Abstract
Lymphocyte depletion has been shown to control costimulation blockade–resistant rejection (CoBRR) but in some settings exacerbate antibody-mediated rejection (AMR). We have used alemtuzumab, which depletes T and B cells, combined with belatacept and rapamycin, and previously reported control of both CoBRR and AMR. To evaluate this regimen’s effect on B cell signatures, we investigated 40 patients undergoing this therapy. B cell counts and phenotypes were interrogated using flow cytometry and serum was analyzed for total IgG, IgM, and donor-specific alloantibody (DSA). Alemtuzumab induction produced pan-lymphocyte depletion; B cells repopulated faster and more completely than T cells. Reconstituting B cells were predominantly naïve, and memory B cells were significantly reduced (p=0.001) post repopulation. Two B cell populations with potential immunomodulatory effects—regulatory (CD38hiCD24hiIgMhiCD20hi) and transitional B cells (CD19+CD27−IgD+CD38hi)—were enriched posttransplantation (p=0.001). Total serum IgG decreased from baseline (p=0.016) while IgM levels remained stable. Five patients developed DSA within 36 months posttransplantation but none developed AMR. Baseline IgG levels in these patients were significantly higher than in patients without DSA. These findings suggest that belatacept and rapamycin together limit homeostatic B cell activation following B cell depletion and may lessen the risk of AMR. This regimen warrants prospective, comparative study.
Keywords: Costimulation blockade, alemtuzumab, B cell
Introduction
Belatacept, a B-7 costimulation-specific fusion protein, has been introduced(1–2) as an alternative to calcineurin inhibitor (CNI)-based maintenance immunosuppressive regimens, which are associated with numerous on- and off-target side effects(3,4). However, initial studies have shown that when belatacept is used as labeled, combined with non-depletional induction with basiliximab and maintenance with steroids and mycophenolate mofetil (MMF), it is associated with substantially higher incidences of acute rejection, now termed costimulation blockade resistant rejection (CoBRR), compared to CNI-based regimens(5). Indeed, CoBRR has been associated with numerous mature T cell subsets, such as terminally differentiated CD4+CD57+ cells(6), Th17 cells(7), and/or CD28 expressing effector memory cells(8) sensitive but belatacept resistant.
Perioperative T cell depletion has emerged as one potential approach toward mitigating the effects of belatacept resistant T cell subsets, with recent reports successfully combining either thymoglobulin that are CNI(9) or alemtuzumab(10) with belatacept and rapamycin maintenance regimens. Importantly, these preliminary studies have not only reported control of CoBRR, but have also reported low rates of antibody mediated rejection (AMR). This second observation is notable, as depletional induction when combined with more conventional maintenance regimens have in some cases been associated with enhanced de novo donor-specific alloantibody (DSA) formation(11–12). The favorable outcome in our prior study suggests that the presence of belatacept and rapamycin during lymphocyte repopulation influences the lymphocyte subsets that counter this tendency. We have recently reported on the T cell subsets in a group of patients undergoing alemtuzumab-mediated depletion followed by belatacept and rapamycin maintenance therapy –– the ABR regimen –– with specific attention toward the prevention of T cell–mediated COBRR in kidney transplantation(10,13). In this study, we have focused on the B cell subsets in these patients.
B cells, as major precursors of antibody producing plasma cells, play a critical role in DSA-formation and although T cells have typically remained a central focus in the study of transplant tolerance(14), an increasing number of studies have demonstrated that specific B cell signatures are associated with transplant tolerance in kidney transplant patients after withdrawing maintenance immunosuppression(15–17). Specifically, B cell subsets with immune regulatory functions are now well associated with allograft survival(18–19), and the lack of these specific B cell subsets is associated with rejection(20–22). In general, conventional immunosuppressive regimens, including those using polyclonal lymphocyte depletional induction, leave B cells relatively unaltered(23). The ABR regimen, in contrast, leads to profound B cell depletion(10,13), and is designed to block costimulation signals between T and B cells in the germinal center(24–25), and suppress B cell proliferation by mTOR inhibition(26–27), resulting in promotion of B cell population skewing toward naïve subsets and subsets with potential immunoregulatory function.
Herein, we have longitudinally evaluated the dynamics of reconstituting B cell subsets in a cohort of 40 consecutive patients who received the ABR regimen. We find that alemtuzumab induction produces profound B cell depletion followed by rapid B cell reconstitution, creating repertoires with predominantly naïve B cells and reduced frequencies and absolute counts of memory B cell subsets. Importantly, two B cell populations with surface phenotypes suggesting regulatory function are enriched and both general IgG and DSA levels are well controlled.
Materials and Methods
Patients, immunosuppressive regimen, and follow-up
This study included 40 patients, 20 to 70 years of age, enrolled under an institutional review board–approved, US Food and Drug Administration–sponsored clinical trial (ClinicalTrials.gov - NCT00565773) following informed consent. All patients were seropositive for Epstein-Barr virus (EBV) antibodies as determined by the Emory clinical laboratory. All patients were negative for DSA at baseline, and the calculated panel reactive antibody (PRA) was ≤ 20% at enrollment in 37 patients, and ≥ 20% in 3 patients. Thirty patients received their kidney allografts from living donors, while 10 patients received kidney allografts from deceased donors. No donors were HLA identical. Preliminary clinical outcomes and flow cytometry data have been previously reported in the first 20 patients(10,13).
The immunosuppressive regimen included a single 500mg dose of methylprednisolone followed by a single 30mg dose of alemtuzumab, both given intravenously during the transplant procedure. Belatacept (Nulojix, Bristol Myers Squibb) was initiated on postoperative day 1 at 10mg/kg and dosed thereafter as described previously(10). Oral rapamycin (Sirolimus, Wyeth) was started on postoperative day 1 at 2mg daily and dosed to a trough level of 8–12 ng/ml. Nine of the first 20 patients enrolled in the study were randomized to receive a single infusion of unfractionated donor bone marrow, as previously described(10). The marrow infusions were eliminated from the protocol in the second group of 20 patients, as there was no evidence that the bone marrow influenced the clinical outcome(10). Patients who met prespecified criteria one year after transplantation were offered the opportunity to wean off rapamycin and continue with belatacept monotherapy (Figure 1). Individuals who experienced rapamycin related side effects had their rapamycin discontinued and replaced with mycophenolate mofetil, 1gm twice daily, and prednisone, 10mg daily. All patients received prophylactic valganciclovir for at least 6 months or until their absolute lymphocyte count exceeded 500, whichever was later.
Figure 1: Patient demographics. Clinical trial conduct with regard to donors, donor bone marrow infusion, and maintenance immunosuppressive regimen.
