Abstract
“Senile osteoporosis” is defined as significant aging-associated bone loss, and is accompanied by increased fat in the bone marrow. The proportion of adipocytes in bone marrow is inversely correlated with bone formation, and is associated with increased risk of fracture. NF-κB is a transcription factor that functions as a master regulator of inflammation and bone remodeling. NF-κB activity increases during aging; furthermore, constitutive activation of NF-κB significantly impairs skeletal development in neonatal mice. However, the effects of NF-κB activation using a skeletally mature animal model have not been examined. In the current study, an osteoprogenitor (OP)-specific, doxycycline-regulated NF-κB activated transgenic mouse model (iNF-κB/OP) was generated to investigate the role of NF-κB in bone remodeling in skeletally mature mice. Reduced osteogenesis in the OP-lineage cells isolated from iNF-κB/OP mice was only observed in the absence of doxycycline in vitro. Bone mineral density in the metaphyseal regions of femurs and tibias was reduced in iNF-κB/OP mice. No significant differences in bone volume fraction and cortical bone thickness were observed. Osmium-stained bone marrow fat was increased in epiphyseal and metaphyseal areas in the tibias of iNF-κB/OP mice. These findings suggest that targeting NF-κB activity as a therapeutic strategy may improve bone healing and prevent aging-associated bone loss in aged patients.
Keywords: Senile osteoporosis, NF-κB, osteoprogenitors, bone remodeling, bone marrow fat
Lay Summary
“Senile osteoporosis” denotes significant aging-associated bone loss from the axial and peripheral skeleton, and is accompanied by increased fat in the bone marrow. This imbalance in osteogenesis and adipogenesis is associated with an increased incidence of fragility fractures of the spine, hip, knee, shoulder and wrist. NF-κB is a key regulator of bone remodeling. Increased NF-κB activity was found in many organs during natural aging process. Clarification of the specific effect of increased NF-κB activity on osteoprogenitors during aging will delineate novel therapeutic approaches to mitigate the adverse effects of chronic inflammation and suppressed bone formation in aging-associated osteoporosis.
1. Introduction
“Senile osteoporosis” is defined as significant aging-associated bone loss from the axial and peripheral skeleton, and is accompanied by increased fat in the bone marrow [1, 2]. After the age of 60, the lifetime risk of fragility fractures due to senile osteoporosis is approximately 44%- 65% in women and 25–42% in men [3], and has become a major health concern in our aging society.
Osteoblasts are differentiated from mesenchymal stem cells (MSCs), which have multilineage differentiation capabilities, including bone, adipose tissue, muscle, and cartilage. During osteoblastic differentiation, MSCs are first developed into osteoprogenitors (OPs), which express Runx2, a master regulator of osteoblastogenesis, followed by Osterix (Osx). Thereafter, OPs differentiate into pre-osteoblasts with the expression of mature markers such as alkaline phosphatase (ALP) and type I collagen. Finally, pre-osteoblasts differentiate into mature osteoblasts, which are located on the bone surface and express markers such as osteocalcin [4]. Mature osteoblasts deposit a mineralized bone matrix and then become osteocytes, which account for almost 95% of all bone cells.
NF-κB is a master transcription factor that regulates the response to stress and immune-associated target gene expression. Ongoing chronic inflammation and the presence of increased levels of reactive oxygen species are hallmarks of the aging process, and also enhance NF-κB activity [5–8]. We recently reported that NF-κB activity is increased in MSCs and OP-lineage cells during the natural aging process, and is associated with reduced osteogenesis in vitro [8]. Excess bone formation was observed in transgenic mice with defective NF-κB signaling, suggesting an essential role of NF-κB in bone formation and remodeling [9, 10]. Interestingly, constitutively active NF-κB in osteoblast-lineage cells significantly impairs skeletal development in neonatal mice [11]. However, the biological environment for bone formation and remodeling are different in younger mice (which are frequently used in investigations of bone healing) versus older mice. Furthermore, there is a limited amount of bone marrow fat at an early stage of life [12, 13]. Therefore, examining the bone remodeling process and the balance between bone and fat in the bone marrow in skeletally mature animals is crucial for the investigation of potential strategies to mitigate senile osteoporosis.
In the current study, we have generated a doxycycline-regulated transgenic mouse model that activates NF-κB signaling in OP-lineage cells upon doxycycline withdrawal in skeletally mature mice. The effects of NF-κB activation on bone formation and the balance between bone and fat in the marrow fat were examined.
2. Materials and Methods
2.1. Generation of OP-specific inducible NF-kB activation transgenic mice
Stanford’s Administrative Panel on Laboratory Animal Care (APLAC) approved this animal protocol (APLAC 30038). Male transgenic mice (iNF-kB/OP, Cre+/− & floxed/−) with inducible NF-kB activation in OPs were generated by breeding the Tet-Osx-Cre male mice (Cre +/−, Jackson Laboratory, 006361, C57BL/6) with aIKK-EGFP female mice (floxed/floxed, Jackson laboratory, 008242, C57BL/6). The litter mates (Cre−/− & floxed/−) were used as control mice. To delay the activation of NF-kB in OPs until bone development is mature, the mice were supplied with doxycycline in their drinking water at a dose of 10 μg/ml from breeding period until 12 weeks of age. Each group included 6 male mice. We initially chose male mice for these experiments in order to investigate the effects of NF-κB signaling in an environment that did not include female hormones. The doxycycline treatments were then removed for 24 weeks and the mice were euthanized at 36 weeks of age.
