Abstract
Objectives
The aim of the DiaFu study was to evaluate effectiveness and safety of negative pressure wound therapy (NPWT) in patients with diabetic foot wounds in clinical practice.
Design
In this controlled clinical superiority trial with blinded outcome assessment patients were randomised in a 1:1 ratio stratified by study site and ulcer severity grade using a web-based-tool.
Setting
This German national study was conducted in 40 surgical and internal medicine inpatient and outpatient facilities specialised in diabetes foot care.
Participants
368 patients were randomised and 345 participants were included in the modified intention-to-treat (ITT) population. Adult patients suffering from a diabetic foot ulcer at least for 4 weeks and without contraindication for NPWT were allowed to be included.
Interventions
NPWT was compared with standard moist wound care (SMWC) according to local standards and guidelines.
Primary and secondary outcome measures
Primary outcome was wound closure within 16 weeks. Secondary outcomes were wound-related and treatment-related adverse events (AEs), amputations, time until optimal wound bed preparation, wound size and wound tissue composition, pain and quality of life (QoL) within 16 weeks, and recurrences and wound closure within 6 months.
Results
In the ITT population, neither the wound closure rate (difference: n=4 (2.5% (95% CI−4.7% – 9.7%); p=0.53)) nor the time to wound closure (p=0.244) was significantly different between the treatment arms. 191 participants (NPWT 127; SMWC 64) had missing endpoint documentations, premature therapy ends or unauthorised treatment changes. 96 participants in the NPWT arm and 72 participants in the SMWC arm had at least one AE (p=0.007), but only 16 AEs were related to NPWT.
Conclusions
NPWT was not superior to SMWC in diabetic foot wounds in German clinical practice. Overall, wound closure rate was low. Documentation deficits and deviations from treatment guidelines negatively impacted the outcome wound closure.
Trial registration numbers
NCT01480362 and DRKS00003347.
Keywords: negative pressure wound therapy, wound healing, benefit assessment, wound treatment, diabetic foot, wound care
Strengths and limitations of this study.
The DiaFu study included patients with diabetic foot ulcers both with peripheral neuropathy and peripheral arterial occlusive disease, which corresponds to the typical mixed patient population in real-life clinical practice. This allows a general statement on effectiveness and safety of negative pressure wound therapy (NPWT) in the typical medical care situation, but including patients with peripheral artery occlusive disease and clinical signs of inflammation (suspected infection) had a potentially negative effect on the treatment outcome wound closure.
The study does not provide any information on the effectiveness of NPWT in specific patient groups.
In this health services research study, hospitals and outpatient facilities were selected by means of a qualification checklist, and clinical investigators were obliged to provide patients with the best clinical practice in compliance with all relevant diagnostic and treatment guidelines, but there was no active monitoring of the implementation of these guidelines.
To ensure the best quality of local wound treatment and to achieve optimal baseline conditions, the study sites were trained for both NPWT and standard moist wound care, but treatment application was at the discretion of the clinical investigators.
A high number of missing endpoint documentations, premature termination of NPWT and unauthorised therapy changes negatively impacted the treatment outcome wound closure and may have led to bias in the results.
Background
More than 400 million people worldwide suffer from diabetes,1 2 and about 15% of all these patients will develop a diabetic foot ulcer (DFU) during their lifetime.3 4 Approximately 50%–70% of all lower limb amputations are due to diabetes.4 DFUs represent complex chronic wounds with a major impact on patients’ morbidity, mortality and quality of life (QoL). Beside an optimal diabetes and infection control, pressure-relieving strategies and restoring pulsatile blood flow, effective local wound care is part of the holistic approach necessary to optimally treat patients with DFUs. Only a few modern moist wound dressings and topical agents have been convincingly shown to achieve higher wound closure rates compared with traditional wet gauze dressings in patients with diabetic foot wounds.5 Also, for other ulcer types, there is uncertainty as to which dressings and topical agents are most effective for treatment.6 Negative pressure wound therapy (NPWT) is an innovative treatment option and one of the most commonly used and well-established technologies with the aim to promote wound healing.7 The first use of vacuum sealing was described in 1993 by Fleischmann et al,8 and the commercially available product was developed later in the 1990s.9 10 Positive effects of NPWT on wound healing have been suggested in various basic studies.10 11 At the time of planning the DiaFu study, the clinical evidence largely consisted of clinician perception, case reports and series, small cohort studies and weakly powered or low-quality randomised trials that documented broad use of NPWT in various clinical settings and constituted a substantial number of publications but an overall small amount of evidence.12–15 Two randomised controlled trials (RCTs) performed by Armstrong and Lavery16 and Blume et al 17 provided a solid basis for planning a study.
