Abstract
Background
Uterine leiomyomas, also referred to as myomas or fibroids, are benign tumours arising from the smooth muscle cells of the myometrium. They are the most common pelvic tumour in women. The estimated rate of leiomyosarcoma, found during surgery for presumed benign leiomyomas, is about 0.51 per 1000 procedures, or approximately 1 in 2000.
Treatment options for symptomatic uterine leiomyomas include medical, surgical, and radiologically‐guided interventions. Laparoscopic myomectomy is the gold standard surgical approach for women who want offspring, or otherwise wish to retain their uterus. A limitation of laparoscopy is the inability to remove large specimens from the abdominal cavity through the laparoscope. To overcome this challenge, the morcellation approach was developed, during which larger specimens are broken into smaller pieces in order to remove them from the abdominal cavity via the port site. However, intracorporeal power morcellation may lead to scattering of benign tissues, with the risk of spreading leiomyoma or endometriosis. In cases of unsuspected malignancy, power morcellation can cause unintentional dissemination of malignant cells, and lead to a poorer prognosis by upstaging the occult cancer.
A strategy to optimise women's safety is to morcellate the specimens inside a bag. In‐bag morcellation may avoid the dissemination of tissue fragments.
Objectives
To evaluate the effectiveness and safety of protected in‐bag extracorporeal manual morcellation during laparoscopic myomectomy compared to intra‐abdominal uncontained power morcellation.
Search methods
On 1 July 2019, we searched; the Cochrane Gynaecology and Fertility Group Specialized Register of Controlled Trials, CENTRAL, MEDLINE, Embase, PsycINFO, CINAHL, LILACS, PubMed, Google Scholar, and two trials registers.
We reviewed the reference lists of all retrieved full‐text articles, and contacted experts in the field for additional and ongoing trials.
Selection criteria
We included all randomised controlled trials comparing in‐bag extracorporeal manual morcellation versus intracorporeal uncontained power morcellation during laparoscopic myomectomy in premenopausal women.
Data collection and analysis
We followed standard Cochrane methods.
Two review authors independently reviewed the eligibility of trials, extracted data, and evaluated the risk of bias. Data were checked for accuracy.
The summary measures were reported as risk ratios (RR) or mean differences (MD) with 95% confidence interval (CI).
The outcomes of interest were a composite of intraoperative and postoperative complications, operative times, ease of morcellation, length of hospital stay, postoperative pain, conversion to laparotomy, and postoperative diagnosis of leiomyosarcoma. Results for the five main outcomes follow.
Main results
We included two trials, enrolling 176 premenopausal women with fibroids, who underwent laparoscopic myomectomy.
The experimental group received in‐bag manual morcellation, during which each enucleated myoma was placed into a specimen retrieval bag, and manually morcellated with scalpel or scissors. In the control group, intracorporeal uncontained power morcellation was used to reduce the size of the myomas.
No intraoperative complications, including accidental morcellation of the liver, conversion to laparotomy, endoscopic bag disruption, bowel injury, bleeding, accidental injury to any viscus or vessel, were reported in either group in either trial.
We found very low‐quality evidence of inconclusive results for total operative time (MD 9.93 minutes, 95% CI ‐1.35 to 21.20; 2 studies, 176 participants; I² = 35%), and ease of morcellation (MD ‐0.73 points, 95% CI ‐1.64 to 0.18; 1 study, 104 participants). The morcellation operative time was a little longer for the in‐bag manual morcellation group, however the quality of the evidence was very low (MD 2.59 minutes, 95% CI 0.45 to 4.72; 2 studies, 176 participants; I² = 0%). There were no postoperative diagnoses of leiomyosarcoma made in either group in either trial.
We are very uncertain of any of these results. We downgraded the quality of the evidence due to indirectness and imprecision, because of limited sites in high‐income settings and countries, small sample sizes, wide confidence intervals, and few events.
Authors' conclusions
There are limited data on the effectiveness and safety of in‐bag morcellation at the time of laparoscopic myomectomy compared to uncontained power morcellation. We were unable to determine the effects of in‐bag morcellation on intraoperative complications as no events were reported in either group. We are uncertain if in‐bag morcellation improves total operative time or ease of morcellation compared to control. Regarding morcellation operative time, the quality of the evidence was also very low and we cannot be certain of the effect of in‐bag morcellation compared to uncontained morcellation. No cases of postoperative diagnosis of leiomyosarcoma occurred in either group. We found only two trials comparing in‐bag extracorporeal manual morcellation to intracorporeal uncontained power morcellation at the time of laparoscopic myomectomy. Both trials had morcellation operative time as primary outcome and were not powered for uncommon outcomes such as intraoperative complications, and postoperative diagnosis of leiomyosarcoma.
Large, well‐planned and executed trials are needed.
Plain language summary
In‐bag manual versus uncontained power morcellation for laparoscopic myomectomy
Review question
Cochrane authors reviewed the effectiveness and safety of in‐bag manual morcellation compared to uncontained power morcellation during laparoscopic myomectomy. The primary outcome of the review was a composite of intraoperative (during surgery) complications.
Background
Myomectomy is a surgical procedure to remove uterine fibroids — also called leiomyomas. These common, non‐cancerous growths usually develop in the uterus during childbearing years, but they can occur at any age. In laparoscopic myomectomy, which is a minimally invasive procedure, the surgeon accesses and removes the fibroids through several small abdominal incisions.
Laparoscopic myomectomy is the gold standard for women with uterine leiomyomas, but it is difficult to remove large fibroids through the small incisions and tubes (laparoscopes). To overcome this challenge, surgeons developed a technique to break the large fibroids into smaller pieces (morcellation).
However, power morcellation (using a drill‐like instrument to cut up the fibroids) inside the abdomen may lead to the scattering of the tissues. These tissues may be benign (such as fibroids or endometriosis), but they may also be tissues from an undiagnosed cancer. A strategy to optimise women's safety is to cut up larger fibroids with a scalpel or scissors, inside a bag.
Study Characteristics
We included two randomised controlled trials, which enrolled 176 premenopausal women undergoing laparoscopic myomectomy; they were randomised to receive either in‐bag manual morcellation (87 women) or uncontained power morcellation (89 women). The literature was searched to 1 July 2019.
Key results
Neither study reported complications during or after surgery, including no diagnoses of leiomyosarcoma, for women in either group.
We are very uncertain whether in‐bag morcellation reduces the total time of the operation, or improves the ease of morcellation. The evidence suggests that the morcellation operative time was slightly longer when the surgeon used in‐bag morcellation. However, the quality of the evidence is very low and we cannot be certain of any of these results.
Quality of the evidence
The evidence was of very low quality. The main limitations were indirectness (both trials took place in high‐income settings and countries, therefore, our findings are limited to this type of setting), and imprecision (both trials had small sample sizes and wide confidence intervals).
Summary of findings
Summary of findings 1. Any type of in‐bag morcellation versus uncontained power morcellation during laparoscopic myomectomy.
| Any type of in‐bag morcellation versus uncontained power morcellation during laparoscopic myomectomy | ||||||
| Patient or population: premenopausal women undergoing laparoscopic myomectomy for uterine fibroids Setting: university hospitals in Italy Intervention: any type of in‐bag morcellation Comparison: uncontained power morcellation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with uncontained power morcellation | Risk with any type of in‐bag morcellation | |||||
| Composite intraoperative complications | No intraoperative complications occurred in either group in either trial. | 176 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | The evidence is very uncertain about the effect of in‐bag morcellation on composite intraoperative complications. | ||
|
Total operative time (minutes) |
The mean total operative time for the control group was 94.41 minutes | The mean total operative time for the intervention group was 105.1 minutes (ranging from 96.96 to 113.24 minutes) | MD 9.93 (‐1.35 to 21.20) | 176 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,c | The evidence is very uncertain about the effect of in‐bag morcellation on total operative time |
|
Morcellation operative time (minutes) |
The mean morcellation operative times for the control group was 10.26 minutes | The mean morcellation operative times for the intervention group was 12.83 minutes (ranging from 9.47 to 16.18 minutes) | MD 2.59 (0.45 to 4.72) | 176 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,c | The evidence is very uncertain about the effect of in‐bag morcellation on morcellation operative time |
|
Ease of morcellation (scale 1 to 10; 1 = very difficult, 10 = very easy) |
The mean ease of morcellation score for the control group was 7.5 | The mean ease of morcellation score for the intervention group was 6.77 | MD ‐0.73 (‐1.64 to 0.18) | 104 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c | The evidence is very uncertain about the effect of in‐bag morcellation on ease of morcellation |
| Postoperative diagnosis of leiomyosarcoma | There were no postoperative diagnoses of leiomyosarcoma made in either group in either trial. | 176 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | The evidence is very uncertain about the effect of in‐bag morcellation on postoperative diagnosis of leiomyosarcoma. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean Difference | ||||||
| GRADE Working Group grades of evidence High certainty. We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty. We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty. Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty. We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
adowngraded once for indirectness; all the included trials took place in high‐income settings and countries bdowngraded twice for very serious imprecision; zero events, small sample size cdowngraded twice for very serious imprecision; small sample size, wide confidence interval
Background
Description of the condition
Uterine leiomyomas, also called myomas, or fibroids, are benign tumours arising from the smooth muscle cells of the myometrium. They are the most common pelvic tumour in women. Uterine fibroids arise in women of reproductive age; and symptoms include menorrhagia, pain, miscarriage, and infertility (Wallach 2004). The estimated rate of leiomyosarcoma found at surgery for presumed benign fibroids is about 0.51 per 1,000 procedures, or approximately 1 in 2000 (Pritts 2015).
Uterine fibroids are described according to their location in the uterus, although many fibroids have more than one location designation. The International Federation of Gynecology and Obstetrics (FIGO) classification system for fibroid location is as follows (Munro 2011):
intramural myomas (FIGO type 3, 4, 5);
submucosal myomas (FIGO type 0, 1, 2);
type 0: completely within the endometrial cavity;
type 1: extend less than 50% into the myometrium;
type 2: extend 50% or more into the myometrium;
subserosal myomas (FIGO type 6, 7);
