Abstract
Since the 1960s, heart and lung transplantation has remained the optimal therapy for patients with end-stage disease, extending and improving quality of life for thousands of individuals annually. Expanding donor organ availability and immunologic compatibility is a priority to help meet the clinical demand for organ transplant. While effective, current immunosuppression is imperfect as it lacks specificity and imposes unintended adverse effects such as opportunistic infections and malignancy that limit the health and longevity of transplant recipients. In this review, we focus on donor macrophages as a new target to achieve allograft tolerance. Donor organ-directed therapies have the potential to improve allograft survival while minimizing patient harm related to global suppression of recipient immune responses. Topics highlighted include the role of ontogenically distinct donor macrophage populations in ischemia–reperfusion injury and rejection, including their interaction with allograft-infiltrating recipient immune cells and potential therapeutic approaches. Ultimately, a better understanding of how donor intrinsic immunity influences allograft acceptance and survival will provide new opportunities to improve the outcomes of transplant recipients.
Keywords: basic (laboratory) research/science, heart (allograft) function/dysfunction, heart disease: immune/inflammatory, heart transplantation/cardiology, immunosuppressant, immunosuppression/immune modulation, lung (allograft) function/dysfunction, lung disease: immune/inflammatory, translational research/science
1 |. INTRODUCTION
Organ transplant continues to represent the optimal therapy for individuals with end-stage organ failure since the first lung transplant in 1963 and heart transplant in 1967. Given increased demand for organ transplant and enhanced regulatory scrutiny, waitlist times have increased1 out of proportion to donor availability.2 Expanding the donor organ pool and enhancing immunologic compatibility have become priorities within the transplant community. In addition, limiting posttransplant complications, including ischemia–reperfusion injury (IRI), primary graft dysfunction, acute cellular rejection (ACR), antibody-mediated rejection (AMR), graft vasculopathy, and chronic airway rejection, remains an area of intense focus.
Current immunosuppression strategies globally suppress recipient immune responses and as a direct result lead to many complications (renal failure, infections, malignancy). Balancing allograft protection against risks of infection and malignancy is a problematic and challenging issue. These observations highlight the clinical need to explore alternative options to reduce rejection and improve allograft survival.
Recent paradigm shifting studies have uncovered that macrophages represent heterogeneous populations composed of distinct cell types with differing developmental origins, recruitment dynamics, and functions. Several timely studies have investigated the role of macrophages in solid organ transplant.3–5 Macrophages have di-chotomous roles in inducing allograft injury and promoting allograft survival.5 These contrasting functions highlight the complex and dynamic functions of monocytes and macrophages. Macrophages within the heart and lung can be initially divided into tissue resident macrophages and infiltrating monocyte-derived macrophages.6–8 In the context of transplant, this subclassification is particularly informative as tissue resident macrophages and monocyte-derived macrophages originate from the donor and recipient, respectively. In the following sections, we provide a comprehensive discussion of tissue resident macrophage composition within the heart and lung, review the functions of tissue resident macrophages, and highlight known and presumptive roles for tissue resident macrophages in allograft rejection and tolerance.
2 |. HEART AND LUNG MACROPHAGE POPUL ATIONS ARE DIVERSE WITH DIVERGENT FUNCTIONS
Macrophages are an essential component of the innate immune system. Although there are many approaches to categorize macrophage subtypes, division into tissue resident and infiltrating monocyte-derived populations is widely accepted and highly applicable to the transplant field. Tissue resident macrophages typically seed organs during embryonic or early postnatal development and exist throughout life largely independent of monocyte input.6,7,9 Many tissue resident macrophage populations (heart, lung, liver, kidney, brain) are derived from early hematopoietic progenitors located within the yolk sac and/or fetal liver.10–12 However, some tissue resident macrophages originate from peripheral monocytes (stomach, intestine, colon) and are later maintained through local proliferation.13,14 A general theme from studies of macrophage ontogeny is that tissue resident macrophages acquire unique functions based on the organ in which they reside.15 Thus, macrophage phenotype is influenced by both ontogeny and environmental cues. Current work in this space is focused on delineating the mechanistic basis by which developmental and environmental information is integrated at the signaling, transcriptional, and epigenetic levels.
The heart contains distinct macrophage populations with divergent origins and functions.16–18 Cardiac tissue resident macrophages are readily distinguished based on the expression of C-C chemokine receptor 2 (CCR2).6,9 CCR2A and CCR2B are widely conserved among mammals17,19,20 and are expressed on the cell surface of monocytes, dendritic cells, and some T cells,21 and their primary ligands are CCL2 and CCL7.22 Under resting conditions, the adult heart contains CCR2+MHC-IIhi macrophages, CCR2−MHC-IIlo macrophages, and CCR2−MHC-IIhi macrophages. Monocytes are differentiated from cardiac macrophages because they are CCR2+MHC-IIlo and lack the expression of MertK.7
CCR2− macrophages are derived from embryonic hematopoietic progenitors, seed the heart during fetal development, are long lived, and are maintained independent of monocyte input throughout life via local proliferation. CCR2− macrophages promote coronary angiogenesis and cardiomyocyte proliferation and have anti-inflammatory effects potentially by secreting interleukin (IL)-10 and transforming growth factor (TGF)-β.7,18,23–25 CCR2− macrophages suppress neutrophil and monocyte recruitment. CCR2+ macrophages are derived from circulating monocytes, seed the heart during postnatal life, and are maintained through a combination of gradual monocyte recruitment and local proliferation. CCR2+ macrophages are enriched in proinflammatory genes, and their activation represents a mechanism driving inflammation.18,24 CCR2+ macrophages orchestrate neutrophil and monocyte recruitment. Removal of CCR2+ macrophages is sufficient to reduce infarct area and adverse cardiac remodeling following myocardial infarction.26 Recently, it was discovered that the human heart contains macrophage populations that are developmentally and functionally analogous to CCR2− and CCR2+ macrophages found in the mouse heart (Table 1).16
TABLE 1.
Surface phenotype, origin, and presumed function of donor and recipient macrophages and monocytes in the heart transplant
| Heart | ||||
|---|---|---|---|---|
| Donor | Recipient | |||
| Donor CCR2+ macrophage | Donor CCR2− macrophage | Classical recipient monocyte | Nonclassical recipient monocyte | |
| Surface phenotype | CD11b+ CD64+ CCR2+ MHC-IIhi | CD11b+ CD64+ CCR2− MHC-IIhi or lo | CD11b+ Ly6G− CD64int Ly6Chi CCR2+ | CD11b+ Ly6G− NK1.1− SiglecF− CD64− Ly6Clo MHCIIlo |
| Origin | Blood monocytes | Extraembryonic progenitors | Blood monocytes | Blood monocytes |
| Function | Orchestrate recipient neutrophil and monocyte recruitment | Suppress recipient neutrophil and monocyte recruitment | Differentiate into macrophages and dendritic cells. Contribute to both rejection and tolerance | Unknown. It is thought that nonclassical recipient monocytes do not give rise to tissue macrophages |
The lung contains 2 distinct macrophage populations: alveolar and interstitial macrophages.27,28 They constitute 80% and 20% of the lung resident macrophage pool, respectively. Alveolar macrophages line the surface of alveoli and are long-lived lung-resident cells. Alveolar macrophages are derived from embryonic hematopoietic progenitors located predominantly within the fetal liver.29 However, recent studies show that alveolar macrophages can also be derived from circulating bone marrow–derived monocytes, which can play a role in the development of lung fibrosis.30 Interstitial macrophages are localized in the narrow space between the alveolar epithelium and vascular endothelium, largely originate from circulating blood monocytes, and are maintained through monocyte turnover.27 Some investigators have suggested that a small population of interstitial macrophages may be derived from yolk sac hematopoietic progenitors (Table 2).31
TABLE 2.
