Abstract
This review aimed to assess whether the FINDRISC, a risk score for type 2 diabetes mellitus (T2DM), has been externally validated in Latin America and the Caribbean (LAC). We conducted a systematic review following the CHARMS (CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies) framework. Reports were included if they validated or re-estimated the FINDRISC in population-based samples, health facilities or administrative data. Reports were excluded if they only studied patients or at-risk individuals. The search was conducted in Medline, Embase, Global Health, Scopus and LILACS. Risk of bias was assessed with the PROBAST (Prediction model Risk of Bias ASsessment Tool) tool. From 1582 titles and abstracts, 4 (n=7502) reports were included for qualitative summary. All reports were from South America; there were slightly more women, and the mean age ranged from 29.5 to 49.7 years. Undiagnosed T2DM prevalence ranged from 2.6% to 5.1%. None of the studies conducted an independent external validation of the FINDRISC; conversely, they used the same (or very similar) predictors to fit a new model. None of the studies reported calibration metrics. The area under the receiver operating curve was consistently above 65.0%. All studies had high risk of bias. There has not been any external validation of the FINDRISC model in LAC. Selected reports re-estimated the FINDRISC, although they have several methodological limitations. There is a need for big data to develop—or improve—T2DM diagnostic and prognostic models in LAC. This could benefit T2DM screening and early diagnosis.
Keywords: type 2 diabetes mellitus, prognostic models, diagnostic models, low- and middle-income countries, FINDRISC
Introduction
With an increasing load in terms of prevalence,1 disability and mortality,2–4 as well as economic burden,5 type 2 diabetes mellitus (T2DM) is a global threat to population health and health systems, especially in low-income and middle-income countries.1–5 Although universal health coverage should secure treatment for all patients, this goal may not be realistic where there is not universal screening and where resources are limited to identify all at-risk populations. Therefore, inexpensive yet reliable screening tools could be useful to identify T2DM cases or high-risk people. Risk scores, both diagnostic and prognostic, help identify people at high risk of having or developing T2DM. This way, these people could undergo further diagnostic tests, primary prevention or receive pharmacological treatment as needed. Nonetheless, risk scores need to be tested, and possibly adapted (ie, recalibrated), to produce accurate estimates to inform health decisions. Several T2DM risk scores have been developed,6–9 although very few for Latin America and the Caribbean (LAC), where those available exhibit major limitations hindering their implementation across countries or their endorsement by policies or guidelines.10 A well-known T2DM risk score is the FINDRISC,11 which is also acknowledged by the Latin American diabetes guidelines as an available diabetes screening tool;12 yet it is unknown if this model has been appropriately adapted in LAC. Consequently, we aimed to describe and assess if external validations of the FINDRISC model in LAC were conducted following adequate methods.13 14 We will complement the available evidence about T2DM risk scores in LAC10 and inform regional guidelines,12 while also pinpointing research priorities and policies for T2DM screening and early diagnosis through risk stratification.15 16
Methods
Protocol
This systematic review and critical appraisal of the scientific literature adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (online supplementary material). We followed the CHARMS (CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies) methodology to formulate the review framework, research question and strategy (table 1).17 18
Table 1.
CHARMS criteria to define research question and strategy
| Concept | Criteria |
| Prognostic or diagnostic? | The review focused on FINDRISC regardless if it was studied as a diagnostic or prognostic model for T2DM. |
| Scope | To inform physicians, researchers and the general population whether they are likely to have T2DM (ie, diagnostic) or will be likely to have T2DM (ie, prognostic). FINDRISC models could be used for research, screening and treatment allocation in primary prevention. |
| Type of prediction modeling studies |
|
| Target population to whom the prediction model applies | General adult population in LAC. |
| Outcome to be predicted | T2DM. |
| Time span of prediction | Prognostic models will not be included/excluded based on prediction time; that is, it could be short term (eg, next 2.5 years) or long term (eg, next 10 years). |
| Intended moment of using the model | FINDRISC models to be used in asymptomatic adults of LAC to assess their probability to have T2DM (ie, diagnostic) or their probability to develop T2DM in a predefined period (ie, prognostic). |
Based on the CHARMS checklist.18
CHARMS, CHecklist for critical Appraisal and data extraction for systematic Reviews of prediction Modelling Studies; LAC, Latin America and the Caribbean; T2DM, type 2 diabetes mellitus.
bmjdrc-2019-001169supp001.pdf (346.1KB, pdf)
Information sources
The search strategy was conducted in five search engines: Embase, Medline and Global Health through OVID, and also in Scopus and LILACS. The search was conducted on September 28, 2019. The search terms are available in the online supplementary material.
