Abstract
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal progressive neurological disorder characterised by a selective degeneration of motor neurons (MNs). Stem cell transplantation is considered as a promising strategy in neurological disorders therapy and the possibility of inducing bone marrow cells (BMCs) to circulate in the peripheral blood is suggested to investigate stem cells migration in degenerated ALS nerve tissues where potentially repair MN damage. Granulocyte-colony stimulating factor (G-CSF) is a growth factor which stimulates haematopoietic progenitor cells, mobilises BMCs into injured brain and it is itself a neurotrophic factor for MN. G-CSF safety in humans has been demonstrated and many observations suggest that it may affect neural cells. Therefore, we decided to use G-CSF to mobilise BMCs into the peripheral circulation in patients with ALS, planning a clinical trial to evaluate the effect of G-CSF administration in ALS patients compared with placebo.
Methods and analysis
STEMALS-II is a phase II multicentre, randomised double-blind, placebo-controlled, parallel group clinical trial on G-CSF (filgrastim) and mannitol in ALS patients. Specifically, we investigate safety, tolerability and efficacy of four repeated courses of intravenous G-CSF and mannitol administered in 76 ALS patients in comparison with placebo (indistinguishable glucose solution 5%). We determine increase of G-CSF levels in serum and cerebrospinal fluid as CD34+ cells and leucocyte count after treatment; reduction in ALS Functional Rating Scale-Revised Score, forced vital capacity, Scale for Testing Muscle Strength Score and quality of life; the adverse events/reactions during the treatment; changes in neuroinflammation biomarkers before and after treatment.
Ethics and dissemination
The study protocol was approved by the Ethics Committee of Azienda Ospedaliera Universitaria ‘Città della Salute e della Scienza’, Torino, Italy. Results will be presented during scientific symposia or published in scientific journals.
Trial registration number
Eudract 2014-002228-28.
Keywords: amyotrophic lateral sclerosis, randomised clinical trial, GCS-F, haematopoietic stem cells
Strengths and limitations of this study.
We planned a phase II multicentre, randomised double-blind, placebo-controlled, parallel group clinical trial on granulocyte-colony stimulating factor (G-CSF) and mannitol in amyotrophic lateral sclerosis (ALS) patients.
Safety, tolerability and efficacy of G-CSF treatment versus placebo are carefully evaluated.
Chemokines and cytokines involved in inflammatory biochemical changes are also assessed and correlated with demographic and clinical parameters.
The mass of biological data longitudinally collected could lead to important information on the trend of neuroinflammation in ALS.
Since nutritional and respiratory status may influence the progression and survival of ALS, the protocol requires a homogeneous approach to maintain respiratory function and nutritional status.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal severe progressive neurological disorder characterised by a selective degeneration of spinal, bulbar and cortical motor neurons (MNs). The mean annual ALS incidence rate is 3/100 000 population.1 The disease includes a sporadic form (85%–90% of cases) and familial variants (10%–15% of cases). Several mechanisms have been proposed to explain the progressive degeneration of MN, such as oxidative stress, neurofilament damage, mitochondrial alterations, glutamate-induced excitotoxicity and hypoxia response alteration.
The goals of ALS therapy are to find treatments that can reduce or block neuronal loss and/or to reconstruct damaged neuronal circuits. In the past, some experimental drugs showed to delay ALS progression in preclinical animal models but failed in human clinical trials or are still in phase I–III trials.2
Currently, the approved therapy for ALS is riluzole, an inhibitor of neuronal glutamate release, which prolongs survival by 9%.3 Lately, the antioxidant drug edaravone has been shown to decrease, although slightly, ALS progression measured with the ALS Functional Rating Scale-Revised (ALS-FRS-R) Score during early stages and has been approved in Japan, South Korea and USA.4
Recently, stem cell transplantation has been suggested as a promising strategy in neurological disorders therapy.5 In addition to offer a possible treatment for patients with ALS, it is always necessary to keep careful surveillance on the possible side effects, linked to procedures that involve delicate steps, such as collection, in vitro manipulation and reinfusion of bone marrow cells (BMCs).
The possibility of inducing medullary cells to leave the osteomedullary environment and circulate in the peripheral blood has long been known. This phenomenon, known as ‘mobilisation’, occurs after administration of chemotherapy and/or cytokines.6 7 BMCs mobilisation procedure has been used for many years in bone marrow transplantation treatments for haematological diseases and involves the transit of very immature medullary elements in the peripheral bloodstream.8 The recirculation phenomena in the peripheral blood of plentiful quantities of BMCs can be exploited to try to induce stem cells to migrate and localise in damaged areas of non-haematopoietic tissues. In particular, cytokine-induced BMCs mobilisation could be used in an attempt to repair the degenerated areas of nerve tissues in ALS. In this regard, it should be remembered that circulating BMCs are able to reach the central nervous system (CNS) through the blood–brain barrier (BBB).
A BMCs mobilisation procedure could therefore favour the stem cells passage into the nervous tissue. Here, BMCs could replace degenerate neurons, through transdifferentiation and reconstruction of functional circuits. Alternatively, BMCs could differentiate into glial or microglial cells that preserve the MNs still present at spinal and cortex level. Anyhow, the advantage of this approach is represented by the extent to which the BMCs can reach different levels of the disease, that is, cervical and lumbar spinal cord, medulla oblongata and pons, motor cortex.
