Abstract
Background
Prospective evidence supports active surveillance/watchful waiting (AS/WW) as an efficacious management option for low-risk prostate cancer that avoids potential treatment toxicity. AS/WW schedules require regular follow-up and adherence, and it is unknown to what extent patient socioeconomic status (SES) may impact management decisions for AS/WW. We sought to determine whether AS/WW use in the United States differs according to patient SES.
Design
Using the Surveillance, Epidemiology, and End Results Prostate with AS/WW Database, all adult men diagnosed with localized low-risk prostate cancer (clinical T1–T2a, Gleason 6, and prostate-specific antigen <10 ng/mL) and managed with either AS/WW, radical prostatectomy, or radiotherapy were identified between 2010 and 2015. SES tertile was measured by the validated Yost Index (low: 0–10,901; middle: 10,904–11,469; high: 11,470–11,827). AS/WW trends were defined across SES tertiles from 2010 to 2015. Logistic multivariable regression defined adjusted odds ratios (aOR) for receipt of AS/WW by SES tertile.
Results
In 50,302 men, AS/WW use was higher with increasing SES tertile (24.6, 25.3, and 30.5% for low, middle, and high SES tertiles, respectively; PTrend (SES) <0.001). From 2010 to 2015, AS/WW use in the low, middle, and high SES tertiles increased from 11.2 to 37.3%, 14.1 to 45.8%, and 17.6 to 46.4%, respectively (PTrends <0.001). By 2015, likelihood of AS/WW became comparable among the middle vs. high SES tertiles (aOR 0.96, 95% confidence interval (CI): 0.83–1.11, P = 0.55), but remained lower among the low vs. high SES tertile (aOR 0.73, 95% CI: 0.64–0.83, P < 0.001).
Conclusions
AS/WW use for low-risk prostate cancer in the US differs by SES. Despite increases in AS/WW across SES from 2010 to 2015, patients from low SES received significantly lower rates of AS/WW compared with higher SES groups. SES may therefore influence management decisions, where factors associated with low SES might act as a barrier to AS/WW, and may need to be addressed to reduce any disproportionate risk of unnecessary treatment to lower SES patients.
Introduction
Active surveillance/watchful waiting (AS/WW) is an efficacious management approach to low-risk prostate cancer that avoids potential treatment toxicity [1–3]. As such, national guidelines recommend AS/WW as the preferred management approach to low-risk prostate cancer [4]. Previous studies report that rates of AS/WW in the United States have increased significantly from 2010 to 2015 [5, 6].
Still, selecting patients who are most likely to have a good outcome with AS/WW compared to definitive treatment is controversial [7]. AS/WW schedules require regular follow-up via physical exams, prostate-specific antigen (PSA) monitoring, and sometimes repeat biopsy or imaging [4]. Thus, there is concern that patients with certain socioeconomic barriers to health care (e.g., those who cannot afford reliable transportation or adequate sick leave from work for multiple AS/WW follow-up appointments) might be at higher risk for AS/WW non-adherence [8, 9]. However, the impact of socioeconomic status (SES) on AS/WW use in the US is poorly understood. Therefore, we examined the recent rates of AS/WW over time across SES in the United States, using a novel and contemporary cohort of patients with quality-assured data.
Methods
Study Cohort
The US Surveillance, Epidemiology, and End Results (SEER) Prostate with AS/WW [10] Database (accessed through SEER’s custom data group) identified 50,302 men, aged ≥18 years, diagnosed with localized (N0M0) low-risk prostate cancer (clinical T1–T2a, Gleason 6, and PSA <10 ng/mL) [4] between 2010 and 2015, and “actively managed” with either initial AS/WW, radical prostatectomy, or radiation therapy (including external beam radiotherapy and/or brachytherapy) [5]. This patient cohort excluded men with either unknown T stage, Gleason score, or PSA (N = 6601), and men who were not “actively managed” (N = 10,203)—including men with unknown treatment status, men who were recommended active management but refused it, and men whose physicians decided not to actively manage (e.g., due to the presence of comorbidities). Compared with men who underwent AS/WW, men who were not actively managed would not have received regular PSA testing or routine follow-up for the monitoring of cancer-related symptoms.
