Abstract
Objectives:
Decreased muscle mass is known to be associated with several serious medical conditions. We analyzed the Fifth Korean National Health and Nutrition Examination Survey (KNHANES V, 2010–2011) to estimate the heritability of muscle mass in Korean parent-offspring pairs.
Study Design:
Cross-sectional.
Main outcome measures:
A total of 1233 parents (average age 57.67 ± 8.50 years) and 917 offspring (average age 29.10 ± 7.57 years) from 743 families were included in the analysis. Muscle mass was estimated based on three different indices: appendicular skeletal muscle mass (ASM) measured with a dual-energy X-ray absorptiometry (DXA), weight-adjusted ASM (SMI), and height-adjusted ASM (RASM). The heritability was estimated by employing the maximum-likelihood variance components implemented in Sequential Oligogenic Linkage Analysis Routines (SOLAR). The best-fitting model was determined out of four polygenic models. Pearson’s partial correlation coefficient was also calculated using the muscle mass indices to further study the association between father or mother and son or daughter pairs.
Results:
The heritability estimates of the muscle mass indices ranged from 55% to 80% (all p < 0.01). The correlation coefficient of father and offspring ranged from 0.11 to 0.40, while that of mother and offspring ranged from 0.23 to 0.43 (all p < 0.01).
Conclusions:
The heritability estimates of muscle mass in Koreans are large and significant, suggesting that parental muscle mass is an important predictor of the offspring’s muscle mass. The result implies that there may be a genetic factor partly determining muscle mass.
Keywords: Muscle mass, Heritability, Sarcopenia, KNHANES, SOLAR
1. Introduction
One of the most notable changes in the elderly is a decrease in muscle mass. When this is accompanied with a decrease in muscle function, sarcopenia can be diagnosed [1]. Sarcopenia can increase the risk of osteoporotic fractures, which affect mortality, morbidity, and quality of life [2]. Even without evident fracture, sarcopenia can lead elderly persons into the frailty cycle [3], which in turn can result in multi-organ functional impairment. Sarcopenia is also linked to accumulation of visceral fat mass, increasing the risk of obesity and cardiometabolic conditions such as diabetes, hypertension and cardiovascular diseases [4]. Several studies have researched the determinants of muscle mass, in an attempt to prevent or slow the progression of sarcopenia [5,6]. However, none of the pharmacologic interventions proposed is sufficiently effective and safe; currently, exercise with or without nutritional intervention is the only intervention applicable [7].
Studies of the genetic influence on body composition have been performed in different settings. In particular, the heritability of body mass index (BMI) and obesity is well established [8], and a strong genetic influence on bone mineral density and fat mass has also been demonstrated [2,9–12]. Although the genetic influence on muscle mass and muscle strength has been highlighted, along with other aspects of body composition [13,14], most of these studies had small samples and were done in Caucasian or African populations. Considering the specificities of the Asian population in terms of body size and lifestyle, studies of this population can aid understanding of the genetic influence on the muscle mass.
There is a consensus that muscle mass and muscle strength should be considered together when defining sarcopenia, but there is no concrete operational definition for sarcopenia or a standardized intervention program in this regard [1]. According to the Korean Sarcopenic Obesity Study (KSOS), the prevalence of sarcopenia in women aged over 60 years is 4.1% or 14.2%, depending on the definition of muscle mass [15]. Given that there is no consensus on the definition of sarcopenia, a variety of muscle mass indices should be used.
To our knowledge, this is the first study of the heritability of muscle mass measured with different muscle mass indices using a nationwide cohort in an Asian country. We investigated the genetic influence on muscle mass measured with various indices in the Korean population, by analyzing the pedigree set obtained from the Fifth Korean National Health and Nutrition Examination Survey (KNHANES V, 2010–2011) database.
2. Materials and methods
2.1. Subjects
This study employed the KNHANES V (2010–2011) database, which is a nationwide, cross-sectional survey conducted by the Korean Ministry of Health and Welfare to obtain a representative data regarding the health and nutrition of the non-institutionalized Korean civilian population. To target the Korean civilian population, statistics from the 2009 Resident Registration by the Ministry of Government Administration and Home Affairs were extracted and a multi-stage clustered sampling method was used to select 3800 households, a total of 31,596 individuals (10,938 in 2010, 10,589 in 2011, 10,069 in 2012).
