Abstract
Objective
A 5% change in weight is a significant predictor for frailty and obesity. We ascertained how self-reported weight change over the lifespan impacts rates of frailty in older adults.
Methods
We identified 4,984 subjects ≥60 years with body composition measures from the National Health and Nutrition Examination Survey. An adapted version of Fried's frailty criteria was used as the primary outcome. Self-reported weight was assessed at time current,1 and 10 years earlier and at age 25. Weight changes between each time point were categorized as ≥ 5%, ≤5% or neutral. Logistic regression assessed the impact of weight change on the outcome of frailty.
Results
Among 4,984 participants, 56.5% were female, mean age was 71.1 years, and mean BMI was 28.2kg/m2. A weight loss of ≥ 5% had a higher association with frailty compared to current weight, age 25 (OR 2.94 [1.72,5.02]), 10 years ago (OR 1.68 [1.05,2.69]), and 1 year ago (OR 1.55 [1.02,2.36]). Weight gain in the last year was associated with increased rate of frailty (1.59 [1.09,2.32]).
Conclusion
There is an association between frailty and reported weight loss over time while only weight gain in the last year has an association with frailty.
Key words: Obesity, frailty, sarcopenia, pre-frailty
Abbreviations
- BMI
body mass index
- NHANES
National Health and Nutrition Examination Survey
Introduction
Frailty is the result of physical, psychological and social factors that contribute to a decline in the body's physiological reserve and its reduced ability to maintain homeostasis among life's stressors (1, 2). While a standardized pragmatic definition of frailty is still debated, Fried's landmark study operationalized frailty as a phenotype defined by a set of variables: unintentional weight loss of ≥10 lbs, self-reported exhaustion, slow gait speed, low energy expenditure and weak grip strength (frail ≥3, pre-frail 1 or 2, robust =0) (3). Frailty is strongly associated with functional losses, disability, increased healthcare utilization and higher cost of healthcare (1, 4, 5, 6, 7, 8).
Weight status is commonly assessed in healthcare settings; however, it may be underutilized as a metric to indicate current and future adverse health outcomes (9, 10). Changes in body composition occur with each decade of life and include a peaking of fat mass in the 7th decade followed by subsequent decline in both skeletal muscle and fat (11, 12) making the most commonly used metric, body mass index (BMI), a highly insensitive measure of weight status in older adults(13). Some older adults are at risk for what is known as sarcopenic obesity or a disproportionate loss of lean mass to gain of fat mass which is shown to be associated with higher rates of morbidity and mortality outside of both sarcopenia or obesity alone (14, 15). Long-term changes of loss or gain in body weight are associated with the highest mortality rates among persons in the general population while mortality is the lowest among those with modest weight changes(16). Weight cycling, or gaining/losing a similar amount of weight repeatedly, is known to be associated with higher disability and mortality rates(17). This harmful cycle emphasizes the importance of obtaining a weight history in clinical practice as even lower percent changes may be significant in those with frailty (18, 19, 20, 21, 22).
Gaining an understanding of longitudinal weight measures over a lifespan could be helpful for predicting future risk of disease and functional status. Such measures are easily captured using most outpatient electronic medical records. As both frailty and obesity are associated with similar adverse outcomes (4, 5, 6, 7, 8, 23, 24, 25), weight change trajectories may help clinicians assess for the development of frailty and other adverse health outcomes in older adulthood. The purpose of this study was to evaluate the relationship between self-reported weight change over a lifespan and frailty in a representative sample of US older adults.
Methods
Study Design and Participants
Participants included in the analysis were community dwelling older adults identified from 1999–2004 National Health and Nutrition Survey (NHANES) data. NHANES is a multistage probability survey conducted by the National Center for Health Statistics designed to assess the health and nutritional status of adults and children in the United States. The survey oversamples Non-Hispanic blacks, Mexican Americans, persons greater than 60 years of age. Results are therefore generalizable to the United States population. All manuals, procedures and data files are publicly available at http://www.cdc.gov/nchs/nhanes.html.
NHANES screened 38 077 individuals, interviewed 31 125, and then examined 29 402 in a mobile examination unit, with exams conducted by trained medical personnel. For this secondary analysis of data, we included participants aged 60 years older with body composition measures and frailty variables for a final analytical cohort of N =4 984. The local Institutional Review Board at Dartmouth College exempted this study from review due to the de-identified nature of all NHANES data in the database.
Baseline Characteristics
Self-reported sociodemographic characteristics including age, race, sex, physical activity levels, smoking status and co-morbid conditions were obtained from questionnaires completed by participants or their primary caregivers. Age was stratified into three categories as performed in our previous analyses: 60–69, 70–79, 80+ years (13, 26, 27). Race was reported as non-Hispanic White, non-Hispanic Black, and Hispanic American. Co-morbid conditions were self-reported using the question, “Has a doctor or other health professional ever told you that you have [medical condition]?” Smoking status was classified as current smoker, former smoker or never smoker. Physical activity was categorized as sitting, walking, performing light loads or heavy work using the question, “Please tell me which of these four sentences best describes your usual daily activities?”