This is the first SEER dataset to include a validated SES variable, the Yost Index [11], which incorporates a composite analysis of census tract-level median household income, median house value, median rent, percent below 150% of poverty line, education index, percent working class, and percent unemployed. The Yost Index is a continuous score where higher values correspond to higher SES. For the purposes of this study, SES was categorized into tertiles (Yost Index Range—low: 0–10,901; middle: 10,904–11,469; high: 11,470–11,827) [11].
AS/WW data was collected and quality assured by SEER starting in 2010 [10]; cases were coded as a single variable (“AS/WW”) given that a clinical distinction between AS and WW was not made until 2014 [12].
Statistical analyses
Descriptive statistics summarized baseline characteristics stratified by SES tertile. Kruskal–Wallis one-way analysis of variance (ANOVA) and χ2 tests compared the distribution of continuous and categorical variables, respectively, across SES tertiles.
AS/WW use over time was defined in each SES tertile from 2010 to 2015; trends in AS/WW over time and across increasing SES tertile were assessed using Cochrane-Armitage and Cuzick’s tests for trend, respectively. Multivariable logistic regressions defined adjusted odds ratios (aORs) and corresponding 95% confidence intervals (CI) for the receipt of AS/WW, with SES tertile as the primary independent variable of interest. Other variables included were age at diagnosis, race, PSA, number of positive biopsy cores, insurance status, and year of diagnosis. Interactions terms (SES tertile*Year of diagnosis) were tested in multivariable regression models to assess for any varying trends in AS/WW use over time across SES tertiles. For subgroup analyses, trends in AS/WW across increasing SES tertile were repeated after stratification by insurance status (insured [private or Medicare] vs. uninsured/Medicaid-insured) and race (Black vs. non-Black). On multivariable analysis, an exploratory interaction term (SES tertile*black race) was included to test for any effect modification in the receipt of AS/WW. A variance inflation factor (VIF) analysis was performed to assess for any collinearity in our multivariable regression model.
Two-sided P values with α = 0.05 were applied for all tests. Analyses were performed with Stata/SE 15.1 (Stata-Corp). The Dana-Farber/Harvard Cancer Center institutional review board granted a waiver of informed consent for this study.
Results
Baseline characteristics of the study cohort (N = 50,302) are described in Table 1.
Table 1.
Baseline characteristics across patient socioeconomic status (SES) tertiles, among 50,302 men diagnosed with low-risk prostate cancer (clinical T1–T2a, Gleason 6, and PSA <10 ng/mL) in the custom SEER with AS/WW Database and actively manageda from 2010 to 2015
| Characteristic | Low SES tertile (N = 18,678) |
Middle SES tertile (N = 14,487) |
