Abstract
Purpose of review:
In perilacunar/canalicular remodeling (PLR), osteocytes dynamically resorb, and then replace, the organic and mineral components of pericellular extracellular matrix. Given the enormous surface area of the osteocyte lacuna-canalicular network (LCN), PLR is important for maintaining homeostasis of the skeleton. The goal of this review is to examine the motivations and critical considerations for the analysis of PLR, in both in vitro and in vivo systems.
Recent findings:
Morphological approaches alone are insufficient to elucidate the complex mechanisms regulating PLR in the healthy skeleton and in disease. Understanding the role and regulation of PLR will require the incorporation of standardized PLR outcomes as a routine part of skeletal phenotyping, as well as the development of improved molecular and cellular outcomes. Current PLR outcomes assess PLR enzyme expression, the LCN, and bone matrix composition and organization, among others.
Summary:
Here, we discuss current PLR outcomes and how they have been applied to study PLR induction and suppression in vitro and in vivo. Given the role of PLR in skeletal health and disease, integrated analysis of PLR has potential to elucidate new mechanisms by which osteocytes participate in skeletal health and disease.
Keywords: osteocyte, perilacunar/canalicular remodeling, lacuna-canalicular network
INTRODUCTION
Osteocytes are highly differentiated cells derived from osteoblasts. In some ways, they represent the last frontier of bone cell biology. With a phenotype specialized for cellular function within a mineralized extracellular matrix (ECM), osteocytes have been difficult targets for standard cellular and molecular biology approaches. Nonetheless, technical and scientific advances have provided insight into these cells and their essential roles in skeletal and systemic homeostasis and disease [1,2], including the process by which they continually remodel the surrounding ECM. To further understand these dynamic cells, and their role in health and disease, we review the current approaches used to investigate osteocyte perilacunar/canalicular remodeling, and discuss key considerations for each of these outcomes.
Perilacunar/Canalicular Remodeling (PLR)
One of the unique characteristics of osteocytes is their formation of a highly organized network within a mineralized matrix [3]. Dendrites projecting from osteocyte cell bodies form gap junctions with dendrites from neighboring osteocytes, as well as with bone lining osteoblasts and osteoclasts, and vasculature. These dendrites extend through canaliculi to form the lacuna-canalicular network (LCN). Fluid flow through the perilacunar and canalicular spaces supports osteocyte cellular functions. Osteocytes, in turn, respond to chemical, biological, and physical cues carried by this fluid with changes in cellular behavior. These changes include secretion of osteoclast and osteoblast regulating molecules [4–6], by which osteocytes indirectly impact bone remodeling, as well as activation or suppression of osteocyte intrinsic perilacunar/canalicular remodeling (PLR). The latter is the process by which osteocytes directly resorb and replace the local bone ECM. In PLR, osteocytes resorb the organic and mineral components of perilacunar and pericanalicular ECM through a combination of matrix metalloproteases (MMPs), ATPase proton pumps, and other enzymes, such as cathepsin K and carbonic anhydrases [7,8].
Our early understanding of PLR derived almost entirely from morphologic analyses. Light and electron microscopy first revealed ‘osteocyte osteolysis’, with enlarged lacunae and rough borders around osteocytes in bone infection, rickets, or osteomalacia [9–12]. Visualization of perilacunar and pericanalicular fluorochrome labels in bone from parathyroid hormone (PTH) treated rats [13] provided early functional evidence for the formation of new mineralized tissue by osteocytes [14]. Morphological evidence in bone from numerous species, including humans, snakes, egg-laying hens, rodents, and hibernating bats and ground squirrels [15–18], highlights the extensive conservation of osteocyte PLR, especially in the context of metabolic stress.
Although review of these pioneering studies clearly implicates PLR in skeletal homeostasis and disease, interest in PLR was supplanted by the idea that osteoclasts are the primary bone resorbing cell type [15]. In 1977, Parfitt published a paper disputing ideas advanced by Krook and Belanger in 1970. In addition to discrediting the idea of ‘bone flow’, Parfitt argued that “The belief that osteocytes resorb substantial amounts of bone rests on invalid conclusions from indirect techniques, various artifacts of specimen processing and unawareness of the microscopic characteristics of woven bone.” Given Parfitt’s stature in the field, his skepticism was sufficient to suppress research on the role of osteocytes in bone remodeling for years.
During the time in which PLR was largely overlooked, osteoclast and osteoblast biology advanced tremendously through the use of genetically modified mouse models and other molecular and cellular approaches, leaving our knowledge of osteocyte PLR far behind. More recent application of these tools to investigate osteocytes has largely resolved the critiques raised by Parfitt, which were significantly founded on methodologic limitations. Nonetheless, the field sill wrestles with the need to identify the most robust PLR outcome measures. Traditional morphological approaches alone are insufficient to probe the complex biological mechanisms involved in this process. Several new PLR outcomes have been described, but which of these are most useful to rigorously and efficiently evaluate PLR remains unclear. Likewise, given the many questions about PLR itself (Box 1), results must be carefully interpreted in light of each technical approach. In this review, we will examine current PLR methods and how these have been applied to study PLR induction and suppression in vitro and in vivo.
Box 1: Major questions about PLR.
Resorption vs. Deposition: What are the mechanisms controlling the deposition of new ECM around osteocytes, and how is this coupled to osteocytic resorption?
Lacunae vs. Canaliculi: Do distinct or common processes regulate osteocytic remodeling at lacunae and at canaliculi?
Acid vs. Proteases: Which aspects of PLR depend on acidification of the osteocyte microenvironment, and which require proteolysis?
Mineral vs. Organic: Do distinct or common processes control the resorption of mineral and organic components of the ECM around osteocytes?
Motivation to Investigate PLR
Several lines of evidence motivate incorporation of PLR outcomes as a standard part of skeletal analyses. First, PLR participates in the crosstalk among osteocytes, osteoblasts, and osteoclasts. Many of the same stimuli already known to regulate osteoclast and osteoblast function also regulate PLR, including glucocorticoids [19–22], vitamin D [23], ovariectomy [24–28], PTH [15,29,30], and calcium or phosphate deficiency [7,14,31]. In addition, PLR may be mechanosensitive. Sclerostin and TGFβ, both of which are required for the anabolic response of bone to mechanical load, stimulate PLR [32–35]. Mice exposed to microgravity may remodel the ECM in a mechanosensitive, MMP10-dependent manner [36]. However, in mice with immobilized hind limbs, lacunar properties (size, shape, orientation) are not altered, despite the corresponding decrease in bone volume and load to fracture [7,37,38]. Careful investigation utilizing a range of PLR outcomes may help to unravel these apparently contradictory findings.
Second, the study of PLR will elucidate mechanisms controlling metabolic and mechanical homeostasis in the skeleton. Osteocytes utilize PLR to respond to metabolic stress. The lactation model is currently used as a gold standard model to study PLR, since osteocytes stimulate local mineral resorption to meet the calcium demands of milk production [7,39]. PLR occurs in both male and female mouse bone [4], though the sex-dependent mechanisms that control baseline PLR remain to be defined. PLR also plays a critical role in maintaining the material quality of the bone matrix, in part by controlling ECM mineralization and collagen organization [40,41]. Though much is known about the biological control of bone mass, many questions remain about the mechanisms that control this important aspect of bone quality and the ability of bone to resist fracture.
Third, understanding PLR may elucidate new mechanisms of musculoskeletal disease. As with any essential homeostatic process, deregulation of PLR is a product as well as a driver of degenerative disease processes. As for remodeling by osteoblasts and osteoclasts, too much or too little remodeling by osteocytes can be problematic (Figure 1). PLR is induced in rickets and osteomalacia [31,42], but suppressed in aging [43,44]. Relative to young bone, bone from aging humans and mice has many hallmarks of suppressed osteocyte PLR, including decreased lacunar volume, a diminished LCN, hypermineralization, and increased bone fragility [45–47]. This bone fragility is not fully explained by a loss of bone mass [48] and is attributed in part to impaired bone extracellular matrix material properties. Mounting evidence implicates an age-dependent defect in osteocyte PLR, which may in turn further impact the regulation of osteoblast and osteoclast function [46,49–52]. Although the extent to which PLR is a driver or a side effect of age-related bone fragility is yet unclear, PLR suppression may play a causal role in many diseases of bone and bone quality. For example, in glucocorticoid-induced osteonecrosis, even prior to other symptoms, glucocorticoid treatment suppresses PLR with disruption of the LCN, collagen organization, and matrix mineralization [21]. These changes in mice parallel those in subchondral bone from patients with end stage glucocorticoid-induced osteonecrosis of the femoral head [53]. Given a role for PLR in disease and aging, an improved understanding of PLR may also lead to the identification of new cellular targets for musculoskeletal therapeutics.
Figure 1: Homeostatic control of bone mass and quality by osteoblasts, osteoclasts, and osteocytes.
