Abstract
During a 2-year period, 157 consecutive episodes of respiratory virus infections that occurred in 130 patients with upper or lower respiratory tract infection were analyzed for respiratory viruses. A respiratory virus was identified in 75 episodes (48%), and several viruses were found in 13 episodes: there were a total of 56 influenza A virus infections, 14 respiratory syncytial virus infections, 8 adenovirus infections, 8 infections with parainfluenza virus types 1 or 3, and 7 enterovirus infections. On multivariate analysis, the only variable that predicted progression to pneumonia in patients with an upper respiratory tract infection was the presence of respiratory syncytial virus, whereas lymphocytopenia had a nonsignificant trend. Also, among the 38 patients who had pneumonia at any time during the episode, both respiratory syncytial virus and lymphocytopenia were commonly found. For both epidemiological and therapeutic considerations, frequent screening for respiratory viruses should be incorporated into the routine diagnostic study of patients with hematologic malignancies.
Respiratory viruses have been recognized as potential causes of severe pneumonia in patients with hematologic malignancies [1,2,3,4,5,6–7]. In this patient population, these viruses cause upper respiratory tract infections (URTIs), as in the general population; however, in patients with hematologic malignancies, these infections appear to have a higher tendency to progress to severe lower respiratory tract infections (LRTIs). Respiratory syncytial virus (RSV), influenza A and B viruses, and parainfluenza viruses have been well described as causes of severe respiratory morbidity and mortality, especially among recipients of hematopoietic stem cell transplants (HSCTs) [1,2,3,4,5,6–7]. Adenoviruses cause both isolated respiratory infections and disseminated visceral syndromes [8].
Despite an increasing number of reports, there have been few prospective studies of respiratory virus infections in adults with hematologic malignancies. We performed a prospective 2-year study to assess the role of respiratory virus infections in the pathogenesis of URTI and LRTI and risk factors for severe LRTI in an adult clinical hematology department.
Patients and Methods
This prospective study was performed at the Division of Clinical Hematology of the Hospital de la Santa Creu i Sant Pau (Barcelona, Spain) from 1 October 1999 through 31 May 2001. All adult patients with a hematologic malignancy (including HSCT recipients) who had signs and symptoms of a URTI or LRTI in the inpatient and outpatient settings underwent a detailed clinical evaluation, and samples from the upper and/or lower respiratory tracts were screened for the presence of respiratory viruses. Patients with symptoms of URTI underwent nasopharyngeal aspiration, whereas patients with LRTI (i.e., pneumonia) underwent bronchoalveolar lavage (BAL), when clinically possible. Patients with pneumonia but no signs of URTI did not undergo nasopharyngeal aspiration. Patients (both outpatients and inpatients) whose nasopharyngeal aspirate (NPAs) specimens tested positive for respiratory viruses were asked to return to the clinic (or undergo an inpatient sampling) at least weekly for further clinical assessment and microbiological analysis until all symptoms resolved and the virus was not detected in a NPA.
Microbiological methods. All samples were processed for respiratory viruses by antigen detection with use of direct immunofluorescence and culture. For direct immunofluorescence, the specimens were spotted onto glass slides and processed by use of standard techniques [9]. The presence of viral antigen in respiratory cells was indicated by the appearance of characteristic intracellular apple-green fluorescence in ⩾1 cell. We used labeled antibodies to influenza A and B viruses; parainfluenza viruses 1, 2, and 3; RSV; and adenoviruses. If the samples did not contain respiratory epithelial cells, the finding was considered to be inconclusive. For viral culture, specimens were inoculated into each of 4 cell lines: human fibroblasts (MRC5), human epithelial cells (Hep-2 and A-549), and Madin-Darby canine kidney cells. Cultures were incubated for 2 weeks (4 weeks for detection of cytomegalovirus) on a roller drum at 35°C. Viruses were identified on the basis of cytopathic effect in cell cultures and confirmed by staining with fluorescein-conjugated monoclonal antibodies [10]. BAL samples were also processed for routine aerobic, anaerobic, mycobacterial, and fungal culture and for parasite examination.