High SES tertile (N = 17,137) |
P valueb |
|---|---|---|---|---|
| Age at diagnosis (median [IRQ]) | 63 (57–68) | 63 (57–67) | 62 (57–67) | 0.0015 |
| Race | ||||
| Non-Black | 14,752 (79.0) | 12,497 (86.3) | 15,536 (90.1) | <0.001 |
| Black | 3926 (21.0) | 1990 (13.7) | 1601 (9.3) | <0.001 |
| Insurance status | ||||
| Privately- or Medicare-Insured | 16,980 (90.9) | 12,875 (88.9) | 15,472 (90.3) | <0.001 |
| Medicaid | 808 (4.3) | 426 (2.9) | 388 (2.3) | <0.001 |
| Uninsured | 212 (1.1) | 157 (1.1) | 138 (0.8) | 0.004 |
| Unknown | 678 (3.6) | 1029 (7.1) | 1139 (6.6) | <0.001 |
| Initial management type | ||||
| AS/WW | 4594 (24.6) | 3668 (25.3) | 5222 (30.5) | <0.001 |
| Radical Prostatectomy | 7803 (41.8) | 6080 (42.0) | 6629 (38.7) | <0.001 |
| Radiation Therapy | 6281 (33.6) | 4739 (32.7) | 5286 (30.8) | <0.001 |
| PSA (median [IRQ]) | 5.4 (4.4–6.8) | 5.3 (4.3–6.6) | 5.2 (4.3–6.6) | <0.0001 |
| Number of positive biopsy cores | ||||
| ≤2 Cores | 8360 (44.8) | 6721 (46.4) | 8.176 (47.7) | <0.001 |
| ≥3 Cores | 5968 (32.0) | 4423 (30.5) | 5372 (31.4) | 0.02 |
| Unknown | 4350 (23.3) | 3343 (23.1) | 3589 (20.9) | <0.001 |
AS/WW active surveillance/watchful waiting, IQR interquartile range (reported as 25th–75th percentiles), PSA prostate-specific antigen, SEER Surveillance, Epidemiology, and End Results, SES socioeconomic status
Active management was defined as initial management with either AS/WW, radical prostatectomy, or radiation therapy. Men who were not actively managed (N = 10,203) were excluded from the patient cohort, and comprised 18.6% (N = 4261), 16.3% (N = 2815), and 15.4% (N = 3127) of all men in the low, middle, and high SES tertiles, respectively (P < 0.001)
P values represent χ2 and ANOVA tests for categorical and continuous variables, respectively
AS/WW rates were higher with increasing SES tertile (24.6%, 25.3%, and 30.5% for low, middle, and high SES tertiles, respectively; PTrend (SES) <0.001). On multivariable analysis (including adjustment for insurance status), compared with high SES, there was a lower likelihood of AS/WW for both low (aOR 0.65, 95% CI: 0.62–0.68, P < 0.001) and middle SES (aOR 0.80, 95% CI: 0.76–0.85, P < 0.001).
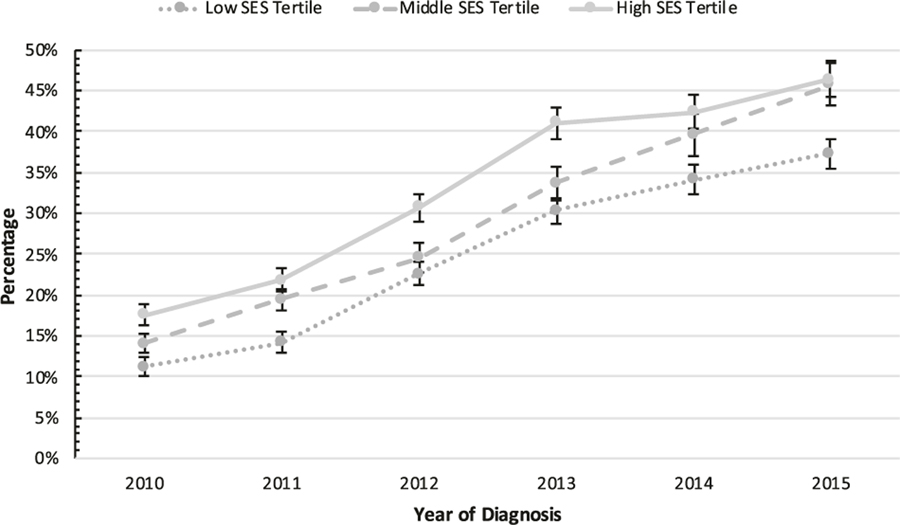
From 2010 to 2015, AS/WW rates in the low, middle, and high SES tertiles increased from 11.2 to 37.3% (+26.1%; aOR 1.41, 95% CI: 1.38–1.44, P <0.001 [per year increase]), 14.1 to 45.8% (+31.7%; aOR 1.40, 95% 1.36–1.43, P < 0.001 [per year increase]), and 17.6 to 46.4% (+28.8%; aOR 1.35, 95% 1.33–1.38, P <0.001 [per year increase]), respectively (PTrend(time) <0.001; Fig. 1). Rates of radical prostatectomy among the high, middle, and low SES tertiles decreased between 2010 and 2015 from 46.4 to 28.1% (−18.3%), 49.9 to 30.3% (−19.6%), and 45.9 to 34.1% (−11.8%), respectively. Similarly, rates of radiation therapy decreased from 36.0 to 25.5% (−10.5%), 36.0 to 24.0% (−12.0%), and 42.9 to 28.6% (−14.3%), respectively.