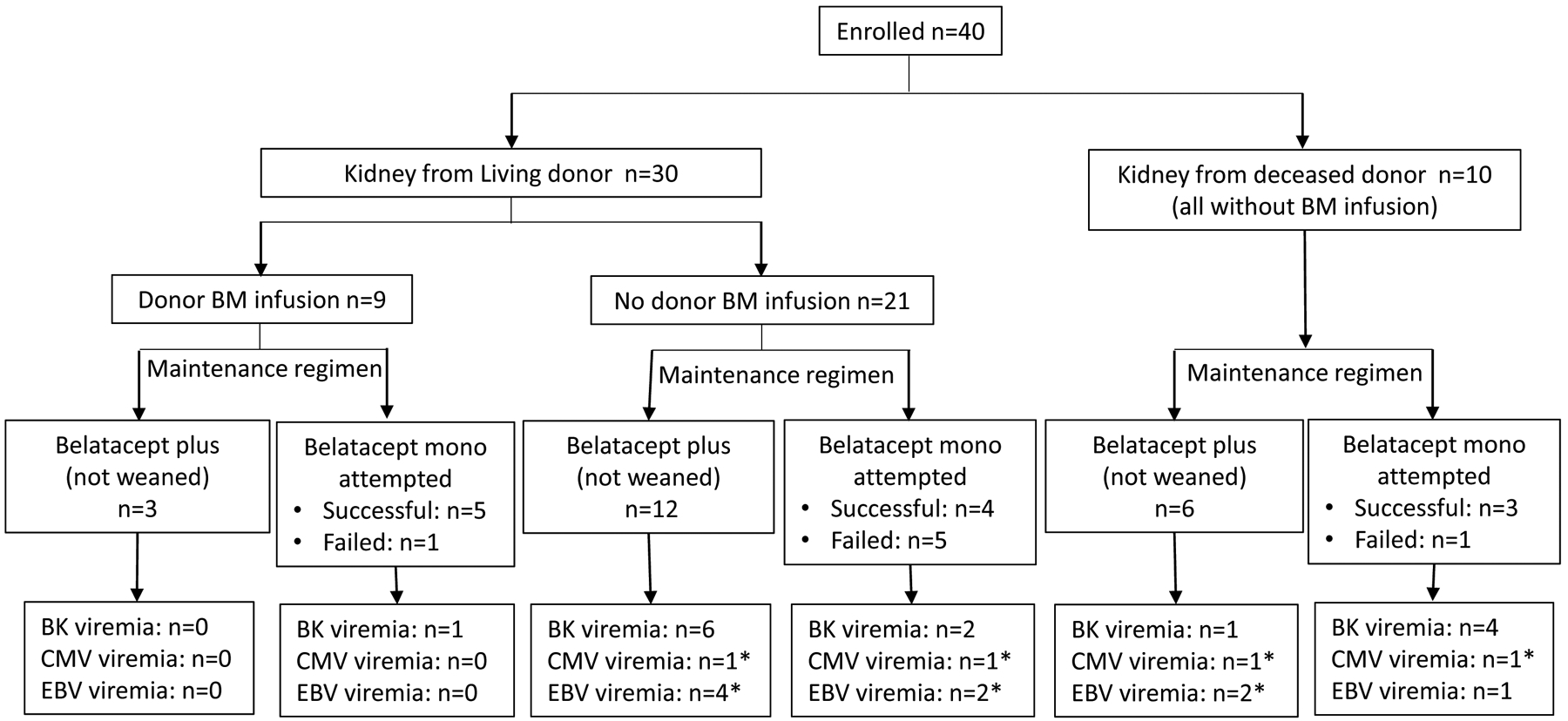
All patients received alemtuzumab induction followed by belatacept maintenance therapy, plus sirolimus (or MMF in patients who were intolerant to sirolimus). The opportunity to wean off sirolimus (or MMF) followed by belatacept monotherapy was offered to patients who met prespecified criteria at 12 months posttransplantation. Unsuccessfully weaning was determined by evidence of rejection (clinical or subclinical) on biopsy. No patient had clinical rejection in the first year. * All episodes of CMV or EBV were transient low positive defined as values < 300 copies/mL.
Blood samples were collected pre-operatively and at post-transplant months 1, 6, 12, 18, 24, 30 and 36. Protocol biopsies were performed at the time of reperfusion and post-operatively at 6 months, 1 year and 3 years, and as a condition of rapamycin weaning. Biopsies were evaluated by hematoxylin and eosin stain and immunohistochemical staining for C4d deposition.
Cells, reagents and flow cytometry
Blood samples were collected into BD Vacutainer CPT Tubes (BD Biosciences, Franklin Lakes, NJ), and peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation. PBMCs were harvested and washed three times with phosphate-buffered saline (PBS) and resuspended in PBS-FACS buffer containing 1% fetal bovine serum. PBMCs were transferred into 5 mL testing tubes, and at least 105 PBMCs were surface stained with mAbs, protected from light for 15 minutes at room temperature, and then washed with FACS buffer followed by fixation with 200-μL Stabilizing Fixative solution (BD Bioscience). Cells were analyzed using LSR II polychromatic flow cytometry. Data analysis was performed using FlowJo software (Tree Star, San Carlos, CA).
The fluorochrome-labeled mAb specific for human CD3-Alexa 700, CD4-V450, CD4-PE, CD8-PacBlue, CD16-FITC, CD20-PECy7, CD19-V450, CD20-APC Cy7, CD45-PerCP, CD56-APC, IgM-FITC, and IgD-PE, and stain buffer were purchased from BD Biosciences. Anti-CD8-Alexa 780, anti-CD27-Alexa Fluor 700, and anti-CD38-eFluor 650NC were obtained from eBioscience (San Diego, CA). Anti-CD24-PEcy7 mAbs were purchased from BioLegend (San Diego, CA). BD Trucount (BD Biosciences) analysis was performed to evaluate absolute counts for B and T cell subsets according to the manufacturer’s instruction. Briefly, 50 μL blood was added into a BD Trucount tube followed by incubation with mAbs specific to CD3, CD4, CD8, CD16, CD20, CD45 and CD56 at room temperature for 15 minutes. 1mL of High-Yield Lysing Solution (Invitrogen, Carlsbad, CA) was added to each tube and incubated at 37°C for 10 minutes. Cells were analyzed using LSR II polychromatic flow cytometry (BD Biosciences), and the data analysis was performed using FlowJo software.
Measurement of serum antibodies
Serum samples collected from patients were evaluated through the Duke Clinical Immunology Laboratory using their clinical enzyme-linked immunosorbent assay for total IgM and IgG levels. Serum PRA levels were routinely screened pre- and posttransplantation and reported by the Emory Clinical Histocompatibility Laboratory. Serum DSA was detected by a microparticle-based FACS analysis, and the specificities of DSA to HLA class I and II was determined by a clinical Luminex-based assay by the Emory Clinical Laboratory as described previously(10).
Measurement of plasma BAFF
Increased serum levels of B cell activating factor of the FNF family (BAFF) was detected post-alemtuzumab induction in patients initially enrolled in this pilot trial (13). To confirm our previous observation, the serial plasma samples from the second cohort of trial patients (n=20) were tested in duplicate by a standard enzyme-linked immunosorbent assay (ELISA) using a human BAFF ELISA kit (R&D System, Minneapolis, MN) according to manufacturer’s protocol.
Statistical analysis
To quantify the changes in absolute counts or frequencies of B cell subsets over time and account for repeated measures for the same patient, we used longitudinal generalized estimating equation (GEEs) specifying linear models with an exchangeable correlation structure and times as covariates. The main effects of time were tested, and the phenotype measurements at each post-transplantation time point were compared to the previous time point. A two-sided significance level of 0.05 was used for all statistical tests. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Two-sample Student’s t-test was performed to determine the statistical significance for IgM and IgG levels, and naïve and memory subsets in patients with or without DSA before and after transplantation. Student’s t-test was performed to determine the statistical significance for plasma BAFF concentrations before and after transplantation. A p-value of less than 0.05 was considered statistically significant.
Results
The ABR regimen effectively prevents costimulation blockade resistant rejection.
There was no graft or patient loss within the first 36 months. The ABR regimen effectively prevented acute rejection during the first year of the study. Specifically, no patient had clinical rejection in the first year and excellent posttransplant renal allograft function was observed over time as determined by sustained and improving estimated glomerular filtration rate (data not shown) consistent with previous reports(5,10). Protocol maintenance therapy was tolerated by 28 of 40 patients. Twelve patients required conversion from rapamycin to MMF and prednisone for rapamycin intolerance, including mouth ulcers, wound healing issues, and/or arthralgias. For 6 patients the conversion was transient and patients were able to return to rapamycin at a lower dose, while 6 patients required permanent removal from rapamycin. Nineteen patients underwent protocol directed rapamycin weaning beginning at 12 months posttransplantation. Twelve of these patients were successfully maintained with belatacept monotherapy (Figure 1). Seven patients were unsuccessfully weaned from rapamycin, based on protocol biopsy findings of subclinical rejection, or formation of de novo DSA, and were returned to maintenance therapy with belatacept/rapamycin (n=6) or belatacept/mycophenolate mofetil (MMF) (n=1) immunotherapy thereafter. No sustained reactivations of cytomegalovirus or EBV were detected despite monthly surveillance in these patients (Figure 1), consistent with previous reports(10–13). There was no late rejection for patients remaining on protocol therapy. One patient was withdrawn from the trial after unplanned pregnancies and subsequent abortions required transfer to conventional immunosuppression, and precipitated subsequent allosensitization and graft loss.
The ABR regimen induces profound B cell depletion followed by rapid B cell repopulation to levels exceeding pre-depletion levels.
Consistent with prior reports describing the effects of alemtuzumab induction in transplant patients receiving calcineurin- or mTOR-inhibitor monotherapies(11,28), all patients in this study experienced rapid, pan-lymphocyte depletion. The bulk lymphocyte effects in this expanded cohort were consistent with the effects observed in our previously reported pilot (10,13). Here we focused specifically on the B cell depletion and reconstitution. As shown in Figure 2, peripheral blood B cells were profoundly depleted post– alemtuzumab induction as determined by absolute cell counts. Unlike the absolute T cell counts, which we have shown remain depressed for at least 3 years(13), B cell counts rebounded relatively rapidly. This differential rate of repopulation was observed at all time points post-depletion, and by 6 months, the B cell counts exceeded those present pre-transplantation (131.2±89.7 k/μl vs. 138.5±89.9 k/μl). Thereafter, the absolute B cell counts remained significantly above pre-transplant levels (p=0.0001).
