Abstract
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease often associated with atopic comorbidities and has significant impact on children and their families. There is a lack of robust and longitudinal long-term data on disease characteristics and typical clinical practice with currently available treatments in children with moderate-to-severe AD. Hence, an observational study is needed to evaluate AD characteristics and progression in paediatric patients with moderate-to-severe AD.
Methods and analysis
Pediatric Study in Atopic Dermatitis (PEDISTAD) is a prospective, observational, longitudinal study in paediatric patients with moderate-to-severe AD who are currently receiving systemic or topical treatment and whose disease is not adequately controlled by topical prescription therapies or for whom those therapies are not medically advisable. 1300 children at 100–150 sites in approximately 20 countries worldwide will be enrolled and followed for 5 years. AD therapy is at the discretion of the investigator. Data collected will include: AD disease characteristics and comorbidities; current therapy for AD and initiation of new treatments/changes in current treatment; patient-reported/caregiver-reported outcomes; days missed from school/work for the patient/caregiver; healthcare professional visits; safety and biomarkers.
Ethics and dissemination
This study is conducted in accordance with the principles established by the 18th World Medical Assembly and all subsequent amendments and the guidelines for Good Epidemiology Practice. Each individual country assures that ethics approval has been received and local regulatory requirements are met. Ethics approval has been obtained in all countries currently participating in PEDISTAD. Study data will be disseminated in manuscripts submitted to peer-reviewed medical journals as well as in abstracts submitted to congresses and in the resulting posters and presentations.
Trial registration number
NCT03687359; pre-results.
Keywords: atopic dermatitis, observational, paediatric, systemic treatment
Strengths and limitations of this study.
Paediatric Study in Atopic Dermatitis is a multinational, observational, longitudinal study in a large cohort of paediatric patients with moderate-to-severe atopic dermatitis (AD) that will collect long-term data on patient and disease characteristics, progression of disease, selected atopic comorbidities, real-world treatment patterns, efficacy and safety.
Previous observational studies in patients with AD have not focused on moderate-to-severe disease leading to a gap in knowledge that will be addressed by this study.
The observational nature of the study limits the robustness of the collected data compared with that obtained from blinded studies with control groups.
Challenges of the study include patient recruitment in multiple countries and retention of patients through the observation period of 5 years, both of which can be difficult in young children.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease often associated with atopic comorbidities.1 2 Due to lack of standardised diagnostic criteria and outcome measures of disease severity, there is variability in the reported prevalence rates of AD in children.
AD profoundly affects the quality of life of children and family members.3 Itching can affect mood and sleep quality, and the chronic relapsing nature of AD has a detrimental impact on the quality of life of the family.3 Children with AD may also have symptoms of anxiety and depression.4
Limited treatment options are available for children with moderate-to-severe AD and primarily include topical corticosteroids, topical calcineurin inhibitors, topical crisaborole and systemic immunosuppressants.5–8 Most systemic agents are broadly immunosuppressive, used off-label and are not currently approved for use in children. In general, they do not provide a favourable long-term benefit–risk profile for paediatric patients with AD inadequately controlled by topical therapies. Furthermore, disease can often rebound after cessation of systemic therapy, especially after administration of systemic cyclosporine.9
There is a lack of robust and longitudinal long-term data related to disease characteristics and typical clinical practice with currently available treatments in children. Hence, an observational study is necessary to evaluate the characteristics of paediatric patients with moderate-to-severe AD whose disease is not adequately controlled with topical therapies or when those therapies are not medically advisable.
The Pediatric Study in Atopic Dermatitis (PEDISTAD) aims to address the substantial need for a better understanding of AD characteristics and progression, including patient and caregiver burden, in paediatric patients with moderate-to-severe AD who initiate, or are candidates for, systemic therapy. The study will document patient characteristics, patient-reported and caregiver-reported outcomes, AD progression and atopic comorbidities and assess the effectiveness and safety of therapies (systemic and topical) while describing real-world treatment patterns over a 5-year period. A biomarker substudy will analyse the association between biomarkers and disease state and time course of AD in a subset of PEDISTAD study participants. Here, we describe the objectives, design and endpoints of the PEDISTAD.
Methods and analysis
PEDISTAD is an international, multicentre, longitudinal, prospective, non-interventional study designed to describe the disease life course and comorbidities of paediatric patients with moderate-to-severe AD whose disease is not adequately controlled with topical therapies or for whom those therapies are not medically advisable (NCT03687359, study OBS15333, protocol version 1, 01 May 2018 and sub-study LPS15496, protocol version 1, 10 April 2018).