Study Variables
Frailty: We defined frailty according to the phenotypic model (3), using participant self-reported and objectively measured data. This phenotypic definition consists of five criteria derived from the Cardiovascular Health Study (3, 28) as follows: unintentional weight loss of 10 pounds or more in a year; self-reported exhaustion; weakness defined by grip strength; slow walking speed; and low physical activity. We adapted the criteria to define each variable(26), respectively, using data available in NHANES: low body mass index (BMI)<18.5kg/m2 [59 (1.3%)]; difficulty walking between rooms [586 (10%)]; difficulty lifting or carrying 10 pounds [1,455 [27.1%)], gait speed <0.8 m/s [1,865 (31.13%)]; and self-reported perception of reduced physical activity compared to others [735 (14.1%)]. Frailty was defined as meeting three or more of the five following criteria and pre-frailty was defined as meeting 1 or 2 criteria. Individuals not meeting any criteria were classified as robust.
Anthropometric Measures: Weight was assessed using a self-reported questionnaire. Participants were asked to report their current weight, weight one year ago, weight 10 years ago, and weight at age 25. Participants were asked to provide their specific weight in kg and if not known to give their best guess. Additional questions included “During the past 12 months, have you tried to lose weight?” and “During the past 12 months have you done anything to keep from gaining weight?” Objective weight was measured on the right side of the body to the nearest tenth of a centimeter on an electronic digital scale (calibrated in kilograms), and height was measured using a stadiometer.
Statistical Analysis
All data were downloaded in September 2015 into a single dataset. Weight history data was combined in November 2017 following NHANES standard operating procedures, accounting for weighting, strata, primary sampling unit, and cluster. Descriptive statistics are presented as means ± standard errors, and counts (weighted percentages). Comparisons between groups were conducted using t-tests and chi-square tests of independence. We calculated self-reported percent weight change as the quotient of the difference between baseline year and year in question (1 year prior, 10 years prior or at age 25 years old). Meaningful weight loss/gain is categorized as ± a change of 5% or more (29). We created three categories: ≥5% weight loss; ≥5% weight gain; or no change in weight (−5 to +5% weight change). The latter category is represented as the referent in our models. A 5% change in weight loss or gain was used since it has been used as a significant predictor for both frailty and obesity in past studies (30, 31). Slope for each individual change was calculated as the participant's age at each of the three time points (quotient of Weight Time1.-Weight Time2 and Δ Age) and is represented as the change in weight per year. Multiple models were constructed to evaluate the effect of weight change (primary predictor — gain/loss of 5%) on the presence of frailty (primary outcome). Gait speed was not assessed in NHANES 2003–2004 therefore imputation by mean was used conditional on covariates to account for missing values using R (v 3.3.2) and the package mice for 3,645 participants(http://www.r-project.org). The package creates plausible data values from a distribution specifically designed for each data point; five imputed data sets are generated using predictive mean matching. The correction variables used were age, sex, education, race, diabetes, arthritis, congestive heart failure, cancer, and lean mass percent. The five data sets were averaged, resulting in a final imputed data set used for analysis. Analyses were run on the full imputed data set as well as a subset excluding the imputed variables to test the quality. The data presented in our results is based on full imputed data alone; data excluding imputed variables is not shown and presented elsewhere (26).
We constructed three incremental logistic regression models adjusting for co-variates: age, gender (Model 1); Model 1 co-variates plus race, education, smoking (Model 2); Model 2 co-variates plus diabetes, arthritis, coronary artery disease, and cancer (Model 3). Data are not shown for Model 1 and Model 2 but results did not change as we adjusted for additional variables. All logistic regression models assessed the impact of weight change (gain, loss, no change) for two different frailty outcomes (Frailty vs. Pre-Frailty/Robust, and Pre-Frailty vs. Robust). Visual representation of change in weight over time was plotted per individual with a LOESS local regression line (span = 0.7). All analyses were conducted using STATA v.14 (College Station, Texas) and R v3.5 (http://www.r-project.org). P values were considered statistically significant if they were less than the criterion level of 0.05.
Results
Table 1 presents the baseline characteristics of participants. Of 4,984 participants, 56.5% were female, mean age was 71.1 years and BMI was 28.2 kg/m2. Prevalence of pre-frailty and frailty was 40.1% and 9.1%, respectively. Robust participants were more likely to be non-Hispanic white and have a higher education level (p=<0.001). Frail patients were more likely to have comorbidities such as arthritis, diabetes and coronary artery disease (p=<0.001) but not more likely to have cancer (p=0.48). Nearly all frail patients met the criterion of weakness (96.9%) and the most common criterion identified for pre-frailty was slowness of gait (59.1%).
Table 1.
