Abstract
BACKGROUND:
The rate of decline in language in Primary Progressive Aphasia (PPA) is highly variable and difficult to predict at baseline. The severity of diffuse white matter disease (leukoaraiosis), a marker of overall brain health, may substantially influence the rate of decline.
AIMS:
To test the hypothesis that leukoaraiosis is associated with a steeper decline in naming in PPA.
METHODS AND PROCEDURES:
In this longitudinal, observational study, 29 individuals with PPA (all variants) were administered the Boston Naming Test (BNT) at baseline and 1 year later. Two raters evaluated leukoaraiosis on baseline MRI, using the Cardiovascular Health Study scale. We evaluated the effects of leukoaraiosis severity, age, education, and baseline BNT on decline measured by change in BNT accuracy with multivariable linear regression. We also evaluated the effects of these variables on the dichotomized outcome of faster decline in BNT (worst 50%) versus slower decline (best 50%) using logistic regression.
RESULTS:
Together, leukoaraiosis, age, education, and baseline BNT score predicted change in BNT score (F(3, 25) = 8.12; p=0.0006). Change in BNT score was predicted by severity of leukoaraiosis (t =−3.81; p=0.001) and education (t= −2.45; p=0.022), independently of the other variables. When we dichotomized outcome into upper 50th percentile versus lower 50th percentile (faster decline), faster decline was predicted by all variables together (chi squared = 13.91; p = 0.008). However, only leukoaraiosis independently predicted outcome (OR=2.80; 95%CI: 1.11 to 7.03). For every 1 point increase on the CHS rating scale, there was 2.8 times higher chance of showing faster decline in naming.
CONCLUSION:
Severity of leukoaraiosis is associated with steeper decline in naming in PPA. This imaging marker can aide in prognosis and planning by caregivers and stratification of participants in clinical trials.
Keywords: primary progressive aphasia, leukoaraiosis, white matter disease, prognosis
Introduction
Primary progressive aphasia is a clinical syndrome caused by neurodegenerative disease, characterized by progressive decline in language out of proportion to other cognitive functions (Mesulam, 1982). The rate of decline is quite variable, ranging from a slow, protracted decline over many years to a rapid decline over months (Machulda et al., 2013; Sebastian et al., 2018). One study identified rapid decliners and slow decliners in all three of the major variants of PPA – logopenic variant (lvPPA), semantic variant PPA (svPPA), and nonfluent agrammatic variant PPA (nfaPPA) (Sebastian et al., 2018). These variants are distinguished by speech and language characteristics, and generally reflect distinct areas of greatest atrophy and different underlying neurodegenerative diseases (Gorno-Tempini et al., 2011). Another study was able to identify subtypes of logopenic variant PPA (lvPPA) based on severity and rate of progression (Machulda et al., 2013). The variability in rate of decline in all variants presents a challenge to clinicians in counseling caregivers and in planning levels of care.
There are a few studies that shed light on the variety of factors that are associated with the rate of decline in PPA. Sebastian and colleagues showed that the highest rates of decline in naming – one of the most common deficits in all variants -- occurred in nfaPPA, followed by svPPA, and then lvPPA (Sebastian et al., 2018). The same study found that female sex, longer symptom duration, higher baseline naming score, and any speech-language rehabilitation were associated with slower decline. Other studies have identified some baseline imaging markers that are associated with faster or slower decline. Faria and colleagues found that increased resting state functional connectivity between frontal, temporal, fusiform, insula, and basal ganglia correlated with slower decline in naming in PPA (Faria, Meyer, Tippett, Friedman, & Hillis, 2017). Olm et al. (2016) showed that reductions in perfusion on Arterial Spin Labelling MRI are associated with faster rate of progression in PPA. However, much of the variability in rate of decline in PPA has yet to be explained.