Definitions. A first episode of infection was defined as the period during which the patient had symptoms of URTI and/or LRTI, regardless of whether a respiratory virus was isolated. A further episode(s) of infection required the presence of a symptom-free period and negative results of testing of the NPA for any respiratory viruses isolated during the previous episode. A polymicrobial infection was defined as the isolation of >1 respiratory virus during the same episode or another clinically relevant pathogen from a BAL fluid specimen or a lung sample. A diagnosis of nosocomial respiratory virus infection was considered if a patient had been in the hospital for ⩾3 days and if symptoms of the infection developed during hospitalization. A URTI was defined by the new onset of nasal, pharyngeal, or laryngeal irritation, and an LRTI (or pneumonia) was defined by the development of a new pulmonary infiltrate in patients with signs and symptoms of LRTI (cough, rales, wheezing). Death due to pneumonia was defined as death due to respiratory failure during the episode of respiratory virus infection. Progression of a URTI to an LRTI was defined as onset of pneumonia in patients with a prior or concurrent URTI, whereas patients who had pneumonia without a URTI were considered to have an isolated LRTI.
Treatment of respiratory virus infections. The recommended therapy for LRTI due to RSV was inhaled ribavirin (6 g per day for 5 days); for URTI and LRTI due to influenza A virus, the recommended therapy was orally administered amantadine (100 mg t.i.d. for 7 days). However, physicians were free to decide whether to treat patients who were ambulatory and had an isolated URTI, according to their own clinical judgement. For inpatients, strict isolation was done in all cases to avoid spread of the virus.
Statistical analysis. Univariate analyses of the risk factors for development of an LRTI were done with use of the χ2 test or Fisher's exact test, for discontinuous variables, and Student's t test or the Mann-Whitney U test, for continuous variables. Multivariate analysis of variables predictive of development of an LRTI was done by logistic regression. Age and neutrophil and lymphocyte counts were included as continuous variables and as binary variables (more than or less than a fixed value). The other factors that were analyzed were sex, underlying disease, disease status (early vs. advanced), type of therapy (receipt of an allogeneic HSCT vs. other therapies), nosocomial infection (yes vs. no), use of corticosteroids (defined as ⩾1 mg/kg of prednisone per day or equivalent for >1 week; yes vs. no), and virus isolated (influenza virus vs. other/none and RSV vs. other/none). Tests of significance were 2-sided, and P <.05 was considered to be statistically significant.
Results
During the study period, 157 episodes of infection were studied in 130 patients (17 patients had >1 independent episode). Patient characteristics are shown in table 1. The study period was divided into 3 periods: first winter, summer, and second winter. As expected, most episodes were identified during winter months. Ninety-seven patients (62%) had received autologous or allogeneic HSCTs, whereas most other patients were treated with intensive chemotherapy for leukemia, lymphoma, or myeloma. At the onset of infection, 23 patients (15%) had neutropenia, and 70 (45%) had lymphocyte counts of <109 lymphocytes/L.
Table 1.
Characteristics of patients in study of respiratory virus infections in adults with hematologic malignancies.
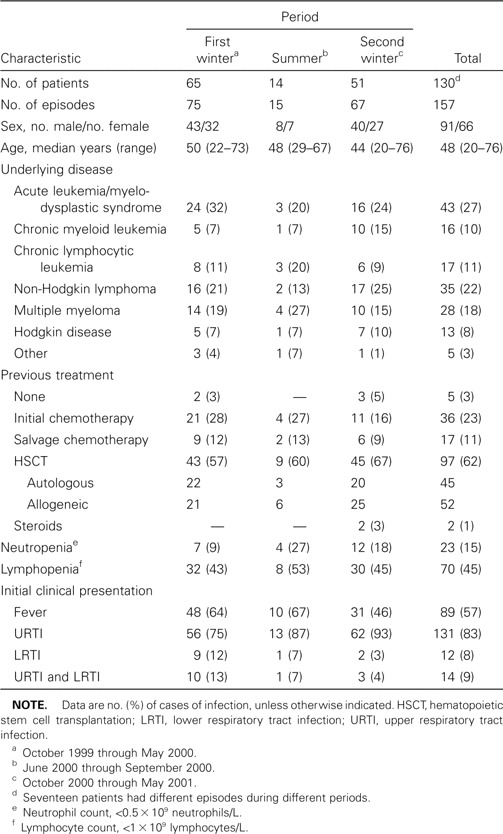
Eighty-nine patients (57%) had fever at the onset of the infection. Most patients (131 [83%]) had only symptoms of URTI at inclusion in the study, 14 (9%) had both URTI and LRTI, and 12 (8%) had only pneumonia without clear symptoms of a URTI. Thus, 145 patients (92%) had symptoms of URTI at inclusion in the study.