Figure 2: Repopulation of peripheral blood CD20+ B cells after renal allograft transplantation (n=40).
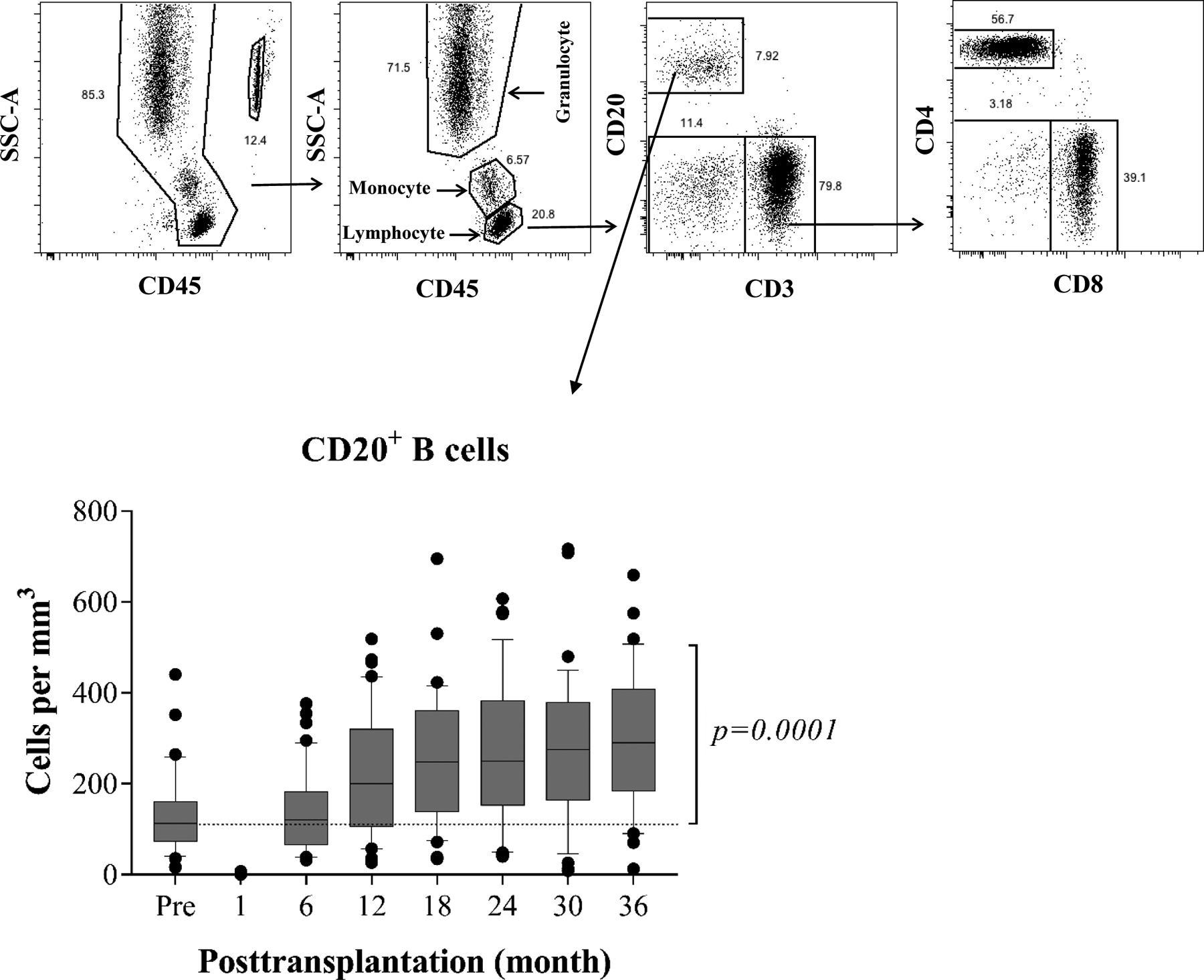
The absolute lymphocyte cell counts and subsets are analyzed by polychromatic flow cytometry, and a representative dot plots of gating strategy for B cell analysis (top). Profound T cell and B cell (CD3−CD20+) depletion is achieved with alemtuzumab induction followed by rapid repopulation to baseline level at 6 months, then significantly exceeding baseline values thereafter (bottom). The box borders indicate 75th and 25th percentile, and the line within box indicates median. Upper and lower whiskers represent 90th and 10th percentiles. The dots represent fifth and 95th percentiles.
B cell reconstitution under the ABR regimen leads to a disproportionately naïve B cell repertoire.
The phenotypical dynamics of the repopulating B cells in these patients were longitudinally examined and characterized using both the CD19/27/IgD(29) and Bm1 through Bm5 (CD19/CD38/IgD)(30) classification systems. CD19+ B cells consist of four distinct subsets based on the surface expression of CD27 and IgD (Figure S1a). As shown in Figure 3a, the frequency of repopulating naïve subsets (CD27−IgD+) significantly increased from baseline levels (58.3±18.5%) to 88.5±6.3% at 6 months, and constantly maintained high levels for 36 months. The absolute numbers of naïve cells also markedly exceeded the pre-transplant levels after 6 months post-transplantation (from 76.7±66.8 to 126.0±81.9). Unlike the naïve populations, the frequencies of memory B cell subsets, including switched (CD27+IgD−), unswitched (CD27+IgD+), and exhausted (CD27−IgD−), were markedly reduced at all time points and remained below pre-transplant levels for up to 36 months (Figure 3b). The absolute cell counts for both switched and exhausted, but not unswitched memory cells, were also significantly reduced through 36 months (Figure 3b).
Figure 3. Postdepletional reconstitution of naïve and memory B cell subsets after kidney transplantation (n=40).