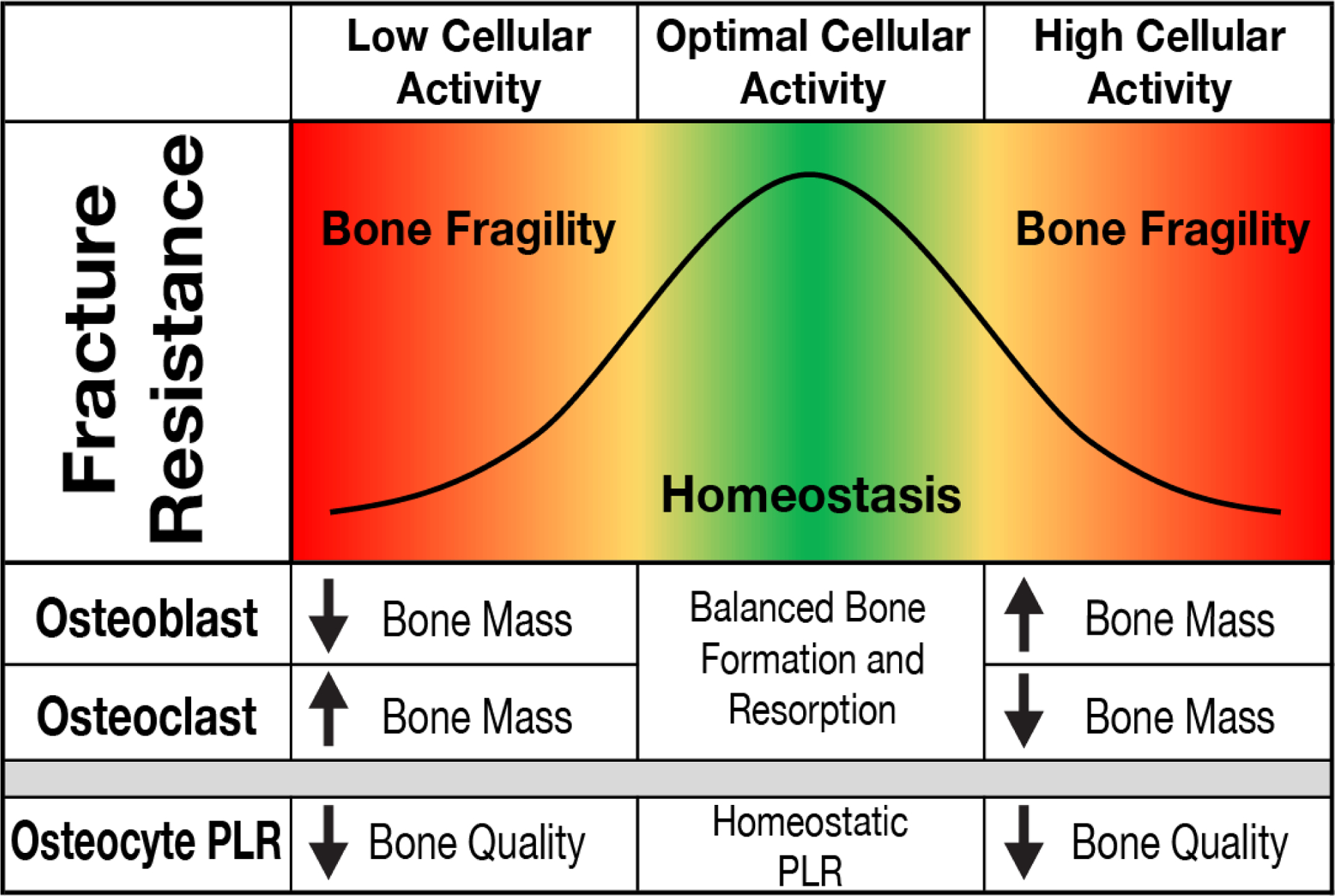
During homeostasis (green region), optimal cellular activity of osteoblasts and osteoclasts maintains bone resistance to fracture. Unopposed changes in osteoblast or osteoclast function lead to changes in bone mass that can cause bone fragility (red regions). Osteocyte PLR also contributes to the mechanical homeostasis of bone. As for osteoblasts and osteoclasts, the effect of osteocyte PLR on bone quality is non-linear. Too much or too little osteocytic PLR can compromise bone quality.
Many questions remain about PLR in homeostatic conditions and how it differs with developmental stage, sex, aging, and disease. Systematic evaluation of PLR in these conditions is needed to more fully understand the integral role of osteocytes in skeletal homeostasis and disease.
Critical Considerations for the Study of PLR
Although aspects of PLR are apparent throughout the skeleton, the LCN varies among species, anatomical sites, and bone types. For example, the LCN appears vastly different when comparing the sparse canaliculi in elderly human trabecular bone to the robust network of canaliculi in young mouse cortical bone (Figure 2, A,F). In addition to age, several other biological factors influence the appearance of the LCN. Cortical bones in humans, as well as other large mammals, are composed of Haversian systems, or osteons. Osteocytes within osteons arrange themselves in rings around the central vascular canal, with their canaliculi often perpendicular to the rings. Smaller rodents, in contrast, do not have Haversian systems and their long bones resemble a single large osteon around the marrow cavity [54], often with internal rings of slightly less organized lamella and osteocyte canaliculi [55]. The effect of structural differences in bone organization on the LCN are likewise apparent in trabecular bone, where the diminished lamellar organization corresponds to less aligned canaliculi (Figure 2, C,F). Accordingly, the LCN is less aligned in the mouse mandible, which is dominated by trabecular bone, relative to cortical bone of the mouse femur [19], where osteocytes are spatially aligned perpendicular to the lamellar plane [19] (Figure 2, E,F). Even within one bone, proximal to distal and medial to lateral variations in the LCN are apparent. Therefore, care must be taken to consistently analyze LCN parameters at the same region of interest in each bone.
Figure 2: Diversity in the histological appearance of the lacuna-canalicular network (LCN) across species and bones.
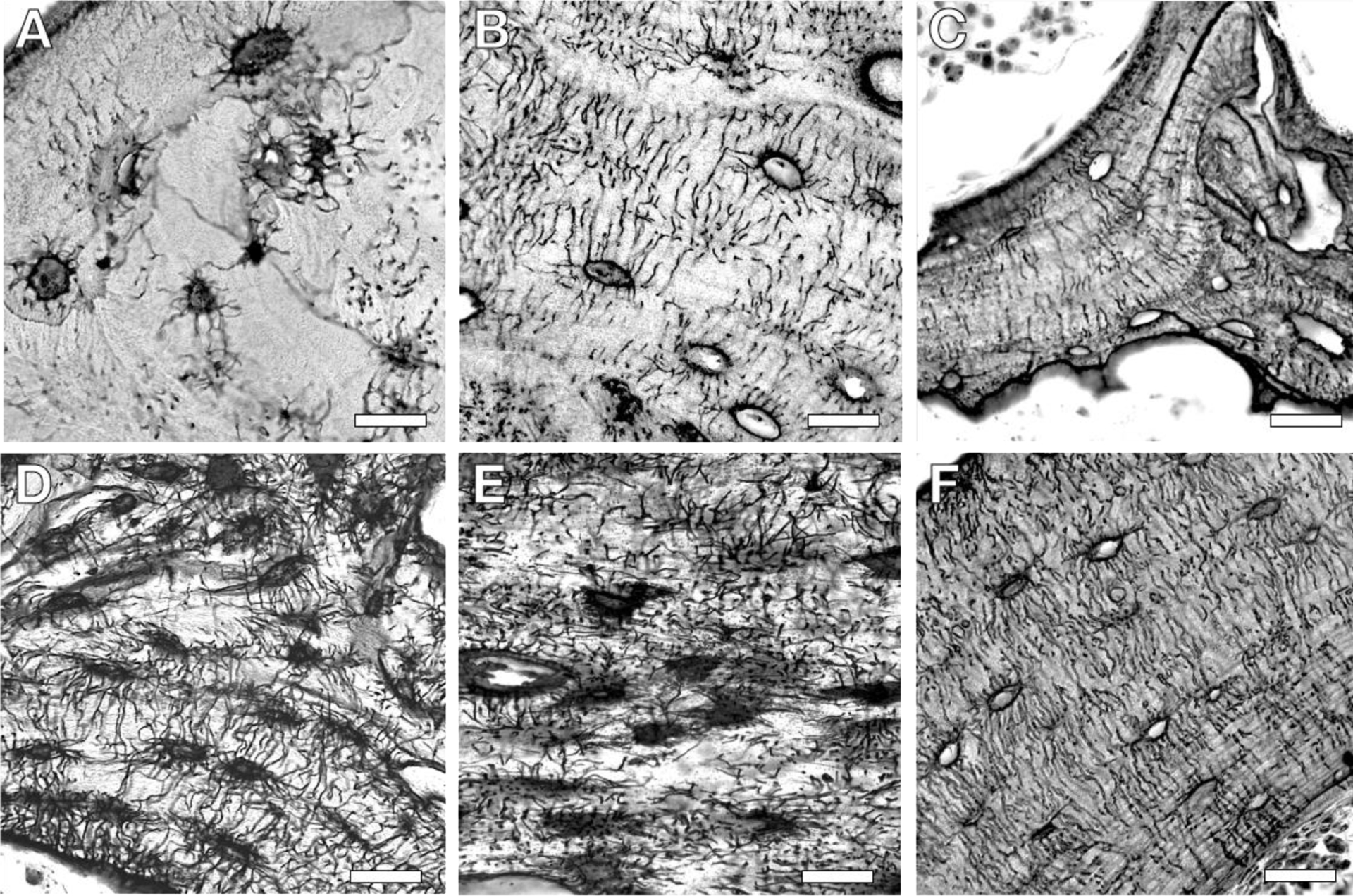
Differences in the LCN are apparent in silver nitrate stained sections of human (A), rabbit (B), and mouse (C) trabecular bone. LCN variation also appears among bones, as shown from mouse cortical bone of the cochlea (D), mandible (E), and femur (F). Scale bar, 20 μm.