Eligibility criteria
We sought FINDRISC models aiming to inform about the current (diagnostic) or future (prognostic) risk of T2DM in LAC populations. Selected original reports could have developed a new model using the same (or very similar) predictors as in the original FINDRISC11; similarly, they could have performed an independent external validation in LAC populations. The outcome of the diagnostic or prognostic FINDRISC models was T2DM. The outcome should have been ascertained with at least one biomarker (eg, fasting glucose, hemoglobin A1c (HbA1c) or oral glucose tolerance test). Thus, we did not include studies where the outcome relied entirely on self-reported diagnosis. We focused on adult men and women.
Study selection
Original scientific reports were excluded if the study population only included people with a disease (eg, patients with hypertension) or based on a risk factor (eg, smokers). Similarly, studies with LAC populations in countries outside LAC were excluded (eg, Hispanics in the USA). Conversely, reports were included if they followed a probabilistic population-based sampling approach, were based on primary care settings, or were based on health or claims registries or administrative data. The original work should have focused on the FINDRISC model, regardless of whether they developed an identical new model, a very similar model, or independently externally validated the FINDRISC model. Studies were included if they followed a cross-sectional or prospective observational design.
Data collation process
We used EndNote and Rayyan19 to remove duplicates from the search. First, we used Rayyan19 to screen titles and abstracts, which were screened by two reviewers independently (pairwise combinations between RMC-L and DJA-G or JRM); discrepancies were solved by consensus. Then, two reviewers independently (pairwise combinations between RMC-L and DJA-G or JRM) studied the full text of those reports selected in the screening phase; discrepancies were solved by consensus. If consensus could not be reached, discrepancies were solved by a third party (AB-O).
The authors developed a data extraction form based on international guidelines for systematic reviews of prognosis models17 18 and on a previous systematic review on the subject.10 The data extraction form was not modified during data collation. Information was extracted as presented in the original reports by two reviewers independently (pairwise combinations between RMC-L and DJA-G or JRM); discrepancies were solved by consensus.
Risk of bias of individual studies
Using the PROBAST (Prediction model Risk of Bias ASsessment Tool) tool for risk of bias assessment of prognosis models,20 21 two reviewers (DJA-G and JRM) independently assessed the risk of bias of the selected reports. If there were any discrepancies, these were solved by consensus or by a third party (RMC-L).
Synthesis of results
Because of the limited numbers of results and the great heterogeneity among them, only a qualitative synthesis was conducted.
Results
Reports selection
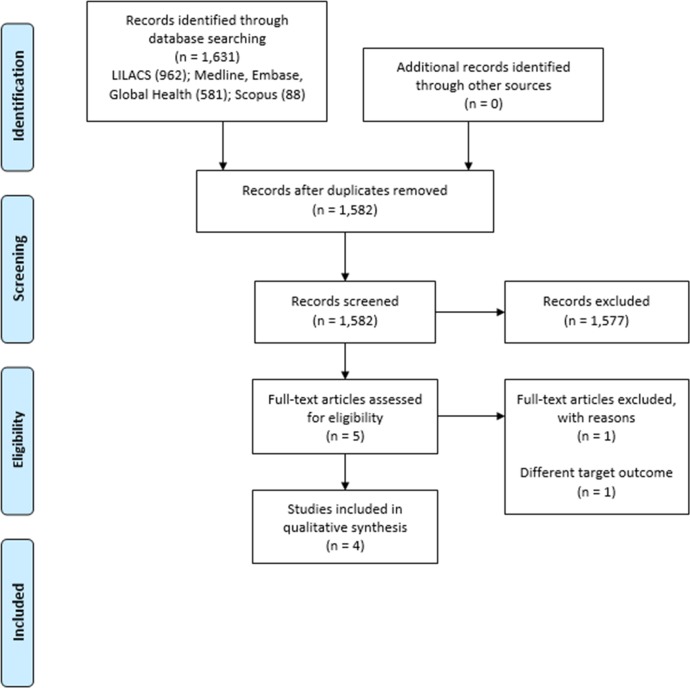
The screening process included 1582 titles and abstracts, of which 1577 were excluded. Therefore, five reports were studied in full text. One report was excluded because they assessed a different outcome.22 Finally, four reports (n=7502) were included in the qualitative review (figure 1).23–26
Figure 1.