Baseline Characteristics of Participants
| Robust N= 2,246 (50.8%) | Pre-frail N= 2,195 (40.1%) | Frail N=541 (9.1%) | p-value | |
|---|---|---|---|---|
| Age, years | 68.7±0.22 | 73.3±0.23 | 74.9±0.5 | <0.001 |
| Women n(%) | 949 (47.2) | 1244 (65.6) | 336 (68.1) | <0.001 |
| Body Mass Index kg/m2 | 27.8±0.12 | 28.3±0.18 | 30.7±0.49 | <0.001 |
| Fat Mass % | 35.9±0.13 | 38.3±0.20 | 40.0±0.46 | <0.001 |
| Lean Mass % | 61.7±0.13 | 59.3±0.20 | 57.8±0.47 | <0.001 |
| Education | ||||
| >12 years | 986 (50.4) | 585 (32.3) | 105 (23.4) | <0.001 |
| Race | ||||
| Hispanic | 533 (5.9) | 522 (8.1) | 146 (10.8) | |
| Non-Hispanic White | 1387 (86.2) | 1203 (77.4) | 256 (70.5) | <0.001 |
| Non-Hispanic Black | 281 (5.4) | 403 (10.3) | 127 (15.8) | |
| Other | 45 (2.4) | 67 (4.2) | 12 (3.0) | |
| Smoking Status | 0.02 | |||
| Former | 1004 (44.2) | 1052 (49.1) | 271 (50.3) | |
| Never | 948 (43.9) | 889 (39.6) | 198 (35.4) | |
| Current | 288 (11.9) | 254 (11.4) | 69 (14.3) | |
| Comorbidities | ||||
| Diabetes | 356 (13.2) | 499 (21.0) | 205 (34.5) | <0.001 |
| Coronary Artery Disease | 297 (14.3) | 421 (20.6) | 152 (30.9) | <0.001 |
| Cancer | 418 (22.1) | 395 (20.8) | 103 (22.9) | 0.48 |
| Arthritis | 786 (38.3) | 1228 (59.8) | 363 (73.9) | <0.001 |
| Experience Confusion or Memory Problems | 145 (5.8) | 363 (15.9) | 201 (34.2) | <0.001 |
| # of Frailty Variables | ||||
| Low body mass index (BMI)<18.5kg/m2 | — | 39 (2.2) | 20 (3.7) | <0.001 |
| Difficulty walking between rooms | — | 150 (6.0) | 436 (79.0) | <0.001 |
| Difficulty lifting or carrying 10 pounds | — | 929 (44.9) | 526 (96.9) | <0.001 |
| Gait speed <0.8 m/s | — | 1,419 (59.1) | 446 (81.6) | <0.001 |
| Reduced physical activity compared to others your age | — | 367 (19.1) | 368 (79.7) | <0.001 |
Values represented are means± standard errors or counts (weighted percentages).
Table 2 outlines participants' self-reported weights and weight changes over time. Weight increased in all groups over time, with individuals with frailty losing weight, as compared to the pre-frail or robust groups within the past year or 10 years. Weight gain of ≥5% in the past year was associated with higher rates of frailty among those classified in other categories. When comparing each participant's weight at age 25 years old to his/her current weight, fewer individuals with frailty gained clinically significant weight and most had a greater than 5% weight loss. Changes in weight and rates of combination of frailty, pre-frailty and robust percentages can be found in Supplementary Table 1.
Table 2.
Weight Change and Rates of Classification Along Frailty Spectrum
| Robust | Pre-Frail | Frail | p-value | |
|---|---|---|---|---|
| Self-Reported Weight | ||||
| Current Weight, kg | 78.6±0.36 | 75.6±0.55 | 79.5±1.53 | <0.001 |
| 1 year ago, kg | 78.9±0.40 | 76.4±0.60 | 81.0±1.6 | 0.002 |
| 10 years ago, kg | 75.7±0.36 | 73.5±0.55 | 78.7±1.40 | <0.001 |
| At age 25 years, kg | 65.6±0.29 | 62.8±0.36 | 65.9±0.99 | <0.001 |
| Tried to lose weight, % | 556 (30.1) | 508 (27.3) | 100 (20.9) | <0.001 |
| Tried not to gain weight, % | 839 (42.0) | 660 (33.6) | 121 (26.4) | <0.001 |
| Self-Reported Weight Change,% | ||||
| 1 year to current weight | -0.6±0.16 | -1.2±0.20 | -2.2±0.6 | 0.002 |
| 10 years to current weight | 3.2±0.2 | 1.6±0.46 | -1.2±1.2 | <0.001 |
| Age 25 to current weight | 15.4±0.29 | 15.0±0.57 | 14.2±1.5 | 0.59 |
| 1 year to 10 years ago | 3.6±0.19 | 2.7±0.44 | 0.76±1.0 | 0.006 |
| 1 year to 25 years old | 15.7±0.3 | 15.8±0.56 | 16.0±1.4 | 0.97 |
| 10 years to 25 years old | 12.5±.32 | 13.2±0.46 | 14.7±0.9 | 0.052 |
| Weight Δ: 1 year to Current Weight | ||||
| ≥5% weight loss | 351 (15.6) | 429 (20.1) | 153 (27.1) | |
| No weight A (−5 to +5%) | 1628 (74.5) | 1425 (67.1) | 270 (56.9) | <0.001 |