In the recent years, a specific review for the use of NPWT in diabetic foot wounds performed by Dumville et al in 2013,18 an assessment in the home setting by Rhee et al in 201419 and a health technology assessment particularly issued for the evaluation of NPWT for managing DFUs20 in 2014, as well as the most recent work of Liu et al in 201721 22 all concluded that although NPWT may have a positive effect, the trials that have been performed have methodological flaws and sufficient, unbiased evidence of whether wounds heal better or worse with NPWT than with conventional treatment is still missing.
In Germany, the issue of evidence for effectiveness and safety of NPWT in acute and chronic wounds was first addressed in 2002 when the German Federal Joint Committee (German: Gemeinsamer Bundesausschuss (G-BA)) needed to decide whether NPWT could be reimbursed without restrictions in outpatient care.
Finally, in 2007 taking into account all available evidence the G-BA decided that the benefits of the treatment method NPWT should be evaluated in a so-called model project. The project was intended to include the conduct of clinical studies for which the G-BA defined basic requirements. This essentially concerned a study hypothesis that supports G-BA’s overall question if NPWT can be reimbursed in German outpatient care without any limitation, the selection of a comparator that represents the current treatment standard in Germany, and implementation of all measures to ensure a sufficient certainty of the results.
Following the announcement of the G-BA, the German statutory health insurance funds initiated an overall project through a European tender. The DFU was chosen to be the representative for chronic wounds in an RCT comparing NPWT and standard moist wound care (SMWC) in clinical practice.
Methods
Aim of the study
The aim of the DiaFu study was to evaluate whether the effectiveness and safety of NPWT is superior to SMWC in German real-life clinical practice.
Study design
The DiaFu study was a multicentre, randomised controlled clinical superiority trial with blinded assessment of wound closure, wound size and wound tissue qualities using photographs. This German national study was conducted both in hospital departments and outpatient facilities with a special qualification for diabetic foot care. Study sites were selected based on their qualifications and experiences using a prestudy qualification checklist and annual quality reports of the respective institution (if available). Study treatment was allowed to be started both in inpatient and outpatient care and should be continued outpatient whenever possible. More detailed information on the study design can be found in the study protocol publication that is available open access.23
Patient and public involvement
Patients were not involved in the design, recruitment or conduct of the study. The results of this study will not be disseminated directly to study participants.
Participants
Following a pragmatic approach with the aim to include a patient population best representing real-life clinical practice, inclusion and exclusion criteria were selected based on manufacturers' contraindications and US Food and Drug Administration (FDA) warnings, the necessity to exclude patients in need of protection and who are unable to give their consent, and the intention to avoid general study-related and treatment specific influences on the results.
Adult patients (age >18 years) with at least 4-week-old chronic DFUs corresponding to Wagner 2–4 were screened for study participation by the local investigators. Before inclusion, the study protocol required either a debridement or, if necessary, an amputation of foot parts, or a thorough wound cleansing, depending on the individual needs of the patients. Thus, chronic diabetic foot wounds after adequate wound pretreatment as well as postsurgical amputation wounds below the upper ankle joint were eligible for inclusion. The initially planned minimum ulcer age of 6 weeks was reduced to 4 weeks during the course of the study. As in clinical practice, the assessment of patients’ suitability for a specific wound therapy with the aim of complete wound closure and (due to randomisation) for both study treatment arms (NPWT and SMWC) was at the discretion of the treating physicians (clinical investigators of the study). Particular attention was to be paid to the diagnosis and therapy of concomitant diseases.