Current participating sites are listed in the Clinicaltrials.gov record.
Patients
This study will enrol a balanced number of patients aged <2 years, ≥2–<6 years and ≥6–<12 years at baseline. Enrolment quotas with respect to treatment types (systemic vs topical) may be imposed to ensure target numbers of patients in each treatment category. To minimise patient selection bias, all eligible patients at each selected site should be invited to participate in this registry until the global enrolment goal or the site enrolment limit is met.
AD therapy prescribed to patients who are enrolled in the study is not dictated per study protocol, and the therapeutic drug prescription is decided by the medical judgement of the study investigator. Patients may begin treatment with therapies that become commercially available during the course of the study.
Inclusion and exclusion criteria are reported in table 1. Briefly, patients are eligible for the study if they are <12 years of age at baseline, have investigator-assessed moderate-to-severe disease and are either receiving systemic treatment for AD (including biologics (currently used off-label), ultraviolet therapy and immunomodulators such as cyclosporine, azathioprine, methotrexate, mycophenolate mofetil and corticosteroids) or are currently on topical treatment but would otherwise be candidates for systemic therapy (systemic therapies do not include systemic antihistamines).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
|
| Exclusion criteria |
|
AD, atopic dermatitis.
Patient and public involvement
Patients and the public were not involved in the design of this study.
Study locations and timings
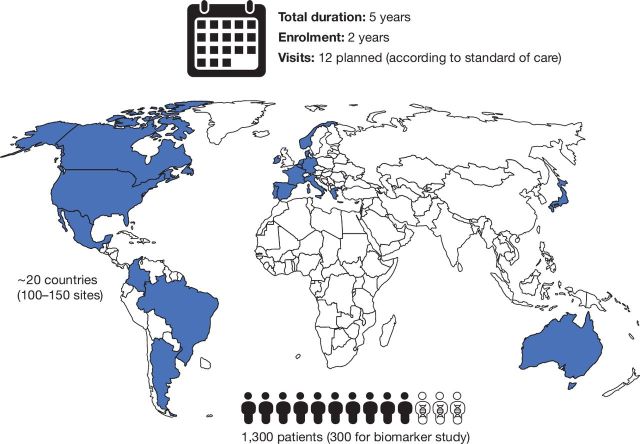
Approximately 100–150 sites in approximately 20 countries are expected to enrol 1300 patients (figure 1). Patient enrolment is expected to take 2 years, and the study duration for each patient is 5 years. A total of 12 visits are planned for each enrolled patient. The study began on 28 September 2018 and is expected to be completed in September 2025.
Figure 1.
Patients and study locations.
Study endpoints and data collection
The primary and secondary objectives of the PEDISTAD are reported in table 2.
Table 2.
Primary and secondary objectives
| Primary objectives |
|
| Secondary objectives |
|
AD, atopic dermatitis;
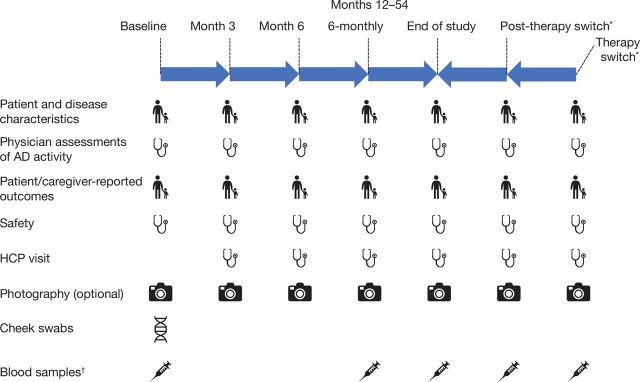
Data collected will include: demographics; AD disease characteristics at baseline; the presence of selected comorbidities at baseline; time course of conditions, including AD; current therapy for AD and initiation of new treatments/changes in current treatment over time; severity of disease at baseline and over the follow-up period (as assessed by Eczema Area and Severity Index (EASI) and Body Surface Area (BSA) percentage affected by AD, which the study investigators can use to assess disease severity at baseline); patient-reported/caregiver-reported outcomes at baseline and over the follow-up period; days missed from school for the patient and days missed from work for the primary caregiver due to AD; visits to healthcare professionals; disease state and evolution of selected atopic comorbid conditions and photography of a representative area affected by AD at select centres and safety. The association between biomarkers and disease state and time course of AD will be examined by a biomarker substudy collecting blood samples for analysis of protein and RNA biomarkers and cheek swabs for DNA genomic biomarkers. The data being collected in PEDISTAD are summarised in table 3, and the timings for data collection are shown in figure 2.