cervical myomas (FIGO type 8).
The estimated incidence of uterine fibroids is about 20% to 80% in women of reproductive age (Donnez 2015). The incidence rates of fibroids are typically found to be two to three times greater in black women than in white women (Baird 2003). Other risk factors include reproductive and endocrine factors, nulliparity, early menarche, prenatal exposure to diethylstilbestrol, and obesity (Pavone 2018). Use of long‐acting progestin‐only contraceptives (e.g. depot medroxyprogesterone) appear to protect against the development of leiomyoma (Wise 2004).
Relief of symptoms is the major goal in the management of women with significant symptoms. Asymptomatic myomas can be managed by reassurance and careful follow‐up. It is estimated that at least 50% of fibroids are asymptomatic (Divakar 2008).
The type of intervention is individualised, based on the type and severity of symptoms, size and location of myomas, patient age, reproductive plans, and obstetric history.
Description of the intervention
Interventions for symptomatic uterine fibroids include medical therapy, interventional radiology, and surgery (Donnez 2015; Giarrè 2019). Surgical approach includes myomectomy or hysterectomy. Myomectomy is a surgical procedure to remove uterine fibroids. In laparoscopic myomectomy, a minimally invasive procedure, the surgeon accesses and removes the fibroids through several, small, abdominal incisions (Donnez 2015).
Compared with a laparotomy, laparoscopic myomectomy is associated with significantly less subjectively reported postoperative pain, lower postoperative fever, and shorter hospital stay (Bhave Chittawar 2014).
A limitation of laparoscopy is the inability to remove large specimens from the abdominal cavity through the laparoscope. To overcome this challenge, the morcellation approach was developed, during which larger specimens are broken into smaller pieces, in order to remove them from the abdominal cavity (Senapati 2015). Until 2014, intra‐abdominal, or intracorporeal power morcellation (also known as electromechanical morcellation), was the primary method of uterine fibroid morcellation used during laparoscopic myomectomy. With power morcellator, electrical energy is transformed into mechanical power, which cuts the specimen into many small pieces, which then can be easily removed through a 12 mm to 20 mm laparoscopic access port (Glaser 2018). However, to reduce the risk of injury to other structures, this method should only be used by experienced operators.
Following a highly publicised case of morcellation, where a woman was postoperatively diagnosed with leiomyosarcoma, the US Food and Drug Administration (FDA) released a warning statement, in 2014, discouraging the use of power morcellation, in order to reduce the risk of unintentionally spreading undiagnosed leiomyosarcoma cancer cells throughout the abdominal cavity (Wright 2015). The FDA updated the warning in 2020. In the 2020 safety communication, the FDA recommends performing laparoscopic morcellation for myomectomy or hysterectomy only with a tissue containment system, and performing these procedures only in appropriately selected patients (FDA 2020).
Distributed between December 2014 and February 2015, a survey of the American Association of Gynecologic Laparoscopists Minimally Invasive Gynecologic Surgery Fellowship program faculty, showed that intra‐abdominal power morcellation was used commonly before the FDA warning; after 2014, gynaecologists adapted their management strategies (Desai 2015). For example, there are several reports of morcellation performed in a contained isolation system, such as an insufflated bag (Siedhoff 2017).
How the intervention might work
Preoperative diagnosis of uterine sarcoma is very difficult, and currently, its diagnostic accuracy is not satisfactory. Although the use of multiple predictors, including age, serum lactate dehydrogenase (LDH) values, LDH5/LDH1 ratio ultrasound, magnetic resonance imaging (MRI), and endometrial cytology, is beneficial in the clinical setting, no test can definitely exclude leiomyosarcoma preoperatively (Mollo 2018).
Performing intracorporeal power morcellation at the time of laparoscopic myomectomy may lead to a scattering of benign tissues, with the risk of spreading leiomyoma or endometriosis. Dispersed tissue fragments can implant on abdominal organ surfaces and lead to several complications, including inflammation and infection (Siedhoff 2017).
In cases of unsuspected malignancy, power morcellation can cause unintentional dissemination of malignant cells and a poorer prognosis by upstaging the occult cancer. It is worth noting that cancer cells may be spread during open myomectomy and with laparoscopic myomectomy, even before morcellation.
A strategy to optimise women's safety is to morcellate the specimens inside a bag. In‐bag morcellation may avoid the dissemination of tissue fragments at the time of laparoscopic myomectomy, compared to intra‐abdominal uncontained power morcellation.
Why it is important to do this review
For women who require myomectomy, the laparoscopic approach is considered the gold standard, because of intraoperative and postoperative benefits, and the minimal access route. Since currently, there are no accurate diagnostic modalities to differentiate benign from malignant uterine tumours, it is important to validate alternative surgical techniques for the safe removal of the specimens during laparoscopic myomectomy.
This review aims to investigate the potential benefits and safety of in‐bag manual morcellation versus uncontained power morcellation for women undergoing laparoscopic myomectomy.
Objectives
To evaluate the effectiveness and safety of protected in‐bag extracorporeal manual morcellation during laparoscopic myomectomy compared to intra‐abdominal uncontained power morcellation.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCT) comparing in‐bag extracorporeal manual morcellation versus intracorporeal uncontained power morcellation at the time of laparoscopic myomectomy. We did not include trials evaluating procedures other than laparoscopic myomectomy, such as hysteroscopic myomectomy.
Types of participants
We included premenopausal women undergoing laparoscopic myomectomy.
Types of interventions
The experimental intervention was extracorporeal in‐bag manual morcellation.
The comparator was intracorporeal uncontained power morcellation.
Types of outcome measures
Primary outcomes
-
Composite intraoperative complications included at least one of the following:
accidental morcellation of the liver;
conversion to laparotomy;
endoscopic bag disruption;
bowel injury;
bleeding;
accidental injury to any viscus or vessel.
Secondary outcomes
Total operative time, in minutes
Morcellation operative time, in minutes. This was defined as the time from when the myoma was clamped and subjected to intracorporeal morcellation, or positioned within a bag for extracorporeal in‐bag morcellation, to the moment when the last fragment of myoma was removed from the surgical field.
Ease of morcellation, defined by the surgeon as one (very difficult) to 10 (very easy)
Postoperative length of stay, in days
Postoperative pain expressed on a visual analogue scale (VAS), from one (little pain) to 10 (intense pain)
Conversion to laparotomy (related only to morcellation or bag issues)
Postoperative diagnosis of leiomyosarcoma
-
Postoperative complications, including:
abscesses
ileus
sepsis
blood transfusion
Search methods for identification of studies
We searched for all published and unpublished RCT, without language restrictions, and in consultation with the Gynaecology and Fertility (CGF) Group Information Specialist.
Electronic searches
We searched the following electronic databases, trial registers, and websites:
The CGF Group Specialized Register of Controlled Trials; PROCITE platform (searched 1 July 2019; Appendix 1);
Cochrane Central Register of Controlled Trials; Ovid (CENTRAL; 2019, Issue 5) (searched 1 July 2019; Appendix 2);
MEDLINE; Ovid – Epub ahead of print, In‐process & Other non‐indexed citations platform (1946 to 1 July 2019; Appendix 3);
Embase; Ovid (1980 to 1 July 2019; Appendix 4);
PsycINFO; Ovid (1806 to 1 July 2019; Appendix 5);
CINAHL (Cumulative Index to Nursing and Allied Health Literature; EBSCO; 1961 to 1 July 2019; Appendix 6).
We combined the MEDLINE with the Cochrane highly sensitive search strategy for identifying randomised trials, from Chapter 6 of the Cochrane Handbook of Systematic Reviews of Interventions (Lefebvre 2011). We combined the Embase, PsycINFO and CINAHL searches with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN; sign.ac.uk/search-filters.html).
Search terms were: endobag, containment bag, morcellation bag, bowel bag, containment system, myoma, myomata, fibroids, myomectomy, laparoscopy.
Searching other resources
Other electronic sources of trials included the following.
-
Trial registers for ongoing and registered trials:
ClinicalTrials.gov (clinicaltrials.gov);
The World Health Organization International Trials Registry Platform search portal (apps.who.int/trialsearch/).
LILACS and other Spanish and Portuguese language databases: Latin American and Caribbean Health Science Information database (1982 to June 2019), found in the Virtual Health Library Regional Portal (VHL; pesquisa.bvsalud.org/portal/).
PubMed and Google Scholar, for recent trials not yet indexed in the major databases.
We handsearched reference lists of all retrieved full‐text articles and all relevant conference proceedings. We personally contacted experts in the field requesting additional relevant data, so unpublished studies could be included. We also handsearched relevant journals and conference abstracts that are not covered in the CGF register, in liaison with the Information Specialist.
We did not apply any language or date restrictions to the searches. We included a PRISMA flow chart to present the results of the search and the process of screening and selecting studies for inclusion in the review.
Data collection and analysis
We conducted data collection and analysis in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019).
Selection of studies
We used Covidence software for selection of studies, data extraction, and assessment of risk of bias of included studies (Covidence). Two review authors (FZ, GS) independently conducted an initial screen of titles and abstracts identified by the search. We excluded studies with clearly irrelevant titles or abstracts. We retrieved full‐text articles of all potentially eligible studies; two review authors (FZ, GS) independently assessed them. We recorded the reason for exclusion for any study that we excluded following a review of the full text. Disagreements or doubts were resolved by discussion with a third review author (RV).
Data extraction and management
For eligible studies, two review authors (FZ, GS) extracted data using a data extraction form in Covidence (Covidence). We resolved discrepancies through discussion with a third review author (RV).
FZ and RV are co‐authors of one of the trials included in the full review (Venturella 2016). FZ and RV were not involved in data extraction and management of this trial; it was independently assessed by GS and AR.
Data extracted included study characteristics and outcome data. We entered data into Review Manager 5 software and checked for accuracy (Review Manager 2014). When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (GS, FZ) independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). We resolved any disagreement by discussion and by involving an additional assessor (RV).
FZ and RV are co‐authors of one of the trials included in the full review (Venturella 2016). FZ and RV were not involved in assessment of risk of bias of this trial; it was independently assessed by GS and AR.
We reported the assessment of risk of bias graphically, as a summary of risk of bias for each trial, and as percentages across all included studies. For details of the domains we assessed for risk of bias, see Appendix 7.
