Abstract
In light of the sophisticated communication abilities of females in ant societies and their associated chemical ecology and sensory physiology, male ants are largely ignored and accordingly, little is known about their olfactory sensory capabilities. To address this, we explored peripheral odor sensitivities in male Harpegnathos saltator by measuring the electrophysiological activity of olfactory sensory neurons housed within antennal trichoid and coeloconic sensilla using an extracellular recording technique. In an initial trial against a panel of 46 compounds, sensilla trichodea responded strongly to two alarm pheromone components, while a limited number of general odorants elicited strong responses in sensilla coeloconica and both sensillar types were indifferent to 31 cuticular hydrocarbons (CHCs). In a dedicated search for CHC-responding sensilla and a smaller panel of six compounds, we found some sensilla that responded to a synthetic CHC and CHCs from virgin queen postpharyngeal glands which are potentially used for close range mate recognition. Olfactometric bioassays of male ants with 15 general odors corroborated the sensory responsiveness with respective behavioral responses. Overall, when comparing olfactory responses between H. saltator males and females, we found that sensilla coeloconica and basiconica of workers showed stronger responses and broader selectivity to all delivered compounds. The rarity of CHC-responding trichoid sensilla in Harpegnathos males hints to a specific role in sexual communication compared to females that use CHCs in a broader communication and identification context and that have sensilla responding robustly to a broad range of CHCs.
Keywords: Harpegnathos saltator, male, antennal sensilla, odor coding, electrophysiology, behavioral bioassay
Introduction
In the life of social insects, accurate and efficient communication plays a key role in colony integrity and maintenance. In ants, the majority of communication is mediated by olfactory cues occurring in the colony (Hölldobler and Wilson, 1990). For instance, behaviors such as exploiting a profitable food source, evading a possible danger, and finding a compatible mate are principally driven by volatile trail, alarm, and sex pheromones (Tumlinson et al., 1971; Hernández et al., 1999; Moser et al., 2004). Furthermore, distinct cuticular hydrocarbon (CHC) profiles of different castes impart information regarding sex, age, colony and reproductive status, that is sensed and subsequently decoded by a well-developed peripheral olfactory system in advance of an appropriate behavioral response (Peeters et al., 1999; Liebig et al., 2000; Cuvillier-Hot et al., 2001; Ozaki et al., 2005; Leonhardt et al., 2007; Liebig, 2010; van Zweden and d’Ettorre, 2010).
The responsiveness of female antennal sensilla to single CHCs or CHC mixtures has been already investigated in a number of ant species. It has been suggested that antennal sensilla of female Camponotus japonicus and Formica yessensis respond in an all-or-none manner to the CHC extracts of foreign conspecific ants implying that discrimination of foreign ants from colony members occurs at the sensillar level of the antennae (Ozaki et al., 2005; Kidokoro-Kobayashi et al., 2012). However, similar binary response profiles have not been found in other species such as Camponotus floridanus leading to more conventional??, integrative odor coding models (Brandstaetter and Kleineidam, 2011; Sharma et al., 2015). Moreover, we recently found that the responsiveness of the peripheral olfactory system in workers of Harpegnathos saltator varies in regards to reproductive status (Ghaninia et al., 2017). In this species, workers act either as helpers and raise brood or they may become reproductive individuals, so-called gamergates. These gamergates displayed reduced responses to long-chained hydrocarbons while their sensitivity to other compounds was unchanged relative to non-reproductive nestmates. In other ants, electroantennogram (EAG)-based studies demonstrated the sensitivity to single long-chained hydrocarbons (D’Ettorre et al., 2004; Holman et al., 2013). Using calcium imaging technique, the antennal lobe (AL) activity profile of nestmate and non-nestmate CHC extract recognition was examined in the ant C. floridanus (Brandstaetter and Kleineidam, 2011). In this study while the spatial pattern of odor-activated ALglomeruli was identical for both nestmate and non-nestmate CHC extracts, the olfactory sensory neurons (OSNs) treated with nestmate extract elicited a significantly stronger response in those glomeruli which may reflect differences in the relative abundance of individual components of these two otherwise similar CHC profiles (Brandstaetter and Kleineidam, 2011).
In H. saltator, males mate with workers inside the colony and, subsequently, fly out to mate with alate queens outside the colony (Peeters et al., 2000). Alate queens that are ready for the mating flight develop a distinct CHC profile that is rich in olefins which is not present after nest foundation (Liebig et al., 2000). The development and subsequent loss of the olefin-rich CHC profile in alate queens suggests an involvement in mate recognition in this species. Therefore, we expect males of H. saltator to be able to perceive alkenes and alkedienes present in the CHC profile of alate queen, although males may not require the ability to detect CHCs inside the nest. Despite the fact that ants rely heavily on chemical communication and produce a large number of hydrocarbons, our knowledge regarding the perception of these chemicals by the peripheral olfactory system of male ants is still scant.
The recent sequencing of the H. saltator genome (Bonasio et al., 2010), and the subsequent discovery of a large number of differentially expressed chemosensory receptors between female and male H. saltator (Zhou et al., 2012), have renewed interest in the investigation of functional characteristics of the candidate receptors expressed in the chemosensory neurons. Although male antenna mostly show much lower transcript abundance levels of chemosensory receptors compared to females, there is almost always some expression of any receptor (Zhou et al., 2012) which are likely to encode hydrocarbon receptors. This opens up the possibility that males still are sensitive to hydrocarbons. To address this, we have investigated the physiological responses of trichoid and coeloconic sensilla and associated responses of male H. saltator to various compounds that include CHCs and general odors and compared them with female-associated sensilla coeloconica. To our knowledge, these are the first electrophysiological recordings from the antennal sensilla of males of any ant species.
Methods and Materials
Animals
The ants used in this study were the derived from colonies originating from India (Liebig et al., 1998). The colonies were maintained in the laboratory at 25 °C with a 12L:12D period and housed in plastic container with plaster covered floors. See Haight (2012) for further details regarding colony maintenance.
Scanning electron microscopy
The isolated antennae of adult male H. saltator were mounted on a specimen holder, and coated with gold-palladium using a Technics Hummer II sputter-coater. The specimens were imaged with a scanning electron microscope (JEOL JSM-6300) operated at 15 kV. Brightness and contrast adjustments were made using Adobe Photoshop CS5.