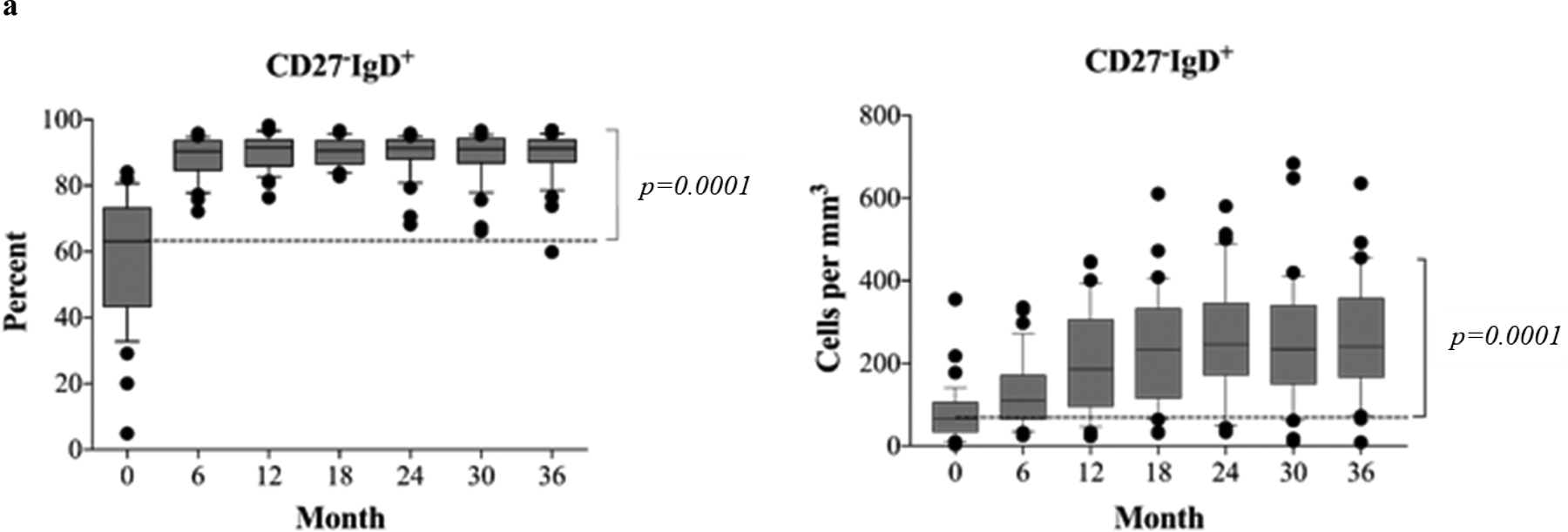
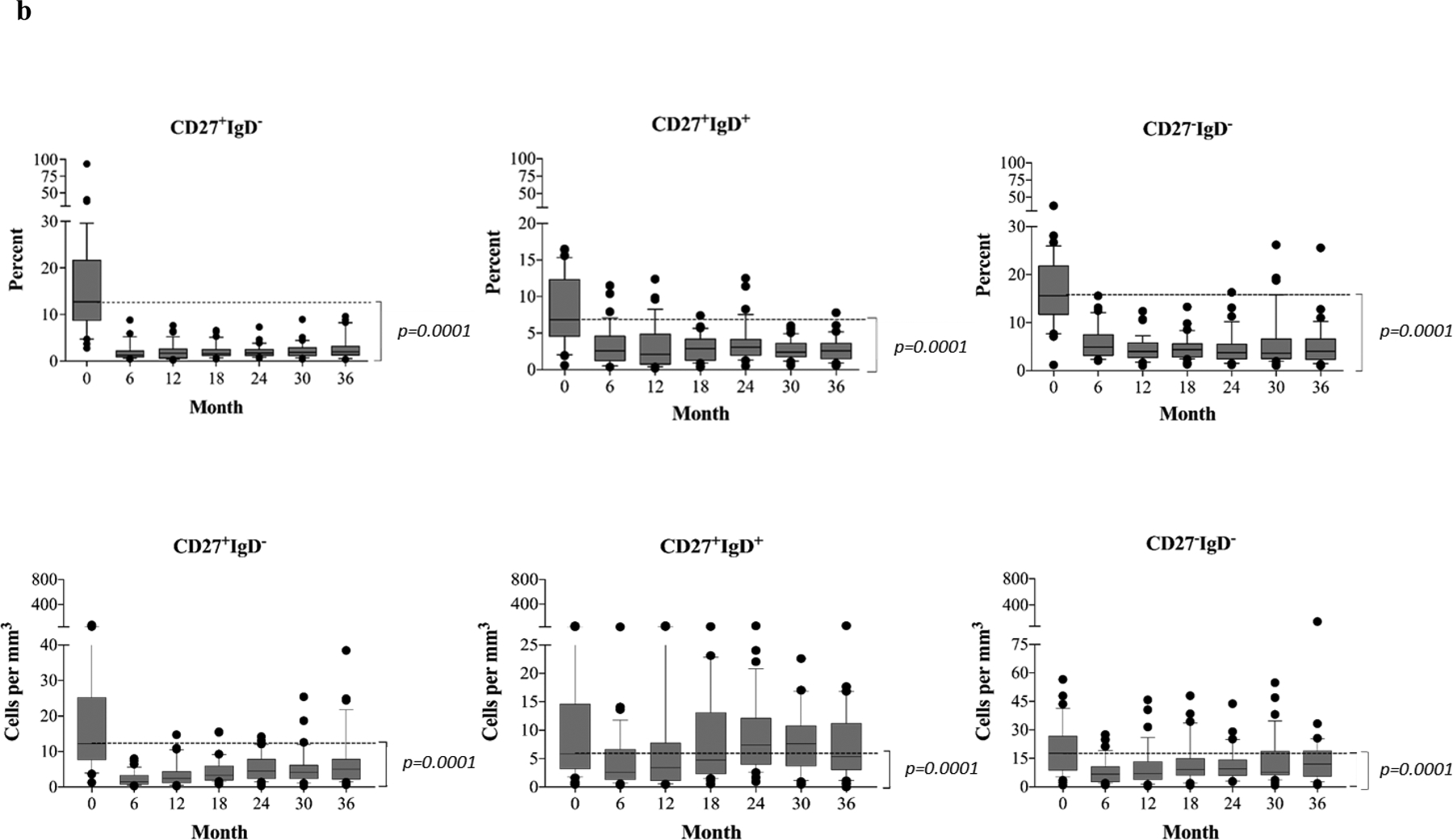
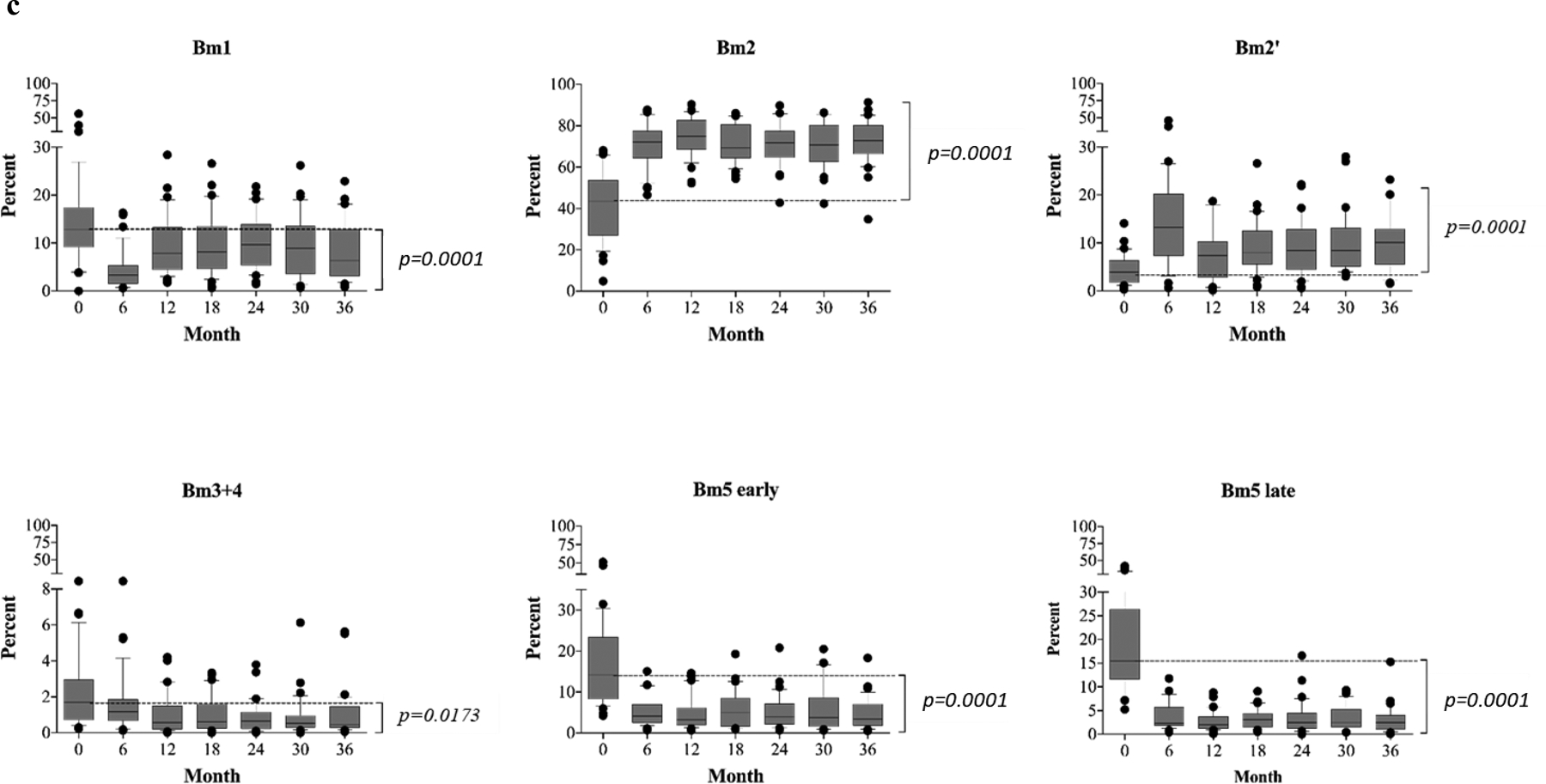
(a) Absolute numbers and frequencies over time of CD27−IgD+ naïve B cells showing rapid repopulation post–alemtuzumab induction. (b) Absolute numbers and frequencies over time of memory B cells, including CD27+IgD− (switched), CD27+IgD+ (unswitched), and CD27−IgD− (exhausted) memory subsets. Alemtuzumab induction and belatacept-based immunotherapy significantly inhibits the repopulation of memory B cells. (c) Analysis of naïve and memory B cells using Bm1-Bm5 classification based on CD38/IgD expression shows that repopulating B cells are arrested at the Bm2 stage, consisting largely of naïve B cells. The Bm2’ subset, considering mainly regulatory/transitional cells, increases significantly over time during B cell repopulation. The box borders indicate 75th and 25th percentile, and the line within box indicates median. Upper and lower whiskers represent 90th and 10th percentiles. The dots represent fifth and 95th percentiles.
