ABSTRACT
To determine whether temporal artery biopsy (TABx) or Doppler ultrasound (US) of the temporal artery is the preferred confirmatory test for giant cell arteritis, an online survey of ophthalmologists and neurologists in North America, Europe and Israel was conducted in 2019; Canadian rheumatologists were also included. There were 406 survey participants with an estimated survey response rate of 18%. Ninety-four per cent of North American practitioners preferred TABx compared with 74% of their European counterparts. Two per cent of North American practitioners preferred Doppler US versus 24% of European physicians. Regional differences were statistically significant (p < .001).
KEYWORDS: Giant cell arteritis, temporal artery biopsy, doppler ultrasound, preferred practice patterns, survey
Introduction
Giant cell arteritis (GCA) is one of the most important neuro-ophthalmic emergencies. Temporal artery biopsy (TABx) has long been acknowledged as the “gold standard” confirmatory test in patients with suspected GCA.1–6 However, TABx is an invasive test with potential for facial nerve palsy, haemorrhage, infection, untoward scarring and rarely stroke.
In 2018 the European League Against Rheumatism (EULAR) guidelines suggested that at centres with appropriate equipment and sufficient radiologic expertise Doppler ultrasound (US) of the temporal artery or magnetic resonance imaging (MRI) may be first line investigations for suspected GCA.7 The British Society of Rheumatology guidelines for GCA 2019 revision draft proposed the use of a confirmatory test for GCA which can either be, “a temporal artery biopsy at least 1 cm in length, or an ultrasound of the temporal and axillary arteries, or both.”8 Given the above recommendations, our objective was to determine if ophthalmologists and neurologists (neuro-ophthalmologists) currently prefer US or TABx as their test of choice to confirm GCA. There is debate between the use of the two confirmatory tests9,10 and a systematic review has questioned the reliability of US in comparison to TABx.11
Materials and methods
Research ethics board approval was obtained from Michael Garron Hospital, and the research was compliant with Declaration of Helsinki. An online survey of ophthalmologists and neurologists in North America, Europe and Israel was conducted in May and June 2019. Canadian rheumatologists were also canvassed.
The survey instrument was Survey Planet (https://surveyplanet.com/). The three study questions were: 1) What test do you currently use to confirm the diagnosis of giant cell arteritis? 2) Where do you work? and 3) What is your primary specialty? The available responses to each question are shown in Appendix A and at https://s.surveyplanet.com/UJ2kjVmw6. The survey did not advance until all questions were answered, and the software prevented double entries from the same computer or internet protocol (IP) address.
Practitioners with membership in neuro-ophthalmology (European Neuro-ophthalmology Society, North American Neuro-ophthalmology Society) and oculoplastic surgery societies (American Society of Oculoplastic and Reconstructive Surgery, Canadian Society of Oculoplastic and Reconstructive Surgery) were targeted as these specialists were most likely to encounter patients with GCA. The survey was also sent to a group of Canadian rheumatologists from Ontario, Canada. To optimise the response rate, the survey was kept anonymous, was designed to be completed in 25 s, incorporated a logo, and avoided questions about age or years in practice.12 Also, on the internet lines that allowed mass emailing, requests for survey responses were canvassed at least twice on two separate dates. Respondents were allowed to free text additional details, and their email address if they desired. The results of the European and Israeli physicians were pooled as a group.
The survey margin of error (x) was determined using the calculator from https://www.surveysystem.com/sscalc.htm and reported as (± x)95%CI with the superscript denoting a ninety-five per cent confidence interval. (see Appendix C)
Results
In total 406 surveys were completed in a median time of 22 s. Our estimated survey response rate was 18% (see Appendix B).
There were 253 (62.3%) ophthalmology and neurology respondents (O&N) from North America, 82 (20.2%) O&N participants from Europe, and 71 (17.5%) Canadian rheumatologists.
The overall results from the O&N group showed that 303 (90.5 ± 2.9%)95%CI of survey participants preferred TABx as their confirmatory test for GCA, while 22 (6.6 ± 2.4%)95%CI favoured Doppler US. Out of the O&N practitioners that preferred TABx, 268 (88.4 ± 3.3%) 95%CI indicated they use TABx exclusively, and 35 (11.6 ± 3.3%)95%CI ordered both TABx and US, but preferred TABx. Ten of the 335 O&N participants (3.0 ± 1.8%)95%CI indicated they did not order TABx or US for their GCA suspects; one used MRI head exclusively, one endorsed US of the central retinal artery, three deferred work-up decisions to their group’s neuro-ophthalmologist, and the remaining five respondents did not provide a reason.