Electrophysiology
For preparation, electrophysiology, and odor presentation we followed a Single Sensillum Recording (SSR) protocol established by (Ghaninia et al., 2017). Briefly, the whole body of a male individual including antennae was gently mounted on a thin layer of modeling clay already flattened on a glass slide. The preparation was then put on the mounting plate of an Olympus microscope to view the sensilla of interest at 750X for extracellular recordings. A sharpened capillary glass (ground) electrode was inserted into the thorax. Another sharpened (recording) electrode was mounted on a motorized micromanipulator (World Precision Instruments, FL, USA) by which fine movement towards sensilla of interest was facilitated. Silver wires were slipped over both electrodes which had been filled with insect ringer solution. Upon the insertion of the recording electrode into the sensillum a contact with neurons residing inside was made. The spontaneous activity of the neurons was then amplified, digitized, and eventually visualized on a PC computer screen (Lenovo with windows XP). In total, 51 stimuli consisting of insect hydrocarbons, general odor, and controls were used in this study (46 odorants, 5 controls). The identities and other characteristics of the compounds are presented in Table S1. Hydrocarbons heavier than C23 were delivered by applying a ≥0.5 s heat shock using a micro-torch (BernzOmatic, NY, USA). The hydrocarbons remain intact with this procedure (Ghaninia et al., 2017). Sensillar responses were considered as non-responding when the increase in the firing frequency was ≤10 spike/s. The detailed spike counting procedure and analysis of electrophysiological responses are presented in (Ghaninia et al., 2017).
Two series of male SSR were conducted. We started with the full 46 compound panel. Two years later, we examined the responses of male trichoid sensilla to a reduced odorant panel consisting of 4-methyl-3-heptanone, 4-methyl-3-heptanol, 6-methyl-5-hepten-2-one, ethyl acetate, heptacosane (C27) and postpharyngeal gland extracts of alate H. saltator queens. In this recording series, we focused on the search for CHC-responding sensilla by first delivering C27 and PPG extracts. Only if the current sensillum responded to either of these compounds on a subjective scale, the panel was completed. After analyzing these data, only sensillum recordings that showed more than 30 spikes/s for either C27 or PPG extracts were retained. In this series, we separated recordings for long and short sensilla trichodea.
Extraction and chemical analysis of postpharyngeal glands (PPGs)
The PPGs of six virgin alate queens were gently dissected out and placed in separate 2ml glass vials containing 800μl of hexane (Sigma-Aldrich, St. Louis, MO). After five minutes the glands were removed and the extracts were concentrated down to 200μl using N2 gas. The concentrated extracts were then used for the SSR experiments and chemical analyses to verify the presence of alkenes and alkadienes. For the chemical analyses, 1μl of the individual extracts was injected into an Agilent 6890N gas-chromatograph (GC) (Agilent, Santa Clara, CA, USA) equipped with a DB-MS1 (J&W Scientific) non-polar capillary column (30m × 0.25mm × 0.25μm), connected to an Agilent 5975 mass spectrometer under the following conditions: the injection port was set to 280° and the transfer line to 300°. Helium was used as carrier gas with a constant flow of 1ml/min. Oven temperature was set to 60°C for 2 min before was rising with 40°C/min to 200°C and then increasing with 5°C /min to 320°C which was held for 20 min. Samples were injected in the splitless mode with a splitless time of 30sec. Electron impact mass spectra were measured at 70eV, with a source temperature of 230°C.
Behavioral assay
The behavioral activity of the general odors that elicited electrophysiological responses in the OSNs of H. saltator males were examined using a one-way olfactometer comprised of a 9 cm long and 1 cm diameter glass tube whose one end was connected to a continuous airflow (40ml/min) was used. The tube was covered by metal and fabric mesh at the upwind and downwind ends, respectively. Eleven individuals, each belonging to a distinct colony, were chosen for this experiment. Prior to a trial, each individual was placed in the olfactometer until it settled. Subsequently a panel of 15 general odors (Table 1) was individually air-puffed (with a 0.5s air pulse) through a hole located at the upwind end of the glass tube. Stimuli order was randomized for each trial. Thirty seconds after a stimulus delivery, a blank air puff was delivered as a control for any spontaneous agitations. In case of agitation due to prior odor delivery, subsequent odor presentation was done 30 s after the animal was settled. For each delivered compound, ants were given a discrete response score in real time ranging from 0 to 3; no physical response (0); antennal movement only (1); antennal movement coupled with elevation of anterior end of the body and minor movements less than half a body length (2); displacement of at least half a body length in any direction (3). Given the clear behavioral response in preliminary tests and the lack of a clear expectation of the odorant effect, the behavioral assessment was done without blinding.
Results
Gross features of antennal trichoid and coeloconic sensilla
The filiform antenna of male H. saltator possesses 11 flagellomeres which are decorated with different types of sensilla (Figure 1A, B). Cuticular, hairshaped sensilla consist largely of sensilla trichodea which appear evenly distributed over the antenna. These sensilla, which taper distally, are further subdivided into two types: long (~40μm long) with sharp tips and short (~15μm long) with slightly blunter tips (Figure 1A, B). In contrast, sensilla coeloconica (also known as pitted pegs) are hollow structures with a rim of about 3–4μm diameter from which a grooved peg projects and are much less abundant (Figure 1C). We did not find any sensilla basiconica that frequently occur in female antenna (Ghaninia et al., 2017).
Figure 1:
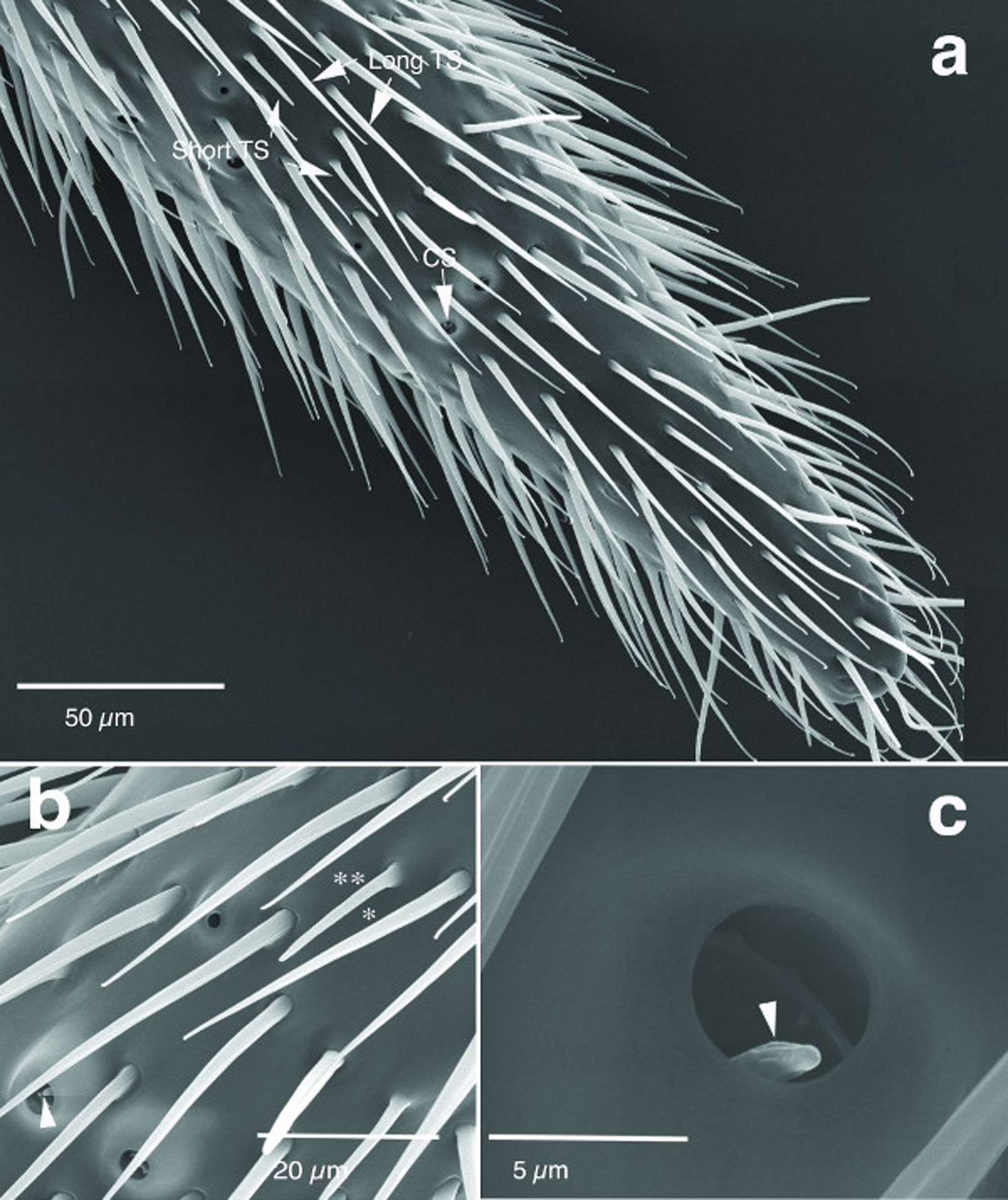
Scanning electron micrographs of antennal sensilla of H. saltator. A and B) Long (single asterisk) and short (double asterisk) trichoid sensilla (TS), and coeloconic (arrowhead) sensilla (CS) of a male. C) A coeloconic sensillum of a female (worker).