Surface phenotype, origin, and presumed function of donor and recipient macrophages and monocytes in the lung transplant
| Lung | ||||
|---|---|---|---|---|
| Donor | Recipient | |||
| Donor alveolar macrophage | Donor interstitial macrophage | Donor nonclassical monocyte | Classical recipient monocyte | |
| Surface phenotype | CD11b+ | CD11b+ | CD11b+ | CD11b+ Ly6G− Ly6Chi CCR2+ |
| CD64+ SiglecF+ CD11c+ | 0D64+ | Ly6G− | ||
| MHC-IIhi | NK1.1− SiglecF− CD64− Ly6Clo MHCIIlo | |||
| CD11c+ | ||||
| Origin | Embryonic progenitors or blood monocytes | Embryonic progenitors or blood monocytes | Blood monocytes | Blood monocytes |
| Function | Cytokine/chemokine production |
Unknown | Orchestrate recruitment of recipient neutrophils and classical monocytes | Differentiate into macrophages or dendritic cells |
| Promote extravasation of neutrophils | ||||
Lung classical monocytes survey the parenchyma and are recruited to sites of inflammation by CCL2, CX3CL1, and CCR5 ligands.32,33 Classical monocytes can differentiate into interstitial macrophages, alveolar macrophages, and dendritic cells. Nonclassical monocytes patrol the endothelium in a crawling-type motility and are recruited to sites of inflammation by CX3CL1.32,34,35
3 |. ALLOGR AFT MACROPHAGE POPUL ATIONS FOLLOWING HEART AND LUNG TR ANSPL ANT
The transplanted heart contains a compilation of myeloid cells consisting of “donor-derived” CCR2+ and CCR2− macrophages and infiltrating recipient neutrophils, monocytes, monocyte-derived macrophages, and monocyte-derived dendritic cells.16,36,37 Classical recipient monocytes derive from blood monocytes and differentiate into macrophages and dendritic cells and contribute to both rejection and tolerance.38 In murine models, we can separate donor from recipient cells using cell-tracing strategies such as congenic (i.e., CD45.1/CD45.2) or reporter (i.e., GFP/RFP) mice. It is also possible to distinguish donor and recipient immune cells in humans by analyzing sex-mismatched transplant recipients or allelic HLA expression. For example, we analyzed endomyocardial biopsy samples (average 8.8 years after transplant, no active rejection, normal allograft function) involving a female donor and a male recipient. Donor-versus-recipient origin was based on the presence or absence of a “Y” chromosome.16 Combined in situ hybridization and immunostaining revealed the presence of a Y chromosome in a very small percentage of CCR2− macrophages (<2%). In contrast, a Y chromosome was detected in 30% of CCR2+ macrophages, suggesting that cardiac CCR2+ macrophages represent a compilation of donor- and recipient-derived cells. Little is known regarding whether donor- and recipient-derived CCR2+ macrophages are functionally distinct in humans. However, mouse studies suggest that recipient-derived CCR2+ macrophages express higher levels of inflammatory chemokines (CXCL11, CXCL2, CCL2, CCL7, CCL19), cytokines (IL1-β, IL-10), and adverse cardiac remodeling genes (AREG, EREG, GDF3) compared with donor-derived CCR2+ macrophages. Donor-derived CCR2+ macrophages expressed higher levels of type I interferon responsive genes.26
After lung transplant, the vast majority of alveolar macrophages in the allograft are donor derived.39,40 Donor-derived alveolar macrophages were positive for Ki67, suggesting they have the capacity to proliferate locally. Although donor-derived alveolar macrophages are the predominant macrophage for at least 2 to 3 years after transplant, the contribution of recipient monocyte recruitment is less well understood. Conflicting data exist regarding whether monocytes contribute to alveolar macrophages in the lung allograft over extended transplant durations.39,40 Collectively, these observations indicate that macrophages resident within the donor heart and lung exist within the graft for extended periods of time and bring to light the possibility that donor macrophages constitute unique and functionally important cell types that likely contribute to allograft health and longevity (Figure 1).
FIGURE 1.
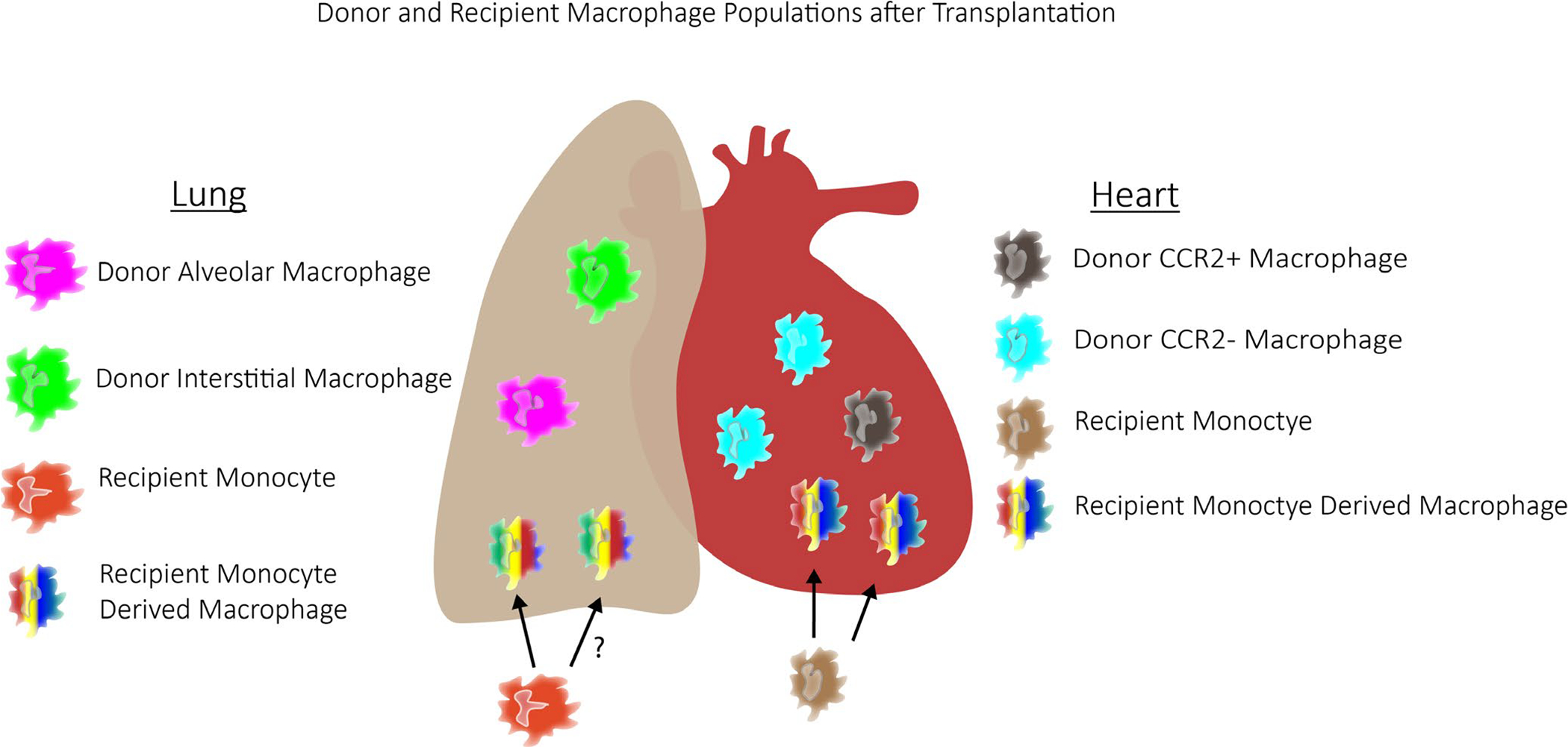
Schematic of donor-vs recipient-derived macrophages after lung and heart transplant. Lung donor-derived macrophages consist of donor alveolar and donor interstitial macrophages. Circulating monocytes can infiltrate the lung and differentiate into macrophages, including recipient monocyte-derived alveolar macrophages and recipient monocyte-derived interstitial macrophages. Similarly, after cardiac transplant, the heart consists of donor CCR2+ macrophages, donor CCR2− macrophages, in addition to recipient monocyte-derived macrophages. Some of these recipient monocyte-derived macrophages are CCR2+
4 |. ROLE OF DONOR MACROPHAGES IN IRI AND PRIMARY GR AFT DYSFUNCTION
A critical goal during organ transplant is to minimize ischemic time. Longer ischemic times are associated with poor clinical outcomes after heart41,42 and lung transplant.43,44 IRI is thought to be the predominant mechanism of primary graft dysfunction, a major cause of early allograft loss and mortality.45,46 Recently, the United Network for Organ Sharing modified the heart and lung allocation system to promote broader organ sharing and increase allocation to sicker individuals. Although comprehensive data are not yet available, it is clear that the new allocation system prolongs allograft ischemic time due to increased distances between donor procurement sites and transplant centers. As such, there is growing interest to improve our understanding of the mechanisms that contribute to allograft IRI.