Diffuse white matter disease, or leukoaraiosis, has been associated with cognitive decline in aging in otherwise healthy older adults (Black, Gao, & Bilbao, 2009; Vermeer et al., 2003) and after stroke (Arsava et al., 2009; Debette et al., 2010; Kissela et al., 2009), although its direct role in cognitive impairment remains controversial. Leukoaraiosis is a non-specific imaging finding that seems to reflect the health of the brain tissue. It is strongly associated with hypertension, diabetes, aging, and hypercholesterolemia (Longstreth et al., 2005; Manolio et al., 1994), as well as atherosclerosis (Smith, 2010). More severe leukoaraiosis is also associated with more severe hemispatial neglect after right hemisphere stroke (Bahrainwala, Hillis, Dearborn, & Gottesman, 2014) and is associated with poorer naming outcome after left hemisphere stroke (Wright et al., 2018). These reported effects of leukoaraiosis on outcome have been observed independently of infarct volume and other demographic variables associated with worse outcome. Therefore, we aimed to test the hypothesis that severity of leukoaraiosis is associated with a steeper rate of decline in naming in PPA. Because leukoariosis is a common cerebral white matter lesion, especially among those over age 65 years, and because in naming difficulty is a core symptom in lvPPA and svPPA (Gorno-Tempini et al, 2011), we studied leukoaraiosis and naming irrespective of variant and independently of other baseline characteristics.
METHODS:
Participants
A series of 29 individuals with PPA were included in the study. PPA and the variant was determined by the senior author (AH) using consensus criteria (Gorno-Tempini, et al., 2011). Participants were part of a larger prospective study of 94 participants (Sebastian et al., 2018); those who did not have baseline T2 sequences or follow-up testing at one year (n=65) were excluded. No participant had previous stroke or TIA. All participants or their care providers (in cases of impaired comprehension) provided informed consent to participate, using consent forms and protocols approved by the Johns Hopkins Institutional Review Board. Participants were diagnosed using neuropsychological testing, history, and MRI to exclude alternative diagnoses. We included 12 participants with logopenic variant PPA, 5 with nonfluent agrammatic variant PPA, 11 with semantic variant PPA, and one who was unclassifiable because he did not have the core features of any of the variants, but had progressive impairment in naming and spelling. The mean age was 66.6 (±6.8) years; the mean education was 16.8 (±2.4) years; 15 (51.7%) were women. Demographics of participants in each variant are provided in Table 1.
Table 1.
Age, Sex, and Education for PPA Variants and for Participants Overall
| Variant | Age (years) Mean (SD) |
Education (years) Mean (SD) |
Sex (F) N (%) |
|---|---|---|---|
| lvPPA (n=12) | 69.2 (6.6) | 17.0 (2.5) | 6 (50) |
| nfaPPA (n=5) | 65.2 (3.8) | 17.0 (2.7) | 3 (60) |
| svPPA (n=11) | 63.8 (7.3) | 16.2 (2.7) | 6 (55) |
| Unclassified (n=1) | 75 | 18 | 0 |
| Overall (n=29) | 66.7 (6.9) | 16.8 (2.5) | 15 (52) |
F, female; SD, standard deviation; yrs, years; mos, months; lvPPA, logopenic primary progressive aphasia; nfaPPA, nonfluent agrammatic primary progressive aphasia; svPPA semantic variant primary progressive aphasia
Language
A battery of language and cognitive tests was administered to make the diagnosis of PPA. Testing was completed based on participant tolerance and included the following: Word Reading Test; Semantic Word-Picture Matching Test (Rogalsky, Love, Driscoll, Anderson, & Hickok, 2011); Semantic Associates Test; JHU Anagram Test; Sentence Repetition Test; Noun and Verb Naming Tests; Sentence Reading Test; Boston Naming Test (BNT) (Kaplan, Goodglass, & Weintraub, 2001; Mack, Freed, Williams, & Henderson, 1992); Hopkins Assessment of Naming Actions (HANA; Breining, Tippett et al., 2015), Pyramids and Palm Trees Test (PPTT; Breining, Lala et al., 2015; Howard & Patterson, 1992); Benson Figure Copy and Recall; Forward and Backward Digit Span; Verbal Fluency Task including both letter (FAS) (Loonstra, Tarlow, & Sellers, 2001) and action (verb) (Woods et al., 2005) word fluency; Spelling to Dictation Test; a Picture Word Verification Test (Caramazza & Hillis, 1990); and Kissing and Dancing Test (Bak & Hodges, 2003). This battery (including unpublished subtests) is an expansion of the National Alzheimer’s Coordinating Center’s Frontotemporal Dementia Battery, from the National Institute on Aging (NIA, a US Government Health Institute). Some patients were also administered the Apraxia Battery for Adults (Dabul, 2000); in others assessment of speech and limb praxis was done as a part of the comprehensive neurological examination that included a motor speech examination. The test that was most commonly administered longitudinally