ABSTRACT
Observing optic disc pallor during a patient’s first visit frequently raises a diagnostic challenge, particularly in regards to whether the cause is due to glaucoma or another form of optic neuropathy. Bruch’s membrane opening (BMO) was recently discovered as the anatomical border of the optic disc. BMO minimum rim width (BMO-MRW) seems to be a reliable representation of the neuroretinal rim. In our study, we demonstrate the ability of BMO-MRWs to differentiate between glaucomatous and non-glaucomatous. Additionally, we propose an MRW ratio which may allow discrimination of open angle glaucoma from either non-arteritic anterior ischaemic optic neuropathy or compressive optic neuropathy.
KEYWORDS: Glaucomatous neuropathy, non-glaucomatous neuropathy, minimal rim width, non-arteritic anterior ischaemic optic neuropathy, compressive optic neuropathy
Introduction
Optic disc atrophy is a common end point for many optic neuropathies. Despite there being several distinct pathophysiological mechanisms and agents that may injure the optic nerve, the optic nerve head tends to respond in a relatively monotonous pattern.1 Small variations in the morphology of the optic nerve head may point towards a specific aetiology, however, these are frequently misleading.1,2
Glaucomatous neuropathy is a term used to describe a group of optic disc degenerative diseases, frequently associated with intraocular hypertension and specific forces on the lamina cribosa, that lead to the death of retinal ganglion cells and loss of axons. The underlying mechanism produces characteristic morphological changes, namely an excavation of the optic disc, with enlarged cup-to-disc ratio and posterior displacement and thinning of the lamina cribosa.2–4 However, some of these changes are not specific to glaucomatous neuropathies and may be seen in non-glaucomatous eyes.1,5,6
Non-arteritic anterior ischaemic optic neuropathy (NAION) is caused by an acute reduction of the blood flow to the anterior portion of optic nerve. NAION is associated with abrupt vision loss and optic disc oedema in the acute phase, followed by diffuse or segmental disc atrophy.1,7 However, a small proportion of eyes may develop optic disc cupping, as seen in the glaucomatous neuropathies, leading to misdiagnosis.1,5
Compressive optic neuropathy (CON) is a neuro-ophthalmological entity in which a lesion of the optic nerve is caused by compression of the anterior visual pathway (from the optic nerve head to the lateral geniculate body). The compression may be intrinsic or extrinsic, nonetheless, in both cases, there is retrograde axonal degeneration and nerve fibre loss that culminates in optic disc atrophy.6,7 In some cases, the atrophy may be segmental and reflect the area of optic pathway involved. In other cases, there may be an enlarged cup-to-disc ratio similar to that seen in glaucomatous neuropathies which may mask a potentially life-threatening condition.6
The differential diagnosis of these entities is crucial for the correct management of the underling disease and prevention of further damage, involvement of the fellow eye and, specifically in CON, identification of a central nervous system (CNS) tumour.
Optical coherence tomography (OCT) is a powerful diagnostic tool that plays an important role in the management of optic neuropathies.1,7–9 OCT of the optic nerve allows for a detailed study of its morphology and the assessment of the peripapillary retinal nerve fibre layer (pRNFL). Technological advances and a growing number of clinical studies have allowed the search for specific changes that can assist in the differentiation of these conditions.8 The Bruch’s membrane opening (BMO) was recently found to be the true anatomical border of the optic rim, through which retinal ganglion cell axons travel.10 Bruch’s membrane opening minimum rim width (BMO-MRW) is a recent parameter for the neuroretinal rim, that measures optic disc cupping and has proven reproducibility and diagnostic ability in glaucomatous neuropathies.3,10–14 This parameter may also play a role in differentiating between other optic neuropathies and glaucoma. Previous studies have reported preservation of the BMO-MRW in eyes with NAION, in which it is similar to that of healthy optic discs and greater than that of glaucomatous ones.15,16 In CON, however, despite the frequent appearance of cupping, BMO-MRW changes are currently poorly characterised.
The purpose of this study was to evaluate the discriminative capability of BMO-MRW in different optic neuropathies and to access the diagnostic ability of a new index, based on the relationship between MRW and pRNFL loss, for these different optic neuropathies.
Materials and methods
In this retrospective, observational, cross-sectional study, we reviewed the medical records of the patients who attended our ophthalmology department from January 2018 to January 2019 and were diagnosed with NAION, CON or open angle glaucoma (OAG). Their complete medical and ophthalmological history, as well as visual field testing and spectral domain OCT (SD-OCT) imaging were analysed. This study respected the tenets of the Declaration of Helsinki. Since this was a retrospective and anonymous study, the patients were not required to provide a written consent.
Inclusion and exclusion criteria
Patients in the NAION group were selected according to the following inclusion criteria:
Unilateral NAION with more than 6 months of follow-up
Documented optic disc oedema in the acute phase
Optic disc pallor on funduscopy
pRNFL loss documented on optic disc scanning with SD-OCT.
In this group of patients only the involved eye was included.
For the CON group, we recruited patients with:
Optic disc atrophy associated with direct compression of the anterior optic pathway by a space-occupying lesion (SOL)
SOL documented on magnetic resonance imaging (MRI) or surgically resected at least one year ago
Optic disc pallor present on funduscopy
A visual field defect corresponding with the SOL location
pRNFL loss documented on SD-OCT.
The most affected eye was selected.
For the OAG group, patients were selected with respect to age and the following criteria:
(1) A diagnosis of OAG with ocular hypertension that required topical medication
(2) Glaucomatous loss of the pRNFL, documented on SD-OCT
(3) A glaucomatous visual field defect
(4) No previous glaucoma surgery.
For the control group, healthy subjects, that had undergone a complete ophthalmological examination plus optic disc and macular SD-OCT scanning, were recruited. In the OAG and control groups, age-matching was performed by randomly selecting subjects with no more than three years of age difference than that of patients in the NAION and CON groups. One eye of each patient in these two groups was randomly selected.
Exclusion criteria were the following:
(1) A spherical equivalent (SE) greater than 3 dioptres (D) or an astigmatism greater than 3D
(2) A history of intraocular surgery in the last six months
(3) A significant corneal or lens opacities;
(4) Any other inflammatory or degenerative ophthalmological pathology
(5) SD-OCT scans with segmentation errors, poor centring or poor quality (signal strength <20 dB).
Additionally, in the NAION, CON and control groups, patients with ocular hypertension (intraocular pressure (IOP) greater than 21 mmHg) were excluded.