Fig. 1.
Rates of active surveillance/watchful waiting (AS/WW), as compared with definitive treatment with either radical prostatectomy or radiotherapy, from 2010 to 2015 by patient socioeconomic status (SES) tertile, among N = 50,302 men diagnosed with low-risk prostate cancer (clinical T1–T2a, Gleason 6, and PSA <10 ng/mL) in the custom SEER with AS/WW Database. Error bars represent 95% confidence intervals
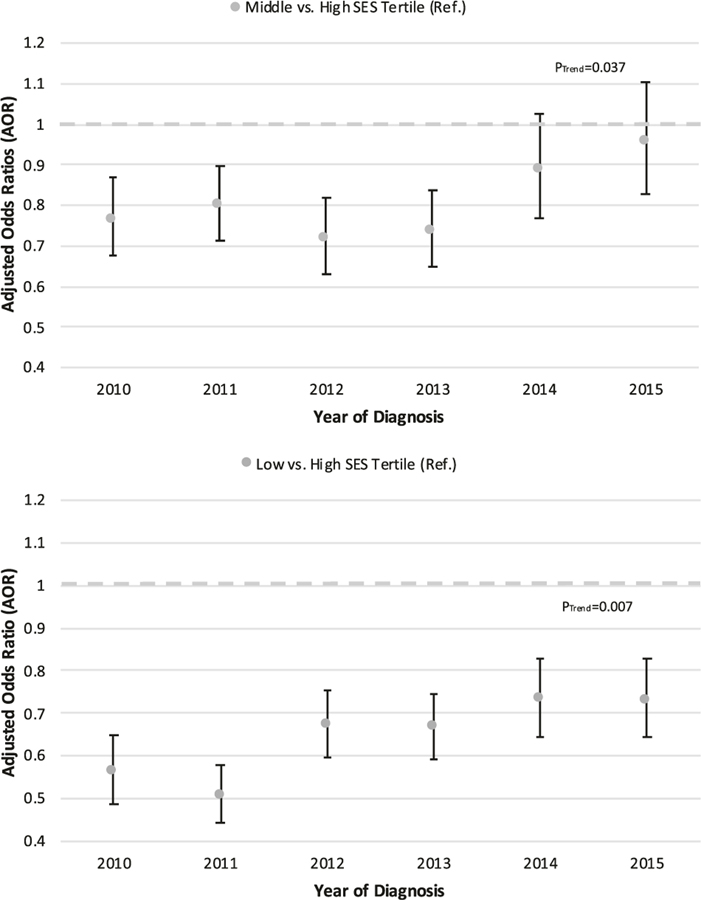
There were greater increases in AS/WW for the lower two SES tertiles compared with the high tertile such that differences in receipt of AS/WW across SES tertiles decreased over time (PInteraction = 0.007). Specifically, relative to the high SES tertile, the aOR for receipt of AS/WW for the middle tertile went from 0.77 in 2010 (95% CI: 0.67–0.87, P < 0.001) to 0.96 in 2015 (95% CI: 0.83–1.11, P = 0.55) (PTrend = 0.037; Fig. 2), and from 0.56 in 2010 (95% CI: 0.49–0.65, P < 0.001) to 0.73 in 2015 (95% CI: 0.64–0.83, P < 0.001) in the low SES tertile (PTrend = 0.007); still there was a lower odds of AS/WW for low compared with high SES throughout the study period (Fig. 2).