Patients estimated to be at risk of non-compliance with study requirements, with wounds with necrotic tissue present that could not be removed by debridement or amputation, with exposed blood vessels within or directly surrounding the wound not possible to be sufficiently covered or with an increased risk of bleeding with haemodynamic consequences (mainly relevant for posterior tibial artery dorsalis pedis artery), and outpatients receiving anticoagulation therapy or suffering from a high-grade impaired clotting function with a heightened risk of bleeding with haemodynamic consequences were excluded from the DiaFu study. The use of NPWT devices on the study wound within 6 weeks prior to study start represented an exclusion criterion in order to demonstrate a clear therapeutic effect of each treatment arm.
Written informed consent was obtained from every participant after being informed about all aspects of the trial, and before randomisation and any trial-related procedure. As the statutory health insurance funds provided integrated care contracts for outpatient NPWT, only patients who were members of a participating health insurance fund were allowed to be enrolled.
Randomisation and masking
Patients were randomly allocated to the treatment arms in a 1:1 ratio using a computer-generated list located on a centralised web-based tool. The randomisation list consisted of permuted blocks of variable length which were randomly arranged. Patients were stratified by study site and by Wagner-Armstrong stage within each site (<Wagner-Armstrong stage 2C and ≥Wagner-Armstrong stage 2C). The randomisation lists were generated with the help of a self-created Java program and integrated into the study database. Each registered investigator received individual access to the randomisation tool via the study website but without knowledge of future treatment assignment, which provided adequate allocation concealment. The investigators were responsible for adequately implementing the assigned therapy. Due to the physical differences between the treatment regimens, it was not possible to blind either participant or physician to the treatment assignment. Verification of complete wound closure was performed by independent, blinded assessment of wound photographs. Determination of wound size and percentage wound tissue quality was also performed by central, blinded outcome assessors based on the wound photographs using the Wound Healing Analyzing Tool (W.H.A.T.). The determination of sufficient wound bed conditioning and the indication for surgical closure was carried out by the treating physician, as in clinical practice. The treating physician was not blinded to treatment allocation.
Procedures
Basic data were collected for all patients considered for study participation during screening and were updated during the randomisation visit. Patients received an extensive examination of overall health status, specific diabetes associated disorders and relevant influence factors on wound healing during screening with an update at the randomisation visit. Neuropathy and vascular diagnostics were performed according to the German National Health Care Guidelines for Type 2 Diabetes Foot Complications.24 After anamnesis and general diagnostics (physical examination), this care guideline recommends the following further vascular diagnostics: ankle–arm index (‘Ankle-Brachial-Index’) and additional assessment of the Doppler frequency spectrum (due to the possible falsifying of the results by Media sclerosis) and, if necessary, additional hydrostatic toe pressure measurement (pole test) or a transcutaneous oxygen measurement (tcPO2), duplex sonography to determine the extent and distribution pattern of a potential peripheral artery occlusive disease (PAOD) (including the lower leg arteries if necessary). In case of inconclusive findings, contrast agent-enhanced MR angiography and intra-arterial digital subtraction angiography were considered. No detailed examination results of the vascular diagnostics but the final diagnosis of PAOD and critical limb ischaemia (CLI) were to be documented in the electronic case report form (eCRF) by the clinical investigators. Infection diagnosis comprised clinical evaluation and laboratory testing. In case of suspected diabetic foot osteomyelitis, a probe to bone test and a stepwise approach to imaging modalities were applied in order to confirm the clinical diagnosis and to determine the best treatment regimen for the study participants.
Before randomisation and start of study treatment, all patients underwent one or more of the following no longer than 6 hours before randomisation: amputation, debridement or thorough wound cleansing. Study therapy was allowed to be started either in-hospital or as outpatient and was intended to be continued in outpatient care whenever possible.
