The discovery of interferon (IFN), as an antiviral substance, by Isaacs and Lindenmann in 1957 [1], attracted the attention of a number of investigators, including Pieter De Somer at the Rega Institute in Leuven (Belgium) and Tom Merigan at Stanford University (California). De Somer’s original attempts were aimed at determining the mode of action of IFN [2], whereas Merigan’s work focused on trying to understand the role of IFN in human viral diseases [3], based on the use of the mouse as the animal model
Of pioneering importance was the demonstration by Merigan [4] in 1967 that IFN could be induced in mice by a synthetic polyanion called maleic divinyl ether copolymer (or pyran copolymer). In further experiments, pyran copolymer was also shown to induce IFN in humans [5], although the serum IFN titers obtained in humans were considerably lower than those in mice and, moreover, were accompanied by a considerable raise in body temperature
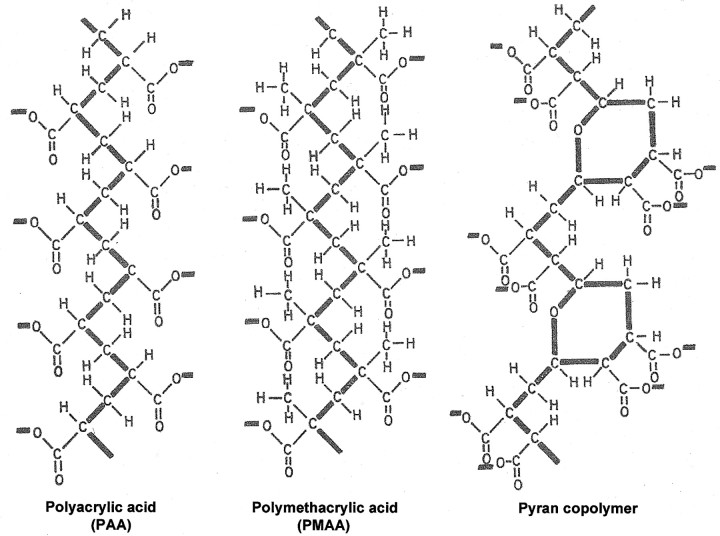
Meanwhile, in De Somer’s laboratory, I had identified 2 other polyanions, polyacrylic acid (PAA) and polymethacrylic acid, as antiviral agents [6, 7] and attributed their antiviral action to either the induction of IFN or a direct interference with virus adsorption to the cells. Overall, the structures of PAA and polymethacrylic acid are quite similar to that of pyran copolymer (figure 1), the prominent features being the —C-C-C-C-C-C- or —C-C-C-O-C-C- backbone and the negatively charged carboxylic acids mounted on this backbone
Figure 1.
_Structures of polyacrylic acid, polymethacrylic acid, and pyran copolymer
In writing about the potential role of IFN in clinical medicine in 1967, Merigan [8] had added an addendum: “recently, a significant report appeared describing the induction of IFN and antiviral effects in animals with double-stranded synthetic ribonucleotide homopolymers.” This report [9], by Maurice Hilleman’s group at Merck, was the first of the induction of IFN by what later could be hailed as the most potent inducer of IFN-β ever described, poly(I)·poly(C)
DOUBLE-STRANDED RNA INDUCERS OF IFN
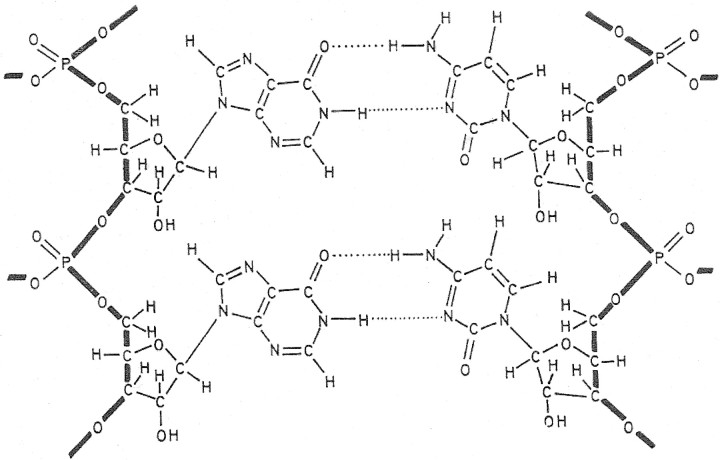
Guided by the discovery of poly(I)·poly(C) (figure 2) as a potent inducer of IFN [9], we further examined the structural requirements to which polyribonucleotides should adhere, in order to induce IFN production and resistance to viral infection. Our initial studies indicated that a stable secondary, preferably multistranded, structure was required for antiviral activity [10]
Figure 2.