| ≥5% weight gain | 236 (10.0) | 265 (12.7) | 83 (16.0) | |
| Weight Δ: 10 years to Current Weight | ||||
| ≥5% weight loss | 410 (15.6) | 602 (26.1) | 202 (36.7) | |
| No weight A (−5 to +5%) | 900 (42.4) | 700 (33.7) | 122 (25.8) | <0.001 |
| ≥5% weight gain | 897 (42.0) | 761 (40.1) | 171 (37.4) | |
| Weight Δ: Age 25 to Current Weight | ||||
| ≥5% weight loss | 159 (6.1) | 249 (11.6) | 105 (18.9) | |
| No weight A (−5 to +5%) | 317 (14.2) | 272 (13.0) | 49 (8.2) | <0.001 |
| ≥5% weight gain | 1684 (79.7) | 1480 (75.3) | 319 (72.9) | |
| Weight Δ: 10 years ago to One year ago | ||||
| ≥5% weight loss | 280 (10.5) | 421 (18.4) | 143 (25.2) | |
| No weight A (−5 to +5%) | 1069 (48.9) | 895 (42.9) | 189 (38.9) | <0.001 |
| ≥5% weight gain | 851 (40.5) | 743 (38.7) | 153 (35.8) | |
| Weight Δ: Age 25 year to One year ago | ||||
| ≥5% weight loss | 145 (5.5) | 219 (10.4) | 77 (14.7) | |
| No weight A (−5 to +5%) | 1175 (54.3) | 1036 (50.8) | 233 (47.5) | <0.001 |
| ≥5% weight gain | 821 (40.2) | 728 (38.8) | 149 (37.8) | |
| Weight Δ: Age 25 years to 10 years ago | ||||
| ≥5% weight loss | 101 (4.3) | 132 (6.6) | 44 (7.8) | |
| No weight A (−5 to +5%) | 441 (18.6) | 433 (21.8) | 92 (17.3) | 0.03 |
| ≥5% weight gain | 1619 (77.1) | 1422 (71.6) | 332 (74.9) | |
This was an ANOVA test and all values represented are mean ± standard error or counts (weighted percentage).
Multivariable logistic regression models evaluating the relationship between weight change during a specific time interval, and presence of frailty or pre-frailty are presented in Table 3. Weight loss of ≥5% was strongly associated with presence of frailty compared to pre-frailty/robust and the relationship strengthens with longer time intervals. A ≥5% weight gain only was associated with frailty if the gain occurred within the last year. Similar associations were seen with pre-frailty when comparing pre-frailty to robust status, albeit weaker than the association seen with frailty.
Table 3.
Association of Weight Change and Frailty Status
| Time Period | |||||||
|---|---|---|---|---|---|---|---|
| One year ago to Current Weight | 10 years ago to Current Weight | Age 25 years to Current Weight | 10 years ago to 1 year ago | Age 25 years to 1 year ago | Age 25 years to 1 year ago | ||
| Frailty vs. Pre-Frailty/Robust | ≥5% loss | 1.55 [1.02,2.36] | 1.68 [1.05,2.69] | 2.94 [1.72,5.02] | 1.40 [1.00, 1.96] | 2.03 [1.41,2.92] | 1.34 [0.75,2.41] |
| No weight A (−5 to +5%) | Ref | Ref | Ref | Ref | Ref | Ref | |
| ≥5% gain | 1.59 [1.09,2.32] | 1.26 [0.89,1.78] | 1.35 [0.90,2.02] | 1.08 [0.78, 1.48] | 1.16 [0.85,1.59] | 1.02 [0.71,1.45] | |
| Pre-Frailty vs. Robust | ≥5% loss | 1.35 [1.05,1.74] | 1.58 [1.19,2.09] | 1.64 [1.17,2.31] | 1.43 [1.05,1.95] | 1.91 [1.36,2.67] | 1.13 [0.77,1.66] |
| No weight A (−5 to +5%) | Ref | Ref | Ref | Ref | Ref | Ref | |
| ≥5% gain | 1.27 [1.03,1.57] | 1.26 [1.03,1.54] | 0.96 [0.73,1.25] | 1.22 [0.99,1.50] | 1.21 [0.98,1.50] | 0.69 [0.54,0.88] | |
Data are represented as odds ratio [95% Confidence interval]. Multivariable logistic regression models (referent category: no change in weight) are represented as odds ratios (95% confidence intervals). The primary predictor was weight change (gain ≥5%, loss of 5%, or no change in weight) during the time period. Separate multivariable models were created for the outcomes of Frailty vs. Pre-Frailty/Robust, and Pre-Frailty vs. Robust (each yes/no). Models presented were adjusted for: age (years as continuous variable), sex (female=1 male=0), race (non-Hispanic white (ref), non-Hispanic black, Hispanic and Other) education status >12 years (yes=1, no=0), smoking status (former, current=1, never=0) self-reported diabetes (yes=1, no=0) , arthritis (yes=1, no=0), coronary artery disease (yes=1, no=0), cancer (yes=1, no=0).