Initial findings of microbiological studies. The initial samples analyzed were NPAs in 146 cases, a BAL sample in 9 cases, and both NPA and BAL fluid samples in 2 cases. Table 2 details the results of the initial sample analysis. Overall, a respiratory virus was identified in 75 episodes (48%) for the initial samples, whereas 75 samples tested negative and 7 NPA samples had insufficient cells and were considered to be unevaluable. The most frequently identified virus was influenza A virus, which was found in 51 cases. The isolation of this virus was strongly associated with its epidemic presence in the community. As seen in figure 1, 29 (52%) of 56 cases of influenza A virus infection occurred during January and February 2000, in conjunction with a large epidemic of infection in the community. The detailed clinical characteristics of the 75 episodes for which a respiratory virus was isolated from the initial sample are shown in table 3. The only apparent difference between these virus groups was the probability of dying of pneumonia, which was 10% among persons with influenza A virus infection, 20% among those with parainfluenza virus 1 or 3 infection, and 0% among those infected with other viruses.
Table 2.
Findings of viral studies of the first samples analyzed in a study of respiratory virus infections in adults with hematologic malignancies.
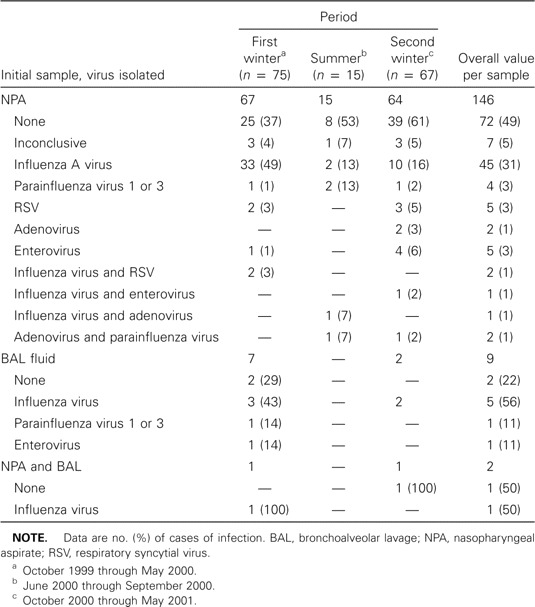
Figure 1.
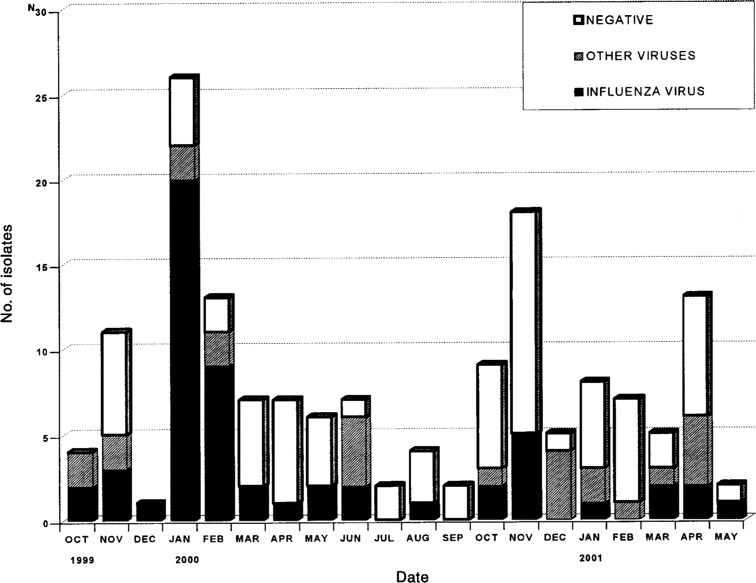
Rates of isolation of influenza viruses and other respiratory viruses in 157 episodes studied during October 1999 through May 2001
Table 3.