On a regional basis, 242/253 (95.7 ± 2.31%)95%CI of North American O&N preferred TABx as their confirmatory test, compared with 61/82 (74.4 ± 7.7%)95%CI of their European counterparts. Doppler US was the favoured test in 2/253 (0.8 ± ~1.13%)95%CI of North American O&N versus 20/82 (24.4 ± 7.6%)95%CI of European physicians. The regional differences were statistically significant as the confidence intervals do not overlap; also the non survey-weighted two-sample test of proportions, as well as Pearson chi2 test, showed p < .001. (see Appendix D)
With regards to test preference and our survey physicians, 34/37 (91.9%) neurologists, 269/298 (90.3%) ophthalmologists and 64/71 (90.1%) rheumatologists endorsed TABx as their confirmatory test for GCA, with no statistically significant difference on repeated two-sample test of proportions. (the p values ranged from 0.75 to 0.97, Appendix E)
Seventy-one Canadian rheumatologists were surveyed, and 64/71 (90.1 ± 6.0%)95%CI preferred TABx, 4/71 (5.6 ± 4.6%) 95%CI preferred Doppler US, and 3/71 (4.2 ± 3.9%) 95%CI ordered neither. One rheumatologist from the latter group endorsed MRI head as their preferred investigation. We canvassed EULAR but did not receive results from European rheumatologists.
Discussion
Of the imaging techniques described for GCA including MRI, magnetic resonance angiography, computed tomographic angiography and positron emission tomography. EULAR has recommended US of the temporal ± axillary arteries as the first imaging modality in patients with suspected predominantly cranial GCA.7
As of July 2019, the majority of O&N clinicians in North America and Europe prefer TABx to US in the work-up of GCA. The greater proportionate use of US in Europe versus North America may be because of the EULAR guidelines. The advantages of US over TABx include its non-invasive nature, and lower cost. US can assess both the temporal and axillary arteries and increase the diagnostic yield,13 and serial US can help monitor the effect of treatment.14 However, US is highly examiner-dependent technique. Systematic review of articles comparing imaging and pathology showed that the hypoechoic halo sign on temporal artery Doppler US had 68% (57%,78%)95%CI sensitivity and 81% (75%,86%)95%CI specificity compared to a positive TABx.11 Conditions such as atherosclerosis can cause false positive halo signs on Doppler US.15 The low 39% sensitivity for TABx in comparison to US in the Role of Ultrasound Compared to Biopsy of Temporal Arteries in the Diagnosis and Treatment of Giant Cell Arteritis (TABUL) study,13 may be attributable to factors such as i) Seven per cent of the TABUL biopsies did not retrieve a temporal artery, but instead structures such as veins, fat, muscle or nerve, ii) At least 43% of the TABUL TABx specimens were less than 1 cm, and iii) The ACR classification non-biopsy criteria were not intended for the diagnosis of GCA. Even the EULAR task force conceded that TABx “should be performed in all cases, where GCA cannot be confirmed or excluded based on clinical, laboratory and imaging results.”16
A potential weakness of our survey is the 18% survey response rate. This likely was an underestimate as some members had multiple listings on the same society membership, retired members were still listed on the internet line, and members who belonged to both neuro-ophthalmology and the oculoplastic societies were double-counted, and paediatric subspecialists would be unlikely to encounter patients with GCA. Our report of 95% confidence intervals accounts for the response rate. Furthermore, the direct correlation between response rate and study validity has been questioned.17 Some surveys with low response rates, even as low as 20%, may yield more accurate results than studies with response rates of 60% to 70%.18 Investigations with low response rates may be only marginally less accurate than those with higher response rates.19
The results of this physician survey may elucidate geographic and physician specialty trends in the work-up of GCA, and perhaps aid in the development of future preferred practice patterns. The use of clinical prediction rules,20 in conjunction with continuously improving imaging techniques, and possibly forthcoming genetic tests, may decrease the reliance on TABx in the future.