2.2. Western blot
The mouse femurs were dissected and decalcified by EDTA before protein extraction [14]. The decalcified bone samples were chopped into small pieces and homogenized with TissueLyser 5mm stainless steel beads (Qiagen, Redwood City, CA) in the RIPA lysis buffer containing the proteinase inhibitor cocktail (ThermoFisher Scientific, Waltham, MA). Protein concentrations were measured with the BCA protein reagent (ThermoFisher Scientific). The p65 (D14E12) and phospho-p65 (93H1) were stained by the antibodies from Cell Signaling Technology (Danvers, MA). The horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology) was used and the signals were detected by adding the enhanced chemiluminescence western blotting detection reagents (ThermoFisher Scientific). The quantification of western blotting results was done by Image Lab statistic software (Bio-Rad). The images were quantified by using ImageJ.
2.3. In vitro osteogenesis assay
The methods of isolating murine bone marrow derived MSCs have been described previously (APLAC 17566) [15]. In brief, bone marrow was collected from the femurs and tibias of 8 weeks old transgenic or control male mice (Cre−/− & floxed/−). Institutional guidelines for the care and use of laboratory animals were observed in all aspects of this project. The bone marrow cells collected from femurs and tibias were carefully suspended and passed through a 70 μm strainer, spun down, and resuspended in α-minimal essential medium (α-MEM) supplied with 10% MSC certified fetal bovine serum (FBS, Invitrogen) and antibiotic antimycotic solution (100 units of penicillin, 100 μg of streptomycin, and 0.25 μg of Amphotericin B per ml; Hyclone, ThermoFisher Scientific). The fresh media was replaced the next day to remove the unattached cells (passage 1). The isolated MSCs were differentiated in osteogenic medium (α-MEM supplemented with 10% FBS, 100 nM dexamethasone, 10 mM β-glycerol phosphate and 50 μM ascorbate-2-phosphate) for 3 weeks. Bone mineralization was quantified by Alizarin red stain.
2.4. In vivo bone analysis
The mice were scanned by μCT using a GE RS150 μCT scanner with 50 μm resolution. The distal femurs and proximal tibias were used to create the Regions of Interest (ROI) at the epiphysis (4×4×1mm3), metaphysis (4×4×3mm3), and diaphysis (4×4×3mm3). The ROI in the trabecular bone region was generated using the advanced 3D spline tool (the interior line of endosteum was generated every 5 slices among 40 slices between 1–3 mm from the end,). The bone mineral density (BMD), bone volume fraction (BVF), and cortical thickness were quantified, and the 3D images were reconstructed using GEMS MicroView (threshold: 700 HU).
2.5. Quantification of bone marrow fat
Femurs and tibias were dissected and decalcified in EDTA for 2 weeks. The bone marrow fat was stained with 1% osmium tetroxide/2.5% potassium dichromate [16] for 48 hours at room temperature and washed in distilled water for 2 hours before being scanned by μCT (50μm resolution, threshold: 2000HU). The percentage of osmium in mm3/total volume, and the stained osmium density at the epiphysis, metaphysis, and diaphysis were quantitated and compared.
2.6. RNA extraction and Quantitative PCR
MSC isolated from the iNF-κB/OP or control mice were cultured in osteogenic medium or adipogenic medium (α-MEM supplemented with 10% FBS, 1 μM dexamethasone, 0.5 μM IBMX, 2 μg/ml insulin, and 2 μM rosiglitazone) for one week. Cellular RNAs were extracted by using RNeasy RNA purification kit (Qiagen, Valencia, CA). RNAs were reverse transcribed into complementary DNA (cDNA) using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). Probes for 18s rRNA, Runx2, osteocalcin, PPAR-γ, and C/EBP were purchased from Applied Biosystems. Reverse-transcriptase polymerase chain reaction (RT-PCR) was performed in an ABI 7900HT Sequencing Detection System (Applied Biosystems), using the 18s rRNA as the internal control. The −ΔΔCt relative quantitation method was used to evaluate gene expression level.
2.7. Statistical analysis
Non-paired t tests were performed for data with two groups, and a one-way Analysis of Variance (ANOVA) with Tukey’s posthoc test was performed for data with 3 or more groups. The statistical analysis was conducted using Prism 7 (GraphPad Software, San Diego, CA). Data are reported as mean ± standard deviations. P<0.05 was chosen as the threshold of statistical significance.
