Maguire MG, Martin DF, Shaffer J, Ying GS, Grunwald JE, et al. Angiographic cystoid macular edema and outcomes in the Comparison of Age-related macular degeneration Treatments Trials. Ophthalmology 2016;123(4):858-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Toth CA, Daniel E, Grunwald JE, Maguire MG, Ying GS, et al. Macular morphology and visual acuity in the second year of the Comparison of Age-related macular degeneration Treatments Trials. Ophthalmology 2016;123(4):865-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth CA, Decroos FC, Ying GS, Stinnett SS, Heydary CS, Burns R, et al. Identification of fluid on optical coherence tomography by treating ophthalmologists versus a reading center in the Comparison of Age-Related Macular Degeneration Treatments Trials. Retina 2015;35(7):1303-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby AS, Ying GS, Toth CA, Maguire MG, Burns RE, Grunwald JE, et al, Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Subretinal hyperreflective material in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2015;122(9):1846-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying GS, Kim BJ, Maguire MG, Huang J, Daniel E, Jaffe GJ, et al. Sustained visual acuity loss in the Comparison of Age-related macular degeneration Treatments Trials. JAMA Ophthalmology 2014;132(8):915-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying GS, Maguire MG, Daniel E, Ferris FL, Jaffe GJ, Grunwald JE, et al. Association of baseline characteristics and early vision response with 2-year vision outcomes in the Comparison of AMD Treatments Trials (CATT). Ophthalmology 2015;122(12):2523-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying GS, Maguire MG, Daniel E, Grunwald JE, Ahmed O, Martin DF, Comparison of Age-Related Macular Degeneration Treatments Trials Research Group. Association between antiplatelet or anticoagulant drugs and retinal or subretinal hemorrhage in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016;123(2):352-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
CLEAR‐IT 2 2011b {published data only}
- Brown DM, Heier JS, Ciulla T, Benz M, Abraham P, Yancopoulos G, et al. Primary endpoint results of a phase II study of vascular endothelial growth factor trap-eye in wet age-related macular degeneration. Ophthalmology 2011;118(6):1089-97. [DOI] [PubMed] [Google Scholar]
- *.Heier JS, Boyer D, Nguyen QD, Marcus D, Roth DB, Yancopoulos G, et al. The 1-year results of CLEAR-IT 2, a phase 2 study of vascular endothelial growth factor trap-eye dosed as-needed after 12-week fixed dosing. Ophthalmology 2011;118(6):1098-106. [DOI] [PubMed] [Google Scholar]
El‐Mollayess 2012 {published data only}
- El-Mollayess GM, Mahfoud Z, Schakal AR, Salti HI, Jaafar D, Bashshur ZF. Fixed-interval versus OCT-guided variable dosing of intravitreal bevacizumab in the management of neovascular age-related macular degeneration: a 12-month randomized prospective study. American Journal of Ophthalmology 2012;153(3):481-9. [DOI] [PubMed] [Google Scholar]
GMAN 2015 {published data only}
- Mahmood S, Roberts SA, Aslam TM, Parkes J, Barugh K, Bishop PN. Routine versus as-needed bevacizumab with 12-weekly assessment intervals for neovascular age-related macular degeneration: 92-week results of the GMAN Trial. Ophthalmology 2015;122(7):1348-55. [DOI] [PubMed] [Google Scholar]
HARBOR 2013 {published data only}
- Busbee BG, Ho AC, Brown DM, Heier JS, Suner IJ, Li Z, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013;120(5):1046-56. [DOI] [PubMed] [Google Scholar]
- Frenkel RE, Fung AE, Shapiro H, Stoilov I. Low luminance vision improves nearly twice as much as standard vision with ranibizumab for neovascular age-related macular degeneration. Investigative Ophthalmology & Visual Science 2015;56(7):5358. [Google Scholar]
- Frenkel RE, Shapiro H, Stoilov I. Predicting vision gains with anti-VEGF therapy in neovascular age-related macular degeneration patients by using low-luminance vision. British Journal of Ophthalmology 2016;100(8):1052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AC, Busbee BG, Regillo CD, Wieland MR, Everen SA, Li Z, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014;2014(121):2181-92. [DOI] [PubMed] [Google Scholar]