Optic disc scanning and measurements
All participants underwent optic disc scanning with SD-OCT (Spectralis®, Heidelberg Engineering GmbH, Heidelberg, Germany), using the Glaucoma Module Premium (GMP) Edition software (version 6.0; Heidelberg Engineering). For each patient the Anatomic Position System (APS) was used to align the scans with respect to the fovea-BMO axes. Two different sets of scans were obtained: one for the neuroretinal rim assessment and a circumferential peripapillary scan for pRNFL thickness (pRNFLT) evaluation. Regarding neuroretinal rim assessment, 24 radial high-resolution B-scans, centred on the BMO, every 15°, were obtained. In each B-scan, the BMO point and the internal limiting membrane (ILM) were automatically identified by the software, and the BMO-MRW was defined as the shortest distance between the BMO and the ILM. For the pRNFL evaluation, a 12° circular scan was used to measure pRNFLT. The results obtained from both scans were compared with a normative database, adjusted for BMO size and age, and presented in a Garway-Heath sector format. Additionally, the results were classified as “within normal”, “borderline” or “outside normal limits”, based on the percentiles of the normative database. An experienced observer (JB) inspected all of the scans for segmentation errors and malposition of the BMO or ILM and manually corrected scans when necessary.
Visual field testing
The visual field (VF) testing for the patients included in this study was performed using the automated VF analyser (Humphrey Field Analyser; Carl Zeiss Meditec, Inc., Dublin, CA, USA) with a 24–2 pattern and a size III white stimulus using the Swedish Interactive Threshold Algorithm (SITA) standard strategy. Reliable tests were defined as <20% fixation loss rate, <15% false-positive rate and <15% false-negative rate. The mean deviation (MD) result of each test was collected. The VF tests with non-reliable test results were not considered in the data analysis. Some patients included in this study were not able to perform the Humphrey SITA test due to lack of cooperation or very low vision acuity, in which case, kinetic Goldmann perimetry was performed.
Statistical analysis
Statistical analysis was performed using the SPSS Statistics® software, version 25.0 (IBM, New York, NY, USA). The Kolmogorov Smirnov and the Shapiro-Wilk tests were used to verify the normal distribution of the BMO-MRW and pRNFL thickness values in each group. One-way-ANOVA data analysis, with the Kruskal-Wallis test and post-hoc analysis (with Dunn’s pairwise tests), was employed. For the pairwise comparison between groups (Dunn’s tests) the Bonferroni correction was applied and the adjusted significance value was considered. When possible (after testing for the required assumptions), the ANCOVA test was applied, using a univariate general linear model, to test for the effect of MD in the distribution of BMO-MRW and pRNFLT values between groups. The Chi-square test was used for categorical variables (classification as “within normal”, “borderline” or “outside normal limits”). The correlation between BMO-MRW and pRNFLT values was tested using the Pearson correlation coefficient. A new variable, the MRW/pRNFLT ratio, was defined. This ratio was calculated as the fraction between the MRW and pRNFLT values for global, nasal and temporal sectors (G-MRW/pRNFLT; N-MRW/pRNFLT and T-MRW/pRNFLT, respectively). The diagnostic ability of these ratios was evaluated using a receiver operating characteristic (ROC) curve analysis. The cut-off values were determined by the best sensitivity-specificity balance. A p-value of less than 0.05 was considered statistically significant. The best ROC curve was defined by the highest area under the curve (AUC) and the lowest p-value.
Results
A total of 100 patients (100 eyes) were included; 25 patients (25 eyes) in each group, with a mean age of 64.23 standard deviation (SD) 10.5 years. There was no significant difference in the mean age of patients in each group (ANOVA, p = .983).
In the group of patients with NAION, the mean follow-up time was 37 SD 21.88 months (range 6–77 months). In the CON group, 13 patients had a pituitary macroadenoma (12 of them surgically resected), two had craniopharyngioma (surgically resected) and 10 had meningiomas (six of them surgically resected). When performed, the mean follow-up time after surgical resection was 8.05 SD 4.49 years (range 2–15 years). Of the 25 patients included in OAG group, 20 had primary OAG, four had pseudoexfoliation syndrome and one had corticosteroid-induced glaucoma.
Descriptive data of optic disc parameters of the eyes in each group is summarised in Table 1. The mean BMO-area of the eyes was 2.02 SD 0.43 mm2, without significant difference between the four groups (ANOVA, p = .472). MRW was lower in the temporal sector of each group compared with the other sectors. However, pRNFLT was lower in the temporal sector of each group except in the NAION group, in which the lowest value of pRNFLT was observed in the nasal sector. In the intergroup analysis, the control group showed the highest MRW and pRNFLT values. The lowest MRW values were observed in the OAG group, whereas the lowest pRNFLT mean values were observed in the NAION group. Compared with the healthy controls, the relative reduction of the mean MRW and pRNFLT values was calculated for each sector of the study groups (Figure 1). The superior and inferotemporal sectors of the OAG group had the greatest reduction of MRW, while the superonasal and nasal sectors of the NAION group revealed the greatest reduction of pRNFLT.
Table 1.
Descriptive data for the four groups.
| Group | Control | NAION | CON | OAG |
|---|---|---|---|---|
| Age (years) | 64.16 ± 10.78 | 64.64 ± 10.18 | 63.56 ± 10.43 | 64.56 ± 10.50 |
| Age range | 44–91 | 45–88 | 44–83 | 43–88 |
| MD (n) | n = 23 | n = 18 | n = 24 | |
| 21.20 ± 9.47 | 11.27 ± 9.59 | 9.17 ± 7.92 | ||
| BMO area (mm2) | 2.02 ± 0.39 | 1.96 ± 0.39 | 1.97 ± 0.35 | 2.13 ± 0.56 |
| BMO-MRW (µm) | ||||
| Global | 318.68 ± 50.33 | 276.88 ± 66.21 | 232.92 ± 49.67 | 188.28 ± 79.42 |
| Superotemporal | 301.60 ± 59.24 | 260.28 ± 78.86 | 206.84 ± 53.21 | 160.92 ± 91.01 |
| Temporal | 227.32 ± 44.89 | 224.64 ± 68.54 | 159.84 ± 32.61 | 145.72 ± 58.99 |
| Inferotemporal | 337.96 ± 61.31 | 288.52 ± 94.90 | 233.84 ± 52.67 | 179.92 ± 102.81 |
| Inferonasal | 389.88 ± 76.13 | 348.60 ± 93.58 | 299.96 ± 92.68 | 229.52 ± 105.14 |
| Nasal | 354.64 ± 63.17 | 288.40 ± 76.90 | 266.04 ± 69.71 | 216.44 ± 98.04 |
| Superonasal | 353.12 ± 68.62 | 290.44 ± 86.40 | 266.04 ± 70.42 | 201.52 ± 85.05 |
| pRNFLT (µm) | ||||
| Global | 98.68 ± 9.58 | 54.68 ± 19.76 | 62.44 ± 15.98 | 64.68 ± 18.59 |
| Superotemporal | 123.68 ± 24.40 | 69.20 ± 54.15 | 85.32 ± 29.54 | 74.72 ± 28.20 |
| Temporal | 67.12 ± 11.31 | 45.76 ± 26.86 | 42.60 ± 12.91 | 48.04 ± 12.27 |
| Inferotemporal | 148.76 ± 18.81 | 85.40 ± 36.92 | 101.24 ± 32.72 | 90.96 ± 37.45 |
| Inferonasal | 121.56 ± 25.09 | 66.00 ± 22.91 | 71.12 ± 28.23 | 77.00 ± 31.11 |
| Nasal | 83.04 ± 11.42 | 41.72 ± 19.11 | 49.72 ± 18.00 | 55.96 ± 19.63 |
| Superonasal | 116.52 ± 24.85 | 54.20 ± 31.15 | 71.76 ± 30.84 | 78.24 ± 26.56 |
NAION – non-arteritic anterior ischaemic optic neuropathy. CON – compressive optic neuropathy. OAG – open angle glaucoma. BMO – Bruch’s membrane opening. BMO-MWR – Bruch’s membrane opening minimum rim width. MD – mean deviation. pRNFLT – peripapillary retinal nerve fibre layer thickness. ± – standard deviation.