Among the factors that contribute to these anatomic differences in the LCN are site-specific expression and activity of the enzymes required for PLR. For example, MMP2 expression varies across the skeleton, with high levels in the calvaria and low levels in the long bones [56]. This expression pattern likely explains the observation that MMP2-deficient mice have dramatic alterations of the LCN in calvaria compared to moderate disruption of the LCN in long bones. Likewise, the LCN, collagen organization, and bone matrix mineralization were most affected in mid-cortical bone of MMP13-deficient mice, which was the site of highest MMP13 expression [56]. Similar differences in PLR enzyme expression may contribute to hypermineralization in the endocortical region compared to periosteal region of aging cortical bone [45]. Post-translational control of PLR enzymes may also determine where and how PLR is regulated, since cochlear bone expresses high levels of MMP13 protein, but the hearing and cochlear bone of MMP13-deficient mice was normal [57]. This observation is consistent with the presence of unique mechanisms to control bone remodeling by osteoblasts and osteoclasts in the cochlea. Undoubtedly, a sophisticated network of transcriptional and post-transcriptional mechanisms control bone remodeling by osteocytes, as it does for osteoblasts and osteoclasts.
Variations in the LCN and PLR within and between bones highlight the heterogeneity of the osteocyte population. Though the sources and the reasons for this heterogeneity remain largely unknown, intriguing possibilities include differences in the embryonic origin, exposure to physical stimuli, metabolic supply/demand, innervation, and others. For example, elongated lacunae are observed in cortical bones, such as the tibia, that undergo loading during locomotion [58,59], compared to calvaria [60]. This suggests that loading and lacunar shape may share a functional relationship, which potentially contributes to the LCN differences along the cortical regions of long bones [55]. Mechanistic studies are needed to establish the causality of these and other observations. Meanwhile, these observations have important practical implications for the study of PLR. Attention to species-specific and anatomically distinct differences in bone must be considered when studying PLR, especially when extrapolating findings from rodent models to understand human clinical conditions.
A number of mechanistic questions about the regulation of PLR further complicate our understanding of this process (Box 1). Most work focuses on the resorption of perilacunar and pericanalicular bone, while much less is known about the deposition of new ECM. In some cases, the induction or repression of PLR enzymes corresponds to changes in lacunar size or shape; whereas in others, lacunar size is unchanged, but canalicular networks are altered. The mechanisms that determine when, where, and how proteolysis or acidification exert their effects in the mineral and/or organic phases of the osteocyte microenvironment remain unclear. While these processes initially appeared to be coupled, more recent studies suggest that perilacunar remodeling is distinct from pericanalicular remodeling [7,34].
Furthermore, the approaches used to study PLR may impact the interpretation of the results. If distinct mechanisms control the resorption of the mineral and organic components of the bone matrix, then the appearance of the LCN may differ depending on whether the outcome is performed on mineralized bone (i.e. quantitative backscatter electron imaging) or demineralized bone (i.e. histological staining of the LCN). Efforts of the scientific community to elucidate the role of PLR in bone homeostasis, and how PLR is regulated normally and pathologically will require attention to the sensitivities of the methods used, as well as to the diverse regulation of PLR throughout the skeleton, across species, and with age. The following discussion considers these issues in light of a number of currently used PLR outcome measures: PLR enzyme expression and osteocyte acidification, the geometry of the LCN, and the management of the organic and mineral containing portions of the bone ECM.
Cell Models to Investigate PLR
Developing a mechanistic understanding of PLR will benefit from in vitro, cellular, and molecular approaches. In vitro analysis of PLR requires an appropriate cell culture model as well as in vitro PLR outcome measures that correspond to PLR outcomes in vivo. An ideal cell culture model would recapitulate the mature osteocyte phenotype and respond to growth factors, hormonal, or physical signals that regulate PLR. The advantages and limitations of currently available osteocyte-like cell lines have been detailed in other reviews [61,62]. The most commonly used osteocyte-like cell lines are MLO-Y4, MLO-A5, IDG-SW3, OCY454, and SaOS2 cells. MLO-Y4 and MLO-A5 cells are most often used as early osteocytes and post-osteoblast/pre-osteocyte cells, respectively [62–64]. MLO-Y4 cells are highly sensitive to fluid flow stress, as demonstrated by the increased release of PGE2 and ATP, and are valuable for evaluating the effects of fluid flow on signaling pathways [62]. MLO-A5 cells, on the other hand, are useful to investigate the osteoblast to osteocyte transition and in mineralization studies [62]. However, neither of these cells express late osteocyte markers such as Sclerostin, which also regulates PLR [32]. Therefore, alternative cell lines that better represent mature osteocytes may be more relevant for studies investigating PLR.
IDG-SW3, OCY454, and SaOS2 cell lines express late osteocyte markers, including Sclerostin. Advantages of IDG-SW3 cells include expression of Sclerostin and FGF-23 [65], possible interaction with artificial 3D collagen matrices, and retention of viability over a 21-day period that may be useful for long-term PLR analyses [65]. OCY454 are osteocyte-like cells that respond to mechanical (fluid flow shear stress), cytokine (PGE2), and hormonal (PTH) stimuli and express high levels of Sclerostin, even without added differentiation factors [66]. SaOS2, a human osteosarcoma cell line, exhibits osteocyte-like characteristics, such as high Sclerostin expression, PTH responsiveness, and in vitro mineralization [67]. A new osteocyte cell line, OmGFP66 cells, recapitulates important aspects of the osteocytic phenotype and behavior, including the formation of three-dimensional bone spicules. The cells are derived from mice that expressed a membrane tagged GFP variant under control of the DMP1 promoter, facilitating visualization of cells embedded within the surrounding mineral [48]. Since these cells extend dendrites and form lacunar structures in vitro, they could be useful tools for the study of PLR. Although each of these immortalized cell lines have previously been used to study osteocyte behavior, only MLO-Y4 and OCY454 cells have reportedly been used to investigate outcomes related to PLR.
All of these cell lines have specific limitations and none completely recapitulate osteocyte behavior. For example, MLO-Y4 and MLO-A5 cells are not fully differentiated, OCY454 and IDG-SW3 cells need multiple weeks to fully differentiate [65,66], and SaOS2 cells are derived from human osteosarcoma and therefore maintain inherent differences to endogenous, healthy osteocytes. As a result of these limitations, more investigators are now using a gold-standard of primary osteocytes, which are more representative of in vivo osteocyte behavior [68]. Several studies demonstrate the ability of human and mouse primary osteocytes to mimic in vivo osteocyte behaviors, including aging and cell behavior in 3D, that are otherwise difficult to capture with the immortalized lines [30,50,69–72]. Thus, despite the labor intensive and challenging isolation protocol, primary osteocytes and other similar cell lines, such as human primary osteocyte-like cells [32], fulfill many criteria for an ideal in vitro model to study PLR. A balanced approach might test mechanistic hypotheses using more tractable osteocyte cell lines, which could then be validated with primary osteocytes.
Molecular and In Vitro PLR Assays
Osteocytes dynamically resorb the mineralized and organic components of the bone extracellular matrix through secretion of proteases and acidification of the microenvironment. These cell-intrinsic behaviors provide suitable outcomes to study PLR in vitro, specifically by monitoring the expression of enzymes implicated in PLR and pH in osteocytic cell lines or in primary osteocytes.
PLR Enzyme Expression:
Numerous enzymes have been functionally implicated in PLR through in vivo studies. The ‘PLR enzymes’ are expressed at the time and site of PLR, and their expression responds to stimuli that regulate PLR. In many cases, ablation of these enzymes in mice causes disruption of the LCN. Specifically, the long bones from lactating mice have increased mRNA expression of cathepsin K, TRAP (ACP5/Acp5), and carbonic anhydrase 1 and 2 [7,32,73]. Similar to osteoclasts, osteocytes simultaneously express acid catalyzed proteases and ATP proton pumps, implying a coordinated effort by the osteocyte to 1) establish extracellular pH gradients within the LCN and 2) activate proteolytic events to resorb the surrounding bone matrix [7,18,34,74,75]. Other proteases, such as MMP2, MMP13 and MMP14, are also expressed by osteocytes and play a critical role in maintaining the LCN [56,76,77]. In cortical bone from mice with an osteocyte-specific deletion of the TGFβ type II receptor, qPCR revealed a coordinated repression of multiple PLR enzymes. The same bones had several features consistent with suppressed PLR, including diminished canalicular networks and bone quality deficits [34]. Other conditions that induce or suppress PLR (i.e. lactation or glucocorticoid treatment, respectively) also show coordinated induction or repression of PLR genes [7,21], suggesting that PLR enzymes may share some common regulatory mechanisms. Since many of these PLR genes are expressed by bone resorbing osteoclasts and other cell types, parallel use of immunohistochemistry is needed to definitively demonstrate that gene expression differences in osteocyte-enriched cortical bone mRNA are primarily due to osteocytes.