Characteristics of 75 episodes of respiratory virus infections, according to initial virus isolated.
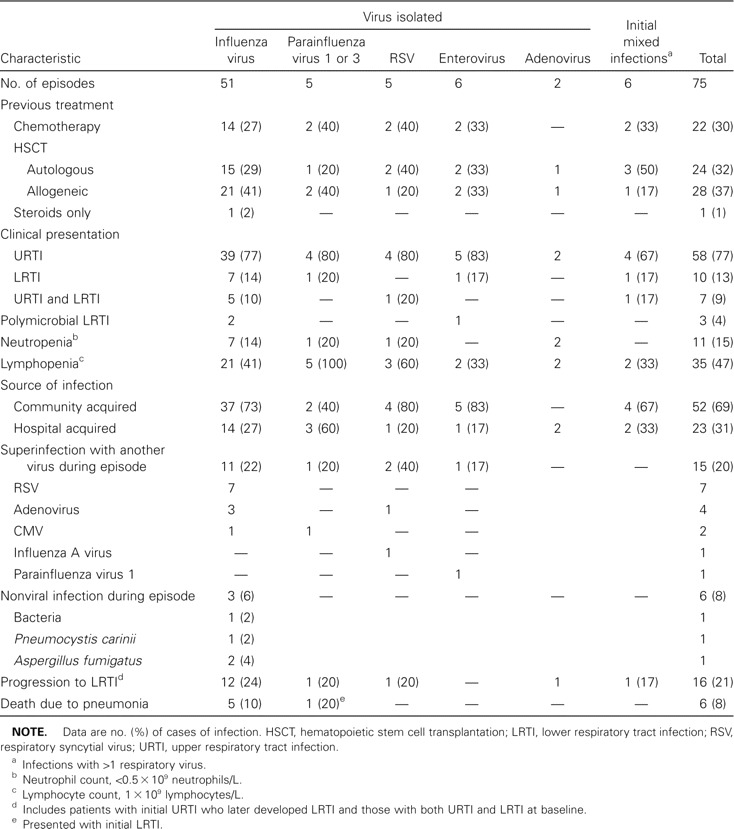
Findings of follow-up microbiological studies. Other viral and nonviral pathogens were isolated from the lower respiratory tract or a follow-up NPA sample for several patients (table 3, figure 2). The same virus was isolated again from 16 (30%) of 54 for a median of 2 weeks. Patients who were treated with amantadine for influenza A virus infection and who provided follow-up samples (n = 24) shed the virus longer (median, 3 weeks; range, 1–9 weeks) than did those who were not treated and who had follow-up samples (n = 18; median, 1 week; range, 1–4 weeks; P =.03). However, it should be emphasized that this was a prospective observational study and not a controlled treatment study, and, thus, it is probable that physicians were more prone to subjectively treat patients who were more ill or at higher risk.
Figure 2.