Function of the male antennal sensilla
In order to describe the general olfactory abilities of male antennae, we investigated the responsiveness of OSNs housed in sensilla trichodea (regardless of morphological subtype, see Figure 1) and sensilla coeloconica to an odorant panel that includes general odors and long-chained hydrocarbons. With the exception of prominent responses to 6-methyl-5-hepten-2-one and 4-methyl-3-heptanol, only minor or no responses (below an average of 11 spikes/s) were observed in the OSNs associated with trichoid sensilla in response to the delivery of the rest of the panel (Figure 2A). OSNs housed in sensilla coeloconica similarly responded with excitation and inhibition to a narrow subset of odorants - they were strongly excited by 2,3-butanedione, propanal, formic acid, and acetic acid, whereas 1-hexenol elicited a slight inhibition (Figure 2B).
Figure 2:
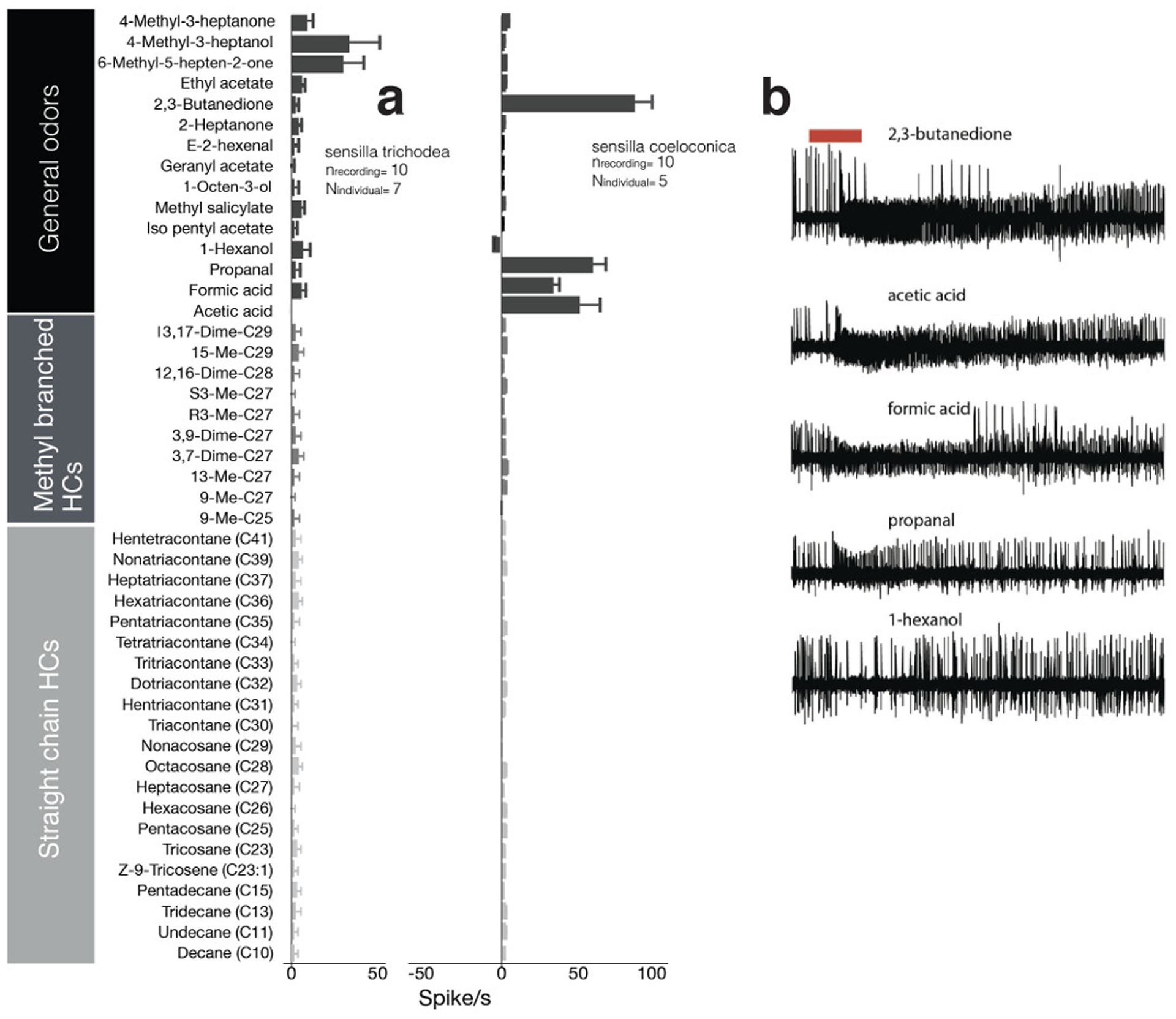
A) Response patterns of trichoid and coeloconic sensilla of male H. saltator, and B) Single sensillum recordings made from coeloconic sensilla showing excitation and inhibition of the associated neurons upon a 0.5 s stimulus delivery (red bar). Data associated with both long and short trichoid sensilla in panel A are pooled because initially we did not separate the two morphological subtypes.