Granulocyte-colony stimulating factor (G-CSF) is a growth factor which stimulates proliferation, differentiation and survival of haematopoietic progenitor cells.9 It is being used extensively in clinical practice to accelerate recovery of patients from neutropenia after cytotoxic therapy and on healthy subjects as bone marrow donors to mobilise and collect cells for transplantation.10
G-CSF induces a transient mobilisation of haematopoietic progenitor cells from bone marrow to peripheral blood and mobilises, among others, a population of CD34+ haematopoietic stem cells. G-CSF has also been shown to mobilise BMCs into the injured brain improving neural plasticity.11 12 Some studies have shown the potential use of osteomedullary derivation cells, in particular of the most immature stem cells, in order to repair CNS damage such as those found in MNs of ALS patients.13 14 In superoxide dismutase 1 transgenic ALS mice models, this approach with BMCs has been shown to modify the tissue micro-environment of the nervous system generating microglia and glial cells,15–17 slowing disease progression and prolonging survival.18 Moreover, in cerebrospinal fluid (CSF) and blood of patients before/after the treatment with G-CSF and placebo, relevant information about the course of ALS may be obtained by the assessment of chemoattractant protein-1 (MCP-1), interleukin-17 (IL-17), IP10 (CXCL10) and NADPH oxidase 2 (NOX2). MCP-1, IL-17 and CXCL10 are chemokines and cytokines involved in inflammatory biochemical changes,19 20 while NOX2 is the main reactive oxygen species (ROS)-producing enzyme, that controls key neuronal functions and neuroinflammatory processes. NOX2 inappropriate activation has been hypothesised to be damaging in neurodegenerative disorders.21
Furthermore, there are also indications that G-CSF is by itself a neurotrophic factor for MN,12 and it is thought to exert neuroprotective actions through the inhibition of apoptosis and inflammation and the stimulation of neurogenesis. The direct neuroprotective effect of G-CSF has been demonstrated in preclinical models of stroke,11 22 Parkinson’s disease23 and ALS.24 25
G-CSF goes through the intact BBB, allowing peripheral delivery of this protein for the treatment of neurological conditions.24 26 This molecule has a strong anti-apoptotic effect on neurons through two cellular pathways: (a) activation of STAT3, ERK1/2 and ERK5, recently implicated as factors favouring neuronal survival,27 28 and (b) activation of PI3K/Akt, a kinase with a powerful regulatory role on neuronal survival.29 Furthermore, the G-CSF would appear to perform neuroprotective action in mice with familial ALS, stimulating the recruitment of microglia in the damaged areas.30 The receptor (G-CSF-R) is expressed on CNS neurons and glia and its expression is induced by different pathogenic noxae.31 32 G-CSF-R is present on adult stem cells and induces their differentiation in neurons. It also plays a role as a factor involved in the plasticity of the vasculature.9
In the spinal cord, G-CSF has been found expressed in reactive astrocytes of eight cases of ALS but not in six non-neurological controls, whereas in ALS cases the expression of G-CSF-R was significantly reduced in spinal motoneurons.33
The downregulation of the G-CSF-R could therefore play a role in the pathogenesis of ALS.
Based on this, we planned a double-blind, placebo-controlled, parallel group multicentre clinical trial to determine whether the treatment with G-CSF and mannitol is safe and significantly slows down the rate of progression of ALS.
Methods and analysis
Objectives
The main objectives of the study are:
To evaluate the safety and tolerability of G-CSF treatment in patients with ALS.
To evaluate the efficacy of G-CSF treatment versus placebo in patients with ALS.
To evaluate the changes in CSF/blood inflammatory parameters (CD34+ cells and leucocyte count, chemokines, cytokines, NOX2 activity) in patients treated with G-CSF compared with those treated with placebo.
Study design
The acronym of the study is STEMALS-II.
STEMALS-II is a multicentre, phase II randomised, double-blind, placebo-controlled, parallel group, no-profit clinical trial on G-CSF (filgrastim) intravenous treatment in ALS patients.
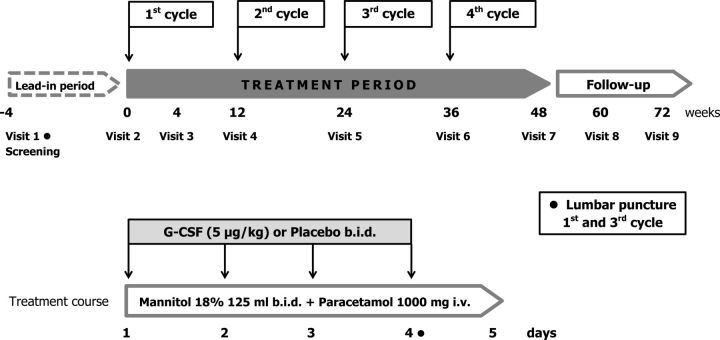
The design of the study is reported in figure 1.
Figure 1.
Design of STEMALS-II study. G-CSF, granulocyte-colony stimulating factor.
The expected duration of the study for each patient is maximum 76 weeks: a screening period (up to 4 weeks), 48 weeks of treatment and 24 weeks of follow-up.
In particular, our aim is to evaluate safety, tolerability and efficacy of four repeated courses of G-CSF and mannitol administered intravenously at 3-month intervals in 76 patients with ALS, assessing the clinical disease progression during the 48 weeks of BMCs mobilisation cycles and a subsequent 24 weeks of follow-up period.
We determine:
The increase of G-CSF levels in serum and CSF after treatment, as CD34+ cells and leucocyte count.
If a significant reduction has occurred in mean decrease in ALS-FRS-R Score, forced vital capacity (FVC), Scale for Testing Muscle Strength (MRC) Score and quality of life (QoL, measured with McGill QoL Questionnaire).
The adverse events (AEs)/reactions that may occur in patients treated with drug and placebo, and if they are transitory.
The changes in chemokines/cytokines levels and NOX2 activity in CSF and blood as an in-depth study in case of positive response to the treatment.
Since nutritional and respiratory status may influence the progression and survival of ALS, the protocol requires a homogeneous approach to maintain respiratory function and nutritional status, through researchers’ explicit adhesion to the European Federation of Neurological Societies (EFSN) guidelines.34
Participating centres
The study is performed in seven Italian ALS centres with haematological laboratories and with experience in the use of G-CSF:
Torino (Promoter and Coordinating Centre)—CRESLA (ALS Regional Expert Centre)—Neurology 2, ‘Rita Levi Montalcini’ Department of Neuroscience, University of Turin and ‘Azienda Ospedaliero Universitaria (AOU) Città della Salute e della Scienza’ of Turin.