Co‐authors of included trials were not involved in the assessment of eligibility or risk of bias of those trials.
Measures of treatment effect
We expressed dichotomous data as Mantel‐Haenszel risk ratio (RRs) with 95% confidence intervals (CIs), using the random‐effects model of DerSimonian and Laird, and combined them in meta‐analyses using Review Manager 5 software.
Graphicaly, we displayed increased risk of a particular outcome in the meta‐analyses to the right of the centre line, and decreased risk of an outcome to the left of the centre line.
For continuous outcomes, we extracted means (with or without standard deviation) and imported the values into Review Manager 5. We reported the summary measures as summary mean difference (MD) with 95% confidence intervals (CIs), using the random‐effects model of DerSimonian and Laird. If similar outcomes were reported on different scales, we calculated the standardized mean difference (SMD).
Unit of analysis issues
The analysis was per women randomly assigned. We conducted intention‐to‐treat (ITT) analysis when possible.
Dealing with missing data
For missing data from any of the included studies, we contacted the study authors to supply relevant missing information. If missing data were not provided, we conducted ITT analysis, if possible. For dichotomous data, the denominator represented the number of women entering the trial, and we assumed that the outcome did not occur. If data for continuous outcomes were lacking, we only used the available data because we were unable to impute missing values.
Assessment of heterogeneity
We assessed the characteristics of included studies to decide whether the participants, interventions, and outcomes were sufficiently similar for meta‐analysis to be appropriate.
We used the I² statistic to assess heterogeneity in the meta‐analysis. We interpreted results of the I² statistic as follows (Higgins 2019):
• 0% to 40%: might not be important; •30% to 60%: may represent moderate heterogeneity; •50% to 90%: may represent substantial heterogeneity; •75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We did not investigate reporting biases (such as publication bias) because we included fewer than 10 studies in the meta‐analysis.
Data synthesis
We performed statistical analysis using Review Manager 5.3 (Review Manager 2014). We aimed to combine the data in the following comparisons, using a random‐effects model:
any type of in‐bag morcellation versus uncontained power morcellation;
in‐bag morcellation using a 10 mm bag versus uncontained power morcellation;
in‐bag morcellation using a 15 mm bag versus uncontained power morcellation.
For comparisons 2 and 3, 10 mm and 15 mm refer to the size of the port.
Subgroup analysis and investigation of heterogeneity
Where data were available, we aimed to conduct subgroup analyses for the comparison 'any type of in‐bag morcellation versus uncontained power morcellation' according to the following categories:
participants with myoma(s) of less than 5 cm versus those with myoma(s) of 5 cm or greater;
participants with single myoma versus those with multiple myomas;
participants younger than 45 years versus those aged 45 years and older.
Sensitivity analysis
To examine the stability of study results, we planned to conduct the following sensitivity analyses for the primary outcomes for the comparison 'any type of in‐bag morcellation versus uncontained power morcellation':
excluding trials published only as an abstract;
restricting the analyses to trials with low risk of bias in the first two domains only (i.e. selection bias, see Appendix 7);
using fixed‐effect instead of random‐effects model.
Overall quality of the body of evidence: 'Summary of findings' table
We used the GRADE approach to assess the quality of the body of evidence relating to the following outcomes for the comparison 'any type of in‐bag morcellation versus uncontained power morcellation':
intraoperative complications;
total operative time;
morcellation operative time;
ease of morcellation;
postoperative diagnosis of leiomyosarcoma.
The GRADE approach uses five considerations (study limitations (e.g. risk of bias), consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from high quality by one level for serious (or by two levels for very serious) limitations. We used GRADEpro GDT software to import data from Review Manager 5 in order to generate 'Summary of findings' tables (GRADEpro GDT). A summary of the intervention effect and a measure of quality for each of the above outcomes is presented.
Results
Description of studies
Results of the search
After the initial abstract screening (387 records screened), we assessed five papers for eligibility; we classified two as ongoing trials (NCT02777203 and NCT03281460); we included two trials, reported in three papers (Frascà 2018; Venturella 2016).
We included two trials in the meta‐analysis, including 176 participants (87 in the intervention group and 89 in the control group; Frascà 2018; Venturella 2016). See: Figure 1.
1.
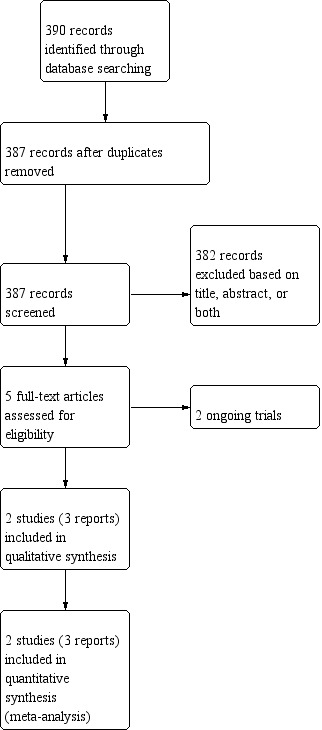
Study flow diagram
Included studies
The two trials included women undergoing laparoscopic myomectomy who were randomised to receive either uncontained or in‐bag morcellation.
In the control group, intracorporeal uncontained power morcellation was performed with a reusable power morcellator (Rotocut G1, Storz).
In the intervention group, each enucleated myoma was placed into a specimen retrieval bag. The fibroid was tightly grasped with Schroeder tenaculum and extracorporeal manual morcellation was performed with scalpel or scissors, avoiding bag damage.
In both included trials, the primary outcome was the comparison of morcellation operative time, and the final diagnosis of fibroid was obtained after postoperative histological examination of all tissue samples.
Setting
Both included trials were based in Italian university hospital settings, and were single centre trials.
Participants
Venturella 2016 included premenopausal women with heavy menstrual bleeding or patients already diagnosed with fibroids from referral sources and undergoing a laparoscopic myomectomy. Inclusion criteria were: age between 18 and 40 years, body mass index (BMI) 18 kg/m² to 40 kg/m², heavy menstrual bleeding, and the presence of at least one myoma measuring 4 cm or more in diameter (but no myoma measuring >10 cm, according to local practice on eligibility for laparoscopy).
Frascà 2018 included premenopausal patients aged between 18 and 50 years, with an ultrasonographic diagnosis of at least one myoma measuring between 4 cm and 10 cm in mean diameter, and presenting with heavy menstrual bleeding or infertility as indications to laparoscopic myomectomy.
Intervention and comparisons
In Venturella 2016, in the intervention group, each enucleated myoma was placed into a non‐rip nylon specimen bag (Endo Catch Gold Auto Suture 10 mm or 15 mm, Covidien), which could hold 220 mL or 1000 mL, according to the size chosen. The central lower 10 mm trocar incision was increased to 30 mm, and a 65‐mm reusable sterile pessary was placed inside of the bag, between the myoma and pelvic wall, to create a barrier between the morcellated portion of the myoma and the bag. In this way, the pessary protected the bag from the coring rotational movements of either the knife or the scissors and allowed a more manageable coring. After exteriorisation of the fibroid's surface with the aid of Alexis retractors, it was grasped with Schroeder tenaculum, double tooth, or Backhaus towel forceps and subjected to gradual manual morcellation with scalpel or scissors by cautious C‐coring. The surgeons achieved adequate traction on the fibroid by using different instruments, depending on the myoma consistency.
In the control group of Venturella 2016, intracorporeal uncontained morcellation using a power morcellator (Rotocut G1, Storz) was performed.
In the intervention group of Frascà 2018, each enucleated myoma was placed into a specimen retrieval bag (Endo Catch II Auto Suture 10 mm or 15 mm, Covidien). The edges around the bag's opening were then pulled out through the lower central 10 mm trocar incision, previously enlarged to 20 mm, along with the abdominal fascia. The fibroid was tightly grasped with Schroeder tenaculum and extracorporeal manual morcellation was performed with scalpel or scissors, while the first assistant carefully pulled on the edges of the bag to move it away from the blade, avoiding bag damage. At the end of morcellation, the endoscopic bag was retrieved through the port incision and filled with water to identify bag disruptions.
In the control group of Frascà 2018, intra‐abdominal uncontained power morcellation was performed with the reusable power morcellator Rotocut G1, Storz.
Outcomes
Primary outcome
Both included trials reported on the primary outcome of composite intraoperative complications.
Secondary outcomes
Both included trials reported on total operative time, morcellation operative time, ease of morcellation, postoperative length of stay, postoperative pain, conversion to laparotomy, postoperative diagnosis of leiomyosarcoma, and postoperative complications.
Funding
Neither trial disclosed their funding sources.
Declarations of interest
Both trials declared that none of the authors had any conflicts of interest.
Excluded studies
We did not exclude any studies after full‐text review.
Ongoing studies
NCT02777203 and NCT03281460 were reported as completed on clinicaltrials.gov, but we were unable to find any published data. It was unclear if the intervention group was manual in‐bag morcellation, or in‐bag morcellation with device. We were unable to contact the principal investigator in order to obtain more information on this trial.
Risk of bias in included studies
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Overall, the trials were at low risk of bias, with low risk of bias in random sequence generation, incomplete outcome data, and selective reporting. However, blinding was not feasible in either trial given the intervention, resulting in our assessment of a high risk of bias in performance bias and detection bias.
Allocation
Random sequence generation
We considered risk of selection bias to be low for random sequence generation, because both included trials used computer‐generated sequences.
Allocation concealment
Venturella 2016 used sealed opaque envelopes for allocation concealment; Frascà 2018 did not report any information regarding allocation concealment, therefore, we assessed unclear risk of bias for this domain.
Blinding
Both trials were unblinded. Women and staff were aware of the intervention to which they were allocated due to its nature. Therefore, we assessed this domain at high risk
Incomplete outcome data
We assessed both trials at low risk of attrition bias because they reported no losses to follow‐up.
Selective reporting
We considered the risk of reporting bias as low in Venturella 2016. We did not note any deviations from the original protocol.
Frascà 2018 was not registered in a trial register, or was the original protocol available; therefore, we considered it as unclear risk of bias for reporting bias.
Other potential sources of bias
Venturella 2016 and Frascà 2018 did not provide enough information to enable us to adequately assess the presence of other forms of bias; as such, we deemed them as unclear risks of bias.
Effects of interventions
See: Table 1
See Table 1 for the main comparison ''any type of in‐bag morcellation versus uncontained power morcellation''.
Comparison 1. Any type of in‐bag morcellation versus uncontained power morcellation
Both trials compared extracorporeal in‐bag manual morcellation versus intracorporeal uncontained power morcellation (Frascà 2018; Venturella 2016).
Primary Outcome
1.1. Composite intraoperative complications
No intraoperative complications, including accidental morcellation of the liver, conversion to laparotomy, endoscopic bag disruption, bowel injury, bleeding, or accidental injury to any viscus or vessel, occurred in either group in either trial (Analysis 1.1; Figure 4).Therefore meta‐analysis was not possible.
1.1. Analysis.