Flow chart of the study selection process.
General characteristics
All the reports were from South America: one from Peru,24 one from Venezuela,26 and two from Colombia.23 25 Bernabe-Ortiz et al24 as well as Nieto-Martínez et al26 studied population-based samples while Gomez-Arbelaez et al25 and Barengo et al23 analyzed data from health centers (online supplementary material).
The largest sample size was studied by Nieto-Martínez et al (n=3061); this was also a national representative sample.26 The smallest sample was studied by Gomez-Arbelaez and colleagues (n=772).25 The studied samples tended to include slightly more women than men, except for one report (58.3% men).25 The mean age ranged from 29.5 to 49.7 years (table 2, online supplementary material).24 25
Table 2.
General characteristics
| Study | Outcome prevalence (%) | Mean age (years) | Men (%) |
Outcome details | Baseline sample size | Outcome events (n) | Outcome events per candidate predictors |
| Gomez-Arbelaez et al25 | 2.59 | 58.34 | 29.53 | The diagnosis of type 2 diabetes mellitus was established when fasting plasma glucose ≥126 mg/dL, OGTT ≥200 mg/dL and/or HbA1c ≥6.5%. | 772 | 20 | |
| Bernabe-Ortiz et al24 FINDRISC Simplified, FINDRISC and LA-FINDRISC | 4.70 | 48.20 | 49.70 | Individuals who were not aware of having type 2 diabetes mellitus and had fasting glucose ≥126 mg/dL (≥7.0 mmol/L) or 2-hour plasma glucose ≥200 mg/dL (≥11.1 mmol/L). | 1609 | 71 | 5.92 |
| Nieto-Martinez et al26 LA-FINDRISC and FINDRISC | 3.30 | 39.90 | 46.97 | Diabetes was defined if the fasting plasma glucose was ≥126 mg/dL or if the 2-hour OGTT glucose was ≥200 mg/dL. | 3061 | 101 | |
| Barengo et al23 ColDRISC and FINDRISC | 5.10 | 47.20 | 38.00 | Individuals who had fasting plasma glucose ≥126 mg/dL or 2-hour plasma glucose ≥200 mg/dL were classified as having T2DM. | 2060 | 105 | 11.67 |
HbA1c, hemoglobin A1c; OGTT, oral glucose tolerance test.
Across reports, T2DM was ascertained with a combination of fasting plasma glucose ≥126 mg/dL, HbA1c ≥6.5% or 2-hour plasma glucose ≥200 mg/dL (table 2, online supplementary material).23–26 Undiagnosed T2DM prevalence was largest in the report by Barengo et al (5.10%; n=105),23 followed by the study in Peru (4.70%; n=71),24 the report from Venezuela (3.30%; n=101)26 and the work by Gomez-Arbelaez et al25 (2.59%; n=20) (table 2, online supplementary material).
Predictors and modeling
None of the studies conducted an independent external validation of the FINDRISC model. Conversely, they used the same (or very similar) predictors to fit a new model.23–26 In so doing, they all produced new coefficients and baseline risks.23–26 As in the original FINDRISC,11 numeric variables were categorized. The modeling strategy was consistently logistic regression, and complete-case analyses were conducted (online supplementary material).
Two authors developed new risk scores.23 24 Barengo et al23 started with nine candidate predictors to develop a Colombian version of the FINDRISC with six predictors; predictor selection was based on univariate analysis (online supplementary material). Bernabe-Ortiz et al24 developed a simplified version of the FINDRISC including 5 predictors, yet there were 12 candidate predictors selected through stepwise backward elimination (online supplementary material).