Supplementary Material
Appendix A. Survey Questions
Appendix B: Estimation of Survey Response Rate
Calculations
NANOS members: 627 American + 55 Canadian = 682
ASOPRS members: 669 American + 28 Canadian = 697
Overlaps in NANOS & ASOPRS estimated at 5%: (682 + 697)*.95 = 1,310
Ont-Eye internet line ophthalmologists: 463-16-14 = 433
2018 communication with website moderator: 16 NANOS members overlap in Ont-Eye;
14 ASOPRS overlaps in Ont-Eye
EUNOS member: 244–4 NA = 240 recipients
Ontario rheumatologists: 270 according to College of Physicians and Surgeons website
Best denominator estimate = 2,253
406 (NA + Europe&Israel) respondents/2253 = 18.0% response rate overall
Abbreviations
NANOS = North American Neuro-Ophthalmology Society
ASOPRS = American Society of Ophthalmic Plastic & Reconstructive Surgery
Ont-Eye = Eye Physicians and Surgeons of Ontario
EUNOS = European Neuro-ophthalmology Society
NA = North American
CPSO = College of Physicians and Surgeons of Ontario
Appendix C: Calculation of Survey 95% Confidence Intervals
Calculation of survey 95% confidence intervals was done using the online tool: https://www.surveysystem.com/sscalc.htm
| Result | Sample Size Calculator |
|---|---|
| 303/335 O&N NA + Europe (90.5% prefer TABx). Total estimated 1,983 O&N NA + Europe 95% CI is ±2.9% |
 |
| A total of 303 O&N in NA & Europe preferred TABx. 88.4% used TABx exclusively 95% CI is ±3.32% |
 |
| 253 North American O&N/total 1743 O&N in North America with 95.58% choosing TABx 95% CI is ±2.31% |
 |
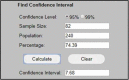
| 82 European O&N/total 240 O&N in Europe with 74.39% choosing TABx 95% CI is ±7.7% |
 |
| 24.39 ± 7.56% of op& neuro in Europe prefer US | |
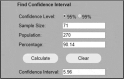
| 64/71 rheumatologists in survey (90.14% prefer TABx). There are 270 provincially registered rheum 95%CI is ± 6.0% |
 |
Appendix D: Statistical Tests for Regional Differences in O&N Preference for TABx
Chi-Square Test
| Region |
|||
|---|---|---|---|
| Europe | North America | Total | |
| Neither | 1 | 9 | 10 |
| TABx | 61 | 242 | 303 |
| US | 20 | 2 | 22 |
| Total | 82 | 253 | 335 |
Pearson Chi2 = 56.7492 Pr = <0.001
Conventional 2 Sample Tests of Proportions
Although the test is not adjusted for survey weighting, given the 21% difference and survey confidence intervals, there would be no change in the inference.
| x: Number of obs = 253 |
||||||
|---|---|---|---|---|---|---|
| Two-sample test of proportions |
y: Number of obs = 82 |
|||||
| Mean | Std. Err. | z | P>|z| | [95% Conf. Interval] | ||
| x | .9565217 | .012821 | .931393 | .9816505 | ||
| y | .7439024 | .0482007 | .6494307 | .8383742 | ||
| diff | .2126193 | .0498768 | .1148626 | .310376 | ||
| under Ho: | .0373514 | 5.69 | 0.000 | |||
diff = prop(x) – prop(y); z = 5.6924
Ho: diff = 0
Ha: diff < 0; Ha: diff != 0; Ha: diff > 0
Pr(Z < z) = 1.0000; Pr(|Z| > |z|) = 0.0000; Pr(Z > z) = 0.0000
Appendix E: Statistical Tests for Specialty Differences in Preference for TABx

Declaration of interest
The authors have no conflicts of interest. The authors do not have any commercial enterprises involving the diagnosis or treatment of temporal arteritis.