To further characterize the developmental stages of repopulating peripheral B cells posttransplantation, CD19+ CD38 and IgD expression (Figure S1b). As shown in Figure 3c, B cell repopulation led to a skewed distribution of B cell maturation phenotypes compared to baseline. Cells in the Bm1 subset expanded but failed to return to baseline levels. However, marked accumulation of cells occurred in both the Bm2 (predominantly naïve cells) and the Bm2’ (germinal center founder cells were segregated into Bm1 to Bm5 subsets based on surface cells containing a significant portion of transitional and regulatory B cells)(31–32) subpopulations. In contrast, later stage cells remained under-represented following B cell reconstitution, with the frequency of memory subsets including Bm3+4 cells, Bm5-early, Bm5-late, remaining depressed below baseline for 36 months post-transplantation (Figure 3c). Thus, B cell repopulation proceeded rapidly leading to bulk numbers of B cell that exceeded baseline counts, though there was evidence of marked maturational arrest leading to accumulation of cells at the Bm2/2’ stage.
The infusion of donor bone marrow (BM) was performed in nine patients enrolled in the initial patient cohort and this practice was eliminated in the last 20 patients due to the lack of notable effects in influencing the clinical outcome when compared with patients without donor BM infusion (13). To determine the effects of donor BM infusion on naïve B cell recovery, naïve B cells in patients with donor BM were evaluated in comparison with patients without BM infusion, and the naïve B cell reconstitution in these patients was comparable with patients without BM infusion pre- and posttransplantation (Figure S2). Additionally, the repopulating naïve and memory B cells in patients requiring conversion from rapamycin to MMF due to rapamycin intolerance was also comparable to patients without rapamycin conversion (Figure S3).
Transitional and regulatory B cell populations expand under the ABR regimen.
B cells are well known as precursors of antibody producing plasma cells and are also known to foster T cell activation by presenting antigen with concomitant costimulation signals. However, increasing evidence suggests that B cells play critical roles in regulating the acquired cellular immune response as well(33–34), and some specific B cell subsets, such as regulatory(31–34) and transitional B cells(16–18, 21–22), are identified as components of immune signatures associated with immune tolerance limiting both allo- and autoimmune activity. We have hypothesized that alemtuzumab-mediated B cell depletion may promote the development of these subsets following rapid B cell reconstitution in the context of B7 costimulation blockade. We therefore focused on two subsets: transitional B cells defined as CD19+CD27−IgD+CD38hi and regulatory cells characterized as CD19+CD24hiD38hi (Figure S4). As shown in Figure 4a, both the frequencies and absolute counts of phenotypically regulatory B cells were low prior to kidney transplantation. After depletion, this subset differentially expanded, and by 6 months post-depletion was significantly (p<0.0001) elevated above pre-transplant levels, a change that persisted for all 36 months of the study. Further analysis demonstrated significantly higher percentages and absolute counts of the CD27− IgD+CD38hi transitional subset when compared with baseline levels during B cell repopulation (Figure 4b), a finding corresponding with the observation for the Bm2’ subpopulation.
Figure 4. Alemtuzumab induction and belatacept/rapamycin-based immunotherapy promotes transitional/regulatory B cell repopulation after kidney transplantation (n=40).
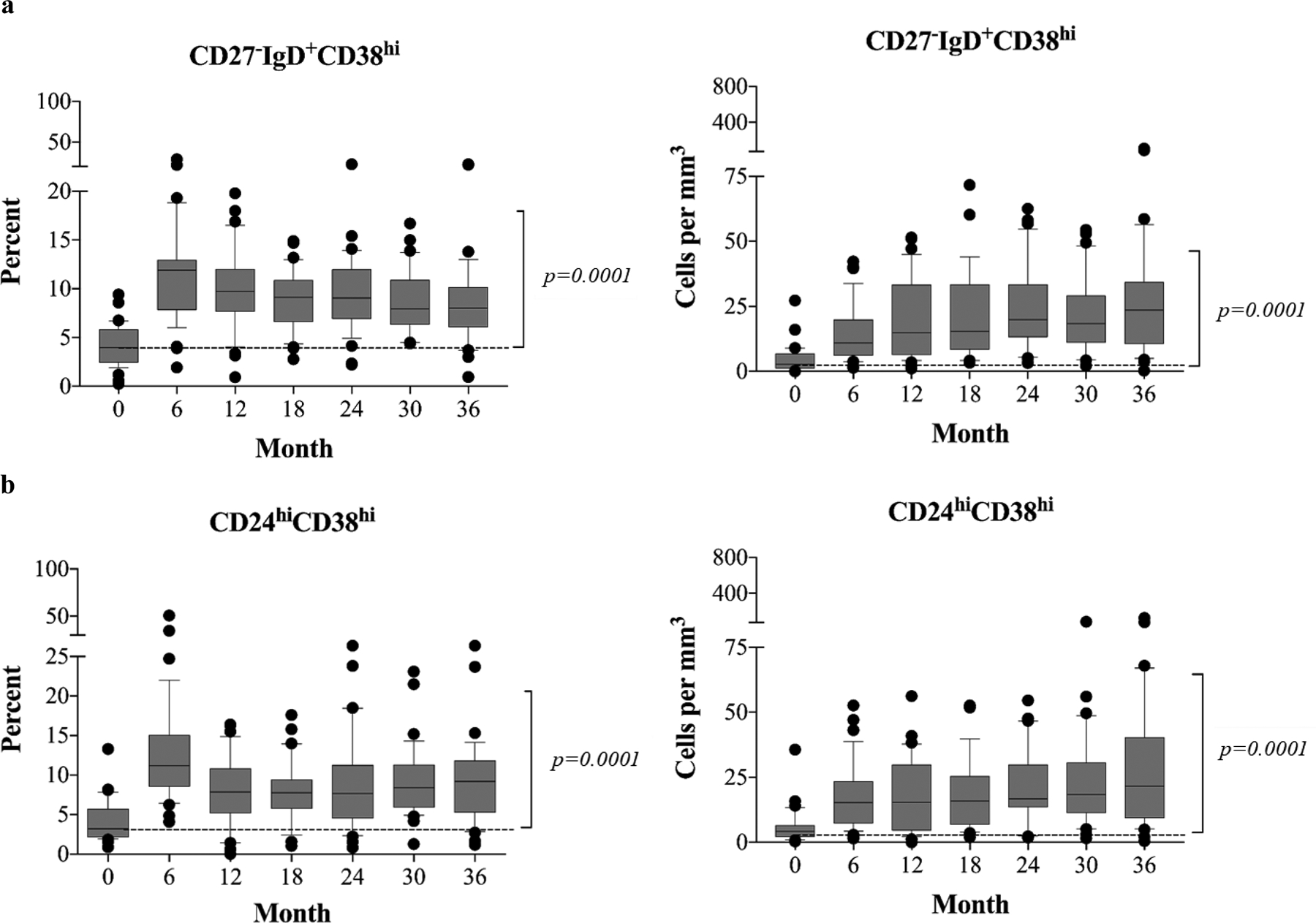
(a) Transitional B cells characterized as CD27−IgD+CD28hi subset demonstrate rapid expansion postdepletion induction. (b) CD24hiCD38hi regulatory B cells rapidly repopulate over baseline levels, posttransplantation, and maintain significant high levels through 36 months of study when compared with baseline. The box borders indicate 75th and 25th percentile, and the line within box indicates median. Upper and lower whiskers represent 90th and 10th percentiles. The dots represent fifth and 95th percentiles.
The ABR regimen alters IgG production and suppresses de novo DSA formation.
Alemtuzumab induction has been shown to alter B cell activity in a BAFF-dependent fashion, leading to accentuated antibody production initially demonstrated increased serum BAFF levels posttransplantation(11–12). Indeed, the first 20 patients enrolled in this initially demonstrated increased serum BAFF levels posttransplantation(13), and ELISA analysis confirmed increased BAFF levels in last 20 patients enrolled in this study (Figure S5). To determine the effects of the ABR regimen on B cell activity in vivo, serum samples were longitudinally acquired and measured for total IgM and IgG levels. As shown in Figure 5a, total serum IgM levels remained unchanged throughout the 36 months of the analysis (overall time effect p=0.500). In contrast, serum total IgG levels after transplantation were significantly reduced from baseline. This was first evident by 1 year and persisted as significant (p=0.016) for the remainder of the 36-month study (Figure 5a). Thus, the rapid B cell reconstitution was not associated with a concomitant increase in overall IgG production, but rather appeared to be associated with a generalized reduction in production of class-switched antibody, a finding consistent with the observed arrest in the Bm2 stage of development.