_Structure of poly(I)·poly(C)
This antiviral activity of these polyribonucleotides could be dramatically enhanced by thermal activation [11, 12], a remarkable phenomenon that has remained unexplored after all these years
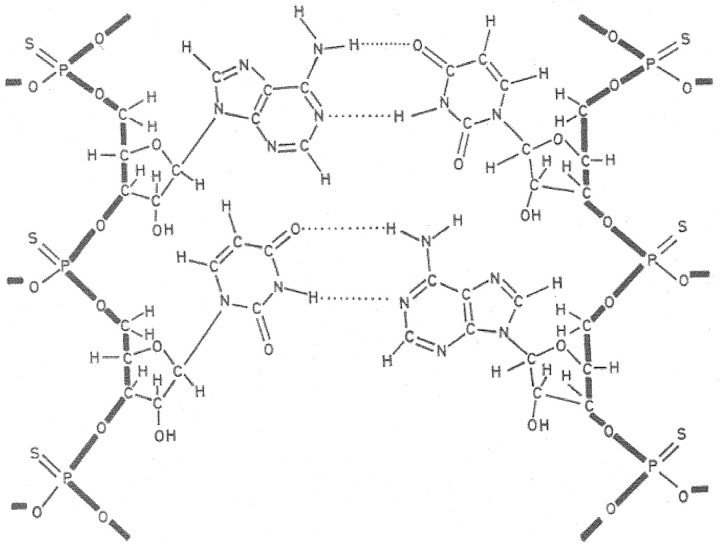
Starting from the alternating double-stranded RNA poly(A-U), we were able to significantly increase the IFN-inducing ability through substitution of the thiophosphate for the phosphate moieties [13]. The resulting poly(sA-sU) (figure 3) also gained increased resistance toward degradation by nucleases compared with that of its parent compound, poly(A-U), but the initial expectation that this modified RNA would ever aid in the fight against viral diseases [14] was eventually not fulfilled
Figure 3.
_Structure of thiophosphate analogue of poly r(A-U)
With poly(I)·poly(C) as the double-stranded RNA inducer of IFN, we [15] demonstrated that in an animal model for virus-induced encephalitis (i.e., on intranasal challenge of mice with vesicular stomatitis virus [VSV], a rhabdovirus), the whole protective effect conferred by the double-stranded RNA could be accounted for by IFN production. In these early days of IFN (and IFN inducers) research, we also developed an animal (mouse) model for induction of tumors by Moloney murine sarcoma virus, which is still used today to study the in vivo antiretroviral activity and which allowed us to demonstrate the inhibitory effect of poly(I)·poly(C) on Moloney murine sarcoma virus—induced tumor formation [16]
Later on, it was shown that of the 2 strands of poly(I)·poly(C), the poly(I) strand plays the predominant role, and that for the induction of IFN, poly(I) and poly(C) may be added in sequential order—that is, poly(I) followed by poly(C) [17]—assuming that under these conditions, the double-stranded poly(I)·poly(C) complex would be assembled at the cell surface. Also, modifications in the poly(I) strand (i.e., 7-N substitution by a CH group) could be introduced without detrimental effects on the IFN-inducing ability of the resulting double-stranded RNA complex [18]. Moving into the other direction, by introducing modifications in the poly(C) strand (i.e., interruption of this strand every twelfth or thirteenth cytidine residue by uridine), poly(I)·poly(C) analogues such as poly(I)·poly(C12U) were constructed that still induced IFN while being apt to a faster degradation by nucleases [19]. This type of “mismatched” double-stranded RNA still survives today under the name Ampligen
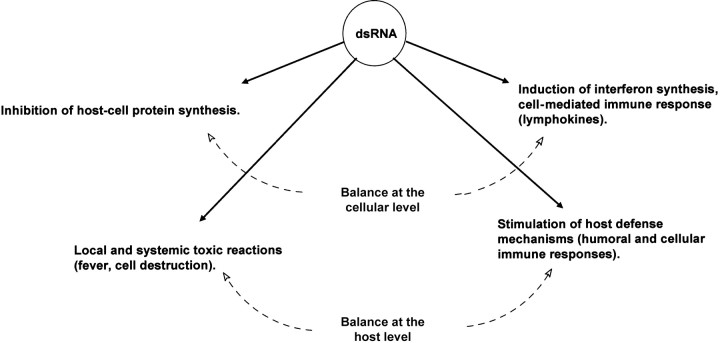
Double-stranded RNA has a double-modulatory role (figure 4), as originally postulated by Carter and De Clercq [20]. At the cellular level, it induces IFN synthesis but inhibits host cell protein synthesis, and at the host level, it stimulates host defense mechanisms but induces both local and systemic toxic side effects [20]
Figure 4.
