Abstract
The peptide kisspeptin and its receptor, Kiss1r, act centrally to stimulate reproduction. Evidence indicates that kisspeptin signaling is also important for body weight (BW) and metabolism. We recently reported that Kiss1r KO mice develop obesity, along with reduced metabolism and energy expenditure, independent of estradiol levels. Outside the brain, Kiss1r is expressed in several metabolic tissues, including brown adipose tissue (BAT), but it is unknown which specific tissue is responsible for the metabolic phenotype in Kiss1r KOs. We first determined that global Kiss1r KO mice have significant alterations in body temperature and BAT thermogenic gene expression, perhaps contributing to their obesity. Next, to test whether kisspeptin signaling specifically in BAT influences BW, metabolism, or body temperature, we used Cre/ lox technology to generate conditional Kiss1r knockout exclusively in BAT (BAT-Kiss1r KO). Unlike global Kiss1r KOs, BAT-Kiss1r KOs (lacking Kiss1r in just BAT) were not hypogonadal, as expected. Surprisingly, however, BAT-Kiss1r KOs of both sexes displayed significantly lower BW and adiposity than controls. This novel BAT-Kiss1r KO phenotype was of greater magnitude in females and was associated with improved glucose tolerance, increased metabolism, energy expenditure, and locomotor activity, along with increased body temperature and BAT gene expression, specifically Cox8b. Our findings suggest that the previously observed obesity and decreased metabolism in global Kiss1r KOs reflect impaired kisspeptin signaling in non-BAT tissues. However, the novel finding of increased metabolism and body temperature and lower BW in BAT-Kiss1r KOs reveal a previously unidentified role for endogenous kisspeptin signaling in BAT in modulating metabolic and thermogenic physiology.
Keywords: adipose, BAT, fat, GPR54, Kiss1, Kiss1r, kisspeptin, metabolism, obesity, temperature
1 |. INTRODUCTION
Kisspeptin (encoded by the gene Kiss1) is a neuropeptide that binds the receptor, Kiss1r (previously termed GPR54). In mammals, including humans, kisspeptin and its receptor regulate reproduction by acting in the brain to directly stimulate gonadotropin-releasing hormone (GnRH) secretion, supported by the fact that humans and rodents with disrupted kisspeptin signaling are infertile, have undeveloped gonads, and have diminished reproductive hormone secretion (reviewed in1,2). In addition to being expressed in GnRH neurons in the brain,6–8 Kiss1r is also located in several peripheral tissues, such as adipose tissue, pancreas, and gonads.9–14 Likewise, kisspeptin is expressed in several peripheral tissues, including liver, pancreas, gonad, and placenta.12–14 This peripheral expression of both kisspeptin and its receptor suggests that kisspeptin signaling likely has additional roles outside of reproductive regulation. However, until recently, non-reproductive roles of kisspeptin have been largely overlooked.
We recently reported that, in addition to stimulating the reproductive axis, the kisspeptin system is also an important player in regulating body weight (BW), energy balance, and glucose homeostasis. Specifically, compared with WT littermates, global Kiss1r knockout (KO) female mice— lacking kisspeptin signaling in all tissues—displayed dramatically higher BWs beginning in early adulthood, weighing as much as 30% more than control females.15,16 In addition to becoming obese, adult Kiss1r KO females also had increased adiposity, higher leptin levels, and substantially impaired glucose tolerance, both on standard chow and high-fat diets.15,16 This obesity phenotype was sexually dimorphic, as Kiss1r KO males had normal BW and glucose regulation out to 6 months of age. Despite their obesity, adult Kiss1r KO females did not have increased food intake, but they did display significantly reduced metabolic parameters, including lower respiratory rates and energy expenditure, suggesting that their obesity reflects, at least in part, lower daily energy expenditure.15,16 Importantly, the BW and metabolic phenotype in Kiss1r KO females were not solely reflective of absent gonadal sex steroids, in particular ovarian estradiol (E2), as long-term ovariectomized Kiss1r KO females still developed obesity, hyperleptinemia, reduced metabolism, and glucose intolerance versus similarly ovariectomized WT females (ovaries removed before puberty to permanently eliminate ovarian-derived E2 in both genotypes) .15,16
The findings above in Kiss1r KOs demonstrated that globally absent kisspeptin signaling can be an important factor in BW, adiposity, glucose tolerance, and metabolism, particularly in females. However, because Kiss1r was absent from all cells in the body in those mice, it remains unknown which specific tissues and cell types are responsible for kisspeptin’s influence on the various metabolic phenotypes. Indeed, peripheral Kiss1r is present in several metabolic-related peripheral tissues, suggesting a number of potential candidate target sites for its ability to alter metabolism and energy balance. Furthermore, it is possible that different phenotypic alterations observed in the global Kiss1r KOs may reflect absent kisspeptin signaling in different tissues. For example, glucose intolerance might reflect absent Kiss1r in the pancreas, whereas altered adiposity or energy expenditure could hypothetically reflect absent Kiss1r in white or brown adipose tissue (BAT), with the net effect of these tissue-specific changes resulting in the overall obese phenotype.
The present study had two primary goals. First, we tested whether body temperature and thermogenic gene expression in BAT are altered in global Kiss1r KO mice, correlating with their previously reported changed BW and metabolism. Second, we sought to use a Kiss1r flox mouse line to selectively ablate Kiss1r from just one target cell type, thereby isolating potential effects of kisspeptin signaling on BW and metabolism. We chose BAT because we found that our global Kiss1r KOs displayed altered body temperature and expression levels of uncoupling protein-1 (Ucp1), a thermogenic protein in the mitochondria of BAT. Thus, we used Cre/lox technology to generate specific deletion of Kiss1r in just BAT to test the hypothesis that endogenous kisspeptin may normally modulate metabolism or body temperature by acting directly in BAT.
2 |. MATERIALS AND METHODS
2.1 |. Animals
Experiments used either “global” (whole-body) Kiss1r KO mice, the same mouse line as in our previous reports,15–18 or a new conditional KO mouse line generated with Cre/lox technology: a BAT-specific Kiss1r KO line (termed BAT cKO). In all cases, littermate sibling controls (WTs or Cre−) were used to compare directly to the KO animals.
To study the role of endogenous Kiss1r specifically in BAT, we generated mice lacking Kiss1r exclusively in cells expressing Ucp-1, which are highly localized to BAT. Female Ucp1-Cre mice (Jackson Labs) were crossed with our recently created male Kiss1rfl/fl mice13,19 to generate Ucp1-Cre−/−/Kiss1rfl/wt female progeny, which were then backcrossed with male Kiss1rfl/fl mice to generate BAT Kiss1r KOs (Ucp1-Cre−/−/Kiss1rfl/fl; termed “BAT cKO”) and control littermates (Ucp1-Cre−/−/Kiss1rfl/wt and Ucp1-Cre−/−/Kiss1rfl/fl). For confirmation of proper Cre/flox removal of Kiss1r, multiple tissues were dissected from cKO mice. DNA was extracted from each tissue and tested for recombination using PCR. To further confirm the expression pattern of Ucp1-Cre, which was previously reported to be in BAT only, a cohort of cKO mice were crossed with Cre-dependent tdTomato reporter mice to generate offspring that express the fluorescent tdTomato reporter gene wherever Cre recombination has occurred. Sections of BAT and brain were then collected and fixed in 4% paraformaldehyde for microscopy analysis of tdTomato reporter expression.
Mice from all lines were genotyped and sexed using PCR of DNA obtained from toe samples at postnatal day 7 (PND7) or tail DNA at weaning. All mice were weaned at PND21 and housed 2–3 per cage with mixed genotype in a 12-hour light/ 12-hour dark cycle, with ad libitum water and standard rodent chow containing 3.5 kcal/g, 45.2% carbohydrates, 11.4% fat, and 17.2% crude protein. All experiments were approved by the Institutional Animal Care and Use Committee from the University of California San Diego and by the Animal Ethics Committee from the University of Western Australia.
2.2 |. Ovariectomies
In the global Kiss1r KO studies, some mice of each genotype (detailed in the results) were ovariectomized (OVX) to remove any influence of circulating sex steroids (E2) on the metabolic measures. Mice were briefly anesthetized with isoflurane, the ventral skin shaved and sterilized, and a single midline incision made in the skin and abdominal musculature. Both left and right ovaries were identified and cut out, and the muscle wall sutured with chromic gut. The skin cut was then closed with surgical wound clips. Mice were given analgesic (buprenorphine) via s.c. injection.
2.3 |. Reproductive characteristics
Global Kiss1r KO mice are well known to have extremely underdeveloped gonads, reduced anogenital distance (AGD; an indirect measure of testosterone exposure) in males, and correspondingly low circulating estrogen and testosterone levels. For the newly generated conditional KO mouse line (BAT cKO), reproductive developmental status for females and males were quantified by measuring AGD at week 12, and gonadal weights at sacrifice. For the former, mice were briefly anesthetized with isoflurane and distance measurement was taken from the anus opening to the base of the reproductive organ. In addition, fertility was assessed in the BAT cKO mouse line by pairing adult female cKOs with control males.
2.4 |. Body weight assessment and body composition analyses
Mice of both sexes from the BAT cKO line were analyzed for their BW at multiple ages. Control and experimental littermates were weighed once every 2 weeks, starting at 4 weeks of age and ending around 4–5 months of age. Lean mass and fat mass of BAT cKO and control mice were analyzed using the EchoMRI-3 instrument (EchoMRI LLC, Houston, TX), a quantitative nuclear magnetic resonance (qNMR) imaging system for whole-body composition analysis of unanesthetized small animals, at 19- to 22-weeks old. qNMR body composition analysis with EchoMRI instrumentation has been proposed to be “gold standard” methodology for metabolic studies in the mouse. After calibration of the EchoMRI 3-in-1 instrument, each mouse was positioned into the EchoMRI Chamber. The instrument calculated the lean mass and fat mass.