Figure 1 represents the change in weight as a function of age plotted with a LOESS smoothed line by frailty status. Graphically, a period of increased rate of weight gain is noted prior to a steep decline in weight for individuals who were frail at time of study inclusion; conversely, this was not observed in those with pre-frail or robust status. Additionally, frail individuals had a higher peak weight (87.3 kg), occurring earlier in life (58.4 years), relative to both pre-frail (peak weight 79.9 kg at age 59.9 years) and robust (79.0 kg peak weight at 63.0 years) individuals. This suggests that the earlier one reaches their peak weight in life, the higher incident frailty.
Figure 1.
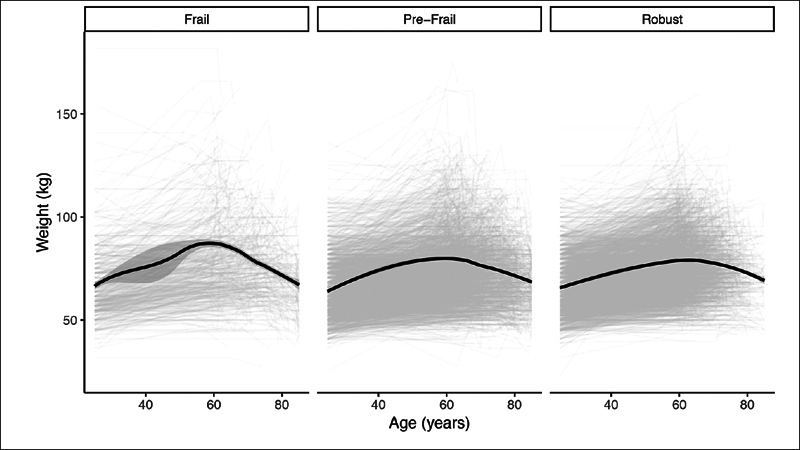
Change in Weight by Frailty Status. This figure represents a LOESS smoothed line demonstrating the change in weight as a function of age by frailty status
Discussion
Our findings demonstrate the importance of dynamic weight changes over a lifetime in the future development of frailty. While frailty is classically defined by weight loss (3), here we show that significant changes in weight gain or loss over a lifetime were associated with frailty progression making this a more complicated relationship than was previously thought. We created two separate analyses looking at frailty versus pre-frail and robust participants and pre-frail vs robust participants alone. Pre-frailty has individually been associated with an increase in overall mortality and cardiovascular mortality (26). Here we demonstrate that significant weight loss or weight gain is strongly associated with frailty and has a weaker, but statistically significant, association with pre-frailty supporting the significance of each step along the frailty spectrum.
While weight loss' association with frailty is well accepted, the relationship of weight gain with frailty is less understood. A 22 year follow-up study demonstrated obesity was associated with higher rates of pre-frailty and frailty compared to robust individuals, suggesting obesity could be a contributing factor to progression along the frailty spectrum (32, 33). Unsurprisingly, we showed that older adults with a ≥5% weight loss compared to 1 year ago, 10 years ago, and age 25 have significantly increased odds of having frailty suggesting that weight loss is a strong indicator of potential frailty development. Yet, a ≥5% weight gain only increased odds of frailty when this occurred within the last year, but not compared to 10 years ago or age 25. This seems to suggest that a sudden increase in weight may not be marker of health, but a weight trajectory trending more toward the concept of sarcopenic obesity (34).
Prior work has shown that when evaluating weight gain and loss, more lean mass is lost than is gained over repeated fluctuations suggesting weight cycling could accelerate sarcopenia in older adults and contribute to sarcopenic obesity (35). Weight cycling leads to greater central body fat and increased mortality (22). Some theorize that the trajectory of pathological aging is to move from robust status to sarcopenic obesity to frailty then to disability and mortality (36). Our previous data suggest that late adulthood weight changes over a 10 year period are predictive of sarcopenia development and that there is a healthy, natural propensity to gain weight over a life course (37). Our current findings demonstrate a similar trend as robust participants were more likely than those pre-frail or frail to have gained ≥5% weight over the course of the prior 10 years.
A natural, healthy trend toward weight gain could explain why the classification of overweight status in older adults, in part, has been noted as somewhat “protective” (38). Overweight status could be beneficial for reducing disability and functional loss with reduced osteoporosis and injuries from falls (39). An ability to gain or maintain weight demonstrates a reduced vulnerability to stressors from comorbid health conditions (40).
This study is not without limitations. First, the analysis relied on self-reported weights which may be impacted by recall bias but studies looking at self-reported weight accuracy and actual weight are reasonably comparable (41), however we were not able to assess the accuracy of recall in those with cognitive impairment included in the study. This was not done due to concurrent limitations in how those with cognitive impairment would be identified from NHANES variables (41). In clinical practice, weight 10 years prior and at age 25 would most likely be ascertained by recall, and therefore, use of self-report is ecologically valid. Second, the sample was comprised of community dwelling adults. Individuals living in facilities were not included, which limits the ability to generalize these findings to the older adult population as a whole. To operationalize Fried's frailty criteria in NHANES some of the original definitions were modified. Those labeled frail could be considered those at “high likelihood of frailty” by our modified metrics. However the accuracy of our results are further supported by the fact the prevalence of each component is comparable to those observed in other studies who did not require modification (3, 42, 43). As walking speed was missing for 3,645 patients, multiple imputations needed to be performed to maximize the number of participants with appropriate data. Multivariate imputation by chained equations, a robust method that generates multiple predictions for each missing value, taking the uncertainty of the imputations into account and yielding accurate standard errors (44), was used to handle missing data. The reasons for unintentional weight loss in participants was unknown therefore the implications of these factors behind their weight loss are also unknown. As with all cross-sectional studies, we are not able to make causal inferences. Lastly, the true relationship between weight change and incident to frailty may not necessarily be linear.