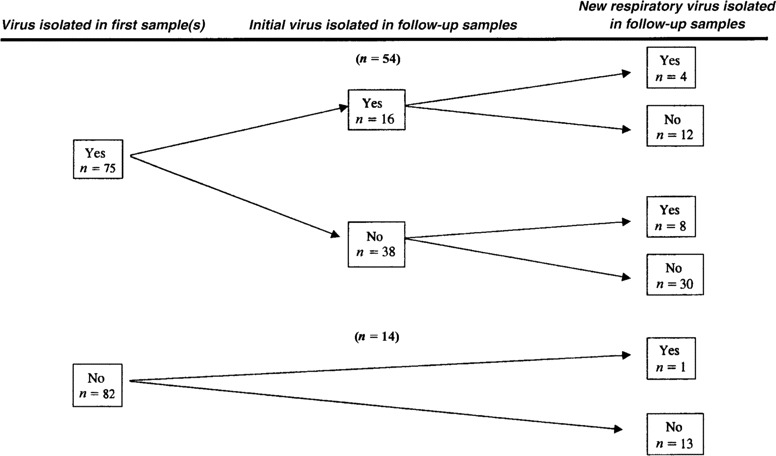
Results of follow-up virological studies for 157 episodes of symptomatic lower or upper respiratory tract infection
A new respiratory virus was isolated during the same clinical episode in 13 cases; these include 7 RSVs and 4 adenoviruses (table 3, figure 2). Thus, the total number of infections with the different respiratory viruses during the 157 episodes studied (including initial polymicrobial infections and later superinfections) was as follows: influenza A virus, 56 infections; RSV, 14 infections; adenoviruses, 8 infections; parainfluenza virus type 1, 4 infections; parainfluenza virus type 3, 4 infections; and enteroviruses, 7 infections.
Other pathogens. Clinically relevant pathogens were isolated from BAL samples obtained from 5 patients with LRTI: cytomegalovirus isolates were recovered from 2 patients, and isolates of Pseudomonas aeruginosa, Pneumocystis carinii, and Aspergillus fumigatus were recovered from 1 patient each. Two of these patients died of pneumonia.
Outcome of LRTI. Of the 157 patients, 26 (17%) had LRTI at the time of inclusion in the study (12 had only LRTI and 14 had both URTI and LRTI). Twelve additional patients who initially had only a URTI later developed LRTI (9% of the 131 cases of URTI). Thus, overall, 38 patients (24%) developed LRTI during the study period. As specified above, 5 of these patients had a copathogen identified, 21 had only a respiratory virus identified as the possible etiologic agent, and there was no etiologic diagnosis for 12. Seven (27%) of 26 patients with LRTI in whom a respiratory virus was found died of pneumonia, as opposed to 2 (17%) of 12 for whom no respiratory virus was identified (P =.1).
Risk factors for progression to LRTI. Twenty-six (18%) of the 145 patients who presented with symptoms of a URTI either had pneumonia or had progression to pneumonia during the follow-up period. Age, sex, underlying disease, receipt of an allogeneic HSCT, neutropenia, use of corticosteroids, and infection with influenza virus were not predictive of progression to LRTI. Inpatients had a higher risk of having progression to pneumonia on univariate analysis (P =.04). The presence of lymphocytopenia (<109 lymphocytes/L) was of borderline significance on univariate (P =.06) and multivariate (P =.1) analyses (15 [25%] of 61 patients with vs. 11 [13%] of 84 patients without lymphocytopenia). RSV infection was the only variable that predicted the progression to pneumonia on univariate (P =.01) and multivariate (P =.02) analyses (6 [46%] of 13 patients with vs. 20 [15%] of 132 patients without RSV infection). Subanalyses of allogeneic HSCT recipients and of patients with any respiratory viruses isolated during the episode revealed no significant variables, but the same trend was observed for RSV infection (data not shown). Among the 97 episodes in HSCT recipients, we found no correlation between the time after transplantation (analyzed a continuous variable) and the risk of progression to pneumonia.
Univariate and multivariate analyses were performed for all 157 episodes to analyze the characteristics of patients who had pneumonia any time during the study (n = 38). Two variables were associated with pneumonia on univariate and multivariate analyses: lymphocytopenia (<109 lymphocytes/L; P =.05 on multivariate analysis) and RSV infection (P =.02). Lymphocytopenia was present in 24 (63%) of 38 episodes in patients with pneumonia and in 47 (39%) of 119 episodes in patients without pneumonia, whereas RSV infection was present in 8 episodes (21%) in patients with pneumonia and 7 (6%) in patients without pneumonia.