Comparison 1: Any type of in‐bag morcellation versus uncontained power morcellation, Outcome 1: Composite intraoperative complications
4.

Forest plot of comparison: 1 Any type of in‐bag morcellation versus uncontained power morcellation, outcome: 1.1 Composite intraoperative complications
Secondary Outcomes
1.2. Total operative time
The results for total operative time were inconclusive between the in‐bag and uncontained morcellation groups (mean difference (MD) 9.93 minutes, 95% confidence interval (CI) ‐1.35 to 21.20; 2 studies, 176 participants; I² = 35%; very low‐quality evidence; Analysis 1.2; Figure 5). The evidence suggests that if mean total operative time with uncontained morcellation is 94 minutes, the mean total operative time with in‐bag morcellation is 9.93 minutes longer (1.35 minutes shorter to 21.20 minutes longer).
1.2. Analysis.

Comparison 1: Any type of in‐bag morcellation versus uncontained power morcellation, Outcome 2: Total operative time
5.

Forest plot of comparison: 1 Any type of in‐bag morcellation versus uncontained power morcellation, outcome: 1.2 Total operative time
1.3. Morcellation operative time
The morcellation operative time was longer in the in‐bag manual morcellation group than in the uncontained power morcellation group, however the quality of the evidence was very low and we cannot be certain of the effect. (MD 2.59 minutes, 95% CI 0.45 to 4.72; 2 studies, 176 participants; I² = 0%; very low‐quality evidence; Analysis 1.3; Figure 6). The evidence suggests that if mean morcellation operative time with uncontained morcellation is 10 minutes, the mean morcellation operative time with in‐bag morcellation is 2.59 minutes longer (between 0.45 minutes and 4.72 minutes longer)
1.3. Analysis.

Comparison 1: Any type of in‐bag morcellation versus uncontained power morcellation, Outcome 3: Morcellation operative time
6.

Forest plot of comparison: 1 Any type of in‐bag morcellation versus uncontained power morcellation, outcome: 1.3 Morcellation operative time
1.4. Ease of morcellation
The results for ease of morcellation were inconclusive between the in‐bag and uncontained morcellation groups (MD ‐0.73 points, 95% CI ‐1.64 to 0.18; 1 study, 104 participants; very low‐quality evidence; Analysis 1.4). The evidence suggests that if the mean score for the ease of uncontained morcellation group is 7.5 points, then the mean score for ease of in‐bag morcellation is ‐0.73 points lower (1.64 points lower to 0.18 points higher).
1.4. Analysis.

Comparison 1: Any type of in‐bag morcellation versus uncontained power morcellation, Outcome 4: Ease of morcellation
1.5. Postoperative length of stay in days
The results for length of postoperative length of stay were inconclusive between the in‐bag morcellation and uncontained morcellation groups (MD 0.03 days, 95% CI ‐0.42 to 0.49; 2 studies, 176 participants; I² = 75%; Analysis 1.5). The evidence suggests that if the mean postoperative length of stay with uncontained morcellation is 3 days, then the mean postoperative length of stay with in‐bag morcellation is 0.03 days longer (0.42 days shorter to 0.49 days longer).
1.5. Analysis.

Comparison 1: Any type of in‐bag morcellation versus uncontained power morcellation, Outcome 5: Postoperative length of stay
1.6. Postoperative pain
The results for postoperative pain were inconclusive between the in‐bag morcellation and uncontained morcellation groups (MD 0.22 points, 95% CI ‐0.50 to 0.94; 2 studies, 176 participants; I² = 63%; Analysis 1.6). The evidence suggests that if the mean postoperative pain score for the uncontained morcellation group is 2 points, the mean postoperative pain for the in‐bag morcellation group is 0.22 points higher (0.50 points lower to 0.94 points higher).
1.6. Analysis.