The general tuning of male insect antennae to female pheromones lead to the suggestion that males may only benefit from detecting certain CHCs (alkenes and alkadienes) at specific times, e.g., when seeking mates, as they have been observed to leave the colony concurrently with virgin alate queens for their mating flights (Liebig et al., 2000). Given that virgin queens presumably develop a CHC profile for mate attraction (Liebig et al., 2000), we collected hexane extracts of postpharyngeal glands (PPG), which are known to have high resemblance to the CHC profile (e.g. (Leonhardt et al., 2007), of virgin queens for subsequent delivery to the male sensilla. The CHC profile of these glands consisted of 90.53 ± 2.02 % (N=5, mean plus SD) of alkenes and alkadienes that are not present in PPG or cuticular extracts of other females (Figure 3, (Liebig et al., 2000).
Figure 3:
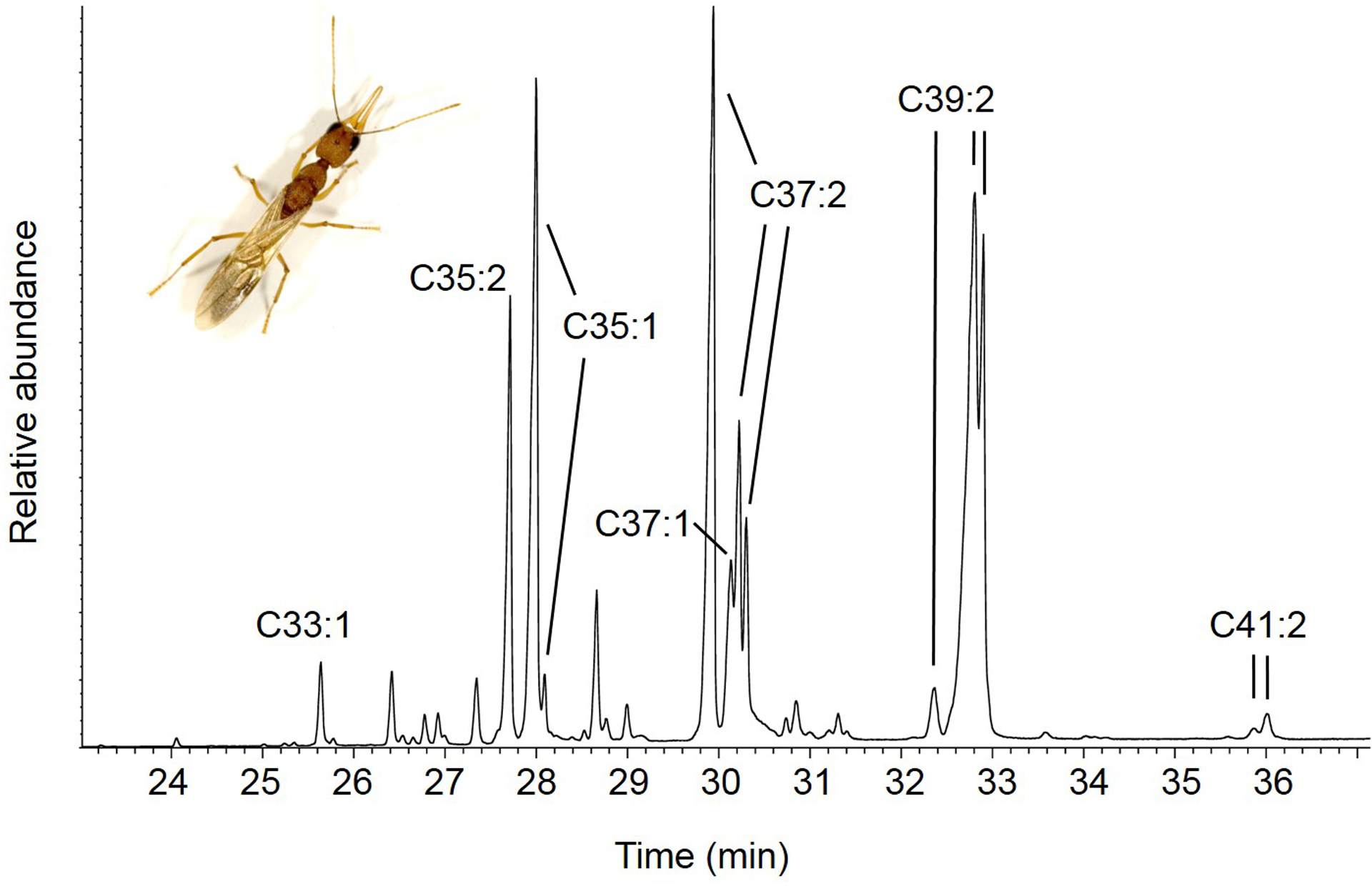
Representative chromatogram of PPG extracts of alate queens. Olefin peaks are indicated with chain length and number of double bonds. Minor tricosane and pentacosane peaks are not included in the time frame of the chromatogram selection.
The fact that CHC-sensitive olfactory receptors are expressed at some level in the antenna of H. saltator males suggests that males should be able to detect long-chained CHCs (Zhou et al., 2012; Pask et al., 2017; Slone et al., 2017). For the purpose of finding CHC responding sensilla, C27 and PPG extracts were first delivered and only if a response to either of the two was detected, the complete set of odorants was delivered. We investigated the response profiles of the short sharp and long sharp sensilla trichodea subtypes of males to these stimuli (Figure 1). We found that the two sensilla subtypes indeed contained sensilla that responded to either C27 or PPG extracts or both (Figure 4).