The KNHANES V has three parts: the health interview survey, the health examination survey, and the nutrition survey. About 80% of sampled individuals participated in all three surveys, and among them dual-energy X-ray absorptiometry (DXA) measurements were available in 9800 individuals (7043 in 2010 and 2757 in 2011). Exclusion criteria for DXA included any participants (1) who refused the test, (2) who was pregnant or suspected to be pregnant, (3) who was unable to lie down on the table, (4) who weighed over 159 kg, or (5) who had been administered a contrast agent within the previous week. All participants signed an informed consent form (IRB registration number: 2010–02CON-21-C, 2011–02CON-06-C). A detailed report of the KNHANES is available on http://knhanes.cdc.go.kr/.
Among the 9800 participants involved in all three surveys and who had DXA measurements, we excluded those aged < 20years (n = 1412), and those from single-membered families (n = 3173), families with unrelated members (n = 9), or families consisting of either siblings or couples only (n = 3056). Finally, 253 families consisting of either a father or a mother and their offspring (one or more), and 490 families with both parents and their offspring (one or more) were included in this study (total 1233 parents and 917 offspring, 2150 individuals).
2.2. Data collection and measurements
A set of four mobile examination centers (MECs) with the required equipment visited each district; participants were surveyed in the mobile center. The health interview survey of the KNHANES included information on age, gender, education level, and marital status. Participants’ body weight was measured to the nearest 0.1 kg while they wore light clothing. Height was measured to the nearest 0.1 cm.
All instruments were calibrated each morning. BMI was calculated as weight/height2 (kg/m2). The nutrition survey was carried out a week after the health interview using the 24-h dietary recall method. Daily energy intake (in kcal/day) and daily protein intake (in g/day) were estimated based on the Standard Korean Food Composition Table [16].
Appendicular skeletal muscle mass (ASM, in kg), defined as the total skeletal muscle mass of the four limbs, was measured with the Discovery-W bone densitometer (Hologic, USA). Daily quality control was done according to the manufacturer’s protocol. Skeletal muscle index (SMI, in %) was calculated as ASM divided by body weight and multiplied by 100, while relative appendicular skeletal muscle mass (RASM, in kg/m2) was defined as ASM divided by the square of the height.
Current smokers were defined as those who were smoking at the time of the survey, and had smoked more than five packs of cigarettes during their lifetime. Drinking was categorized into three levels: heavy drinker (women, ≥7 units/week; men, ≥14 units/week), social drinker (women, 1–6 units/week; men, 1–13 units/week), or none. Exercise types were categorized as resistance exercise, such as push-ups, crunches, chin-ups, or weightlifting, or as flexibility exercise, such as stretches or free gymnastics; the frequency of each type of exercise was checked. The Korean version of International Physical Activity Questionnaire Short-Form (IPAQ-SF) was used to levels of physical activity [17]. Participants were put in one of three categories of some physical activity, or a residual category (minimal physical activity) if they responded ‘no’ to all three items: they were placed in the high physical activity group if they performed intensive physical activity for over 20 min, more than 3 days a week; in the moderate physical activity group if they performed moderate physical activity for over 30 min, over 5 days a week; or in the low physical activity group if they walked for over 30 min, more than 5 days a week.
Information regarding disease history, such as hypertension, diabetes, cardiovascular disease, osteoarthritis, rheumatoid arthritis, thyroid disease, chronic renal failure, depression, or cancer was examined through face-to-face interview by a member of the KNHANES staff. The participants were assumed to have the disease if they were currently on medication or had been diagnosed by a doctor.
2.3. Definition of heritability and four polygenic models
Heritability (h2) is defined as the proportion of the phenotypic variance in a trait that is attributable to genetic effects [2]. We used the maximum-likelihood variance components method implemented in the SOLAR (Sequential Oligogenic Linkage Analysis Routines) software (SOLAR Eclipse version 8.1.1, Texas Biomedical Research Institute) to estimate the heritability of the variables of interest. Heritability of ASM, SMI, and RASM were estimated in four polygenic models, with adjustment for increasing numbers of covariates, to measure the genetic contribution to the variance in the muscle mass measures. Model 1 was adjusted for age and sex; model 2 incorporated model 1 plus adjustments for demographic covariates, such as home income, education level, and marital status; model 3 incorporated model 2 plus adjustments for lifestyle, such as BMI, daily energy intake, daily protein intake, smoking/drinking habit, and physical activity; and model 4 incorporated model 3 plus adjustments for the presence of disease history, such as hypertension, diabetes, stroke, MI/angina, osteoarthritis, rheumatoid arthritis, thyroid disease, chronic renal failure, depression, or cancer. For each analysis, only significant covariates (p ≥ 0.1) were included in the final model.
2.4. Statistical analysis
The sample weights provided by KNHANES were applied to all analyses to produce weighted variables to represent the Korean population by accounting for the complex survey design, survey non-response and post-stratification [18]. Continuous variables such as age, waist circumference, daily energy or protein intake, ASM and its derivatives were presented as mean and standard error (SE). Discrete variables were described as weighted percentages. To compare groups (father vs. mother, son vs. daughter), independent t-tests and chi-square tests were used for continuous and discrete variables, respectively.