Comparison 1: Any type of in‐bag morcellation versus uncontained power morcellation, Outcome 6: Postoperative pain
1.7. Conversion to laparotomy
There were no laparotomy conversions reported for either group in either trial. Therefore meta‐analysis was not possible
1.8. Postoperative diagnosis of leiomyosarcoma
There were no postoperative diagnoses of leiomyosarcoma reported for either group in either trial. Therefore meta‐analysis was not possible
1.9. Postoperative complications
The results for postoperative complications were inconclusive between the in‐bag morcellation and uncontained morcellation groups (risk ratio (RR) 0.80, 95% CI 0.16 to 4.12; 2 studies, 176 participants; I² = 0%; Analysis 1.9). If the chance of postoperative complications in the uncontained morcellation group is assumed to be 3%, the chance of postoperative complications in the in‐bag morcellation group would be between 1% and 14%.
1.9. Analysis.

Comparison 1: Any type of in‐bag morcellation versus uncontained power morcellation, Outcome 9: Postoperative complications
Subgroup analyses
1. Participants with myoma(s) of less than 5 cm versus those with myoma(s) of 5 cm or greater
Neither of the included trials reported stratified data on myoma(s) of less than 5 cm versus those with myoma(s) of 5 cm or greater.
2. Participants with single myoma versus those with multiple myomas
Neither of the included trials reported stratified data on participants with single myoma versus those with multiple myomas.
3. Participants younger than 45 years versus those aged 45 years and older
Neither of the included trials reported stratified data on participants younger than 45 years versus those aged 45 years and older.
Sensitivity analyses
1. Excluding trials published only as an abstract
Neither of the included trials were published only as an abstract.
2. Restricting the analyses to trials with low risk of bias in the first two domains only
We only assessed one trial at low risk of selection bias (Venturella 2016). For morcellation operative time, the results were inconclusive between the in‐bag morcellation and uncontained morcellation groups (MD 1.83, 95% CI ‐1.23 to 4.89; 1 study, 104 participants; low‐quality evidence).
The analysis did not lead to a change in conclusions for the other outcomes for which there were data.
3. Using fixed‐effect instead of random‐effects model
Using a fixed‐effect model, uncontained morcellation showed a shorter total operative time than in‐bag morcellation (MD 9.52, 95% CI 0.53 to 18.51; 2 studies, 176 participants; I² = 35%; very low‐quality evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: Sensitivity analysis: fixed‐effect model, Outcome 1: Total operative time
Using a fixed‐effect model did not lead to different results in the other outcomes for which there were data.
Comparison 2. In‐bag manual morcellation using a 10‐mm bag versus uncontained power morcellation
Both of the included trials used both 10‐mm and 15‐mm bags.
Neither of them reported stratified data on in‐bag manual morcellation using a 10‐mm bag versus uncontained power morcellation. We found no trials evaluating in‐bag manual morcellation using only a 10‐mm bag.
Comparison 3. In‐bag manual morcellation using a 15‐mm bag versus uncontained power morcellation
Both of the included trials used both 10‐mm and 15‐mm bags.
Neither of them reported stratified data on in‐bag manual morcellation using a 15‐mm bag versus uncontained power morcellation. We found no trials evaluating in‐bag manual morcellation using only a 15‐mm bag.
Discussion
Summary of main results
This Cochrane Review aimed to evaluate the effectiveness and safety of protected in‐bag extracorporeal manual morcellation compared to intracorporeal uncontained power morcellation during laparoscopic myomectomy in premenopausal women with uterine fibroids.
We only found two trials that met our inclusion criteria. Both trials measured morcellation operative time as a primary outcome and were not powered for uncommon outcomes, such as intraoperative complications, or postoperative diagnosis of leiomyosarcoma.
Our review showed there are limited data on the effectiveness and safety of protected in‐bag morcellation at the time of laparoscopic myomectomy compared to uncontained power morcellation. We were unable to determine the effects of in‐bag morcellation on intraoperative complications.
Morcellation operative time was longer in the in‐bag group, but only by about 2.5 minutes. However, the quality of the evidence was very low and we cannot be certain of the effect (Analysis 1.3).
The results were inconclusive for ease of morcellation, postoperative length of stay, postoperative pain, and postoperative complications. There were no reports of intraoperative complications, conversions to laparotomy, or postoperative diagnosis of leiomyosarcoma.
Given the very low‐quality evidence in the review it is difficult to draw any conclusion on effectiveness and safety of in‐bag compared to uncontained power morcellation during laparoscopic myomectomy.
Our findings are summarised in Table 1 for the main comparison (i.e. any type of in‐bag morcellation versus uncontained power morcellation).
We found no trials that looked at in‐bag morcellation using a 10‐mm bag or a 15‐mm bag versus uncontained power morcellation.
Overall completeness and applicability of evidence
Based on our findings, it is evident that there is a paucity of data for effectiveness and safety of protected in‐bag compared to uncontained power morcellation during laparoscopic myomectomy.
Both included trials used in‐bag extracorporeal manual morcellation; neither used in‐bag power morcellation. There were no trials comparing intracorporeal in‐bag morcellation with intracorporeal uncontained morcellation.
All of the included trials took place in high‐income settings and countries, therefore, our findings are limited to this type of setting.
Overall, even though the findings of the review draw into question the use of in‐bag morcellation at the time of laparoscopic myomectomy, we feel that the overall quality of the evidence is too low to support one method over another.
Further high‐quality research is required to draw definitive conclusions.
Quality of the evidence
In this Cochrane Review, we identified and included data originating from two randomised controlled trials, involving a total of 176 participants (premenopausal women undergoing laparoscopic myomectomy).
The two included randomised studies were both relatively small, and blinding was not possible. Frascà 2018 was not registered in a trial register; see Figure 2 and Figure 3 for a summary of the risks of bias.
For the comparison 'any type of in‐bag morcellation versus uncontained power morcellation', we assessed the evidence to be very low quality. We assessed indirectness as serious, and imprecision as very serious. We downgraded the evidence for indirectness because all the included trials took place in high‐income settings and countries, therefore, our findings are limited to this type of setting; and for imprecision because both trials had small sample size and wide confidence intervals, with no events in the primary outcome (Table 1).
Quality of the evidence was also limited by the substantial statistical heterogeneity for two outcomes, postoperative length of stay (I² = 75%), and postoperative pain (I² = 63%).
Potential biases in the review process
We aimed to reduce the risk of publication bias by conducting systematic searches of multiple databases as well as trials registries to identify unpublished and ongoing studies. We contacted trial authors for (more) information when necessary. However, it is possible that our searches did not identify all unpublished studies. We were unable to develop a funnel plot due to the small number of included studies.
FZ and RV are co‐authors of one of the included trials (Venturella 2016). They were not involved in the assessment of eligibility or risk of bias for this trial. It was assessed by GS and AR.
Agreements and disagreements with other studies or reviews
The evidence presented in this review may be the first in the literature comparing in‐bag and intracorporeal uncontained power morcellation during laparoscopic myomectomy. To the best of our knowledge there are no other reviews evaluating this topic.
Authors' conclusions
Implications for practice.
This review raises important questions regarding the use of in‐bag morcellation at the time of laparoscopic myomectomy. There are limited data on the effectiveness and safety of in‐bag morcellation compared to uncontained power morcellation.
We were unable to determine the effects of in‐bag morcellation on intraoperative complications as no events were reported in either group. We are uncertain if in‐bag morcellation increases total operative time or ease of morcellation. Morcellation operative time was slightly longer in the in‐bag morcellation group, but the quality of the evidence was very low and we cannot be certain of the results. No cases of postoperative diagnosis of leiomyosarcoma were reported in either group.
Implications for research.
This review found limited evidence on the effectiveness and safety of protected in‐bag morcellation compared to intracorporeal uncontained power morcellation during laparoscopic myomectomy, and encourages further research. Future trials should look at core clinical outcomes, including intraoperative and postoperative complications, postoperative pain, and postoperative diagnosis of leiomyosarcoma. Future studies should include a clear protocol for in‐bag morcellation so that it can be easily evaluated and replicated. They should be larger, multicentred, and high‐quality.
History
Protocol first published: Issue 6, 2019 Review first published: Issue 5, 2020
Acknowledgements
As part of the pre‐publication editorial process, this review was commented on by five peers. The authors are grateful to the following peer reviewers: Rik van Eekelen, Anne Lethaby, Mbaka Fon Nji (consumer), Sarah Armstrong, Elena Kostova (Managing editor).
Appendices
Appendix 1. Cochrane Gynaecology and Fertility specialised register search strategy
Searched 1 July 2019
PROCITE platform