Milano—NEMO (Neuromuscular Omnicentre) Clinical Center, Serena Onlus Foundation.
Genova—Neurological Clinic I—IRCCS AOU San Martino IST Genova.
Novara—ALS Regional Expert Centre, Neurological Clinic AOU Maggiore della Carità.
Modena—Operating Unit Neurology, New Civil Hospital S. Agostino-Estense of Modena, University of Modena and Reggio Emilia.
Palermo—Regional Reference Centre for Motor Neuron Diseases-ALS, Department of Emergencies, Urgencies and Neurosciences, AOU Policlinic ‘P Giaccone’.
Bari—University Hospital Consortium Polyclinic of Bari ‘Aldo Moro’—Department of Basic Medical Sciences, Neuroscience and Sensory Organs.
Each centre is expected:
To randomise at least four patients fulfilling including and excluding criteria in a period of 48 weeks (including 4 weeks of screening).
To administer the treatment for the 48 weeks planned and perform the follow-up visits.
To provide one principal investigator (PI) (treating neurologist), not aware of treatment arm and of haematological values (to ensure the ‘double blind’ of the study), in order to evaluate inclusion and exclusion criteria, provide detailed information on all aspects of the trial, obtain signed informed consent, administer the treatment and evaluate the end-points.
To provide a laboratory physician/biologist able to obtain (always in blind of treatment) the haematological values within 2 hours after blood collection and send the results by mail to the coordinating centre; the same must also verify the carrying out of the other tests, in particular the monitoring of circulating CD34+ cells.
To evaluate the FVC in a sitting position.
To formally adhere to the EFSN guidelines for the management of patients, with particular regard to respiratory support and nutrition.
To manage with its own pharmacy the methods of provision and management of the drug/placebo and verify to provide all the material necessary for the conduct of the study in accordance with the protocol.
Patients
Subjects eligible for the study are adult individuals of both sexes, diagnosed with both spinal and bulbar onset ALS. All patients must adhere to inclusion and exclusion criteria reported in table 1.
Table 1.
Inclusion and exclusion criteria for STEMALS-II trial
| Inclusion criteria | Exclusion criteria |
|
|
Randomisation
All eligible patients are randomised to receive G-CSF or matching placebo, with 1:1 allocation in two groups of 38 subjects each. They receive a unique identification number at screening visit when signing the informed consent. A block stratified randomisation model was used with small block size, with stratification for type of onset; one-half of the patients in each block are assigned to each treatment through random allocation. The randomisation procedure involved the generation of two lists, one for patients with bulbar onset and one for patients with spinal onset. Cases with only bulbar signs or symptoms in the first 3 months of diagnosis, with subsequent appearance of spinal signs, are considered bulbar-onset ALS. All others cases are considered spinal-onset ALS. The randomisation lists are informatically generated and are available on the www.randomization.com site. Patients should be randomised and treatment initiated within 4 weeks of screening visit (V1).
Treatment
Patients are treated in hospital during each cycle of G-CSF/placebo administration. Filgrastim (Tevagrastim, kindly provided by Teva, Italy) is a recombinant human G-CSF whose biological activity is very similar to that of endogenous G-CSF. Filgrastim or the matching placebo (indistinguishable glucose solution 5%) are administered intravenously at a dose of 5 µg/kg two times per day for four consecutive days (one cycle). Each course is repeated after 12 weeks (times 0, 12, 24 and 36 weeks), for a total of four cycles of treatment (48 weeks).
Administration should be as provided in the Summary of Product Characteristics (SPC): intravenous infusion administered in 30 min.
For safety reasons, G-CSF administration has to be interrupted if the leucocyte count is ≥50 x 109/L.
Co-treatment
From the first day of each cycle, mannitol 18% is administered intravenously, 125 mL two times per day for 5 days, in order to permeabilise the BBB35 and promote cells entry into the CNS.
Since filgrastim treatment may cause flu-like disorders, in order to maintain the blindness of the study, all patients receive prophylactic treatment with paracetamol 1000 mg intravenously for each day of the treatment cycle, plus other 1000 mg intravenously as needed for up to three times per day.
Treatment scheme
From day 1 to day 4
Mannitol 18% 125 mL at least 30 min before the first filgrastim/placebo infusion.
First administration of filgrastim/placebo in 5% glucose solution by intravenous infusion over 30 min.
Approximately 3 hours after the first infusion of filgrastim/placebo, administration of paracetamol 1000 mg intravenously.
At least 30 min before the second infusion of filgrastim/placebo, administration of mannitol 18% 125 mL.
6 hours after the first infusion, second administration of filgrastim/placebo in 5% glucose solution by intravenous infusion over 30 min.
Day 5
Mannitol 18% 125 mL two times per day about 6 hours apart and paracetamol 1000 mg intravenously between the two mannitol infusions
Procedures and evaluations
Clinical assessments
The study flowchart is reported in table 2.
Table 2.
Study flowchart
| Evaluation | Visit | ||||||||
| V1 | V2 | V3 | V4 | V5 | V6 | V7 | V8 | V9 | |
| Time (weeks) | 0 | 4 | 12 | 24 | 36 | 48 | 60 | 72 | |
| Informed consents | X | ||||||||
| Medical history | X | ||||||||
| Physical examination | X | X | X | X | X | X | X | X | X |
| Vital signs | X | X | X | X | X | X | X | X | X |
| Blood chemistry | X | X | X | X | X | X | X | X | X |
| ALS-FRS-R | X | X | X | X | X | X | X | X | X |
| Neurological examination | X | X | X | X | X | X | X | ||
| MRC | X | X | X | X | X | X | X | ||
| Spirometry | X | X | X | X | X | X | |||
| McGill QoL Scale | X | X | X | X | |||||
| ECAS | X | X | X | X | X | ||||
| Bone marrow aspirate | X | ||||||||
| Treatment | X | X | X | X | X | ||||
| Lumbar puncture | X | X | X | ||||||
| DNA collection | X | ||||||||
| Concomitant therapies | X | X | X | X | X | X | X | ||
| Other procedures* | X | X | X | X | X | X | X | X | |
| Screening | Treatment | Follow-up | |||||||
| 4 weeks max | 48 weeks | 24 weeks | |||||||
*Include enteral or parenteral feeding (eg, percutaneous endoscopic gastrostomy) and/or respiratory support (non-invasive ventilation, tracheostomy, intubation).