Figure 5. Measurement of serum total IgM and IgG concentrations before and after transplantation.
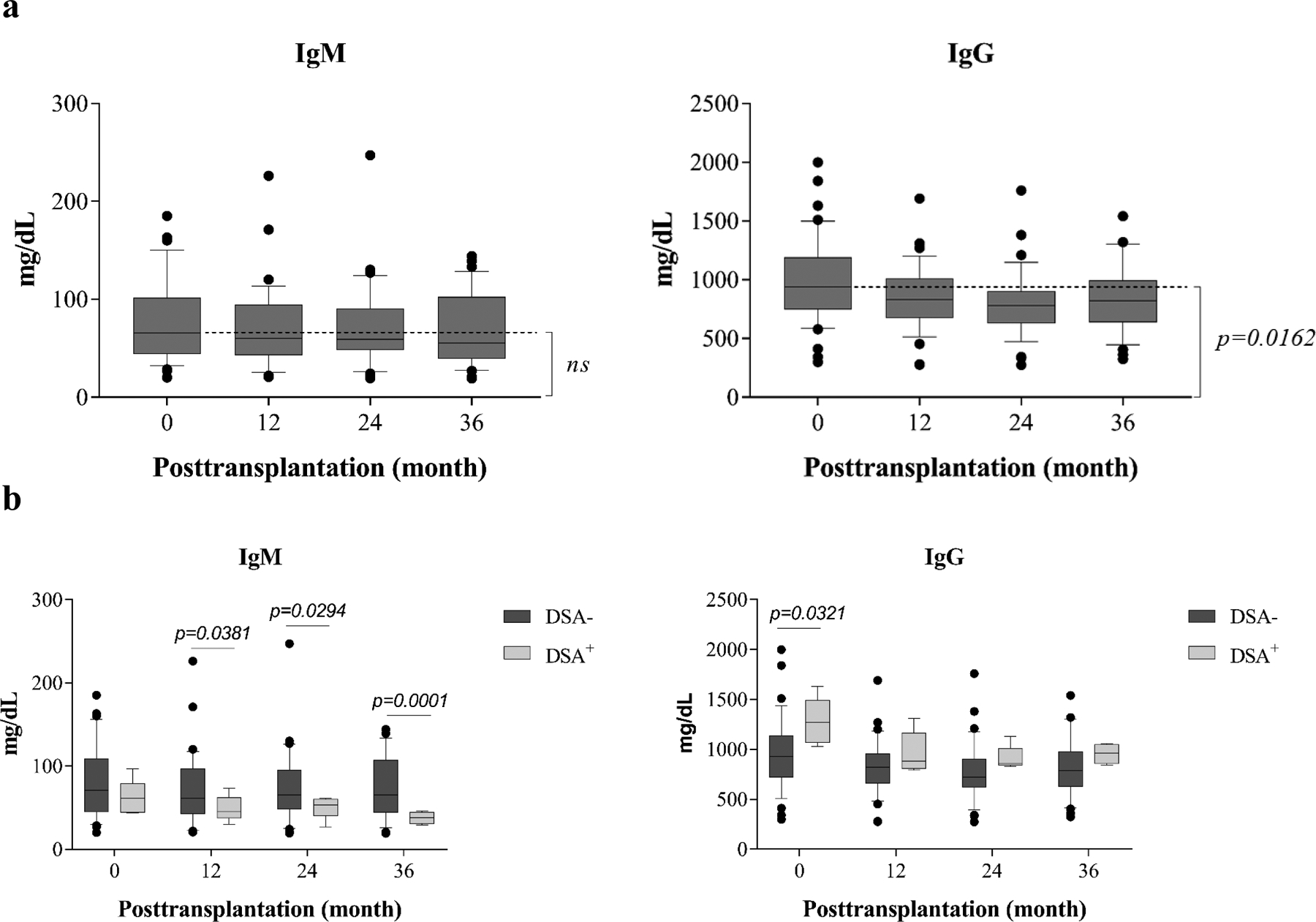
Serum samples longitudinally collected from patients (n=40) were tested for total IgM and IgG levels as measured by ELISA. (a) Patients undergoing depletion induction followed by belatacept-based immunotherapy demonstrate reduction of IgG but not IgM over 36 months of study. (b) Patients with detectable donor-specific alloantibody (DSA) demonstrated lower IgM levels after transplantation, and higher IgG levels before transplantation when compared with patients without DSA. The box borders indicate 75th and 25th percentile, and the line within box indicates median. Upper and lower whiskers represent 90th and 10th percentiles. The dots represent fifth and 95th percentiles.
To determine whether de novo alloantibody formation was inhibited, serum samples were routinely screened to detect DSA, and the samples with detectable DSA were further evaluated for the specificities to donor HLA class I and II epitopes. Thirty-five of 40 patients demonstrated no de novo DSA at any time during post-transplantation. DSAs specific for donor class I antigens were detected in three patients, and DSAs specific for donor class II antigens were detected in two patients within 36 months post-transplantation. All five of these patients deviated from the protocol ABR therapy prior to the development of DSA. One patient was withdrawn from therapy after unplanned pregnancy mandated trial withdrawal. One patient was weaned from belatacept due to development of Kaposi’s Sarcoma (KS). The KS resolved and the patient was maintained on rapamycin monotherapy. Three patients were unable to tolerate protocol rapamycin therapy and required reduction of rapamycin. There were no significant differences in B cell subsets that distinguished the patients with or without de novo DSA (Figure S6). However, analysis of serum IgM and IgG levels in patients with (n=5) or without (n=35) de novo DSA revealed that patients who developed DSA post-transplant not only deviated from protocol therapy, but also had significantly lower IgM levels at month 12 (p=0.038), 24 (p=0.0294), and 36 (p=0.0001) and significantly (p=0.0321) higher IgG levels prior to transplantation than patients without DSA (Figure 5b). There were no significant differences in B cell subpopulations or immunoglobulin levels that distinguished individuals who successfully weaned to belatacept monotherapy (data not shown).
Discussion
Belatacept-based maintenance immunosuppressive therapy is incompletely effective in preventing CoBRR when use with a nondepletional induction regimen(5). Depletional induction has been investigated as a solution for this deficit with encouraging results(9–10), but the mechanisms by which CoBRR is avoided remain unknown in humans. We have investigated the role of alemtuzumab, previously showing that when used with the ABR regimen, it facilitates T cell depletion followed by a repopulation of naïve, largely CD28 positive T cells that are well controlled by belatacept(13). However, alemtuzumab-mediated B cell depletion has, in prior studies using rapamycin or cyclosporine but not belatacept, been associated with B cell activation, high levels of BAFF, and consequent production of DSA and higher rates of AMR(11,12). As our patients have not experienced AMR, we have been interested in B cell repopulation in the presence of belatacept. The patients reported herein, now expanded to 40 patients from prior studies, are the first B cell depleted patients to be explicitly studied on a belatacept-based regimen. We find that the B cell signature that emerges on this regimen has many appealing characteristics, and several aspects of this deserve mention.
Alemtuzumab induction produces profound but remarkably transient B cell depletion which is in contrast to the prolonged, and in the case of CD4 cells, indefinite depletion seen in T cells. Thus, mechanisms of homeostatic repopulation remain intact for these patients. However, the ABR regimen markedly alters the B cell subset phenotypes, characterized by a dramatic increase in naïve B cell frequency and absolute numbers. Indeed, all memory B cell compartments remained significantly reduced after transplantation. The rapid reconstitution of peripheral B cells with a predominantly naïve phenotype has been suggested to play a role in directly promoting the expansion of regulatory T cells during early T cell reconstitution(35–36) and the interplay between regulatory T and B cell elements may be important in the control of rejection seen in these patients.
One of the most striking features of our patients is the remarkably consistent maturational arrest seen at the Bm2 phase of development. This is in stark contrast to the data from non-belatacept treated patients(12) and suggests that B7 plays a role in the germinal center requirements for B cell maturation(37). Our finding that the patients who stayed on this regimen experienced significant and sustained reductions in IgG levels provides further evidence that B7 derived costimulatory signals are involved in IgM to IgG class switching. Furthermore, the lack of DSA formation in patients on protocol therapy also speaks to the role of costimulation in germinal center affinity maturation.