- Khanani A, Hill L, Tuomi L. Improvement in visual acuity following ranibizumab treatment in neovascular age-related macular degeneration patients with large pigment epithelial detachments: a subgroup analysis of the HARBOR study. Investigative Ophthalmology & Visual Science 2015;56(7):5363. [Google Scholar]
- Penden MC, Frenkel RE, Shapiro H, Stoilov I. Visual acuity impairment under low luminance conditions at baseline is a powerful predictor of visual acuity response to ranibizumab therapy in patients with neovascular age-related macular degeneration. Investigative Ophthalmology & Visual Science 2015;56(7):5362. [Google Scholar]
- Regillo CD, Busbee BG, Ho AC, Ding BM, Haskova Z. Baseline predictors of 12-month treatment response to ranibizumab in patients with wet age-related macular degeneration. American Journal of Ophthalmology 2015;160(5):1014-23. [DOI] [PubMed] [Google Scholar]
- Sarraf D, London NJ, Khurana RN, Dugel PU, Gene S, Hill L, et al. Ranibizumab treatment for pigment epithelial detachment secondary to neovascular age-related macular degeneration: post hoc analysis of the HARBOR study. Ophthalmology 2016;123(10):2213-24. [DOI] [PubMed] [Google Scholar]
- Stoller GL, Kokame GT, Dreyer RF, Shapiro H, Tuomi LL. Patterns of early and delayed visual response to ranibizumab treatment for neovascular age-related macular degeneration. JAMA Ophthalmology 2016;134(5):545-53. [DOI] [PubMed] [Google Scholar]
IVAN 2012b {published data only}ISRCTN92166560
- Chakravarthy U, Harding SP, Rogers CA, Downes S, Lotery AJ, Dakin HA, et al. A randomised controlled trial to assess the clinical effectiveness and cost-effectiveness of alternative treatments to Inhibit VEGF in Age-related choroidal Neovascularisation (IVAN). Health Technology Assessment 2015;19:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, on behalf of the IVAN study investigators. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013;383(9900):1258-67. [DOI] [PubMed] [Google Scholar]
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012;119(7):1399-411. [DOI] [PubMed] [Google Scholar]
- Foss AJ, Scott LJ, Rogers CA, Reeves BC, Ghanchi F, Gibson J, et al. Changes in intraocular pressure in study and fellow eyes in the IVAN trial. British Journal of Ophthalmology 2016;100(12):1662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotery A, Scott L, Harding SP, Reeves B, Rogers C, Chakravarthy U. Visual acuity by responder status in the IVAN clinical trial. Investigative Ophthalmology & Visual Science 2014;55(13):867. [Google Scholar]
Lushchyk 2013 {published data only}
- Lushchyk T, Amarakoon S, Martinez-Ciriano JP, Born LI, Baarsma GS, Missotten T. Bevacizumab in age-related macular degeneration: a randomized controlled trial on the effect of injections every 4 weeks, 6 weeks and 8 weeks. Acta Ophthalmologica 2013;91(6):e456-61. [DOI] [PubMed] [Google Scholar]
NATTB 2012 {published data only}
- Li X, Hu Y, Sun X, Zhang J, Zhang M. Neovascular Age-Related Macular Degeneration Treatment Trial Using Bevacizumab (NATTB). Bevacizumab for neovascular age-related macular degeneration in China. Ophthalmology 2012;1119(10):2087-93. [DOI] [PubMed] [Google Scholar]
Sarraf 2013 {published data only}
- Sarraf D, Chan C, Rahimy E, Abraham P. Prospective evaluation of the incidence and risk factors for the development of RPE tears after high- and low-dose ranibizumab therapy. Retina 2013;33(8):1551-7. [DOI] [PubMed] [Google Scholar]
TREND 2017 {published data only}
- Mones J, Silva R, Larsen M, Clemenes A, Muscianisi E, Feller C, et al. Ranibizumab 0.5 mg treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: one-year results from the trend study. Ophthalmic Research 2017;58:3. [Google Scholar]
- Silva R, Berta A, Larsen M, Macfadden W, Feller C, Mones J. Treat-and-extend versus monthly regimen in neovascular age-related macular degeneration: results with ranibizumab from the TREND Study. Ophthalmology 2018;125(1):57-65. [DOI] [PubMed] [Google Scholar]
TREX‐AMD 2015 {published data only}
- Abdelfattah NS, Al-Sheikh M, Pitetta S, Mousa A, Sadda SR, Wykoff CC. Macular atrophy in neovascular age-related macular degeneration with monthly versus treat-and-extend ranibizumab: findings from the TREX-AMD trial. Ophthalmology 2017;124(2):215-23. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Brown DM, Croft D, Wang R, Clark L, Payne JF. Multicenter, prospective trial comparing treat & extend to monthly dosing in neovascular AMD management: TREXAMD 12-month outcomes. Investigative Ophthalmology & Visual Science 2015;56(7):5371. [Google Scholar]
- Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L, et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology 2015;122(12):2514-22. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Croft DE, Brown DM, Wang R, Payne JF, Clark L, et al. Prospective trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: TREX-AMD 1-year results. Ophthalmology 2015;122(12):2514-22. [DOI] [PubMed] [Google Scholar]
VIEW 2012 {published data only}
- Freund KB, Hoang QV, Saroj N, Thompson D. Intraocular pressure in patients with neovascular age-related macular degeneration receiving intravitreal aflibercept or ranibizumab. Ophthalmology 2015;122(9):1802-10. [DOI] [PubMed] [Google Scholar]
- Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012;119:2537-48. [DOI] [PubMed] [Google Scholar]
- Jaffe GJ, Kaiser PK, Thompson D, Gibson A, Saroj N, Vitti R, et al. Differential response to anti-VEGF regimens in age-related macular degeneration patients with early persistent retinal fluid. Ophthalmology 2016;123(9):1856-64. [DOI] [PubMed] [Google Scholar]
- Korobelnik JF, Morton R, Katz TA, Slakter JS, Sowade O, Wolf S. Baseline characteristics of the fellow eye in patients with wet age-related macular degeneration: post-hoc analysis of the VIEW studies. Investigative Ophthalmology & Visual Science 2014;55(13):3965. [DOI] [PubMed] [Google Scholar]
- Mitchell P, VIEW Study Group. Intravitreal aflibercept versus ranibizumab for neovascular age-related macular degeneration: 52-week subgroup analyses from the combined VIEW studies. Clinical & Experimental Ophthalmology 2012;40(Suppl 1):41-56. [Google Scholar]
- Moshfeghi DM, Hariprasad SM, Marx JL, Thompson D, Soo Y, Gibson A, et al. Effect of fluid status at week 12 on visual and anatomic outcomes at week 52 in the VIEW 1 and 2 trials. Ophthalmic Surgery Lasers and Imaging Retina 2016;47(3):238-44. [DOI] [PubMed] [Google Scholar]
- Moshfeghi DM. Changes in neovascular activity following continuous anti-vascular endothelial growth factor administration in the view studies. Investigative Ophthalmology & Visual Science 2016;57(12):516. [Google Scholar]
- Ogura Y, Terasaki H, Gomi F, Yuzawa M, Iida T, Honda M, et al. Efficacy and safety of intravitreal aflibercept injection in wet age-related macular degeneration: outcomes in the Japanese subgroup of the VIEW 2 study. British Journal of Ophthalmology 2015;99(1):92-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard G, Mones J, Wolf S, Korobelnik JF, Guymer R, Goldstein M, et al. Scheduled versus pro re nata dosing in the VIEW trials. Ophthalmology 2015;122(12):2497-503. [DOI] [PubMed] [Google Scholar]
- Schmidt-Erfurth U, Kaiser PK, Korobelnik J-F, Brown DM, Chong V, Nguyen QD, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration. Ninety-six week results of the VIEW studies. Ophthalmology 2014;121(1):193-201. [DOI] [PubMed] [Google Scholar]
- Shah C. Impact of retinal pigment epithelial elevation at baseline on visual outcomes in the view studies. Investigative Ophthalmology & Visual Science 2014;55(13):3934. [Google Scholar]
- Simader C, Waldstein SM, Larsen M, Mitchell P, Staurenghi G, Jaffe GJ, et al. Structure–function correlation of optical coherence tomography features and visual outcome in a fixed and a PRN regimen in neovascular AMD. Investigative Ophthalmology & Visual Science 2014;55(13):868. [Google Scholar]
- Singer M. Long-term visual outcomes by baseline subgroup characteristics of intravitreal aflibercept injection for neovascular age-related macular degeneration after the VIEW1 study. Investigative Ophthalmology & Visual Science 2015;56(7):4607. [Google Scholar]
- Waldstein SM, Simader C, Staurenghi G, Chong NV, Mitchell P, Jaffe GJ, et al. Morphology and visual acuity in aflibercept and ranibizumab therapy for neovascular age-related macular degeneration in the VIEW trials. Ophthalmology 2016;123(7):1521-9. [DOI] [PubMed] [Google Scholar]
- Waldstein SM, Simader C, Staurenghi G, Larsen M, Mitchell P, Schmidt-Erfurth U. Antiangiogenic efficacy of intravitreal aflibercept versus ranibizumab in a fixed and a PRN-guided regimen in the VIEW2 trial. Investigative Ophthalmology & Visual Science 2014;55:3959. [Google Scholar]