Model performance
None of the studies reported any calibration metrics (online supplementary material).23–26 Conversely, they all focused on discrimination (area under the receiver operating curve) and other classification metrics, including sensitivity, specificity, and positive and negative predictive values. The area under the receiver operating curve was consistently above 65.0% (table 3, online supplementary material).
Table 3.
Performance metrics
| First author, and assessed model | Discrimination (%) | Classification measures |
| Gomez-Arbelaez et al25 | 74.77 (95% CI 57.22 to 92.32) (men) and 71.75 (95% CI 58.68 to 84.81) (women) | At a cut-off of ≥14. Men: sensitivity=66.7; specificity=75.2; positive predictive value=6.8; negative predictive value=98.8; Youden’s index=0.419. Women: sensitivity=71.4; specificity=62.6; positive predictive value=4.8; negative predictive value=98.8; Youden’s index=0.340. |
| Bernabe-Ortiz et al,24 FINDRISC Simplified | 71.10 | At a cut-off of 3: sensitivity=0.859; specificity=0.467; positive predictive value=0.074; negative predictive value=0.985; likelihood ratio positive=1.6; likelihood ratio negative=0.3; diagnostic OR=5.3. |
| Bernabe-Ortiz et al,24 FINDRISC | 69.00 | At a cut-off of 11: sensitivity=0.690; specificity=0.668; positive predictive value=0.094; negative predictive value=0.978; likelihood ratio positive=2.1; likelihood ratio negative=0.5; diagnostic OR=4.5. |
| Bernabe-Ortiz et al,24 LA-FINDRISC | 68.00 | At a cut-off of 10: sensitivity=0.704; specificity=0.591; positive predictive value=0.079; negative predictive value=0.970; likelihood ratio positive=1.7; likelihood ratio negative=0.5; diagnostic OR=3.4. |
| Nieto-Martinez et al,26 LA-FINDRISC | 72.2 (95% CI 66.8 to 77.5) (men) and 72.40 (95% CI 63.9 to 81.0) (women) | For men at a cut-off of 9: sensitivity=72.2; specificity=62.2; positive likelihood ratio=1.91. For women at a cut-off of 10: sensitivity=71.4; specificity=65.4; positive likelihood ratio=2.06. |
| Nieto-Martinez et al,26 FINDRISC | 72.90 (95% CI 67.6 to 78.1) (men) and 73.20 (95% CI 64.8 to 81.6) (women) | |
| Barengo et al,23 ColDRISC | 74 (95% CI 70 to 79) | At a cut-off of 4: sensitivity=0.73; specificity=0.67; positive predictive value=0.106; negative predictive value=0.979. |
| Barengo et al,23 modified FINDRISC | 73 (95% CI 69 to 78) | At a cut-off of 10: sensitivity=0.72; specificity=0.60; positive predictive value=0.084; negative predictive value=0.984. |
Risk of bias
All studies exhibited high risk of bias mainly due to limitations in the analytical approach, for example limited number of outcome events. In this line, a complete-case analysis was consistently preferred versus multiple imputation. Most importantly, calibration metrics were consistently not reported. On the other hand, participants, predictors, and outcome criteria showed low risk of bias. There was low applicability concern (table 4, online supplementary material).
Table 4.
Risk of bias assessment of individual diagnostic/prediction models (PROBAST)20 21
| First author and assessed model | RoB | Applicability | Overall | ||||||
| Participants | Predictors | Outcome | Analysis | Participants | Predictors | Outcome | RoB | Applicability | |
| Barengo et al,23 ColDRISC | + | + | + | − | + | + | + | − | + |
| Barengo et al,23 modified FINDRISC | + | + | + | − | + | + | + | − | + |
| Bernabe-Ortiz et al,24 FINDRISC Simplified | + | + | + | − | + | + | + | − | + |
| Bernabe-Ortiz et al,24 FINDRISC | + | + | + | − | + | + | + | − | + |
| Bernabe-Ortiz et al,24 LA-FINDRISC | + | + | + | − | + | + | + | − | + |
| Gomez-Arbelaez et al25 | + | + | + | − | + | + | + | − | + |
| Nieto-Martínez et al,26 LA-FINDRISC | + | + | + | − | + | + | + | − | + |
| Nieto-Martínez et al,26 FINDRISC | + | + | + | − | + | + | + | − | + |
+ indicates low RoB/low concern regarding applicability; − indicates high RoB/high concern regarding applicability.