2.5 |. Metabolic and energy expenditure analyses
To measure metabolic rates, adult BAT cKO and control littermates were analyzed at 20–24 weeks of age with indirect calorimetry using equal flow Comprehensive Laboratory Animal Monitoring System (CLAMS) calorimeter system (CLAMS; Columbus Instruments, Columbus, OH). The mice were individually housed 2–3 days prior to the experiment for habituation to being single housed. Food in the CLAMS cages was Tekland diet 2018 (18% protein, 6% fat, and 42% carbohydrate). CLAMS data were collected in clear respiratory chambers (20 × 10 × 12.5 cm) equipped with a sipper tube delivering water and a food tray connected to a balance. The consumption of O2 and production of CO2 was measured by having sample air sequentially passed through O2 and CO2 sensors (Columbus Instruments) for determination of O2 and CO2 content, from which measures of oxygen consumption (VO2) and carbon dioxide production (VCO2) were estimated. Room air was passed through chambers at a flow rate of 0.5 L/min. Exhaust air from each chamber was sampled at 18-min intervals for 1 minutes. Outdoor air reference values were sampled after every eight measurements. Gas sensors were calibrated prior to the onset of experiments with primary gas standards containing known concentrations of O2, CO2, and N2 (Airgas Puritan Medical, Ontario, CA). Respiratory exchange ratio (RER) was calculated as the ratio of carbon dioxide production (VCO2) to oxygen consumption (VO2). Per standard convention, energy expenditure (EE; heat formation) was corrected for each mouse’s lean mass. Total locomotor activity was measured by photosensor beam breaks. Data were recorded under ambient room temperature (~24°C) for up to 7 days.
2.6 |. Body temperature assessment
Telemeters were implanted into the abdominal cavity of adult mice to measure their core body temperature every 18 min. The sterile transmitters (Emitter 15.5 × 6.5 mm, 1.1 grams, sterilized in Spor-Klenz Cold Sterilant) were implanted into the peritoneal cavity and sutured by dissolvable suture thread to the cavity wall. VetBond surgical glue was used to close the external skin cut. A 1-week recovery period was given to the mice before starting measurement under ambient room temperature (~24°C) for several days. In a separate study, global Kiss1r KO and WT female mice with telemeters for core body temperature recording were exposed to a cold challenge where they were transferred to a cold room (4°C) for 6 hours, after which they were returned to ~22°C for 1 hour. In that experiment, body temperature was recorded every 30 minutes to track changes over time in the cold.
2.7 |. Glucose tolerance tests
At 18–20 weeks of age, BAT cKO mice and their control littermates of both sexes underwent glucose tolerance testing (GTT). Mice of both genotypes and sexes were fasted for 6 hours before starting the glucose measurements, with free access to water throughout the experiment. Blood glucose was measured just before glucose injection using a handheld glucometer. Then, at time 0, mice were given an IP glucose injection; 2 g/kg BW dissolved in saline. Blood glucose levels were then re-measured at 15, 30, 45, 60, 90, and 120 minutes after glucose injection.
2.8 |. qPCR of Kiss1r in multiple tissues and thermogenic genes in BAT
Adult female Kiss1r KOs show an obese and metabolically impaired phenotype. To determine which tissues express Kiss1r in normal adult females, we used RT-PCR to assess presence in multiple metabolic and non-metabolic tissues. To verify these initial results and provide more quantitative analysis, we next performed qPCR to detect Kiss1r mRNA in various tissues from normal adult (8-weeks old) females. For the Kiss1r gene, we used the QuantiTect Primer Assay using Mm_Kiss1r_1_SG (catalog number QT00140427).
To determine whether thermogenic-related genes were altered in the BAT of Kiss1r KO and BAT cKO females, BAT tissue was collected and tested with real-time qPCR for expression of several genes of interest including Ucp1 (fwd AGGC TTCCAGTACCATTAGGT, rev CTGAGTGAGGCAAAGCT GATTT,) Coxb8 (fwd TGTGGGGATCTCAGCCATAGT; rev AGTGGGCTAAGACCCATCCTG, and PRDM16 (fwd CCAAGGCAAGGGCGAAGAA, rev CCAAGGCAAGGGCG AAGAA). For all assays, total RNA was isolated using the phenol/chloroform method and converted to cDNA via reverse transcription (Promega, Sydney, Australia). qRT-PCR was performed in 10 μl reaction volumes and samples were tested in duplicate using a Rotorgene 3000 (Corbett Life Science, US). Reactions used either BioRad IQ SYBR Green Supermix (Biorad, Australia) or Qiagen SYBR Green PCR Kit (Qiagen, Australia). Samples were compared to a standard curve (10-fold dilution) and relative gene expression was normalized using a GE Norm algorithm of reference genes peptidylprolyl isomerase A (Ppia), succinate hydrogenase (Sdha), and TATA box binding protein (Tbp).17
2.9 |. Statistical analyses
All data are presented as mean ± SEM. For data at single points (non-repeated measures), single comparisons were made using one- or two-tailed t tests, as appropriate, and multiple comparisons were performed using one-way ANOVA with Tukey’s post-hoc test. For repeated measures (BWs, GTTs, and body temperature during cold exposure), repeated measures ANOVA was performed, with Bonferroni post-hoc tests directly comparing genotypes at specific points for three groups or t tests for two groups. Statistical significance was P < .05.
3 |. RESULTS
3.1 |. Body temperature is decreased in global Kiss1r KO female mice
Given the decreased metabolism and energy expenditure of global Kiss1r KOs and Kiss1r is expressed in BAT, we asked whether absent kisspeptin signaling might alter body temperature, and if so, whether this correlated with changes in BAT size or gene expression. We first determined that core body temperature is significantly lower in global Kiss1r KO females compared to WT littermates, especially during the dark phase of the light-dark cycle when mice are most active (P < .05, Figure 1). This lower body temperature was true for both gonad-intact (P < .05, Figure 1A) and OVX mice (P < .05, Figure 1B), indicating that the genotype difference is not simply due to differences in circulating ovarian steroid (E2) levels. We further tested whether an acute cold challenge to normal body temperature would be impacted by loss of kisspeptin signaling. In a cold challenge at 4°C, global Kiss1r KOs showed a sharper rate of body temperature drop than WTs, with a significantly lower mean body temperature after 6 hours (P < .05, Figure 1C). Collectively, these findings suggest that the ability to maintain a normal core body temperature is impaired in the absence of normal kisspeptin signaling.
FIGURE 1.
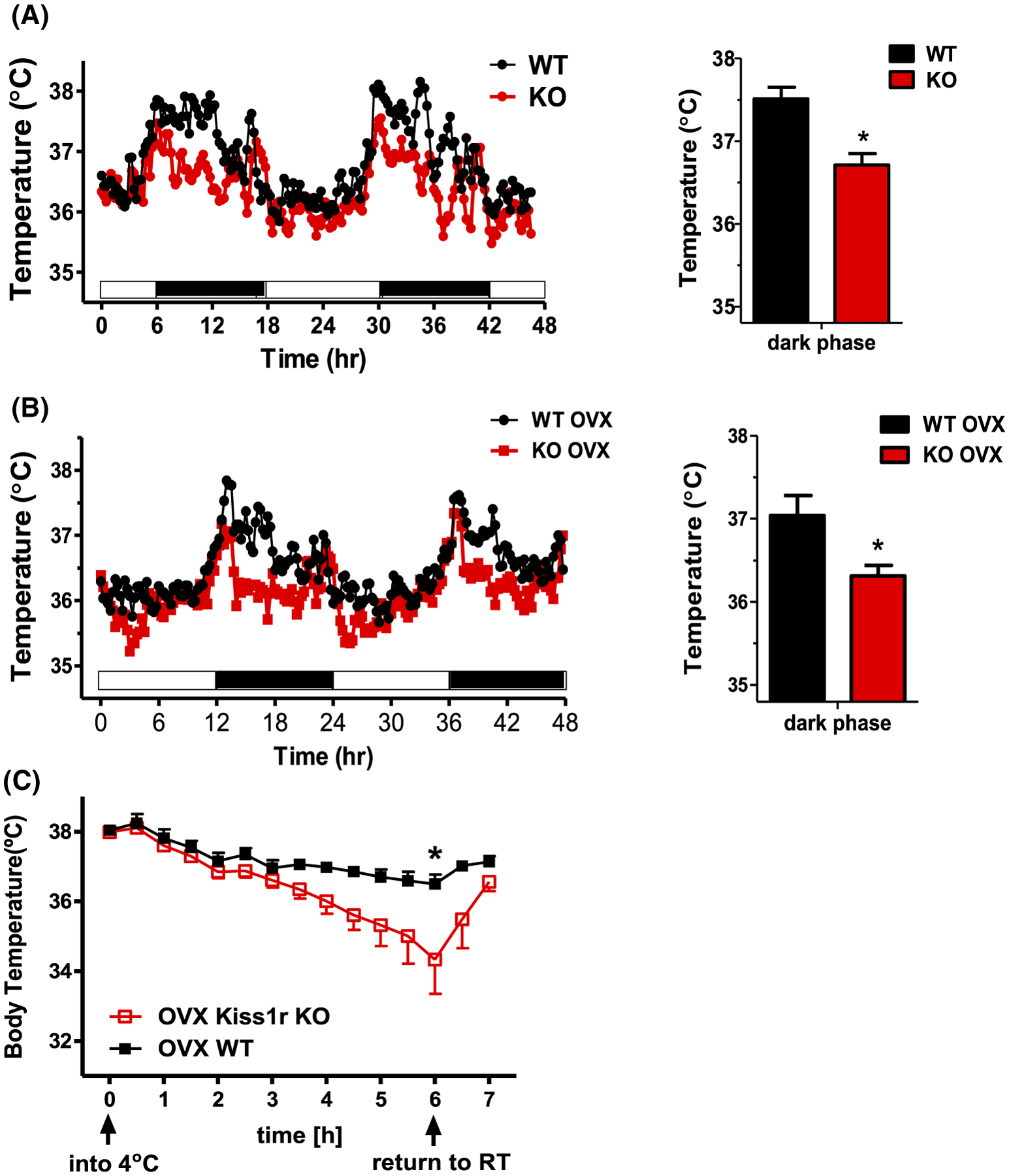
Core body temperature is lower in mice globally lacking kisspeptin signaling. A, In gonad-intact (n = 4/group) and B, chronically OVX mice (n = 5–8/group), global Kiss1r KO females have lower body temperature than WT littermates, primarily during the dark phase of the light cycle. C, Cold exposure at 4°C for 6 hours causes a sharper drop in body temperature in OVX global Kiss1r KOs than OVX WT controls (n = 6/group). *P < .05
3.2 |. BAT gene expression is significantly altered in global Kiss1r KO female mice
Because BAT modulates thermogenesis and metabolic rate, we next tested whether thermogenic-related genes were altered in the BAT of global Kiss1r KO females. We found that even though overall BAT weight was not different between genotypes, BAT expression of Ucp1 (uncoupling protein in the BAT mitochondria, used to generate heat by non-shivering thermogenesis), Coxb8 (polypeptide of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport), and Prdm16 (transcriptional coregulator promoting thermogenic processes in BAT) were all significantly lower in gonad-intact global Kiss1r KO females versus controls (P < .05, Figure 2), perhaps contributing to the lower body temperature in the KOs. To exclude an influence of E2 levels on this finding, we also measured BAT gene expression in OVX global Kiss1r KO and littermate controls. As with the gonad-intact mice, OVX Kiss1r KOs had significantly lower Ucp1 and Cox8b gene expression in their BAT than OVX WTs (P < .05, Figure 2), correlating with their lower body temperature (Figure 1B).