Figure 1.
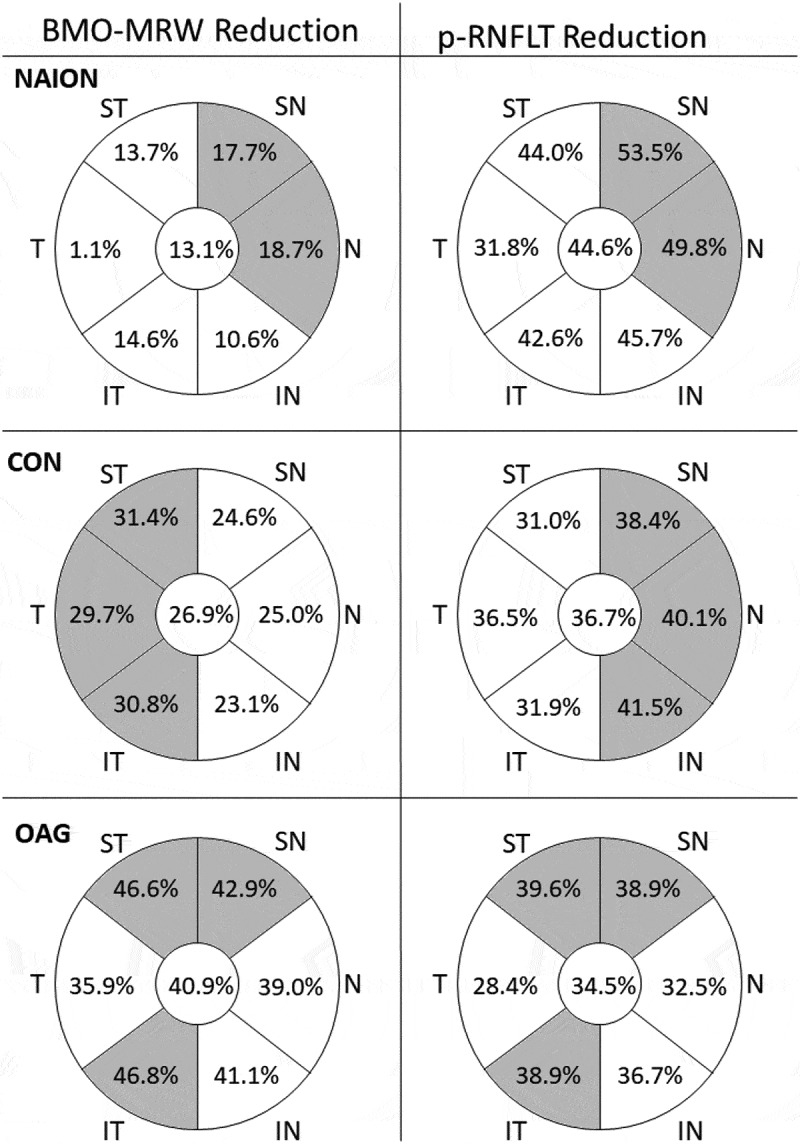
Relative reduction of the mean sectorial values of MRW and pRNFLT in each study group compared with healthy controls. The sectors with the greatest reduction for each group are highlighted in grey. NAION – non-arteritic anterior ischaemic optic neuropathy. CON – compressive optic neuropathy. OAG – open angle glaucoma. BMO – Bruch’s membrane opening. BMO-MWR – Bruch’s membrane opening minimum rim width. pRNFLT – peripapillary retinal nerve fibre layer thickness. ST – superotemporal. T – temporal. IT – inferotemporal. IN – inferonasal. N – nasal. SN – superonasal.
Available VF testing of patients in the study groups were analysed. There was a statistical difference in the mean MD between groups (Kruskall-Wallis, p < .001). Patients from the NAION group had significantly larger visual field deficits than those from CON and OAG groups (Kruskall-Wallis, p = .037 and p = .001, respectively). There was no difference in the MD between the CON and OAG groups (Kruskal-Wallis p = 1.000), however, we observed that the mean MD in CON was greater than in OAG (11.27 vs 9.17, respectively). For this reason, we performed an ANCOVA test for the comparison of these two groups, with correction to the covariate MD value.
Figures 2 and 3 show the distribution of the Global-MRW (G-MRW) and Global-pRNFLT (G-pRNFLT) classifications based on the percentiles of the normative database for each group. The eyes with OAG had a significantly increased proportion of G-MRW ‘outside normal limits’ and a reduced proportion of G-MRW ‘within normal limits’ compared with the eyes of the NAION and CON groups (CHI2, p < .001 and p = .013, respectively). Regarding the G-pRNFLT classification, there was no significant difference between the study groups (CHI2, p = .216 and p = .661, respectively).
Figure 2.
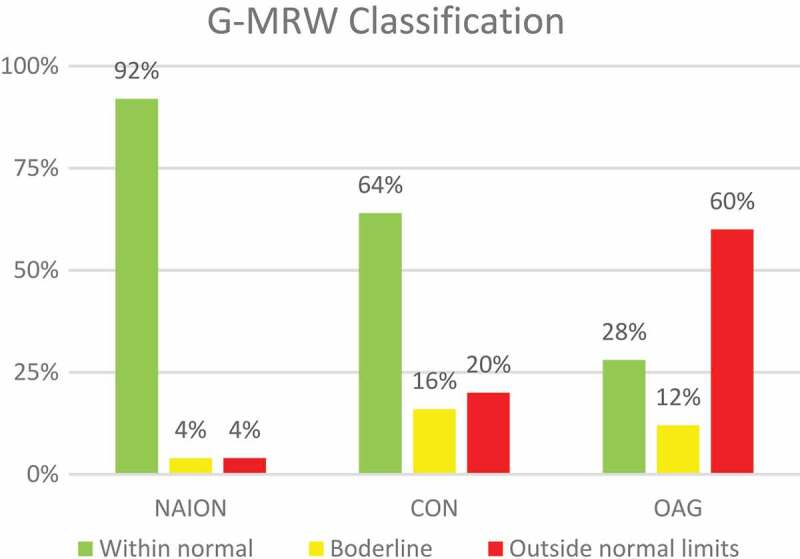
Frequency of normative percentiles-based classification of the G-MRW on the OCT in the three study groups. NAION – non-arteritic anterior ischaemic optic neuropathy. CON – compressive optic neuropathy. OAG – open angle glaucoma.
Figure 3.