Table 3.
Data being collected in PEDISTAD
| Type | Collected data |
| Patient and disease characteristics |
|
| Patient-reported/caregiver-reported outcomes |
|
| Physician assessments of AD disease activity |
|
| Safety data |
|
| Other data |
|
| Biomarker data |
|
AD, atopic dermatitis; BSA, body surface area; CGAD, Caregiver Global Assessment of Disease; CDLQI, Children's Dermatology Life Quality Index; DFI, Dermatitis Family Impact; EASI, Eczema Area and Severity Index; HCP, healthcare professional; IDQOL, Infant's Dermatitis Quality of Life; NRS, Numerical Rating Scale; POEM, Patient-Oriented Eczema Measure; TNSS, Total Nasal Symptom Score.
Figure 2.
Data collection schedule. AD, atopic dermatitis; HCP, healthcare professional. *Therapy switch visits will take place at each initiation of a new systemic therapy and post-therapy switch will take place approximately 1 month later. Therapy switch visits are not required if the patient stays on topical therapy, stays on the same systemic drug through the study or if the visit overlaps with another scheduled visit.†Blood samples are collected every 12 months.
Statistical analysis
Sample size
To ensure that approximately 930 patients complete the 5-year follow-up, approximately 1300 patients will be enrolled (about 435 in each age group). A sample of approximately 310 participants per age group will ensure a maximum width for the CI of 11% for the estimates based on binary endpoints (table 4). This sample size needed requires that a total of 930 patients complete the study. The biomarker substudy will be conducted in approximately 300 participants (100 participants within each age cohort), whose sample size is based empirically on the results of a previous biomarker study with similar objectives.10
Table 4.
Precision estimates for the overall population
| Width of the 95% CI | Overall population n=930 |
33% of overall population n=310 |
| Binary data–widest width | 6.4% | 11.1% |
| Normal data | 0.13 SD | 0.22 SD |
Analysis
All statistical analyses will be performed descriptively with no hypothesis testing. Patient-reported and care-giver reported outcomes will be summarised within each age cohort, as many are age-based assessments. Continuous data will be described using summary statistics, including arithmetic mean, SD, median and range, whereas categorical data will be summarised using counts and percentages.
Ethics and dissemination
This study is being conducted in accordance with the principles established by the 18th World Medical Assembly and all subsequent amendments and in accordance with the guidelines for Good Epidemiology Practice. Each participating country should locally ensure all necessary regulatory submissions (eg, Institutional Review Board/Institutional Ethics Committee) are performed in accordance with local regulations, including local data protection regulations. Ethics approval has been obtained in all countries currently participating in PEDISTAD. A list of all ethics approvals received as of 20 December 2019 is provided as an online supplementary file.
bmjopen-2019-033507supp001.xlsx (27.1KB, xlsx)
The study team and the study steering and scientific committees are responsible for study reporting and interpretation, including interim data analyses and subgroup analyses. The data from the PEDISTAD study will be disseminated in manuscripts for submission to peer-reviewed medical journals as well as in abstracts for submission to congresses and in the resulting posters and presentations. The final decision to publish will be made by the study scientific steering committee after prior notice to the sponsor to allow for its internal review and comments.
Discussion
The PEDISTAD aims to address the lack of robust and longitudinal long-term data related to AD characteristics, disease progression, development of comorbidities and the typical clinical practice with currently available treatments for children with AD. By collecting information about clinical characteristics, including patient-reported and caregiver-reported outcomes, physician-assessed clinical severity, safety of currently used medicines and photographs of a representative area affected by AD (at select centres), over time, the PEDISTAD study aims to bridge this knowledge gap. A biomarker substudy of PEDISTAD will analyse the association between biomarkers and disease state and time course of AD in a subset of PEDISTAD study participants.
There are various disease trajectories in paediatric patients with AD, and clinical presentation varies with different ages of onset and development of comorbid atopic conditions.11 12 Hence, patients in this study will be recruited into three age cohorts (<2 years, ≥2–<6 years and ≥6–<12 years) and followed for 5 years.