Keywords CONTAINS "uterine fibroids" or "uterine leiomyomas" or "uterine myoma" or "uterine myomas" or "myoma" or "myomas" or "myomata" or "Leiomyoma" or "leiomyomata" or "fibroids" or "myomatous uterus" or "fibroid size" or "fibroid volume" or "fibroids Symptoms" or "fibroids symptoms‐scores" or "Leiomyoma‐size" or "fibroid" or Title CONTAINS "uterine fibroids" or "uterine leiomyomas "or "uterine myoma" or "uterine myomas" or "myoma" or "myomas" or "myomata" or "Leiomyoma" or "leiomyomata" or "fibroids" or "myomatous uterus" or "fibroid size" or "fibroid volume" or "fibroids Symptoms" or "fibroids symptoms‐scores" or "Leiomyoma‐size" or "fibroid"
AND
Keywords CONTAINS "morcellation" or "morcellator" or "myomectomy" or "Laparoendoscopic single‐site myomectomy" or "laparoscopic" or "laparoscopic myomectomy" or "laparoscopic procedure" or "laparoscopic surgical treatment" or "laparoscopic surgery" or "laparoscopy" or Title CONTAINS "morcellation" or "morcellator" or "myomectomy" or "Laparoendoscopic single‐site myomectomy" or "laparoscopic" or "laparoscopic myomectomy" or "laparoscopic procedure" or "laparoscopic surgical treatment" or "laparoscopic surgery" or "laparoscopy"
(252 records)
Appendix 2. CENTRAL search strategy
Searched Issue 5 'May 2019' on 1 July 2019
Ovid platform
1 exp leiomyoma/ or exp myoma/ (611) 2 leiomyoma*.tw. (457) 3 myoma*.tw. (654) 4 fibroid*.tw. (958) 5 fibroma*.tw. (122) 6 fibromyoma*.tw. (21) 7 hysteromyoma*.tw. (144) 8 myomectom*.tw. (666) 9 Uterine Myomectomy/ (47) 10 (minimally invasive adj2 procedure*).tw. (514) 11 or/1‐10 (2851) 12 Morcellation/ (5) 13 (Morcellat* or Morcelat*).tw. (124) 14 morcellement.tw. (1) 15 morselli?*.tw. (7) 16 morseli?*.tw. (14) 17 morcelli?*.tw. (6) 18 morceli?*.tw. (0) 19 in bag.tw. (2117) 20 spill free.tw. (0) 21 uncontained power.tw. (3) 22 manual.tw. (13656) 23 endobag*.tw. (11) 24 containment bag*.tw. (1) 25 containment system*.tw. (4) 26 bowel bag*.tw. (3) 27 or/12‐26 (15831) 28 11 and 27 (68)
Appendix 3. MEDLINE search strategy
Searched from 1946 to 1 July 2019
Ovid platform
1 exp leiomyoma/ or exp myoma/ (22530) 2 leiomyoma*.tw. (13292) 3 myoma*.tw. (5704) 4 fibroid*.tw. (6159) 5 fibroma*.tw. (11713) 6 fibromyoma*.tw. (717) 7 hysteromyoma*.tw. (75) 8 myomectom*.tw. (3383) 9 Uterine Myomectomy/ (797) 10 (minimally invasive adj2 procedure*).tw. (6286) 11 or/1‐10 (48521) 12 Morcellation/ (223) 13 (Morcellat* or Morcelat*).tw. (1075) 14 morcellement.tw. (18) 15 morselli?*.tw. (169) 16 morseli?*.tw. (404) 17 morcelli?*.tw. (104) 18 morceli?*.tw. (20) 19 in bag.tw. (304) 20 spill free.tw. (1) 21 uncontained power.tw. (8) 22 manual.tw. (88018) 23 endobag*.tw. (160) 24 containment bag*.tw. (18) 25 containment system*.tw. (194) 26 bowel bag*.tw. (28) 27 or/12‐26 (90354) 28 11 and 27 (670) 29 randomized controlled trial.pt. (484622) 30 controlled clinical trial.pt. (93136) 31 randomized.ab. (447277) 32 randomised.ab. (89197) 33 placebo.tw. (204219) 34 clinical trials as topic.sh. (187463) 35 randomly.ab. (313513) 36 trial.ti. (200806) 37 (crossover or cross‐over or cross over).tw. (80777) 38 or/29‐37 (1285063) 39 exp animals/ not humans.sh. (4593621) 40 38 not 39 (1182132) 41 28 and 40 (32)
Appendix 4. Embase search strategy
Searched from 1980 to 1 July 2019
Ovid platform
1 exp leiomyoma/ or exp myoma/ (34112) 2 leiomyoma*.tw. (16126) 3 myoma*.tw. (7327) 4 fibroid*.tw. (10415) 5 fibroma*.tw. (11013) 6 fibromyoma*.tw. (283) 7 hysteromyoma*.tw. (156) 8 myomectom*.tw. (5982) 9 exp myomectomy/ (6601) 10 or/1‐9 (52909) 11 exp morcellation/ (878) 12 (Morcellat* or Morcelat*).tw. (2428) 13 morcellement.tw. (15) 14 (morselli?* or morseli?*).tw. (725) 15 (morcelli?* or morceli?*).tw. (162) 16 in bag.tw. (421) 17 spill free.tw. (2) 18 uncontained power.tw. (15) 19 containment system*.tw. (242) 20 endobag*.tw. (496) 21 containment bag*.tw. (43) 22 bowel bag*.tw. (75) 23 manual.tw. (117201) 24 or/11‐23 (121583) 25 10 and 24 (1414) 26 Clinical Trial/ (949870) 27 Randomized Controlled Trial/ (552149) 28 exp randomization/ (82999) 29 Single Blind Procedure/ (35550) 30 Double Blind Procedure/ (159063) 31 Crossover Procedure/ (59560) 32 Placebo/ (322777) 33 Randomi?ed controlled trial$.tw. (205207) 34 Rct.tw. (32809) 35 random allocation.tw. (1885) 36 randomly.tw. (409623) 37 randomly allocated.tw. (32619) 38 allocated randomly.tw. (2442) 39 (allocated adj2 random).tw. (804) 40 Single blind$.tw. (22835) 41 Double blind$.tw. (191972) 42 ((treble or triple) adj blind$).tw. (956) 43 placebo$.tw. (285428) 44 prospective study/ (528556) 45 or/26‐44 (2266761) 46 case study/ (62005) 47 case report.tw. (374321) 48 abstract report/ or letter/ (1061265) 49 or/46‐48 (1487891) 50 45 not 49 (2215343) 51 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5737606) 52 50 not 51 (2061191) 53 25 and 52 (124)
Appendix 5. PsycINFO search strategy
Searched form 1806 to 1 July 2019
Ovid platform
1 gynecological disorders/ (541) 2 (leiomyoma* or myoma*).tw. (50) 3 (fibroid* or fibrom*).tw. (3259) 4 hysteromyoma*.tw. (2) 5 myomectom*.tw. (11) 6 or/1‐5 (3828) 7 (Morcellat* or Morcelat*).tw. (2) 8 morcellement.tw. (3) 9 morselli?*.tw. (66) 10 morseli?*.tw. (1) 11 in bag.tw. (21) 12 or/7‐11 (93) 13 6 and 12 (1)
Appendix 6. CINAHL search strategy
Searched from 1961 to 1 July 2019
EBSCO platform
S45 S21 AND S44 37 S44 S43 NOT S42 572,660 S43 S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 598,030 S42 S40 NOT S41 159,074 S41 MH (human) 1,942,888 S40 S37 OR S38 OR S39 179,734 S39 TI (animal model*) 2,762 S38 MH (animal studies) 103,970 S37 MH animals+ 83,361 S36 AB (cluster W3 RCT) 288 S35 MH (crossover design) OR MH (comparative studies) 216,690 S34 AB (control W5 group) 91,629 S33 PT (randomized controlled trial) 86,878 S32 MH (placebos) 11,346 S31 MH (sample size) AND AB (assigned OR allocated OR control) 3,668 S30 TI (trial) 92,572 S29 AB (random*) 259,181 S28 TI (randomised OR randomized) 90,464 S27 MH cluster sample 3,814 S26 MH pretest‐posttest design 38,773 S25 MH random assignment 55,270 S24 MH single‐blind studies 12,602 S23 MH double‐blind studies 41,522 S22 MH randomized controlled trials 83,612 S21 S7 AND S20 302 S20 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 53,377 S19 TX (bowel bag*) 272 S18 TX (containment system*) 1,287 S17 TX (containment bag*) 81 S16 TX endobag* 42 S15 TX (manual) 38,598 S14 TX (in bag) 13,113 S13 TX (spill free) 97 S12 TX (uncontained power) 15 S11 TX morcelli?* or TX morceli?* 38 S10 TX morselli?* or TX morseli?* 178 S9 TX Morcellat* or TX Morcelat* 481 S8 TX morcellement 2 S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 6,831 S6 TX Myomectom* 1,108 S5 TX hysteromyoma* or TX fibroma* 1,276 S4 TX fibroid* or TX fibromyoma* 2,007 S3 TX leiomyoma* or TX myoma* 4,457 S2 (MM "Myoma+") 223 S1 (MM "Leiomyoma") 2,806
Appendix 7. 'Risk of bias' domains
(1) Random sequence generation (selection bias)
For each included study, we described the method used to allocate participants to interventions. We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e. g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias (insufficient information about the process of sequence generation).
(2) Allocation concealment (selection bias)
For each included study, we described the method used to conceal allocation to interventions prior to assignment. We assessed the method as:
low risk of bias (telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias (insufficient information about the process of allocation concealment).
(3.1) Blinding of participants and personnel (performance bias)
For each included study, we described the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We assessed the method as:
low risk of bias (blinding of participants, care providers, and researchers);
high risk of bias (no blinding of participants, care providers, and researchers);
unclear risk of bias (insufficient information about the process of blinding participants, care providers, and researchers).
(3.2) Blinding of outcome assessment (detection bias)
For each included study, we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed the method as:
low risk of bias (blinding of outcome assessors);
high risk of bias (no blinding of outcome assessors);
unclear risk of bias (insufficient information about the process of blinding outcome assessors).
(4) Incomplete outcome data (attrition bias)
For each included study, we described the completeness of data, including attrition and exclusions from the analysis. We assessed the method as:
low risk of bias (no missing data, or missing data with clear reasons);
high risk of bias (missing data or no reasons given for missing data);
unclear risk of bias (insufficient information about the completeness of outcome data).
(5) Selective reporting (reporting bias)
For each included study, we described the completeness of outcomes measured. We assessed the method as:
low risk of bias (all prespecified outcomes in the protocol reported in the published article);
high risk of bias (not all prespecified outcomes in the protocol reported in the published article);
unclear risk of bias (insufficient information about the process of outcome reporting).
(6) Other bias (bias not covered by (1) to (5) above).
For each included study, we described any important concerns we had about other possible sources of bias. We assessed these as:
low risk of bias (study free of other bias (e.g. baseline imbalance of groups, blocked randomisation in unblinded trials);
high risk of bias (other biases present (these were specified));
unclear risk of bias (insufficient information about other sources of bias).
Data and analyses
Comparison 1. Any type of in‐bag morcellation versus uncontained power morcellation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Composite intraoperative complications | 2 | 176 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.2 Total operative time | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 9.93 [‐1.35, 21.20] |
| 1.3 Morcellation operative time | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 2.59 [0.45, 4.72] |
| 1.4 Ease of morcellation | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.5 Postoperative length of stay | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.42, 0.49] |
| 1.6 Postoperative pain | 2 | 176 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.50, 0.94] |
| 1.7 Conversion to laparotomy | 2 | 176 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.8 Postoperative diagnosis of leiomyosarcoma | 2 | 176 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 1.9 Postoperative complications | 2 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.16, 4.12] |
1.7. Analysis.