FIGURE 2.
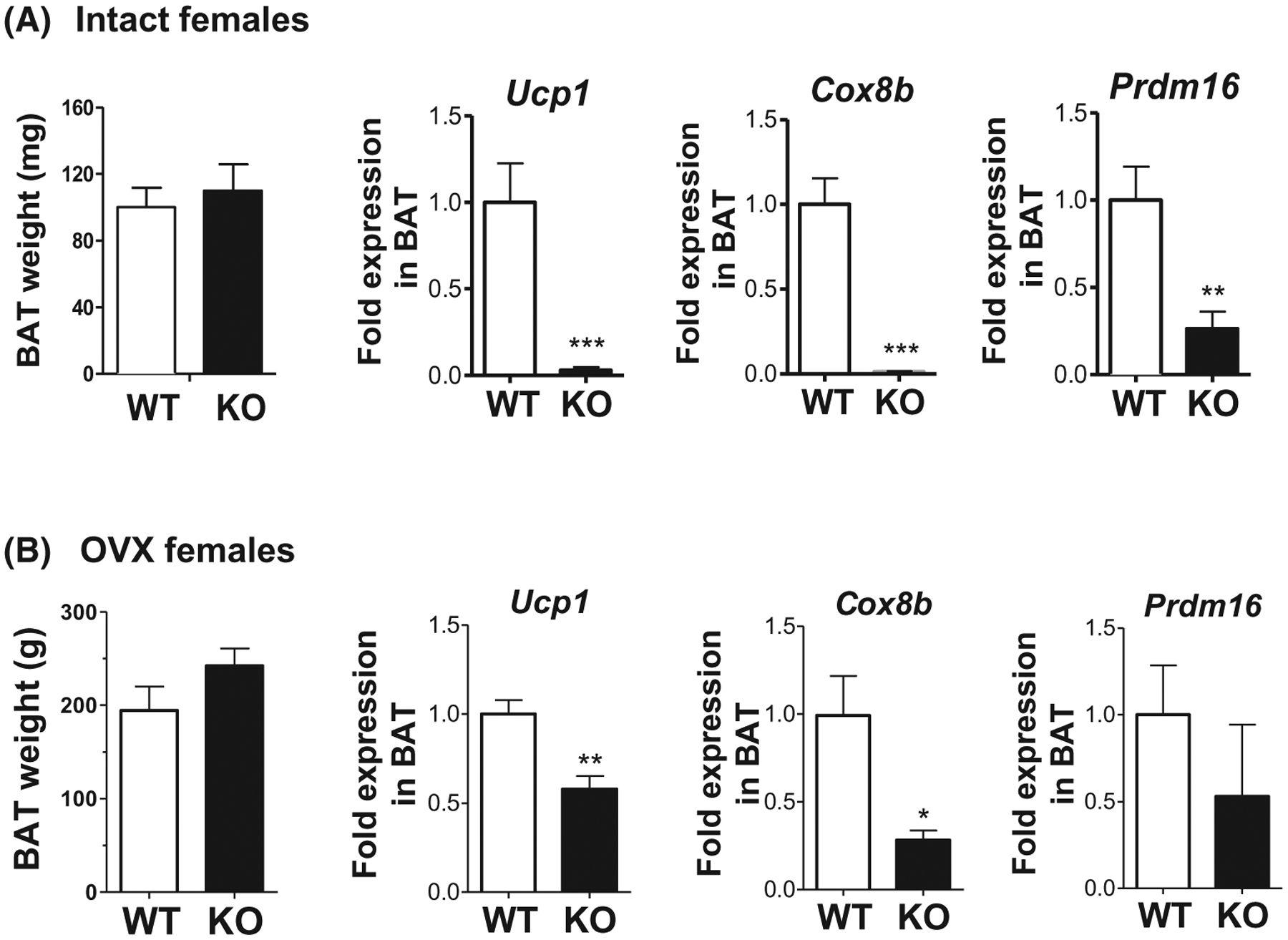
Thermogenic gene expression is decreased in BAT of global Kiss1r KO mice. A, In ovary-intact mice, BAT weight is normal but mRNA levels of Ucp1 (protein in the BAT mitochondria used to generate heat by non-shivering thermogenesis), Cox8b (polypeptide of cytochrome c oxidase, the terminal oxidase in mitochondrial electron transport), and Prdm16 (transcriptional coregulator promoting thermogenic processes in BAT) are significantly decreased in global Kiss1r KOs compared to WT littermates (n = 10–12/group). B, In chronically OVX females, BAT weight remains normal but Ucp1 and Cox8b expression are still reduced in global Kiss1r KOs versus WT controls (n = 6–8/group). *P < .05; **P < .01, ***P < .001
3.3 |. Body temperature and BAT gene expression are normal in global Kiss1r KO males
We previously reported that, unlike global Kiss1r KO females, global Kiss1r KO males do not exhibit a significant BW phenotype.16 We therefore tested whether there is also a sex difference in body temperature or BAT thermogenic gene expression in the global KOs. Indeed, we found that unlike global KO females (Figures 1 and 2), male global KOs have normal body temperature and BAT Ucp1 levels compared to WT littermates (Figure 3), matching the KO males’ normal BW.
FIGURE 3.