- Yuzawa M, Fujita K, Wittrup-Jensen KU, Norenberg C, Zeitz O, Adachi K, et al. Improvement in vision-related function with intravitreal aflibercept: data from phase 3 studies in wet age-related macular degeneration. Ophthalmology 2015;122(3):571-8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arnold 2015 {published data only}
- Arnold J, Markey C, Baird P, Richardson A, Kurstjens N, Guymer R. Baseline characteristics of the fluid study patients: a randomised clinical trial investigating the need for complete resolution of sub-retinal fluid (SRF) in ranibizumab-treated patients with neovascular age-related macular degeneration (NAMD) using a treat and extend (T&E) regimen. Clinical and Experimental Ophthalmology 2015;43:108. [Google Scholar]
Arnold 2016 {published data only}
- Arnold JJ, Markey CM, Kurstjens NP, Guymer RH. The role of sub-retinal fluid in determining treatment outcomes in patients with neovascular age-related macular degeneration – a phase IV randomised clinical trial with ranibizumab: the FLUID study. BMC Ophthalmology 2016;16:31. [DOI] [PMC free article] [PubMed]
Avery 2016 {published data only}
- Avery RL. Re: Berg et al.: comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol (Ophthalmology 2015;122:146-52). Ophthalmology 2016;123(2):e14-6. [DOI] [PubMed] [Google Scholar]
Barikian 2015b {published data only}
- Barikian A, Mahfoud Z, Abdulaal M, Safar A, Bashshur ZF. Induction with intravitreal bevacizumab every two weeks in the management of neovascular age-related macular degeneration. American Journal of Ophthalmology 2015;159(1):131-7. [DOI] [PubMed] [Google Scholar]
Berg 2016 {published data only}
Bishop 2014 {published data only}
- Bishop PN, Roberts S, Aslam T, Charles S, Stanga PE, Parkes J, et al. Randomised controlled trial over 92 weeks using bevacizumab for the treatment of neovascular age-related macular degeneration comparing a treatment regime with 12 weekly regular injections to one with injections on an as-needed basis. Investigative Ophthalmology & Visual Science 2014;55(13):1646. [Google Scholar]
Eldem 2015 {published data only}
- Eldem BM, Muftuoglu G, Topbas S, Cakir M, Kadayifcilar S, Ozmert E, et al. A randomized trial to compare the safety and efficacy of two ranibizumab dosing regimens in a Turkish cohort of patients with choroidal neovascularization secondary to AMD. Acta Ophthalmologica 2015;93(6):e458-64. [DOI] [PubMed]
Enseleit 2017 {published data only}
- Enseleit F, Michels S, Sudano I, Stahel M, Zweifel S, Schlager O, et al. SAVE-AMD: safety of VEGF Inhibitors in age-related macular degeneration. Ophthalmologica 2017;238(4):205-16. [DOI] [PMC free article] [PubMed]
EXCITE 2011 {published data only}
- Golbaz I, Ahlers C, Stock G, Schutze C, Schriefl S, Schlanitz F, et al. Quantification of the therapeutic response of intraretinal, subretinal, and subpigment epithelial compartments in exudative AMD during anti-VEGF therapy. Investigative Ophthalmology & Visual Science 2011;52(3):1599-605. [DOI] [PubMed] [Google Scholar]
- Mayr-Sponer U, Waldstein SM, Kundi M, Ritter M, Golbaz I, Heiling U, et al. Influence of the vitreomacular interface on outcomes of ranibizumab therapy in neovascular age-related macular degeneration. Ophthalmology 2012;120(12):2620-9. [DOI] [PubMed] [Google Scholar]
- *.Schmidt-Erfurth U, Eldem B, Guymer R, Korobelnik JF, Schlingemann RO, Axer-Siegel R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology 2011;118(5):831-9. [DOI] [PubMed] [Google Scholar]
- Simader C, Ritter M, Bolz M, Deak GG, Mayr-Sponer U, Golbaz I, et al. Morphologic parameters relevant for visual outcome during anti-angiogenic therapy of neovascular age-related macular degeneration. Ophthalmology 2014;121(6):1237-45. [DOI] [PubMed] [Google Scholar]
Feltgen 2014 {published data only}
- Feltgen N, Pfeiffer S, Goerlitz A, Hennig H, Bretag M, Neunhoffer H, et al. Efficacy of intravitreal ranibizumab given bimonthly versus PRN in patients with neovascular age-related macular degeneration: 1 year results of a prospective, randomized clinical trial. Investigative Ophthalmology & Visual Science 2014;55(13):3930. [Google Scholar]
Feltgen 2017 {published data only}