_Modulatory role of double-stranded (ds) RNA (modified from [20])
Recently, double-stranded as well as single-stranded RNAs have been postulated to interact with toll-like receptors 3 and 7 (see, e.g., [21]), but the authenticity of these interactions remains to be further established
IFN-α IN THE TREATMENT OF HEPATITIS B AND C
A landmark observation, which laid the basis for the later use of IFN-α in the treatment of hepatitis B, was made by Merigan’s group in 1976 [22], when they showed that parenteral (human leukocyte) IFN administration at a dosage between 6.0 × 103 and 17 × 104 U/kg/day was associated with a rapid and reproducible fall in all Dane particle markers in 3 patients with chronic active hepatitis B. Long-term IFN therapy was associated with a marked fall in hepatitis B surface antigen in 2 of 3 patients and a disappearance of e antigen in 2 of 2 patients. It was concluded that IFN may be useful in limiting carrier infectivity or eradicating chronic hepatitis B virus (HBV) infection
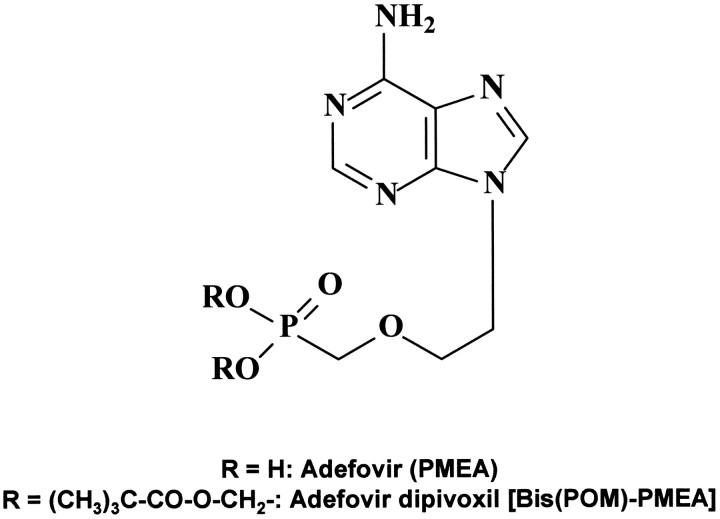
Four drugs have been formally approved for the treatment of chronic HBV infections: pegylated IFN-α, lamivudine, adefovir dipivoxil [23], and entecavir. It has not been established, but deserves further exploration, whether combinations of these drugs provide incremental benefit in the treatment of hepatitis B. Adefovir dipivoxil corresponds to the bis(pivaloyloxymethyl)ester of 9-(2-phosphonomethoxyethyl)adenine (PMEA; figure 5), a compound that was first mentioned for its antiviral properties in 1986 [24]. Adefovir dipivoxil has proved to be efficacious in the treatment of both e antigen—negative and e antigen—positive chronic hepatitis B [25, 26]. It has been firmly established that adefovir acts as a chain terminator in the reverse transcriptase (RNA-dependent DNA polymerase) reaction [27], and this by itself could explain the reductions in HBV DNA titers achieved in vivo by the therapeutic doses used for adefovir dipivoxil (10 mg daily orally)
Figure 5.
_Structure of adefovir and adefovir dipivoxil. PMEA, 9-(2-phosphonomethoxyethyl)adenine
It should be mentioned in this context that adefovir (PMEA) has also been shown to enhance natural killer cell activity and IFN production, at least in mice [28, 29], and more recent studies with N6-substituted PMEA derivatives [30] have indicated that this type of compound can also stimulate the secretion of cytokines and chemokines. Whether such potential side effects could contribute to the efficacy of adefovir dipivoxil in the treatment of chronic hepatitis B has not been demonstrated, however
IFN-α (in its pegylated form), in combination with ribavirin, has become the standard treatment for chronic hepatitis C virus (HCV) infections. In a recent study, Hadziyannis et al. [31] demonstrated that patients infected with HCV genotype 1 required treatment with pegylated IFN-α2a (180 μg/week) plus ribavirin (1000 or 1200 mg/day) for 48 weeks, whereas patients with HCV genotypes 2 or 3 seemed to be adequately treated for only 24 weeks with the same dose of pegylated IFN and a lower dose (800 mg/day) of ribavirin. Therefore, at least in the long term, hepatitis C may be better manageable by IFN and ribavirin when due to HCV genotypes 2 or 3 rather than genotype 1. The mechanistic basis for this differential behavior remains to be unraveled
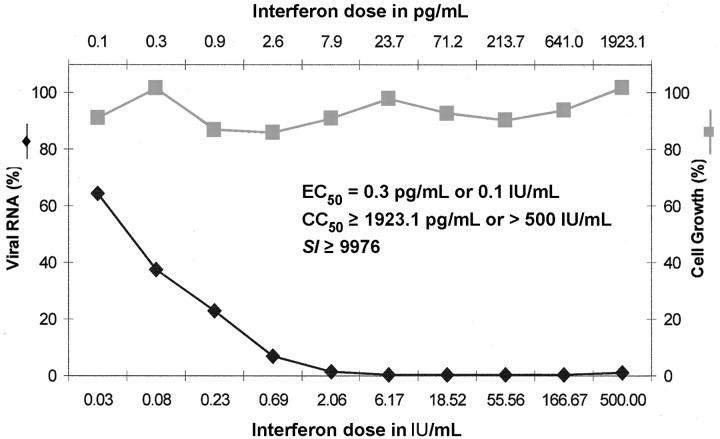
Of note, IFN has a strong antiviral effect on HCV, as we have demonstrated in the HCV replicon system in Huh-7 cells, in which IFN-α (intron A) was found to inhibit the replication of HCV (genotype 1b) at an EC50 of 0.3 pg/mL, whereas no cytotoxicity for the host cells was noted at 10,000-fold higher concentration (figure 6). Ribavirin had relatively weak antiviral activity in the HCV (genotype 1b) replicon system (we have not yet examined the effects of IFN and ribavirin in HCV replicon systems with other genotypes). Thus, I surmise that IFN-α, which mainly acts as an immunosuppressant in the treatment of chronic hepatitis B, primarily acts as an antiviral in the treatment of hepatitis C, whereas ribavirin, which is best known for its antiviral properties, may be assumed to primarily act as an immunosuppressant in the case of hepatitis C
Figure 6.