Macrophages have been shown to have dynamic roles on allograft function after IRI in kidney and liver transplant models.47–49 Distinct macrophage populations contribute to early proinflammatory responses50 and later postinjury resolution.51 The precise identity of these macrophage subsets and mechanisms that orchestrate their activation and effector responses in the context of allograft IRI remain incompletely defined. Improved understanding of these topics could have profound clinical impact in this new era of more widespread organ sharing, as mitigating the effects of prolonged ischemic time may improve transplant outcomes and further increase the donor pool.
5 |. IRI AFTER HEART TR ANSPL ANT
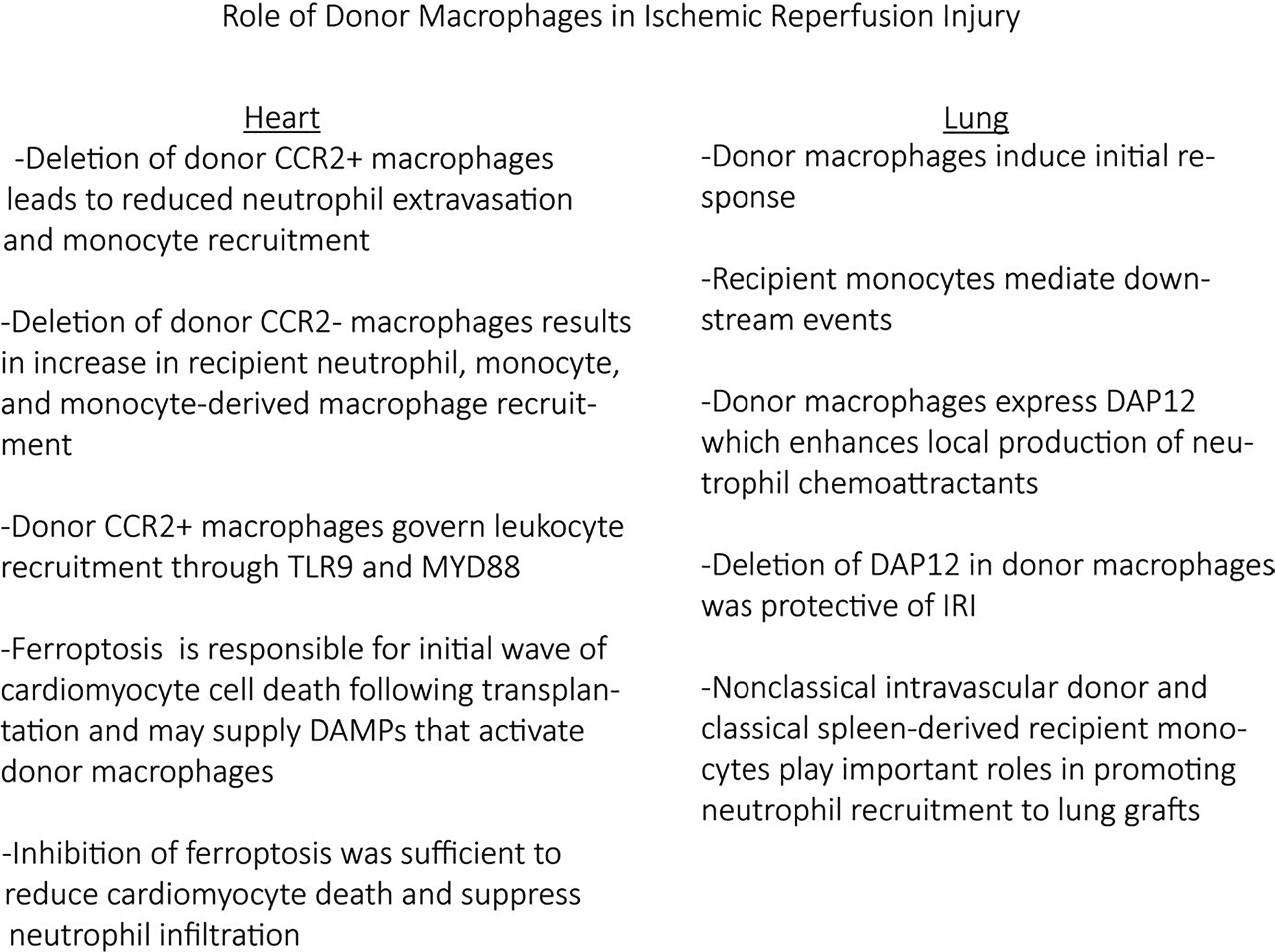
Incorporation of cold ischemic time into mouse heart transplant models allows investigators to dissect mechanisms that contribute to allograft IRI. These studies have observed evidence of cardiomyocyte cell death and infiltration of recipient neutrophils, monocytes, and monocyte-derived macrophages into the donor heart.26 Intriguingly, macrophage populations resident within the donor heart differentially orchestrate graft inflammation following IRI. Depletion of tissue resident CCR2+ from the donor heart leads to reduced recipient neutrophil extravasation and monocyte recruitment into the donor heart following transplant. Within this context, donor CCR2+ macrophages governed recipient leukocyte recruitment through the generation of neutrophil and monocyte chemokines through a Toll-like receptor (TLR)9- and MYD88-dependent mechanism.26,52 Conversely, depletion of tissue resident CCR2− macrophages from the donor heart resulted in a marked increase in recipient neutrophil, monocyte, and monocyte-derived macrophage recruitment. Intriguingly, single-cell mRNA sequencing uncovered remarkable diversity among infiltrating monocytes and monocyte-derived macrophages and revealed that donor macrophages play pivotal roles in monocyte fate decisions.26 These findings implicate the importance of donor macrophages in allograft inflammation after IRI. In theory, pretreating donor macrophages before procurement may ameliorate IRI-associated allograft injury and reduce the incidence and severity of primary graft dysfunction. Consistent with this concept, studies assessing the feasibility of treating the procured donor heart have shown a significant role for macrophages on graft survival.53,54
Release of damage-associated molecular patterns (DAMPs) and alarmins from injured and/or dying cells is thought to constitute the signal mechanistically linking IRI to immune cell activation. Necrosis, necroptosis, and ferroptosis are among the various forms of cell death implicated in DAMP and alarmin release. Although necroptosis has previously been implicated in allograft immune cell activation,55 increasing evidence suggests that ferroptosis is responsible for the initial wave of cardiomyocyte and cardiac fibroblast cell death after IRI. The treatment of donor hearts with ferrostatin-1 (specific inhibitor of ferroptosis) is sufficient to dramatically reduce initial cardiomyocyte and fibroblast cell death and suppress early neutrophil graft infiltration. Inhibition of necroptosis had minimal effect on cardiomyocyte cell death and neutrophil extravasation within 4 hours after transplant.56 The exact identity of DAMPs/alarmins released by ferroptotic cardiomyocytes and fibroblasts remains to be elucidated. However, it is likely that these mediators engage TLRs. The deletion of TLR4 in donor endothelial cells was sufficient to prevent adhesion of neutrophils to venous endothelial cells, a critical upstream step in neutrophil extravasation. Mechanistically, TLR4 activation was transduced through a TRIF and type I interferon-dependent pathway.56 Deletion of TLR9 and MYD88 in donor macrophages did not affect the initial adhesion of neutrophils to endothelial cells but instead resulted in impaired transendothelial migration.52 These findings highlight the possibility that DAMPs/alarmins released by dying myocardial cells initiate allograft inflammation via signaling to multiple donor resident cell types, including endothelial cells and macrophages. Future studies will be required to further delineate additional cell types and signaling mechanisms governing IRI-induced allograft inflammation and resultant allograft dysfunction.