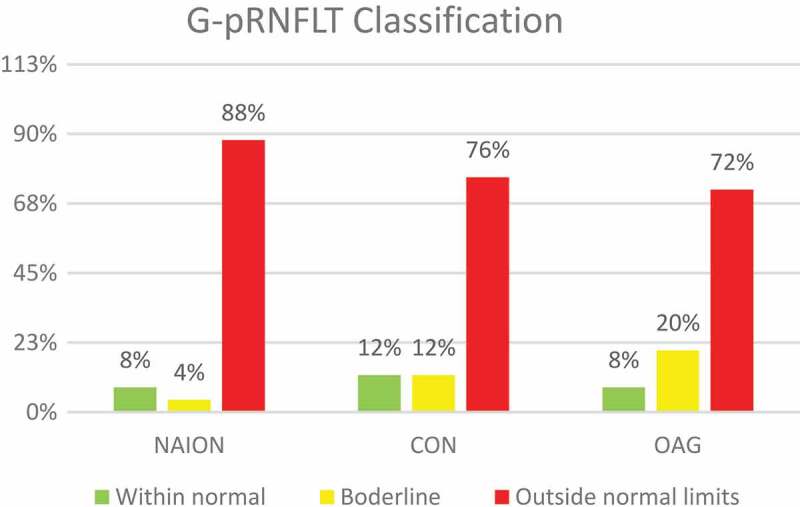
Frequency of normative percentiles-based classification of the G-pRNFLT on the OCT in the three study groups. NAION – non-arteritic anterior ischaemic optic neuropathy. CON – compressive optic neuropathy. OAG – open angle glaucoma.
The mean values of the global and sectorial MRW were significantly different in the 4 groups (Kruskal-Wallis, p < .001). The pairwise comparison (see Table 2) showed that the MRW values of the eyes with NAION were similar to those of the control group, and were significantly higher than those of the OAG group (Dunn’s tests, p < .01 for all sectors) and the temporal sector of the discs in the CON group (Dunn’s tests, p = .001) (Figure 4). The MRW values of the CON and OAG groups were significantly lower than that of the control group (Dunn’s tests, p < .01 for all sectors) without significant difference between each other. However, when the comparison was performed with adjustment for the MD value, the MRW was significantly greater in CON compared with OAG in all sectors, except the temporal one (see Table 2 ANCOVA test).
Table 2.
Pairwise comparison of the MRW and pRNFLT between groups. Kruskal-Wallis test with Dunn-Bonferroni post-hoc and ANCOVA test between CON and OAG adjusted for the covariate MD.
| Pairwise comparison | G | ST | T | IT | IN | N | SN |
|---|---|---|---|---|---|---|---|
| MRW | |||||||
| NAION vs Control | 0.266 | 0.300 | 1.000 | 0.184 | 0.945 | 0.051 | 0.095 |
| CON vs Control | <0.001* | <0.001* | <0.001* | <0.001* | 0.017* | 0.003* | 0.007* |
| OAG vs Control | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* |
| NAION vs CON | 0.201 | 0.157 | 0.001* | 0.323 | 0.685 | 1.000 | 1.000 |
| NAION vs OAG | <0.001* | 0.002* | <0.001* | 0.001* | 0.001* | 0.009* | 0.006* |
| OAG vs CON | 0.254 | 0.927 | 1.000 | 0.357 | 0.148 | 0.137 | 0.083 |
| ANCOVA CON vs OAG | 0.021* | 0.038* | 0.286 | 0.030* | 0.014* | 0.036* | 0.008* |
| pRNFLT | |||||||
| NAION vs Control | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* |
| CON vs Control | <0.001* | 0.002* | <0.001* | <0.001* | <0.001* | <0.001* | <0.001* |
| OAG vs Control | <0.001* | <0.001* | 0.001* | <0.001* | <0.001* | <0.001* | 0.003* |
| NAION vs CON | 1.000 | 0.068 | 1.000 | 1.000 | 1.000 | 1.000 | 0.405 |
| NAION vs OAG | 0.642 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.052 |
| OAG vs CON | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
| ANCOVA CON vs OAG | 0.823 | 0.127 | 0.431 | 0.167 | 0.959 | 0.608 | 0.690 |
Significant value p < 0.05. * – statistical significance. NAION – non-arteritic anterior ischaemic optic neuropathy. CON – compressive optic neuropathy. OAG – open angle glaucoma. G – global. ST – superotemporal. T – temporal. IT – inferotemporal. IN – inferonasal. N – nasal. SN – superonasal.
Figure 4.
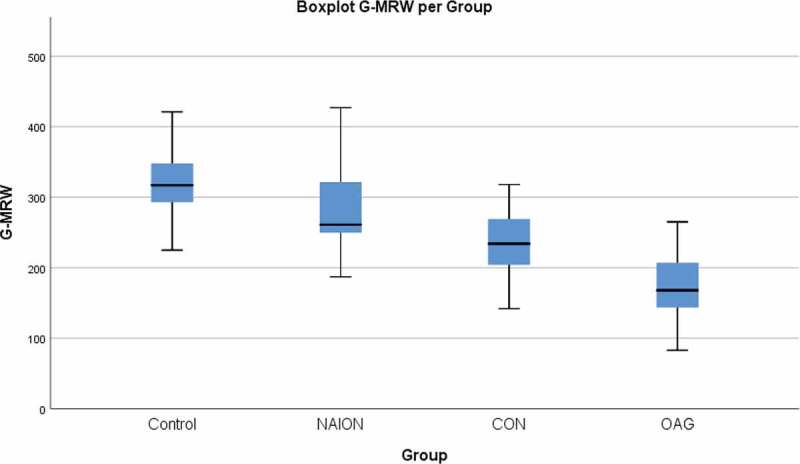
Boxplot of the global MRW value for each of the four groups. NAION – non-arteritic anterior ischaemic optic neuropathy. CON – compressive optic neuropathy. OAG – open angle glaucoma.
The mean values of the global and sectorial pRNFLT were significantly different in the four groups (Kruskal-Wallis, p < .001). In all three study groups, the pRNFLT values were significantly lower than in the control group (Dunn’s tests, p < .001 in all sectors), however there were no significant differences between the three study groups in any sector (Figure 5).
Figure 5.

Boxplot of the global pRNFLT value for each of the four groups. NAION – non-arteritic anterior ischaemic optic neuropathy. CON – compressive optic neuropathy. OAG – open angle glaucoma.
The Pearson correlation coefficient (ρ) revealed a positive correlation between MRW and pRNFLT in the study groups, in the following order of increasing strength: NAION, CON and OAG (ρMRW,pRNFLT = 0.417, p = .038; ρMRW,pRNFLT = 0.592, p = .002; ρMRW,pRNFLT = 0.720, p < .001). In the control group, there was no correlation between MRW and the pRNFLT (ρMRW,pRNFLT = 0.188, p = .369).