Discussion
We identified a respiratory virus in 48% of adult patients who had a hematologic malignancy present and signs and symptoms of URTI. Influenza A virus was by far the most common virus found (56 episodes), followed by RSV and parainfluenza virus. These respiratory viruses are the most commonly found viruses in studies that involve HSCT recipients [1,2,3,4,5,6–7], although their relative proportions vary between studies, probably because of the epidemic situation in the population during the study period. A large epidemic of influenza A virus infection occurred during our study period, and the isolates recovered from patients closely reflected the respiratory viruses isolated in the community, as shown in figure 1. The 7 enteroviruses found in our study confirm that these viruses also cause LRTI and URTI in these immunocompromised hosts [7, 11, 12]. However, we found no cases of infection with rhinoviruses or coronaviruses. The viral culture technique used in the study can detect infections with rhinoviruses but not coronavirus infections [6, 7, 10]. Because rhinoviruses are commonly implicated in the common cold, we have no explanation for not having isolated any such virus in our study.
On the other hand, a significant proportion of our patients (52%) with clear signs of URTI or LRTI had no respiratory viruses identified. Many of these cases may be of noninfectious origin or nonviral origin, or they may have been caused by other undetected viral pathogens, whereas other cases may represent technical flaws in sample processing or lack of sensitivity to detect involved respiratory viruses. In a recent study, PCR-based techniques were found to increase the rate of respiratory virus identification in a similar patient population to 35% (compared with 19% for culture) [12]. Thus, had we used such an approach, we might have found even more cases of respiratory virus infections. With respect to the clinical applicability of the microbiological methods used in our study for clinical decision making, findings for the direct immunofluorescence technique were available within 6–12 h after the sample arrived in the laboratory, but viral cultures usually took 7–10 days for results.
An interesting finding in our study was the isolation of >1 respiratory virus in 19 episodes (12%). Two or more respiratory viruses were found in the initial sample for 6 patients, whereas, in 13 cases, a new respiratory virus was later isolated during the same clinical episode, either in addition to (n = 4) or in the absence of (n = 9) the initial virus. These observations show that a patient can be superinfected with a new respiratory virus, even if they have not recovered from a prior infection.
Among the 145 patients with URTI at the time of inclusion in the study, 26 (18%) developed LRTI at some time during follow-up of the episode. An LRTI occurred in 16 (21%) of 75 episodes in which a respiratory virus was identified and in 10 (14%) of 70 in which no respiratory virus was found, but the presence of any respiratory virus was not found to carry a higher risk of progression to pneumonia on multivariate analysis. Of the specific respiratory viruses found, influenza A virus did not carry a higher risk of progression of infection to LRTI (23%) on univariate and multivariate analyses. RSV infection, however, appeared to have a higher risk of LRTI (46% among RSV-positive patients and 15% among the rest; P =.01). This finding is not surprising, because both RSV and parainfluenza virus have been found to lead to LRTI in 30%–60% of immunosuppressed HSCT recipients with URTI [3,4–5, 13,14–15]. Parainfluenza viruses 1 and 3 have also been described as carrying a high risk of producing severe LRTI [2], but we had too few cases of infections with these viruses for meaningful analysis. Of note, all 4 patients with parainfluenza virus 3 infection developed pneumonia, whereas none of those infected with parainfluenza virus 1 had an LRTI.
We found a trend of lymphocytopenia being a risk factor for progression to LRTI, as was previously found in a European multicenter study [5]. In all 157 episodes, this variable was also more frequently found among patients with pneumonia than among those with an isolated URTI. This finding, however, requires confirmation in other series of patients. Allogeneic HSCT recipients are usually found to be at the highest risk for developing severe respiratory virus infections [1,2,3,4,5,6–7]. However, in our study, which included 97 HSCT recipients (52 of whom received allogeneic transplants), we did not find that receipt of an allogeneic HSCT was a risk factor for progression to pneumonia (20% vs. 17% for progression to LRTI).