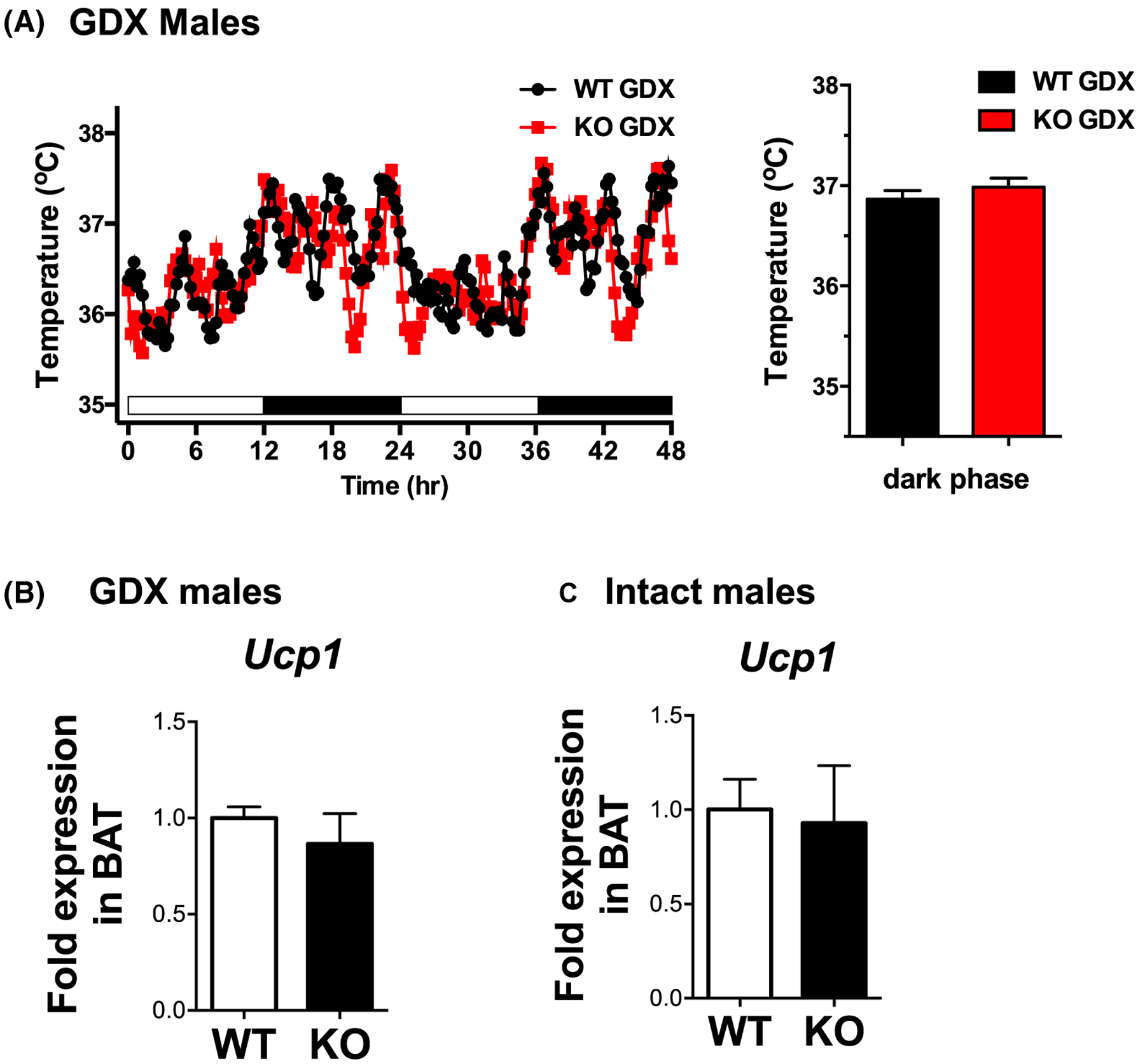
Core body temperature and Ucp-1 gene expression levels are normal in adult male mice globally lacking kisspeptin signaling. A, Global Kiss1r KO males (n = 4–5/group) have similar body temperature as WT littermate males. B, BAT mRNA levels of Ucp1 are normal in global Kiss1r KO males, regardless of gonadal status (n = 7–9/group)
3.4 |. Kiss1r is expressed in various metabolic tissues, including BAT
Given the profound metabolic phenotype in global Kiss1r KO females, we assessed where Kiss1r is normally expressed in normal adult female mice. RT-PCR analysis detected Kiss1r transcript in hypothalamus (as expected) as well as several metabolic-related tissues, including BAT, WAT, adrenal, and liver (Figure 4). Kiss1r was also detected in ovary and kidney, and a very faint band was observed in skeletal muscle. We verified this with a more quantitative assessment of Kiss1r levels in normal adult females using qPCR. qPCR analysis demonstrated that, in addition to the hypothalamus, Kiss1r was expressed in both BAT and WAT, with slightly lower levels detected in liver (Figure 4). Matching the faint band in the RT-PCR assay, very little to no expression was detected in skeletal muscle with qPCR.
FIGURE 4.
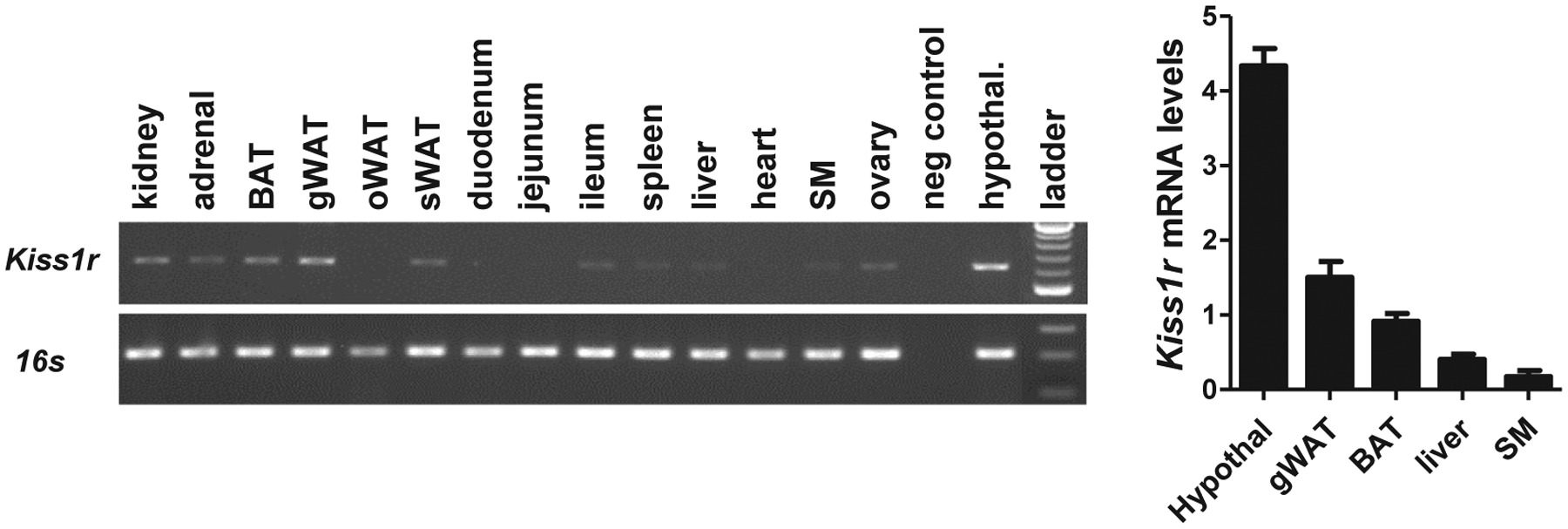
Kiss1r expression in BAT and other tissues of normal adult female mice. A, Gel image of RT-PCR detection of Kiss1r expression in hypothalamus and several metabolic tissues, including BAT, WAT, adrenal, and liver. B, qPCR analysis of Kiss1r levels in several metabolic tissues and brain (hypothalamus) of adult WT females (n = 5). Kiss1r was expressed in both BAT and WAT, and at slightly lower levels in liver. Very low expression was detected in skeletal muscle (SM)
3.5 |. Generation and validation of a BAT-specific Kiss1r KO model
The findings above suggest that impaired kisspeptin signaling somewhere in the body lowers BAT gene expression and core body temperature, likely contributing to the development of the observed obesity and metabolic phenotype. To determine what specific tissues/cell types might be involved in mediating this effect of kisspeptin signaling, we sought to use Cre/lox technology to selectively knock out Kiss1r from specific tissues, starting with BAT. To target BAT cells, we bred Ucp1-Cre mice to our Kiss1r flox mice, thereby generating selective knockout of Kiss1r only in Ucp1-expressing cells (Figure 5).
FIGURE 5.
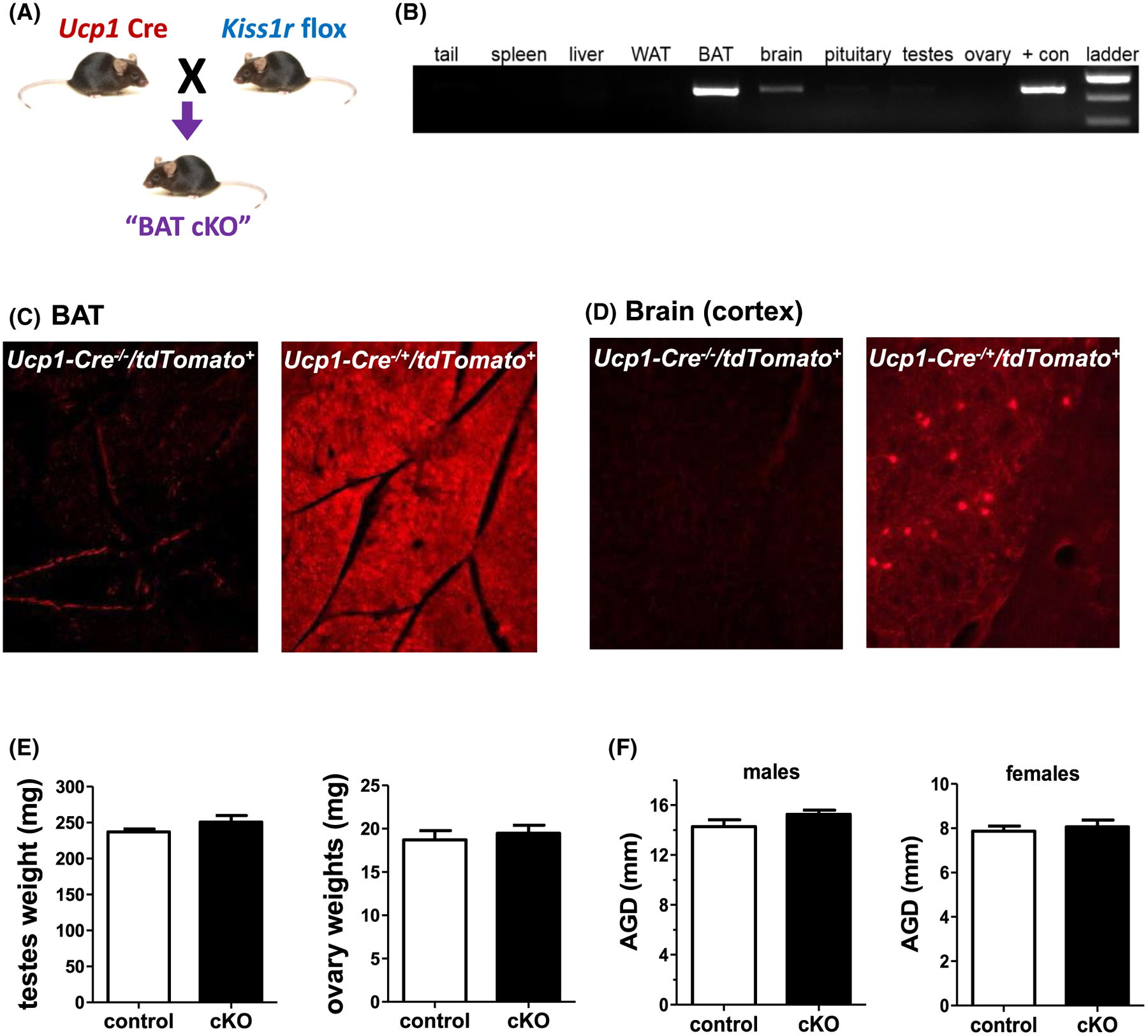
Generation and validation of BAT-specific KO of Kiss1r mice (“BAT cKO”). A, Strategy for selectively knocking out Kiss1r from BAT tissue by crossing Ucp1-Cre and Kiss1r floxed mouse lines. B, PCR product of different tissues collected from Ucp1-Cre+/Kiss1rfl/fl (BAT cKO) mice tested for Kiss1r gene recombination. C, Cross section of BAT from Ucp1-Cre−/Tdtomato+/− (control) and Ucp1-Cre+/TdTomato+/− (recombined) mice demonstrating fluorescent reporter only in the latter. D, Cross section of brain tissue from Ucp1-Cre−/Tdtomato+/− (control) and Ucp1-Cre+/Tdtomato+/− (recombined) mice demonstrating a few fluorescent cells in the cortex of the latter (a region of the brain where Kiss1r is not expressed). E, Gonad weights from adult BAT cKO males and females (n = 5–8/group for males and n = 11/group for females). F, AGD in adult BAT cKO males and females (n = 7–11/group for males and n = 12–13/group for females)
To validate this new conditional KO mouse line, we tested multiple tissues from BAT cKOs for Kiss1r recombination. Recombination was strongly detected in BAT but not in WAT, liver, gonads, or tail (Figure 5B). Very faint recombination was also detected in the brain (Figure 5B). To determine exactly where the brain recombination was occurring, we bred our Ucp1-Cre line to a Cre-dependent tdTomato reporter mouse line to assess the neuroanatomical location of Ucp1-driven Cre in brain, as well as confirm Cre action in BAT. As expected, Cre expression, identified via tdTomato fluorescence, was present throughout the entire BAT in Ucp1-Cre+/tdTomato+ mice and completely lacking in the BAT of control Ucp1-Cre−/ tdTomato+ mice (Figure 5C). In the brain, a few scattered fluorescent cells were present in Ucp1-Cre+/ tdTomato+ mice but not in Cre−/tdTomato+ controls (Figure 5D). Specifically, some fluorescent cells were observed in the dorsal lateral septum, medial paraventricular thalamus, cortical amygdaloid nucleus, ventromedial hypothalamus, and the cortex, all brain areas in which Kiss1r is not expressed in mice.7 Thus, the brain expression of Ucp1-Cre is not expected to remove any neural Kiss1r expression in our BAT cKOs.