Tables 3 and 4 describe the MRW/pRNFLT ratios (G-, T- and N-) calculated for each group. Figure 6 illustrates the mean G-MRW/pRNFLT ratio obtained for each group. G-, T- and N-MRW/pRNFLT ratios were significantly higher in the NAION group compared with the control and OAG groups (Kruskal-Wallis, p ≤ .001 for all). In the OAG group, there was no significant difference compared with controls in any sector (Dunn’s tests, p = .886, p = 1.000 and p = 1.000 for G-, N- and T-MRW/pRNFLT, respectively). In the CON group, MRW/pRNFLT was higher than in the OAG group in all three sectors (ANCOVA test, p = .006, p = .016 and p = .010 for G-, N-, and T-MRW/pRNFLT, respectively); and N-MRW/pRNFLT was significantly higher than in the controls (Kruskal-Wallis, p = .041). G-MRW/pRNFLT was higher in the NAION group than in the CON group, however, this difference was not present in the nasal and temporal sectors (N- and T-MRW/pRNFLT, p = .600 and p = .149, respectively).
Table 3.
Descriptive data of MRW/pRNFLT ratios and ROC curve.
| Group | Control | NAION | CON | OAG |
|---|---|---|---|---|
| G-MRW/pRNFLT | ||||
| mean ± SD | 3.25 ± 0.56 | 5.50 ± 1.85 | 3.88 ± 0.98 | 2.91 ± 0.79 |
| ROC Curve | AUC | 0.856 | 0.508 | 0.864 |
| p value | <.001 | .906 | <.001 | |
| N-MRW/pRNFLT | ||||
| mean ± SD | 4.32 ± 0.84 | 8.61 ± 5.84 | 5.93 ± 2.88 | 4.08 ± 1.75 |
| ROC Curve | AUC | 0.784 | 0.553 | 0.835 |
| p value | <.001 | .455 | <.001 | |
| T-MRW/pRNFLT | ||||
| mean ± SD | 3.47 ± 0.81 | 6.12 ± 3.68 | 4.00 ± 1.12 | 3.02 ± 0.80 |
| ROC Curve | AUC | 0.802 | 0.512 | 0.814 |
| p value | <.001 | .862 | <.001 |
Significant value p < .05. AUC – area under the curve. G – MRW/pRNFLT: ratio in the global sector. N-MRW/pRNFLT: ratio in the nasal sector. T-MRW/pRNFLT: ratio in the temporal sector. NAION – non-arteritic anterior ischaemic optic neuropathy. CON – compressive optic neuropathy. OAG – open angle glaucoma.
Table 4.
Pairwise comparison of the MRW/pRNFLT ratio, in each sector, between groups. Kruskal-Wallis test with Dunn-Bonferroni post-hoc and ANCOVA test between CON and OAG adjusted for the covariate mean deviation.
| MRW/pRNFLT |
|||
|---|---|---|---|
| G- | N- | T- | |
| NAION vs Control | <0.001* | <0.001* | 0.001* |
| CON vs Control | 0.250 | 0.041* | 0.702 |
| OAG vs Control | 0.886 | 1.000 | 1.000 |
| NAION vs CON | 0.046* | 0.600 | 0.149 |
| NOIAN vs OAG | <0.001* | <0.001* | <0.001* |
| OAG vs CON | 0.003* | 0.002* | 0.022* |
| ANCOVA OAG vs CON | 0.006* | 0.010* | 0.0016* |
Significant value p < .05. * – statistical significance- G- global. N- nasal. T-temporal. NAION – non-arteritic anterior ischaemic optic neuropathy. CON – compressive optic neuropathy. OAG – open angle glaucoma.
Figure 6.
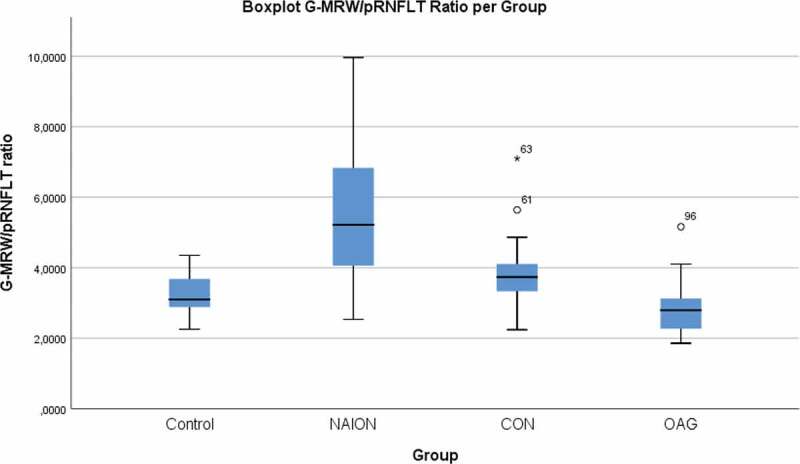
Boxplot of the G-MRW/pRNFLT ratio value for each of the four groups. NAION – non-arteritic anterior ischaemic optic neuropathy. CON – compressive optic neuropathy. OAG – open angle glaucoma.
ROC curves were calculated to evaluate the ability to diagnose each pathology among the eyes of the three study groups, in each sector. The obtained AUC and p values are described in Table 3. The best curve obtained was for the G-MRW/pRNFLT ratio, in the diagnosis of OAG among the eyes of the study groups. A G-MRW/pRNFLT ratio value ≤ 3.72 had a sensitivity of 84% and a specificity of 72% for the diagnosis of OAG, and a value ≥ 3.80 had a sensitivity of 88% and a specificity of 70% for the diagnosis of NAION. The ROC curves for the diagnosis of CON among study groups were not statistically significant in any sector.
We then tested the ability to differentiate NAION from OAG and CON from OAG, in the sectors with the best ROC Curves. Once the CON cases had been excluded, to determine the ability to differentiate NAION from OAG, the ROC curve of the G-MRW/pRNFLT ratio revealed an AUC of 0.922 (p > .001), with 88% sensitivity and 88% specificity for the cut-off value of 3.80 (see Figure 7). The ROC curve for the ability of the N-MRW/pRNFLT ratio to distinguish CON from OAG (Figure 8) had an AUC of 0.797 (p < .001). For an N-MRW/pRNFLT ≥ 4.24, the sensibility was 84% and the specificity 72%.
Figure 7.
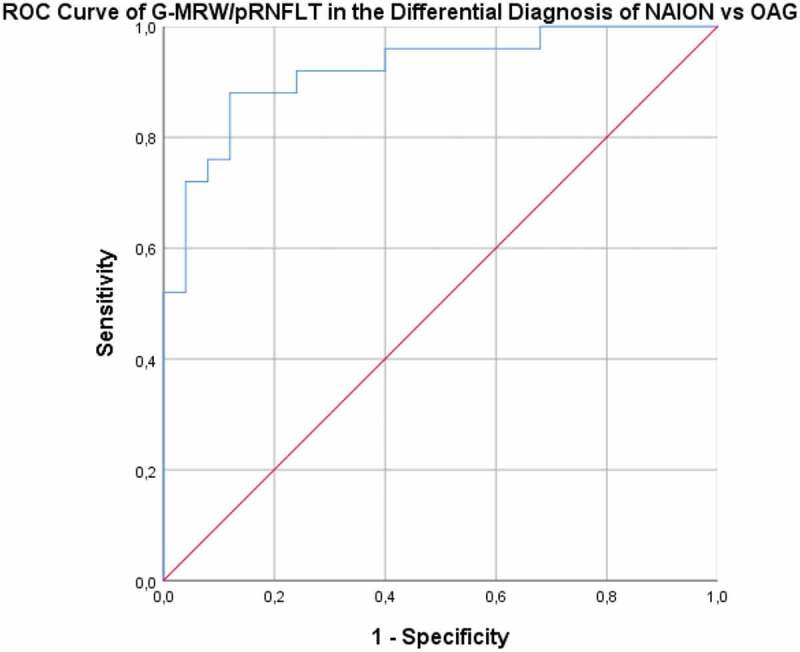
ROC curve – Ability of the G-MRW/pRNFLT to distinguish NAION from OAG. NAION – non-arteritic anterior ischaemic optic neuropathy. OAG – open angle glaucoma.