was the 30 item form of the Boston Naming Test (BNT; Kaplan, Goodglass, Weintraub, Segal, & van Loon-Vervoorn, 2001; Mack, Freed, Williams, & Henderson, 1992), and so we chose this test to measure rate of progression. Furthermore, impaired object naming is a core criterion for classification of lvPPA and svPPA (Gorno-Tempini et al., 2011). Although impaired naming is not a core criterion in nfaPPA, this deficit is likely under-recognized in this variant. It is well-documented that impaired action naming frequently precedes impaired object naming in this variant (Bak & Hodges, 2003; Hillis, Tuffiash, & Caramazza, 2002; Hillis, Oh, & Ken, 2004; Silveri & Ciccarelli, 2007; Silveri & Ciccarelli, 2007; Thompson, Lukic, King, Mesulam, & Weintraub, 2012), but see (Cotelli et al., 2006) for divergent findings. Participants in this study were administered the short form of the BNT at baseline and approximately one year later. Rate of decline was measured by change in BNT, after controlling for other variables, including baseline BNT. We scored accuracy (correct or incorrect), rather than specific error types, as the error types vary across variants (Budd et al., 2010). A response was considered correct if it included all phonemes of the target name in the correct order, even if the phonemes were distorted, as long as they were recognizable. For example, for a picture of a volcano, semantic paraphasias (e.g., “mountain”), phonemic paraphasias (e.g., “talcano,” rhyming with volcano), non-responses (e.g. “I don’t know”), circumlocutions (e.g. “a mountain erupting”), and visual errors (e.g. “a teepee”) would all be scored as incorrect, unless immediately self-corrected without prompting. There was no time limit for responding. Responses given after cuing were not considered correct.
Imaging analysis
Two raters (masked to behavioral data) evaluated leukoaraiosis on baseline T2 sequences (or Fluid Attenuation Inversion Recovery, FLAIR, a derivative of T2), using the Cardiovascular Health Study scale (Longstreth et al., 2005). Participants’ images were compared to templates corresponding to each rating on the CHS scale, from 1 (minimal) to 8 (severe and confluent white matter changes). Images with no white matter hyperintensities were graded as 0, and those with more extensive white matter hyperintensities than the 8 template were rated as 9. We evaluated inter-rater reliability between two raters using Kappa.
Statistical analysis
We used Stata version 13 for Windows (College Station, TX) for all analyses. We evaluated differences between outcome groups with respect to demographic variables using t-tests (for continuous variables) and Fisher’s Exact tests (for dichotomous variables). The effects of leukoaraiosis severity, age, education, and baseline BNT on decline (measured by baseline percent accuracy on BNT minus follow-up percent accuracy on BNT) were evaluated with multivariable linear regression. Multivariable linear regression (also called multiple linear regression) is used to assess relationships between a single continuous outcome (e.g. decline on BNT) and multiple, independent continuous variables (age, education, baseline BNT score). We also evaluated the effects of these variables on the dichotomized outcome of faster vs slower decline in BNT using multivariable logistic regression. Multivariable logistic regression is used to assess relationships between a single dichotomous outcome (e.g. worst decline on BNT) and multiple independent variables that can be continuous (age, education, baseline BNT score) or categorical (sex, PPA variant, speech and language treatment) while adjusting for potential confounders (Hidalgo & Goodman, 2012). Finally, we evaluated the association between dichotomized severity of leukoaraiosis (minimal-to-mild leukoaraiosis (CHS <5) versus moderate-to-severe leukoaraiosis (CHS ≥5) versus dichotomized rate of decline in naming using Fisher’s Exact.
RESULTS:
The two independent raters had excellent reliability in rating scans using the CHS scale (Kappa= 0.75). All participants had some degree of leukoaraiosis (CHS score range 1-7).
The mean decline in BNT was 15.1 (SD 26.6) percentage points; 72.4% showed decline, and the remainder showed no change or improvement. None of the dichotomous independent variables (sex, PPA variant, speech and language treatment) were associated with outcome (Table 2), and were therefore not entered in the logistic regression. Although the fast and slow decline groups were not significantly different for age and education, we included age and education in the multivariable (linear and logistic) regressions because these variables have been associated with leukoaraiosis (Grueter & Schulz, 2012; Jokinen, Melkas, Madureira, Verdelho, Ferro, Fazekas … & Inzitari, 2016), and we wanted to confirm that any effect of leukoaraiosis was independent of these variables.
Table 2.