Increasing evidence suggests a correlation between specific B cell signatures and T cell hyporesponsiveness in patients showing operational transplant tolerance(16–19). Recent studies have suggested that a reduction of peripheral transitional B cell subsets may be associated with allograft rejection and deterioration of kidney allograft function(21–22). Furthermore, depletional induction with alemtuzumab(38), but not thymoglobulin,(22) prior to kidney transplantation may promote a protolerance B cell signature, and the presence of belatacept even in non-depletional settings may foster a trend of increased naïve and transitional B cells when compared with CNI-based immunotherapy(39). The present study suggests that this potentially salutary phenotype can be more consistently achieved through the combination of alemtuzumab with belatacept. Our study finds a rapid, significant increase in the proportion and absolute numbers of both transitional B cells and B cells with a surface phenotype suggestive of regulatory function, with consistently high levels of these B cell subsets through 36 months post-transplant. These changes appear to be transient in patients who underwent alemtuzumab induction followed by CNI-based immunotherapy(38). Although the ABR regimen did not induce operational tolerance(10), the belatacept and rapamycin–based maintenance regimen effectively skews repopulating B cells toward the regulatory phenotype that may directly participate in inhibitory regulation of T cell–mediated responses(31–32,40) through soluble mediators such as IL-10(34,41) and TGF-β(19). It is still unclear to what degree the ABR regimen directly alters the B cell receptor repertoire during B cell repopulation, and whether this is directly associated with the apparent donor hyporesponsiveness seen posttransplantation. It is important to acknowledge that our observations are based on surface phenotype, and thus cannot directly assess regulatory behavior. Nevertheless, the change in phenotype is consistent and encouraging.
Antibody-producing allospecific B cells play a central role in antibody-mediated allograft rejection(14), particularly chronic renal allograft nephropathy(42–44). A recent report suggests that mTOR inhibition not only suppresses alloprimed B cells but also antibody-suppressing CD8+ cells(26). However, mTOR inhibitor, when use as monotherapy following alemtuzumab induction, does not prevent de novo DSA and antibody mediated rejection(11). In contrast, this cohort study demonstrates that B7 costimulation blockade effectively inhibits de novo DSA. Indeed, 12/40 patients with low risk factors were successfully maintained with belatacept monotherapy without demonstrating de novo DSA formation, a robust demonstration of a costimulation sensitive state. The inhibition of de novo DSA was also found in 7/20 patients with low risk factors but who failed rapamycin weaning. We found de novo DSA developed after 12 months posttransplantation in 5 patients, all of whom had identified risk factors, such as multiple failed posttransplant pregnancies, discontinuation of belatacept due to Kaposi’s sarcoma, or a required reduction of rapamycin due to side effects. The repopulating B cell phenotype in these patients was similar to patients was similar to patients without de novo DSA (Figure S5).
Herein, our data characterize the dynamics and phenotypic repertoires of reconstituting B cells longitudinally in a sizable cohort of patients receiving ABR, and demonstrate repertoires with predominantly naïve but not memory B cell subsets. We find a significant increase of two unique B cell subsets characterized as potentially regulatory occurring over 36 months of study, consistent with findings seen in kidney transplant patients with functional immune tolerance. This novel immunotherapy effectively prevents CoBRR and de novo DSA. Our findings suggest that lymphocyte depletion and a belatacept/rapamycin-based maintenance regimen achieves its antirejection effects by promoting naïve and immunomodulatory B cell subsets while suppressing memory B cells and de novo DSA production posttransplantation. This regimen warrants formal, prospective, comparative clinical trial study.
Supplementary Material
Figure S1. Phenotype of naïve and memory B cells. (a) CD19+ naïve and memory B cells were defined based on CD27 and IgG expression. (b) CD19+ cells were segregated into 6 subsets of Bm1, Bm2, Bm2’, Bm4+5, Bm5 early, and Bm late based on CD38 and IgD expression.
Figure S2. Repopulating naïve B cells (CD27−IgD+) in patients with donor bone marrow (BM) infusion were comparable to patients without donor BM infusion. The box borders indicate 75th and 25th percentile, and the line within box indicates median. Upper and lower whiskers represent 90th and 10th percentiles. The dots represent fifth and 95th percentiles.
Figure S3. Repopulating naïve and memory B cells in patients requiring conversion from rapamycin to MMF were comparable to patients without conversion. The box borders indicate 75th and 25th percentile, and the line within box indicates median. Upper and lower whiskers represent 90th and 10th percentiles. The dots represent fifth and 95th percentiles.
Figure S4. Phenotypical analysis of transitional and regulatory B cells. Singlets of CD19+CD27− cells were segregated by the expression of CD38 and IgD into naïve, intermediate, and transitional subsets. Regulatory cells were defined as CD19+CD24hiCD38hiCD20hi cells.
Figure S5. Plasma BAFF concentrations before and after transplantation in patients enrolled in phase II of study (n=20) measured by a standard enzyme-linked immunosorbent assay. *** p=0.001
Figure S6. Repopulating naïve and memory B cells in patients developing DSA (n=5) posttransplantation were similar to patients without development of DSA (n=35).
ACKNOWLEDGMENTS
The authors thank the members of the Emory Transplant Center and Duke Transplant Center Biorepositories for sample collection and storage. We also acknowledge the Duke Transplant Center Flow Cytometry Core for technical assistance in flow cytometry. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002553.
This work was funded in part by grants from the United States Food and Drug Administration (1R01 FD003539-01, ADK), the National Institutes of Health (R01 AI097423, ADK) and a Roche Organ Transplant Research Foundation grant (346678023, HX).
Abbreviations:
- ABR
alemtuzumab induction followed by belatacept/rapamycin maintenance immunotherapy
- AMR
antibody mediated rejection
- ANOVA
analysis of variance
- CNI
calcineurin inhibitor
- COBRR
costimulation resistant rejection
- DSA
donor-specific alloantibody
- EBV
Epstein–Barr virus
- ELISA
enzyme-linked immunosorbent assay
- mAb
monoclonal antibody
- MMF
mycophenolate mofetil
- PBS
phosphate-buffered saline
- PMBC
peripheral blood mononuclear cell
- PRA
panel reactive antibody
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Trial Registration. ClinicalTrials.gov - NCT00565773
References
- 1.Vicenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005; 353:770–781. [DOI] [PubMed] [Google Scholar]
- 2.Vicenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016; 374:333–343. [DOI] [PubMed] [Google Scholar]
- 3.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003; 349:931–940. [DOI] [PubMed] [Google Scholar]
- 4.Fisher R. Cytomegalovirus infection and disease in the new era of immunosuppression following solid organ transplantation. Transpl Infect Dis. 2009; 11:199–225. [DOI] [PubMed] [Google Scholar]
- 5.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Dario P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant 2010; 10:535–546. [DOI] [PubMed] [Google Scholar]
- 6.Espinosa J, Herr F, Tharp G, et al. CD57+CD4 T cells underlie belatacept-resistant allograft rejection. Am J Transplant 2016; 16:1102–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krummey SM, Cheeseman JA, Gonger JA, et al. High CTLA-4 expression on Th17 cells results in increased sensitivity to CTLA-4 coinhibition and resistance to belatacept. Am J Transplant 2014; 14:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortes-Cerisuelo M, Laurie SJ, Mathews DV, Winterberg PD, Larsen CP, Adams AB, Ford ML. Increased pretransplant frequency of CD28+CD4+TEM predicts belatacept-resistant rejection in human renal transplant recipients. Am J Transplant 2017; 17:2350–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson R, Grinyó J, Vincenti F, Kaufman DB, Woodle ES, Marder BA, Citterio F, Marks WH, Agarwal M, Wu D, Dong Y, Garg P. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant. 2011; 11:66–76. [DOI] [PubMed] [Google Scholar]
- 10.Kirk A, Guasch A, Xu H, Cheeseman J, Mead S, Ghali A, et al. Renal transplantation using belatacept without maintenance steroids or calcineurin inhibitors. Am J Transplant. 2014; 14(5)1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knechtle SJ, Pirsch JD, Fechner JH Jr, Backer BN, Friedl A, Colvin RB, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant 2003; 3:722–730. [DOI] [PubMed] [Google Scholar]