Figure 8.
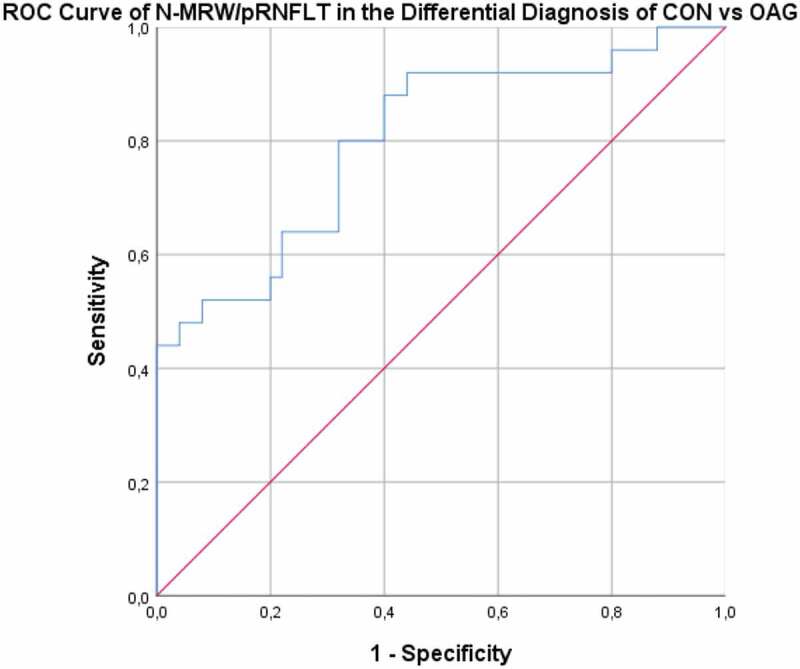
ROC curve – Ability of the N-MRW/pRNFLT to distinguish CON from OAG. CON – compressive optic neuropathy. OAG – open angle glaucoma.
Discussion
In this study, we evaluated optic disc parameters of eyes with optic atrophy caused by NAION, CON and OAG and of eyes from an age-matched healthy control group. The eyes from the three study groups had diffuse pRNFL loss, as expected considering that optic disc pallor on the funduscopy was one of the inclusion criteria, and there were no differences in the mean pRNFLT values between groups. However, this homogeneity did not translate into a similarity in the severity of disease among the study groups. In fact, the severity of the VF defects was not among the inclusion/exclusion criteria of this study, which resulted in wide variation of visual field deficit between roups. The low mean MD in the NAION group may be in accordance with the low pRNFLT seen in this group, despite the absence of statistical significance. A larger sample, with different subgroups of balanced disease severity and structural damage, would enable a more detailed evaluation of the MRW/pRNFLT changes with the extent of disease. Nonetheless, the absence of a significant difference in the pRNFLT values was important for the purpose of the study, as it allowed us to report different BMO-MRW variations.
Patients with NAION have been reported to have smaller optic discs, with lower disc areas and thicker neuroretinal rims than OAG patients and healthy controls.17,18 However, other studies using BMO-based disc areas found no significant difference between NAION affected eyes, unaffected fellow eyes and healthy controls eyes.15,19–21 These findings suggest that BMO is not as relevant as the scleral opening for the bottleneck effect suffered by the axons in the disc-at-risk phenotype of eyes with NAION. On the other hand, Nakanishi et al. alerted to the association between axial length (AL), BMO area and MRW. Eyes with larger AL had also larger BMO areas and thinner MRW.21 In our study, despite the lack of AL measurements, the applied exclusion criteria provided a small range of refractive errors among patients, and there was no significant difference in the BMO area between the four groups. This suggests that the higher MRW observed in the NAION group was not due to a smaller optic disc, but was more likely related to a truly thicker neuroretinal rim in these patients.
The software classification of MRW as “within normal limits”, “borderline” and “outside normal limits” showed statistically significant differences between the groups. Eyes with NAION and CON, frequently had an MRW classified as “within normal limits”, opposed to a pRNFLT classified as “outside normal limits”. This classification is promptly available and can be easily observed on OCT examinations.
The pairwise comparison also revealed a relative preservation of MRW in NAION eyes, which was similar to that of healthy controls. Pérez-Sarriegui et al. have demonstrated that, in patients with NAION, the affected eyes have thinner MRWs, while the unaffected fellow eyes have thicker MRWs than healthy controls.15 Previous studies suggested that a thicker MRW and thicker peripapillary choroid thickness may, in fact, be involved in the pathophysiology of the disease.15,19 In our study, the MRW in the NAION group was lower than in the control group, which denotes a reduction of the neuroretinal rim after NAION, however, there was no statistically significant difference.
The MRW in OAG eyes was significantly reduced compared with that of NAION and healthy control eyes. This intergroup analysis of the mean MRW values is in accordance with previously reported studies and reflects the underlying mechanisms of disease in each pathology.3,4,16 In NAION, the abrupt reduction of blood flow and ischaemia in the anterior portion of the optic nerve are responsible for the death of the nerve fibres inside the nerve head, leading to optic disc atrophy without significant cupping.15,16,19,22 However, in glaucoma the pathophysiology involves pressure forces to the nerve head, which lead to progressive cell death, cupping and remodelling. In this process, the lamina cribosa seems to be the primary point of lesion.2,12,22,23 In fact, substantial MRW thinning may precede detectable visual field loss.14
In CON, there is retrograde axonal degeneration caused by a compression of the retrobulbar anterior visual pathway. The pathophysiology may involve both a mechanical lesion and ischaemia of the axons. Intracranial lesions often simulate the appearance of the optic disc in glaucoma, with the presence of cupping. Although it may comprise loss of the glial tissue, the exact mechanism is unknown. In previous studies, 50% of CON cases presented with a cup-to-disc ratio ≥0.8 on stereoscopic photographs, and 44% were classified as having a glaucoma-like disc on the Heidelberg Retinal Tomography (HRT).6,7 Hata et al. evaluated the disc morphology in CON and OAG patients using HRT and radial OCT scans of the optic disc and identified a subgroup of CON patients (15 of 34 patients), with glaucoma-like optic discs, in which the cup/disc area ratio was not different from glaucomatous optic discs.6 Their results suggest that the depth of the lamina cribosa and the maximum cup depth are more useful for the differentiation of these two entities.6 In our results, the pairwise comparison did not reveal a significant difference in MRW between the CON and OAG groups. However, the adjusted comparison, that accounted for the unbalanced disease severity between the groups, showed that MRW values were significantly greater in the CON group, in all sectors, except the temporal one, when compared with the OAG group, without significant difference in the pRNFLT values. Therefore, we believe that an OAG group with more advanced damage (with a MD value similar to that of the CON group, or even the NAION group) would present an even more accentuated thinning of the MRW.