Not only are these PLR enzymes robust measures of PLR in vivo, but they also respond to PLR regulatory stimuli in vitro. Analysis of PLR enzyme expression using MLO-Y4, OCY454, and human osteocyte-like cells (hOCy) is a useful indicator of the extent of PLR induction or suppression [32,34]. For example, TGFβ induces MMP13 and cathepsin K expression in MLO-Y4 and OCY454; whereas recombinant human Sclerostin induces ACP5, cathepsin K, and carbonic anhydrase 2 expression in MLO-Y4 and hOCy [32,34]. In our laboratory, initial analyses of gene and protein expression typically focus on MMP13 and cathepsin K, followed by MMP2, MMP14, carbonic anhydrase 2, and various ATPases (i.e. ATP6V1G1 and ATP6V0D2) if initial results suggest a role in PLR regulation [7,34]. In some cases, carbonic anhydrase 2 and the ATPases seem to be regulated distinctly from the expression of the proteases, suggesting the possible presence of compensatory mechanisms between the acidification and proteolytic functions of PLR.
Intracellular Osteocyte Acidification:
During PLR, osteocytes undergo acidification using cellular and molecular mechanisms that parallel those used by osteoclasts [7,18,30,34,74,75]. This results in changes in intracellular (pHi) as well as extracellular pH [30]. However, the mechanisms by which osteocytes tolerate an acidic microenvironment remain unknown. Since both osteocytes and osteoclasts resorb bone using similar mechanisms, osteocytes may also employ mechanisms that are well known in osteoclasts [78–80], such as HCO3−/Cl− exchange [81], to withstand this challenging microenvironment. Other cellular adaptations to the acidic environment include skin cells, which endure acidic conditions that promote attachment of protective resident bacterial flora [82], fibroblasts, which resist the inflammatory acidic microenvironment that activates tissue remodeling [83], and cells in the tumor microenvironment that resist acidification during metastasis [84].
Quantification of this acidification process in cultured osteocytes may provide a valuable functional measure of PLR. Though quantification of pH changes in the culture media has proven challenging, intracellular pH (pHi) changes in osteocytic cell lines represent a useful PLR surrogate. In the pHi assay, cells loaded with a pH-sensitive dye reveal quantitative changes in pHi through changes in fluorescence measurements. Previous studies using pHi assays revealed that MLO-Y4 osteocytes decreased pHi in a cell-intrinsic manner [30,32,34] in response to Sclerostin and TGFβ, both of which induce PLR in vivo [34]. To our knowledge, this is currently the best available functional in vitro PLR outcome, particularly when combined with an analysis of PLR enzyme gene expression. Nonetheless, an important limitation of this approach is that pHi is affected by multiple processes, including hypoxia and mitochondrial respiration [85,86]. While hypoxia and mitochondrial function are also related to PLR [47,87], more specific assays will be needed to distinguish whether changes to pH in response to these processes are independent of or coupled to active PLR.
Visualization of the Lacuna-canalicular Network
Two-dimensional Histological Analysis of the LCN:
Morphological examination of the LCN provided the first evidence of PLR utilizing histological stains to visualize the LCN. Common histological stains include H&E, Alizarin red, basic fuschin [88–90], toluidine blue [20,73] and ploton silver nitrate stain [13,19–21,31,32,57,73]. For example, H&E and toluidine blue can be used to distinguish empty lacunae from lacunae containing osteocytes in the section plane, while ploton silver nitrate stain can be used for qualitative two-dimensional visualization of both lacuna and canaliculi.
Semi-quantitative lacunae and canaliculi measurements have been performed and reported in various ways. For example, lacunae measurements have been reported as percent lacuna-canalicular volume [19], number of lacunae occupied by osteocytes per bone area [47,91,92], number of empty lacunae per bone area [46,47,91,93], lacunae area [3,13,19,91], and lacunae area per osteocytes [31]. Canalicular measurements also have been reported as number of canaliculi per osteocytes [31,47], canalicular connectivity [31], canalicular branching [31], and canalicular length per osteocyte [34,93]. Lacunar and canalicular measurements have also been summarized and reported as lacuna-canalicular area [3,57] as well as other various parameters [3]. Due to the variations of these measurable LCN outcomes, there is a need for an established set of lacunar and canalicular parameters when reporting these results. There is also wide variation in osteocyte analysis on histological sections with often low sample size and low number of images per section which may lead to misinterpretation of the LCN. These issues are critical to address since PLR changes to the LCN can be subtle and may be localized. The variable nature of histological stains for LCN visualization has slowed the development of accurate automatic measuring software, which has limited the field to manual measurements that are time consuming and subjective to the grader – adding to uncontrolled consistency across experiments. Nonetheless, a standardized approach for reproducible staining, consistent selection of regions of interest, adequate biological and technical replicates, and blinded grading help to overcome these limitations and significantly increase the rigor of LCN histological outcomes.
Three-dimensional Visualization of the LCN:
Traditional two-dimensional methods of visualizing morphological features of the LCN are planar and ignore the three-dimensional nature of the osteocyte network. Reconstructing and understanding the complex geometries of the osteocyte LCN is critical as it supplies needed clues to the health, arrangement, and activity of osteocytes involved in PLR. The reliable imaging and reconstruction of the LCN is a naturally challenging endeavor due to the density of the canalicular network and the nanoscale dimensions of these processes within an optically opaque, three-dimensional material.
Several methods, previously and more extensively reviewed [20,60,94], have focused on capturing the LCN in three dimensions. With the advent of confocal microscopy, it has become more feasible to view the LCN in three dimensions [46,95], while advancements in tissue clearing are pushing the limits of tissue penetration [96,97]. Confocal methods have allowed for the reliable collection of osteocyte lacunar parameters, including lacunar density, lacunar shape [51], osteocyte surface area and volume [98], volume fraction of the LCN [99], canalicular density, canalicular number per osteocyte, canalicular volume, and canalicular length [100], among many others. Light microscopy, however, is limited by diffraction aberrations that may skew quantitative efforts especially at the nanoscale dimensions of osteocyte canaliculi, so care must be taken when observing these features [101]. Multi-photon microscopy has been employed to overcome some of these limitations and has also been successful in further increasing the penetration depth of light microscopy [98,102]. Despite advancements, these techniques are time consuming, data-expensive, and only capture a small window of the LCN. Even with these drawbacks, light-based microscopy remains one of the most common and best used tools in the study of the osteocyte LCN in three dimensions and provides a practical and manageable tool with which to study PLR-related changes in osteocyte function [58,103,104].
Given the broad range of parameters reported about the LCN in both two and three dimensions we suggest a standardization to the reporting of these details [105]. A term representative of the whole LCN should be first supplied, i.e. “LCN area / bone area” in 2D and “LCN volume fraction” in 3D. Terms dissecting the differences of the osteocyte cell bodies or lacunae from the canaliculi are also ideal and include lacunar area (2D) and volume (3D), lacunar number densities (2D/3D), canalicular length (2D/3D) and tortuosity (3D), and canaliculi per osteocyte or canalicular density.
Evaluation of the Bone Extracellular Matrix
PLR plays a critical role in maintaining bone quality, at least in part through its effects on the organic and mineral components of the bone extracellular matrix [106]. The unique physical features of collagen and mineral afford opportunities to monitor their composition and organization in bone and how they are affected by PLR induction or suppression.
Organic Extracellular Matrix:
Observation of collagen organization provides clues to osteocytic action. Collagen birefringence is a property that can be assessed using polarized light microscopy because of collagen’s naturally ordered structure. Staining with picrosirius red, an anionic dye that binds to cationic collagen fibers, enhances this birefringence under polarized light [107,108]. Using this technique, we and others find that PLR suppression due to MMP-deficiency, glucocorticoid treatment, or aging [21,56] disrupt the normally aligned lamellar arrangement of collagen. In some but not all cases, PLR suppression in mouse models or in human disease correlates with reduced collagen alignment and reduced bone quality [34]. Indeed, these changes in collagen organization with PLR suppression are apparent even in situations where remodeling by osteoclasts and osteoblast is sufficiently intact to retain normal bone mass. The causal relationship between PLR and collagen organization remains an area of active investigation.