- Feltgen N, Bertelmann T, Bretag M, Pfeiffer S, Hilgers R, Callizo J, et al. Efficacy and safety of a fixed bimonthly ranibizumab treatment regimen in eyes with neovascular age-related macular degeneration: results from the RABIMO trial. Albrecht von Graefes Archiv fur Klinische und Experimentelle Ophthalmologie [Graefe's Archive for Clinical and Experimental Ophthalmology] 2017;255(5):923-34. [DOI] [PubMed]
FLUID 2016 {published data only}
- Arnold JJ, Markey CM, Kurstjens NP, Guymer RH. The role of sub-retinal fluid in determining treatment outcomes in patients with neovascular age-related macular degeneration--a phase IV randomised clinical trial with ranibizumab: the FLUID study. BMC Ophthalmol 2016;16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymer RH, Markey CM, McAllister IL, Gillies MC, Hunyor AP, Arnold JJ, et al. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology 2019;126(5):723-34. [DOI] [PubMed] [Google Scholar]
Mahmood 2015 {published data only}
- Mahmood S, Roberts SA, Aslam TM, Parkes J, Barugh K, Bishop PN. Routine versus as-needed bevacizumab with 12-weekly assessment intervals for neovascular age-related macular degeneration: 92-week results of the GMAN trial. Ophthalmology 2015;122(7):1348-55. [DOI] [PubMed] [Google Scholar]
Mori 2017 {published data only}
RIVAL 2014 {published data only}
- Gillies M, Hunyor A, Woodcock C, Walsh M, Kurstjens N. Design and rationale of a clinical trial to measure the development of geographic atrophy in patients undergoing anti-vascular endothelial growth factor treatment for neovascular (WET) age-related macular degeneration: the RIVAL study. 46th Annual Scientific Congress of the Royal Australian and New Zealand College of Ophthalmologists; 2014 Nov 22-26; Brisbane, Australia 2014.
SAVE 2013 {published data only}
- Wykoff CC, Brown DM, Chen E, Major JC, Croft DE, Mariani A, et al. SAVE (Super-dose anti-VEGF) trial: 2.0 mg ranibizumab for recalcitrant neovascular age-related macular degeneration: 1-year results. Ophthalmic Surgery, Lasers & Imaging 2013;44(2):121-6. [DOI] [PubMed] [Google Scholar]
- Wykoff CC, Brown DM, Croft DE, Wong TP. Two year SAVE outcomes: 2.0 mg ranibizumab for recalcitrant neovascular AMD. Ophthalmology 2013;120(9):1945-6. [DOI] [PubMed] [Google Scholar]
SEVEN‐UP 2016 {published data only}
- Brijesh T, Shorya A. Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. American Journal of Ophthalmology 2016;162:200. [DOI] [PubMed] [Google Scholar]
Takayama 2017 {published data only}
- Takayama K, Kaneko H, Sugita T, Maruko R, Hattori K, Ra E, et al. One-year outcomes of 1 + pro re nata versus 3 + pro re nata intravitreal aflibercept injection for neovascular age-related macular degeneration. Ophthalmologica 2017;237(2):105-10. [DOI] [PubMed] [Google Scholar]
Tempelaar 2015 {published data only}
- Tempelaar S, Stephens S, Schouten J. Cost-effectiveness of treatments for wet age-related macular degeneration (WAMD) in the Netherlands, the case of intravitreal aflibercept (IVT-AFL) and ranibizumab. Value in Health 2015;18(7):A420. [Google Scholar]
Waldstein 2016 {published data only}
- Waldstein SM, Wright J, Warburton J, Margaron P, Simader C, Schmidt-Erfurth U. Predictive value of retinal morphology for visual acuity outcomes of different ranibizumab treatment regimens for neovascular AMD. Ophthalmology 2016;123(1):60-9. [DOI] [PubMed] [Google Scholar]
Wijeyakumar 2015 {published data only}
- Wijeyakumar W, Zhu M, Broadhead G, Hong TH, Chang AA. Comparison of aflibercept treatment regimes for treatment resistant neovascular age-related macular degeneration: 8 weekly versus an 'as-needed' regime. Investigative Ophthalmology & Visual Science 2015;56(7):4620. [Google Scholar]
References to studies awaiting assessment
Kon‐Jara 2007 {published data only}
- Kon-Jara V, Torres-Soriano M, Diaz-Rubio J, Hernandez-Rojas M, Guerrero-Naranjo J, Fromow-Guerra J, et al. Efficacy of retreatments with bevacizumab in choroidal neovascularization. Investigative Ophthalmology & Visual Science 2007;48(13):3349. [Google Scholar]
MATE 2015 {published data only}
- Airody A, Dorey T, Mankowska A, Balaskas K, Mukherjee R, Empeslidis T, et al. The MATE study: treating neovascular age related macular degeneration with aflibercept: a pilot, 24 month randomised controlled trial comparing standard care with individualised treat and extend regimen. Investigative Ophthalmology and Visual Science 2018;59(9):ARVO E-abstract 798.