Demographics of Participants in Each Outcome Group
| Slower Decline/ Improvement Naming |
Faster Decline in Naming |
Significance of Difference |
|
|---|---|---|---|
| Logopenic Number (%) |
4 (25%) | 8 (75%) | FE: p=0.14 |
| Nonfluent Number (%) |
4 (80%) | 1 (20%) | FE: p=1 |
| Semantic Number (%) |
6 (54.5%) | 5 (45.5%) | FE: p=1 |
| Unclassifiable Number (%) |
1 (100%) | 0 (0%) | |
| Age Mean (SD) |
66.9 (5.5) | 66.4 (8.3) | t=0.22, p=0.83 |
| Education Mean (SD) |
16.2 (2.7) | 17.4 (2.1) | t= −1.3, p=0.21 |
| Sex: Number Female (%) |
7 (46.7%) | 8 (57.1%) | FE: p=0.72 |
| Received SLT (%) |
3 (25%) | 3 (37.5%) | FE: p=1 |
|
CHS Rating Mean (SD) |
2.4 (1.2) | 3.9 (1.8) | t=−2.7; p=0.01 |
SLT= Speech and language treatment
Bolded values are statistically significant
FE: Fisher’s Exact
In multivariable linear regression, leukoaraiosis rating, age, education, and baseline BNT score predicted change in BNT score (F(3, 25) = 8.12; p=0.0006). Change in BNT score was predicted by severity of leukoaraiosis (t = −3.66; p=0.001) and education (t= −2.46; p=0.022) independently of the other variables (Table 3).
Table 3.
Results of Multivariable Linear Regression: Predictors of Degree of Decline
| Change in BNT | Coefficient | t | p-value| | 95% Conf. Interval |
|---|---|---|---|---|
| Age | −0.45 | −0.77 | 0.451 | −1.66 to 0.76 |
| Education | −5.20 | −2.46 | 0.022 | −9.38 to −0.81 |
| CHS Rating | −9.62 | −3.66 | 0.001 | −15.05 to −4.20 |
| Baseline BNT | −0.071 | −0.44 | 0.666 | −0.41 to 0.26 |
CHS Rating= Cardiovascular Health Scale Rating of Leukoaraiosis
Bolded values are statistically significant
When we dichotomized outcome into worst 50th percent (faster decline; n=14) versus slower decline or improvement (n=15), and evaluated the influence of leukoaraiosis rating, age, and education, outcome was predicted by all variables together (chi squared = 13.91; p=0.008; pseudo r2=0.35; Table 4). However, only leukoaraiosis independently predicted faster decline (OR=2.80; 95%CI: 1.11 to 7.03). For every 1 point increase on the CHS rating scale, there was 2.8 times higher chance of showing faster decline on the BNT.
Table 4.
Results of Multivariable Logistic Regression: Predictors of Decline on BNT
| Variable | Odds Ratio | z | p-value | 95% Conf. Interval |
|---|---|---|---|---|
| Age | 0.96 | −0.49 | 0.63 | 0.81 to 1.13 |
| Education | 1.48 | 1.28 | 0.20 | 0.81 to 2.69 |
| Baseline BNT | 1.02 | 0.94 | 0.35 | 0.98 to 1.06 |
| CHS | 2.80 | 2.19 | 0.029 | 1.11 to 7.03 |
| Constant | 0.00029 | −0.93 | 0.352 | 1.06e-11 to 8019 |
CHS Rating = Cardiovascular Health Scale Rating of Leukoaraiosis
Furthermore, when we dichotomized leukoaraiosis into moderate-to-severe leukoaraiosis (CHS score≥5; n=7) versus minimal-to-mild (CHS score <5; n=22), we found that moderate-to-severe leukoaraiosis was associated with faster decline in naming (Fisher’s Exact: p=0.035).
Figure 1 shows the univariate correlation between decline in BNT and severity of leukoaraiosis, with each variant of PPA indicated by a letter. Decline in BNT was measured by baseline percent accuracy on BNT minus follow-up percent accuracy on BNT (so positive values indicate greater decline). The Pearson correlation between decline in BNT and CHS rating was r=0.51 (p=0.0048). Figure 1 indicates that the correlation reflected a strong correlation across all variants. Correlations were similar across variants (lvPPA: r=0.50 (p=0.10); nfaPPA: r=0.40, p=0.50; svPPA: 0.50; p=0.12), but not statistically significant because of the small number in each variant.
Figure 1.