Comparison 1: Any type of in‐bag morcellation versus uncontained power morcellation, Outcome 7: Conversion to laparotomy
1.8. Analysis.

Comparison 1: Any type of in‐bag morcellation versus uncontained power morcellation, Outcome 8: Postoperative diagnosis of leiomyosarcoma
Comparison 2. Sensitivity analysis: fixed‐effect model.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Total operative time | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | 9.52 [0.53, 18.51] |
| 2.2 Morcellation operative time | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | 2.59 [0.45, 4.72] |
| 2.3 Postoperative length of stay | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.25, 0.15] |
| 2.4 Postoperative pain | 2 | 176 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.27, 0.37] |
| 2.5 Postoperative complications | 2 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.16, 3.68] |
2.2. Analysis.

Comparison 2: Sensitivity analysis: fixed‐effect model, Outcome 2: Morcellation operative time
2.3. Analysis.

Comparison 2: Sensitivity analysis: fixed‐effect model, Outcome 3: Postoperative length of stay
2.4. Analysis.

Comparison 2: Sensitivity analysis: fixed‐effect model, Outcome 4: Postoperative pain
2.5. Analysis.

Comparison 2: Sensitivity analysis: fixed‐effect model, Outcome 5: Postoperative complications
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Frascà 2018.
| Study characteristics | ||
| Methods | Unblinded single‐centre randomised controlled trial, conducted at the Gynecology and Human Reproduction Physiopathology of Sant'Orsola‐Malpighi University Hospital of Bologna, Bologna, Italy | |
| Participants | Premenopausal women aged between 18 and 50 years, with an ultrasonographic diagnosis of at least one myoma measuring between 4 cm and 10 cm in mean diameter, and presenting a heavy menstrual flow or infertility as indications to laparoscopic myomectomy. | |
| Interventions | Sample size: 72 women (34 in the intervention group and 38 in the control group) In the control group, intra‐abdominal uncontained power morcellation was performed with the reusable power morcellator Rotocut G1, Storz. In the intervention group (extracorporeal in‐bag manual morcellation), each enucleated myoma was placed within a specimen retrieval bag (Endo Catch II Auto Suture 10‐mm or 15‐mm, Covidien). The edges around the bag's opening were then pulled out through the lower central 10‐mm trocar incision, previously enlarged to 20 mm, along with the abdominal fascia. The fibroid was tightly grasped with Schroeder tenaculum and manual morcellation was performed with scalpel or scissors, while the first assistant carefully pulled on the edges of the bag to move it away from the blade, avoiding bag damage. At the end of morcellation, the endoscopic bag was retrieved on the edges of the bag to move it away from the blade, avoiding bag damage, retrieved through the port incision, and filled with water to identify eventual bag disruptions. |
|
| Outcomes | The primary outcome was the comparison of morcellation operative times. | |
| Notes | Dates: November 2015 to October 2016 Conflict of interest: none Funding source: not reported Clinical Trial Registration: not registered. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | The study was not registered on ClinicalTrials.gov or on any other trial registry, and original protocol was not available |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias |
Venturella 2016.
| Study characteristics | ||
| Methods | Unblinded single‐centre randomised controlled trial, conducted at the Department of Obstetrics and Gynecology, University ‘‘Magna Graecia’’ of Catanzaro, Catanzaro, Italy | |
| Participants | Sample size: 104 women (53 in the intervention group and 51 in the control group) Premenopausal women with heavy menstrual bleeding or women already diagnosed with fibroids from referral sources and undergoing a laparoscopic myomectomy. Inclusion criteria were the following: age between 18 and 40 years, body mass index (BMI) 18 kg/m² to 40 kg/m², heavy menstrual bleeding, and the presence of at least one myoma measuring 4 cm or more in diameter (but no myoma measuring >10 cm, according to local practice on eligibility for laparoscopy). |
|
| Interventions | In the intervention group (extracorporeal in‐bag manual morcellation), each enucleated myoma was placed within a rip‐stop nylon specimen bag (Endo Catch Gold Auto Suture 10‐mm or 15‐mm, Covidien), which could hold 220 mL or 1000 mL according to the size chosen. The central lower 10‐mm trocar incision was increased to 30 mm, and a 65‐mm reusable sterile pessary was placed inside the bag, between the myoma and pelvic wall, to create a barrier between the morcellated portion of the myoma and the bag. In this way, the pessary protected the bag from the coring rotational movements of either the knife or the scissors and allowed a more manageable coring. After exteriorisation of the fibroid's surface with the aid of Alexis retractors, it was grasped with Schroeder tenaculum, double tooth, or Backhaus towel forceps and subjected to gradual morcellation with scalpel or scissors by cautious C‐coring. Fibroid adequate traction was allowed by using different instruments, depending on the myoma consistency. In the control group, intracorporeal un‐contained morcellation using a power morcellator (Rotocut G1, Storz) was performed. |
|
| Outcomes | The primary outcome was the comparison of morcellation operative times. | |
| Notes | Dates: March 2014 to January 2015 Conflict of interest: none Funding source: Department of Obstetrics and Gynecology, University ‘‘Magna Graecia’’ of Catanzaro Clinical Trial Registration: NCT02086435 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No deviation from the original protocol |
| Other bias | Unclear risk | Insufficient reporting to determine presence of other forms of bias |
Characteristics of ongoing studies [ordered by study ID]
NCT02777203.
| Study name | Power morcellation systems for laparoscopic hysterectomy and myomectomy |
| Methods | Open‐label, single centre randomised trial conducted in Illinois, USA |
| Participants | Premenopausal women undergoing robotic or laparoscopic total or supracervical hysterectomies or myomectomies for the indication of symptomatic uterine fibroids |
| Interventions | In‐bag morcellation with specimen morcellated in the EcoSac400 ECO‐T bag |
| Outcomes | Primary outcome: egg albumin leakage |
| Starting date | May 2016 to July 2019 |
| Contact information | Charles Miller, Advocate Health Care |
| Notes | On ClinicalTrials.gov, the trial is reported as completed. It is not clear if the intervention group was manual in‐bag morcellation, or in‐bag morcellation with device. We could not contact the principal investigator in order to obtain more information on this trial. |
NCT03281460.
| Study name | Efficacy of in‐bag morcellation (FIBROSAC) |
| Methods | Open‐label, single centre randomised trial conducted in Bron, France |
| Participants | Women aged 18 years or more undergoing laparoscopic myomectomy or laparoscopic subtotal hysterectomy |
| Interventions | In‐bag morcellation with More‐cell‐Safe (AMI bag morcellation) |
| Outcomes | Primary outcome: smooth muscular cells in the peritoneal fluid after morcellation (after morcellation, there will be cytology and immunohistochemistry of peritoneal washing with 500 cc of saline serum) |
| Starting date | September 2017 to February 2019 |
| Contact information | Gautier Chene, MD. Gynaecology Department, Hôpital Femme Mère Enfant, HCL |