Conclusions
The results demonstrate an association between frailty and weight change over time. Weight loss over a lifespan is strongly associated with frailty while weight gain in the last year also is association with higher rates of frailty and can therefore be a marker of declining health. A natural trend toward weight gain and overweight status in a lifetime could actually be a sign of metabolic health and longevity. These findings demonstrate the clinical value in weight trends obtained at most clinical visits and highlight potential trends that may warrant closer evaluation for syndromes like frailty that require additional intervention to help curb poorer health outcomes.
Funding: Dr. Batsis receives funding from the National Institute on Aging of the National Institutes of Health under Award Number K23AG051681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Batsis has also received honoraria from the Royal College of Physicians of Ireland, Endocrine Society, and Dinse, Knapp, McAndrew LLC, legal firm. Dr. Mackenzie: none. Dr. Cook: none. Alexander Titus' research reported in this publication was supported in part by the National Institutes of Health under Award Number T32LM012204. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Mr. Petersen's research reported in this publication was supported by the Burroughs-Wellcome/Dartmouth Big Data in the Life Sciences Training Program. Dr. Crow's research reported in this publication was supported by The Dartmouth Center for Health and Aging and the Department of Medicine. Dr. Stevens' research is supported by National Institute of Mental Health (T32 MH073553, PI: Bruce, Fellow: Stevens). Support was also provided by the Dartmouth Health Promotion and Disease Prevention Research Center supported by Cooperative Agreement Number U48DP005018 from the Centers for Disease Control and Prevention. The findings and conclusions in this journal article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The authors acknowledge Friends of the Norris Cotton Cancer Center at Dartmouth and NCI Cancer Center Support Grant 5P30 CA023108-37 Developmental Funds.
Disclosures: There are no conflicts of interest pertaining to this manuscript
Acknowledgements
The authors would like to acknowledge Meaghan A. Kennedy MD, MPH who helped with editing and reviewing the transcript as well as support from the National Institute on Aging of the National Institutes of Health, Burroughs-Wellcome/Dartmouth Big Data in the Life Sciences Training Program, National Institute of Mental Health, Dartmouth Health Promotion and Disease Prevention Research Center, The Dartmouth Center for Health and Aging and the Dartmouth Hitchcock Medical Center Department of Medicine.
Footnotes
Electronic Supplementary Material
Supplementary material is available for this article at https://doi.org/10.14283/jfa.2019.44 and is accessible for authorized users.
Electronic supplementary material
Supplementary Table 1: Changes in Weight and Rates of Combination of Frailty, Pre-Frailty and Robust
References
- 1.Chong E, Ho E, Baldevarona-Llego J, Chan M, Wu L, Tay L. Frailty and Risk of Adverse Outcomes in Hospitalized Older Adults: A Comparison of Different Frailty Measures. J Am Med Dir Assoc. 2017;18(7):638.e7–638.e11. doi: 10.1016/j.jamda.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. PubMed PMID: 23395245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 4.Robinson TN, Wu DS, Stiegmann GV, Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg. 2011;202(5):511–514. doi: 10.1016/j.amjsurg.2011.06.017. PubMed PMID: 21890098, PMCID 3346286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bock JO, Konig HH, Brenner H, Haefeli WE, Quinzler R, Matschinger H, et al. Associations of frailty with health care costs—results of the ESTHER cohort study. BMC Health Serv Res. 2016;16:128. doi: 10.1186/s12913-016-1360-3. PubMed PMID: 27074800, PMCID 4831082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makizako H, Shimada H, Doi T, Tsutsumimoto K, Suzuki T. Impact of physical frailty on disability in community-dwelling older adults: a prospective cohort study. BMJ Open. 2015;5(9):e008462. doi: 10.1136/bmjopen-2015-008462. PubMed PMID: 26338685, PMCID 4563225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comans TA, Peel NM, Hubbard RE, Mulligan AD, Gray LC, Scuffham PA. The increase in healthcare costs associated with frailty in older people discharged to a post-acute transition care program. Age Ageing. 2016;45(2):317–320. doi: 10.1093/ageing/afv196. PubMed PMID: 26769469. [DOI] [PubMed] [Google Scholar]