3. Results
3.1. Characterization of the doxycycline-inducible transgenic mice model
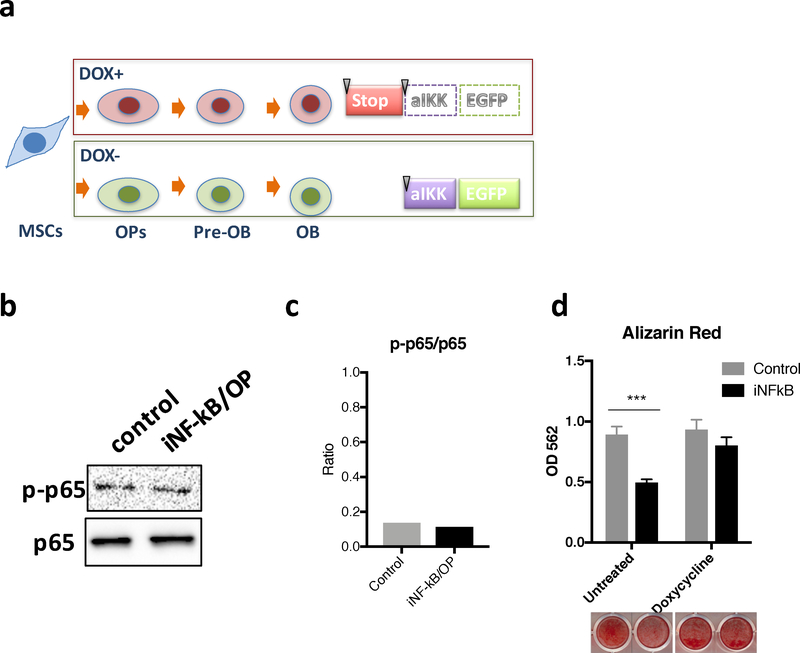
The regulation of NF-κB activation by the doxycycline-regulated system in the transgenic iNF-κB/OP and control mice is illustrated in Fig.1a. The constitutively active mutant IκB kinase β (IKKβ) is expressed in OPs and downstream lineage cells in the iNF-κB/OP mice, but not in control mice, after doxycycline withdrawal. The mice were treated with doxycycline until skeletally mature at week 12 to maintain NF-κB activity in the femurs from the iNF-κB/OP mice at a basal level (Fig.1b & c).
Fig.1. Illustration of the OP-specific inducible NF-kB activation transgenic mice (iNF-kB/OP).
(a) The STOP sequence is knocked-out by Cre recombinase under Osx promoter regulation in OPs, which turns on active IKK (alKK) and EGFP expression in OPs (Runx2+/Osx+), Pre-osteoblasts (Pre-OBs, ALP+/Calcitonin+), and osteoblasts (OBs, osteocalcin+). (b) NF-kB activity in the femur from iNF-kB/OP mice (heterozygous) and control mice before doxycycline withdrawal (week 12). Phosphorylated and non-phosphorylated p65 were detected by western blot to confirm no leakage on NF-kB activation before doxycycline withdrawal. (c) Quantification of western blot by ImageJ. (d) Decreased osteogenesis in MSCs/OPs with constitutive NF-kB activation. MSCs isolated from the iNF-kB/OP or control mice were differentiated in osteogenic media with/without doxycycline (100ng/ml). Bone mineralization was quantified by Alizarin red staining at day 21. ***p<0.005
Bone marrow-derived MSCs were isolated from the transgenic mice for the in vitro osteogenesis assay. The bone mineralization was reduced 44.4% in the cells isolated from iNF-κB/OP mice compared to the controls (Fig.1d). The reduction was reversed by continuous doxycycline treatment, which inhibited Cre recombinase expression and NF-κB activation in OPs.
3.2. Reduced bone mineral density in the femurs and tibias of iNF-kB/OP mice
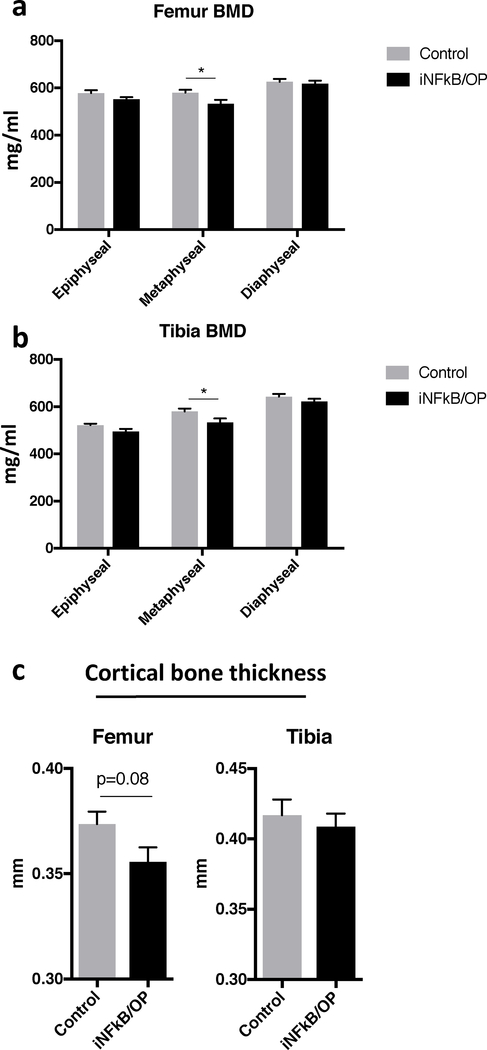
The metaphyseal BMD in the femurs and tibias was reduced in iNF-κB/OP mice at 36 weeks of age (24 weeks after doxycycline withdrawal, Fig.2a & b). No significant differences in BMD were observed in the epiphysis and diaphysis of femurs and tibias. The iNF-κB/OP mice showed a trend towards decreased diaphyseal cortical thickness (p=0.08) in femurs, but no differences were observed in the tibias (Fig.2c).
Fig.2. Bone analysis in iNF-kB/OP transgenic and control mice at 36 weeks of age (24 weeks after doxycycline withdrawal).