6 |. POSTTR ANSPL ANT ISCHEMIC LUNG INJURY
IRI causes severe graft dysfunction in up to 20% of lung transplant recipients.57 IRI after lung transplant has been described as a biphasic process in which pulmonary macrophages play an important role. Donor macrophages were suggested to induce an initial response (independent of recipient neutrophils), and circulating leukocytes were thought to mediate downstream events.58
In a rat lung transplant model, alveolar macrophages contribute to local inflammation via induction of cytokines (e.g., TNF-α).59–61 These findings were corroborated in a mouse model of pulmonary IRI.61 Consistent with a role for donor macrophages in generating inflammatory responses, the injection of gadolinium chloride (inhibitor of macrophage phagocytic and inflammatory responses) 24 hours before rabbit lung reperfusion resulted in improved oxygenation at 30 minutes.62
We have shown that neutrophils play a critical role in mediating IRI and can augment alloimmunity.63,64 Interestingly, macrophage and monocyte populations in donors and recipients potentiate IRI by regulating trafficking of neutrophils into pulmonary grafts. For example, we have shown that lung-resident macrophages express the cell membrane–associated protein DAP12, which contributes to the local production of proinflammatory cytokines and neutrophil chemoattractants, in part by regulating the survival of macrophages.8 In a mouse lung transplant model, we demonstrated that deficiency of DAP12 in the donor reduces neutrophil extravasation and protects against IRI. Intravital 2-photon imaging experiments have revealed that circulating monocytes facilitate extravasation of neutrophils into reperfused lung grafts.65 Subsequent work demonstrated that spleen-derived recipient classical CCR2+ monocytes promote neutrophil entry in injured lung tissue through MyD88-dependent production of IL-1β.33,66 Recent evidence also suggests that intravascular nonclassical monocytes of donor origin (carried over during transplant despite flushing of the pulmonary vessels) also regulate neutrophil recruitment to the graft through MyD88/Trif-dependent production of the neutrophil chemokine CXCL2. Interestingly, donor nonclassical monocytes also regulate the recruitment of recipient classical monocytes. Notably, depletion of nonclassical monocytes in donor lungs results in amelioration of lung transplant–mediated IRI (Figure 2).
FIGURE 2.
Roles of donor macrophages after ischemia–reperfusion injury
7 |. MACROPHAGES AND ALLOGR AFT REJECTION
Given their persistence after organ transplant, it is possible that donor macrophages contribute to allograft rejection. There is growing evidence that macrophages play a role in both ACR67 and AMR.68–70 In the context of ACR, macrophages contribute to graft injury and myocardial fibrosis through cytokine and reactive oxygen species production.71–73 Within the rejecting human kidney, macrophages account for 38%-60% of infiltrating leukocytes.5,74,75 Examination of human heart transplant recipients (n = 25) with ACR revealed increased abundance of CD16+ monocytes/macrophages compared with healthy controls.71 CD16+ intermediate monocytes are considered a proinflammatory population with elevated expression of TNF-α and IL-1β. Consistent with a contribution of infiltrating monocytes, the authors observed increased expression of HLA-DR and CD54 within circulating CD16+ intermediate monocytes, suggesting an activated state with increased migratory potential.76,77 Despite the recognition that macrophages accumulate within the allograft during ACR, the discrete roles of macrophage subpopulations and their origins are incompletely understood.78
A hallmark of AMR is the perivascular and intravascular accumulation of neutrophils and macrophages. The clinical diagnosis is in part made by histopathologic evidence of intravascular CD68+ monocytes/macrophages.79 Macrophages contribute to cardiac allograft injury in acute AMR.80 Intravascular monocytes/macrophages displaying a proinflammatory phenotype contribute to AMR through antigen processing/presentation, cytokine production, and tissue remodeling.4,68,81–84 The signals responsible for recruiting intravascular monocytes/macrophages are not well established.
Donor macrophages contribute to allograft injury after lung transplant and may contribute to the development of chronic rejection. Because of their longevity in the lung allograft, donor alveolar macrophages serve as a long-term source of donor antigens. Donor alveolar macrophages secrete proinflammatory cytokines after stimulation with donor-specific antibodies, suggesting that donor alveolar macrophages contribute to antibody-mediated lung rejection.39 Specifically, induction of zinc finger and BTB domain containing protein 7a, a transcription factor that helps regulate development of lymphocytes and tissue resident macrophages, in alveolar macrophages is a critical step in donor-specific antibody–induced chronic rejection.85 Elimination of zinc finger and BTB domain containing protein 7a in alveolar macrophages was associated with decreased bronchiolar occlusion and chronic rejection.39 Future studies will be required to define additional mechanisms by which donor macrophages participate in allograft rejection. Potential mechanisms include regulation of monocyte/neutrophil trafficking, monocyte fate specification, antigen presentation, and cytokine production.
8 |. MACROPHAGES AND ALLOGR AFT TOLER ANCE
The gold standard of transplant immunology is graft acceptance or graft tolerance. To date, tolerogenic protocols have targeted T cells, given their prominent role in graft rejection.86,87 Substantial evidence exists that macrophage subpopulations contribute to tolerance. Braza et al show that graft infiltrating macrophages expressed pattern recognition receptors dectin-1 and TLR4 (in response to DAMPs) and genetically deleting these proteins in recipient mice decreased recipient inflammatory Ly6chi macrophages and promoted graft tolerance with accumulation of Ly6clo macrophages.87–89 Interestingly, in this model, costimulatory blockade with CD40 inhibition (T cell receptor) was required to induce long-term tolerance, suggesting T cells may modulate macrophage phenotypes.
In addition, TIMD4+ and DC-SIGN+ macrophages suppress T cell activation, increase regulatory T cell abundance, and promote allograft tolerance.88,90,91 Although TIMD4+ macrophages represent long-lived tissue resident macrophages, DC-SIGN+ macrophages appear to be of monocytic origin. Blockade of the CD40L-CD40 costimulatory pathway promotes the differentiation of monocytes into DC-SIGN+CD169+ suppressive macrophages capable of secreting IL-10 and suppressing CD8 T cell activation.88 Treatment of CD169-DTR recipient mice with diphtheria toxin was sufficient to deplete DC-SIGN+ macrophages and prevent allograft tolerance.88 Conditional deletion of mammalian target of rapamycin (mTOR) in recipient myeloid cells provided further support for a role of monocyte-derived macrophages in tolerance. Deletion of mTOR in recipient monocytes and macrophages led to increased numbers of intragraft Foxp3+ T cells, long-term allograft survival, and reduced myocardial or vascular injury. Using high-density lipo-protein nanobiologic targeting the mTOR, Braza et al87 were able to more selectively target myeloid cells and inhibit T cell proliferation and induce expansion of tolerogenic Foxp3 regulatory T cells suggesting an ability to promoting tolerogenic Ly6clo macrophages. Mechanistically, mTOR-deficient graft infiltrating macrophages upregulated programmed cell death 1 ligand and blockade of programmed cell death 1 ligand resulted in rapid graft rejection.92 Although these findings identify a role for monocytes and macrophages in establishing allograft tolerance, the exact role for donor macrophages remains to be clarified. An intriguing possibility is that donor macrophages may influence the ability of graft infiltrating monocytes to differentiate into macrophage subsets with regulatory activity.