- 8.Avila-Funes JA, Amieva H, Barberger-Gateau P, Le Goff M, Raoux N, Ritchie K, et al. Cognitive impairment improves the predictive validity of the phenotype of frailty for adverse health outcomes: the three-city study. J Am Geriatr Soc. 2009;57(3):453–461. doi: 10.1111/j.1532-5415.2008.02136.x. PubMed PMID: 19245415. [DOI] [PubMed] [Google Scholar]
- 9.Meltzer AA, Everhart JE. Unintentional weight loss in the United States. Am J Epidemiol. 1995;142(10):1039–1046. doi: 10.1093/oxfordjournals.aje.a117557. PubMed PMID: 7485049. [DOI] [PubMed] [Google Scholar]
- 10.Willett WC. Weight loss in the elderly: cause or effect of poor health? Am J Clin Nutr. 1997;66(4):737–738. doi: 10.1093/ajcn/66.4.737. PubMed PMID: 9322545. [DOI] [PubMed] [Google Scholar]
- 11.Beaufrere B, Morio B. Fat and protein redistribution with aging: metabolic considerations. Eur J Clin Nutr. 2000;54(Suppl 3):S48–S53. doi: 10.1038/sj.ejcn.1601025. PubMed PMID: 11041075. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1985;83(1):229–239. doi: 10.1152/jappl.1997.83.1.229. 1997. [DOI] [PubMed] [Google Scholar]
- 13.Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes (Lond) 2016;40(5):761–767. doi: 10.1038/ijo.2015.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi KM. Sarcopenia and sarcopenic obesity. Korean J Intern Med. 2016;31(6):1054–1060. doi: 10.3904/kjim.2016.193. PubMed PMID: 27809450, PMCID 5094937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cauley JA. An Overview of Sarcopenic Obesity. J Clin Densitom. 2015;18(4):499–505. doi: 10.1016/j.jocd.2015.04.013. PubMed PMID: 26141163. [DOI] [PubMed] [Google Scholar]
- 16.Andres R, Muller DC, Sorkin JD. Long-term effects of change in body weight on all-cause mortality, A review. Ann Intern Med. 1993;119(7):737–743. doi: 10.7326/0003-4819-119-7_part_2-199310011-00022. PubMed PMID: 8363208, Pt 2. [DOI] [PubMed] [Google Scholar]
- 17.Oh TJ, Moon JH, Choi SH, Lim S, Park KS, Cho NH, et al. Body-Weight Fluctuation and Incident Diabetes Mellitus, Cardiovascular Disease, and Mortality: A 16-Year Prospective Cohort Study. J Clin Endocrinol Metab. 2019;104(3):639–646. doi: 10.1210/jc.2018-01239. PubMed PMID: 30500906. [DOI] [PubMed] [Google Scholar]
- 18.Payette H, Coulombe C, Boutier V, Gray-Donald K. Weight loss and mortality among free-living frail elders: a prospective study. J Gerontol A Biol Sci Med Sci. 1999;54(9):M440–M445. doi: 10.1093/gerona/54.9.m440. PubMed PMID: 10536646. [DOI] [PubMed] [Google Scholar]
- 19.Somes GW, Kritchevsky SB, Shorr RI, Pahor M, Applegate WB. Body mass index, weight change, and death in older adults: the systolic hypertension in the elderly program. Am J Epidemiol. 2002;156(2):132–138. doi: 10.1093/aje/kwf019. PubMed PMID: 12117704. [DOI] [PubMed] [Google Scholar]
- 20.Tully CL, Snowdon DA. Weight change and physical function in older women: findings from the Nun Study. J Am Geriatr Soc. 1995;43(12):1394–1397. doi: 10.1111/j.1532-5415.1995.tb06620.x. PubMed PMID: 7490392. [DOI] [PubMed] [Google Scholar]
- 21.Alibhai SM, Greenwood C, Payette H. An approach to the management of unintentional weight loss in elderly people. CMAJ. 2005;172(6):773–780. doi: 10.1503/cmaj.1031527. PubMed PMID: 15767612, PMCID 552892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy RA, Patel KV, Kritchevsky SB, Houston DK, Newman AB, Koster A, et al. Weight change, body composition, and risk of mobility disability and mortality in older adults: a population-based cohort study. J Am Geriatr Soc. 2014;62(8):1476–1483. doi: 10.1111/jgs.12954. PubMed PMID: 25039391, PMCID 4134405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dee A, Kearns K, O'Neill C, Sharp L, Staines A, O'Dwyer V, et al. The direct and indirect costs of both overweight and obesity: a systematic review. BMC Res Notes. 2014;7:242. doi: 10.1186/1756-0500-7-242. PubMed PMID: 24739239, PMCID 4006977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visscher TL, Seidell JC. The public health impact of obesity. Annu Rev Public Health. 2001;22:355–375. doi: 10.1146/annurev.publhealth.22.1.355. PubMed PMID: 11274526. [DOI] [PubMed] [Google Scholar]
- 25.Hirani V, Naganathan V, Blyth F, Le Couteur DG, Seibel MJ, Waite LM, et al. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. Age Ageing. 2016 doi: 10.1093/ageing/afw214. [DOI] [PubMed] [Google Scholar]