- 12.Todeschini M, Cortinovis M, Perico N, Poli F, Innocente A, Aparecida R, et al. In kidney transplant patients, alemtuzumab but not basiliximab/low-dose rabbit anti-thymocyte globulin induces B cell depletion and regeneration, which associated with a high incidence of de novo donor-specific anti-HLA antibody development. J Immunol. 2013; 191:2818–2828. [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Samy KP, Guasch A, Mead SI, Ghali A, Mehta AK, Stempora L, Kirk AD. Postdepletion lymphocyte reconstitution during belatacept and rapamycin treatment in kidney transplant recipients. Am. J. Transplant 2016; 16: 550–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 2012; 8:348–357. [DOI] [PubMed] [Google Scholar]
- 15.Pallier A, Hillion S, Danger R, Giral M, Racape M, Degauque N et al. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int 2010;78:503–513. [DOI] [PubMed] [Google Scholar]
- 16.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 2010;120:1836–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newell KA, Asare A, Sanz I, Wei C, Rosenberg A, Cao Z, et al. Longitudinal studies of a B cell-derived signature of tolerance in renal transplant recipients. Am J Transplant 2015;15:2908–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shabir S, Girdlestone J, Briggs D, Kaul B, Smith H, Daga S, et al. Transitional B lymphocytes are associated with protection from kidney allograft rejection: a prospective study. Am J Transplant 2015;15:1384–1391. [DOI] [PubMed] [Google Scholar]
- 19.Lee KM, Stott R, Zhao G, SooHoo J, Xiong W, Lian MM, et al. TGF-β-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J Immunol 2014; 44:1728–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherukuri A, Rothstein DM, Clark B, Carter CR, Davison A, Hernandez-Fuentes M et al. Immunologic human renal allograft injury associates with an altered IL-10/TNF-alpha expression ratio in regulatory B cells. J Am Soc Nephrol 2014; 25:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherukuri A, Salama AD, Carter CR, Landsittel D, Arumugakani G, Clark B et al. Reduced human transitional B cell T1/T2 ratio is associated with subsequent deterioration in renal allograft function. Kidney Int 2017;91:183–195. [DOI] [PubMed] [Google Scholar]
- 22.Svachova V, Sekerkova A, Hruba P, Tycova I, Rodova M, Cecrdlova E, et al. Dynamic changes of B-cell compartments in kidney transplantation: lack of transitional B cells is associated with allograft rejection. Transpl Int 2016; 29:540–548. [DOI] [PubMed] [Google Scholar]
- 23.Kho MML, Bouvy AP, Cadogan M, Kraaijeveld R, Baan CC, Weimar W. The effect of low and ultra-low dosages thymoglobulin on peripheral T, B and NK cells in kidney transplant recipients. Transplant Immunology 2012; 26:186–190. [DOI] [PubMed] [Google Scholar]
- 24.Kim EJ, Kwun J, Gibby AC, Hong JJ, Farris AB 3rd, Iwakoshi NN et al. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant 2014;14:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Wang Q, Yin D, Vu V, Sciammas R, Chong AS. Cutting Edge: CTLA-4Ig Inhibits Memory B Cell Responses and Promotes Allograft Survival in Sensitized Recipients. J Immunol 2015;195:4069–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avila CL, Zimmerer JM, Elzein SM, Pham TA, Abdel-Rasoul M, Bumgardner GL. mTOR inhibition suppresses posttransplant alloantibody production through direct inhibition of alloprimed B cells and sparing of CD8+ antibody-suppressing T cells. Transplantation 2016; 100:1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traitanon O, Mathew JM, La Monica G, Xu L, Mas V, Gallon L. Differential Effects of Tacrolimus versus Rapamycin on the Proliferation, Activation and Differentiation of Human B Cells. PLoS One 2015;10:e0129658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirk A, Hale D, Mannon R, Kleiner D, Hoffmann S, Kampen R, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (campath-1H). Transplantation 2003; 76:120–129. [DOI] [PubMed] [Google Scholar]
- 29.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+D+ peripheral B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med 1998; 188:1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bohnhorst J, Bjorgan M, Thoen J, Natvig J, Thompson K. Bm1-Bm5 classification of peripheral B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in B cell subpopulations in patients with primary Sjogren’s syndrome. J Immunol 2001; 167:3610–3618. [DOI] [PubMed] [Google Scholar]
- 31.Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, Mauri C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting Th1 and Th17 differentiation. Sci Transl Med 2013; 5:173ra23. [DOI] [PubMed] [Google Scholar]
- 32.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR. Mauri C. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 2010; 32:129–140 [DOI] [PubMed] [Google Scholar]
- 33.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity 2015; 42:607–612. [DOI] [PubMed] [Google Scholar]
- 34.Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunol Rev 2014:259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Jensen P. Direct expansion of human allospecific Foxp3+CD4+ regulatory T cells with allogeneic B cells for therapeutic application. J Immunol 2009; 183:4094–4102. [DOI] [PubMed] [Google Scholar]
- 36.Reichardt P, Dombach B, Rong S, Beissert S, Gueler F, et al. Naïve B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood 2007; 110:1519–1529. [DOI] [PubMed] [Google Scholar]
- 37.Liu YJ, Arpin C. Germinal center development. Immunol Rev 1997; 156:111–126 [DOI] [PubMed] [Google Scholar]
- 38.Heidt S, Hester J, Shankar S, Friend PJ, Wood KJ. B cell repopulation after alemtuzumab induction-transient increase in transitional B cells and long-term dominance of naïve B cells. Am J Transplant 2012;12:1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leibler C, Matignon M, Pilon C, Montespan F, Bigot J, Lang P, et al. Kidney transplant recipients treated with belatacept exhibit increased naïve and transitional B cells. Am J Transplant 2014;14:1173–1182. [DOI] [PubMed] [Google Scholar]
- 40.Bigot J, Pilon C, Matignon M, Grodin C, Leibler C, Aissat A, et al. Transcriptomic signature of CD24hiCD38hi transitional B cells associated with an immunoregulatory phenotype in renal transplant recipients. Am J Transplant 2016;16:3430–3442. [DOI] [PubMed] [Google Scholar]
- 41.Yanaba K, Bouazia JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008; 28:639–650. [DOI] [PubMed] [Google Scholar]
- 42.Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, et al. Evidence for antibody-mediated injury as major determinant of late kidney allograft failure. Transplantation 2010; 90:68–74. [DOI] [PubMed] [Google Scholar]
- 43.DeVos JM, Gaber AO, Knight RJ, Land GA, Suki WN, Gaber LW, Patel SJ. Donor-specific HLA-DQ antibodies may contribute to poor graft outcome after renal transplantation. Kidney Int 2017; 82:598–604. [DOI] [PubMed] [Google Scholar]
- 44.Willcombe M, Brookes P, Sergeant R, Santos-Nunez E, Steggar C, Jack G, et al. De novo DQ donor-specific antibodies are associated with a significant risk of antibody-mediated rejection and transplant glomerulopathy. Transplantation 2012; 94:172–177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Phenotype of naïve and memory B cells. (a) CD19+ naïve and memory B cells were defined based on CD27 and IgG expression. (b) CD19+ cells were segregated into 6 subsets of Bm1, Bm2, Bm2’, Bm4+5, Bm5 early, and Bm late based on CD38 and IgD expression.
Figure S2. Repopulating naïve B cells (CD27−IgD+) in patients with donor bone marrow (BM) infusion were comparable to patients without donor BM infusion. The box borders indicate 75th and 25th percentile, and the line within box indicates median. Upper and lower whiskers represent 90th and 10th percentiles. The dots represent fifth and 95th percentiles.
Figure S3. Repopulating naïve and memory B cells in patients requiring conversion from rapamycin to MMF were comparable to patients without conversion. The box borders indicate 75th and 25th percentile, and the line within box indicates median. Upper and lower whiskers represent 90th and 10th percentiles. The dots represent fifth and 95th percentiles.
Figure S4. Phenotypical analysis of transitional and regulatory B cells. Singlets of CD19+CD27− cells were segregated by the expression of CD38 and IgD into naïve, intermediate, and transitional subsets. Regulatory cells were defined as CD19+CD24hiCD38hiCD20hi cells.
Figure S5. Plasma BAFF concentrations before and after transplantation in patients enrolled in phase II of study (n=20) measured by a standard enzyme-linked immunosorbent assay. *** p=0.001
Figure S6. Repopulating naïve and memory B cells in patients developing DSA (n=5) posttransplantation were similar to patients without development of DSA (n=35).