_Effect of intron A on the replication of hepatitis C virus (genotype 1b) replicon in Huh-7 cells. Unpublished data (average values for 2 experiments) from Jan Paeshuyse, Erik De Clercq, and Johan Neyts (2004). SI, selectivity index
IFN-β IN THE TREATMENT OF MULTIPLE SCLEROSIS
In 1980, we succeeded in cloning the human IFN-β and bringing it to expression through DNA recombination technology [32, 33]. Now, 20 years later, IFN-β has become the standard treatment for multiple sclerosis. It was shown to be an effective treatment, in a dose-related manner, for relapsing-remitting multiple sclerosis in terms of relapse rate, defined disability, and all magnetic resonance imaging outcome measures [34]
In a recent overview, Revel [35] noted that the role of IFN-β for the treatment of relapsing-remitting multiple sclerosis is now well established, and its efficacy has been demonstrated unequivocally in large-scale clinical trials. Recent trials underline the importance of both dose and dosing frequency and indicate that for improved efficacy in relapsing-remitting multiple sclerosis, IFN-β therapy should be administered frequently at the highest tolerable and thus most effective dose
The mechanism of action of human IFN-β in the treatment of multiple sclerosis has not been firmly established but may more likely be mediated by an immunosuppressant rather than antiviral effect. Human IFN-β has been traditionally used in the treatment of multiple sclerosis and human IFN-α in the treatment of chronic hepatitis B and C. There are no scientific reasons to believe that it may not work the other way around, but direct comparative studies with IFN-α versus IFN-β in the treatment of either hepatitis B or C or multiple sclerosis have, to the best of my knowledge, not been carried out
IFN IN THE POTENTIAL TREATMENT OF SEVERE ACUTE RESPIRATORY SYNDROME (SARS)
IFN-α, -β and, -γ have been found in vitro to be effective in inhibiting the replication of the SARS coronavirus (SCV) [36]. IFN-α effectively inhibited SCV replication, but with a selectivity index 50–90 times lower than that of IFN-β. IFN-γ was slightly better than IFN-α in Vero cell cultures but was completely ineffective in Caco2 cell cultures
In vivo, prophylactic treatment of SCV-infected macaques with pegylated IFN-α was found to significantly reduce viral replication and excretion, viral antigen expression in type 1 pneumocytes, and pulmonary damage [37]. Pegylated IFN-α may therefore be considered a candidate drug for the prophylaxis and therapy of SARS
IFN IN THE POTENTIAL TREATMENT OF POXVIRUS INFECTIONS
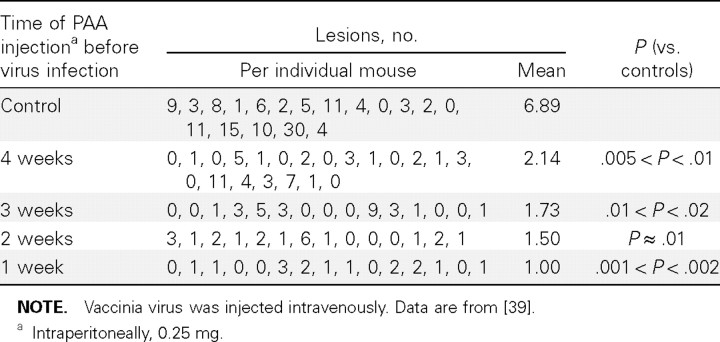
Should smallpox ever pose a threat following a bioterrorist attack with variola virus [38], IFN and its inducers may be considered, among many other compounds (such as cidofovir [23]), as a possible means to counteract such attack. In this perspective, we had already shown in 1968 [39] that IFN and its inducers are able to strongly act prophylactically against poxvirus infections. In the vaccinia virus tail lesion model in mice, IFN and PAA were able to markedly suppress poxvirus-induced lesions, the most remarkable finding being that a single injection of PAA, 4 weeks before challenge with virus, was able to significantly reduce vaccinia virus—induced tail lesions (table 1) [39]
Table 1.
_Effect of time of a single polyacrylic acid (PAA) injection on the formation of vaccinia virus tail lesions
IFN IN THE POTENTIAL TREATMENT OF FILOVIRUS INFECTIONS