Nunes 2014 {published data only}
- Nunes RP, Rodrigues E, Hirai FE, Barroso LF, Novais E, Badaro E, et al. Efficacy and cost-effectiveness of anti-VEGF treatments for age-related macular degeneration (AMD). Investigative Ophthalmology & Visual Science 2014;55(13):4939. [Google Scholar]
Ohnaka 2017 {published data only}
- Ohnaka M, Ohji M, Okada A, Terano Y, Kobayashi M, Takahashi K. Randomised, open-label study to evaluate 2 intravitreal aflibercept treat-and-extend dosing regimens in wet age-related macular degeneration: 52-week outcomes from ALTAIR. 49th Annual Scientific Congress of the Royal Australian and New Zealand College of Ophthalmologists; 2017 Oct 27-Nov 1; Perth, Australia 2017.
References to ongoing studies
Foss 2015 {published data only}
- Foss AJ, Childs M, Reeves BC, Empeslidis T, Tesha P, Dhar-Munshi S, et al. Comparing different dosing regimens of bevacizumab in the treatment of neovascular macular degeneration: study protocol for a randomised controlled trial. Trials 2015;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AAO 2015
- American Academy of Ophthalmology. Age-related macular degeneration. eyewiki.aao.org/Age-related_macular_degeneration (accessed 1 September 2015).
Abedi 2014
- Abedi F, Wickremasinghe W, Islam AF, Inglis KM, Guymer RH. Anti-VEGF treatment in neovascular age-related macular degeneration. Retina 2014;34(8):1531-8. [DOI] [PubMed] [Google Scholar]
ANCHOR 2009
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T, et al. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 2009;116(1):57-65. [DOI] [PubMed] [Google Scholar]
Berg 2017
- Berg K, Roald AB, Navaratnam J, Bragadóttir R. An 8-year follow-up of anti-vascular endothelial growth factor treatment with a treat-and-extend modality for neovascular age-related macular degeneration. Acta Ophthalmologica 2017;95:796-802. [DOI] [PubMed] [Google Scholar]
Bourne 2014
- Bourne RR, Jonas JB, Flaxman SR, Keeffe J, Leasher J, Naidoo K, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990-2010. British Journal of Ophthalmology 2014;98(5):629-38. [DOI] [PubMed] [Google Scholar]
Bressler 2009
- Bressler SB. Introduction: understanding the role of angiogenesis and antiangiogenic agents in age-related macular degeneration. Ophthalmology 2009;116(10 Suppl):S1-7. [DOI] [PubMed] [Google Scholar]
Brown 2005
- Brown GC, Brown MM, Sharma S, Stein JD, Roth Z, Campanella J, et al. The burden of age-related macular degeneration: a value-based medicine analysis. Transactions of the American Ophthalmological Society 2005;103:173-84. [PMC free article] [PubMed] [Google Scholar]
Brown 2006
- Brown MM, Brown GC, Sharma S, Stein JD, Roth Z, Campanella J, et al. The burden of age-related macular degeneration: a value-based analysis. Current Opinion in Ophthalmology 2006;17(3):257-66. [DOI] [PubMed] [Google Scholar]
Casten 2004
- Casten RJ, Rovner BW, Tasman W. Age-related macular degeneration and depression: a review of recent research. Current Opinion in Ophthalmology 2004;15(3):181-3. [DOI] [PubMed] [Google Scholar]
CATT 2012
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al, Comparison of Age-Related Macular Degeneration Treatments Trial (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119(7):1388-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chin‐Yee 2016
- Chin-Yee D, Eck T, Fowler S, Hardi A, Apte RS. A systematic review of as needed versus treat and extend ranibizumab or bevacizumab treatment regimens for neovascular age-related macular degeneration. British Journal of Ophthalmology 2016;100:914-7. [DOI] [PubMed] [Google Scholar]
Christen 1996
- Christen WG, Glynn RJ, Manson JE, Ajani UA, Buring JE. A prospective study of cigarette smoking and risk of age-related macular degeneration in men. JAMA 1996;276(14):1147-51. [PubMed] [Google Scholar]
CLEAR‐IT 2 2011a
- Heier JS, Boyer D, Nguyen QD, Marcus D, Roth DB, Yancopoulos G, et al. The 1-year results of CLEAR-IT 2, a phase 2 study of vascular endothelial growth factor trap-eye dosed as-needed after 12-week fixed dosing. Ophthalmology 2011;118(6):1098-106. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 17 January 2019.Available at www.covidence.org.