PROBAST, Prediction model Risk of Bias ASsessment Tool; RoB, risk of bias.
Discussion
Main findings
This review did not find any independent external validations of the original FINDRISC model in LAC, as the four reports herein described re-estimated the FINDRISC model; in other words, authors computed new coefficients and baseline risks instead of using the original ones to test the model performance in a new population with subsequent recalibration if needed. While the analyzed reports exhibited methodological limitations, including reduced number of outcome events and not reporting calibration metrics, they showed acceptable discrimination performance. In LAC, risk prediction research needs to be improved to generate reliable tools for risk stratification, which could offer a cost-effective approach in the cascade to identify new and future T2DM cases.27
Limitations of the review
Although we followed a comprehensive methodology, there are still limitations to be acknowledged. First, we did not search gray literature; however, we would not expect results from these sources, if any, to substantially change the main findings or conclusions of this review. Second, the focus of this work was on LAC; whether our findings apply to other world regions mostly hosting low-income and middle-income countries deserves further verification.
Limitations of the selected reports
We have previously pinpointed several methodological limitations of T2DM risk scores in LAC,10 and these would also apply to those herein studied. Although there is literature addressing good methods for development and validation of risk scores,13 14 the most recurrent pitfall herein identified is the limited number of outcome events, which may allow including few predictors or could lead to overfitting of the prediction model. We understand that (big) data with enough outcome events may be scarce in LAC; thus, we value and acknowledge the available research. Recently, methods have been developed to define sample size for risk prediction models with binary outcomes.28 Where possible, researchers could adhere to these standards.
We strongly believe there is a great need to look for (big) data, for example, national surveys (eg, WHO STEPwise approach to Surveillance (STEPS) or Demographic and Health Surveys (DHS)). These surveys are available in many countries, and pooling them, following adequate techniques, could generate a rich database to develop a T2DM risk score for LAC. Finally, it is also worrying that none of the studies reported calibration metrics such as calibration slope, calibration in the large or calibration plots.13 14 Calibration refers to the agreement between observed and predicted events.13 14 Therefore, it provides information to understand whether the model is underestimating (observed > predicted) or overestimating (observed < predicted) the outcome. As risk prediction research further penetrates in LAC, standard and sound methods should be adopted and reported appropriately; thereby, robust tools will be available to be incorporated in health policies and guidelines.13 14 29
The summarized reports also provided metrics usually available for diagnostic tools, including positive/negative predictive values and positive/negative likelihood ratios. Of these additional metrics, the negative predictive value was the largest, consistently above 97%. This refers that a subject with a negative test is in fact disease-free. In other words, of 100 people who take the test and have a negative result, over 97 of them would not have the disease. This metric depends on the prevalence in the underlying population; that is, this is not an intrinsic property of the model. Thus, this metric would not be useful in generalizing the accuracy of the test across populations with different prevalences. Nonetheless, this could suggest that for people with a very low score or a score below a established threshold, further tests are not needed because they are most likely not to have diabetes.
Clinical and public health relevance
The American30 and Canadian31 T2DM guidelines include risk scores to identify people who would need further laboratory tests to confirm T2DM;30 31 these documents suggest specific risk scores such as the Canadian Diabetes Risk Assessment Questionnaire.31 The LAC guidelines, on the other hand, support the use of risk scores for screening purposes, without advocating for any tool in particular, although they acknowledge the FINDRISC as a relevant and useful tool.12 Probably the LAC guidelines do not support a risk score in particular due to the dearth of tools and the limitations of the few available ones.10 Following the example of the US and Canadian guidelines, LAC T2DM institutions should support and foster the development of a strong T2DM risk score, which could benefit from national survey data or large pooling data endeavors.