To estimate the heritability of ASM and RASM, inverse transformation was applied to normalize the data, since the residual kurtosis exceeded the normal range. After normalization, as per SOLAR’s requirement, the chi-square test was performed to estimate the heterogeneity of genetic variance in model 3 and model 4 of ASM and RASM (those shown with asterisks (*) in Table 2). If the standard deviation of the estimated trait was less than 0.5, we multiplied the trait with the scaling factor recommended by SOLAR, and the scaled trait was then used in the analysis [10]. The heritability estimates were not affected by the scaling factor.
Table 2.
a. Heritability of ASM, SMI, and RASM.
| ASM | SMI | RASM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| h2 | SE | p-value | h2 | SE | p-value | h2 | SE | p-value | |
| From parents to offspring (son or daughter) | |||||||||
| Model 1 | 0.60 | 0.04 | <0.01 | 0.67 | 0.04 | <0.01 | 0.59 | 0.04 | <0.01 |
| Model 2 | 0.59 | 0.04 | 0.66 | 0.04 | 0.59 | 0.04 | |||
| Model 3 | 0.67* | 0.04 | 0.73 | 0.05 | 0.71* | 0.04 | |||
| Model 4 | 0.66* | 0.04 | 0.72 | 0.04 | 0.70* | 0.04 | |||
| From parents to son | |||||||||
| Model 1 | 0.59 | 0.06 | <0.01 | 0.57 | 0.05 | <0.01 | 0.55 | 0.06 | <0.01 |
| Model 2 | 0.58 | 0.06 | 0.57 | 0.05 | 0.55 | 0.06 | |||
| Model 3 | 0.71* | 0.06 | 0.65 | 0.06 | 0.68 | 0.07 | |||
| Model 4 | 0.71* | 0.06 | 0.64 | 0.06 | 0.67 | 0.07 | |||
| From parents to daughter | |||||||||
| Model 1 | 0.60 | 0.05 | <0.01 | 0.77 | 0.05 | <0.01 | 0.61 | 0.05 | <0.01 |
| Model 2 | 0.60 | 0.05 | 0.77 | 0.05 | 0.61 | 0.05 | |||
| Model 3 | 0.65* | 0.05 | 0.80 | 0.05 | 0.71 | 0.05 | |||
| Model 4 | 0.64* | 0.05 | 0.79 | 0.05 | 0.71 | 0.05 | |||
Model 1: adjusted for age and sex.
Model 2: adjusted for age, sex, home income, education level, and marital status.
Model 3: adjusted for age, sex, home income, education level, marital status, BMI, daily energy intake, daily protein intake, smoking/drinking habit, exercise, and physical activity.
Model 4: adjusted for all the covariates mentioned in model 3 and whether the participants had certain medical conditions, such as hypertension, diabetes, stroke, MI/angina, osteoarthritis, rheumatoid arthritis, thyroid disease, chronic renal failure, depression, or cancer.
For the maximum-likelihood variance components method, the heritability only from parents to offspring is calculated. Pearson’s partial correlation analysis was employed to evaluate the association between the muscle mass indices of fathers and offspring, and that of mothers and offspring. Adjustment was made for age and sex. The SAS 9.1 statistics package (SAS institute, Cary, NC, USA) was used for the analysis.
3. Results
A total of 1233 parents and 917 offspring were included in the analysis. Their demographic characteristics are shown in Table 1. The average age of the parents was 57.67 ± 8.50 years and the average BMI of parents was 23.90 ± 3.01 kg/m2. While the mean BMIs of fathers and mothers were not significantly different, daily energy intake was higher in fathers than in mothers. The ASM, SMI, and RASM were higher in fathers than in mothers. Fathers performed resistance exercise more frequently than mothers did, while the frequency of flexibility exercise was similar in the two groups.
Table 1.