The ROI at the epiphysis (4×4×1mm3), metaphysis (4×4×3mm3), and diaphysis (4×4×3mm3) in iNF-kB/OP transgenic (n=6) and control mice (n=6) were generated to analyze the BMD in femurs (a) and tibias (b). The diaphyseal cortical thickness (c) was evaluated by μCT scan and analysis. *p<0.05
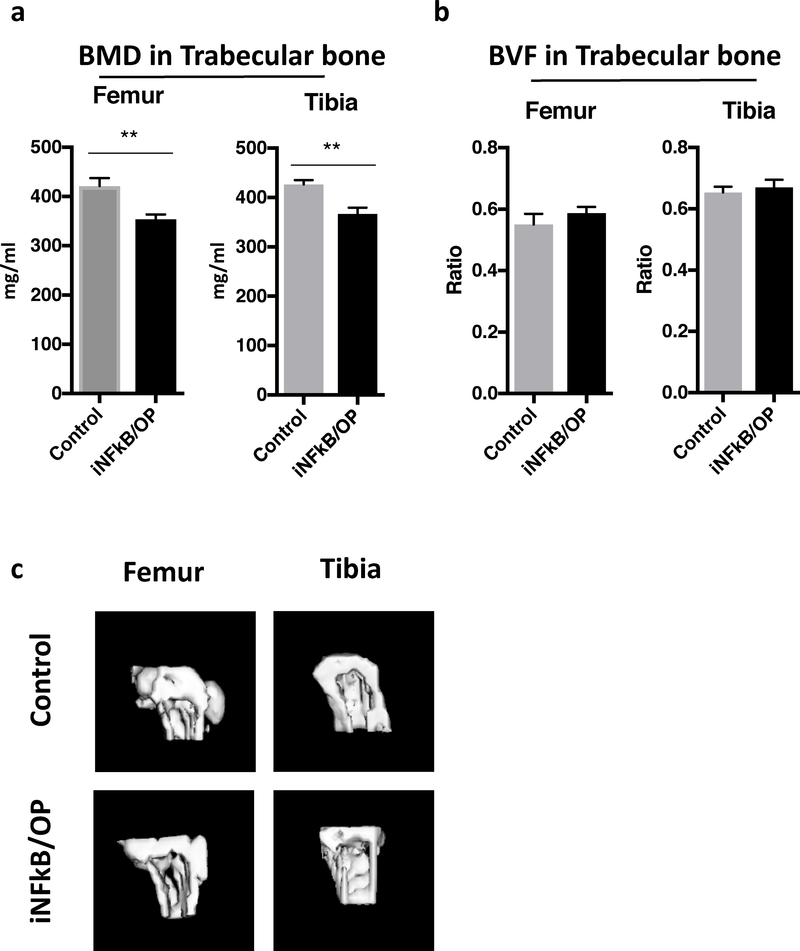
The trabecular bone BMD in the femurs and tibias was reduced in the iNF-κB/OP mice at 36 weeks of age (24 weeks after doxycycline withdrawal, Fig.3a & c). No significant differences in BVF were observed in the ROI of trabecular bones (Fig.3b).
Fig.3. Trabecular bone analysis in iNF-kB/OP transgenic and control mice at 36 weeks of age (24 weeks after doxycycline withdrawal).
The ROI (40 slices between 1–3 mm from the end) in the trabecular bone at the metaphysis in iNF-kB/OP transgenic (n=6) and control mice (n=6) is generated to analyze the trabecular BMD (a) and BVF (b) in femurs and tibias. Trabecular bone structure was reconstructed into 3D images (c). **p<0.01
3.3. Increased bone marrow fat in the iNF-kB/OP mice
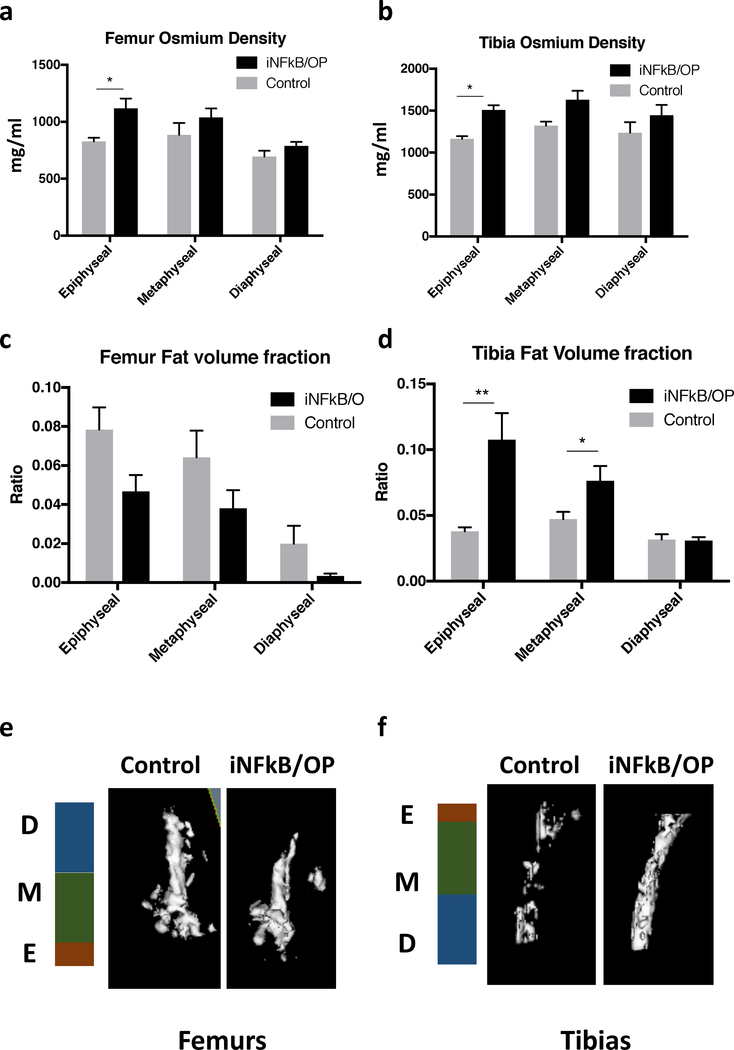
The osmium (bone marrow fat) density in iNF-κB/OP mice was increased at the epiphysis in femurs and tibias (Fig.4a & b). The fat volume fraction (fat/total volume) was increased at the epiphysis and metaphysis in tibias but not in femurs from iNF-κB/OP mice (Fig.4c & d). No differences in osmium density and fat volume fraction were observed in the diaphysis of femurs and tibias. The 3D reconstructed images of bone marrow fat are shown in Fig.4e & f.