To assess reproductive characteristics of BAT cKOs, we first measured gonad weights. Unlike the underdeveloped gonads of global KOs, gonad weights of adult BAT cKOs of each sex were normal and not significantly different than control littermates (Figure 5E), as expected. Additionally, in contrast to the global Kiss1r KO models, AGD in male BAT cKOs was normal and comparable to WTs (Figure 5F). This normal gonadal development was supported by completely normal fertility in BAT cKO females (data not shown). Thus, as expected, the Ucp1-Cre did not target GnRH neurons or other reproductive cell types where Kiss1r is normally expressed, ensuring that the selective knockout of endogenous Kiss1r signaling was exclusive to BAT cells.
3.6 |. Body weight and adiposity are lower in BAT-specific Kiss1r KO mice of both sexes
To assess potential metabolic consequences of selective deletion of kisspeptin signaling from BAT, BWs of BAT cKO and control mice were taken every 2 weeks, starting at 4 weeks of age. Surprisingly, unlike global Kiss1r KO females which become obese in adulthood, BAT cKO females showed significantly lower BWs compared to control littermates (P < .05, Figure 6A). Starting around week 8, cKO females started to gain significantly less weight over time compared to female controls. By 22 weeks of age, cKO females weighed a marked 20% less than their littermate controls (P < .05, Figure 6A). Likewise, BAT cKO males showed a similar, though less robust, pattern of decreased BW in adulthood which started later than BAT cKO females (~ 16 wks vs 8 wks of age) (P < .05 vs control males, Figure 6B). By week 22 of age, BAT cKO males weighed 10% less than control males (P < .05, Figure 6B). Matching their lower BWs, adult BAT cKOs of both sexes showed significantly lower absolute fat mass and decreased percent fat mass compared to control littermates of the same sex (P < .01, Figure 6C and D). Female BAT cKOs also showed a minor but significant decrease in lean mass (P < .05, Figure 6C).
FIGURE 6.
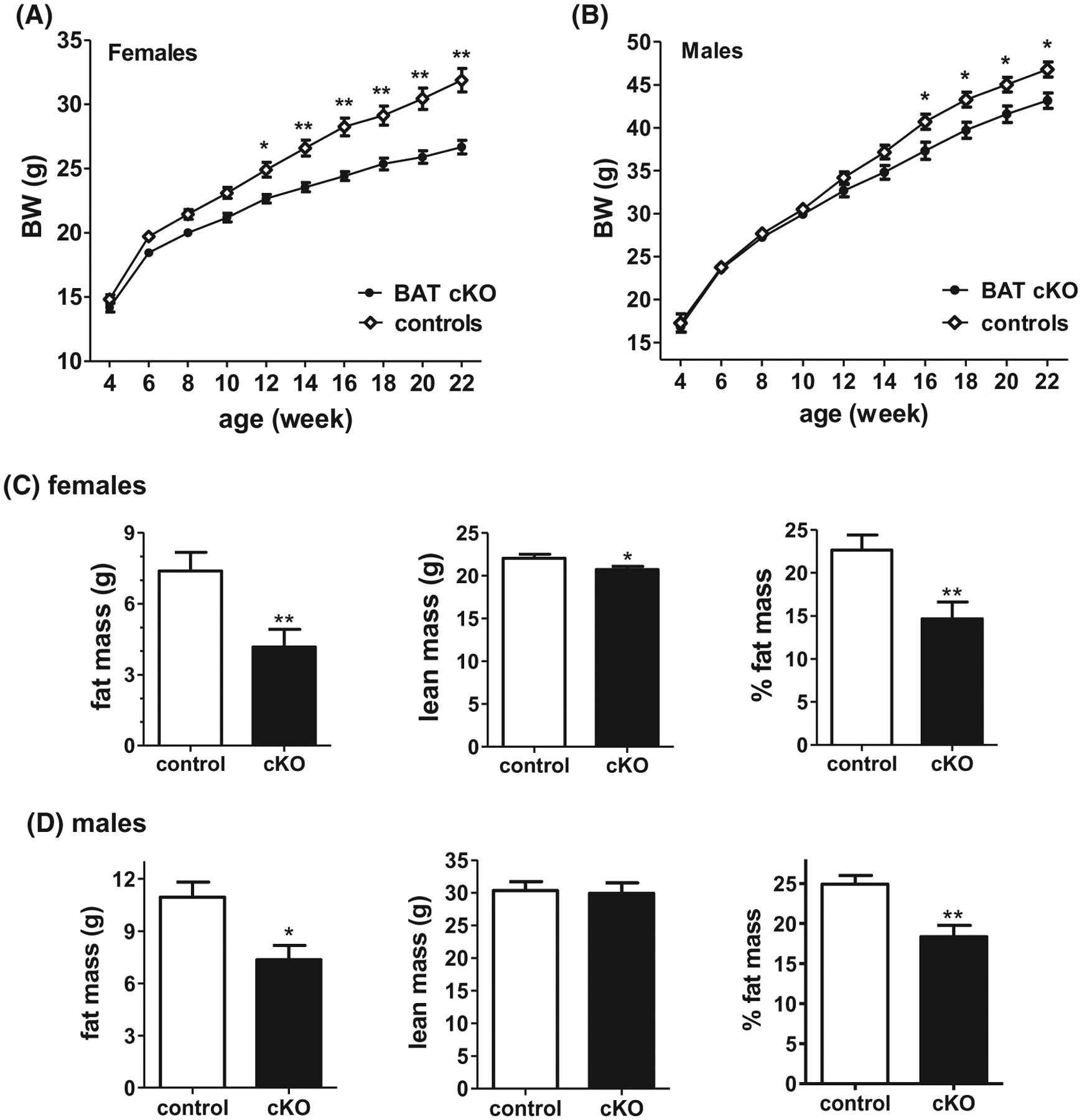
BW of adult BAT cKO mice is decreased compared to control littermates in both A, females (n = 31–40/group) and B, males (n = 21–25/group). C and D, Mean body composition (fat mass, lean mass, and % fat mass) is also altered in BAT cKO females (n = 14/group) and males (n = 5–7/group), respectively, as measured by EchoMRI. *P < .05; **P < .01
Because glucose homeostasis can be influenced by BW, with impaired glucose regulation often observed in heavier or obese animals, ip GTTs were performed in adult BAT cKOs. BAT cKO females showed slightly improved glucose tolerance compared to control females, as evidenced by significantly lower blood glucose levels at 30 minutes after glucose injection (P < .01, Figure 7A) as well as significantly lower AUC for the 2-h testing period (P < .05, Figure 7A). Matching their more moderate BW phenotype compared to the females, BAT cKO males displayed no significant difference in glucose tolerance compared to control males (Figure 7B).
FIGURE 7.