- 26.Crow RS, Lohman MC, Titus AJ, Bruce ML, Mackenzie TA, Bartels SJ, et al. Mortality Risk Along the Frailty Spectrum: Data from the National Health and Nutrition Examination Survey 1999 to 2004. J Am Geriatr Soc. 2018;66(3):496–502. doi: 10.1111/jgs.15220. PubMed PMID: 29368330, PMCID 5849536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rippberger PL, Emeny RT, Mackenzie TA, Bartels SJ, Batsis JA. The association of sarcopenia, telomere length, and mortality: data from the NHANES 1999–2002. Eur J Clin Nutr. 2018;72(2):255–263. doi: 10.1038/s41430-017-0011-z. PubMed PMID: 29238037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K, Yashin AI. Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study. J Am Geriatr Soc. 2008;56(5):898–903. doi: 10.1111/j.1532-5415.2008.01656.x. PubMed PMID: 18363679, PMCID 2703425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2013;63(25):2985–3023. doi: 10.1016/j.jacc.2013.11.004. PubMed PMID: 24239920, 2014. [DOI] [PubMed] [Google Scholar]
- 30.Painter SL, Ahmed R, Hill JO, Kushner RF, Lindquist R, Brunning S, et al. What Matters in Weight Loss? An In-Depth Analysis of Self-Monitoring. J Med Internet Res. 2017;19(5):e160. doi: 10.2196/jmir.7457. PubMed PMID: 28500022, PMCID 5446667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bieniek J, Wilczynski K, Szewieczek J. Fried frailty phenotype assessment components as applied to geriatric inpatients. Clin Interv Aging. 2016;11:453–459. doi: 10.2147/CIA.S101369. PubMed PMID: 27217729, PMCID 4853008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stenholm S, Strandberg TE, Pitkala K, Sainio P, Heliovaara M, Koskinen S. Midlife obesity and risk of frailty in old age during a 22-year follow-up in men and women: the Mini-Finland Follow-up Survey. J Gerontol A Biol Sci Med Sci. 2014;69(1):73–78. doi: 10.1093/gerona/glt052. PubMed PMID: 23640762. [DOI] [PubMed] [Google Scholar]
- 33.Strandberg TE, Sirola J, Pitkala KH, Tilvis RS, Strandberg AY, Stenholm S. Association of midlife obesity and cardiovascular risk with old age frailty: a 26-year follow-up of initially healthy men. Int J Obes (Lond) 2012;36(9):1153–1157. doi: 10.1038/ijo.2012.83. [DOI] [PubMed] [Google Scholar]
- 34.Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999–2004. Nutr Res. 2015;35(12):1031–1039. doi: 10.1016/j.nutres.2015.09.003. PubMed PMID: 26472145, PMCID 4825802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82(4):872–878. doi: 10.1093/ajcn/82.4.872. PubMed PMID: 16210719, quiz 915–6. [DOI] [PubMed] [Google Scholar]
- 36.Cederholm T. Overlaps between Frailty and Sarcopenia Definitions. Nestle Nutr Inst Workshop Ser. 2015;83:65–69. doi: 10.1159/000382063. PubMed PMID: 26484770. [DOI] [PubMed] [Google Scholar]
- 37.Batsis JA, Petersen CL, Crow RS, Cook SB, Stevens CJ, Lillian SM, et al. Weight Change and Risk of Sarcopenia: Data from the National Health and Nutrition Examination Surveys 1999–2004 [Abstract]. 2018 International Frailty & Sarcopenia Conference, Miami Beach, FL. 2018. https://frailty-sarcopenia.com/docs/abstracts-2018pdf [accessed 18 September 2018].
- 38.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi: 10.1001/jama.2012.113905. PubMed PMID: 23280227, PMCID 4855514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowen ME. The relationship between body weight, frailty, and the disablement process. J Gerontol B Psychol Sci Soc Sci. 2012;67(5):618–626. doi: 10.1093/geronb/gbs067. PubMed PMID: 22967933, PMCID 3536552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12(6):913–920. doi: 10.1038/oby.2004.111. PubMed PMID: 15229329. [DOI] [PubMed] [Google Scholar]
- 41.Luo J, Thomson CA, Hendryx M, Tinker LF, Manson JE, Li Y, et al. Accuracy of self-reported weight in the Women's Health Initiative. Public Health Nutr. 2019;22(6):1019–1028. doi: 10.1017/S1368980018003002. PubMed PMID: 30449294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12(2):719–736. doi: 10.1016/j.arr.2012.03.001. PubMed PMID: 22426304. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Garrido J, Ruiz-Ros V, Buigues C, Navarro-Martinez R, Cauli O. Clinical features of prefrail older individuals and emerging peripheral biomarkers: a systematic review. Arch Gerontol Geriatr. 2014;59(1):7–17. doi: 10.1016/j.archger.2014.02.008. PubMed PMID: 24679669. [DOI] [PubMed] [Google Scholar]
- 44.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20(1):40–49. doi: 10.1002/mpr.329. PubMed PMID: 21499542, PMCID 3074241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Changes in Weight and Rates of Combination of Frailty, Pre-Frailty and Robust