ALS-FRS-R, Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised; ECAS, Edinburgh cognitive and behavioral ALS screen; MRC, Scale for Testing Muscle Strength; QoL, quality of life.
During the visit of screening before entering the study (V1), the patient must provide the report of the following tests, performed in the last 30 days: an ECG, a chest X-ray, an ultrasound scan of neck, with evaluation of nodal sites, abdomen, with spleen volume assessment, and inguinal region. These tests are repeated later only if clinically indicated.
Only at the screening, a bone marrow aspiration with morphological and cytogenetic evaluation and in vitro cultures is performed. AEs were assessed at each visit.
Evaluation of BMCs mobilisation into peripheral blood and CSF
Complete blood cell counts and determination of circulating CD34+ cells to evaluate dismissal of BMCs are performed daily throughout all the cycles of G-CSF administration (5 days) and at every visit before the infusion. The evaluation of the presence of BMCs in the CSF is assessed with a lumbar tap at Day 4 of the first (V2) and of the third (V5) cycle of the G-CSF administration, and compared with a basal evaluation performed during the screening (V1).
End-points
Primary end-point (efficacy analysis): assessment of change in the rate of progression of total disability score (ALS-FRS-R Score) over the 72 weeks of the study (48 weeks of treatment + 24 weeks of follow-up) in a series of ALS patients compared with placebo, calculated as follows: ALS-FRS-R slope = [score ALS-FRS-R (time 0) − score ALS-FRS-R (time 72)]/72 Day 4.
-
Secondary end-points (secondary efficacy analyses and safety and tolerability analyses) include:
Modification of progression rates of MRC Score, respiratory function (FVC) and QoL (measured with McGill QoL Questionnaire) during all the study, calculated with the same formula reported for ALS-FRS-R.
Time to death or tracheostomy (survival analysis) or use of non-invasive ventilation ≥18 hours/day.
Interruption of treatment due to disease progression or to an AE.
Safety and tolerability analysis: comparison between serious adverse event (SAE) in patients treated with drug and placebo.
Statistical analysis
The statistical analysis plan includes descriptive statistics for the assessment of the comparability of the two treatment groups and the safety of active treatment and placebo, and the use of ad-hoc statistical tests (univariable and multivariable) for the efficacy analysis.
Statistical analysis is performed in the following study populations:
All randomised patients receiving at least one dose of the study medication (‘intention-to-treat population’) (primary analysis).
Study completers: patients completing the 12-month observation period (secondary analysis).
Study completers and compliers; patients completing the 12-month observation period and taking at least 75% of assigned drug dose (secondary analysis).
The primary statistical analysis includes the evaluation of change in the rate of disease progression in the treatment period (from T0 to T72) using the global score of the functional scale ALS-FRS-R, comparing the velocity averages in the two treatment groups. The analysis will be performed as intention-to-treat, including all patients recruited for the study and who have performed at least one course of treatment.
Patients leaving the study for any reason will not be replaced.
The secondary statistical analyses include the evaluation of the change in the rate of progression of muscle strength (upper limbs MRC, lower limbs MRC, overall MRC), respiratory function (FVC) and the overall score of the McGill Scale (QoL).
A Student’s t-test is employed to compare the progression rate averages of the various parameters (slopes of the ALS-FRS-R, MRC, QoL Scores and FVC) between the two treatment groups.
For patients who do not complete the trial (leave the study before the 72 weeks provided by the protocol for death or withdrawal of consent), slopes are calculated using the last observation performed, before the exit from the study, divided by the number of weeks of observation.
Survival is assessed using the Kaplan-Meier method with death or tracheostomy as end-points and log-rank test. Cox’s proportional hazard model is employed to adjust for any unbalanced distribution among prognostic factors. Statistical significance is considered at a level of 0.05 with two-tailed tests.
About end-points related to security, all patients undergoing at least one course of treatment are included in the safety analysis. Any alterations of vital signs or laboratory tests are detected during the course of the study. All data are tabulated including incidence and severity of AE.
Sample size and study power
According to the data of our ALS centre, calculated on 850 patients prospectively followed-up during the last 10 years, the mean score of ALS-FRS-R shows a 11.3 points decrease with a SD of 8.2 points during the first year after the diagnosis (1 year of follow-up). With a two-sided alpha set at 0.05 and a power (beta) of 0.9, we can calculate that in order to find a difference of 6.7 points in the ALS-FRS-R Score (ie, a reduction of 6.7 points in the ALS progression) we will need 38 patients in each arm (drug vs placebo). Secondary objectives are to investigate cytokine/chemokine levels and NOX2 activity in the CSF and blood of patients before and after the treatment using multiplexed fluorescent bead-based immunoassay and oxidative burst assay. This will allow to implement the interesting data obtained from our preliminary studies which indicated significant and selective changes of specific cytokines in the CSF and serum of G-CSF treated patients showing a trend to slowing down the disease progression.
Pharmacovigilance
All centres participate in the pharmacovigilance programme.
The definitions of AEs, SAE, adverse reaction (AR), serious adverse reaction (SAR) and suspected unexpected serious adverse reaction (SUSAR) are those reported in the The Medicines for Human Use-Clinical Trials-Regulations (2004).
Criteria of seriousness for SAE, SAR and SUSAR
Any AE or AR that, irrespective of the dose, corresponds to one or more of the following criteria is considered serious:
Has a fatal outcome.
Endangers the life of the subject.
Requires hospitalisation or prolongs the admission in progress.