The filoviruses, Marburg and Ebola, are classified as category A biowarfare agents by the US Centers for Disease Control and Prevention. Most known human infections with these viruses have been fatal, and no vaccines or effective therapies are currently available [40]. The filovirus disease syndrome resembles that caused by other hemorrhagic fever viruses, necessitating studies in a biocontainment laboratory to confirm the diagnosis. Some progress has been made in developing vaccines and antiviral drugs, but efforts are hindered by the limited number of maximum-containment laboratories
As mentioned by Bray et al. [41], the recombinant B/D chimeric form of human IFN-α has proven to be highly protective against Ebola virus in mice: a single dose given on the day of challenge only delayed death, but a 5- to 7-day course of the same dosage, begun on day 0, 1, or 2 after infection, was highly protective. However, a recent trial of IFN-α2b therapy in Ebola virus—infected cynomolgus monkeys resulted only in a delay in the onset of viremia, fever, and illness; all animals finally died of the infection [42]
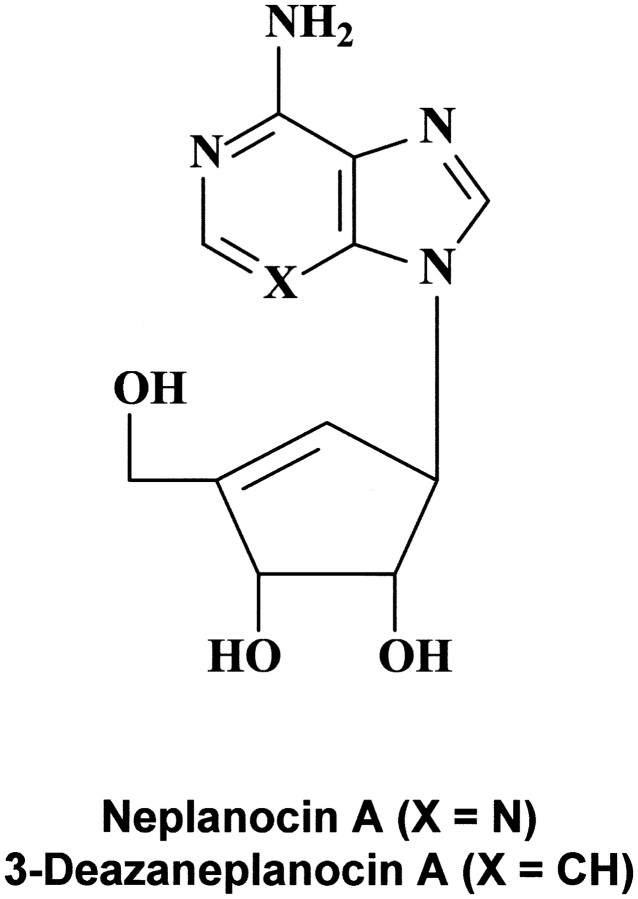
That IFN may be effective in the treatment of filovirus (i.e., Ebola virus) infections could be anticipated from our initial results [43] on the protective effects obtained with IFN and IFN inducers (such as PAA) in newborn mice infected with VSV, a rhabdovirus related to the filoviruses. In fact, VSV could be considered a surrogate virus of the filoviruses, in that anti-VSV activity may be predictive of activity against filoviruses. This premise has been borne out with S-adenosylhomocysteine hydrolase inhibitors such as 3-deazaneplanocin A (figure 7); on the basis of its activity against VSV [44], it was tested in treating Ebola virus infections in mice, and a single dose protected the mice against a lethal challenge with Ebola virus [41]
Figure 7.
_Structure of neplanocin A and 3-deazaneplanocin A
How might 3-deazaneplanocin A exert its antiviral action against Ebola virus in vivo? On one hand, 3-deazaneplanocin is a potent inhibitor of S-adenosylhomocysteine hydrolase [45]; on the other hand, 3-deazaneplanocin has been shown to massively stimulate the production of IFN-α in Ebola virus—infected mice [46]. Considering the unequaled potency of double-stranded RNAs to induce IFN, I hypothesize that as an S-adenosylhomocysteine hydrolase inhibitor, 3-deazaneplanocin leads to the accumulation of S-adenosylhomocysteine, which, being a product and inhibitor of methyltransferase reactions using S-adenosylmethionine as methyl donor, will inhibit these methylation reactions, including those that are required for the 5′-capping of the viral mRNA [45]. In the case of negative-stranded RNA viruses (such as Ebola), it means that the positive-stranded RNA (transcribed from the minus strand by the RNA replicase) is not processed and remains attached to the negative-stranded RNA, thus resulting in increased double-stranded RNA formation and, consequently, IFN induction. Further experiments should be undertaken to validate this hypothesis
IFN AND ITS INDUCERS IN THE POTENTIAL TREATMENT OF FLAVIVIRUS-INDUCED ENCEPHALITIS AND COXSACKIE B3 VIRUS—INDUCED MYOCARDITIS
IFN-α2b, pegylated IFN-α2b, poly(I)·poly(C), and poly(I)·poly(C12U) (Ampligen) have been evaluated against Modoc virus encephalitis in a mouse model for flavivirus infections. All compounds significantly delayed virus-induced morbidity (paralysis) and mortality (due to progressive encephalitis). Virus load was significantly reduced by 80%–100% in serum, brain, and spleen of mice that had been treated with either IFN-α2b, pegylated IFN-α2b, poly(I)·poly(C), or Ampligen (figure 8) [47]. IFN and double-stranded RNA inducers of IFN may therefore be considered for further studies for their potential in the therapy or prophylaxis of flavivirus infections in humans
Figure 8.