EuroQol 1990
- EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. [DOI] [PubMed] [Google Scholar]
Evans 2005
- Evans JR, Fletcher AE, Wormald RP. 28,000 cases of age-related macular degeneration causing visual loss in people aged 75 years and above in the United Kingdom may be attributable to smoking. British Journal of Ophthalmology 2005;89(5):550-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrara 2004
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocrine Reviews 2004;25(4):581-611. [DOI] [PubMed] [Google Scholar]
Fiske 2009
- Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annual Review of Clinical Psychology 2009;5:363-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friberg 2012
- Friberg TR, Bilonick RA, Brennen PM. Risk factors for conversion to neovascular age-related macular degeneration based on longitudinal morphologic and visual acuity data. Ophthalmology 2012;119(7):1432-7. [DOI] [PubMed] [Google Scholar]
Friedman 1999
- Friedman DS, Katz J, Bressler NM, Rahmani B, Tielsch JM. Racial differences in the prevalence of age-related macular degeneration: the Baltimore Eye Survey. Ophthalmology 1999;106(6):1049-55. [DOI] [PubMed] [Google Scholar]
Friedman 2004
- Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, Jong PT, et al. Prevalence of age-related macular degeneration in the United States. Archives of Ophthalmology 2004;122(4):564-72. [DOI] [PubMed] [Google Scholar]
GEFAL 2013
- Kodjikian L, Souied EH, Mimoun G, Mauget-Faysse M, Behar-Cohen F, Decullier E, et al. Ranibizumab versus bevacizumab for neovascular age-related macular degeneration: results from the GEFAL non-inferiority randomized trial. Ophthalmology 2013;120(11):2300-9. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 5 April 2019. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.Available at gradepro.org.
Gunda 2013
- Gunda V, Sudhakar YA. Regulation of tumor angiogenesis and choroidal neovascularization by endogenous angioinhibitors. Journal of Cancer Science and Therapy 2013;5(12):417-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
HARBOR 2014
- Ho AC, Busbee BG, Regillo CD, Wieland MR, Van Everen SA, Li Z, et al. Twenty-four-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2014;121(11):2181-92. [DOI] [PubMed] [Google Scholar]
Harper 2010
- Harper RA. Chronic visual loss. In: Basic Ophthalmology. 9th edition. San Francisco (CA): American Academy of Ophthalmology, 2010:47-71. [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
IVAN 2012a
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al, Inhibition of VEGF in Age-related choroidal Neovascularization (IVAN) Study Investigators. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012;119(7):1399-411. [DOI] [PubMed] [Google Scholar]
Kim 2016
- Kim LN, Mehta H, Barthelmes D, Nguyen V, Gillies MC. Metaanalysis of real-world outcomes of intravitreal ranibizumab for the treatment of neovascular age-related macular degeneration. Retina 2016;36:1418-31. [DOI] [PubMed] [Google Scholar]
Klein 1992
- Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 1992;99(6):933-43. [DOI] [PubMed] [Google Scholar]
Leibowitz 1980
- Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, Kahn HA, et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973-1975. Survey of Ophthalmology 1980;24(Suppl):335-610. [PubMed] [Google Scholar]
MANTA 2013
- Krebs I, Schmetterer L, Boltz A, Told R, Vecsei-Marlovits V, Egger S, et al. A randomized double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. British Journal of Ophthalmology 2013;97(3):266-71. [DOI] [PubMed] [Google Scholar]
MARINA 2006
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. New England Journal of Medicine 2006;355(14):1419-31. [DOI] [PubMed] [Google Scholar]
Miller 2013
- Miller JW. Age-related macular degeneration revisited – piecing the puzzle: the LXIX Edward Jackson memorial lecture. American Journal of Ophthalmology 2013;155(1):1-35. [DOI] [PubMed] [Google Scholar]
Moja 2014
- Moja L, Lucenteforte E, Kwag KH, Bertele V, Campomori A, Chakravarthy U, et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011230.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NEI VFQ‐25
- National Eye Institute. National Eye Institute Visual Functioning Questionnaire-25 (VFQ-25), 2000. nei.nih.gov/sites/default/files/nei-pdfs/vfq_sa.pdf (accessed prior to 28 May 2019).