References
- 1.Koster MJ, Warrington KJ.. Giant cell arteritis: pathogenic mechanisms and new potential therapeutic targets. BMC Rheumatol. 2017; 1(2). doi: 10.1186/s41927-017-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frohman L, Wong AB, Matheos K, Leon-Alvarado LG, Danesh-Meyer HV. New developments in giant cell arteritis. Surv Ophthalmol. 2016;61(4):400–421. doi: 10.1016/j.survophthal.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Weyand CM, Goronzy JJ. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med. 2014;371(1):50–57. doi: 10.1056/NEJMcp1214825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ness T, Bley TA, Schmidt WA, Lamprecht P. The diagnosis and treatment of giant cell arteritis. Dtsch Arztebl Int. 2013;110(21):376–386. doi: 10.3238/arztebl.2013.0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danesh-Meyer H. Temporal artery biopsy: skip it at your patient’s peril. Am J Ophthalmol. 2012;154(4):617–619. doi: 10.1016/j.ajo.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Villa-Forte A. Giant cell arteritis: suspect it, treat it promptly. Cleve Clin J Med. 2011;78(4):265–270. doi: 10.3949/ccjm.78a.10131. [DOI] [PubMed] [Google Scholar]
- 7.Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77(5):636–643. doi: 10.1136/annrheumdis-2017-212649. [DOI] [PubMed] [Google Scholar]
- 8.British Society of Rheumatology . BSR Guideline on Diagnosis and Treatment of Giant Cell Arteritis: Executive Summary, Draft Revision. London: British Society of Rheumatology; 2019. https://www.rheumatology.org.uk/Portals/0/Documents/Guidelines/GCA/Open_Consultation_GCA_GL_Executive_Summary.pdf?ver=2019-07-02-152636-127 [Google Scholar]
- 9.González Porto SA, Silva Díaz MT, Reguera Arias A, et al. A comparative study of doppler ultrasound against temporary artery biopsy in the diagnosis of giant cell arteritis. Reumatologia Clinica. 2018;S1699–258X(18)30187–6. doi: 10.1016/j.reuma.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Bilyk JR, Murchison AP, Leiby BT, et al. The utility of color duplex ultrasonography in the diagnosis of giant cell arteritis: a prospective, masked study. (An American ophthalmological society thesis). Trans Am Ophthalmol Soc. 2018;115:T9. [PMC free article] [PubMed] [Google Scholar]
- 11.Rinagel M, Chatelus E, Jousse-Joulin S, et al. Diagnostic performance of temporal artery ultrasound for the diagnosis of giant cell arteritis: a systematic review and meta-analysis of the literature. Autoimmun Rev. 2019;18(1):56–61. doi: 10.1016/j.autrev.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Fan W, Zheng Y. Factors affecting response rates of the web survey: A systematic review. Comput Human Behav. 2010;26(2):132–139. doi: 10.1016/j.chb.2009.10.015. [DOI] [Google Scholar]
- 13.Luqmani R, Lee E, Singh S, et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): a diagnostic accuracy and cost-effectiveness study. Health Technol Assess. 2016;20(90). doi: 10.3310/hta20400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landau K, Savino PJ, Gruber P. Diagnosing giant cell arteritis: Is ultrasound enough? J NeuroOphthalmol. 2013;33(4):394–400. doi: 10.1097/WNO.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 15.De Miguel E, Beltran LM, Monjo I, Deodati F, Schmidt WA, Garcia-Puig J. Atherosclerosis as a potential pitfall in the diagnosis of giant cell arteritis. Rheumatology (Oxford). 2018;57(2):318–321. doi: 10.1093/rheumatology/kex381. [DOI] [PubMed] [Google Scholar]
- 16.Moiseev SV, Smitienko I, Bulanov N, Novikov P. The role of temporal artery biopsy in patients with giant-cell arteritis is debated. Ann Rheum Dis. 2019;78:e31. doi: 10.1136/annrheumdis-2018-213282. [DOI] [PubMed] [Google Scholar]
- 17.Morton SB, Bandara DK, Robinson EM, Atatoa Carr PE. In the 21st Century, what is an acceptable response rate? Aust NZ J Public Health. 2012;36(2):106–108. doi: 10.1111/j.1753-6405.2012.00854.x. [DOI] [PubMed] [Google Scholar]
- 18.Visser PS, Krosnick JA, Marquette J, Curtin M. Mail surveys for election forecasting? An evaluation of the Colombia dispatch poll. Public Opin Q. 1996;60:181–227. doi: 10.1086/297748. [DOI] [Google Scholar]
- 19.Holbrook A, Krosnick J, Pfent A. The causes and consequences of response rates in surveys by the news media and government contractor survey research firms. In: Lepkowski JM, Leae NTJBEDLLJP, eds.. Advances in Telephone Survey Methodology. New York (NY): Wiley; 2007:499–678. [Google Scholar]
- 20.Ing EB, Miller NR, Nguyen A, et al. Neural network and logistic regression diagnostic prediction models for giant cell arteritis: development and validation. Clin Ophthalmol. February 2019;13:421–430. doi: 10.2147/OPTH.S193460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