Mineralized Extracellular Matrix:
Osteocytes also actively engage in the management of mineral. Mineral resorption by osteocytes has been confirmed in studies of lactation, PTH treatment or regulation, and in disease models such as chronic kidney disease, Vitamin D deficiency, and hypophosphatemia [7,31,42,109]. While several enzymes are thought to be active in the process of mineral regulation, most evidence of lacunar resorption focuses on the idea that osteocytes dynamically regulate the pH within the perilacunar fluid between the cell membrane and the bone mineral surface to exert control over the mineral environment [30]. In addition, multiple imaging techniques reveal swelled osteocyte lacunar size during resorption events (i.e. lactation) and lower bone mineral density. These effects are rapidly reversed once resorption pressure is lifted (i.e. weaning), implying osteocyte-specific deposition of mineral [30].
In vivo administration of small fluorescent molecules that associate into newly formed bone has long been used for spatiotemporal visualization of bone mineralization at periosteal and endosteal surfaces. Fluorescent labels also confirm osteocytes’ ability to participate in bone formation [14]. Double fluorochrome labeling has been a useful approach to assess the dynamics of bone formation by osteoblasts and the calculation of the bone formation rate [7,46,55]. However, the timing of fluorochrome administration has yet to be optimized for the reproducible visualization of osteocyte-specific mineral deposition in homeostatic PLR. For example, fluorescent labeling in post-lactating mice show osteocyte-specific deposition of mineral [7]; however, virgin and non-lactating controls did not show double labelling. These findings imply that lactation represents a significant change to osteocyte remodeling behavior and not homeostatic PLR and that this technique may not be tuned enough to observe rapid changes in matrix deposition and resorption.
Other methods that have been used to observe the mineralization state around lacuna and osteocyte canaliculi [7,42] include X-ray computed tomography imaging, X-ray diffraction and absorption, and several forms of electron microscopy. These methods rely on differing attenuation of signal from radiation sources by the different elemental constituents within the bone. Changes in the mineralization of the perilacunar and pericanalicular bone matrix indirectly give important insight into osteocyte function. Nonetheless, these methods are insensitive to changes in cells or matrix that occur only in the organic phase. Care must be taken to integrate information derived from radiographic approaches with that derived from cellular and histological outcomes.
X-ray based imaging is the most common method used to observe changes in the mineralized portions of bone due to its high energy and very low wavelengths. Micro-computed tomography (μCT) scanners with voxel sizes of a few microns can identify osteocyte lacunae. More advanced μCT imaging is beginning to push the resolution of these techniques from the microscale (500 nm) into the nanoscale (50 nm) [110,111], which is needed to resolve individual canaliculi (200–350 nm). These nanoscale approaches are for small specimens (16 μm2 field of view). Synchrotron generated CT (SRμT) techniques can reliably capture larger segments of the LCN (40 mm2) and provide qualitative and quantitative outcomes of matrix mineralization and lacunar size and shape [34,57]. The best SRμT beamlines can resolve detail down to the canalicular level (30–40 nm with a field of view of as much as 75–80 μm2) [112]. However, these SRμT approaches are time consuming, computationally intensive, and require specialized use of synchrotron light sources not accessible to all researchers [20,103,104]. Especially since access to these resources is limited, we advocate adoption of online digital data-sharing practices to make these valuable and large SRμT datasets publicly available through established repositories including the Materials Data Repository hosted by National Institute of Standards and Technology (NIST), or other third-party repositories i.e. TomoBank [113]. This approach would expand access to any investigator to develop and apply new analytical tools to answer the many remaining questions about PLR.
Several other specialized Electron Microscopy and X-Ray techniques have also been used to study the osteocyte LCN. Some of these include ptychographic X-ray CT, transmission X-ray microscopy (TXM) CT, serial-focused ion beam SEM (serial FIB SEM), serial block-face SEM (SBF SEM), back scatter SEM, surface relief (acid etching) SEM, and others as extensively reviewed elsewhere [20,39,60,114,115]. Even as the field advances through application of these approaches, additional investigation is needed to understand how changes in these imaging parameters relate to changes in the cellular function of osteocytes at homeostasis, in metabolic stress, and in disease.
Future Directions
Answering the many questions about osteocytic perilacunar/pericanalicular bone remodeling (Box 1) will require the systematic and integrated application of approaches described here, as well as new and more sophisticated in vivo and in vitro outcomes. In particular, additional research is needed to elucidate the cellular mechanisms responsible for morphological changes that are apparent using histologic and radiographic approaches. Such studies may reveal distinct functional roles for perilacunar vs pericanalicular remodeling, peri-osteocytic acidification vs proteolysis, or remodeling of the mineral vs organic phases of the peri-osteocytic bone matrix.
Improvements to current methods that could be particularly helpful include robust algorithms to facilitate efficient and quantitative analysis of the LCN in 2D and in 3D. Though many groups have worked intensively to achieve automated detection and quantification of the LCN, this goal remains elusive at this time. Standardized use of common LCN parameters would facilitate comparison across studies.
Continued advances in imaging will provide a critical foundation for asking more mechanistic questions. This includes more widespread availability of μCT with nanometer length scale resolution, so that lacunar and canalicular networks can be visualized and quantified across larger fields of view. Utilization of fluorescent stains, reporter proteins [116], and immunofluorescence, along with specimen clearing and advanced microscopy, would improve our ability to relate structural features to important biological outcomes. Approaches to monitor pH at high spatiotemporal resolution in vivo and in vitro would complement current outcomes. These approaches could be applied to discern cellular or molecular changes in vivo upon PLR induction and suppression, particularly in the presence of gain and loss of function perturbations, or diseaseinducing or resolving interventions.
Finally, application of unbiased approaches such as RNAseq and mass spectrometry may be helpful in the identification of specific new RNA or serum markers of PLR. An ideal marker would be osteocyte-specific, have a functional role in matrix remodeling, and be dynamically regulated with PLR. Not only would these markers improve the precision of research efforts to understand PLR, but they could also support the identification of new diagnostics of skeletal disease or therapeutic interventions to prevent it. Given the role of PLR in skeletal metabolism, bone fragility, joint disease, and aging, our collective efforts to better understand osteocytic remodeling could have significant clinical impact.
Footnotes
Several protocols discussed herein may be found at the Alliston Laboratory website: https://allistonlab.ucsf.eduhttps://allistonlab.ucsf.edu/
References:
Papers of particular interest, published recently, have been highlighted as:
• Of major importance
- 1.Bonewald LF. The amazing osteocyte. Journal of Bone and Mineral Research. 2011;26:229–38. Doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teti A, Zallone A. Do osteocytes contribute to bone mineral homeostasis? Osteocytic osteolysis revisited. Bone. 2009;44:11–6. Doi: 10.1016/j.bone.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Buenzli PR, Sims NA. Quantifying the osteocyte network in the human skeleton. Bone. 2015;75:144–50. Doi: 10.1016/j.bone.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251–64. Doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Takayanagi H The Role of NFAT in Osteoclast Formation. Annals of the New York Academy of Sciences. 2007;1116:227–37. Doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 6.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nature Medicine. 2013;19:179 Doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 7.Qing H, Ardeshirpour L, Pajevic P, Dusevich V, Jähn K, Kato S, et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. Journal of Bone and Mineral Research. 2012;27:1018–29. Doi: 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komori T Functions of the osteocyte network in the regulation of bone mass. Cell and Tissue Research. 2013;352:191–8. Doi: 10.1007/s00441-012-1546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rigal A, Vignal W. Recherches experimentales sur la formation ducal et sur les modifcations des tissus dans les pseudarthroses. Archives of Physiology. 1881; Ser.II:419–58. [Google Scholar]
- 10.Belanger LF. Osteocytic osteolysis. Calcified Tissue Research. 1969;4:1–12. [DOI] [PubMed] [Google Scholar]