In the intervention arm commercially available CE-marked NPWT devices of the manufacturers Kinetic Concepts Incorporated (KCI) and Smith & Nephew (S&N) were used in the discretion of the clinical investigator according to clinical routine and manufacturers’ instructions.23 Intermittent and continuous NPWT was allowed to be used with the negative pressure to be adapted as recommended for the dressing applied (V.A.C.-Granufoam Black or Silver; V.A.C.-White Foam; Renassys–F/P; Renassys–G) and adapted to the wound needs. Recommendations for use are available on the manufacturers’ websites. As part of the European tender for the overall project, the German statutory health insurance funds awarded lots for the provision of the medical products by the respective manufacturers. Germany was divided into four supply areas. During the award procedure, S&N received one lot and KCI three lots. Thus, devices and consumables of S&N were used for the north and northern east region of Germany, and for the rest of Germany, the therapy systems of KCI were used. Within the study, NPWT was required to be used for wound bed preparation in order to achieve at least 95% granulation of the wound area. After optimal preparation of the wound, complete closure could be achieved either by secondary intention with dressings or by surgical closure with subsequent removal of the suture.
Control therapy was defined as any SMWC according to local clinical standards and guidelines.25 26 Healthcare providers were obligated to provide patients with best practice. In the control arm, it was permitted to apply any local wound treatment standard used in the respective study site that did not have an experimental status or was NPWT. To ensure the best quality of local wound treatment, the study sites were trained for both the intervention arm by the manufacturers and the control arm by the German Society for Wound Healing and Wound Treatment, which provided parts of its curriculum and experienced instructors.
The maximum study treatment time was 16 weeks after randomisation. Study visits needed to be performed at week 1, 3, 5, 12 and 16, and in the event of end of treatment, hospital discharge, wound closure and for wound closure confirmation after a minimum of 14 days. Study participants were followed up until 6 months after randomisation. The initially planned follow-up period of 12 months was reduced to 6 months in the course of the study. The amendment to the study protocol was endorsed by the Ethics Committee and immediately communicated to all participating study sites.
Outcomes
The primary outcome was wound closure (100% epithelialisation of the wound, no drainage, no suture material and no need for wound dressing or adjuvants) within the maximum study treatment period of 16 weeks. Wound closure could be achieved both by healing by secondary intention and by delayed primary closure. Complete closure of the wound needed to sustain for a minimum of 14 days and to be confirmed by independent blinded observers using wound photographs.
Secondary outcomes were wound closure after 6 months, time until optimal preparation of the wound bed (a minimum of 95% granulation), amputations and resections, wound size and wound tissue composition, pain and QoL within 16 weeks, and recurrence within 6 months. The initial planned secondary endpoint time until wound closure within 6 months was abandoned during the course of the study. It was found that a time-to-event survey was not possible outside the active study treatment period. This was mostly due to the fact that after this 16-week period, weekly study visits were no longer an obligation, and further patient care was no longer bound to the study site.
Minor and major amputations were assessed separately, whereas the disarticulation at the midtarsal joint (Chopart’s amputation) was considered still to be minor. Wound size and wound tissue composition (percentage of granulation tissue, fibrin and necrosis) were monitored at each study visit. QoL was measured using the questionnaire Euro Quol 5D (EQ5D) at inclusion, end of the maximum treatment time or end of the therapy and at the 6-month follow-up visit. At each study visit participants were asked to provide their assessment of wound-associated pain on a numerical rating scale (0–10). The incidence of serious adverse events (SAEs) within 6 months and the incidence of device-related and treatment-related adverse events (AEs) occurring within 16 weeks or until wound closure confirmation were safety endpoints of this trial.