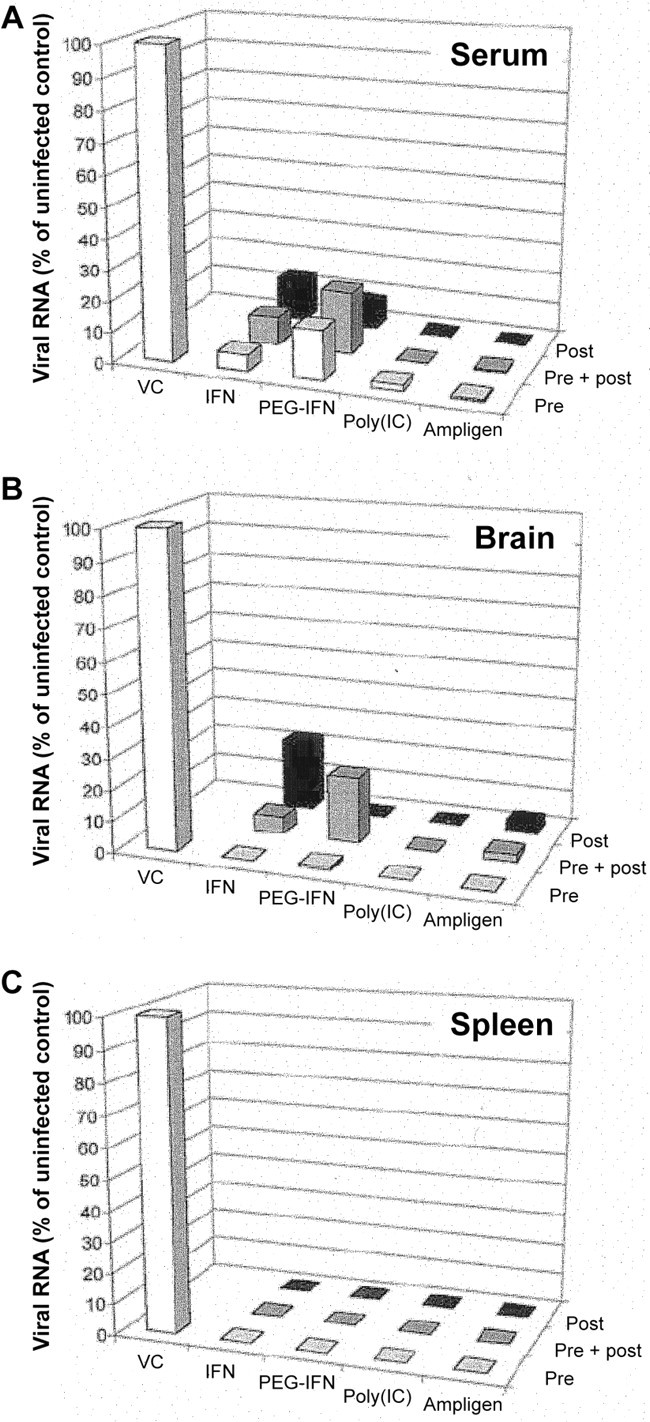
_Effect of treatment with interferon-α2b (IFN), pegylated interferon-α2b (PEG-IFN), poly(I)·poly(C) (polyIC), and Ampligen on virus titers (determined by quantitative reverse transcription—polymerase chain reaction) in serum, brain, and spleen at 7 days after infection of SCID mice with the flavivirus Modoc. Pre or post, before or after infection; VC, virus control (data from [47])
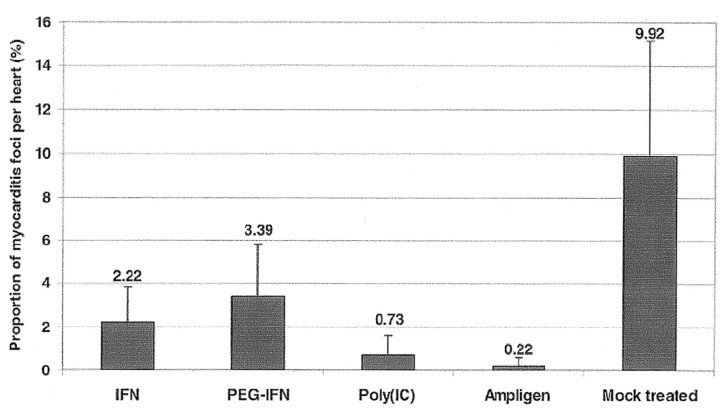
Similarly, IFN and the double-stranded RNA inducers of IFN may seem promising candidate drugs for therapy for or prophylaxis against Coxsackie B virus—induced myocarditis (figure 9) [48]. Ampligen at 20 mg/kg of body weight/day was found to reduce by 98% the severity of Coxsackie B3 virus—induced myocarditis in mice, as assessed by morphometric analysis. When poly(I)·poly(C) was administered at 15 mg/kg/day, it reduced the severity of virus-induced myocarditis by 93%. IFN-α2b and pegylated IFN-α2b were less effective and reduced the severity of virus-induced myocarditis by 78% and 66%, respectively
Figure 9.
_Effect of treatment with interferon-α2b (IFN), pegylated interferon-α2b (PEG-IFN), poly(I)·poly(C) (poly(IC)), and Ampligen on Coxsackie B3 virus—induced myocarditis in C3H/HeNHsd mice (data from [48])
SUMMARY
IFN-α (whether pegylated or not) has acquired a definitive place in the treatment of chronic hepatitis B and C, as has IFN-β in the treatment of multiple sclerosis. Further potential indications for pegylated IFN-α and -β include SARS and for pegylated IFN-α and IFN inducers, such as poly(I)·poly(C) and poly(I)·poly(C12U) (Ampligen), include filovirus, poxvirus, flavivirus, and Coxsackie B virus infections
Acknowledgments
I thank my “old time” tutors and advisers (Pieter De Somer, Thomas C. Merigan) and colleagues (Walter Fiers, Jean Content, Rik Derynck) and “new time” collaborators (Johan Neyts, Pieter Leyssen, Nathalie Charlier, Elizaveta Padalko, Jan Paeshuyse, and Armando De Palma) for the invaluable role they have played (or are still playing) in the unfolding story of IFN and its inducers. Special thanks are also due to Christiane Callebaut for her dedicated editorial assistance
References
- 1.Isaacs A, Lindenmann J. Virus interference I. Interferon. Proc R Soc Lond. 1957;147:258–67. [PubMed] [Google Scholar]
- 2.De Somer, P, Prinzie A, Denys P Jr, Schonne E. Mechanism of action of interferon I. Relationship with viral ribonucleic acid. Virology. 1962;16:63–70. doi: 10.1016/0042-6822(62)90202-7. [DOI] [PubMed] [Google Scholar]
- 3.Merigan TC. Interferons of mice and men. N Engl J Med. 1967;276:913–20. doi: 10.1056/NEJM196704202761608. [DOI] [PubMed] [Google Scholar]
- 4.Merigan TC. Induction of circulating interferon by synthetic anionic polymers of known composition. Nature. 1967;214:416–7. doi: 10.1038/214416a0. [DOI] [PubMed] [Google Scholar]
- 5.Merigan TC, Regelson W. Interferon induction in man by a synthetic polyanion of defined composition. N Engl J Med. 1967;277:1283–7. doi: 10.1056/NEJM196712142772403. [DOI] [PubMed] [Google Scholar]
- 6.De Somer P, De Clercq E, Billiau A, Schonne E, Claesen M. Antiviral activity of polyacrylic and polymethacrylic acids. I. Mode of action in vitro. J Virol. 1968;2:878–85. doi: 10.1128/jvi.2.9.878-885.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Somer P, De Clercq E, Billiau A, Schonne E, Claesen M. Antiviral activity of polyacrylic and polymethacrylic acids. II. Mode of action in vivo. J Virol. 1968;2:886–93. doi: 10.1128/jvi.2.9.886-893.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merigan TC. Interferon’s promise in clinical medicine: fact or fancy? Am J Med. 1967;43:817–21. doi: 10.1016/0002-9343(67)90240-9. [DOI] [PubMed] [Google Scholar]
- 9.Field AK, Tytell AA, Lampson GP, Hilleman MR. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci USA. 1967;58:1004–10. doi: 10.1073/pnas.58.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Clercq E, Merigan TC. Requirement of a stable secondary structure for the antiviral activity of polynucleotides. Nature. 1969;222:1148–52. doi: 10.1038/2221148a0. [DOI] [PubMed] [Google Scholar]