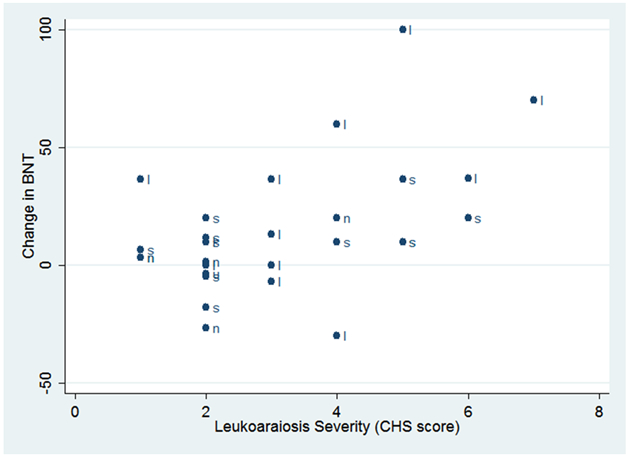
Scatterplot Showing the Pearson Correlation between CHS Leukoaraiosis Score and Decline in BNT
Decline in BNT = Baseline Percent Accuracy on BNT minus Follow-up Percent Accuracy on BNT for each Variant
l=logopenic; s=semantic; n=nonfluent agrammatic; u=unclassifiable
Discussion
Our results indicate severity of leukoaraiosis at baseline is associated with steeper decline in naming over one year in people with PPA, independently of baseline naming score, age, or education (OR=2.80; 95%CI: 1.11 to 7.03). This finding has important implications for clinical prognosis in PPA and enhances our understanding of the highly variable rate of decline in this patient group. The severity of leukoaraiosis was assessed only at baseline in this study; but among those participants with repeat imaging at one year, the severity of leukoaraiosis on the CHS rating did not change. Our results are consistent with other studies indicating that leukoaraiosis may be a marker of worse brain health at baseline. In individuals with PPA, having worse brain health may contribute to language decline or may impede potential compensation for language deficits. For example, individuals with PPA and leukoaraiosis may be less able than those without leukoaraiosis to re-purpose non-atrophied areas of brain to assume the functions of the atrophied areas, at least until those areas show neurodegeneration. Alternatively, leukoaraiosis almost certainly reflects deterioration in white matter tracts that are crucial to language performance. Several studies have reported reduced functional connectivity (Bonakdarpour et al., 2017; Ranasinghe et al., 2017; Sonty et al., 2007) and structural connectivity of specific white matter tracts (Mahoney et al., 2013; Schwindt et al., 2013) in language networks and other networks that correlate with language performance in PPA. Leukoaraiosis likely hastens the deterioration in structural and functional connectivity.
It is unlikely that leukoaraiosis severity is associated specifically with naming. Rather, we used naming as a marker for language, as it is one of the only domains of language that is progressively impaired in most people with PPA, irrespective of the variant. The severity of leukoaraiosis may well be associated with faster decline in cognition more generally, but we were interested primarily in predicting rate of decline in accuracy of naming (a common outcome measure in clinical trials of treatment for PPA) (Meyer, Tippett, & Friedman, 2016; Newhart et al., 2009).
Many studies have shown that PPA is associated with damage to specific white matter tracts. Damage to distinct white matter tracts has been observed in all three PPA variants (Catani et al., 2013; Galantucci et al., 2013; Madhavan et al., 2016; Mahoney et al., 2013; Mahoney et al., 2013; Mandelli et al., 2014; Schwindt et al., 2013). Our study provides complementary evidence indicating that more diffuse white matter disease is a negative prognostic indicator in terms of rate of progression. This measure may complement other imaging markers, from resting state fMRI or ASL.
These results have clinical implications, because they can aide in prognosis and allow more effective planning by caregivers. Additionally, our results suggest that clinical trials for PPA may need to stratify patients into treatment groups based on severity of leukoaraiosis, or otherwise control for severity of leukoaraiosis in evaluating differences in outcome between groups.
In contrast to some studies (e.g. Sebastian et al., 2018), we did not find that sex or PPA variant influenced outcome, but we likely had inadequate numbers of patients to assess the effects of these variables.