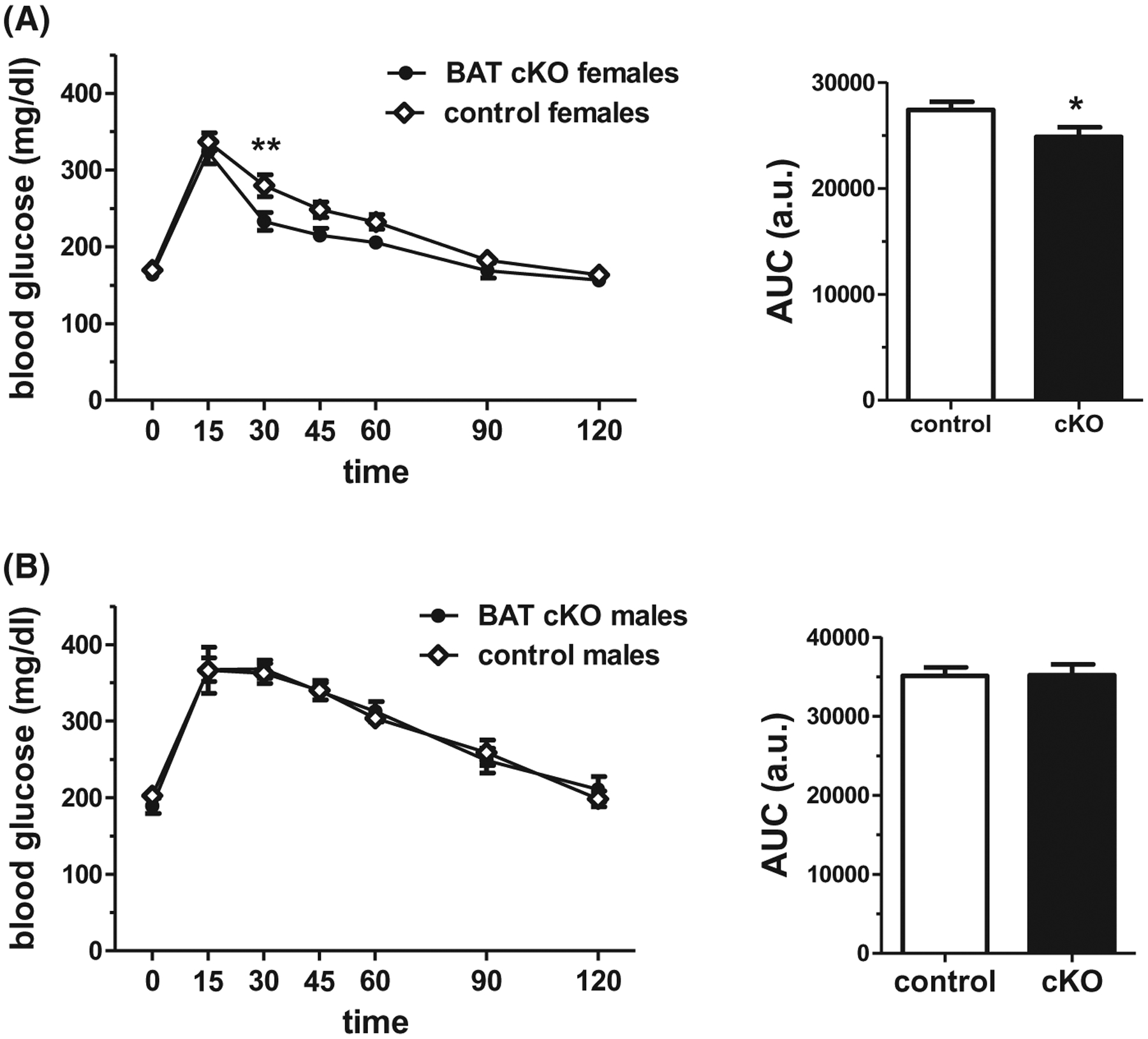
A, BAT cKO females show moderately improved glucose tolerance in an ip glucose tolerance test (GTT) (n = 14/group). B, Male BAT cKO mice show normal GTT responses compared to control males (n = 6–10/group). *P < .05, **P < .01
3.7 |. Metabolic rates and energy expenditure are increased in BAT-specific Kiss1r KO females
Because female BAT cKOs displayed a more pronounced BW, adiposity, and GTT phenotype than males, we next used CLAMS metabolic cages to evaluate if BAT cKO females have increased metabolic rates that might explain their lower BWs. Although BAT cKO females ate the same amount of food as control littermates (data not shown), their metabolic rates were significantly elevated. Specifically, BAT cKO females consumed significantly more O2 (P < .05, Figure 8A) during the dark phase of the light cycle, and showed a near-significant increase (P = .06) in the light phase (when mice are less active). Likewise, BAT cKO females produced more CO2 relative to control females (P < .05, Figure 8B) and displayed increased energy expenditure during both the light and dark phases (P < .01, Figure 8C). Matching the respiratory rates, locomotor activity was elevated in BAT cKO females in the dark phase (P < .05, Figure 8E), though this did not reach significance in the light phase (P = .09). RER was not statistically different between genotypes during either phase of the light cycle (Figure 8D).
FIGURE 8.
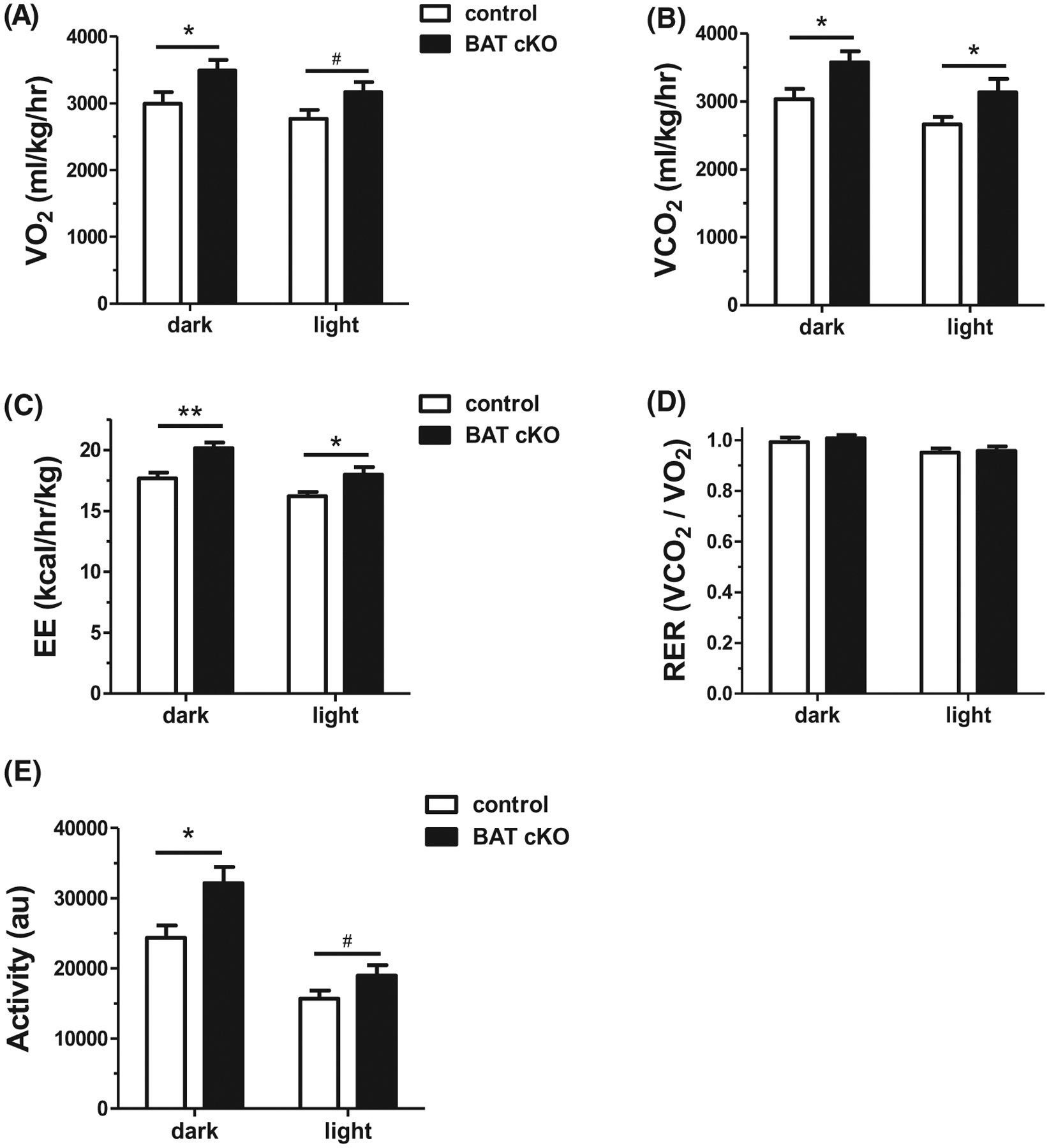
CLAMS assessment of metabolism and energy expenditure in adult females demonstrates that BAT cKO mice have A, increased mean oxygen consumption (VO2) during the dark phase (light phase showed non-significant trend to also be elevated), and increased mean B, carbon dioxide production (VCO2), and C, energy expenditure (heat divided by kg lean mass) during both the dark and light phases. D, Mean RER did not differ between genotypes, whereas E, mean activity levels were significantly elevated in BAT cKOs during the dark phase. n = 14–15/group. *P < .05; **P < .01, #P = .06
3.8 |. Body Temperature and BAT gene expression are elevated in BAT-specific Kiss1r KO mice
Core body temperature was measured in adult BAT cKO females. Matching their increased metabolic rates and energy expenditure, core body temperature was significantly elevated in BAT cKO females compared to control females (Figure 9A). We also determined whether thermogenic gene expression is altered in the BAT of BAT cKOs. While Ucp-1 levels were similar between genotypes (Figure 9B), Cox8b expression levels in BAT were significantly elevated, by nearly 100%, in BAT cKO females (P < .05, Figure 9C), correlating with their elevated body temperature and metabolic rates. BAT weight was not significantly different between genotypes (Figure 9D); however, eWAT weight was trending lower in BAT cKO females versus WT females (P = .06, Figure 9E), matching their lower total adiposity as measured by EchoMRI (Figure 6C).