Fig.4. Quantification of bone marrow fat stained with osmium.
The osmium-stained bone marrow fat scanned by μCT. The osmium density (a & b) and fat volume fraction (c & d) in femurs and tibias at the epiphysis (4×4×1mm3), metaphysis (4×4×3 mm3), and diaphysis (4×4×3 mm3) regions in iNF-kB/OP transgenic (n=6) and control mice (n=6) were quantified. The 3D reconstructed images of osmium-stained bone marrow fat in femurs (e) and tibias (f) were generated. E: epiphysis; M: metaphysis; D: diaphysis*p<0.05, **p<0.01
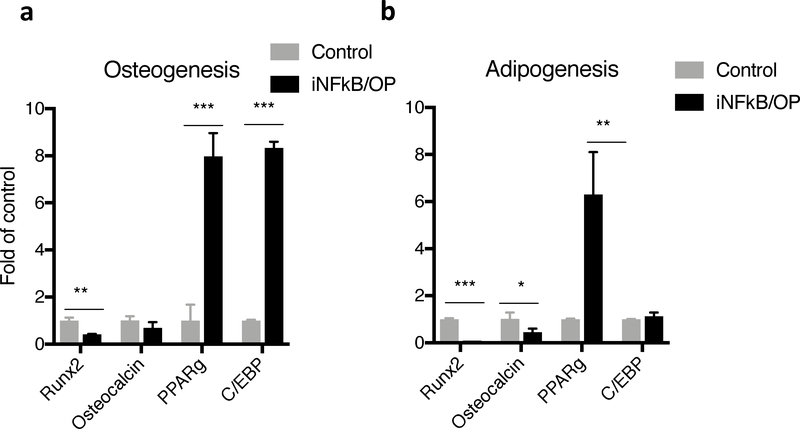
3.4. Impaired osteogenesis and adipogenesis signaling in MSCs from iNFκB/OP mice
We further examined the potential mechanisms of decreased bone mineralization and increased bone marrow fat observed in the iNFκB/OP mice in in vitro cell differentiation models. Decreased osteogenic markers (Runx2 and osteocalcin) and increased adipogenic marker (PPAR-γ and C/EBP) expression were found in MSCs isolated from the iNF-κB/OP transgenic mice during the differentiation assays in vitro (Fig.5a & b).
Fig.5. Decreased osteogenic marker (Runx2 & osteocalcin) and increased adipogenic marker (PPAR-γ & C/EBP) in MSCs isolated from iNF-κB/OP mice.
MSCs were cultured in (a) osteogenic or (b) adipogenic medium, and the expression of Runx2, Osteocalcin, PPAR-γ, and C/EBP was examined at week 1. **p<0.01, ***p<0.005
4. Discussion
The current findings suggest that NF-κB activation in OP-lineage cells reduces their bone-forming ability in vitro and in vivo, and impairs the balance between bone and fat in the marrow. These studies highlight the potential of NF-κB as a therapeutic target in inflammatory diseases of bone and in cases of adverse bone remodeling during the natural aging process.