Limitations of this study include the fact that we did not have measures of atrophy to determine whether or not severity of leukoaraiosis is independent of the degree of cortical atrophy. Atrophy has been measured in a variety of ways in PPA and other neurological diseases, including whole brain volume relative to controls, volume of regions of interest relative to controls, cortical thinning relative to controls (Mesulam, Thompson, Weintraub, & Rogalski, 2015), surface area in regions of interest relative to controls (Meier et al., unpublished data), change in the ratio of brain volume to skull volume for each individual (Wright et al., 2018), or brain volume in each voxel compared to a baseline volume in the same individual (Faria, Sebastian, Newhart, Mori, & Hillis, 2014). Different measures can provide complementary information and are not always correlated with each other (Meier et al., unpublished data). However, all of these indications of atrophy are relatively time-consuming to measure. Rating of leukoaraiosis with the CHS scale is relatively rapid and easy, with high inter-rater reliability. The use of naming scores alone to measure progression might also be seen as a limitation, although change in the BNT correlates with change in core symptoms of each variant. Finally, the small number of participants is a limitation, although we had sufficient power to evaluate our hypothesis that severity of leukoaraiosis is associated with rate of decline in naming, as we obtained statistically significant results using a variety of statistical tests. We had inadequate power to document significant effects of leukoaraiosis on decline in each variant separately, but Figure 1 shows that the correlation between severity of leukoaraiosis and decline in BNT reflected a strong correlation across all variants (r= 0.40-0.50).
Thus, despite its limitations, our study indicates that this simple rating scale of leukoaraiosis can contribute useful prognostic information about the expected rate of decline in naming. This information can be useful to caregivers in planning for future needs and to clinical trialists focusing on PPA.
Acknowledgements:
This work was supported by the National Institutes of Health (National Institute of Deafness and Communication Disorders) through awards R01 DC011317, R01 DC05375, and P50 DC011739.
Footnotes
Declaration of Interest Statement
The authors have no potential conflicts of interest to report.
References
- Arsava EM, Rahman R, Rosand J, Lu J, Smith EE, Rost NS, … Ay H (2009). Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke. Neurology, 72(16), 1403–1410. doi: 10.1212/WNL.0b013e3181a18823 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrainwala ZS, Hillis AE, Dearborn J, & Gottesman RF (2014). Neglect performance in acute stroke is related to severity of white matter hyperintensities. Cerebrovascular Diseases (Basel, Switzerland), 37(3), 223–230. doi: 10.1159/000357661 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak TH, & Hodges JR (2003). Kissing and dancing—a test to distinguish the lexical and conceptual contributions to noun/verb and action/object dissociation. Preliminary results in patients with frontotemporal dementia. Journal of Neurolinguistics, 16(2), 169–181. doi: 10.1016/S0911-6044(02)00011-8 [DOI] [Google Scholar]
- Black S, Gao F, & Bilbao J (2009). Understanding white matter disease: Imaging-pathological correlations in vascular cognitive impairment. Stroke, 40(3 Suppl), S48–52. doi: 10.1161/STROKEAHA.108.537704 [doi] [DOI] [PubMed] [Google Scholar]
- Bonakdarpour B, Rogalski EJ, Wang A, Sridhar J, Mesulam M, & Hurley RS (2017). Functional connectivity is reduced in early stage primary progressive aphasia when atrophy is not prominent. Alzheimer Disease and Associated Disorders, 31(2), 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breining BL, Lala T, Martínez Cuitiño M, Manes F, Peristeri E, Tsapkini K, … Hillis AE (2015). A brief assessment of object semantics in primary progressive aphasia. Aphasiology, 29, 488–505. doi: 10.1080/02687038.2014.973360 [DOI] [Google Scholar]
- Breining BL, Tippett DC, Davis C, Posner J, Sebastian R, Oishie K, … Hillis AE (2015, May). Assessing dissociations of object and action naming in acute stroke. Paper presented at the Clinical Aphasiology Conference, Monterey, CA. [Google Scholar]
- Budd MA, Kortte K, Cloutman L, Newhart M, Gottesman RF, Davis C, … Hillis AE (2010). The nature of naming errors in primary progressive aphasia versus acute post-stroke aphasia. Neuropsychology, 24(5), 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramazza A, & Hillis AE (1990). Where do semantic errors come from? Cortex, 26, 95–122.doi: 10.1016/S0010-9452(13)800 [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam MM, Jakobsen E, Malik F, Martersteck A, Wieneke C, … Weintraub S (2013). A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain, 136(8), 2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotelli M, Borroni B, Manenti R, Alberici A, Calabria M, Agosti C, … Binetti G (2006). Action and object naming in frontotemporal dementia, progressive supranuclear palsy, and corticobasal degeneration. Neuropsychology, 20(5), 558. [DOI] [PubMed] [Google Scholar]