PIER 2010
- Abraham P, Yue H, Wilson L. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. American Journal of Ophthalmology 2010;150(3):315-24. [DOI] [PubMed] [Google Scholar]
PrONTO 2009
- Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. American Journal of Ophthalmology 2009;148(1):43-58. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sarwar 2016
- Sarwar S, Clearfield E, Soliman MK, Sadiq MA, Baldwin AJ, Hanout M, et al. Aflibercept for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD011346.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt‐Erfurth 2011
- Schmidt-Erfurth U, Eldem B, Guymer R, Korobelnik JF, Schlingermann RO, Axer-Siegel R, et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology 2011;118(5):831-9. [DOI] [PubMed] [Google Scholar]
Schmucker 2015
- Schmucker CM, Rucker G, Sommer H, Virgili G, Loke YK, Oeller P, et al. Treatment as required versus regular monthly treatment in the management of neovascular age-related macular degeneration: a systematic review and meta-analysis. PloS One 2015;10(9):e0137866. [DOI] [PMC free article] [PubMed] [Google Scholar]
SECURE 2013
- Silva R, Axer-Siegel R, Eldem B, Guymer R, Kirchhof B, Papp A, et al. The SECURE Study: long-term safety of ranibizumab 0.5 mg in neovascular age-related macular degeneration. Ophthalmology 2013;120(1):130-9. [DOI] [PubMed] [Google Scholar]
Seddon 1996
- Seddon JM, Willett WC, Speizer FE, Hankinson SE. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA 1996;276(14):1141-6. [PubMed] [Google Scholar]
Solomon 2014
- Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD005139.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
SUSTAIN 2011
- Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingermann RO, et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology 2011;118(4):663-71. [DOI] [PubMed] [Google Scholar]
Swaroop 2007
- Swaroop A, Branham KE, Chen W, Abecasis G. Genetic susceptibility to age-related macular degeneration: a paradigm for dissecting complex disease traits. Human Molecular Genetics 2007;16(R2):R174-82. [DOI] [PubMed] [Google Scholar]
TAP 2001
- Bressler NM, Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Archives of Ophthalmology 2001;119(2):198-207. [PubMed] [Google Scholar]
VIP 2001
- Verteporfin In Photodynamic (VIP) Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization – verteporfin in photodynamic therapy report 2. American Journal of Ophthalmology 2001;131(5):541-60. [DOI] [PubMed] [Google Scholar]
VISION 2006
- Chakravarthy U, Adamis AP, Cunningham ET Jr, Goldbaum M, Guyer DR, Katz B, et al, VEGF Inhibition Study in Ocular Neovascularization (VISION) Clinical Trial Group. Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology 2006;113(9):1508.e1-25. [DOI] [PubMed] [Google Scholar]
WHO 2016
- World Health Organization. Causes of blindness and visual impairment. www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed 12 June 2019).
Wormald 2007
- Wormald R, Evans JR, Smeeth LL, Henshaw KS. Photodynamic therapy for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD002030.pub3] [DOI] [PubMed] [Google Scholar]
Yonekawa 2015
- Yonekawa Y, Miller JW, Kim IK. Age-related macular degeneration: advances in management and diagnosis. Journal of Clinical Medicine 2015;4(2):343-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Li 2016
- Li E, Donati S, Virgili G, Lindsley K, Krzystolik MG. Treatment schedules for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD012208] [DOI] [PMC free article] [PubMed] [Google Scholar]