FIGURE 9.
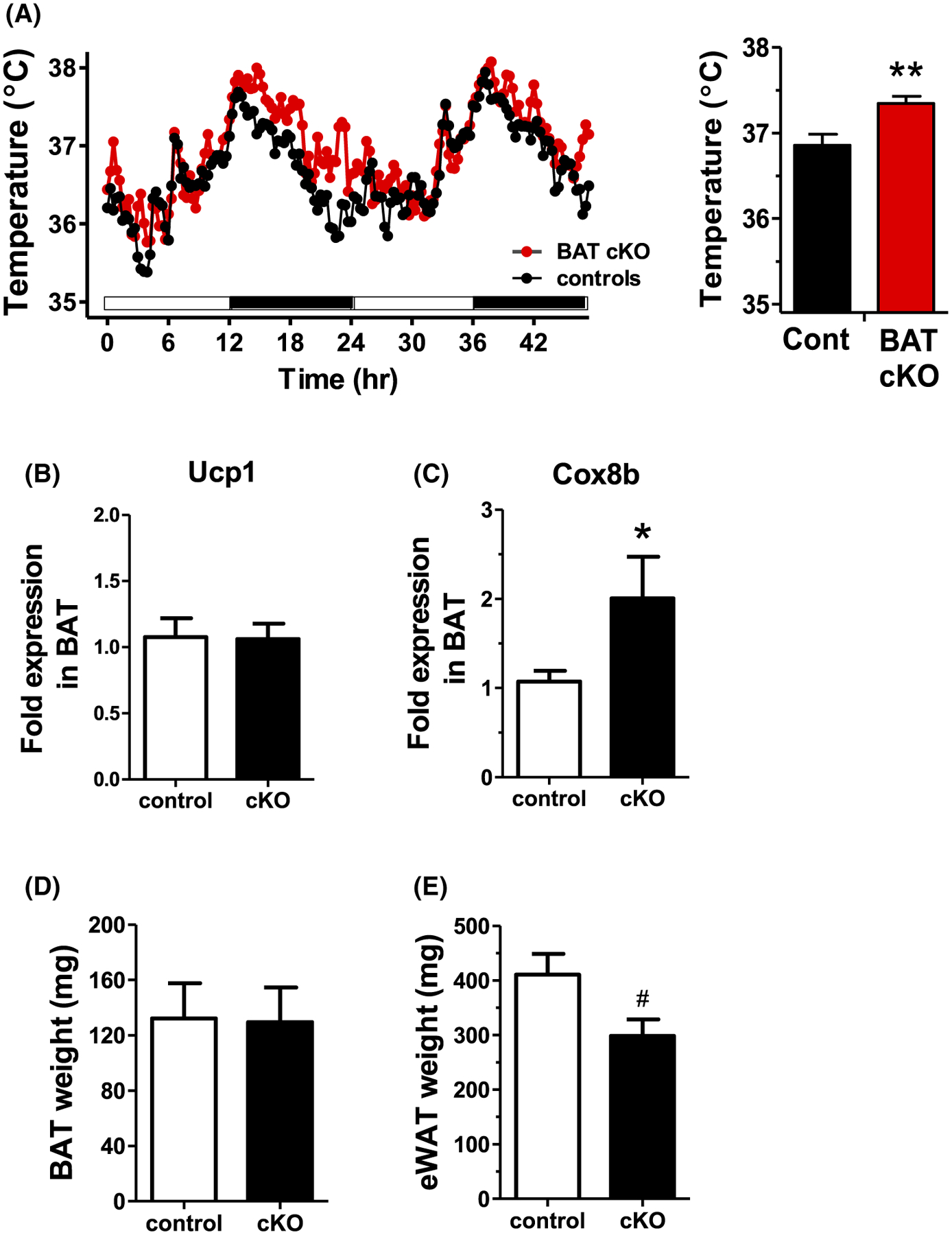
A, Core body temperature of adult BAT cKO females is significantly higher than control female littermates, especially during the dark phase when mice are most active (n = 9–12/group). B, C, BAT gene expression, in particular Cox8b, in BAT cKO females is increased versus controls (n = 8–10/group). D, E, Mean BAT and eWAT weights in adult BAT cKO females versus WT control littermates (n = 5–6/group). *P < .05, **P < .01, #P = .06
4 |. DISCUSSION
Kisspeptin controls reproductive status via its neural actions, stimulating GnRH neurons, which express Kiss1r. However, recent evidence from our group and others demonstrate that kisspeptin signaling is also important for non-reproductive processes, including metabolism and energy balance. We recently reported that global Kiss1r KO females develop obesity, glucose intolerance, and impaired metabolic rates in adulthood which are not due their absent estrogens.15–17 Here we further demonstrate that global Kiss1r KOs have lower body temperature and decreased BAT thermogenic gene expression, correlating with their lower metabolic rates. However, because Kiss1r is expressed in several peripheral tissues, the specific tissue(s) responsible for the observed obese/metabolic phenotype in the global KOs remains undetermined. Here, we demonstrated that the obese phenotype of the global KOs is likely not due to absent kisspeptin signaling in BAT, as BAT-specific Kiss1r KO mice do not become obese or show reduced metabolism. Rather, BAT-specific ablation of kisspeptin signaling increased body temperature, metabolic rates, and energy expenditure, correlating with decreased BWs and lowered adiposity in adulthood. Thus, endogenous kisspeptin signaling in BAT cells may be a previously unrecognized negative regulator of temperature, energy expenditure, and metabolism.
Given the reported obesity and reduced metabolism of global Kiss1r KOs, we analyzed thermogenic gene expression in BAT and found that levels of Ucp1 and Cox8b, two important thermogenic-related BAT genes,20–23 are strongly reduced in Kiss1r KO mice. Moreover, body temperature, which is influenced by UCP1 action and BAT physiology, was significantly lower in global Kiss1r KO females than control females. Additionally, the ability to defend core temperature in global Kiss1r KO females was compromised during a 6-h cold challenge, also indicative of deficient UCP1/BAT thermogenic physiology. These novel thermogenic phenotypes may relate to the reduced metabolism and energy expenditure previously observed in these global Kiss1r KOs, and suggest that endogenous kisspeptin acting somewhere in the body, directly or indirectly, influences thermoregulation and BAT physiology. We also measured body temperature and BAT Ucp1 expression in global Kiss1r KO males, which have normal BWs and do not become overweight in adulthood.16 For both measures, unlike global Kiss1r KO females, global Kiss1r KO males exhibit normal body temperature and Ucp1 levels, matching their previously reported normal BW. We recently compared levels of both Kiss1 and Kiss1r in several peripheral tissues, including BAT, WAT, and liver, of normal mice and found no notable sex differences.24 Thus, at present, the reason for the sex difference in BW, metabolic rates, and body temperature in the global Kiss1r KOs is unknown.
The exact tissues and cell types where kisspeptin signaling influences metabolic and thermogenic parameters remains unknown. Recent data indicate that primary neural energy balance populations, like hypothalamic neuropeptide Y (NPY) and pro-opiomelanocortin (POMC), are relatively normal in global Kiss1r KO females.17 Thus, the underlying mechanism(s) causing obesity and reduced metabolism in those KO mice may instead be occurring outside the brain in one or more peripheral systems. Based on the present findings of altered body temperature and BAT gene expression in the global Kiss1r KOs, along with Kiss1r expression in BAT of normal mice, we hypothesized that absent Kiss1r signaling specifically in BAT may be responsible, in part, for some of the metabolic impairments in the global Kiss1r KOs, perhaps via diminished thermogenesis. To test this possibility, we used Cre/lox technology to conditionally delete Kiss1r from just BAT while maintaining intact Kiss1r elsewhere in the body. In our new BAT cKOs, we confirmed successful recombination of the Kiss1r flox allele in BAT but not other metabolic tissues, such as WAT and liver, or in gonads or pituitary. A very faint level of recombination was detected in the brain. To evaluate whether this was occurring in brain locations that normally express Kiss1r, we also generated Ucp1-Cre mice with a fluorescent tdTomato reporter. tdTomato expression, indicative of Ucp1 Cre-mediated recombination, was highly present throughout BAT, as predicted. In the brain, occasional tdTomato expression was only found in the paraventricular thalamus, cortical amygdaloid nucleus, and ventromedial hypothalamus, and to a lesser extent, the cortex and dorsal lateral septum, which are all brain areas in the mouse that do not express Kiss1r.7 A few previous reports have similarly reported low Ucp1 expression in mouse cortex,25,26 though another study failed to find Ucp1 mRNA or UCP1 protein in the murine brain,27 perhaps because endogenous levels are only in a few neurons at low levels. Regardless, the non-overlapping anatomical patterns of brain Ucp1 and Kiss1r expression in mice indicates that endogenous Kiss1r signaling within the brain is not hindered in our BAT cKOs. To confirm that our BAT cKO model was not altering GnRH function or the reproductive axis, we assessed if there were any reproductive impairments in BAT cKOs. Unlike global Kiss1r KOs, which are hypogonadal and infertile, BAT cKO females and males had normal gonad size and AGD, and the females were completely fertile. Moreover, tdtomato reporter expression of Ucp1 was not present in brain areas containing GnRH neurons. Collectively, these pieces of evidence indicate that Kiss1r signaling in GnRH neurons or other reproductive cell types was not disrupted in the BAT cKOs, as expected if Kiss1r is only functionally deleted from BAT cells.