Fig. 2.
Yearly multivariable-adjusted odds ratios (aOR) for the receipt of active surveillance/watchful waiting (AS/WW) from 2010 to 2015 by patient socioeconomic status (SES): a Middle SES tertile vs. high SES tertile (referent); b low SES tertile vs. high SES tertile (referent). Patient cohort included N = 50,302 men diagnosed with low-risk prostate cancer (clinical T1–T2a, Gleason 6, and PSA <10 ng/mL) and actively managed (active management was defined as initial management with either active surveillance/watchful waiting, radical prostatectomy, or radiation therapy. Men not actively managed were excluded from analysis) in the custom SEER with AS/WW Database. Error bars represent 95% confidence intervals. AS/WW, active surveillance/watchful waiting; SEER, Surveillance, Epidemiology, and End Results; SES, socioeconomic status
On subgroup analysis after stratification by insurance status, AS/WW rates remained higher with increasing SES tertile among both insured (24.7%, 25.1%, and 30.6% for low, middle, and high SES tertiles, respectively; PTrend (SES) <0.001) and uninsured/Medicaid-insured men (19.3%, %, and 36.1%, respectively; PTrend (SES) <0.001). Similarly, after stratification by race, AS/WW rates also remained higher with increasing SES tertile among both Black (23.5%, 23.6%, and 30.7%, respectively; PTrend (SES) <0.001), and non-Black men (24.6%, 25.3%, and 30.8%, respectively; PTrend (SES) <0.001)—there was not a statistically significant interaction between black race and SES tertile (PInteraction = 0.43). Collinearity analysis showed that VIF values were <2.5 for all covariables within our multivariable regression model.
Discussion
This large US population-based analysis demonstrates that AS/WW use for low-risk prostate cancer increased across low, middle, and high SES groups from 2010 to 2015. Relative increases in AS/WW from 2010 to 2015 were greater in middle and low (where rates more than tripled) compared with high SES (where rates more than doubled). By the end of the study period, AS/WW rates became comparable between the middle and high SES groups. However, low SES continued to be associated with lower AS/WW rates when compared with high SES.
These findings are, to our knowledge, the first from a large and representative national cohort to describe population-based differences in AS/WW use and uptake by SES across the United States. These data demonstrate that SES may represent a potential factor that influences management decisions for low-risk prostate cancer, where factors associated with low SES may act as a barrier to receipt of AS/WW. This is concerning because low SES may put patients at increased risk of receiving potentially unnecessary treatment and subsequent treatment related toxicity [3, 13]. Concerted efforts will be needed to ensure that potential barriers to AS/WW are addressed, where creative solutions may be needed—such as programs to aid with transportation, incorporation of virtual PSA monitoring, and more AS/WW resources in plain language [9, 14, 15].
The drivers of these findings are likely multifactorial and complex in nature. One hypothesis is that both patients and providers may be concerned about barriers to adherence to AS/WW follow-up programs that may be introduced by factors associated with low SES [9], and as such may be more likely to proceed with treatment rather than AS/WW. Increasing rates of AS/WW for low SES patients in our study suggest that some of these barriers to AS/WW may be mitigated by the increasing knowledge of the indolent nature of low-risk disease through increased trial-based evidence and national guideline recommendations in support of AS/WW outcomes [1, 2, 4], along with subsequent expansion of clinician support of and familiarity with AS/WW [12]. Additionally, lower levels of education amongst lower SES patients (census tract-level education is incorporated into SEER’s variable for Yost SES), lower health literacy, and possibly greater fear and distrust of the US Health Care System could all contribute to our findings. Specifically, these factors could present barriers to AS/WW for low SES patients. Moreover, low SES patients may face barriers in advocating for their themselves and their healthcare preferences, such as implicit bias. For example, a low SES patient might be less likely to be viewed by healthcare providers as a patient with strong motivation for AS/WW, even if this is the patient’s true conviction. Lastly, another driver of our findings could be that patients with higher SES are more likely to be treated at academic institutions, where physicians might be more likely to manage with AS/WW compared to physicians at non-academic institutions.