Figure 4:
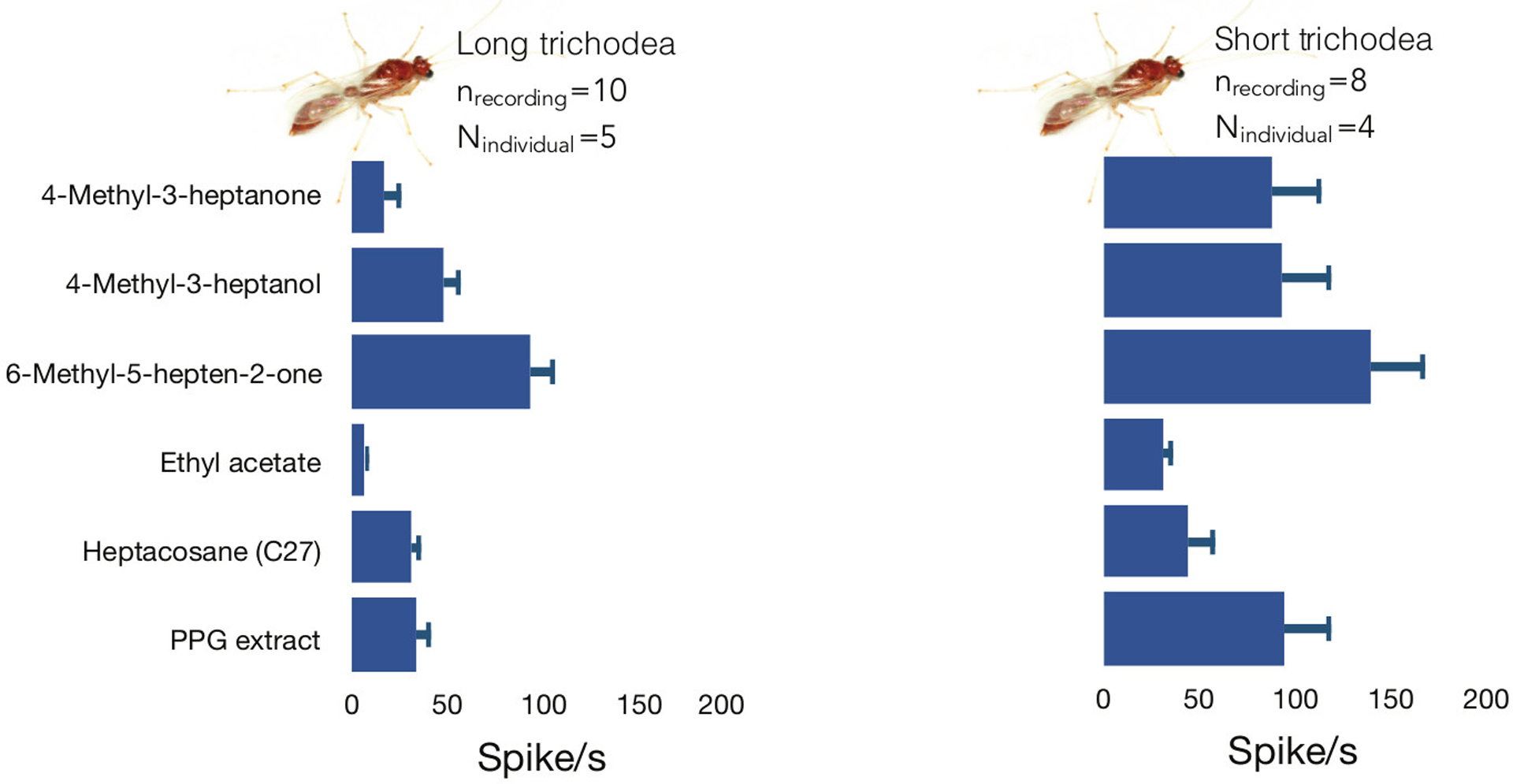
Sensillar response patterns to a panel of six compounds including postpharyngeal (PPG) extracts. (A) Cluster analysis of the responses of short and long male sensilla trichodea. (B) Male sensillar responses to individual compounds. (C) Responses of sensilla trichodea and coeloconica of worker H. saltator to the compound panel.
Function of the female sensilla coeloconica
Because the coeloconic-associated sensilla of males were narrowly tuned to only a few general odors and did not respond to any of the CHCs tested, we examined whether female sensilla coeloconica displayed a similarly narrow response spectrum. Given that we recently found that the OSNs associated with sensilla basiconica display a lowered sensitivity to long-chained hydrocarbons in reproductive worker females, so-called gamergates, compared to non-reproductive workers (Ghaninia et al., 2017), we investigated the sensillum responsiveness separately for these two worker types using the complete set of compounds, excluding 4-methyl-3-heptanone. In contrast to the male-associated sensilla, the sensilla coeloconica of workers and gamergates broadly and strongly responded to all compounds tested except formic acid and acetic acid which elicited weak responses (Figure 5).
Figure 5:
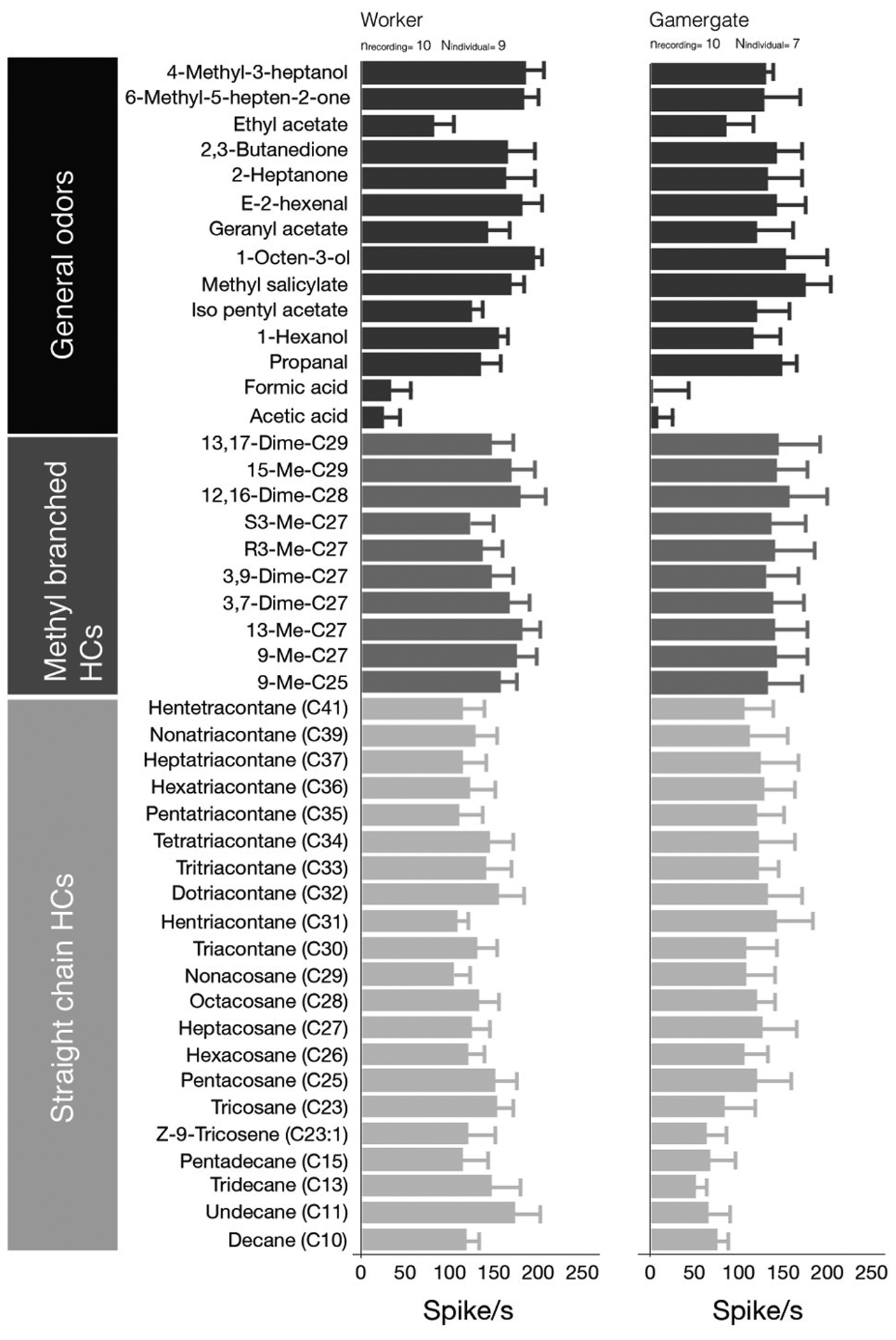
Response patterns of sensilla coeloconica of worker and gamergate H. saltator to a panel of 45 odors.