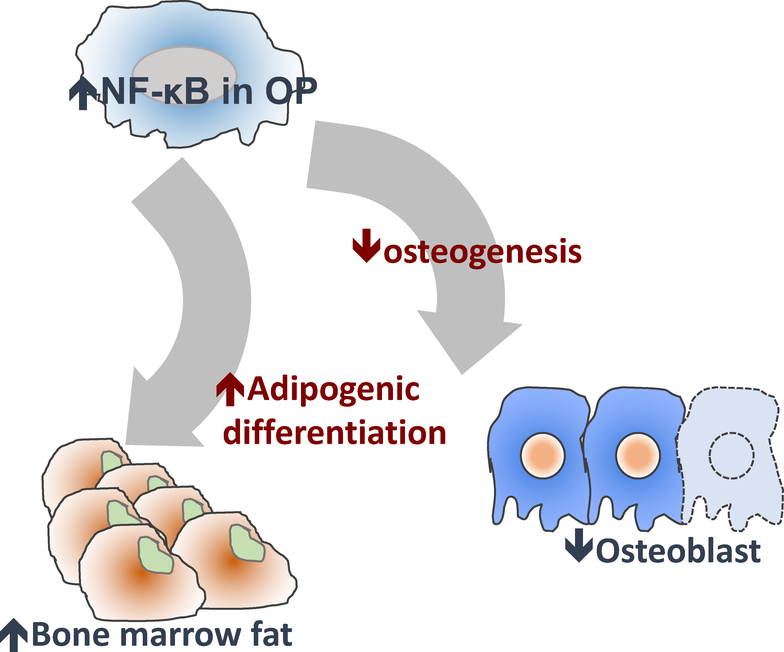
Increased fat in the bone marrow is a hallmark of patients with osteoporosis [1, 2]. The proportion of adipocytes in bone marrow is inversely correlated with bone formation [1] and is associated with increased risk of fracture [2]. Bone marrow fat participates in the regulation of bone remodeling and the development of bone-related diseases [17]. Indeed, formation of bone versus fat within the marrow space is a dynamic process that involves 1) the balance between osteoblastic (new bone formation) and osteoclastic (bone loss) activities, 2) adipogenesis (fat formation) activity, and 3) crosstalk between the various lineage cells in the bone marrow niche (Fig.6).
Fig.6. Potential mechanisms of NF-κB activation interferes with the balance of bone/bone marrow fat in bone.
NF-κB activation in OP-lineage cells could be mediated by 1) impaired osteogenesis with reduced Runx2 expression and 2) increased adipogenesis with the induction of PPAR-γ expression.
Recent studies demonstrated that NF-κB signaling suppressed osteogenic differentiation in MSCs via β-catenin degradation [10, 18]. Our present data shows that NF-κB activation in OP-lineage cells is associated with reduced osteogenesis. However, the potential regulation of OP-lineage cells on osteoclastic activity in the transgenic model remains unclear. We previously showed that Receptor Activator of NF-κB Ligand (RANKL), the NF-κB regulated osteoclast activator, was significantly upregulated in MSC-lineage cells isolated from aged mice, while expression in young MSC was below detectable levels [8]. This suggests that osteoclastic acitivity could be regulated by paracrine factors from MSC-lineage cells during the natural aging process.
While osteogenic differentiation is mediated by induction of Runx2 expression, adipoblastic differentiation by MSCs is initiated by increased expression of CCAAT/enhancer binding protein α (C/EBPα) in pre-adipoblasts [19]. The latter cells then differentiate into adipoblasts and mature into adipocytes with increased expression of peroxisome proliferator activated receptor-γ (PPAR-γ), a master regulator of adipogenesis. Increased bone marrow fat may result from enhanced adipogenesis in parallel with decreased osteogenesis [19–22]. NF-κB activation in OP-lineage cells may impair osteogenesis and favor adipogenic differentiation [23], though we cannot completely exclude the possibility of potential leakage of Cre recombinase expression in non-OP lineage cells in the iNF-κB/OP transgenic mice. Failed osteogenesis is associated with reduced Wnt/β-catenin and TGF-β1 expression and is accompanied by upregulation of the PPAR-γ pathway and adipogenesis [24, 20–22]. Whether this demonstrates a potential for trans-differentiation in the iNFκB/OP transgenic mice is unknown.
NF-κB activation is associated with paracrine factor expression including Wnt/β-catenin and TGF-β1, which have critical roles in the regulation of osteogenesis and adipogenesis [18, 25, 19]. TGF-β1 enhances osteogenesis directly via increasing Runx2 expression in OPs [26], and impairs adipocyte differentiation in vitro and in vivo [27, 28]. The canonical Wnt/β-catenin pathway directly suppresses PPAR-γ mRNA transcription. The non-canonical Wnt/β-catenin pathway represses PPAR-γ transactivation by histone H3K9 methylation on target genes [21]. Activation of NF-κB in OP-lineage cells in transgenic mice could potentially regulate adipoblastic activity via paracrine regulation and increase PPAR-γ expression (Fig.5).
The current studies focus on the effects of NF-κB activation on long bones such as distal femurs and proximal tibias. The bone remodeling changes in the axial skeleton, including spine and hip, have not been examined. Previous studies have indicated that Cre recombinase activity, driven by the Osx promoter, is observed in all skeletal elements in transgenic mice with the β-galactosidase (LacZ) reporter gene [29], suggesting that iNF-κB/OP mice could also be relevant to the study of the axial skeleton. Further studies are also indicated to validate the present findings, performed in aged male mice, for female mice.
The majority of current treatments for patients with osteoporosis are focused on suppression of osteoclast activity by using bisphosphonate or RANKL inhibitors, or by increasing osteoblast activity through injections of recombinant human parathyroid hormone (rhPTH), recombinant modified PTH-related peptide [30], or the selective activator of PTH type 1 receptor (abaloparatide) [31]. While both anti-resorptive and anabolic agents increase bone mass and reduce the risk of fracture, NF-κB targeting therapy provides an alternative approach to modulate bone remodeling by simultaneously increasing osteogenesis and decreasing osteoclast activity. Although the critical functions of NF-κB in the immune system raise concerns of toxicity with systemic treatment, the development of bone-targeting [32, 33] or even bone cell-specific targeting drug delivery vehicles [34–36] further advances the possibility to deliver NF-κB inhibitors as an anabolic therapy with minimal adverse effects. Importantly, targeting NF-κB in inflammatory- or aging-associated bone disorders is unique and different from other anti-inflammatory drugs, given the fact that COX-2 inhibitors suppress bone fracture healing [37].