- 11.De Clercq E, Wells RD, Merigan TC. Increase in antiviral activity of polynucleotides by thermal activation. Nature. 1970;226:364–6. doi: 10.1038/226364a0. [DOI] [PubMed] [Google Scholar]
- 12.De Clercq E, Wells RD, Grant RC, Merigan TC. Thermal activation of the antiviral activity of synthetic double-stranded polyribonucleotides. J Mol Biol. 1971;56:83–100. doi: 10.1016/0022-2836(71)90086-6. [DOI] [PubMed] [Google Scholar]
- 13.De Clercq E, Eckstein F, Merigan TC. Interferon induction increased through chemical modification of a synthetic polyribonucleotide. Science. 1969;165:1137–9. doi: 10.1126/science.165.3898.1137. [DOI] [PubMed] [Google Scholar]
- 14.Modified RNA aids fight against viral diseases. Chem Eng News. 1969;(23 June):17–8. [Google Scholar]
- 15.De Clercq E, Nuwer MR, Merigan TC. The role of interferon in the protective effect of a synthetic double-stranded polyribonucleotide against intranasal vesicular stomatitis virus challenge in mice. J Clin Invest. 1970;49:1565–77. doi: 10.1172/JCI106374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Clercq E, Merigan TC. Moloney sarcoma virus—induced tumors in mice: inhibition or stimulation by poly(rI)·poly(rC) Proc Soc Exp Biol Med. 1971;137:590–4. [Google Scholar]
- 17.De Clercq E, De Somer P. Antiviral activity of polyribocytidylic acid in cells primed with polyriboinosinic acid. Science. 1971;173:260–2. doi: 10.1126/science.173.3993.260. [DOI] [PubMed] [Google Scholar]
- 18.De Clercq E, Edy VG, Torrence PF, Water JA, Witkop B. Antiviral activity of poly(7-deazainosinic acid)—derived complexes in vitro and in vivo. Mol Pharmacol. 1976;12:1045–51. [PubMed] [Google Scholar]
- 19.Carter WA, Pitha PM, Marshall IW, Tazawa I, Tazawa S, Ts’O POP. Structural requirements of the rIn·rCn complex for induction of human interferon. J Mol Biol. 1972;70:567–87. doi: 10.1016/0022-2836(72)90560-8. [DOI] [PubMed] [Google Scholar]
- 20.Carter WA, De Clercq E. Viral infection and host defense. Science. 1974;186:1172–8. doi: 10.1126/science.186.4170.1172. [DOI] [PubMed] [Google Scholar]
- 21.O’Neill LA. Immunology. After the toll rush. Science. 2004;303:1481–2. doi: 10.1126/science.1096113. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg HB, Pollard RB, Lutwick LI, Gregory PB, Robinson WS, Merigan TC. Effect of human leukocyte interferon on hepatitis B virus infection in patients with chronic active hepatitis. N Engl J Med. 1976;295:517–22. doi: 10.1056/NEJM197609022951001. [DOI] [PubMed] [Google Scholar]
- 23.De Clercq E. Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clin Microbiol Rev. 2003;16:569–96. doi: 10.1128/CMR.16.4.569-596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Clercq E, Holý A, Rosenberg I, Sakuma T, Balzarini J, Maudgal PC. A novel selective broad-spectrum anti—DNA virus agent. Nature. 1986;323:464–7. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- 25.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen—negative chronic hepatitis B. N Engl J Med. 2003;348:800–7. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 26.Marcellin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen—positive chronic hepatitis B. N Engl J Med. 2003;348:808–16. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 27.De Clercq E. Potential of acyclic nucleoside phosphonates in the treatment of DNA virus and retrovirus infections. Expert Rev Anti Infect Ther. 2003;1:21–43. doi: 10.1586/14787210.1.1.21. [DOI] [PubMed] [Google Scholar]
- 28.Del Gobbo V, Foli A, Balzarini J, et al. Immunomodulatory activity of 9-(2-phosphonylmethoxyethyl)adenine (PMEA), a potent anti-HIV nucleotide analogue, on in vivo murine models. Antiviral Res. 1991;16:65–75. doi: 10.1016/0166-3542(91)90059-z. [DOI] [PubMed] [Google Scholar]
- 29.Calio R, Villani N, Balestra E, et al. Enhancement of natural killer activity and interferon induction by different acyclic nucleoside phosphonates. Antiviral Res. 1994;23:77–89. doi: 10.1016/0166-3542(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 30.Zídek Z, Potmesil P, Kmoníèková E, Holý A. Immunobiological activity of N-[2-(phosphonomethoxy)alkyl] derivatives of N6-substituted adenines, and 2,6-diaminopurines. Eur J Pharmacol. 2003;475:149–59. doi: 10.1016/s0014-2999(03)02110-1. [DOI] [PubMed] [Google Scholar]