Involves severe or prolonged disability or incapacity.
Involves a congenital anomaly or a birth defect.
New diagnosis of cancer.
Notification and reporting responsibilities of AE, AR, SAE, SAR and SUSAR
AE (non-treatment-related) or AR (treatment-related) are any change in the patient’s clinical condition from baseline that is not due to the expected course of the disease and that has a potential clinical significance or any clinical event presented by the patient who is participating in the study. It therefore includes lesions, toxic effects, hypersensitivity reactions, diseases not related to the study disorder, surgical interventions that occur during the study after the start of the treatment (V2−T0).
All AE and AR that occur in a patient or subject involved in a clinical trial must be collected and reported in the appropriate case report form (CRF).
All AE for which, in the opinion of the clinical trial investigator or promoter, there is a reasonable suspicion that a causal relationship may exist with an investigational medicinal product, are to be considered AR.
In compliance with what reported in the SPC, the events showed in table 3 are called reactions. Reactions defined as very common are not notified if their degree of toxicity is equal to or less than 3 (as per Common Terminology Criteria for Adverse Events V.5.0).
Table 3.
Adverse reactions reported in the summary of product characteristics of G-CSF
| Systems/organs involved | Adverse effect | Frequency |
| Hemolymphopoietic system disorders | Leucocytosis, thrombocytopenia | Very common |
| Spleen disorders | Uncommon | |
| Metabolism and nutrition disorders | High levels of alkaline phosphatase and lactate dehydrogenase | Common |
| Increase in serum glutamic-oxaloacetic transaminase, hyperuricemia | Uncommon | |
| Nervous system disorders | Headache | Very common |
| Vascular disorders | Capillary loss syndrome | Uncommon |
| Musculoskeletal and connective tissue disorders | Musculoskeletal pain | Very common |
| Rheumatoid arthritis exacerbation | Uncommon | |
| General disorders and administration site conditions | Serious allergic reaction | Uncommon |
| Skin and subcutaneous tissue disorders | Sweet syndrome, cutaneous vasculitis | Uncommon |
With regard to the management of reporting, appropriate standard operating procedures are shared with all participating centres. In particular, SAE and SAR must be reported by each PI to coordinating centre and to ethics committee (EC) and health management of the corresponding centre. SUSAR are inserted in the pharmacovigilance system. The notification obligation related to the conduct of the study will cease with its closure.
Withdraw from the study
A subject may voluntarily choose to stop treatment or withdraw consent to participate in the trial at any time. Also the investigator may suspend the treatment or exclude a patient from the study at his discretion in any moment. The withdrawal is to be considered from the moment of the informed consent sign and before the final visit completion. During the 48 weeks of treatment, failure of an entire course of treatment (4 days of filgrastim/placebo administrations) leads to the exclusion from the study.
Blind opening
The pharmacy of each participating centre is authorised to open the envelope to learn about the associated treatment. The participant centre can only open the envelope in case of urgency or when the patient’s clinical conditions require it. In any case, they provide to document the reasons for opening the blind, the person who performed the operation and the methods. This document must be sent immediately by email to the pharmacy of the coordinating centre.
Early termination
Filgrastim treatment must be suspended in case of:
Haematuria/proteinuria.
Pregnancy.
If the maximum spleen diameter is >14 cm (drug administration has, however, to be suspended in all patients in case of rapid and marked increase in the spleen, already detected at palpation).
An AE/SAE or clinically significant condition assessed by the investigator as a significant and unacceptable risk for the subject.
Patients excluded or withdrawn from the study will still be subjected to a 24-week follow-up (two visits every 12 weeks).
Conclusion of the study
The study will be considered concluded when the last randomised patient completes the last follow-up visit foreseen by the protocol and the related data will be included in the CRF.
Regulatory requirements—promoter/researcher obligations
The steering committee of the study consists of the PI of each participating centre or one of its representatives.
The protocol has been discussed and revised according to the suggestions of the data safety and monitoring board, consisting of three independent experts appointed by the promoter of the study, in particular a Professor of Neurology, a Professor of Haematology and a Professor of Pharmacology of the University of Turin.
This study is conducted in accordance with the Helsinki Declaration (Fortaleza, October 2013) and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) E6 guidelines. To this end, with the written approval of this protocol, the researchers agree to fully cooperate allowing access to authorised individuals to all documentation, including hospital records (source documents).
Researchers must strictly adhere to the protocol. If necessary, a written amendment to the protocol will be prepared. The amendment will be approved and signed by the participating parties (Investigators and Promoters) according to defined procedures.
The investigators collect study data by entering them into the trial database through electronic CRF and are responsible for the accuracy of all data entered. The CRF must be available for review by designated representatives in case of monitoring visits. The researchers also allow representatives of regulatory bodies to review the data reported in the CRF and source documents, according to the laws and regulations in force.
The study will be monitored by a certified contract research organisation. The researchers make available all relevant material, including the source documents, for any inspection by the regulatory authorities (audit). The information is considered confidential.
The trial is insured for damages arising from the study and involving the subjects treated with the study drug according to the laws in force. Researchers receive all data concerning the insurance company and the policy number.
Any violation of the protocol will imply, on the side of the promoter, the suspension of the treatment of that patient and the study can be interrupted at any time by the promoter and/or the responsible researcher on the basis of new information regarding the treatment safety. Furthermore, the study can be interrupted if the treatment should give clearly unsatisfactory results.
Patient and public involvement
Patients have not been involved in the drafting of the research protocol and will not be involved in study conduction or in the interpretation of the results. Patient associations will be invited to disseminate the trial, to allow patients to participate in it with maximum adherence and to spread the results of the study.
Ethics and dissemination
The study protocol was approved by the EC of Città della Salute e della Scienza di Torino and by the ECs of participating centres based on the Helsinki declaration (Fortaleza, October 2013). Comitato Etico Interaziendale AOU Città della Salute e della Scienza di Torino – AO Ordine Mauriziano di Torino – ASL TO1, approval ID: CS/260 (signed on 1st July 2015).