9 |. EFFECT OF IMMUNOSUPPRESSION ON MACROPHAGES
Current solid organ immunosuppression regimens consist of calcineurin inhibitors (tacrolimus, cyclosporine), antimetabolites (mycophenolate mofetil [MMF], azathioprine), mTOR inhibitors (sirolimus, everolimus), and glucocorticoids (prednisone). A fraction of patients additionally receive induction therapy with agents such as thymoglobulin and basiliximab. How these agents influence donor macrophage, recipient monocyte, and recipient monocyte-derived macrophage function is an area of interest.
Although the primary mechanism of calcineurin inhibitors is to inhibit T cell receptor activation through reductions in NFAT signaling and cytokine secretion (IL-2, interferon-γ), these agents also influence macrophage behavior.93 Tacrolimus inhibits macrophage calcineurin signaling but activates nuclear factor-κβ signaling and downstream production of IL-12 and TNF-α.93–95 Calcineurin inhibitors are also reported to inhibit TLR signaling and cytokine production (IL-1β, TNF-α, IL-6, IL-10), bacterial phagocytosis, and regulation of macrophage polarization.96–98 The antimetabolite MMF inhibits the synthesis of guanosine nucleotides. In the context of rejection, circulating monocytes treated with MMF produced less IL-1β, IL-10, and TNF-α.99 Healthy human volunteer blood was exposed to tacrolimus or MMF in vitro.100 There was mild inhibition of phosphorylation of CD14+ monocyte activation (p38MAPK with tacrolimus and AKT with MMF) but minimal effects on cytokine production and macrophage differentiation, suggesting that these agents do not dramatically influence macrophage function.100 Glucocorticoids act through many pathways to control antigen presentation, cytokine production, and proliferation of lymphocytes.101 Glucocorticoids are associated with reduced CD14+CD16++ monocytes, increased IL-10 expression, and reduced IL-1, IL-12, and TNF expression.102,103 These effects may be related to the ability of glucocorticoids to influence either macrophage signaling and/or monocyte differentiation.104 Using a zebrafish amputation model for inflammation, Xie et al105 showed that glucocorticoids reduced neutrophil but not macrophage migration (no effect on chemoattractants Ccl2 or Cxcl11aa). They also show with RNA-seq that the glucocorticoid be-clometasone inhibits proinflammatory macrophage differentiation. mTOR inhibitors suppress macrophage CCL2, CCL3, CCL4, CCL5, IL-6, and IL-9 expression106,107 and impair antigen presentation through reduced CD80 expression.108
The influence of induction therapy and biologic agents on donor and recipient macrophage function is less clear. Thymoglobulin induction therapy leads to complement-mediated T cell death and an increase in CD14+ monocytes.109 Belatacept and abatacept (CTLA4-Ig recombinant proteins) prevent T cell activation through inhibition of costimulation: interactions between CD80/CD86 on macrophages and dendritic cells and CD28 molecule on T cells.110 These agents also reduce the production of IL-12 and TNF.111 Whether immunosuppressive agents have differential effects on donor vs recipient macrophages remains unexplored.
10 |. MACROPHAGES AS A THER APEUTIC TARGET
There have been few publications suggesting that depleting macrophage populations, inhibiting their activation, or suppressing their effector mechanisms suppresses IRI, reduces rejection, and improves allograft survival.5,112–115 Although there are substantial gaps in knowledge delineating the exact contributions of donor- and recipient-derived macrophages, emerging data suggest that manipulating donor macrophage subsets could prove efficacious. If indeed the donor subpopulation must be targeted, the CCR2+ macrophages would be the presumptive target in heart transplant. These inflammatory populations could be targeted with nanoparticles or micelles that target short peptides,116,117 standard monoclonal antibodies (i.e., MLN1202), or newer constructs like bispecific or multispecific antibodies where multiple synergistic proteins could be targeted simultaneously or a putative protein with downstream effectors simultaneously such as the CCR2 and CCR5 dual receptor blocker cenicriviroc (TAK-652).118,119 Bispecific or multispecific antibodies can be used for several therapeutic purposes. These reagents can facilitate efficient cell removal by bringing target cells in proximity to activated effector T or natural killer cells. In addition, they can be used to suppress cell signaling on specific cell types, such as donor CCR2+ macrophages.88
Ultimately, there is tremendous potential for developing therapeutics that are applied directly to the donor organ before transplant (during cold storage or normothermic perfusion). Such agents would minimize risks associated with systemic immunosuppression including life-threatening infection and malignancy. Targeting upstream mechanisms that initiate allograft inflammation and rejection or promote the differentiation and survival of infiltrating monocytes and monocyte-derived macrophages with regulatory activity may be particularly advantageous over current approaches that block downstream inflammatory mechanisms.108,120–122
11 |. CONCLUSIONS
Heart and lung transplant have transformed care for patients with end-stage organ failure. To prevent organ rejection, current standard of care requires the use of immunosuppression that lacks specificity and is wrought with untoward toxicities. Macrophages are implicated in many aspects of transplant pathology ranging from IRI to allograft rejection and tolerance. During the past several years, paradigm-shifting studies have provided captivating insights into the extent and functional importance of macrophage diversity. We are now breaking ground into the differential roles and interactions between donor macrophages and recipient monocytes and monocyte-derived macrophages. These studies have raised the possibility that targeting donor macrophages before transplant may be an avenue to improve transplant outcomes. Donor organ–based approaches provide distinct advantages including reductions in therapeutic toxicity. Targeting donor macrophages requires a comprehensive understanding of the respective roles of donor and recipient macrophages over the entire lifespan of the allograft. Large gaps in our knowledge base exist in this area. Key questions to be addressed include, What are the roles of distinct cardiac macrophage subsets in rejection and long-term allograft tolerance? What are the mechanisms that mediate cardiac macrophage activation and monocyte differentiation? How do donor and recruited monocytes and macrophages interact with the adaptive immune system to influence transplant outcomes? It is clear that answering these questions will yield new opportunities and promising targets for safe and effective immunosuppression.
ACKNOWLEDGMENTS
Dr Kopecky was supported by the Principles in Cardiovascular Research Training Grant (T32 HL007081) and the Washington University Physician Scientist Training Program. Dr Lavine is supported by the National Institutes of Health (NIH) K08 HL123519, R01 HL138466, and R01 HL139714, Burroughs Welcome Fund (1014782), Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (CH-II-2015-462 and CH-II-2017-628), and Foundation of Barnes-Jewish Hospital (8038-88). Dr Liu is supported by NIH R01 HL125655 and HL131908. Dr Kreisel is supported by 1P01AI116501 and R01 HL094601, Veterans Administration Merit Review grant 1I01BX002730, and the Foundation for Barnes-Jewish Hospital.
Funding information
Principles in Cardiovascular Research Training, Grant/Award Number: T32 HL007081; Washington University Physician Scientist Training Program; National Institutes of Health (NIH), Grant/Award Number: K08 HL123519, R01 HL138466, R01 HL139714, R01 HL125655, HL131908, 1P01AI116501 and R01 HL094601; Burroughs Welcome Fund, Grant/Award Number: 1014782; Children’s Discovery Institute of Washington University; St. Louis Children’s Hospital, Grant/Award Number: CH-II-2015-462 and CH-II-2017-628; Foundation of Barnes-Jewish Hospital, Grant/Award Number: 8038-88; Veterans Administration Merit Review, Grant/Award Number: 1I01BX002730; Foundation for Barnes-Jewish Hospital
Abbreviations:
- ACR
acute cellular rejection
- AMR
antibody-mediated rejection
- CCR2
C-C chemokine receptor 2
- DAMP
damage-associated molecular pattern
- IL
interleukin
- IRI
ischemia–reperfusion injury
- MMF
mycophenolate mofetil
- mTOR
mammalian target of rapamycin
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- TLR
toll-like receptor
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts in interest to disclose as described by the American Journal of Transplantation.