Characteristics of the parent and offspring groups.
| Parents | Offspring | |||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 1233) | Father (n = 534) | Mother (n = 699) | p-value | Total (n = 917) | Son (n = 467) | Daughter (n = 450) | p-value | |
| Age (years) | 57.67 ± 8.50 | 59.38 ± 8.28 | 56.36 ± 8.45 | <0.01 | 29.10 ± 7.57 | 30.70 ± 8.19 | 27.43 ± 6.46 | <0.01 |
| BMI (kg/m2) | 23.90 ± 3.01 | 23.91 ± 2.77 | 23.89 ± 3.19 | 0.93 | 22.84 ± 3.84 | 24.11 ± 3.74 | 21.52 ± 3.49 | <0.01 |
| Daily energy intake (kcal/day) | 1952.86 ± 782.82 | 2313.2 ± 873.5 | 1698.3 ± 592.4 | <0.01 | 2158.60 ± 1051.72 | 2579.7 ± 1206.4 | 1776.2 ± 695.6 | < 0.01 |
| Daily protein intake (g/day) | 13.99 ± 3.78 | 14.06 ± 3.67 | 13.94 ± 3.85 | 0.61 | 14.98 ± 4.61 | 15.14 ± 4.72 | 14.83 ± 4.50 | 0.36 |
| Appendicular skeletal mass (g) | 17,175.49 ± 4370.72 | 21261.3 ± 3068.9 | 14059.1 ± 2009.3 | <0.01 | 19028.86 ± 5348.42 | 23390.3 ± 3377.3 | 14480.4 ± 2447.7 | < 0.01 |
| SMI (%)a | 27.56 ± 4.16 | 31.47 ± 2.44 | 24.58 ± 2.34 | <0.01 | 29.32 ± 4.28 | 32.57 ± 2.95 | 25.93 ± 2.41 | <0.01 |
| RASM(kg/m2)b | 6.56 ± 1.14 | 7.51 ± 0.86 | 5.83 ± 0.70 | <0.01 | 6.69 ± 1.43 | 7.78 ± 0.95 | 5.55 ± 0.83 | <0.01 |
| Home income in quantiles | ||||||||
| Low | 109 (8.84) | 39 (7.3) | 70 (10.01) | 0.11 | 85 (9.26) | 52 (11.13) | 33 (7.33) | <0.01 |
| Mid-low | 329 (26.68) | 132 (24.71) | 197 (28.18) | 248 (27.04) | 144 (30.83) | 104 (23.11) | ||
| Mid-high | 381 (30.90) | 173 (32.39) | 208 (29.75) | 285 (31.07) | 140 (29.97) | 145 (32.22) | ||
| High | 396 (32.11) | 183 (34.26) | 213 (30.47) | 284 (30.97) | 125 (26.76) | 159 (35.33) | ||
| Education: ≥ high school | 591 (47.93) | 294 (55.06) | 297 (42.49) | <0.01 | 888 (96.84) | 445 (95.29) | 443 (98.44) | <0.01 |
| Marital status: married | 1104 (89.54) | 521 (97.57) | 583 (83.40) | <0.01 | 113 (12.32) | 76 (16.27) | 37 (8.22) | <0.01 |
| Current smoker | 202 (16.48) | 179 (33.58) | 23 (3.32) | <0.01 | 282 (31.06) | 238 (51.40) | 44 (9.89) | <0.01 |
| Drinking | ||||||||
| Heavy drinker | 286 (23.20) | 209 (39.14) | 77 (11.02) | <0.01 | 316 (34.46) | 182 (38.97) | 134 (29.78) | <0.01 |
| Social drinker | 535 (43.39) | 209 (39.14) | 326 (46.64) | 508 (55.40) | 250 (53.53) | 258 (57.33) | ||
| Resistance exercise | ||||||||
| 0–1days/wk | 902 (73.15) | 316 (59.18) | 586 (83.83) | <0.01 | 633 (69.03) | 255 (54.60) | 378 (84.00) | <0.01 |
| 2–3days/wk | 141 (11.44) | 91 (17.04) | 50 (7.15) | 144 (15.70) | 100 (21.41) | 44 (9.78) | ||
| ≥ 4days/wk | 190 (15.41) | 127 (23.78) | 63 (9.01) | 140 (15.27) | 112 (69.44) | 28 (6.22) | ||
| Flexibility exercise | ||||||||
| 0–1days/wk | 556 (45.09) | 227 (42.51) | 329 (47.07) | 0.05 | 408 (44.49) | 211 (45.18) | 197 (43.78) | 0.05 |
| 2–3days/wk | 230 (18.65) | 93 (17.42) | 137 (19.60) | 244 (26.61) | 108 (23.13) | 136 (30.22) | ||
| ≥ 4days/wk | 447 (36.25) | 214 (40.07) | 233 (33.33) | 265 (28.90) | 148 (44.26) | 117 (26.00) | ||
| Physical activity | ||||||||
| Highc | 170 (13.90) | 91 (17.11) | 79 (11.43) | <0.01 | 150 (16.50) | 91 (19.61) | 59 (13.26) | <0.01 |
| Moderated | 135 (11.02) | 53 (9.96) | 82 (11.83) | 0.30 | 91 (10.01) | 61 (13.15) | 30 (6.74) | <0.01 |
| Lowe | 475 (38.74) | 221 (41.46) | 254 (36.65) | 0.09 | 435 (47.85) | 233 (50.22) | 202 (45.39) | 0.15 |
| HTN | 371 (30.09) | 185 (34.64) | 186 (26.61) | <0.01 | 20 (2.18) | 18 (3.85) | 2 (0.44) | <0.01 |
| DM | 197 (15.98) | 76 (14.23) | 121 (17.31) | 0.14 | 19 (2.07) | 15 (3.21) | 4 (0.89) | 0.01 |