There are limitations in the current study. First, the potential leakage of NF-κB activation in other cell types cannot be excluded in the current model. The reporter gene driven by Osx promoter has been detected in the adipocytes and fibroblasts in the bone marrow of the transgenic mice [38]. We choose this doxycycline inducible model since long term treatment of estrogen antagonist in other inducible model (Type 1 collagen/Col1α1-Cre-ERt2 mice) may affect bone homeostasis [39]. Second, we used the protein extracted from the whole bone tissue to examine the regulation of transgene induction. Therefore, NF-κB activation status in specific cell types was not clarified. Finally, we found that NF-κB activation has distinct effects on bone marrow fat homeostasis in the femurs and tibias (Fig.4). A previous study [40] showed that tibial bone marrow is characterized by a fatty acid profile similar to classical white and brown adipose tissues, while the fat in femoral bone marrow showed a high percentage of specific fatty acids (e.g., arachidonic acid and nervonic acid). Though NF-κB activation plays essential roles in the crosstalk with fatty acid metabolism [41], the potential correlation in our observed phenotypes in the femoral and tibial marrow fat fraction remains unclear.
5. Conclusion
Increased NF-κB activity in OP-lineage cells in skeletally mature mice leads to aging-associated osteoporosis-like phenotypes in bone. Targeting NF-κB activity as a therapeutic strategy may improve bone healing and prevent aging-associated bone loss in aged patients and in other inflammatory disorders.
Acknowledgement
This work was supported by NIH grant 1R01AR063717 and the Ellenburg Chair in Surgery at Stanford University. J.P. was supported by a grant from the Jane and Aatos Erkko foundation.
Footnotes
Disclosure of interests
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. Journal of Clinical Pathology. 2002;55(9):693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, Rosen CJ et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. The Journal of Clinical Endocrinology and Metabolism. 2013;98(6):2294–300. doi: 10.1210/jc.2012-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen ND, Ahlborg HG, Center JR, Eisman JA, Nguyen TV. Residual lifetime risk of fractures in women and men. Journal of Bone and Mineral Research. 2007;22(6):781–8. doi: 10.1359/jbmr.070315. [DOI] [PubMed] [Google Scholar]
- 4.Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. Journal of Bone and Mineral Research. 2009;24(5):759–64. doi: 10.1359/jbmr.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–54. [DOI] [PubMed] [Google Scholar]
- 6.Adler AS, Sinha S, Kawahara TL, Zhang JY, Segal E, Chang HY. Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes & Development. 2007;21(24):3244–57. doi: 10.1101/gad.1588507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilstra JS, Clauson CL, Niedernhofer LJ, Robbins PD. NF-kappaB in Aging and Disease. Aging and Disease. 2011;2(6):449–65. [PMC free article] [PubMed] [Google Scholar]
- 8.Lin TH, Gibon E, Loi F, Pajarinen J, Cordova LA, Nabeshima A et al. Decreased osteogenesis in mesenchymal stem cells derived from the aged mouse is associated with enhanced NF-kappaB activity. Journal of Orthopaedic Research. 2017;35(2):281–8. doi: 10.1002/jor.23270. [DOI] [PubMed] [Google Scholar]
- 9.Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nature Medicine. 1997;3(11):1285–9. [DOI] [PubMed] [Google Scholar]
- 10.Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R et al. Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nature Medicine. 2009;15(6):682–9. doi: 10.1038/nm.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swarnkar G, Zhang K, Mbalaviele G, Long F, Abu-Amer Y. Constitutive activation of IKK2/NF-kappaB impairs osteogenesis and skeletal development. PloS one. 2014;9(3):e91421. doi: 10.1371/journal.pone.0091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Iorgi N, Mo AO, Grimm K, Wren TA, Dorey F, Gilsanz V. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. The Journal of Clinical Endocrinology and Metabolism. 2010;95(6):2977–82. doi: 10.1210/jc.2009-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore SG, Dawson KL. Red and yellow marrow in the femur: age-related changes in appearance at MR imaging. Radiology. 1990;175(1):219–23. doi: 10.1148/radiology.175.1.2315484. [DOI] [PubMed] [Google Scholar]
- 14.Choi SE, Hong SW, Yoon SO. Proposal of an appropriate decalcification method of bone marrow biopsy specimens in the era of expanding genetic molecular study. Journal of Pathology and Translational Medicine. 2015;49(3):236–42. doi: 10.4132/jptm.2015.03.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin TH, Sato T, Barcay KR, Waters H, Loi F, Zhang R et al. NF-kappaB Decoy Oligodeoxynucleotide Enhanced Osteogenesis in Mesenchymal Stem Cells Exposed to Polyethylene Particle. Tissue engineering Part A. 2014. doi: 10.1089/ten.TEA.2014.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Styner M, Thompson WR, Galior K, Uzer G, Wu X, Kadari S et al. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone. 2014;64:39–46. doi: 10.1016/j.bone.2014.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermeo S, Gunaratnam K, Duque G. Fat and bone interactions. Current Osteoporosis Reports. 2014;12(2):235–42. doi: 10.1007/s11914-014-0199-y. [DOI] [PubMed] [Google Scholar]