- 11.Baud CA. [Morphology and inframicroscopic structure of osteocytes]. Acta anatomica. 1962;51:209–25. [PubMed] [Google Scholar]
- 12.Recklinghausen F v. Untersuchungen uber Rachitis and Osteomalacia. Jena: Gustav Fischer. 1910. [Google Scholar]
- 13.Tazawa K, Hoshi K, Kawamoto S, Tanaka M, Ejiri S, Ozawa H. Osteocytic osteolysis observed in rats to which parathyroid hormone was continuously administered. Journal of Bone and Mineral Metabolism. 2004;22:524–9. Doi: 10.1007/s00774-004-0519-x. [DOI] [PubMed] [Google Scholar]
- 14.Baylink D, Wergedal J. Bone formation by osteocytes. The American journal of physiology. 1971;221:669–78. Doi: 10.1152/ajplegacy.1971.221.3.669. [DOI] [PubMed] [Google Scholar]
- 15.Wysolmerski JJ. Osteocytic osteolysis: time for a second look? BoneKEy Reports. 2012;1:229 Doi: 10.1038/bonekey.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dallas SL, Prideaux M, Bonewald LF. The Osteocyte: An Endocrine Cell … and More. Endocrine Reviews. 2013;34:658–90. Doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinberg B, Singh I, Mitchell OG. The effects of cold-stress, hibernation, and prolonged inactivity on bone dynamics in the golden hamster, Mesocricetus auratus. Journal of Morphology. 1981;167:43–51. Doi: 10.1002/jmor.1051670105. [DOI] [PubMed] [Google Scholar]
- 18.McGee-Lawrence ME, Carey HV, Donahue SW. Mammalian hibernation as a model of disuse osteoporosis: the effects of physical inactivity on bone metabolism, structure, and strength. American journal of physiology Regulatory, integrative and comparative physiology. 2008;295:R1999–2014. Doi: 10.1152/ajpregu.90648.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alemi SA, Mazur C, Fowler T, Woo JJ, Knott DP, Alliston T. Glucocorticoids cause mandibular bone fragility and suppress osteocyte perilacunar-canalicular remodeling. Bone Reports. 2018;9:145–53. Doi: 10.1016/j.bonr.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciani A, Toumi H, Pallu S, Tsai E, Diaz A, Guizar-Sicairos M, et al. Ptychographic X-ray CT characterization of the osteocyte lacuno-canalicular network in a male rat’s glucocorticoid induced osteoporosis model. Bone Reports. 2018;9:122–31. Doi: 10.1016/j.bonr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fowler TW, Acevedo C, Mazur CM, Hall-Glenn F, Fields AJ, Bale HA, et al. Glucocorticoid suppression of osteocyte perilacunar remodeling is associated with subchondral bone degeneration in osteonecrosis. Scientific Reports. 2017;7:44618 Doi: 10.1038/srep44618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lane NE, Yao W, Balooch M, Nalla RK, Balooch G, Habelitz S, et al. Glucocorticoid-Treated Mice Have Localized Changes in Trabecular Bone Material Properties and Osteocyte Lacunar Size That Are Not Observed in Placebo-Treated or Estrogen-Deficient Mice. Journal of Bone and Mineral Research. 2006;21:466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Busse B, Bale HA, Zimmermann EA, Panganiban B, Barth HD, Carriero A, et al. Vitamin D Deficiency Induces Early Signs of Aging in Human Bone, Increasing the Risk of Fracture. Science Translational Medicine. 2013;5:193ra88–193ra88. Doi: 10.1126/scitranslmed.3006286. [DOI] [PubMed] [Google Scholar]
- 24.Sharma D, Ciani C, Marin PA, Levy JD, Doty SB, Fritton SP. Alterations in the osteocyte lacunar–canalicular microenvironment due to estrogen deficiency. Bone. 2012;51:488–97. Doi: 10.1016/j.bone.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kafantari H, Kounadi E, Fatouros M, Milonakis M, Tzaphlidou M. Structural alterations in rat skin and bone collagen fibrils induced by ovariectomy. Bone. 2000;26:349–53. Doi: 10.1016/S8756-3282(99)00279–3. [DOI] [PubMed] [Google Scholar]
- 26.Ciani C, Sharma D, Doty SB, Fritton SP. Ovariectomy enhances mechanical load-induced solute transport around osteocytes in rat cancellous bone. Bone. 2014;59:229–34. Doi: 10.1016/j.bone.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma D, Larriera AI, Palacio-Mancheno PE, Gatti V, Fritton CJ, Bromage TG, et al. The effects of estrogen deficiency on cortical bone microporosity and mineralization. Bone. 2018;110:1–10. Doi: 10.1016/j.bone.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gatti V, Azoulay EM, Fritton SP. Microstructural changes associated with osteoporosis negatively affect loading-induced fluid flow around osteocytes in cortical bone. Journal of biomechanics. 2018;66 Doi: 10.1016/j.jbiomech.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Divieti P, Inomata N, Chapin K, Singh R, Jüppner H, Bringhurst F. Receptors for the carboxyl-terminal region of pth(1–84) are highly expressed in osteocytic cells. Endocrinology. 2001;142:916–25. Doi: 10.1210/endo.142.2.7955. [DOI] [PubMed] [Google Scholar]
- 30. •.Jähn K, Kelkar S, Zhao H, Xie Y, Tiede-Lewis LM, Dusevich V, et al. Osteocytes Acidify Their Microenvironment in Response to PTHrP In Vitro and in Lactating Mice In Vivo. Journal of Bone and Mineral Research. 2017;32:1761–72. Doi: 10.1002/jbmr.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows how osteocyte acidification is involved during perilacunar remodeling.
- 31.Tokarz D, Martins JS, Petit ET, Lin CP, Demay MB, Liu ES. Hormonal Regulation of Osteocyte Perilacunar and Canalicular Remodeling in the Hyp Mouse Model of X-Linked Hypophosphatemia. Journal of Bone and Mineral Research. 2018;33:499–509. Doi: 10.1002/jbmr.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kogawa M, Wijenayaka AR, Ormsby RT, Thomas GP, Anderson PH, Bonewald LF, et al. Sclerostin Regulates Release of Bone Mineral by Osteocytes by Induction of Carbonic Anhydrase 2. Journal of Bone and Mineral Research. 2013;28:2436–48. Doi: 10.1002/jbmr.2003. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen J, Tang SY, Nguyen D, Alliston T. Load Regulates Bone Formation and Sclerostin Expression through a TGFβ-Dependent Mechanism. PLoS ONE. 2013;8:e53813 Doi: 10.1371/journal.pone.0053813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. •.Dole NS, Mazur CM, Acevedo C, Lopez JP, Monteiro DA, Fowler TW, et al. Osteocyte-Intrinsic TGF-β Signaling Regulates Bone Quality through Perilacunar/Canalicular Remodeling. Cell Reports. 2017;21:2585–96. Doi: 10.1016/j.celrep.2017.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that osteocytes regulate bone quality through PLR in a TGFβ-dependent manner.
- 35.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical Stimulation of Bone in Vivo Reduces Osteocyte Expression of Sost/Sclerostin. Journal of Biological Chemistry. 2008;283:5866–75. Doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 36.Blaber EA, Dvorochkin N, Lee C, Alwood JS, Yousuf R, Pianetta P, et al. Microgravity Induces Pelvic Bone Loss through Osteoclastic Activity, Osteocytic Osteolysis, and Osteoblastic Cell Cycle Inhibition by CDKN1a/p21. PLoS ONE. 2013;8:e61372 Doi: 10.1371/journal.pone.0061372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach-Gansmo F, Wittig N, Brüel A, Thomsen J, Birkedal H. Immobilization and long-term recovery results in large changes in bone structure and strength but no corresponding alterations of osteocyte lacunar properties. Bone. 2016;91:139–47. Doi: 10.1016/j.bone.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Zhang D, Miranda M, Li X, Han J, Sun Y, Rojas N, et al. Retention of osteocytic micromorphology by sclerostin antibody in a concurrent ovariectomy and functional disuse model. Annals of the New York Academy of Sciences. 2019. Doi: 10.1111/nyas.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaya S, Basta-Pljakic J, Seref-Ferlengez Z, Majeska RJ, Cardoso L, Bromage TG, et al. Lactation-Induced Changes in the Volume of Osteocyte Lacunar-Canalicular Space Alter Mechanical Properties in Cortical Bone Tissue. Journal of Bone and Mineral Research. 2017;32:688–97. Doi: 10.1002/jbmr.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vijayan V, Gupta S. Role of osteocytes in mediating bone mineralization during hyperhomocysteinemia. Journal of Endocrinology. 2017;233:243–55. Doi: 10.1530/JOE-16-10.1530/JOE-16-https://doi.org/10.1530/JOE-16-05620562https://doi.org/10.1530/JOE-16-05620562 . [DOI] [PubMed] [Google Scholar]
- 41.Kerschnitzki M, Wagermaier W, Roschger P, Seto J, Shahar R, Duda GN, et al. The organization of the osteocyte network mirrors the extracellular matrix orientation in bone. Journal of Structural Biology. 2011;173:303–11. Doi: 10.1016/j.jsb.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 42.Rolvien T, Krause M, Jeschke A, Yorgan T, Püschel K, Schinke T, et al. Vitamin D regulates osteocyte survival and perilacunar remodeling in human and murine bone. Bone. 2017;103:78–87. Doi: 10.1016/j.bone.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nature genetics. 2006;38:1310–5. Doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milovanovic P, Zimmermann EA, Hahn M, Djonic D, Püschel K, Djuric M, et al. Osteocytic Canalicular Networks: Morphological Implications for Altered Mechanosensitivity. ACS Nano. 2013;7:7542–51. Doi: 10.1021/nn401360u. [DOI] [PubMed] [Google Scholar]
- 45.Busse B, Djonic D, Milovanovic P, Hahn M, Püschel K, Ritchie RO, et al. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell. 2010;9:1065–75. Doi: 10.1111/j.1474-9726.2010.00633.x. [DOI] [PubMed] [Google Scholar]
- 46. •.Tiede-Lewis LM, Xie Y, Hulbert MA, Campos R, Dallas MR, Dusevich V, et al. Degeneration of the osteocyte network in the C57BL/6 mouse model of aging. Aging. 2017. Doi: 10.18632/aging.101308. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows age-related degeneration of lacuna-canalicular network in mice.