| Stroke | 36 (2.92) | 22 (4.12) | 14 (2.00) | 0.03 | 1 (0.11) | 1 (0.21) | 0 (0) | 0.25 |
| MI/angina | 49 (3.97) | 31 (5.81) | 18 (2.58) | <0.01 | 0 (0) | 0 (0) | 0 (0) | - |
| OA | 192 (15.57) | 35 (6.55) | 157 (22.46) | <0.01 | 4 (0.44) | 3 (0.64) | 1 (0.22) | 0.32 |
| RA | 38 (3.08) | 7 (1.31) | 31 (4.43) | <0.01 | 3 (0.33) | 1 (0.21) | 2 (0.44) | 0.54 |
| Thyroid disease | 64 (5.19) | 9 (1.69) | 55 (7.87) | <0.01 | 15 (1.64) | 1 (0.21) | 14 (3.11) | <0.01 |
| CRF | 51 (4.14) | 13 (2.43) | 38 (5.44) | <0.01 | 30 (3.27) | 9 (1.93) | 21 (4.67) | 0.02 |
| Depression | 12 (0.97) | 4 (0.75) | 8 (1.14) | <0.01 | 0 (0) | 0 (0) | 0 (0) | - |
| Cancer | 73 (5.92) | 27 (5.06) | 46 (6.58) | 0.26 | 2 (0.22) | 0 (0) | 2 (0.44) | 0.09 |
BMI, body mass index; ASM, appendicular skeletal muscle mass; SMI, skeletal muscle index; RASM, relative appendicular skeletal muscle mass; DM, diabetes mellitus; MI, myocardial infarction; RA, rheumatoid arthritis; ESRD, end-stage renal disease.
Data are expressed as the mean ± standard error (SE) or weighted percentage (SE).
For home income, drinking, and exercise, likelihood chi-square value was used instead of p-values.
SMI = appendicular skeletal muscle mass/weight.
RASM = appendicular skeletal muscle mass/height2.
High physical activity: intensive physical activity over 20 min s, more than 3 days a week.
Moderate physical activity: moderate physical activity over 30 min s, over 5 days a week.
Low physical activity: walking over 30 min s, more than 5 days a week.
The average age of the offspring was 29.10 ± 7.57 and the average BMI of offspring was 22.84 ± 3.84 kg/m2. The mean BMI of sons was higher than that of daughters. The daily energy intake of sons was also higher than that of daughters. The ASM, SMI, and RASM were significantly higher in sons than in daughters. The sons performed more frequent resistance exercises than did daughters, while the rate of flexibility exercise was similar in the groups. In both parents and offspring, the majority of smokers and heavy drinkers were males.
The heritability estimates (h2) of muscle mass indices and the standard errors (SE) calculated with four polygenic models are shown in Table 2. Heritability of ASM from parents to offspring ranged from 59% to 67%. The h2 of ASM from parents to sons was estimated to be 58% to 71%, depending on the model. The h2 estimates of ASM from parents to daughters ranged from 60% to 65%. For SMI, h2 from parents to offspring was estimated to be between 66% and 73%, 57% to 65% from parents to sons, and 77% to 80% from parents to daughters. The h2 estimate of RASM from parents to offspring was between 59% and 71%, while that from parents to sons was between 55% and 68%, and from parents to daughters 61% to 71%. All estimates were statistically significant with p < 0.01.
Fig. 1 shows the scatter plot analysis of parental and offspring ASM, SMI, and RASM, respectively, and the Pearson’s partial correlation coefficient (r) of muscle mass indices are labeled in the figure. For ASM, r values for daughter-father, daughter-mother, son-father, and son-mother were 0.31, 0.39, 0.28, and 0.31 respectively. For SMI, the r values for daughter-father, daughter-mother, son-father, and son-mother were 0.39, 0.43, 0.40, and 0.29 respectively. For RASM, the r values were 0.31, 0.40, 0.27, and 0.30, in the same order as above. All the correlation analysis was statistically significant with p < 0.01.
Fig. 1.