All patients included in the study from each participating centre must personally sign the informed consent form approved by their EC, after receiving detailed information on all aspects of the trial (see online supplementary file). The researcher must ensure anonymity by using an alphanumeric code for each subject.
bmjopen-2019-034049supp001.pdf (533.7KB, pdf)
No study procedure will be performed before the written informed consent has been provided.
The data of the study are property of the promoter. The results can be presented in scientific conferences or published in scientific journals. All publications are property of the main investigators of all the centres involved in the study. After the trial completion, the project coordinator will prepare a draft manuscript containing the final results of the study. The manuscript will be discussed and approved by all the primary investigators of the centres involved in the study. The publications will respect the privacy of the participating subjects.
Discussion
G-CSF safety in humans has been demonstrated by the wide clinical use in the last 20 years in subjects with leukaemia and in health donors of bone marrow. Many observations suggest that G-CSF may affect the CNS and neural cells in particular. Therefore, we decided to use G-CSF to mobilise BMCs into the peripheral circulation in patients with ALS.
We have previously completed an open-blind phase I study ‘STEMALS’36 37 on 26 patients treated with G-CSF (subcutaneous injection) at the dose of 5 µg/kg two times per day for four consecutive days, with mannitol 18% intravenously, 125 mg, two times per day; the treatment was repeated four times, at 3-month interval. We demonstrated that BMCs mobilisation was constant at each cycle, with a peak of some 40–50 x 109 leucocytes/L. G-CSF treatment was safe and feasible in the multicentre series of ALS patients.
Moreover, the treatment with G-CSF produced a significant reduction of proinflammatory cytokines MCP-1 and IL17 in CSF, and an increase of IP10 in serum, suggesting a central anti-inflammatory response induced by the drug. In this regard, it is of particular interest that the expression of IP10 is significantly reduced in lymphocytes of ALS patients with the C9ORF72 expansion compared with controls and non-C9ORF72 SLA patients. Furthermore, the levels of this cytokine in the CSF of ALS patients are inversely correlated with the progression of the disease, hypothesising a neuroprotective effect of IP10.20
The ROS-producing NOX2 enzyme plays a role in the development of neurological diseases. In our previous studies we verified its activity in blood of patients with ALS, Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) and Parkinson’s disease, in addition to healthy volunteers, finding the strong role of NOX2 in modifying progression in ALS patients.38
Since NOX2 is responsible for the respiratory oxidative burst, the assessment of its activity in granulocytes before and after the treatment with drug/placebo may help us to determine if G-CSF can affect cellular oxidative status.
The most common adverse effects were flu-like symptoms, nausea and asthenia, reported by eight patients over the total course of 12 treatment cycles, and effectively treated with acetaminophen (500 mg two times per day); the only serious adverse effects were a deep venous thrombosis requiring anticoagulation therapy for 4 months in a case and transient hyperprolactinemia in another case (manifested by asthenia and decrease of libido).
The rate of progression of ALS, measured with ALS-FRS-R Score and respiratory function, decreased by 30% comparing the lead-in 4-month period and the 12 months of the study.
In the last years, some new trials with ALS patients treated with subcutaneous injections of G-CSF have been performed. Amirzagar et al 39 have concluded that G-CSF is not a promising option for the treatment of ALS and it may accelerate disease progression in females, while Johannesen et al 40 suggested that G-CSF treatment is feasible and safe and may exert beneficial effects.
In conclusion, these results prompt us to perform ‘STEMALS-II’, a multicentre phase II randomised clinical trial to provide specific information on G-CSF intravenous treatment on the course of ALS in a larger number of cases. Intravenous administration was chosen because it was less irregular, ensuring a more reliable dose of drug. A demonstration of a positive effect on the rate of disease progression will give ALS patients a new therapeutic opportunity to be used with riluzole. This will be very important result for a fatal disorder which currently has no effective therapies. Being G-CSF an already available and used drug, its translation to clinical use in ALS patients does not need extremely long times.
Moreover, the 12-month assessment of chemokines, cytokines and NOX2 activity, both in treated and non-treated (placebo) ALS patients, and its correlation with demographic and clinical parameters, will provide us a mass of biological data longitudinally collected, which could lead to important information on the trend of neuroinflammation in ALS.
The demonstration of specific patterns of neuroinflammation markers could offer new possible targets of other drugs for the treatment of ALS, as well as new markers of disease progression to be used as surrogate markers in future trials on ALS patients.
Supplementary Material
Acknowledgments
The authors acknowledge STEMALS-II Study Group: ‘Rita Levi Montalcini’ Department of Neuroscience, University of Turin, Torino, Italy, and ALS Centre, AOU ‘Città della Salute e della Scienza’, Torino, Italy (A Bombaci, M Brunetti, S Cammarosano, A Canosa, C Calvo, M Daviddi, G De Marco, P Cugnasco, M Grassano, B Iazzolino, A Ilardi, C Lauritano, A Lomartire, U Manera, L Solero, MC Torrieri, R Vasta) Division of Haematology, University of Torino, AOU ‘Città della Salute e della Scienza di Torino’, Torino, Italy (M Gilestro, VE Muccio, P Omedé) NEuroMuscular Omnicentre (NEMO), Fondazione Serena Onlus, Milano, Italy (F Gerardi) Department of Neurosciences, Rehabilitation Ophthalmology, Genetics, Mother and Child Disease, Ospedale Policlinico San Martino, Genova, Italy (C Cabona) Neurological Clinic, IRCCS Ospedale Policlinico San Martino, Genoa, Italy (G Novi) Department of Neurology, Maggiore della Carità Hospital, University of Piemonte Orientale, Novara, Italy (E Bersano, F De Marchi) ALS Clinical Research Centre, Department of Biomedicine, Neuroscience and Advanced Diagnostics, University of Palermo (R Spataro, R. Scimè) Department of Neuroscience, AOU Modena, St. Agostino- Estense Hospital, Modena, Italy (A Fasano, N Fini, A Gessani) Neurology Unit, Department of Basic Medical Sciences, Neurosciences and Sense Organs, University of Bari ‘Aldo Moro’, Bari, Italy (E D’Errico, A Scarafino).