- 18.Chang J, Liu F, Lee M, Wu B, Ting K, Zara JN et al. NF-kappaB inhibits osteogenic differentiation of mesenchymal stem cells by promoting beta-catenin degradation. Proc Natl Acad Sci U S A. 2013;110(23):9469–74. doi: 10.1073/pnas.1300532110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nuttall ME, Shah F, Singh V, Thomas-Porch C, Frazier T, Gimble JM. Adipocytes and the regulation of bone remodeling: a balancing act. Calcified Tissue International. 2014;94(1):78–87. doi: 10.1007/s00223-013-9807-6. [DOI] [PubMed] [Google Scholar]
- 20.Schilling T, Kuffner R, Klein-Hitpass L, Zimmer R, Jakob F, Schutze N. Microarray analyses of transdifferentiated mesenchymal stem cells. Journal of Cellular Biochemistry. 2008;103(2):413–33. doi: 10.1002/jcb.21415. [DOI] [PubMed] [Google Scholar]
- 21.Schilling T, Noth U, Klein-Hitpass L, Jakob F, Schutze N. Plasticity in adipogenesis and osteogenesis of human mesenchymal stem cells. Molecular and Cellular Endocrinology. 2007;271(1–2):1–17. doi: 10.1016/j.mce.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB journal. 2004;18(9):980–2. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 23.Sinha P, Aarnisalo P, Chubb R, Ono N, Fulzele K, Selig M et al. Loss of Gs alpha Early in the Osteoblast Lineage Favors Adipogenic Differentiation of Mesenchymal Progenitors and Committed Osteoblast Precursors. Journal of Bone and Mineral Research. 2014;29(11):2414–26. doi: 10.1002/jbmr.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao B, Huang Q, Lin YS, Wei BY, Guo YS, Sun Z et al. Dose-dependent effect of estrogen suppresses the osteo-adipogenic transdifferentiation of osteoblasts via canonical Wnt signaling pathway. PloS one. 2014;9(6):e99137. doi: 10.1371/journal.pone.0099137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin TH, Sato T, Barcay KR, Waters H, Loi F, Zhang R et al. NF-kappaB decoy oligodeoxynucleotide enhanced osteogenesis in mesenchymal stem cells exposed to polyethylene particle. Tissue Engineering Part A. 2015;21(5–6):875–83. doi: 10.1089/ten.TEA.2014.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. International Journal of Biological Sciences. 2012;8(2):272–88. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clouthier DE, Comerford SA, Hammer RE. Hepatic fibrosis, glomerulosclerosis, and a lipodystrophy-like syndrome in PEPCK-TGF-beta1 transgenic mice. The Journal of Clinical Investigation. 1997;100(11):2697–713. doi: 10.1172/JCI119815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torti FM, Torti SV, Larrick JW, Ringold GM. Modulation of adipocyte differentiation by tumor necrosis factor and transforming growth factor beta. The Journal of Cell Biology. 1989;108(3):1105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133(16):3231–44. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 30.Tella SH, Gallagher JC. Biological agents in management of osteoporosis. European Journal of Clinical Pharmacology. 2014;70(11):1291–301. doi: 10.1007/s00228-014-1735-5. [DOI] [PubMed] [Google Scholar]
- 31.Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA. 2016;316(7):722–33. doi: 10.1001/jama.2016.11136. [DOI] [PubMed] [Google Scholar]
- 32.Cole LE, Vargo-Gogola T, Roeder RK. Targeted delivery to bone and mineral deposits using bisphosphonate ligands. Advanced Drug Delivery Reviews. 2016;99(Pt A):12–27. doi: 10.1016/j.addr.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Dang L, Liu J, Li F, Wang L, Li D, Guo B et al. Targeted Delivery Systems for Molecular Therapy in Skeletal Disorders. International Journal of Molecular Sciences. 2016;17(3):428. doi: 10.3390/ijms17030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang G, Guo B, Wu H, Tang T, Zhang BT, Zheng L et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nature Medicine. 2012;18(2):307–14. doi: 10.1038/nm.2617. [DOI] [PubMed] [Google Scholar]
- 35.Liang C, Guo B, Wu H, Shao N, Li D, Liu J et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nature Medicine. 2015;21(3):288–94. doi: 10.1038/nm.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li CJ, Cheng P, Liang MK, Chen YS, Lu Q, Wang JY et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. The Journal of Clinical Investigation. 2015;125(4):1509–22. doi: 10.U72/JCI77716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon AM, Manigrasso MB, O’Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. Journal of Bone and Mineral Research. 2002;17(6):963–76. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, Strecker S, Wang L, Kronenberg MS, Wang W, Rowe DW et al. Osterix-cre labeled progenitor cells contribute to the formation and maintenance of the bone marrow stroma. PloS one. 2013;8(8):e71318. doi: 10.1371/journal.pone.0071318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14(1):78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 40.Bartelt A, Koehne T, Todter K, Reimer R, Muller B, Behler-Janbeck F et al. Quantification of Bone Fatty Acid Metabolism and Its Regulation by Adipocyte Lipoprotein Lipase. International Journal of Molecular Sciences. 2017;18(6). doi: 10.3390/ijms18061264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13(1):11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]