Our current finding that both body temperature and BAT thermogenic genes were lower in global Kiss1r KOs raised the possibility that endogenous kisspeptin signaling in BAT may alter overall metabolism, energy expenditure, thermo-genesis, or energy balance. To test this, we studied multiple metabolic and energy balance parameters in the BAT cKOs. To our surprise, BAT cKO females had significantly lower BWs in adulthood compared to controls, along with notably reduced fat mass as measured by EchoMRI. BAT cKO males showed a similar, though less robust, pattern of decreased BW in adulthood, which started at a later age than in BAT cKO females; the underlying reasons for the stronger phenotype in the females is not currently known.
We used CLAMS cages to determine whether the reduced adiposity of BAT cKO females is related to altered feeding or metabolism. Opposite to global Kiss1r KOs, which have reduced metabolism, BAT cKOs demonstrated significantly higher O2 and CO2 respiratory rates. Matching this finding, BAT cKO females also had higher energy expenditure than controls, along with a significantly increased core body temperature. Daily food intake was not different between genotypes, suggesting that the observed increases in energy expenditure and metabolism, rather than decreased feeding, contribute to the lower adiposity and BWs. Analysis of BAT gene expression in BAT cKOs found no alteration in Ucp-1 mRNA levels but detected a significant increase in Cox8b levels, which might contribute to the higher body temperature. Whether additional thermogenic or metabolic genes in BAT are similarly altered remains to be determined in future studies. It is also possible that absent kisspeptin signaling alters the functions or actions of these key BAT proteins, in addition to affecting gene expression, but future studies are needed to assess this. Collectively, the present findings suggest that endogenous kisspeptin acting directly in BAT cells normally serves to decrease BAT gene expression and body temperature. Although Cox8b seems to be one affected gene in BAT, the specific cellular signaling pathways underlying this newly identified BAT function of kisspeptin remain to be studied. It also remains to be determined where the kisspeptin signal is coming from. Kisspeptin is made in several peripheral tissues, including liver, pancreas, WAT, gonads, and kidney (but not BAT). Thus, the source of the endogenous kisspeptin for BAT Kiss1r action is likely circulating from another peripheral tissue, such as WAT, liver, and/or pancreas.
Our data clearly demonstrate that peripheral kisspeptin signaling in BAT does not contribute to the obese phenotype seen in global Kiss1r KO mice. On the contrary, BAT cKO females and males display significant weight loss, reduced fat mass, and increased energy expenditure and body temperature. These findings indicate that1 the previously observed obesity and metabolic dysfunction in global Kiss1r KOs are due to impaired kisspeptin signaling in another tissue(s) besides BAT, such as pancreas, white adipose, adrenal, and/or brain. In this case, it is possible that another Kiss1r-expressing tissue, like WAT, is the main driver of the global KO phenotype, but this masks the less robust—and opposing—effects owing to Kiss1r signaling in BAT; and2 endogenous kisspeptin signaling in BAT in “normal” animals may serve to lower body temperature, energy expenditure, and metabolic rates, thereby indirectly modulating BW. While surprising that the global and conditional BAT KOs display opposite metabolic phenotypes, this pattern of results is not unprecedented; a previous study found that deletion of Kiss1r from just the pancreas improved glucose metabolism, whereas global Kiss1r deletion impairs glucose tolerance.13,16 In fact, it is possible that the specific metabolic actions of kisspeptin in BAT may partially counter the greater summated metabolic actions of kisspeptin elsewhere in multiple other tissues. If so, then restoring Kiss1r expression solely in BAT in global Kiss1r KOs might be expected to exacerbate their obese phenotype.
In summary, we demonstrate for the first time that whole-body deletion of Kiss1r from all tissues/cells results in lower body temperature and decreased BAT gene expression, correlating with increased adiposity and BW. In contrast, selective deletion of kisspeptin signaling in just BAT (Ucp1-Cre+/Kiss1rfl/fl; aka BAT cKO) lowers BW and fat mass, and increases body temperature, energy expenditure, and metabolism. These findings reveal a novel, previously unidentified role for endogenous kisspeptin signaling in BAT relating to metabolic and thermogenic physiology. This highlights the complexity and multi-faceted nature of the kisspeptin system throughout the body and emphasizes the need for more tissue-or cell-specific assessments to compare with global whole-body manipulations. Future studies are needed to determine the underlying mechanisms and sites of kisspeptin action that lead to obesity and metabolic dysfunction in global KOs, as well as to further define the specific thermogenic actions of endogenous kisspeptin signaling in BAT cells.
ACKNOWLEDGEMENTS
The authors thank Laurie Nguyen, Chanond Nasamran, Frank Lee, Adriana Esparza, and Melvin Rouse for technical assistance.
Funding information
NIH, Grant/Award Number: R01 HD082567, R01 HD090161, R01 HD068777, R01 DK108773, P30 DK063491, U01 HD066432, K99 HD092894, P50 HD012303, F32 HD076606 and T32 HD007203; American Heart Association, Grant/Award Number: 14SDG19880020
Abbreviations:
- AGD
anogenital distance
- BAT
brown adipose tissue
- BW
body weight
- E2
estradiol
- GnRH
gonadotropin-releasing hormone
- GTT
glucose tolerance test
- OVX
ovariectomized
- WAT
white adipose tissue
Footnotes
CONFLICT OF INTEREST
The authors have nothing to disclose.
REFERENCES
- 1.Kauffman AS. Coming of age in the kisspeptin era: sex differences, development, and puberty. Mol Cell Endocrinol. 2010;324:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colledge WH. Transgenic mouse models to study Gpr54/kisspeptin physiology. Peptides. 2009;30:34–41. [DOI] [PubMed] [Google Scholar]
- 4.Herbison AE. The gonadotropin-releasing hormone pulse generator. Endocrinology. 2018;159:3723–3736. [DOI] [PubMed] [Google Scholar]
- 5.Herbison AE. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat Rev Endocrinol. 2016; 12:452–466. [DOI] [PubMed] [Google Scholar]
- 6.Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbison AE, de Tassigny X, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010; 151:312–321. [DOI] [PubMed] [Google Scholar]
- 8.Koemeter-Cox AI, Sherwood TW, Green JA, et al. Primary cilia enhance kisspeptin receptor signaling on gonadotropin-releasing hormone neurons. Proc Natl Acad Sci USA. 2014;111:10335–10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. [DOI] [PubMed] [Google Scholar]
- 10.Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. [DOI] [PubMed] [Google Scholar]
- 11.Pruszynska-Oszmalek E, Kolodziejski PA, Sassek M, Sliwowska JH. Kisspeptin-10 inhibits proliferation and regulates lipolysis and lipogenesis processes in 3T3-L1 cells and isolated rat adipocytes. Endocrine. 2017;56:54–64. [DOI] [PubMed] [Google Scholar]
- 12.Dudek M, Kolodziejski PA, Pruszynska-Oszmalek E, et al. Effects of high-fat diet-induced obesity and diabetes on Kiss1 and GPR54 expression in the hypothalamic-pituitary-gonadal (HPG) axis and peripheral organs (fat, pancreas and liver) in male rats. Neuropeptides. 2016;56:41–49. [DOI] [PubMed] [Google Scholar]
- 13.Song WJ, Mondal P, Wolfe A, et al. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab. 2014;19:667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muir AI, Chamberlain L, Elshourbagy NA, et al. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. [DOI] [PubMed] [Google Scholar]
- 15.Tolson KP, Garcia C, Delgado I, Marooki N, Kauffman AS. Metabolism and energy expenditure, but not feeding or glucose tolerance, are impaired in young Kiss1r KO female mice. Endocrinology. 2016;157:4192–4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolson KP, Garcia C, Yen S, et al. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J Clin Invest. 2014;124:3075–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Bond JP, Tolson KP, Nasamran C, Kauffman AS, Smith JT. Unaltered hypothalamic metabolic gene expression in Kiss1r knockout mice despite obesity and reduced energy expenditure. J Neuroendocrinol. 2016;28:1–10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novaira HJ, Sonko ML, Hoffman G, et al. Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol Endocrinol. 2014;28:225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva JE, Rabelo R. Regulation of the uncoupling protein gene expression. Eur J Endocrinol. 1997;136:251–264. [DOI] [PubMed] [Google Scholar]
- 21.Seale P Transcriptional control of brown adipocyte development and thermogenesis. Int J Obesity. 2010;34(Suppl 1):S17–S22. [DOI] [PubMed] [Google Scholar]
- 22.Seale P, Kajimura S, Yang W, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Argyropoulos G, Harper ME. Uncoupling proteins and thermoregulation. J Appl Physiol. 2002;92:2187–2198. [DOI] [PubMed] [Google Scholar]
- 24.Tolson KP, Marooki N, Wolfe A, Smith JT, Kauffman AS. Cre/lox generation of a novel whole-body Kiss1r KO mouse line recapitulates a hypogonadal, obese, and metabolically-impaired phenotype. Mol Cell Endocrinol. 2019;498:110559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehler-Wex C, Grunblatt E, Zeiske S, et al. Microarray analysis reveals distinct gene expression patterns in the mouse cortex following chronic neuroleptic and stimulant treatment: implications for body weight changes. J Neural Transm. 2006;113:1383–1393. [DOI] [PubMed] [Google Scholar]
- 26.Lengacher S, Magistretti PJ, Pellerin L. Quantitative rt-PCR analysis of uncoupling protein isoforms in mouse brain cortex: methodological optimization and comparison of expression with brown adipose tissue and skeletal muscle. J Cerebral Blood Flow Metab: Official J Int Soc Cerebral Blood Flow Metab. 2004;24: 780–788. [DOI] [PubMed] [Google Scholar]
- 27.Laursen WJ, Mastrotto M, Pesta D, et al. Neuronal UCP1 expression suggests a mechanism for local thermogenesis during hibernation. Proc Natl Acad Sci USA. 2015;112:1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]