- 47.Kobayashi K, Nojiri H, Saita Y, Morikawa D, Ozawa Y, Watanabe K, et al. Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Scientific Reports. 2015;5:9148 Doi: 10.1038/srep09148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang K, Le L, Chun BM, Tiede-Lewis LM, Shiflett LA, Prideaux M, et al. A Novel Osteogenic Cell Line that Differentiates into GFP-Tagged Osteocytes and forms Mineral with a Bone-like Lacunocanalicular Structure. J Bone Miner Res. 2019. Doi: 10.1002/jbme.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jilka RL, O’Brien CA. The Role of Osteocytes in Age-Related Bone Loss. Current Osteoporosis Reports. 2016;14:16–25. Doi: 10.1007/s11914-016-0297-0. [DOI] [PubMed] [Google Scholar]
- 50.Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nature Medicine. 2017;23:nm.4385 Doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heveran CM, Rauff A, King KB, Carpenter DR, Ferguson VL. A new open-source tool for measuring 3D osteocyte lacunar geometries from confocal laser scanning microscopy reveals age-related changes to lacunar size and shape in cortical mouse bone. Bone. 2018;110:115–27. Doi: 10.1016/j.bone.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carter Y, Thomas DC, Clement JG, Cooper D. Femoral osteocyte lacunar density, volume and morphology in women across the lifespan. Journal of Structural Biology. 2013;183:519–26. Doi: 10.1016/j.jsb.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 53.Weinstein RS. Glucocorticoid-Induced Osteoporosis and Osteonecrosis. Endocrinology and Metabolism Clinics of North America. 2012;41:595–611. Doi: 10.1016/j.ecl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bach-Gansmo F, Irvine S, Brüel A, Thomsen J, Birkedal H. Calcified Cartilage Islands in Rat Cortical Bone. Calcified Tissue International. 2013;92:330–8. Doi: [DOI] [PubMed] [Google Scholar]
- 55. •.Ip V, Toth Z, Chibnall J, McBride-Gagyi S. Remnant Woven Bone and Calcified Cartilage in Mouse Bone: Differences between Ages/Sex and Effects on Bone Strength. PLOS ONE. 2016;11:e0166476 Doi: 10.1371/journal.pone.0166476. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals site specific changes of bone remodeling within cortical bone of mice.
- 56.Tang SY, Herber R, Ho SP, Alliston T. Matrix metalloproteinase–13 is required for osteocytic perilacunar remodeling and maintains bone fracture resistance. Journal of Bone and Mineral Research. 2012;27:1936–50. Doi: 10.1002/jbmr.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jáuregui EJ, Akil O, Acevedo C, Hall-Glenn F, Tsai BS, Bale HA, et al. Parallel mechanisms suppress cochlear bone remodeling to protect hearing. Bone. 2016;89:7–15. Doi: 10.1016/j.bone.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lai X, Price C, Modla S, Thompson WR, Caplan J, Kirn-Safran CB, et al. The dependences of osteocyte network on bone compartment, age, and disease. Bone Research. 2015;3:15009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canè V, Marotti G, Volpi G, Zaffe D, Palazzini S, Remaggi F, et al. Size and density of osteocyte lacunae in different regions of long bones. Calcified Tissue International. 1982;34:558–63. [DOI] [PubMed] [Google Scholar]
- 60.Hemmatian H, Bakker AD, Klein-Nulend J, van Lenthe HG. Aging, Osteocytes, and Mechanotransduction. Current Osteoporosis Reports. 2017;15:401–11. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalajzic I, Matthews BG, Torreggiani E, Harris MA, Pajevic P, Harris SE. In vitro and in vivo approaches to study osteocyte biology. Bone. 2013;54:296–306. Doi: 10.1016/j.bone.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosser J, Bonewald LF. Bone Research Protocols. Methods in molecular biology (Clifton, NJ). 2012;816:67–81. [DOI] [PubMed] [Google Scholar]
- 63.Kato Y, Boskey A, Spevak L, llas, Hori M, Bonewald L. Establishment of an Osteoid Preosteocyte-like Cell MLO-A5 That Spontaneously Mineralizes in Culture. Journal of Bone and Mineral Research. 2001;16:1622–33. Doi: 10.1359/jbmr.2001.16.9.1622. [DOI] [PubMed] [Google Scholar]
- 64.Kato Y, Windle JJ, Koop BA, Mundy GR, Bonewald LF. Establishment of an Osteocyte-like Cell Line, MLO-Y4. Journal of Bone and Mineral Research. 1997;12:2014–23. Doi: 10.1359/jbmr.1997.12.12.2014. [DOI] [PubMed] [Google Scholar]
- 65.Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. Journal of Bone and Mineral Research. 2011;26:2634–46. Doi: 10.1002/jbmr.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spatz JM, Wein MN, Gooi JH, Qu Y, Garr JL, Liu S, et al. The Wnt Inhibitor Sclerostin Is Up-regulated by Mechanical Unloading in Osteocytes in Vitro. J Biol Chem. 2015;290:16744–58. Doi: 10.1074/jbc.M114.628313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prideaux M, Wijenayaka AR, Kumarasinghe DD, Ormsby RT, Evdokiou A, Findlay DM, et al. SaOS2 Osteosarcoma Cells as an In Vitro Model for Studying the Transition of Human Osteoblasts to Osteocytes. Calcified Tissue International. 2014;95:183–93. Doi: [DOI] [PubMed] [Google Scholar]
- 68.Stern A, ern M, Dyke ME, Jähn K, Prideaux M, Bonewald LF. Isolation and culture of primary osteocytes from the long bones of skeletally mature and aged mice. BioTechniques. 2012;52:361–73. Doi: 10.2144/0000113876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikpegbu E, Basta L, Clements DN, Fleming R, Vincent TL, Buttle DJ, et al. FGF-2 promotes osteocyte differentiation through increased E11/podoplanin expression. Journal of Cellular Physiology. 2018;233:5334–47. Doi: 10.1002/jcp.26345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H, Yi J, Li X, Xiao Y, Dhakal K, Zhou J. ALS-associated mutation SOD1G93A leads to abnormal mitochondrial dynamics in osteocytes. Bone. 2018;106:126–38. Doi: 10.1016/j.bone.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun Q, Choudhary S, Mannion C, Kissin Y, Zilberberg J, Lee WY. Ex vivo construction of human primary 3D–networked osteocytes. Bone. 2017;105:245–52. Doi: 10.1016/j.bone.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choudhary S, Sun Q, Mannion C, Kissin Y, Zilberberg J, Lee WY. Hypoxic Three-Dimensional Cellular Network Construction Replicates Ex Vivo the Phenotype of Primary Human Osteocytes. Tissue engineering Part A. 2017;24:458–68. Doi: 10.1089/ten.TEA.2017.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clarke MV, Russell PK, Findlay DM, Sastra S, Anderson PH, Skinner JP, et al. A Role for the Calcitonin Receptor to Limit Bone Loss During Lactation in Female Mice by Inhibiting Osteocytic Osteolysis. Endocrinology. 2015;156:3203–14. Doi: 10.1210/en.2015-1345. [DOI] [PubMed] [Google Scholar]
- 74.Lloyd SA, Loiselle AE, Zhang Y, Donahue HJ. Evidence for the role of connexin 43-mediated intercellular communication in the process of intracortical bone resorption via osteocytic osteolysis. BMC Musculoskeletal Disorders. 2014;15:122. Doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiong J, O’Brien CA. Osteocyte RANKL: New insights into the control of bone remodeling. Journal of Bone and Mineral Research. 2012;27:499–505. Doi: 10.1002/jbmr.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Inoue K, Mikuni-Takagaki Y, Oikawa K, Itoh T, Inada M, Noguchi T, et al. A Crucial Role for Matrix Metalloproteinase 2 in Osteocytic Canalicular Formation and Bone Metabolism. Journal of Biological Chemistry. 2006;281:33814–24. Doi: 10.1074/jbc.M607290200. [DOI] [PubMed] [Google Scholar]
- 77.Delgado-Calle J, Hancock B, Likine EF, Sato AY, McAndrews K, Sanudo C, et al. MMP14 is a novel target of PTH signaling in osteocytes that controls resorption by regulating soluble RANKL production. The FASEB Journal. 2018;32:fj.201700919RRR. Doi: 10.1096/fj.201700919RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teitelbaum SL. Bone Resorption by Osteoclasts. Science. 2000;289:1504–8. [DOI] [PubMed] [Google Scholar]
- 79.Boyle WJ, Simonet SW, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:nature01658 Doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 80.Filvaroff E, Derynck R. Bone remodelling: A signalling system for osteoclast regulation. Curr Biol. 1998;8:R679–82. [DOI] [PubMed] [Google Scholar]
- 81.Teti A, Blair H, Teitelbaum S, Kahn A, Koziol C, Konsek J, et al. Cytoplasmic pH regulation and chloride/bicarbonate exchange in avian osteoclasts. J Clin Invest. 1989;83:227–33. Doi: 10.1172/JCI113863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmetic Sci. 2006;28:359–70. Doi: 10.1111/j.1467-2494.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 83.Riemann A, Ihling A, Thomas J, Schneider B, Thews O, Gekle M. Acidic environment activates inflammatory programs in fibroblasts via a cAMP–MAPK pathway. Biochimica Et Biophysica Acta Bba - Mol Cell Res. 2015;1853:299–307. Doi: 10.1016/j.bbamcr.2014.11.022. [DOI] [PubMed] [Google Scholar]