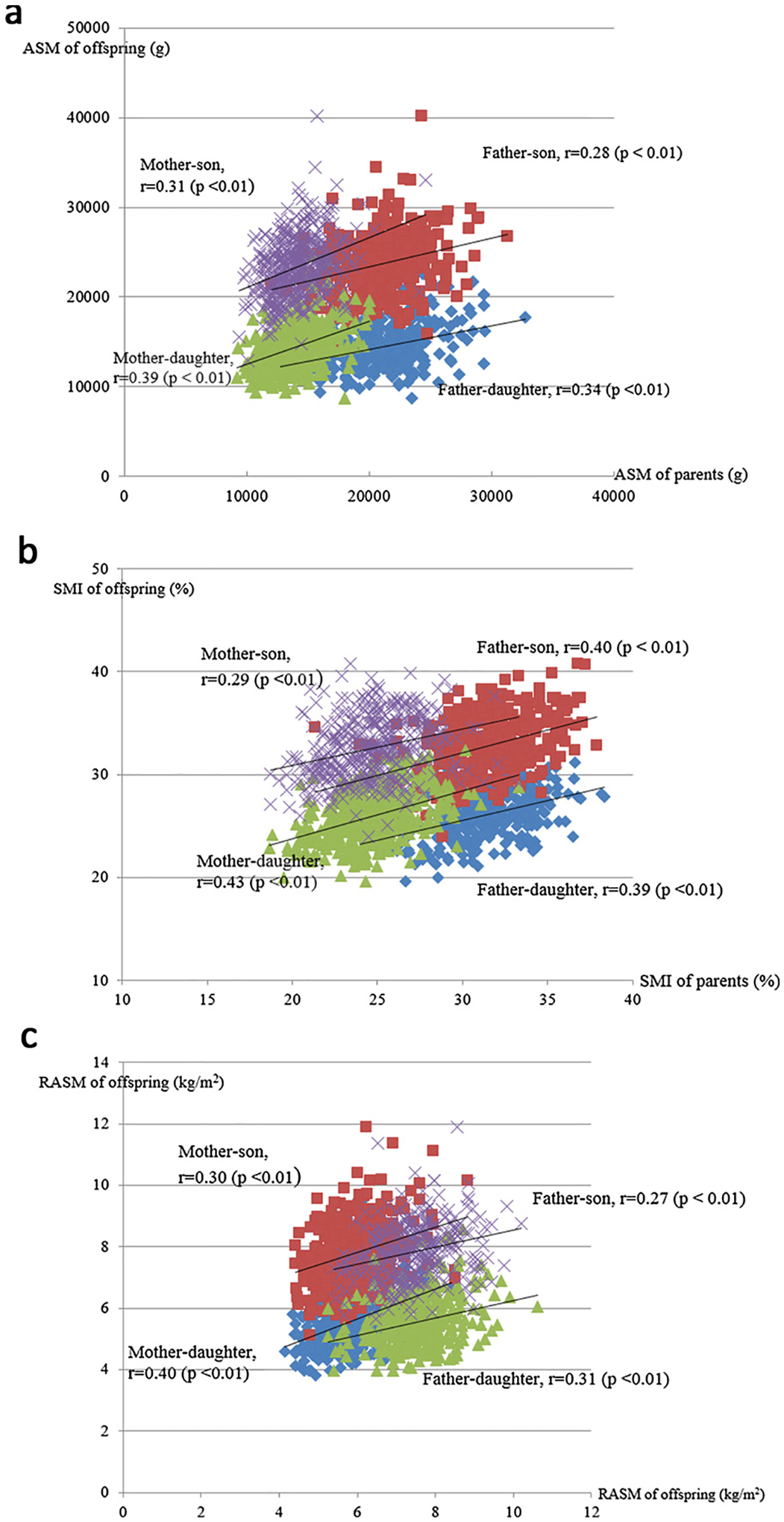
(a) Pearson’s partial correlation of ASMs of parent and offspring. (b) Pearson’s partial correlation of SMIs of parent and offspring. (c) Pearson’s partial correlation of RASMs of parent and offspring.
4. Discussion
In this study, we analyzed a total of 1233 parents and 917 offspring from 743 Korean families to estimate the heritability of muscle mass. Baseline characteristics of fathers vs. mothers and sons vs. daughters showed similar results as previous studies with similar age groups in Korea [19]. The heritability estimates for ASM, weight-adjusted ASM and height-adjusted ASM all showed a strong genetic influence. Even after adjustments for age, sex, demographic covariates, lifestyle factors, and medical history in our polygenic models to eliminate the possible confounding effect of the covariates, heritability of the muscle mass indices remained significant (adjusted h2 ranging from 64% to 79%). Heritability from parents to offspring, from parents to sons, and from parents to daughters were estimated separately; all pairs showed a significant heritable trait. Pearson’s partial correlation analysis was used to investigate the association between the muscle mass of mother or father, and that of son or daughter; r was as high as 0.39 for father-daughter pairs, 0.43 for mother-daughter pairs, 0.40 for father-son pairs, and 0.31 for mother-son pairs. The analysis thus consistently showed a statistically significant positive correlation between father or mother and son or daughter pairs after adjustments were made.