DATA AVAIL ABILIT Y STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Goldstein BA, Thomas L, Zaroff JG, Nguyen J, Menza R, Khush KK. Assessment of heart transplant waitlist time and pre- and post-transplant failure: a mixed methods approach. Epidemiology. 2016;27(4):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khush KK, Zaroff JG, Nguyen J, Menza R, Goldstein BA. National decline in donor heart utilization with regional variability: 1995–2010. Am J Transplant. 2015;15(3):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang X, Tian W, Sung YK, Qian J, Nicolls MR. Macrophages in solid organ transplantation. Vasc Cell. 2014;6(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Li C, Zhuang Q, et al. The evolving roles of macrophages in organ transplantation. J Immunol Res. 2019;2019:5763430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavine KJ, Pinto AR, Epelman S, et al. The Macrophage in cardiac homeostasis and disease: JACC macrophage in CVD series (Part 4). J Am Coll Cardiol. 2018;72(18):2213–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spahn JH, Li W, Bribriesco AC, et al. DAP12 expression in lung macrophages mediates ischemia/reperfusion injury by promoting neutrophil extravasation. J Immunol. 2015;194(8):4039–4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epelman S, Lavine K, Beaudin A, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40(1):91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez Perdiguero E, Schulz C, Geissmann F. Development and homeostasis of “resident” myeloid cells: the case of the microglia. Glia. 2013;61(1):112–120. [DOI] [PubMed] [Google Scholar]
- 11.Schulz C, Perdiguero EG, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. [DOI] [PubMed] [Google Scholar]
- 12.Beattie L, Sawtell A, Mann J, et al. Bone marrow-derived and resident liver macrophages display unique transcriptomic signatures but similar biological functions. J Hepatol. 2016;65(4): 758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bain CC, Scott CL, Uronen-Hansson H, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6(3):498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bain CC, Bravo-Blas A, Scott CL, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15(10):929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bajpai G, Schneider C, Wong N, et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med. 2018;24(8):1234–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dick SA, Macklin JA, Nejat S, et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol. 2019;20(1):29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavine KJ, Epelman S, Uchida K, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc Natl Acad Sci USA. 2014;111(45):16029–16034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurihara T, Bravo R. Cloning and functional expression of mCCR2, a murine receptor for the C-C chemokines JE and FIC. J Biol Chem. 1996;271(20):11603–11607. [DOI] [PubMed] [Google Scholar]
- 20.Boring L, Gosling J, Monteclaro FS, Lusis AJ, Tsou CL, Charo IF. Molecular cloning and functional expression of murine JE (monocyte chemoattractant protein 1) and murine macrophage inflammatory protein 1alpha receptors: evidence for two closely linked C-C chemokine receptors on chromosome 9. J Biol Chem. 1996;271(13):7551–7558. [DOI] [PubMed] [Google Scholar]
- 21.Saederup N, Cardona AE, Croft K, et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS ONE. 2010;5(10):e13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA. 1994;91(7):2752–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SE, Lavine KJ. Primitive embryonic macrophages are required for coronary development and maturation. Circ Res. 2016;118(10):1498–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilgendorf I, Gerhardt LM, Tan TC, et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114(10):1611–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howangyin K-Y, Zlatanova I, Pinto C, et al. Myeloid-epithelial-reproductive receptor tyrosine kinase and milk fat globule epidermal growth factor 8 coordinately improve remodeling after myocardial infarction via local delivery of vascular endothelial growth factor. Circulation. 2016;133(9):826–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajpai G, Bredemeyer A, Li W, et al. Tissue resident CCR2- and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res. 2019;124(2):263–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol. 2013;49(4):503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrne SC, Barrett B, Bhatia R. The impact of diagnostic imaging wait times on the prognosis of lung cancer. Can Assoc Radiol J. 2015;66(1):53–57. [DOI] [PubMed] [Google Scholar]
- 29.Guilliams M, Lambrecht BN, Hammad H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol. 2013;6(3):464–473. [DOI] [PubMed] [Google Scholar]
- 30.Misharin AV, Morales-Nebreda L, Reyfman PA, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214(8):2387–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan SY, Krasnow MA. Developmental origin of lung macrophage diversity. Development. 2016;143(8):1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiu S, Bharat A. Role of monocytes and macrophages in regulating immune response following lung transplantation. Curr Opin Organ Transplant. 2016;21(3):239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsiao HM, Fernandez R, Tanaka S, et al. Spleen-derived classical monocytes mediate lung ischemia-reperfusion injury through IL-1beta. J Clin Invest. 2018;128(7):2833–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng Z, Chiu S, Akbarpour M, et al. Donor pulmonary intravascular nonclassical monocytes recruit recipient neutrophils and mediate primary lung allograft dysfunction. Sci Transl Med. 2017;9(394): pii:eaal4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas G, Tacke R, Hedrick CC, Hanna RN. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Biol. 2015;35(6):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ochando J, Kwan WH, Ginhoux F, Hutchinson JA, Hashimoto D, Collin M. The mononuclear phagocyte system in organ transplantation. Am J Transplant. 2016;16(4):1053–1069. [DOI] [PubMed] [Google Scholar]
- 37.Spahn JH, Li W, Kreisel D. Innate immune cells in transplantation. Curr Opin Organ Transplant. 2014;19(1):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdi R, Means TK, Ito T, et al. Differential role of CCR2 in islet and heart allograft rejection: tissue specificity of chemokine/chemokine receptor function in vivo. J Immunol. 2004;172(2):767–775. [DOI] [PubMed] [Google Scholar]
- 39.Nayak DK, Zhou F, Xu M, et al. Long-term persistence of donor alveolar macrophages in human lung transplant recipients that influences donor-specific immune responses. Am J Transplant. 2016;16(8):2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eguíluz-Gracia I, Schultz HHL, Sikkeland LIB, et al. Long-term persistence of human donor alveolar macrophages in lung transplant recipients. Thorax. 2016;71(11):1006–1011. [DOI] [PubMed] [Google Scholar]
- 41.Ford MA, Almond CS, Gauvreau K, et al. Association of graft ischemic time with survival after heart transplant among children in the United States. J Heart Lung Transplant. 2011;30(11): 1244–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russo MJ, Chen JM, Sorabella RA, et al. The effect of ischemic time on survival after heart transplantation varies by donor age: an analysis of the United Network for Organ Sharing database. J Thorac Cardiovasc Surg. 2007;133(2):554–559. [DOI] [PubMed] [Google Scholar]
- 43.Thabut G, Mal H, Cerrina J, et al. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Respir Crit Care Med. 2005;171(7):786–791. [DOI] [PubMed] [Google Scholar]
- 44.de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167(4):490–511. [DOI] [PubMed] [Google Scholar]
- 45.Kobashigawa J, Zuckermann A, Macdonald P, et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant. 2014;33(4):327–340. [DOI] [PubMed] [Google Scholar]