Whether risk scores are the best method to screen for diabetes is yet to be known. Other alternatives include massive screening with blood tests (eg, random glucose or HbA1c) or screening based on single risk factors (eg, people with severe obesity). The first alternative may not be feasible in low-income and middle-income countries or rural settings, where costs and scarce laboratory facilities may preclude this option. Screening on single risk factors may not be sensible enough, hence the need for risk scores to combine several predictors to compute a more comprehensive probability. Also, there is evidence suggesting that screening with a risk stratification tool, such as a risk score, is a cost-effective approach.27 While other screening methods are being developed and proven better than risk scores, risks scores need to be improved to provide accurate results that can inform public health (eg, number of people at risk in need of tests) and clinical medicine (eg, when to start counseling or treatment). The diabetes guidelines for Latin America do not explicitly recommend using the FINDRISC, yet they signal the FINDRISC as a relevant screening tool.12 In Colombia, on the other hand, clinical guidelines do recommend the FINDRISC.32 Assessing which guidelines recommend the FINDRISC, or other risk scores, is beyond the scope of this work. However, given the limitations herein pinpointed as well as by a previous systematic review on the subject,10 we recommend cautious use of available tools, particularly if they are being used in populations different from those used in developing the model.
Conclusions
There has not been an external validation of the FINDRISC model in LAC, where several re-estimations of this model have been conducted. The available research has benefitted from studies with limited coverage, for example, small cross-sectional studies. This calls to strengthen the use of (big) data or national surveys across LAC to develop—or improve—T2DM diagnostic and prognostic risk scores. This could have large positive impact on T2DM screening and early diagnosis in LAC. Overall, the discrimination accuracy of the FINDRISC in LAC seems adequate, although no evidence is available on calibration metrics.
Footnotes
DJA-G and JRM contributed equally.
Contributors: RMC-L and AB-O conceived the idea. RMC-L, DJA-G and JRM conducted the search and data extraction. DJA-G and JRM conducted the risk of bias. RMC-L wrote the manuscript with input from all coauthors. All authors approved the submitted version.
Funding: The study received funding from Strategic Award, Wellcome Trust-Imperial College Centre for Global Health Research (100693/Z/12/Z), and Imperial College London Wellcome Trust Institutional Strategic Support Fund (Global Health Clinical Research Training Fellowship) (294834/Z/16/Z ISSF ICL). RMC-L is supported by a Wellcome Trust International Training Fellowship (214185/Z/18/Z). The funder had no role in the conception or conduct of this work, neither in the preparation of the results nor in manuscript writing. The authors alone are responsible for the results and opinions in this work.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: No human subjects were studied; thus, this review was classified as of low risk.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data sharing not applicable as no data sets generated and/or analyzed for this study. This is a systematic review of the scientific literature.
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513–30. 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1736–88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2017 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1859–922. 10.1016/S0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018;392:1789–858. 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bommer C, Sagalova V, Heesemann E, et al. Global economic burden of diabetes in adults: projections from 2015 to 2030. Diabetes Care 2018;41:963–70. 10.2337/dc17-1962 [DOI] [PubMed] [Google Scholar]
- 6.Abbasi A, Peelen LM, Corpeleijn E, et al. Prediction models for risk of developing type 2 diabetes: systematic literature search and independent external validation study. BMJ 2012;345:e5900 10.1136/bmj.e5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins GS, Mallett S, Omar O, et al. Developing risk prediction models for type 2 diabetes: a systematic review of methodology and reporting. BMC Med 2011;9:103 10.1186/1741-7015-9-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juarez LD, Gonzalez JS, Agne AA, et al. Diabetes risk scores for Hispanics living in the United States: a systematic review. Diabetes Res Clin Pract 2018;142:120–9. 10.1016/j.diabres.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noble D, Mathur R, Dent T, et al. Risk models and scores for type 2 diabetes: systematic review. BMJ 2011;343:d7163 10.1136/bmj.d7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrillo-Larco RM, Aparcana-Granda DJ, Mejia JR, et al. Risk scores for type 2 diabetes mellitus in Latin America: a systematic review of population-based studies. Diabet Med 2019;36:1573–84. 10.1111/dme.14114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindström J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725–31. 10.2337/diacare.26.3.725 [DOI] [PubMed] [Google Scholar]