Behavioral responses of male H. saltator to general odors
To verify the activity of the electrophysiologically active compounds and to evaluate the response of H. saltator males to general odors, a behavioral experiment was carried out in which the behaviors of males in a one-way olfactometer (Figure 6A) were observed and recorded. Here, males displayed antennal movement (response score 1) mainly in response to 4-methyl-3-heptanone, 2,3-butanedione, methyl salicylate, isopentyl acetate, 1-hexenol, propanal, formic acid, and acetic acid (Figure 6A). Strong agitation (response score 3) was observed when 4-methyl-3-heptanol and 6-methyl-5-hepten-2-one were presented (Figure 6B). Other compounds elicited responses though at a lower intensity (Figure 6B). Hydrocarbons were not tested because we did not get any responses by males or females to hydrocarbon exposure in preliminary experiments.
Figure 6:
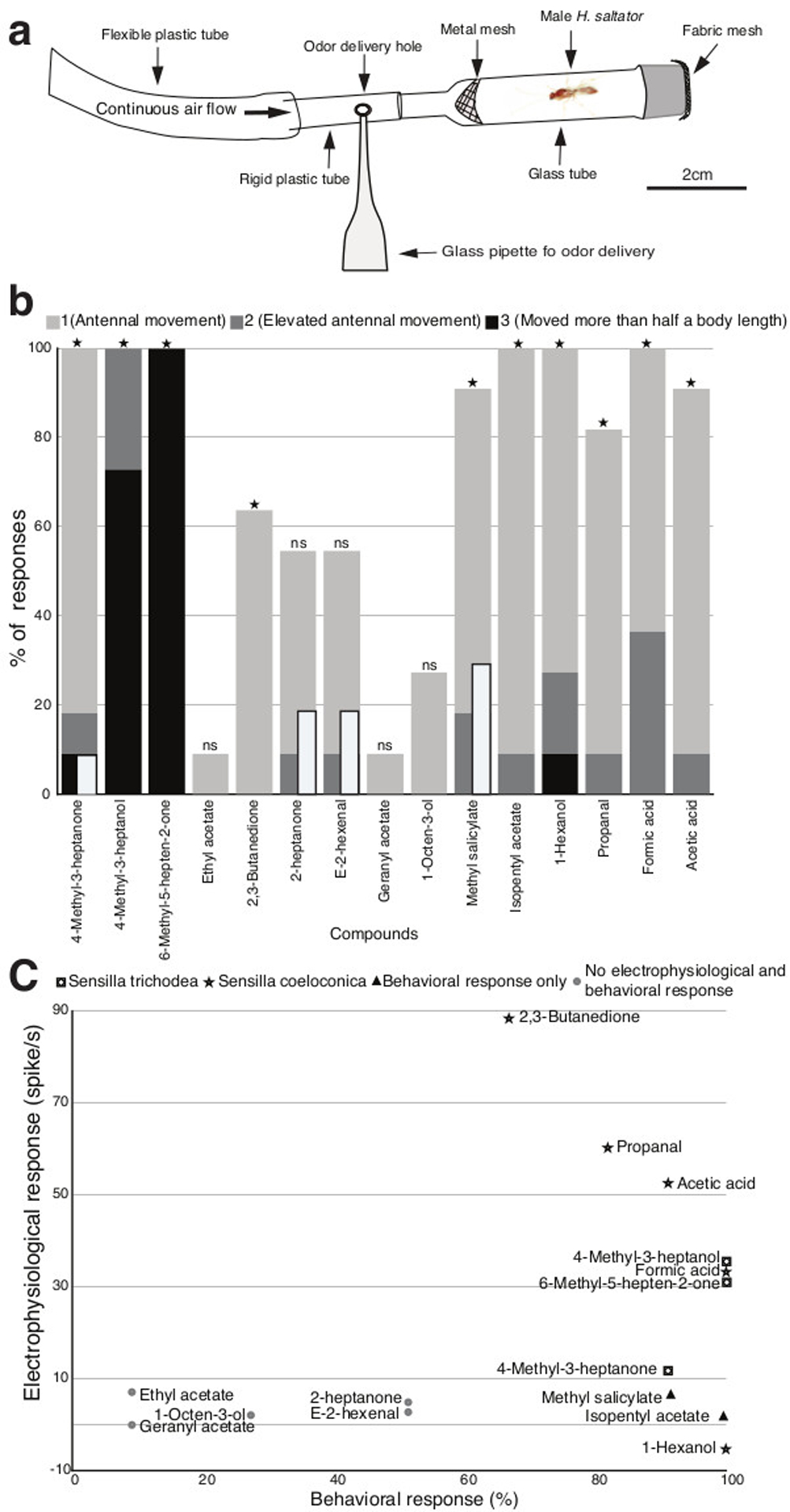
Male responses to an odorant panel and their relationship to SSR results (A) Olfactometric bioassay set up. (B) Olfactometer responses of eleven male H. saltator to a panel of 15 general odors. Responses of ants with no antennal movement were considered as non-responding (response score 0). Responses were classified as “1” when ants displayed only antennal movement, “2” when antennal movement was coupled with anterior elevation and body movement less than half a body length, and “3” when ants moved more than half a body length. The inserted bars indicate responses at level 1 to blank controls. Responses above 1 were not observed for blanks. (Fisher’s exact test for comparisons of number of responses to odorant versus blank control with Holm-Bonferroni correction for multiple comparisons, N = 11, *: P < 0.05, ns: not significant) (C) Plot of sensillar responses to behavioral responses of male H. saltator when confronted with a panel of 15 general odors. The stronger response of trichoid or coeloconic sensilla is displayed. 1-Hexanol is in the negative y-axis range because it elicited an inhibitory response in the OSNs.
We then plotted sensillar responses against behavioral responses and found that all the general odors that evoked electrophysiological responses, including 2,3-butanedione, propanal, acetic acid, 4-methyl-3-heptanol, formic acid, 6-methyl-5-hepten-2-one, 4-methyl 3-heptanone, and 1-hexanol, also elicited behavioral responses in the males (Figure 6C, See also Figure 2). Interestingly, even though methyl salicylate and isopentyl acetate did not on average activate antennal OSNs beyond 7 spikes/s, males exhibited robust antennal movement in response to these compounds. Ethyl acetate, geranyl acetate, 1-octen-3-ol, 2-heptanone, and e-2-hexenal elicited little or no sensillar nor significant behavioral responses (Figure 6C).