We did not find any previous studies comparing MRW in eyes with NAION and CON. Compared with the NAION group (that had significantly worse VF defects), the MRW in the CON group was significantly reduced only in the temporal sector. So, there seems to be a relative preservation of MRW also in CON, although to a smaller degree than in NAION. These neuroretinal rim changes in CON are yet to be understood. In our results, the thinning of the MRW was not in correspondence with the thinning of the pRNFLT. In fact, we observed the greatest reduction of the MRW in the temporal sector, while the greatest reduction of the pRNFLT was observed in the nasal sector (see Figure 1). Given the high proportion of compressive lesions involving the optic chiasm in the CON group, we would predict a more accentuated thinning in the nasal sector.24,25 Lee et al. compared the patterns of macular and pRNFL lesions in CON, glaucomatous and healthy eyes. They reported that in CON the macular RNFL suffered the greatest reduction in the nasal hemiretina, while the peripapillary fibres were most affected in the temporal and nasal sectors. However, in glaucomatous eyes, the superior and inferior sectors showed the greatest thinning of pRNFLT.24 Our results are in accordance with the described patterns of lesions of pRNFL in CON and OAG. Interestingly, the MRW/pRNFLT relation seemed to behave differently in the nasal and temporal sectors of the disc in the CON group. However, since the exact imaging location of these lesions was not evaluated in this study, we cannot establish a relationship between the sectorial reduction of MRW and pRNFLT and the location of the compressive lesion.
The relationship between MRW and pRNFLT in glaucomatous neuropathy was evaluated by Park et al. In their study they demonstrated that MRW and pRNFLT had a linear correlation, which was best represented by a broken-stick model.26 In our study, we found a correlation between the two parameters in the three study groups that was stronger in the OAG group than in the CON and NAION groups (in decreasing order). In the control group, however, there was no correlation found. Given this proven correlation, and the different values of MRW and pRNFLT in each group, we decided to calculate the MRW/pRNFLT ratio in the global, nasal and temporal sectors for each group. This ratio was significantly different between the three study groups, being highest in the NAION group, and lowest in the OAG group.
The calculated highest MRW/pRNFLT ratio in the NAION group, translated the greatest preservation of MRW relative to pRNFL loss in this pathology. While cupping may be present in many CON cases, we also observed a tendency for MRW preservation in this group. Despite being influenced by a higher MD value, we observed higher mean MRW and lower mean pRNFLT values compared with the OAG group, resulting in a significantly greater MRW/pRNFLT for the global, nasal and temporal sectors. In the OAG group, this ratio was the lowest of the study groups, representing the more pronounced MRW thinning relative to pRNFL loss.
These results suggest that a relative preservation of MRW in the presence of pRNFL loss should highly suggests a non-glaucomatous neuropathy. In specific cases, namely in some patients with CON, additional OCT parameters (lamina cribosa depth and retinal ganglion cell analysis) must be considered, with alow threshold for requesting neuroimaging studies in suspicious cases.
The MRW/pRNFLT ratio showed a good diagnostic ability for NAION among the eyes of the study groups, in all three sectors. The capability to distinguish between NAION and OAG was increased once CON cases had been excluded, which, in the clinical setting, can be achieved by the exclusion of an SOL on neuroimaging. A MRW/pRNFLT ratio ≥ 3.80 had a sensitivity of 88%, and a specificity of 88% for the diagnosis of NAION (AUC 0.856). Resch et al., with a stepwise linear regression analysis of the rim-RNFL correlation adjusted to covariates, achieved an AUC of 0.980 for the diagnosis of NAION.16 MRW/pRNFLT, being a simple ratio, may be easily applied in clinical practice, with a reliable diagnostic power. Future studies with subgroups of different damage could obtain higher sensitivity and specificity values for this ratio.
Regarding a diagnosis of CON, our ratio performed poorly in the differentiation of CON cases among the eyes of the three study groups. This is probably due to the fact that the MRW/pRNFLT ratio in CON had an intermediate value between that of NAION and OAG. However, when considering only CON and OAG cases, our ratio was able to distinguish these two entities, using the values of the nasal sector, with a sensitivity of 84% and a specificity of 72% for an N-MRW/pRNFLT value ≥ 4.24. In clinical practice, in the presence of a pale and excavated optic disc, an increased ratio between MRW and pRNFLT in the nasal sector, should be highly suggestive of a compressive cause for the optic atrophy.
This study has several limitations. The small sample size is a major limitation, however it is in line with previous studies.3,4,6,15,18–20,22 The GMP Edition requires good fixation during the image acquisition. In patients with NAION and CON, the quality of the images was frequently compromised, which may have limited our sample size. The lack of significant differences, especially in the comparison between CON and OAG groups, may be due to a low statistical power, rather than a true similarity. Nonetheless, our findings achieved statistical significance in several parameters. Enders et al. found a significant intraday variability of the BMO-MRW and pRNFLT parameters, that was greater in advanced damage of the neuroretinal tissues, and which could be mitigated if the measurements were taken within a five hour time gap.27 These diurnal fluctuations were not considered in our study. Despite these limitations, our study has yielded significant results and we believe it is a good representation of the three entities.
One important strength of this study is that it used only objective data given by the software that was not subject to individual interpretation or manual determination. Furthermore, the MRW/pRNFLT ratio combined two objective parameters that could be obtained simultaneously on the same examination, with increased diagnostic ability.
In conclusion, in non-glaucomatous optic disc atrophy, the MRW is frequently preserved, despite the presence of diffuse pRNFL loss. The MRW/pRNFLT ratio can differentiate between NAION and OAG. Our results suggest that this ratio could be useful in the evaluation of these neuropathies in the clinical setting.
Acknowledgements
The authors would like to acknowledge the orthoptic team of the ophthalmological department of the Centro Hospitalar Vila Nova de Gaia/Espinho for their collaboration in the data collection.
Declaration of interest
The authors declare that there are no conflicts of interest. The authors alone are responsible for the writing and content of the article.