High heritability values of muscle mass in this study correspond with most previous studies on European and African populations; the heritability estimates across these studies were mostly over 50%, which is consistent with our results [11,20–22]. The consistent heritability estimate implies that genetic factors play a key role in determining muscle mass, regardless of the population. Furthermore, the influence of ethnicity on body composition and cardiometabolic risk has been well recognized [23], and the existence of ethnic variability in the heritable trait of metabolic syndrome has been reported [24]. The present study has shown that the high degree of heritability of muscle mass demonstrated in other ethnicities is also present in the Korean population.
Previous studies have focused on the strong genetic influences on body composition, and have revealed that bone mass, fat mass, and lean mass are all highly heritable. Some studies have found a shared genetic component in bone mineral density and lean mass [2,12], corresponding to the recent concept of the bone-muscle unit [25]. However, although the incidence of sarcopenia is increasing as the elderly population has grown, studies that specifically target sarcopenia are lacking. In defining sarcopenia, it has been suggested that both quantitative and qualitative traits of skeletal muscle be considered. The heritability of muscle strength (the qualitative trait) has been estimated to be 30~85% [14]. Given that muscle mass (the quantitative trait) is also heritable, as supported by the result of the present study, there may be heritable trait in sarcopenia itself [14].
For quantitative measurement of muscle mass, ASM, SMI, and RASM have been proposed so far. The European Working Group on Sarcopenia in Older People (EWGSOP) and the Asian Working Group for Sarcopenia (AWGS) have suggested that low muscle mass be defined according to SMI. However, in the Korean population, such a criterion will lead to a comparatively low cutoff point. According to the Korean Sarcopenic Obesity Study (KSOS), the prevalence of sarcopenia in women aged over 60 years is 4.1% or 14.2%, when defined with RASM or SMI respectively [15]. In this study, we looked at all three muscle mass indices for precise analysis, and the heritability estimates of the three values were all significant.
There are several weaknesses in the present study. First, this is an observational study based on a cross-sectional survey. Thus, caution should be exercised in drawing conclusions based on the suggested association. Second, the age group was not stratified. Considering that age could affect the power of heritability, stratification might help better understand the relationship between age and the genetic influence on muscle mass. Lastly, we obtained the medical history of participants from self-reported data instead of standardized lab results or medical records, which may lead to inaccurate and inconsistent results.
However, the study’s strength lies in the large sample size and in the balanced gender distribution of participants. Second, in the analysis, demographic covariates, such as home income, education level, and marital status, as well as other factors known to affect muscle mass, such as dietary protein intake or physical activity, were adjusted. Third, the study was based on the national representative data collected from all parts of the country, eliminating other possible compounding effects.
This was the first study on the heritability of muscle mass in Asia with nationwide cohort using three different muscle mass indices. In conclusion, there may be a strong genetic factor partly determining muscle mass. Further research into the heritability of sarcopenia might help building the devising of strategies for the prevention of sarcopenia and related diseases.
Funding
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, Information and Communications Technology (ICT) & Future Planning (No. 2012R1A1A2041924 to JHK).