Discussion
In what to our knowledge is the first systematic study to elucidate odor responses of antennal OSNs of male ants using an SSR-based approach, we determined the extent of physiological responses of sensilla trichodea and coeloconica of male H. saltator using two sets of biologically relevant odor stimuli, CHCs and general odors. The data indicate that the male ant sensilla trichodea house OSNs that are narrowly tuned to the alarm pheromone components and which do not overlap with the response spectra of sensilla coeloconica that are uniquely tuned to a narrow range of acids, aldehydes, and alcohol. By scanning a large number of trichoid sensilla for responses to long-chained hydrocarbons, we found additional rare CHC-responding trichoid sensilla of both, short and long, subtypes, suggesting that males can perceive CHCs. Furthermore, olfactometric bioassays verified that the male ants display behavioral responses to the electrophysiologically active general odor stimuli which correlate with the life habits of the male ants.
Our finding of CHC-responding trichoid sensilla demonstrates that H. saltator males are not dependent on the presence of basiconic sensilla for CHC detection as they are absent in these males. Basiconic sensilla had been previously implied in the detection of long-chained hydrocarbons typically present in ant CHC profiles (Ozaki et al., 2005) which suggested at that time that males might not be able to perceive CHCs. Our findings are corroborated by the expression of CHC sensitive olfactory receptors in H. saltator male antenna (Pask et al., 2017). In fact, male CHC sensitivity is expected in the context of mate communication. Harpegnathos males leave the colony concurrently with virgin alate queens for their mating flights (Liebig et al., 2000) which may require detection of alkenes and alkadienes, the major CHC compounds of the queens at that time suggesting a role in sexual communication during the mating flights.
In our study, general odors did not elicit a robust response in the OSNs associated with trichoid sensilla. While it is possible that the limited set of compounds tested did not include compounds to which these sensilla can respond, it is also possible that these sensilla are specifically tuned to pheromones produced by virgin queens (Liebig et al., 2000). The response pattern of sensilla in this study indicates excitation of the neurons stimulated with 4-methyl-3-heptanone, 4-methyl-3-heptanol and 6-methyl-5-hepten-2-one. These compounds seem to have bio-ecological importance to males as they are classified as volatile substances of alarm pheromone blend in H. saltator secreted from mandibular glands (do Nascimento et al., 1993; Blum et al., 1994; Jarvis et al., 2004). 4-methyl-3-heptanone is considered as the major constituent of the alarm pheromone comprising 87.3% of the blend which has been shown to elicit a strong typical alarm behavior (do Nascimento et al., 1993; Blum et al., 1994). In the composition of alarm pheromone 4-methyl-3-heptanol and 6-methyl-5-hepten-2-one are among the minorities, comprising 3.7% and 0.2% of the blend, respectively (do Nascimento et al., 1993; Blum et al., 1994). The detection and proper response to alarm pheromone components plays a vital role in the colony cohesion as they alert the colony members of a possible danger (Maschwitz, 1964; Blum, 1969; Crewe, 1973; Alonso and Vander Meer, 1997).
In most insect species, sensilla coeloconica are known to contain thermo/hygro receptors, while an olfactory functionality has been shown in some species (Pophof, 1997; Yao et al., 2005). Here, we show that the sensilla coeloconica of male H. saltator respond strongly to a limited subset of compounds including, e.g., acetic acid, formic acid, propanal, and hexanol. This trend of responses to acids, aldehyde, and alcohol is similar to the response pattern seen in the sensilla coeloconica of Drosophila, which express ionotropic receptors (IRs), another type of olfactory chemoreceptor protein that detects almost exclusively the above named chemicals (Yao et al., 2005; Benton et al., 2009). The expression of IRs was also reported in other insect species, including ants (Benton et al., 2009; Olivier et al., 2011; Zhou et al., 2012; Koch et al., 2013; Pitts et al., 2017). In the ant H. saltator, 23 IR genes were annotated, of which 2 were found over-expressed in males (Zhou et al., 2012). Although currently we do not have molecular evidence that sensilla coeloconica OSNs of males exclusively express IRs, based on their response pattern and their resemblance to Drosophila sensilla coeloconica, it is reasonable to suggest that these sensilla coeloconica-associated OSNs of males express mainly, if not exclusively, IRs.
In contrast to males, the sensilla coeloconica of females (workers and gamergates) were broad spectrum detectors of almost all compounds tested. The vast range of sensitivity to the same panel of compounds was recently reported from sensilla basiconica of female H. saltator with gamergates showing reduced OSN responses to CHCs (Ghaninia et al., 2017). The responsiveness of sensilla trichodea to the CHCs in the second trial stresses the importance of CHC detection in females compared to males.
Antennal movement based bioassays were used to further substantiate the biological relevance of a number of general odors that elicited response in the OSNs of the males of H. saltator. Although the bioassay largely correlated with SSR responses, two odorants (methyl salicylate and isopentyl acetate) triggered male antennal movement despite the lack of electrophysiological activity. The best rationale for this inconsistency is the presence of additional olfactory sensilla that remain uncharacterized.
Our finding of robust peripheral and behavioral responses to only a small spectrum of compounds in male antenna of H. saltator and the rarity of CHC responding sensilla supports the perspective of little benefit of increased olfactory discrimination and identification abilities within and outside colonies in males of this species. This is in contrast with the perception abilities of H. saltator females (Ghaninia et al., 2017) that strongly benefit from excellent broad olfactory discrimination abilities in their various tasks in the colony. Nevertheless, the finding of male sensilla that respond to a synthetic hydrocarbon and CHCs specific to virgin queens that are ready for the mating flight suggests a role of these CHCs in sexual communication which might be the reason why Harpegnathos males detect CHCs despite the lack of need for other communication or identification purposes.
Supplementary Material
Acknowledgment
We thank Kevin Haight (ASU) for assistance with maintaining ant colonies and valuable comments on first draft of the manuscript. David Lowry (SoLS, Electron Microscopy Laboratory, ASU) is acknowledged for assistance with SEM. Kaustubh Gokhale, Brittany Enzmann, and Kelly Dolezal (ASU) are acknowledged for their assistance and discussion of the experiments.