- 46.Nicoara A, Ruffin D, Cooter M, et al. Primary graft dysfunction after heart transplantation: Incidence, trends, and associated risk factors. Am J Transplant. 2018;18(6):1461–1470. [DOI] [PubMed] [Google Scholar]
- 47.Jo SK, Sung SA, Cho WY, Go KJ, Kim HK. Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant. 2006;21(5):1231–1239. [DOI] [PubMed] [Google Scholar]
- 48.Ke B, Shen X-D, Zhang YU, et al. KEAP1-NRF2 complex in ischemia-induced hepatocellular damage of mouse liver transplants. J Hepatol. 2013;59(6):1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22(2):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furuichi K, Wada T, Iwata Y, et al. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J Am Soc Nephrol. 2003;14(10):2503–2515. [DOI] [PubMed] [Google Scholar]
- 51.Yue S, Rao J, Zhu J, et al. Myeloid PTEN deficiency protects livers from ischemia reperfusion injury by facilitating M2 macrophage differentiation. J Immunol. 2014;192(11):5343–5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W, Hsiao H-M, Higashikubo R, et al. Heart-resident CCR2(+) macrophages promote neutrophil extravasation through TLR9/MyD88/CXCL5 signaling. JCI Insight. 2016;1(12): pii:87315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Syrjälä SO, Nykänen AI, Tuuminen R, et al. Donor heart treatment with COMP-Ang1 limits ischemia-reperfusion injury and rejection of cardiac allografts. Am J Transplant. 2015;15(8):2075–2084. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka M, Gunawan F, Terry RD, et al. Inhibition of heart transplant injury and graft coronary artery disease after prolonged organ ischemia by selective protein kinase C regulators. J Thorac Cardiovasc Surg. 2005;129(5):1160–1167. [DOI] [PubMed] [Google Scholar]
- 55.Dhuriya YK, Sharma D. Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflammation. 2018;15(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li W, Feng G, Gauthier JM, et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J Clin Invest. 2019;129(6):2293–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kreisel D, Krupnick AS, Puri V, et al. Short- and long-term outcomes of 1000 adult lung transplant recipients at a single center. J Thorac Cardiovasc Surg. 2011;141(1):215–222. [DOI] [PubMed] [Google Scholar]
- 58.Fiser SM, Tribble CG, Long SM, et al. Lung transplant reperfusion injury involves pulmonary macrophages and circulating leukocytes in a biphasic response. J Thorac Cardiovasc Surg. 2001;121(6):1069–1075. [DOI] [PubMed] [Google Scholar]
- 59.Sekine Y, Bowen LK, Heidler KM, et al. Role of passenger leukocytes in allograft rejection: effect of depletion of donor alveolar macrophages on the local production of TNF-alpha, T helper 1/T helper 2 cytokines, IgG subclasses, and pathology in a rat model of lung transplantation. J Immunol. 1997;159(8):4084–4093. [PubMed] [Google Scholar]
- 60.Sekine Y, Fujisawa T, Saitoh Y, et al. Donor-specific cytotoxic lymphocyte activity from bronchoalveolar lavage during acute canine lung allograft rejection. Eur J Cardiothorac Surg. 1997;11(5):902–909. [DOI] [PubMed] [Google Scholar]
- 61.Zhao M, Fernandez LG, Doctor A, et al. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L1018–1026. [DOI] [PubMed] [Google Scholar]
- 62.Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Kron IL. Pulmonary macrophages are involved in reperfusion injury after lung transplantation. Ann Thorac Surg. 2001;71(4):1134–1138; discussion 1138–1139. [DOI] [PubMed] [Google Scholar]
- 63.Kreisel D, Sugimoto S, Zhu J, et al. Emergency granulopoiesis promotes neutrophil-dendritic cell encounters that prevent mouse lung allograft acceptance. Blood. 2011;118(23):6172–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kreisel D, Sugimoto S, Tietjens J, et al. Bcl3 prevents acute inflammatory lung injury in mice by restraining emergency granulopoiesis. J Clin Invest. 2011;121(1):265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kreisel D, Nava RG, Li W, et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci USA. 2010;107(42):18073–18078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y, Li W, Luehmann HP, et al. Noninvasive imaging of CCR2(+) cells in ischemia-reperfusion injury after lung transplantation. Am J Transplant. 2016;16(10):3016–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jose MD, Ikezumi Y, van Rooijen N, Atkins RC, Chadban SJ. Macrophages act as effectors of tissue damage in acute renal allograft rejection. Transplantation. 2003;76(7):1015–1022. [DOI] [PubMed] [Google Scholar]
- 68.Fedrigo M, Feltrin G, Poli F, et al. Intravascular macrophages in cardiac allograft biopsies for diagnosis of early and late antibody-mediated rejection. J Heart Lung Transplant. 2013;32(4):404–409. [DOI] [PubMed] [Google Scholar]
- 69.Fishbein GA, Fishbein MC. Morphologic and immunohistochemical findings in antibody-mediated rejection of the cardiac allograft. Hum Immunol. 2012;73(12):1213–1217. [DOI] [PubMed] [Google Scholar]
- 70.Xu L, Collins J, Drachenberg C, Kukuruga D, Burke A. Increased macrophage density of cardiac allograft biopsies is associated with antibody-mediated rejection and alloantibodies to HLA antigens. Clin Transplant. 2014;28(5):554–560. [DOI] [PubMed] [Google Scholar]
- 71.van den Bosch TPP, Caliskan K, Kraaij MD, et al. CD16+ Monocytes and skewed macrophage polarization toward M2 type hallmark heart transplant acute cellular rejection. Front Immunol. 2017;8:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Imagawa DK, Millis JM, Seu P, et al. The role of tumor necrosis factor in allograft rejection. III. Evidence that anti-TNF antibody therapy prolongs allograft survival in rats with acute rejection. Transplantation. 1991;51(1):57–62. [DOI] [PubMed] [Google Scholar]
- 73.Roza AM, Cooper M, Pieper G, et al. NOX 100, a nitric oxide scavenger, enhances cardiac allograft survival and promotes long-term graft acceptance. Transplantation. 2000;69(2):227–231. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y, Kloc M, Li XC. Macrophages as effectors of acute and chronic allograft injury. Curr Transplant Rep. 2016;3(4):303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hancock WW, Thomson NM, Atkins RC. Composition of interstitial cellular infiltrate identified by monoclonal antibodies in renal biopsies of rejecting human renal allografts. Transplantation. 1983;35(5):458–463. [DOI] [PubMed] [Google Scholar]
- 76.Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res. 2012;53(1–3):41–57. [DOI] [PubMed] [Google Scholar]
- 77.Belge KU, Dayyani F, Horelt A, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168(7):3536–3542. [DOI] [PubMed] [Google Scholar]
- 78.Wu YL, Ye Q, Eytan DF, et al. Magnetic resonance imaging investigation of macrophages in acute cardiac allograft rejection after heart transplantation. Circ Cardiovasc Imaging. 2013;6(6):965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pajaro OE, Jaroszewski DE, Scott RL, Kalya AV, Tazelaar HD, Arabia FA. Antibody-mediated rejection in heart transplantation: case presentation with a review of current international guidelines. J Transplant. 2011;2011:351950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valenzuela NM, Hong L, Shen XD, et al. Blockade of p-selectin is sufficient to reduce MHC I antibody-elicited monocyte recruitment in vitro and in vivo. Am J Transplant. 2013;13(2):299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cross AR, Glotz D, Mooney N. The role of the endothelium during antibody-mediated rejection: from victim to accomplice. Front Immunol. 2018;9:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Loupy A, Hidalgo L, Duong JP., Response by Loupy et al. to letters regarding article, “gene expression profiling for the identification and classification of antibody-mediated heart rejection”. Circulation. 2017;136(7):698–699. [DOI] [PubMed] [Google Scholar]
- 83.Pabois A, Pagie S, Gérard N, et al. Notch signaling mediates cross-talk between endothelial cells and macrophages via Dll4 and IL6 in cardiac microvascular inflammation. Biochem Pharmacol. 2016;104:95–107. [DOI] [PubMed] [Google Scholar]