- 31.Morgan TR, et al. Peginterferon-α2a and ribavirin combination therapy in chronic hepatitis C. Ann Intern Med. 2004;140:346–55. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 32.Derynck R, Content J, De Clercq E, et al. Isolation and structure of a human fibroblast interferon gene. Nature. 1980;285:542–7. doi: 10.1038/285542a0. [DOI] [PubMed] [Google Scholar]
- 33.Derynck R, Remaut E, Saman E, et al. Expression of human fibroblast interferon gene in Escherichia coli. Nature. 1980;287:193–7. doi: 10.1038/287193a0. [DOI] [PubMed] [Google Scholar]
- 34.PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study, Group Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498–504. [PubMed] [Google Scholar]
- 35.Revel M. Interferon-β in the treatment of relapsing-remitting multiple sclerosis. Pharmacol Ther. 2003;100:49–62. doi: 10.1016/s0163-7258(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 36.Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Treatment of SARS with human interferons. Lancet. 2003;362:293–4. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haagmans BL, Kuiken T, Martina BE, et al. Pegylated interferon-α protects type 1 pneumocytes against SARS coronavirus (SCV) infection in macaques. Nat Med. 2004;10:290–3. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrison SC, Alberts B, Ehrenfeld E, et al. Discovery of antivirals against smallpox. Proc Natl Acad Sci U S A. 2004;101:11178–92. doi: 10.1073/pnas.0403600101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Clercq E, De Somer P. Effect of interferon, polyacrylic acid, and polymethacrylic acid on tail lesions in mice infected with vaccinia virus. Appl Microbiol. 1968;16:1314–9. doi: 10.1128/am.16.9.1314-1319.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bray M. Defense against filoviruses used as biological weapons. Antiviral Res. 2003;57:53–60. doi: 10.1016/s0166-3542(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 41.Bray M, Driscoll J, Huggins JW. Treatment of lethal Ebola virus infection in mice with a single dose of an S-adenosyl-L-homocysteine hydrolase inhibitor. Antiviral Res. 2000;45:135–47. doi: 10.1016/s0166-3542(00)00066-8. [DOI] [PubMed] [Google Scholar]
- 42.Jahrling PB, Geisbert TW, Geisbert JB, et al. Evaluation of immune globulin and recombinant interferon-α2b for treatment of experimental Ebola virus infections. J Infect Dis. 1999;179(suppl 1):S224–34. doi: 10.1086/514310. [DOI] [PubMed] [Google Scholar]
- 43.De Clercq E, De Somer P. Protective effect of interferon and polyacrylic acid in newborn mice infected with a lethal dose of vesicular stomatitis virus. Life Sci. 1968;7:925–33. doi: 10.1016/0024-3205(68)90098-2. [DOI] [PubMed] [Google Scholar]
- 44.De Clercq E, Cools M, Balzarini J, et al. Broad-spectrum antiviral activities of neplanocin A, 3-deazaneplanocin A and their 5′-nor derivatives. Antimicrob Agents Chemother. 1989;33:1291–7. doi: 10.1128/aac.33.8.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Clercq E. Antivirals and antiviral strategies. Nat Rev Microbiol. 2004;2:704–20. doi: 10.1038/nrmicro975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bray M, Raymond JL, Geisbert T, Baker RO. 3-deazaneplanocin A induces massively increased interferon-α production in Ebola virus—infected mice. Antiviral Res. 2002;55:151–9. doi: 10.1016/s0166-3542(02)00018-9. [DOI] [PubMed] [Google Scholar]
- 47.Leyssen P, Drosten C, Paning M, et al. Interferons, interferon inducers, and interferon-ribavirin in treatment of flavivirus-induced encephalitis in mice. Antimicrob Agents Chemother. 2003;47:777–782. doi: 10.1128/AAC.47.2.777-782.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Padalko E, Nuyens D, De Palma A, et al. The interferon inducer Ampligen [poly(I)·poly(C12U)] markedly protects mice against Coxsackie B3 virus—induced myocarditis. Antimicrob Agents Chemother. 2004;48:267–74. doi: 10.1128/AAC.48.1.267-274.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]