- 12.Diabetes ALde. Guías ALAD sobre el Diagnóstico, Control y Tratamiento de la Diabetes Mellitus Tipo 2 con Medicina Basada en Evidencia - Edición, 2019URL Available: http://www.revistaalad.com/guias/5600AX191_guias_alad_2019.pdf
- 13.Riley RD, Dvd W, Croft P, et al. Prognosis research in health care: concepts, methods, and impact Oxford. UK: Oxford University Press, 2019. [Google Scholar]
- 14.Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating: Springer international publishing, 2019. [Google Scholar]
- 15.World Health Organization Noncommunicable diseases and mental health. ncd global monitoring framework. Available: https://www.who.int/nmh/global_monitoring_framework/en/
- 16.Pan American Health Organization / World Health Organization Health policies: regulatory measures for diabetes prevention and control. Available: https://www.paho.org/hq/index.php?option=com_docman&view=list&slug=health-policies-regulatory-measures-for-diabetes-prevention-control-8623&Itemid=270&lang=en
- 17.Debray TPA, Damen JAAG, Snell KIE, et al. A guide to systematic review and meta-analysis of prediction model performance. BMJ 2017;356:i6460 10.1136/bmj.i6460 [DOI] [PubMed] [Google Scholar]
- 18.Moons KGM, de Groot JAH, Bouwmeester W, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the charms checklist. PLoS Med 2014;11:e1001744 10.1371/journal.pmed.1001744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile APP for systematic reviews. Syst Rev 2016;5:210 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moons KGM, Wolff RF, Riley RD, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med 2019;170:W1–33. 10.7326/M18-1377 [DOI] [PubMed] [Google Scholar]
- 21.Wolff RF, Moons KGM, Riley RD, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 2019;170:51–8. 10.7326/M18-1376 [DOI] [PubMed] [Google Scholar]
- 22.Muñoz-González MC, Lima-Martínez MM, Nava A, et al. FINDRISC modified for Latin America as a screening tool for persons with impaired glucose metabolism in Ciudad Bolívar, Venezuela. Med Princ Pract 2019;28:324–32. 10.1159/000499468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barengo NC, Tamayo DC, Tono T, et al. A Colombian diabetes risk score for detecting undiagnosed diabetes and impaired glucose regulation. Prim Care Diabetes 2017;11:86–93. 10.1016/j.pcd.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 24.Bernabe-Ortiz A, Perel P, Miranda JJ, et al. Diagnostic accuracy of the Finnish diabetes risk score (FINDRISC) for undiagnosed T2DM in Peruvian population. Prim Care Diabetes 2018;12:517–25. 10.1016/j.pcd.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez-Arbelaez D, Alvarado-Jurado L, Ayala-Castillo M, et al. Evaluation of the Finnish diabetes risk score to predict type 2 diabetes mellitus in a Colombian population: a longitudinal observational study. World J Diabetes 2015;6:1337–44. 10.4239/wjd.v6.i17.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieto-Martínez R, González-Rivas JP, Ugel E, et al. External validation of the Finnish diabetes risk score in Venezuela using a national sample: the EVESCAM. Prim Care Diabetes 2019;13:574–82. 10.1016/j.pcd.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 27.Khunti K, Gillies CL, Taub NA, et al. A comparison of cost per case detected of screening strategies for type 2 diabetes and impaired glucose regulation: modelling study. Diabetes Res Clin Pract 2012;97:505–13. 10.1016/j.diabres.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 28.Riley RD, Snell KI, Ensor J, et al. Minimum sample size for developing a multivariable prediction model: PART II - binary and time-to-event outcomes. Stat Med 2019;38:1276–96. 10.1002/sim.7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Diabet Med 2015;32:146–54. 10.1111/dme.12654 [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019;42:S13–28. 10.2337/dc19-S002 [DOI] [PubMed] [Google Scholar]
- 31.Diabetes Canada Clinical Practice Guidelines Expert Committee Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2018;42:S1–325.29650079 [Google Scholar]
- 32.Sistema General de Seguridad Social en Salud - Colombia Guia de practica clinica para El diagnostico, tratamiento Y seguimiento de la diabetes mellitus tipo 2 en La poblacion mayor de 18 años. Available: http://gpc.minsalud.gov.co/gpc_sites/Repositorio/Conv_637/GPC_diabetes/DIABETES_TIPO_2_COMPLETA.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-001169supp001.pdf (346.1KB, pdf)