| Notes | On ClinicalTrials.gov, the trial is reported as completed. It is not clear if the intervention group was manual in‐bag morcellation, or in‐bag morcellation with device. We could not contact the principal investigator in order to obtain more information on this trial. |
Differences between protocol and review
We clarified the inclusion criteria as follows:
We included all randomised controlled trials comparing in‐bag extracorporeal manual morcellation versus intracorporeal uncontained power morcellation at the time of laparoscopic myomectomy.
We also analysed the results by adopting a fixed‐effect model.
Contributions of authors
FZ, RV and GS devised the idea, applied for the review.
FZ, RV and GS drafted the protocol.
FZ, RV, AR and GS approved the final edition.
AR and GS performed review and analysis of pertinent and included trials, risk of bias and data extraction.
FZ, RV, AR and GS approved the final version of the review.
Sources of support
Internal sources
-
Cochrane Gynaecology and Fertility Group, Netherlands
Editorial support
External sources
None, Other
Declarations of interest
GS reports no conflict of interest.
AR reports no conflict of interest.
FZ and RV are co‐authors of one of the trials included in the full review (Venturella 2016). They were not involved in the assessment of eligibility or risk of bias for this trial. It was assessed by GS and AR.
New
References
References to studies included in this review
Frascà 2018 {published data only}
- Frascà C, Degli Esposti E, Arena A, Tuzzato G, Moro E, Martelli V, et al. Can in-bag manual morcellation represent an alternative to uncontained power morcellation in laparoscopic myomectomy? A randomized controlled trial. Gynecologic and Obstetric Investigation 2018;83(11):52-6. [DOI] [PubMed] [Google Scholar]
Venturella 2016 {published data only}
- Venturella R, Rocca ML, Lico D, La Ferrera N, Cirillo R, Gizzo S, et al. In-bag manual versus uncontained power morcellation for laparoscopic myomectomy: randomized controlled trial. Fertility and Sterility 2016;105(5):1369-76. [DOI] [PubMed] [Google Scholar]
- Venturella R. Intracorporeal versus extracorporeal morcellation with endobag extraction in patients undergoing laparoscopic myomectomy: clinical efficacy and safety outcomes. Preliminary results of a RCT. Journal of Minimally Invasive Gynecology 2014;21(6):S43. [Google Scholar]
References to ongoing studies
NCT02777203 {published data only}
- NCT02777203. Power morcellation systems for laparoscopic hysterectomy and myomectomy [Safety and efficacy of contained electromechanical power morcellation systems for laparoscopic hysterectomy and myomectomy]. clinicaltrials.gov/ct2/show/NCT02777203 (first posted 19 May 2016).
NCT03281460 {published data only}
- NCT03281460. Efficacy of In-bag morcellation (FIBROSAC). clinicaltrials.gov/ct2/show/NCT03281460 (first posted 13 September 2017).
Additional references
Baird 2003
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. American Journal of Obstetrics and Gynecology 2003;188:100-7. [DOI] [PubMed] [Google Scholar]
Bhave Chittawar 2014
- Bhave Chittawar P, Franik S, Pouwer AW, Farquhar C. Minimally invasive surgical techniques versus open myomectomy for uterine fibroids. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD004638.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed 10 April 2019. Melbourne, Australia: Veritas Health Innovation.Available at covidence.org.
Desai 2015
- Desai VB, Guo XM, Xu X. Alterations in surgical technique after FDA statement on power morcellation. American Journal of Obstetrics and Gynecology 2015;212:685-7. [DOI] [PubMed] [Google Scholar]
Divakar 2008
- Divakar H. Asymptomatic uterine fibroids. Best Practice & Research Clinical Obstetrics & Gynaecology 2008;22:643-54. [DOI] [PubMed] [Google Scholar]
Donnez 2015
- Donnez J, Hudecek R, Donnez O, Matule D, Arhendt HJ, Zatik J, et al. Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertility and Sterility 2015;103:519-27. [DOI] [PubMed] [Google Scholar]
FDA 2020
- FDA. UPDATE: The FDA recommends performing contained morcellation in women when laparoscopic power morcellation is appropriate. https://www.fda.gov/medical-devices/safety-communications/update-fda-recommends-performing-contained-morcellation-women-when-laparoscopic-power-morcellation 2020.
Giarrè 2019
- Giarrè G, Franchini M, Castellacci E, Malune ME, Di Spiezio Sardo A, Saccone G, et al. Ulipristal acetate in symptomatic uterine fibroids. A real-world experience in a multicentric Italian study. Gynecological Endocrinology 2019 Aug 8 [Epub ahead of print];36(2):171-4. [DOI: 10.1080/09513590.2019.1648419] [DOI] [PubMed] [Google Scholar]
Glaser 2018
- Glaser LM, Friedman J, Tsai S, Chaudhari A, Milad M. Laparoscopic myomectomy and morcellation: a review of techniques, outcomes, and practice guidelines. Best Practice & Research: Clinical Obstetrics & Gynaecology 2018;46:99-112. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 10 April 2019. Hamilton (ON): McMaster University (developed by Evidence Prime).Available at gradepro.org.
Higgins 2019
- Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration, 2019. Available from www.training.cochrane.org/handbook.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Mollo 2018
- Mollo A, Raffone A, Travaglino A, Di Cello A, Saccone G, Zullo F, et al. Increased LDH5/LDH1 ratio in preoperative diagnosis of uterine sarcoma with inconclusive MRI and LDH total activity but suggestive CT scan: a case report. BMC Women's Health 2018;18(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munro 2011
- Munro MG, Critchley HO, Broder MS, Fraser IS, FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. International Journal of Gynaecology and Obstetrics 2011;113:3-13. [DOI] [PubMed] [Google Scholar]
Pavone 2018
- Pavone D, Clemenza S, Sorbi F, Fambrini M, Petraglia F. Epidemiology and risk factors of uterine fibroids. Best Practice & Research: Clinical Obstetrics & Gynaecology 2018;46:3-11. [DOI] [PubMed] [Google Scholar]
Pritts 2015
- Pritts EA, Vanness DJ, Berek JS, Parker W, Feinberg R, Feinberg J, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecological Surgery 2015;12:165-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Senapati 2015
- Senapati S, Tu FF, Magrina JF. Power morcellators: a review of current practice and assessment of risk. American Journal of Obstetrics and Gynecology 2015;212:18-23. [DOI] [PubMed] [Google Scholar]
Siedhoff 2017
- Siedhoff MT, Doll KM, Clarke-Pearson DL, Rutstein SE. Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroids: an updated decision analysis following the 2014 Food and Drug Administration safety communications. American Journal of Obstetrics and Gynecology 2017;216:259.e1-259.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallach 2004
- Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstetrics and Gynecology 2004;104:393-6. [DOI] [PubMed] [Google Scholar]
Wise 2004
- Wise LA, Palmer JR, Harlow BL, Spiegelman D, Stewart EA, Adams-Campbell LL, et al. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. American Journal of Epidemiology 2004;159:113-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2015
- Wright JD, Tergas AI, Cui R, Burke WM, Hou JY, Ananth CV, et al. Use of electric power morcellation and prevalence of underlying cancer in women who undergo myomectomy. JAMA Oncology 2015;1:69-77. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Zullo 2019
- Zullo F, Venturella R, Saccone G. In‐bag manual versus uncontained power morcellation for laparoscopic myomectomy. Cochrane Database of Systematic Reviews 2019, Issue 6. [DOI: 10.1002/14651858.CD013352] [DOI] [PMC free article] [PubMed] [Google Scholar]