Footnotes
Twitter: @chrislun76
Collaborators: The STEMALS-II Study Group: A Bombaci; M Brunetti; S Cammarosano; A Canosa; C Calvo; M Daviddi; G De Marco; P Cugnasco; M Grassano; B Iazzolino; A Ilardi; C Lauritano; A Lomartire: U Manera; L Solero; MC Torrieri; R Vasta; M Gilestro; VE Muccio; P Omedé; F Gerardi; C Cabona; G Novi; E Bersano; F De Marchi; R Spataro; R. Scimè; A Fasano; N Fini; A Gessani; E D’Errico; A Scarafino
Contributors: Protocol design: ACh, CT, CM, ACa. Conceptualisation: ACh, CT, CL, CC, LM, VLB, JM, ILS. Data curation: PS, GF, GM, FC, CL, CC, LM, VLB, JM, ILS, CM, ACa, CT, ACh. Formal analysis: PS, FC, GF, ACh. Funding acquisition: ACh, CT. Investigation: PS, GF, GM, FC, CL, CC, LM, VLB, JM, ILS, CM, ACa, CT, ACh. Methodology: GF, FC, CM, ACa. Project administration: GF, PS, CM. Supervision: ACh, CT. Writing – original draft: PS, FC, GF, ACh. Writing – review and editing: CM, ACa, GF, GM, CL, CC, LM, VLB, JM, ILS, CT.
Funding: This work was supported by the Italian Medicines Agency (AIFA, Agenzia Italiana del Farmaco) grant number MRAR08K005.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
the STEMALS-II Study Group:
A Bombaci, M Brunetti, S Cammarosano, A Canosa, C Calvo, M Daviddi, G De Marco, P Cugnasco, M Grassano, B Iazzolino, A Ilardi, C Lauritano, A Lomartire, U Manera, L Solero, MC Torrieri; R Vasta, M Gilestro, VE Muccio; P Omedé, F Gerardi, C Cabona, G Novi, E Bersano, F De Marchi, R Spataro, R. Scimè, A Fasano, N Fini, A Gessani, E D’Errico, and A Scarafino
References
- 1. Chiò A, Mora G, Moglia C, et al. Secular trends of amyotrophic lateral sclerosis: the Piemonte and Valle d'Aosta register. JAMA Neurol 2017;74:1097–104. 10.1001/jamaneurol.2017.1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jaiswal MK. Riluzole and edaravone: a tale of two amyotrophic lateral sclerosis drugs. Med Res Rev 2019;39:733–48. 10.1002/med.21528 [DOI] [PubMed] [Google Scholar]
- 3. Miller RG, Mitchell JD, Lyon M, et al. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev 2007;2 10.1002/14651858.CD001447 [DOI] [PubMed] [Google Scholar]
- 4. Writing Group, Edaravone (MCI-186) ALS 19 Study Group . Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2017;16:505–12. 10.1016/S1474-4422(17)30115-1 [DOI] [PubMed] [Google Scholar]
- 5. Song C-G, Zhang Y-Z, Wu H-N, et al. Stem cells: a promising candidate to treat neurological disorders. Neural Regen Res 2018;13:1294–304. 10.4103/1673-5374.235085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sheridan WP, Begley CG, Juttner CA, et al. Effect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy. Lancet 1992;339:640–4. 10.1016/0140-6736(92)90795-5 [DOI] [PubMed] [Google Scholar]
- 7. Bonig H, Papayannopoulou T. Mobilization of hematopoietic stem/progenitor cells: general principles and molecular mechanisms. Methods Mol Biol 2012;904:1–14. 10.1007/978-1-61779-943-3_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tarella C, Ferrero D, Bregni M, et al. Peripheral blood expansion of early progenitor cells after high-dose cyclophosphamide and rhGM-CSF. Eur J Cancer 1991;27:22–7. 10.1016/0277-5379(91)90052-F [DOI] [PubMed] [Google Scholar]
- 9. Wallner S, Peters S, Pitzer C, et al. The granulocyte-colony stimulating factor has a dual role in neuronal and vascular plasticity. Front Cell Dev Biol 2015;3:48. 10.3389/fcell.2015.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem 2006;99:690–705. 10.1002/jcb.21043 [DOI] [PubMed] [Google Scholar]
- 11. Schäbitz W-R, Kollmar R, Schwaninger M, et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke 2003;34:745–51. 10.1161/01.STR.0000057814.70180.17 [DOI] [PubMed] [Google Scholar]
- 12. Solaroglu I, Jadhav V, Zhang JH. Neuroprotective effect of granulocyte-colony stimulating factor. Front Biosci 2007;12:712–24. 10.2741/2095 [DOI] [PubMed] [Google Scholar]
- 13. Deda H, Inci MC, Kürekçi AE, et al. Treatment of amyotrophic lateral sclerosis patients by autologous bone marrow-derived hematopoietic stem cell transplantation: a 1-year follow-up. Cytotherapy 2009;11:18–25. 10.1080/14653240802549470 [DOI] [PubMed] [Google Scholar]
- 14. Maldonado-Soto AR, Oakley DH, Wichterle H, et al. Stem cells in the nervous system. Am J Phys Med Rehabil 2014;93:S132–44. 10.1097/PHM.0000000000000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beers DR, Henkel JS, Xiao Q, et al. Wild-Type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A 2006;103:16021–6. 10.1073/pnas.0607423103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lasiene J, Yamanaka K. Glial cells in amyotrophic lateral sclerosis. Neurol Res Int 2011;2011:1–7. 10.1155/2011/718987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol 2011;10:253–63. 10.1016/S1474-4422(11)70015-1 [DOI] [PubMed] [Google Scholar]
- 18. Corti S, Locatelli F, Donadoni C, et al. Wild-Type bone marrow cells ameliorate the phenotype of SOD1-G93A ALS mice and contribute to CNS, heart and skeletal muscle tissues. Brain 2004;127:2518–32. 10.1093/brain/awh273 [DOI] [PubMed] [Google Scholar]
- 19. Baron P, Bussini S, Cardin V, et al. Production of monocyte chemoattractant protein-1 in amyotrophic lateral sclerosis. Muscle Nerve 2005;32:541–4. 10.1002/mus.20376 [DOI] [PubMed] [Google Scholar]