The PEDISTAD is a real-world study and, therefore, strict entry criteria are not set. Enrolling physicians are enabled to use their best judgement as to whether a patient meets the inclusion criteria of moderate-to-severe AD using the assessment(s) of their choice. Physician assessment of disease severity will be collected by EASI and BSA percentage affected by AD, which the study investigators can use to assess disease severity at baseline. These assessments can also later be used to assess disease severity in patients over time. Other objective measures of severity may be unfamiliar to clinicians who do not regularly participate in clinical trials in AD, and lack of familiarity may potentially diminish the reliability of their results.
A significant proportion of children have persistent disease12 13; thus, treatment approaches that are effective and tolerated over large time spans are desired. The observational and long-term nature of the PEDISTAD study serves to better understand the long-term evolution of disease burden in patients and caregivers as well as to identify any unmet therapeutic need for moderate-to-severe AD.
Although there are no universal definitions of recalcitrance in patients with AD, expert recommendations mention that failure to respond to adequate topical therapy, a need for prolonged use of high-potency topical steroids or repeated flares are suggestive of recalcitrance and make a patient eligible for systemic therapy.14 This observational study will provide insight into the characteristics of paediatric patients initiated on or who become candidates for systemic therapy to better define when systemic therapy is warranted.
Paediatric AD presents clinically with a high degree of heterogeneity. In addition to the clinical phenotype, biomarkers and endophenotypes are now considered fundamental to stratify complex diseases into subgroups for which more tailored prevention and therapeutic strategies can be developed. Due to the waxing and waning nature of AD and the fact that it can present throughout a lifetime with long periods of remission in some individuals, the ability to predict disease exacerbations or the appearance of associated atopic conditions using biomarkers could have a great impact in the ability to manage the disease for long-term control. For this reason, a biomarker substudy in parallel with the PEDISTAD study will collect blood samples, with the objectives of exploring associations between biomarkers of AD and disease state and time course of AD; disease state and evolution of selected atopic comorbidities and effectiveness of specific AD treatments.
Observational studies in patients with AD to date have been limited by a variety of factors, including number of participants, participating countries and the extent and duration of data collection. The PEDISTAD study aims to address all these issues. Furthermore, the PEDISTAD study will be the largest study to date in a paediatric population with moderate-to-severe AD; by not focusing on moderate-to-severe disease, other observational studies may have underestimated the risk of comorbidities and disease persistence in this patient population.
Limitations of the PEDISTAD study include the open-label observational nature; thus, the collected data may not be as robust as blinded studies with control groups. However, the challenges of running long-term controlled studies, especially in this age group, are well known, and a control group may not be crucial to fulfil the objectives of this study. Therefore, the data from this study are anticipated to be highly valuable. Challenges include patient recruitment in multiple countries and sites and retention of patients in this age group for up to 5 years, which is particularly difficult in very young children. Parental education will be key to keeping patients enrolled in the study and to provide robust and reliable patient-reported, caregiver-reported and physician-reported outcomes at reasonable intervals.
In summary, PEDISTAD will improve our understanding of the long-term evolution of AD, disease burden in patients and caregivers and the impact of therapy on paediatric patients with moderate-to-severe AD and their families.
Supplementary Material
Footnotes
Contributors: ASP, EG-Y, ADI, EB, MdB-W were involved in design of the study and are active steering committee members for the study. SJ contributed to the design and development of the protocol and is the statistical lead. AZ, PM-O, ER and ZEO contributed to the design and development of the protocol and are medical leads. All authors critically revised the manuscript, gave final approval of the manuscript and are accountable for the accuracy and integrity of the manuscript.
Funding: This work was supported by Sanofi and Regeneron Pharmaceuticals, Inc. Medical writing and editorial assistance were provided by Carolyn Ellenberger, PhD, of Excerpta Medica, funded by Sanofi Genzyme and Regeneron Pharmaceuticals, Inc. Investigators will collect consent forms and data. Analysis of the data will be performed by the sponsor. The scientific committee of the study will have full access to the final data allowing for appropriate analysis and reporting of the study results.
Map disclaimer: The depiction of boundaries on the map(s) in this article does not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. The map(s) are provided without any warranty of any kind, either express or implied.