- 84.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nayak DK, Zhou F, Xu M, et al. Zbtb7a induction in alveolar macrophages is implicated in anti-HLA-mediated lung allograft rejection. Sci Transl Med. 2017;9(398). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miller JF. Immunological function of the thymus. Lancet. 1961;2(7205):748–749. [DOI] [PubMed] [Google Scholar]
- 87.Braza MS, van Leent MMT, Lameijer M, et al. Inhibiting inflammation with myeloid cell-specific nanobiologics promotes organ transplant acceptance. Immunity. 2018;49(5):819–828.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Conde P, Rodriguez M, van der Touw W, et al. DC-SIGN(+) macrophages control the induction of transplantation tolerance. Immunity. 2015;42(6):1143–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Braza MS, Conde P, Garcia M, et al. Neutrophil derived CSF1 induces macrophage polarization and promotes transplantation tolerance. Am J Transplant. 2018;18(5):1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thornley TB, Fang Z, Balasubramanian S, et al. Fragile TIM-4-expressing tissue resident macrophages are migratory and immunoregulatory. J Clin Invest. 2014;124(8):3443–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garcia MR, Ledgerwood L, Yang YU, et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest. 2010;120(7):2486–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao Y, Chen S, Lan P, et al. Macrophage subpopulations and their impact on chronic allograft rejection versus graft acceptance in a mouse heart transplant model. Am J Transplant. 2018;18(3):604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kang YJ, Kusler B, Otsuka M, et al. Calcineurin negatively regulates TLR-mediated activation pathways. J Immunol. 2007;179(7):4598–4607. [DOI] [PubMed] [Google Scholar]
- 94.Fric J, Zelante T, Wong AY, Mertes A, Yu HB, Ricciardi-Castagnoli P. NFAT control of innate immunity. Blood. 2012;120(7):1380–1389. [DOI] [PubMed] [Google Scholar]
- 95.Howell J, Sawhney R, Testro A, et al. Cyclosporine and tacrolimus have inhibitory effects on Toll-like receptor signaling after liver transplantation. Liver Transpl. 2013;19(10):1099–1107. [DOI] [PubMed] [Google Scholar]
- 96.Ikezumi Y, Suzuki T, Yamada T, et al. Alternatively activated macrophages in the pathogenesis of chronic kidney allograft injury. Pediatr Nephrol. 2015;30(6):1007–1017. [DOI] [PubMed] [Google Scholar]
- 97.Tourneur E, Ben Mkaddem S, Chassin C, et al. Cyclosporine A impairs nucleotide binding oligomerization domain (Nod1)-mediated innate antibacterial renal defenses in mice and human transplant recipients. PLoS Pathog. 2013;9(1):e1003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weimer R, Melk A, Daniel V, Friemann S, Padberg W, Opelz G. Switch from cyclosporine A to tacrolimus in renal transplant recipients: impact on Th1, Th2, and monokine responses. Hum Immunol. 2000;61(9):884–897. [DOI] [PubMed] [Google Scholar]
- 99.Weimer R, Mytilineos J, Feustel A, et al. Mycophenolate mofetil-based immunosuppression and cytokine genotypes: effects on monokine secretion and antigen presentation in long-term renal transplant recipients. Transplantation. 2003;75(12):2090–2099. [DOI] [PubMed] [Google Scholar]
- 100.Kannegieter NM, Hesselink DA, Dieterich M, et al. The Effect of tacrolimus and mycophenolic acid on CD14+ monocyte activation and function. PLoS ONE. 2017;12(1):e0170806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van den Bosch TP, Kannegieter NM, Hesselink DA, Baan CC, Rowshani AT. Targeting the monocyte-macrophage lineage in solid organ transplantation. Front Immunol. 2017;8:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hodge G, Hodge S, Reynolds PN, Holmes M. Up-regulation of interleukin-8, interleukin-10, monocyte chemotactic protein-1, and monocyte chemotactic protein-3 in peripheral blood monocytes in stable lung transplant recipients: are immunosuppression regimens working? Transplantation. 2005;79(4):387–391. [DOI] [PubMed] [Google Scholar]
- 103.Rogacev KS, Zawada AM, Hundsdorfer J, et al. Immunosuppression and monocyte subsets. Nephrol Dial Transplant. 2015;30(1):143–153. [DOI] [PubMed] [Google Scholar]
- 104.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xie Y, Tolmeijer S, Oskam JM, Tonkens T, Meijer AH, Schaaf MJM. Glucocorticoids inhibit macrophage differentiation towards a pro-inflammatory phenotype upon wounding without affecting their migration. Dis Model Mech. 2019;12(5): pii:dmm037887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin H-H, Chang K-T, Hung C-C, et al. Effects of the mTOR inhibitor rapamycin on monocyte-secreted chemokines. BMC Immunol. 2014;15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Oliveira JG, Xavier P, Sampaio SM, et al. Compared to mycophenolate mofetil, rapamycin induces significant changes on growth factors and growth factor receptors in the early days post-kidney transplantation. Transplantation. 2002;73(6):915–920. [DOI] [PubMed] [Google Scholar]
- 108.Salehi S, Reed EF. The divergent roles of macrophages in solid organ transplantation. Curr Opin Organ Transplant. 2015;20(4):446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sekerkova A, Krepsova E, Brabcova E, et al. CD14+CD16+ and CD14+CD163+ monocyte subpopulations in kidney allograft transplantation. BMC Immunol. 2014;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.de Graav GN, Bergan S, Baan CC, Weimar W, van Gelder T, Hesselink DA. Therapeutic drug monitoring of belatacept in kidney transplantation. Ther Drug Monit. 2015;37(5):560–567. [DOI] [PubMed] [Google Scholar]
- 111.Wenink MH, Santegoets KCM, Platt AM, et al. Abatacept modulates proinflammatory macrophage responses upon cytokine-activated T cell and Toll-like receptor ligand stimulation. Ann Rheum Dis. 2012;71(1):80–83. [DOI] [PubMed] [Google Scholar]
- 112.Qi F, Adair A, Ferenbach D, et al. Depletion of cells of monocyte lineage prevents loss of renal microvasculature in murine kidney transplantation. Transplantation. 2008;86(9):1267–1274. [DOI] [PubMed] [Google Scholar]
- 113.Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119(8):1810–1820. [DOI] [PubMed] [Google Scholar]
- 114.Robinson LA, Nataraj C, Thomas DW, et al. A role for fractalkine and its receptor (CX3CR1) in cardiac allograft rejection. J Immunol. 2000;165(11):6067–6072. [DOI] [PubMed] [Google Scholar]
- 115.Kitchens WH, Chase CM, Uehara S, et al. Macrophage depletion suppresses cardiac allograft vasculopathy in mice. Am J Transplant. 2007;7(12):2675–2682. [DOI] [PubMed] [Google Scholar]
- 116.Wang J, Seo MJ, Deci MB, Weil BR, Canty JM, Nguyen J. Effect of CCR2 inhibitor-loaded lipid micelles on inflammatory cell migration and cardiac function after myocardial infarction. Int J Nanomed. 2018;13:6441–6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mog B, Asase C, Chaplin A, Gao H, Rajagopalan S, Maiseyeu A. Nano-antagonist alleviates inflammation and allows for MRI of atherosclerosis. Nanotheranostics. 2019;3(4):342–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Husain B, Ellerman D. Expanding the boundaries of biotherapeutics with bispecific antibodies. BioDrugs. 2018;32(5):441–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fantuzzi L, Tagliamonte M, Gauzzi MC, Lopalco L. Dual CCR5/CCR2 targeting: opportunities for the cure of complex disorders. Cell Mol Life Sci. 2019;76(24):4869–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Warnecke G, Hutchinson JA, Riquelme P, et al. Postoperative intravenous infusion of donor-derived transplant acceptance-inducing cells as an adjunct immunosuppressive therapy in a porcine pulmonary allograft model. Transpl Int. 2009;22(3):332–341. [DOI] [PubMed] [Google Scholar]
- 121.Riquelme P, Tomiuk S, Kammler A, et al. IFN-gamma-induced iNOS expression in mouse regulatory macrophages prolongs allograft survival in fully immunocompetent recipients. Mol Ther. 2013;21(2):409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shen X-D, Ke B, Zhai Y, et al. Absence of Toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transplant. 2007;13(10):1435–1443. [DOI] [PubMed] [Google Scholar]