References
- 1.Pasol J. Neuro-ophthalmic disease and optical coherence tomography: glaucoma look-alikes. Curr Opin Ophthalmol. 2011;22:124–132. doi: 10.1097/ICU.0b013e328343c1a3. [DOI] [PubMed] [Google Scholar]
- 2.Burgoyne C. The morphological difference between glaucoma and other optic neuropathies. J Neuroophthalmol. 2015;35:S8–S21. doi: 10.1097/WNO.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebolleda G, Pérez-Sarriegui A, Díez-Álvarez L, De Juan V, Muñoz-Negrete FJ. Lamina cribrosa position and Bruch’s membrane opening differences between anterior ischemic optic neuropathy and open-angle glaucoma. Eur J Ophthalmol. 2019;29:202–209. doi: 10.1177/1120672118782101. [DOI] [PubMed] [Google Scholar]
- 4.Rebolleda G, García-Montesinos J, De Dompablo E, Oblanca N, Muñoz-Negrete FJ, González-López JJ. Bruch’s membrane opening changes and lamina cribrosa displacement in non-arteritic anterior ischaemic optic neuropathy. Br J Ophthalmol. 2017;101:143–149. doi: 10.1136/bjophthalmol-2015-307945. [DOI] [PubMed] [Google Scholar]
- 5.Danesh-Meyer HV, Savino PJ, Sergott RC; NOVA . The prevalence of cupping in end-stage arteritic and nonarteritic anterior ischemic optic neuropathy. Ophthalmology. 2001;108:593–598. doi: 10.1016/S0161-6420(00)00602-3. [DOI] [PubMed] [Google Scholar]
- 6.Hata M, Miyamoto K, Oishi A, et al. Comparison of optic disc morphology of optic nerve atrophy between compressive optic neuropathy and glaucomatous optic neuropathy. PLoS One. 2014;9:e112403. doi: 10.1371/journal.pone.0112403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eugenia I, Andreea M, Ramona OM, Dănuţ C. The role of optical coherence tomography in optic neuropathies. Rom J Ophthalmol. 2018;62:3–14. doi: 10.22336/rjo.2018.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan NCY, Chan CKM. The use of optical coherence tomography in neuro-ophthalmology. Curr Opin Ophthalmol. 2017;28:552–557. doi: 10.1097/ICU.0000000000000418. [DOI] [PubMed] [Google Scholar]
- 9.Chwalisz BK, Bouffard MA, Prasad S, Cestari DM. Neuroimaging diagnostic and monitoring approaches in ophthalmology. Curr Opin Neurol. 2018;31:66–73. doi: 10.1097/WCO.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 10.Reis AS, O’Leary N, Yang H, et al. Influence of clinically invisible, but optical coherence tomography detected, optic disc margin anatomy on neuroretinal rim evaluation. Invest Ophthalmol Vis Sci. 2012;53:1852–1860. doi: 10.1167/iovs.11-9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chauhan BC, O’Leary N, AlMobarak FA, et al. Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthalmology. 2013;120:535–543. doi: 10.1016/j.ophtha.2012.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reznicek L, Burzer S, Laubichler A, et al. Structure-function relationship comparison between retinal nerve fibre layer and Bruch’s membrane opening-minimum rim width in glaucoma. Int J Ophthalmol. 2017;10:1534–1538. doi: 10.18240/ijo.2017.10.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rebolleda G, Casado A, Oblanca N, Muñoz-Negrete FJ. The new Bruch’s membrane opening - minimum rim width classification improves optical coherence tomography specificity in tilted discs. Clin Ophthalmol. 2016;10:2417–2425. doi: 10.2147/OPTH.S120237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park KH, Lee JW, Kim JM, Nouri-Mahdavi K, Caprioli J. Bruch’s membrane opening-minimum rim width and visual field loss in glaucoma: a broken stick analysis. Int J Ophthalmol. 2018;11:828–834. doi: 10.18240/ijo.2018.05.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez-Sarriegui A, Muñoz-Negrete FJ, Noval S, De Juan V, Rebolleda G. Automated evaluation of choroidal thickness and minimum rim width thickness in nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2018;38:7–12. doi: 10.1097/WNO.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 16.Resch H, Mitsch C, Pereira I, et al. Optic nerve head morphology in primary open-angle glaucoma and nonarteritic anterior ischaemic optic neuropathy measured with spectral domain optical coherence tomography. Acta Ophthalmol. 2018;96:1018–1024. doi: 10.1111/aos.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito H, Tomidokoro A, Tomita G, Araie M, Wakakura M. Optic disc and peripapillary morphology in unilateral nonarteritic anterior ischemic optic neuropathy and age- and refraction-matched normals. Ophthalmology. 2008;115:1585–1590. doi: 10.1016/j.ophtha.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Saito H, Tomidokoro A, Sugimoto E, et al. Optic disc topography and peripapillary retinal nerve fiber layer thickness in nonarteritic ischemic optic neuropathy and open-angle glaucoma. Ophthalmology. 2006;113:1340–1344. doi: 10.1016/j.ophtha.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 19.Nagia L, Huisingh C, Johnstone J, et al. Peripapillary pachychoroid in nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2016;57:4679–4685. doi: 10.1167/iovs.16-19315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moghimi S, Afzali M, Akbari M, et al. Crowded optic nerve head evaluation with optical coherence tomography in anterior ischemic optic neuropathy. Eye (Lond). 2017;31:1191–1198. doi: 10.1038/eye.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakanishi H, Suda K, Yoshikawa M, et al. Association of Bruch’s membrane opening and optic disc morphology to axial length and visual field defects in eyes with primary open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2018;256:599–610. doi: 10.1007/s00417-017-3874-8. [DOI] [PubMed] [Google Scholar]
- 22.Fard MA, Afzali M, Abdi P, et al. Optic nerve head morphology in nonarteritic anterior ischemic optic neuropathy compared to open-angle glaucoma. Invest Ophthalmol Vis Sci. 2016;57:4632–4640. doi: 10.1167/iovs.16-19442. [DOI] [PubMed] [Google Scholar]
- 23.Xu G, Weinreb RN, Leung CK. Optic nerve head deformation in glaucoma: the temporal relationship between optic nerve head surface depression and retinal nerve fiber layer thinning. Ophthalmology. 2014;121:2362–2370. doi: 10.1016/j.ophtha.2014.06.035. [DOI] [PubMed] [Google Scholar]
- 24.Lee EJ, Yang HK, Kim TW, Hwang JM, Kim YH, Kim CY. Comparison of the pattern of retinal ganglion cell damage between patients with compressive and glaucomatous optic neuropathies. Invest Ophthalmol Vis Sci. 2015;56:7012–7020. doi: 10.1167/iovs.15-17909. [DOI] [PubMed] [Google Scholar]
- 25.Vuong LN, Hedges TR. Ganglion cell layer complex measurements in compressive optic neuropathy. Curr Opin Ophthalmol. 2017;28:573–578. doi: 10.1097/ICU.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 26.Park K, Kim J, Lee J. The relationship between bruch’s membrane opening-minimum rim width and retinal nerve fiber layer thickness and a new index using a neural network. Transl Vis Sci Technol. 2018;7:14. doi: 10.1167/tvst.7.4.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enders P, Bremen A, Schaub F, et al. Intraday repeatability of Bruch’s membrane opening-based neuroretinal rim measurements. Invest Ophthalmol Vis Sci. 2017;58:5195–5200. doi: 10.1167/iovs.17-22812. [DOI] [PubMed] [Google Scholar]