This study has several limitations. First, we did not have data describing the treatment facility setting and were therefore unable to delineate between academic and non-academic institutions. As mentioned above, it is possible that treatment facility settings may act as a driver and confounder for management decisions at varying socioeconomic levels. Second, we were limited by a lack of information on the specific reasoning behind individual management decisions and therefore could not adjust for any of these potential confounders. Third, we lacked data on AS/WW adherence and were also unable to report on rates of AS/WW discontinuation across SES. Fourth, there are no data on median income ranges within the SEER Yost SES Index variable, which could help provide further context to the magnitude of SES differences among the analyzed subgroups. Continued investigation will be needed to determine the drivers for treatment decisions across SES and to monitor AS/WW use in low SES.
Acknowledgements
Dr. Mahal is funded by the Prostate Cancer Foundation-American Society for Radiation Oncology Award to End Prostate Cancer. Dr. Nguyen is funded by the Prostate Cancer Foundation. None of the funders listed above had any input on the design and conduct of the study; None of the funders listed above had any input on the collection, management, analysis, and interpretation of the data; None of the funders listed above had any input on the preparation, review, or approval of the manuscript; None of the funders listed above had any input on the decision to submit the manuscript for publication.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med 2016;375:1415–24. [DOI] [PubMed] [Google Scholar]
- 2.Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015;33:272–7. [DOI] [PubMed] [Google Scholar]
- 3.Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375: 1425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer. Version 2. 2018, 8 March 2018. National Comprehensive Cancer Network, Inc; https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed 1 Jul 2018. [Google Scholar]
- 5.Mahal BA, Butler S, Franco I, Spratt DE, Rebbeck TR, D’Amico AV, et al. Use of active surveillance or watchful waiting for low-risk prostate cancer and management trends across risk groups in the United States, 2010–2015. JAMA 2019;321:704–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeb S, Byrne N, Makarov DV, Lepor H, Walter D. Use of conservative management for low-risk prostate cancer in the veterans affairs integrated health care system from 2005–2015. JAMA 2018;319:2231–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Amico AV. Active surveillance versus treatment of prostate cancer: should metastasis be the primary end point? J Clin Oncol 2017;35:1638–40. [DOI] [PubMed] [Google Scholar]
- 8.Loeb S, Walter D, Curnyn C, Gold HT, Lepor H, Makarov DV. How active is active surveillance? Intensity of followup during active surveillance for prostate cancer in the United States. J Urol 2016;196:721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zapka J, Taplin SH, Price RA, Cranos C, Yabroff R. Factors in quality care—the case of follow-up to abnormal cancer screening tests-problems in the steps and interfaces of care. J Natl Cancer Inst Monogr 2010;2010:58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surveillance, Epidemiology, and End Results Program Prostate with Active Surveillance/Watchful Waiting Database. 2018. https://seer.cancer.gov/seerstat/databases/prostate-ww/index.html. Accessed 28 May 2018.
- 11.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–11. [DOI] [PubMed] [Google Scholar]
- 12.Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen M, D’Amico AV, et al. Prostate cancer, version 2. 2014. J Natl Compr Canc Netw 2014;12:686–718. [DOI] [PubMed] [Google Scholar]
- 13.Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol 2014;65:1046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyajian R, Simeoli A, Orio PF, Martin NE, King MT, Mouw KW, et al. Virtual PSA Monitoring Program Pilot: the integration of technology in post treatment prostate cancer patient care to optimize workflow and increase access to care. Int J Radiat Oncol Biol Phys 2018;102:e101–e102. [Google Scholar]
- 15.Loeb S, Curnyn C, Fagerlin A, Braithwaite RS, Schwartz MD, Lepor H, et al. Informational needs during active surveillance for prostate cancer: a qualitative study. Patient Educ Couns 2018;101:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]