Statistical analysis
Sample size calculation was performed using the expected difference between wound closure rates in both treatment arms based on information extracted from previously published studies by Armstrong and Lavery16 and Blume et al.17 We assumed a complete wound closure rate of 45% for NPWT and 30% in the SMWC group, resulting in a minimum difference of 15% after a treatment time of 16 weeks. Based on a type 1 error of α=0.05 and a type 2 error of β=0.2 (corresponding to a power of 80%), a total sample size of 162 patients per group was calculated. The computer program of Dupont and Plummer was used for sample size calculation.27
We performed all analyses based on a modified intention-to-treat (ITT) population that includes all randomised participants who have a valid baseline and at least one valid post baseline wound assessment. As a secondary approach a per-protocol (PP) analysis was performed excluding patients with any serious protocol deviations, like temporary changes from SMWC to NPWT, permanent wound treatment changes or without valid documentation until wound closure confirmation or end of maximum treatment time (EOMTT). Safety data are presented on an ‘as treated’ basis. Subgroup analysis is presented for small versus large wound subpopulations. There was no interim analysis.
The superiority hypothesis was tested in parallel for the wound closure rate and the time to wound closure within 16 weeks. Incidence of complete wound closure was analysed using Fishers’ exact test comparing the two treatment arms. Time to complete wound closure was compared between the two treatment arms using a log-rank test. The method of Bonferroni-Holm was used for adjustment of the α-error for parallel confirmatory testing of both primary endpoints. Missing values have been incorporated as censored values.
During study planning, the following concomitant diseases and therapeutic measures with a possible influence on the primary study outcome wound closure (confounders) were identified: presence of neuropathy (sensation loss according to the Perfusion, Extent, Depth, Infection and Sensation (PEDIS) classification system28); presence of diabetic neuropathic osteoarthropathy (anatomical classification according to Sanders and Frykberg29 and progression stages according to Levin30); Wagner31 grading of the ulcer; presence of peripheral arterial occlusive disease (Rutherford classification for chronic limb ischaemia32); chronic venous insufficiency (Widmer I–III33); presence of extreme foot deformities and malpositions of toes, foot or the entire limb; untreated or therapy-refractory inflammation in the wound area; chronic anaemia; heel necrosis; presence of a lymphedema; infection; heightened glycated haemoglobin level; dialysis; application of hyperbaric oxygen or normothermal therapy; application of recombinant or autologous growth factors to the study wound; and application of skin or dermal substitutes and with living cells that produce growth factors. These covariates thought to influence wound closure were analysed for their effect on the two primary endpoints. Covariates were excluded from the analysis if the number of missing values was too high. First, the relevant covariates were tested by means of a univariate analysis with regard to their effect on wound closure rate and time without consideration of the treatment arms. If there was a significant influence, the frequency of occurrence in the treatment arms was analysed. Secondary, multivariate analyses were performed for both primary endpoints, taking into account treatment assignment and including all relevant covariates. The multivariate analysis of the primary endpoint wound closure rate was performed with binary logistic regression to describe the influence of the independent covariates (regressors) on the dependent dichotomous variable wound closure. The multivariate analysis of the primary endpoint time to wound closure was performed using a ox regression model.
Safety and secondary endpoints were analysed using conventional univariate testing.
Within an a-priori-planned subgroup analysis, the ITT population was divided into a group of small wounds and a group of large wounds based on the wound surface area documented during the randomisation visit. Wounds smaller than or equal to the total median wound surface (483 mm²) were assigned to the subgroup ‘small wounds’. Patients with wound surface areas larger than the median value were assigned to the subgroup ‘large wounds’. Since no citable scientific definition of a large wound was available at the time of study planning and the clinical experts involved could not make a decision, the median of all wounds was chosen as the criterion for the division into the two subgroups. Confirmatory analysis of primary and secondary endpoints was repeated for the subgroups.