- 84.Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, et al. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013;13:89 Doi: 10.1186/1475-2867-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rotin D, Robinson B, Tannock I. Influence of hypoxia and an acidic environment on the metabolism and viability of cultured cells: potential implications for cell death in tumors. Cancer research. 1986;46:2821–6. [PubMed] [Google Scholar]
- 86.Busa W, Nuccitelli R. Metabolic regulation via intracellular pH. The American journal of physiology. 1984;246:R409–38. Doi: 10.1152/ajpregu.1984.246.4.R409. [DOI] [PubMed] [Google Scholar]
- 87.Loots GG, Robling AG, Chang JC, Murugesh DK, Bajwa J, Carlisle C, et al. Vhl deficiency in osteocytes produces high bone mass and hematopoietic defects. Bone. 2018;116:307–14. Doi: 10.1016/j.bone.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 88.Frost H In vivo osteocyte death. The Journal of bone and joint surgery American volume. 1960;42-A:138–43. Doi: 10.2106/00004623-196042010-00011. [DOI] [PubMed] [Google Scholar]
- 89.Webster DJ, Schneider P, Dallas SL, Müller R. Studying osteocytes within their environment. Bone. 2013;54:285–95. Doi: 10.1016/j.bone.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.bin Ghazali M, Isa M, Hoo P. A Technique for the Simultaneous Staining of Osteocytes and Osteons in Frozen Sections of Decalcified Bone. Stain Technology. 2009;55:47–8. Doi: 10.3109/10520298009067897. [DOI] [PubMed] [Google Scholar]
- 91.Mullender M, van der Meer D, Huiskes R, Lips P. Osteocyte density changes in aging and osteoporosis. Bone. 1996;18:109–13. Doi: 10.1016/8756-3282(95)00444–0. [DOI] [PubMed] [Google Scholar]
- 92.Piemontese M, Onal M, Xiong J, Han L, Thostenson JD, Almeida M, et al. Low bone mass and changes in the osteocyte network in mice lacking autophagy in the osteoblast lineage. Scientific Reports. 2016;6:srep24262. Doi: 10.1038/srep24262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chandra A, Lin T, Young T, Tong W, Ma X, Tseng W, et al. Suppression of Sclerostin Alleviates Radiation-Induced Bone Loss by Protecting Bone-Forming Cells and Their Progenitors Through Distinct Mechanisms. Journal of Bone and Mineral Research. 2017;32:360–72. Doi: 10.1002/jbmr.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cardoso L, Fritton SP, Gailani G, Benalla M, Cowin SC. Advances in assessment of bone porosity, permeability and interstitial fluid flow. Journal of Biomechanics. 2013;46:253–65. Doi: 10.1016/j.jbiomech.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. •.Kamel-ElSayed SA, Tiede-Lewis LM, Lu Y, Veno PA, Dallas SL. Novel approaches for two and three dimensional multiplexed imaging of osteocytes. Bone. 2015;76:129–40. Doi: 10.1016/j.bone.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates a novel in situ imagining technique that can be used to visualize osteocyte cell and lacuna-canalicular network.
- 96.Greenbaum A, Chan KY, Dobreva T, Brown D, Balani DH, Boyce R, et al. Bone CLARITY: Clearing, imaging, and computational analysis of osteoprogenitors within intact bone marrow. Science Translational Medicine. 2017;9:eaah6518 Doi: 10.1126/scitranslmed.aah6518. [DOI] [PubMed] [Google Scholar]
- 97.Berke IM, Miola JP, David MA, Smith MK, Price C. Seeing through Musculoskeletal Tissues: Improving In Situ Imaging of Bone and the Lacunar Canalicular System through Optical Clearing. PLOS ONE. 2016;11:e0150268 Doi: 10.1371/journal.pone.0150268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tokarz D, Cisek R, Wein MN, Turcotte R, Haase C, Yeh S-CA, et al. Intravital imaging of osteocytes in mouse calvaria using third harmonic generation microscopy. PLOS ONE. 2017;12:e0186846 Doi: 10.1371/journal.pone.0186846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Repp F, Kollmannsberger P, Roschger A, Kerschnitzki M, Berzlanovich A, Gruber GM, et al. Spatial heterogeneity in the canalicular density of the osteocyte network in human osteons. Bone Reports. 2017;6:101–8. Doi: 10.1016/j.bonr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Staines KA, Javaheri B, Hohenstein P, Fleming R, Ikpegbu E, Unger E, et al. Hypomorphic conditional deletion of E11/Podoplanin reveals a role in osteocyte dendrite elongation. Journal of Cellular Physiology. 2017;232:3006–19. Doi: 10.1002/jcp.25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang B, Babcock H, Zhuang X. Breaking the Diffraction Barrier: Super-Resolution Imaging of Cells. Cell. 2010;143:1047–58. Doi: 10.1016/j.cell.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sano H, Kikuta J, Furuya M, Kondo N, Endo N, Ishii M. Intravital bone imaging by two-photon excitation microscopy to identify osteocytic osteolysis in vivo. Bone. 2015;74:134–9. Doi: 10.1016/j.bone.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 103.Tanaka T, Hoshijima M, Sunaga J, Nishida T, Hashimoto M, Odagaki N, et al. Analysis of Ca2+ response of osteocyte network by three-dimensional time-lapse imaging in living bone. Journal of Bone and Mineral Metabolism. 2018;36:519–28. Doi: 10.1007/s00774-017-0868-x. [DOI] [PubMed] [Google Scholar]
- 104.Cao R, Xiao W, Wu X, Sun L, Pan F. Quantitative observations on cytoskeleton changes of osteocytes at different cell parts using digital holographic microscopy. Biomedical optics express. 2017;9:72–85. Doi: 10.1364/BOE.9.000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dempster DW, Compston JE, Drezner MK, Glorieux FH, Kanis JA, Malluche H, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. Doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chemical Feng X. and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Current chemical biology. 2009;3:189–96. Doi: 10.2174/187231309788166398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Junqueira L, Bignolas G, Brentani R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. The Histochemical Journal. 1979;11:447–55. Doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 108.Lattouf R, Younes R, Lutomski D, Naaman N, Godeau G, Senni K, et al. Picrosirius Red Staining. Journal of Histochemistry & Cytochemistry. 2014;62:751–8. Doi: 10.1369/0022155414545787. [DOI] [PubMed] [Google Scholar]
- 109.Moysés RM, Schiavi SC. Sclerostin, Osteocytes, and Chronic Kidney Disease – Mineral Bone Disorder. Seminars in Dialysis. 2015;28:578–86. Doi: 10.1111/sdi.12415. [DOI] [PubMed] [Google Scholar]
- 110.Khoury BM, Bigelow EM, ith L, Schlecht SH, Scheller EL, Andarawis-Puri N, et al. The use of nano-computed tomography to enhance musculoskeletal research. Connective Tissue Research. 2015;56:106–19. Doi: 10.3109/03008207.2015.1005211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Hove RP, Nolte PA, Vatsa A, meins C, Salmon PL, Smit TH, et al. Osteocyte morphology in human tibiae of different bone pathologies with different bone mineral density — Is there a role for mechanosensing? Bone. 2009;45:321–9. Doi: 10.1016/j.bone.2009.04.238. [DOI] [PubMed] [Google Scholar]
- 112.Müller BR, Lange A, Harwardt M, Hentschel MP. Synchrotron-Based Micro-CT and Refraction-Enhanced Micro-CT for Non-Destructive Materials Characterisation. Adv Eng Mater. 2009;11:435–40. Doi: 10.1002/adem.200800346. [DOI] [Google Scholar]
- 113.Carlo F, Gürsoy D, Ching DJ, Batenburg JK, Ludwig W, Mancini L, et al. TomoBank: a tomographic data repository for computational x-ray science. Measurement Science and Technology. 2018;29:034004 Doi: 10.1088/1361-6501/aa9c19. [DOI] [Google Scholar]
- 114.Peyrin F, Dong P, Pacureanu A, Langer M. Micro- and Nano-CT for the Study of Bone Ultrastructure. Current Osteoporosis Reports. 2014;12:465–74. Doi: 10.1007/s11914-014-0233-0. [DOI] [PubMed] [Google Scholar]
- 115.Müller R Hierarchical microimaging of bone structure and function. Nature Reviews Rheumatology. 2009;5:373–81. Doi: 10.1038/nrrheum.2009.107. [DOI] [PubMed] [Google Scholar]
- 116.Kalajzic I, Braut A, Guo D, Jiang X, Kronenberg Mina M, et al. Dentin matrix protein 1 expression during osteoblastic differentiation, generation of an osteocyte GFP-transgene. Bone. 2004;35:74–82. Doi: 10.1016/j.bone.2004.03.006. [DOI] [PubMed] [Google Scholar]