References
- Alonso LE, Vander Meer RK, 1997. Source of alate excitant pheromones in the red imported fire antSolenopsis invicta (Hymenoptera: Formicidae). Journal of insect behavior 10:541–555. [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB, 2009. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M, Musthak Ali T, Jones T, Snelling R, 1994. Identification of a chemical relaeser of alarm behavior for workers of Harpegnathos saltator JERD. (Hymenoptera, Formicidae). Memorabilia Zoologica 48:17–22. [Google Scholar]
- Blum MS, 1969. Alarm pheromones. Annual review of entomology 14:57–80. [Google Scholar]
- Bonasio R, Zhang G, Ye C, Mutti NS, Fang X, Qin N, Donahue G, Yang P, Li Q, Li C, 2010. Genomic comparison of the ants Camponotus floridanus and Harpegnathos saltator. science 329:1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstaetter AS, Kleineidam CJ, 2011. Distributed representation of social odors indicates parallel processing in the antennal lobe of ants. Journal of neurophysiology 106:2437–2449. [DOI] [PubMed] [Google Scholar]
- Crewe D, 1973. Ponerine ant secretions: The mandibular gland secretion of Paltothyreus tarsatus Fabr. Journal of the Entomological Society of Southern Africa 37:291–298. [Google Scholar]
- Cuvillier-Hot V, Cobb M, Malosse C, Peeters C, 2001. Sex, age and ovarian activity affect cuticular hydrocarbons in< i> Diacamma ceylonense, a queenless ant. Journal of Insect Physiology 47:485–493. [DOI] [PubMed] [Google Scholar]
- D’Ettorre P, Heinze E, Schulz C, Francke W, Ayasse M, 2004. Does she smell like a queen? Chemoreception of a cuticular hydrocarbon signal in the ant Pachycondyla inversa. Journal of Experimental Biology 207:1085–1091. [DOI] [PubMed] [Google Scholar]
- do Nascimento RR, Billen J, Morgan ED, 1993. The exocrine secretions of the jumping ant< i> Harpegnathos saltator. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry 104:505–508. [Google Scholar]
- Ghaninia M, Haight KL, Berger SL, Reinberg D, Zwiebel LJ, Ray A, Liebig J, 2017. Chemosensory sensitivity reflects reproductive status in the ant Harpegnathos saltator. Sci Rep:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández JV, Cabrera A, Jaffe K, 1999. Mandibular gland secretion in different castes of the leaf-cutter ant Atta laevigata. Journal of chemical ecology 25:2433–2444. [Google Scholar]
- Hölldobler B, Wilson E, 1990. The ants. Cambrigde: Harvard University Press. [Google Scholar]
- Holman L, Leroy C, Jørgensen C, Nielsen J, d’Ettorre P, 2013. Are queen ants inhibited by their own pheromone? Regulation of productivity via negative feedback. Behavioral Ecology 24:380–385. [Google Scholar]
- Jarvis PA, Liebig J, Hölldobler B, Oldham NJ, 2004. Biosynthesis of the insect pheromone (S)-4-methyl-3-heptanone. Chem Commun:1196–1197. [DOI] [PubMed] [Google Scholar]
- Kidokoro-Kobayashi M, Iwakura M, Fujiwara-Tsujii N, Fujiwara S, Sakura M, Sakamoto H, Higashi S, Hefetz A, Ozaki M, 2012. Chemical Discrimination and Aggressiveness via Cuticular Hydrocarbons in a Supercolony-Forming Ant, Formica yessensis. PloS one 7:e46840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SI, Groh K, Vogel H, Hannson BS, Kleineidam CJ, Grosse-Wilde E, 2013. Correction: Caste-Specific Expression Patterns of Immune Response and Chemosensory Related Genes in the Leaf-Cutting Ant, Atta vollenweideri. PloS one 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonhardt SD, Brandstaetter AS, Kleineidam CJ, 2007. Reformation process of the neuronal template for nestmate-recognition cues in the carpenter ant Camponotus floridanus. Journal of Comparative Physiology A 193:993–1000. [DOI] [PubMed] [Google Scholar]
- Liebig J, 2010. Hydrocarbon profiles indicate fertility and dominane status in ant, bee, and wasp colonies In: Blomquist G, Bagnères A, editors. Insect hydrocarbons: biology, biochemistry, and chemical ecology New York: Cambridge University Press; p. 254–280. [Google Scholar]
- Liebig J, Hölldobler B, Peeters C, 1998. Are ant workers capable of colony foundation? Naturwissenschaften 85:133–135. [Google Scholar]
- Liebig J, Peeters C, Oldham NJ, Markstädter C, Hölldobler B, 2000. Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proceedings of the National Academy of Sciences USA 97:4124–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschwitz U, 1964. Gefahrenalarmstoffe und Gefahrenalarmierung bei sozialen Hymenopteren. Zeitschrift für vergleichende Physiologie 47:596–655. [Google Scholar]
- Moser JC, Reeve JD, Bento JMS, Della Lucia TM, Cameron RS, Heck NM, 2004. Eye size and behaviour of day-and night-flying leafcutting ant alates. Journal of Zoology 264:69–75. [Google Scholar]
- Olivier V, Monsempes C, François MC, Poivet E, Jacquin-Joly E, 2011. Candidate chemosensory ionotropic receptors in a Lepidoptera. Insect molecular biology 20:189–199. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Wada-Katsumata A, Fujikawa K, Iwasaki M, Yokohari F, Satoji Y, Nisimura T, Yamaoka R, 2005. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science 309:311–314. [DOI] [PubMed] [Google Scholar]
- Pask GM, Slone JD, Millar JG, Das P, Moreira JA, Zhou X, Bello J, Berger SL, Bonasio R, Desplan C, Reinberg D, Liebig J, Zwiebel LJ, Ray A, 2017. Receptors for cuticular hydrocarbons: Candidate phermones and cues for social insects. Nature Communications:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters C, Liebig J, Hölldobler B, 2000. Sexual reproduction by both queens and workers in the ponerine ant Harpegnathos saltator. Insectes Sociaux 47:325–332. [Google Scholar]
- Peeters C, Monnin T, Malosse C, 1999. Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proceedings of the Royal Society of London Series B: Biological Sciences 266:1323–1327. [Google Scholar]
- Pitts RJ, Derryberry SL, Zhang ZW, Zwiebel LJ, 2017. Variant ionotropic receptors in the malaria vector mosquito Anopheles gambiae tuned to amines and carboxylic acids. Sci Rep 7. doi: 10.1038/srep40297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pophof B, 1997. Olfactory responses recorded from sensilla coeloconica of the silkmoth Bombyx mori. Physiological Entomology 22:239–248. [Google Scholar]
- Sharma KR, Enzmann BL, Schmidt Y, Moore D, Jones GR, Parker J, Berger SL, Reinberg D, Zwiebel LJ, Breit B, 2015. Cuticular hydrocarbon pheromones for social behavior and their coding in the ant antenna. Cell reports 12:1261–1271. [DOI] [PubMed] [Google Scholar]
- Slone JD, Pask GM, Ferguson ST, Millar JG, Berger SL, Reinberg D, Liebig J, Ray A, Zwiebel LJ, 2017. Functional characterization of odorant receptors in the ponerine ant, Harpegnathos saltator. Proc Natl Acad Sci USA:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumlinson J, Silverstein R, Moser J, Brownlee R, Ruth J, 1971. Identification of the trail pheromone of a leaf-cutting ant, Atta texana. [DOI] [PubMed] [Google Scholar]
- van Zweden JS, d’Ettorre P, 2010. Nestmate recognition in social insects and the role of hydrocarbons. Insect hydrocarbons: biology, biochemistry and chemical ecology 11:222–243. [Google Scholar]
- Yao CA, Ignell R, Carlson JR, 2005. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. Journal of Neuroscience 25:8359–8367. doi: 10.1523/jneurosci.2432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Slone JD, Rokas A, Berger SL, Liebig J, Ray A, Reinberg D, Zwiebel LJ, 2012. Phylogenetic and transcriptomic analysis of chemosensory receptors in a pair of divergent ant species reveals caste-specific signatures of odor coding. PLoS Genetics 8:e1002930. doi: 10.1371/journal.pgen.1002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