- 20. Ismail A, Cooper-Knock J, Highley JR, et al. Concurrence of multiple sclerosis and amyotrophic lateral sclerosis in patients with hexanucleotide repeat expansions of C9orf72. J Neurol Neurosurg Psychiatry 2013;84:79–87. 10.1136/jnnp-2012-303326 [DOI] [PubMed] [Google Scholar]
- 21. Gao H-M, Zhou H, Hong J-S. Nadph oxidases: novel therapeutic targets for neurodegenerative diseases. Trends Pharmacol Sci 2012;33:295–303. 10.1016/j.tips.2012.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. England TJ, Sprigg N, Alasheev AM, et al. Granulocyte-Colony stimulating factor (G-CSF) for stroke: an individual patient data meta-analysis. Sci Rep 2016;6:36567 10.1038/srep36567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsai S-T, Chu S-C, Liu S-H, et al. Neuroprotection of granulocyte colony-stimulating factor for early stage Parkinson's disease. Cell Transplant 2017;26:409–16. 10.3727/096368916X694247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pitzer C, Krüger C, Plaas C, et al. Granulocyte-Colony stimulating factor improves outcome in a mouse model of amyotrophic lateral sclerosis. Brain 2008;131:3335–47. 10.1093/brain/awn243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pollari E, Savchenko E, Jaronen M, et al. Granulocyte colony stimulating factor attenuates inflammation in a mouse model of amyotrophic lateral sclerosis. J Neuroinflammation 2011;8:74 10.1186/1742-2094-8-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dittgen T, Pitzer C, Plaas C, et al. Granulocyte-Colony stimulating factor (G-CSF) improves motor recovery in the rat impactor model for spinal cord injury. PLoS One 2012;7:e29880 10.1371/journal.pone.0029880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Watson FL, Heerssen HM, Bhattacharyya A, et al. Neurotrophins use the ERK5 pathway to mediate a retrograde survival response. Nat Neurosci 2001;4:981–8. 10.1038/nn720 [DOI] [PubMed] [Google Scholar]
- 28. Kamezaki K, Shimoda K, Numata A, et al. Roles of STAT3 and ERK in G-CSF signaling. Stem Cells 2005;23:252–63. 10.1634/stemcells.2004-0173a [DOI] [PubMed] [Google Scholar]
- 29. Li L, Klebe D, Doycheva D, et al. G-Csf ameliorates neuronal apoptosis through GSK-3β inhibition in neonatal hypoxia-ischemia in rats. Exp Neurol 2015;263:141–9. 10.1016/j.expneurol.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamasaki R, Tanaka M, Fukunaga M, et al. Restoration of microglial function by granulocyte-colony stimulating factor in ALS model mice. J Neuroimmunol 2010;229:51–62. 10.1016/j.jneuroim.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 31. Schneider A, Krüger C, Steigleder T, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest 2005;115:2083–98. 10.1172/JCI23559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kirsch F, Krüger C, Schneider A. The receptor for granulocyte-colony stimulating factor (G-CSF) is expressed in radial glia during development of the nervous system. BMC Dev Biol 2008;8:32 10.1186/1471-213X-8-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tanaka M, Kikuchi H, Ishizu T, et al. Intrathecal upregulation of granulocyte colony stimulating factor and its neuroprotective actions on motor neurons in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 2006;65:816–25. 10.1097/01.jnen.0000232025.84238.e1 [DOI] [PubMed] [Google Scholar]
- 34. Andersen PM, Abrahams S, Borasio GD, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)--revised report of an EFNS task force. Eur J Neurol 2012;19:360–75. 10.1111/j.1468-1331.2011.03501.x [DOI] [PubMed] [Google Scholar]
- 35. Kiviniemi V, Korhonen V, Kortelainen J, et al. Real-Time monitoring of human blood-brain barrier disruption. PLoS One 2017;12:e0174072 10.1371/journal.pone.0174072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tarella C, Rutella S, Gualandi F, et al. Consistent bone marrow-derived cell mobilization following repeated short courses of granulocyte-colony-stimulating factor in patients with amyotrophic lateral sclerosis: results from a multicenter prospective trial. Cytotherapy 2010;12:50–9. 10.3109/14653240903300682 [DOI] [PubMed] [Google Scholar]
- 37. Chiò A, Mora G, La Bella V, et al. Repeated courses of granulocyte colony-stimulating factor in amyotrophic lateral sclerosis: clinical and biological results from a prospective multicenter study. Muscle Nerve 2011;43:189–95. 10.1002/mus.21851 [DOI] [PubMed] [Google Scholar]
- 38. Marrali G, Casale F, Salamone P, et al. Nadph oxidase (Nox2) activity is a modifier of survival in ALS. J Neurol 2014;261:2178–83. 10.1007/s00415-014-7470-0 [DOI] [PubMed] [Google Scholar]
- 39. Amirzagar N, Nafissi S, Tafakhori A, et al. Granulocyte colony-stimulating factor for amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled study of Iranian patients. J Clin Neurol 2015;11:164–71. 10.3988/jcn.2015.11.2.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johannesen S, Budeus B, Peters S, et al. Biomarker supervised G-CSF (filgrastim) response in ALS patients. Front Neurol 2018;9:971 10.3389/fneur.2018.00971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brooks BR, Miller RG, Swash M, et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–9. 10.1080/146608200300079536 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-034049supp001.pdf (533.7KB, pdf)