Abbreviations:
- ASM
appendicular skeletal muscle mass
- RASM
relative appendicular skeletal muscle mass
- SMI
skeletal muscle index
Footnotes
Ethical approval
All participants of KNHANES V signed an informed consent form (IRB registration number: 2010–02CON-21-C, 2011–02CON-06-C). This work is in accordance with the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- [1].Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. , Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia, J. Am. Med. Dir. Assoc 15 (2) (2014) 95–101. [DOI] [PubMed] [Google Scholar]
- [2].Karasik D, Zhou Y, Cupples LA, Hannan MT, Kiel DP, Demissie S, Bivariate genome-wide linkage analysis of femoral bone traits and leg lean mass: Framingham study, J. Bone Min. Res 24 (4) (2009) 710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ahmed N, Mandel R, Fain MJ, Frailty: an emerging geriatric syndrome, Am. J. Med 120 (9) (2007) 748–753. [DOI] [PubMed] [Google Scholar]
- [4].Kim TN, Choi KM, The implications of sarcopenia and sarcopenic obesity on cardiometabolic disease, J. Cell. Biochem 116 (7) (2015) 1171–1178. [DOI] [PubMed] [Google Scholar]
- [5].Morley JE, Malmstrom TK, Frailty, sarcopenia, and hormones, Endocrinol. Metab. Clin. North Am 42 (2) (2013) 391–405. [DOI] [PubMed] [Google Scholar]
- [6].Ali S, Garcia JM, Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options - a mini-review, Gerontology 60 (4) (2014) 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Phu S, Boersma D, Duque G, Exercise and Sarcopenia, J. Clin. Densitom 18 (4) (2015) 488–492. [DOI] [PubMed] [Google Scholar]
- [8].Huang T, Hu FB, Gene-environment interactions and obesity: recent developments and future directions, BMC Med. Genomics 8 (1) (2015) S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Choi HS, Park JH, Kim SH, Shin S, Park MJ, Strong familial association of bone mineral density between parents and offspring: KNHANES 2008–2011, Osteoporos. Int 28 (3) (2017) 955–964. [DOI] [PubMed] [Google Scholar]
- [10].Ng MY, Sham PC, Paterson AD, Chan V, Kung AW, Effect of environmental factors and gender on the heritability of bone mineral density and bone size, Ann. Hum. Genet 70 (Pt 4) (2006) 428–438. [DOI] [PubMed] [Google Scholar]
- [11].Tarnoki AD, Tarnoki DL, Medda E, Cotichini R, Stazi MA, Fagnani C, et al. , Bioimpedance analysis of body composition in an international twin cohort, Obes. Res. Clin. Pract 8 (3) (2014) e201–298. [DOI] [PubMed] [Google Scholar]
- [12].Medina-Gomez C, Kemp JP, Dimou NL, Kreiner E, Chesi A, Zemel BS, et al. , Bivariate genome-wide association meta-analysis of pediatric musculoskeletal traits reveals pleiotropic effects at the SREBF1/TOM1L2 locus, Nat. Commun 8 (1) (2017) 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Livshits G, Gao F, Malkin I, Needhamsen M, Xia Y, Yuan W, et al. , Contribution of heritability and epigenetic factors to skeletal muscle mass variation in United Kingdom twins, J. Clin. Endocrinol. Metab 101 (6) (2016) 2450–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roth SM, Genetic aspects of skeletal muscle strength and mass with relevance to sarcopenia, Bonekey Rep. 1 (2012) 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim TN, Choi KM, Sarcopenia: definition, epidemiology, and pathophysiology, J. Bone Metab 20 (1) (2013) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].N.I.o.A.S, The Rural Development Administration, Standard Food Composition Table, Rural Development Administration, National Institute of Agricultural Sciences, 2012.
- [17].Chun MY, Validity and reliability of korean version of international physical activity questionnaire short form in the elderly, Korean J. Fam. Med 33 (3) (2012) 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kweon S, Kim Y, Jang MJ, Kim Y, Kim K, Choi S, et al. , Data resource profile: the Korea Health and Nutrition Examination Survey (KNHANES), Int. J. Epidemiol 43 (1) (2014) 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bae EJ, Kim YH, Factors affecting Sarcopenia in Korean adults by age groups, Osong Public Health Res. Perspect 8 (3) (2017) 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Arden NK, Spector TD, Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study, J. Bone Min. Res 12 (12) (1997) 2076–2081. [DOI] [PubMed] [Google Scholar]
- [21].Nabulsi M, Mahfoud Z, El-Rassi R, Al-Shaar L, Maalouf J, El-Hajj Fuleihan G, Gender differences in the heritability of musculoskeletal and body composition parameters in mother-daughter and mother-son pairs, J. Clin. Densitom 16 (2) (2013) 223–230. [DOI] [PubMed] [Google Scholar]
- [22].Hsu FC, Lenchik L, Nicklas BJ, Lohman K, Register TC, Mychaleckyj J, et al. , Heritability of body composition measured by DXA in the diabetes heart study, Obes. Res 13 (2) (2005) 312–319. [DOI] [PubMed] [Google Scholar]
- [23].Wells JC, Ethnic variability in adiposity, thrifty phenotypes and cardiometabolic risk: addressing the full range of ethnicity, including those of mixed ethnicity, Obes. Rev 13 (Suppl. 2) (2012) 14–29. [DOI] [PubMed] [Google Scholar]
- [24].Musani SK, Martin LJ, Woo JG, Olivier M, Gurka MJ, DeBoer MD, Heritability of the severity of the metabolic syndrome in Whites and Blacks in 3 large cohorts, Circ. Cardiovasc. Genet 10 (2) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Reginster JY, Beaudart C, Buckinx F, Bruyere O, Osteoporosis and sarcopenia: two diseases or one? Curr. Opin. Clin. Nutr. Metab. Care 19 (1) (2016) 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