- Dabul B (2000). Apraxia battery for adults – second edition. Austin, TX: Pro-Ed. [Google Scholar]
- Debette S, Beiser A, DeCarli C, Au R, Himali JJ, Kelly-Hayes M, … Seshadri S (2010). Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The Framingham offspring study. Stroke, 41(4), 600–606. doi: 10.1161/STROKEAHA.109.570044 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria AV, Sebastian R, Newhart M, Mori S, & Hillis AE (2014). Longitudinal imaging and deterioration in word comprehension in primary progressive aphasia: Potential clinical significance. Aphasiology, 28(8-9), 948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria A, Meyer A, Tippett D, Friedman R, & Hillis A (2017). Characterization of primary progressive aphasia and prediction of naming decline by multi-modality MRI (N1. 002). Neurology, 88(16 Supplement), N1. 002. [Google Scholar]
- Galantucci S, Agosta F, Canu E, Cappa S, Magnani G, Franceschi M, … Filippi M (2013). Disruption of structural connectivity along the dorsal and ventral language pathways in patients with nonfluent and semantic variant primary progressive aphasia (P06. 070). Neurology, 80(7 Supplement), P06. 070–P06. 070. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, … Grossman M (2011). Classification of primary progressive aphasia and its variants. Neurology, 76(11), 1006–1014. doi: 10.1212/WNL.0b013e31821103e6 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BE, & Schulz UG (2012). Age-related cerebral white matter disease (leukoaraiosis): a review. Postgraduate medical journal, 88(1036), 79–87. [DOI] [PubMed] [Google Scholar]
- Hidalgo B, & Goodman M (2012). Multivariate or multivariable regression?. American journal of public health, 103(1), 39–40. doi:10.2105%2FAJPH.2012.300897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Oh S, & Ken L (2004). Deterioration of naming nouns versus verbs in primary progressive aphasia. Annals of Neurology, 55(2), 268–275. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Tuffiash E, & Caramazza A (2002). Modality-specific deterioration in naming verbs in nonfluent primary progressive aphasia. Journal of Cognitive Neuroscience, 14(7), 1099–1108. [DOI] [PubMed] [Google Scholar]
- Howard D, & Patterson K (1992). The pyramids and palm trees test: A test of semantic access from words and pictures. Cambridge, UK: Pearson [Google Scholar]
- Jokinen H, Melkas S, Madureira S, Verdelho A, Ferro JM, Fazekas F, … & Inzitari D (2016). Cognitive reserve moderates long-term cognitive and functional outcome in cerebral small vessel disease. J Neurol Neurosurg Psychiatry, 87(12), 1296–1302. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S, Segal O, & van Loon-Vervoorn A (2001). Boston naming test. Austin: Pro-ed. [Google Scholar]
- Kissela B, Lindsell CJ, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, … Tsevat J (2009). Clinical prediction of functional outcome after ischemic stroke: The surprising importance of periventricular white matter disease and race. Stroke, 40(2), 530–536. doi: 10.1161/STROKEAHA.108.521906 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth WT Jr, Arnold AM, Beauchamp NJ Jr, Manolio TA, Lefkowitz D, Jungreis C, … Furberg CD (2005). Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: The cardiovascular health study. Stroke, 36(1), 56–61. doi:01.STR.0000149625.99732.69 [pii] [DOI] [PubMed] [Google Scholar]
- Loonstra AS, Tarlow AR, & Sellers AH (2001). COWAT metanorms across age, education, and gender. Applied Neuropsychology, 8, 161–166. doi: 10.1207/S15324826AN0803_5 [DOI] [PubMed] [Google Scholar]
- Machulda MM, Whitwell JL, Duffy JR, Strand EA, Dean PM, Senjem ML, … Josephs KA (2013). Identification of an atypical variant of logopenic progressive aphasia. Brain and Language, 127(2), 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, & Henderson VW (1992). Boston naming test: Shortened versions for use in alzheimer’s disease. Journal of Gerontology, 47(3), P154–P158. [DOI] [PubMed] [Google Scholar]