Competing interests: ASP: AbbVie, AnaptysBio, Eli Lilly, Galderma, Incyte, Janssen, LEO Pharma, Novartis, Regeneron Pharmaceuticals, Inc., Sanofi – investigator; AbbVie, Amgen, Asana, Dermavant, Dermira, Galderma, Eli Lilly, Forte, LEO Pharma, Matrisys Bioscience, Menlo Therapeutics, Morphosys/Galapagos, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi – consultant. EG-Y: AbbVie, Celgene, Eli Lilly, Galderma, GlaxoSmithKline, Glenmark, LEO Pharma, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi – investigator; AbbVie, Anacor, Asana Biosciences, Daiichi Sankyo, DBV, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Glenmark, Kiniksa Pharmaceuticals, Kyowa, LEO Pharma, Menlo Therapeutics, Novartis, Pfizer, Realm, Regeneron Pharmaceuticals, Inc., Sanofi – consultant; AbbVie, Celgene, Dermira, Galderma, Innovaderm, Janssen, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi – research grants. ADI: AbbVie, Chugai Pharma, Genentech, Janssen, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme – consultant. EB: Almirall – speaker; AbbVie, Eli Lilly, Pfizer – investigator; Pierre Fabre Dermatology – investigator, consultant; Regeneron Pharmaceuticals, Inc., Sanofi Genzyme – consultant; Venthera – co-founder, consultant. MdeB-W: Regeneron Pharmaceuticals, Inc., Sanofi Genzyme – investigator, advisory board member, speaker, consultant; AbbVie, Pfizer – investigator, advisory board member; Eli Lilly, UCB – advisory board member SJ, AZ, ER, ZEO: Sanofi – employees, may hold stock and/or stock options in the company. PM-O: Regeneron Pharmaceuticals, Inc. – employee and shareholder.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Shrestha S, Miao R, Wang L, et al. Burden of atopic dermatitis in the United States: analysis of healthcare claims data in the commercial, Medicare, and Medi-Cal databases. Adv Ther 2017;34:1989–2006. 10.1007/s12325-017-0582-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers 2018;4:1. 10.1038/s41572-018-0001-z [DOI] [PubMed] [Google Scholar]
- 3. Ricci G, Bellini F, Dondi A, et al. Atopic dermatitis in adolescence. Dermatol Reports 2012;4:e1 10.4081/dr.2012.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rønnstad ATM, Halling-Overgaard A-S, Hamann CR, et al. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta-analysis. J Am Acad Dermatol 2018;79:448–56. 10.1016/j.jaad.2018.03.017 [DOI] [PubMed] [Google Scholar]
- 5. Wollenberg A, Oranje A, Deleuran M, et al. ETFAD/EADV eczema Task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol 2016;30:729–47. 10.1111/jdv.13599 [DOI] [PubMed] [Google Scholar]
- 6. Sidbury R, Tom WL, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 4. prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol 2014;71:1218–33. 10.1016/j.jaad.2014.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol 2012;26:1176–93. 10.1111/j.1468-3083.2012.04636.x [DOI] [PubMed] [Google Scholar]
- 8. EUCRISA Highlights of prescribing information, FDA, 2017. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/207695s002lbl.pdf [Accessed 4 Jan 2019].
- 9. Sibbald C, Pope E, Ho N, et al. Retrospective review of relapse after systemic cyclosporine in children with atopic dermatitis. Pediatr Dermatol 2015;32:36–40. 10.1111/pde.12367 [DOI] [PubMed] [Google Scholar]
- 10. Thijs JL, Strickland I, Bruijnzeel-Koomen CAFM, et al. Moving toward endotypes in atopic dermatitis: identification of patient clusters based on serum biomarker analysis. J Allergy Clin Immunol 2017;140:730–7. 10.1016/j.jaci.2017.03.023 [DOI] [PubMed] [Google Scholar]
- 11. Irvine AD, Mina-Osorio P. Disease trajectories in childhood atopic dermatitis: an update and practitioner's guide. Br J Dermatol 2019;181:895–906. 10.1111/bjd.17766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paller AS, Spergel JM, Mina-Osorio P, et al. The atopic March and atopic multimorbidity: many trajectories, many pathways. J Allergy Clin Immunol 2019;143:46–55. 10.1016/j.jaci.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 13. Kim JP, Chao LX, Simpson EL, et al. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol 2016;75:681–7. 10.1016/j.jaad.2016.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simpson EL, Bruin-Weller M, Flohr C, et al. When does atopic dermatitis warrant systemic therapy? recommendations from an expert panel of the International eczema Council. J Am Acad Dermatol 2017;77:623–33. 10.1016/j.jaad.2017.06.042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-033507supp001.xlsx (27.1KB, xlsx)