In summary, our prospective study of both ambulatory and hospitalized adults who received treatment for a hematologic malignancy and who had signs and symptoms of URTI and/or LRTI shows that a respiratory virus can be implicated in at least one-half the cases. The isolation of RSV and the presence of lymphocytopenia appear to increase the risk of developing pneumonia, the most dreaded complication of these infections. Therefore, we suggest that screening for these viruses should be incorporated into the routine diagnostic study of patients with hematologic malignancies, both for epidemiological reasons (i.e., strict isolation of hospitalized patients with a respiratory virus infection) and for the use of currently available or emergent treatments in the most debilitated hosts.
Footnotes
Financial support: Fondo de Investigaciones Sanitarias (grant FIS 00/0296).
References
- 1.Ljungman P. Respiratory virus infections in bone marrow transplant recipients: the European perspective. Am J Med. 1997;102(Suppl 3A):44–8. doi: 10.1016/s0002-9343(97)00010-7. [DOI] [PubMed] [Google Scholar]
- 2.Nichols WG, Gooley T, Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biol Blood Marrow Transplant. 2001;7(Suppl):11–5. doi: 10.1053/bbmt.2001.v7.pm11777098. [DOI] [PubMed] [Google Scholar]
- 3.Whimbey E, Champlin RE, Couch RB, et al. Community respiratory virus infections among hospitalized adult bone marrow transplant recipients. Clin Infect Dis. 1996;22:778–82. doi: 10.1093/clinids/22.5.778. [DOI] [PubMed] [Google Scholar]
- 4.Whimbey E, Englund JA, Couch RB. Community respiratory virus infections in immunocompromised patients with cancer. Am J Med. 1997;102(Suppl 3A):10–8. doi: 10.1016/S0002-9343(97)80004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljungman P, Ward KN, Crooks BNA, et al. Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2001;28:479–84. doi: 10.1038/sj.bmt.1703139. [DOI] [PubMed] [Google Scholar]
- 6.Rabella N, Rodriguez P, Labeaga R, et al. Conventional respiratory viruses recovered from immunocompromised patients: clinical considerations. Clin Infect Dis. 1999;28:1043–8. doi: 10.1086/514738. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez Y, Martino R, Rabella N, Labeaga R, Badell I, Sierra J. Community respiratory virus infections in patients with hematologic malignancies. Haematologica. 1999;84:820–3. [PubMed] [Google Scholar]
- 8.La Rosa AM, Champlin RE, Mirza N, et al. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis. 2001;32:871–6. doi: 10.1086/319352. [DOI] [PubMed] [Google Scholar]
- 9.Gardner PS, McQuillin J. Rapid virus diagnosis—application of immunofluorescence. 2nd ed. London: Butterworth; 1980. [Google Scholar]
- 10.Gleaves CA, Hodinka RL, Johnston SLG, Swierkosz EM. Laboratory diagnosis of viral infections. In: Baron EJ, editor. Cumulative techniques and procedures in clinical microbiology. New York: Cumitech; 1994. p. 15A. [Google Scholar]
- 11.González Y, Martino R, Badell I, et al. Pulmonary enterovirus infections in stem cell transplant recipients. Bone Marrow Transplant. 1999;23:511–3. doi: 10.1038/sj.bmt.1701605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Elden LJR, Van Kraaij MGJ, Nihuis M, et al. Polymerase chain reaction is more sensitive than viral culture and antigen testing for the detection of respiratory viruses in adults with hematological cancer and pneumonia. Clin Infect Dis. 2002;34:177–83. doi: 10.1086/338238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowden RA. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102(Suppl 3A):27–30. doi: 10.1016/s0002-9343(97)00007-7. [DOI] [PubMed] [Google Scholar]
- 14.Couch RB, Englund JA, Couch RB. Respiratory viral infections in immunocompetent and immunocompromised persons. Am J Med. 1997;102(Suppl 3A):2–9. doi: 10.1016/S0002-9343(97)00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reusser P. Current concepts and challenges in the prevention and treatment of viral infections in immunocompromised cancer patients. Support Care Cancer. 1998;6:39–45. doi: 10.1007/s005200050130. [DOI] [PubMed] [Google Scholar]