- Madhavan A, Schwarz CG, Duffy JR, Strand EA, Machulda MM, Drubach DA, … Senjem ML (2016). Characterizing white matter tract degeneration in syndromic variants of Alzheimer’s disease: A diffusion tensor imaging study. Journal of Alzheimer’s Disease, 49(3), 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CJ, Malone IB, Ridgway GR, Buckley AH, Downey LE, Golden HL, … Rossor MN (2013). White matter tract signatures of the progressive aphasias. Neurobiology of Aging, 34(6), 1687–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli ML, Caverzasi E, Binney RJ, Henry ML, Lobach I, Block N, … Gorno-Tempini ML (2014). Frontal white matter tracts sustaining speech production in primary progressive aphasia. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 34(29), 9754–9767. doi: 10.1523/JNEUROSCI.3464-13.2014 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA, Kronmal RA, Burke GL, Poirier V, O’Leary DH, Gardin JM, … Bryan RN (1994). Magnetic resonance abnormalities and cardiovascular disease in older adults. The Cardiovascular Health Study. Stroke, 25(2), 318–327. [DOI] [PubMed] [Google Scholar]
- Mesulam M (1982). Slowly progressive aphasia without generalized dementia. Annals of Neurology, 11(6), 592–598. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Thompson CK, Weintraub S, & Rogalski EJ (2015). The wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain : A Journal of Neurology, 138(Pt 8), 2423–2437. doi: 10.1093/brain/awv154 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AM, Tippett DC, & Friedman RB (2016). Prophylaxis and remediation of anomia in the semantic and logopenic variants of primary progressive aphasia. Neuropsychological Rehabilitation, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhart M, Davis C, Kannan V, Heidler‐Gary J, Cloutman L, & Hillis AE (2009). Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology, 23(7-8), 823–834. [Google Scholar]
- Olm CA, Kandel BM, Avants BB, Detre JA, Gee JC, Grossman M, & McMillan CT (2016). Arterial spin labeling perfusion predicts longitudinal decline in semantic variant primary progressive aphasia. Journal of Neurology, 263(10), 1927–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe KG, Hinkley LB, Beagle AJ, Mizuiri D, Honma SM, Welch AE, … Garrett C (2017). Distinct spatiotemporal patterns of neuronal functional connectivity in primary progressive aphasia variants. Brain, 140(10), 2737–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, Love T, Driscoll D, Anderson SW, & Hickok G (2011). Are mirror neurons the basis of speech perception? Evidence from five cases with damage to the purported human mirror system. Neurocase, 7, 178–187. doi: 10.1080/13554794.2010.509318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt GC, Graham NL, Rochon E, Tang‐Wai DF, Lobaugh NJ, Chow TW, & Black SE (2013). Whole‐brain white matter disruption in semantic and nonfluent variants of primary progressive aphasia. Human Brain Mapping, 34(4), 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian R, Thompson CB, Wang N, Wright A, Meyer A, Friedman RB, … Tippett DC (2018). Patterns of decline in naming and semantic knowledge in primary progressive aphasia. Aphasiology, 32(9), 1010–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MC, & Ciccarelli N (2007). Naming of grammatical classes in frontotemporal dementias: Linguistic and non linguistic factors contribute to noun-verb dissociation. Behavioural Neurology, 18(4), 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE (2010). Leukoaraiosis and stroke. Stroke, 41(10 Suppl), S139–43. doi: 10.1161/STROKEAHA.110.596056 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonty SP, Mesulam MM, Weintraub S, Johnson NA, Parrish TB, & Gitelman DR (2007). Altered effective connectivity within the language network in primary progressive aphasia. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 27(6), 1334–1345. doi:27/6/1334 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szolnoki Z (2007). Pathomachenism of leukoaraiosis: a molecular bridge between the genetic, biochemical, and clinical processes (a mitochondrial hypothysis). Neuromolecular Medicine, 9, 21–33. doi: 10.1179/1743132815Y.0000000028 [DOI] [PubMed] [Google Scholar]
- Thompson CK, Lukic S, King MC, Mesulam MM, & Weintraub S (2012). Verb and noun deficits in stroke-induced and primary progressive aphasia: The northwestern naming battery. Aphasiology, 26(5), 632–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, & Breteler MM (2003). Silent brain infarcts and the risk of dementia and cognitive decline. New England Journal of Medicine, 348(13), 1215–1222. [DOI] [PubMed] [Google Scholar]
- Wright A, Tippett D, Saxena S, Sebastian R, Breining B, Faria A, & Hillis AE (2018). Leukoaraiosis is independently associated with naming outcome in poststroke aphasia. Neurology, doi: 10.1212/WNL.0000000000005945 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Sires DA, Grant I, Heaton RK, Tröster AI, & HIV Neurobehavioral Research Center (HNRC) Group. (2005). Action (verb) fluency: Test–retest reliability, normative standards, and construct validity. Journal of the International Neuropsychological Society, 11, 408–415. doi: 10.1017/S1355617705050460 [DOI] [PubMed] [Google Scholar]