Missing values for the following outcome parameters were replaced using the last observation carried forward (LOCF) method: wound closure rate, wound size and wound tissue quality, recurrence and amputation. The outcome parameters time to wound closure and time until optimal preparation of the wound bed did not require data replacement, since missing values are included in the analysis as right-censored values. If wound closure was not confirmed to be closed after a minimum of 14 days, the wound wass considered as an unsustained wound closure. All missing QoL values (EQ5D) were replaced with the overall QoL assessment (visual analogue scale), if available. If there was no QoL assessment, there was no replacement. For missing values of the demographic and baseline characteristics, which are necessary for the estimation of the regression coefficients, no replacement was performed. IBM SPSS Statistics (V.23) was used for all analyses.
This study is registered with ClinicalTrials.gov and in the German Clinical Trial Registry.
A data monitoring committee was formed to oversee overall study performance and safety.
Role of the funding source
Through a European tender, the study was initiated by a consortium of 19 statutory German health insurance funds, which provided integrated care contracts for all study participants and for up to 7000 patients with acute and chronic wounds in Germany, defined basic rules for study design based on the requirements of the German authorities; and provided a critical review of the study protocol and the final report. The study was funded by the manufacturers KCI (Acelity) and S&N. Both companies provided the NPWT devices and associated consumable supplies in the assigned regions of Germany as well as all necessary support and information about the used material. The manufacturers had no role in study design, data collection, data analysis, data interpretation or writing of the report. All authors had full access to all of the data (including statistical reports and tables) in the study and take full responsibility for the accuracy of the data analysis.
Results
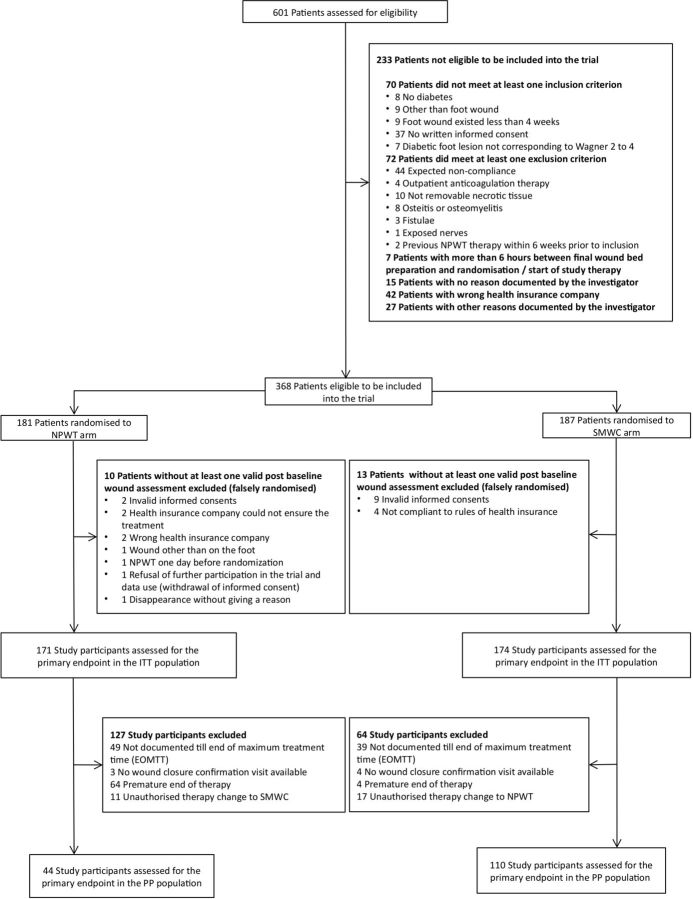
Between 23 December 2011 and 12 August 2014, 386 patients were enrolled and randomly assigned to receive NPWT (181) or SMWC (187) in the DiaFu study (figure 1) in overall 40 study sites, which recruited minimum 1 patient and maximum 76 patients. Thirteen clinical investigators randomised more than 10 patients. Twenty-three study sites enrolled only between one and four patients. Most of these study sites refused further study participation due lack of time and staff for adequately performing the documentation. In the further course of the trial research nurses were hired by the independent scientific institute overseeing the trial in order to support the documentation in the study sites whenever needed.
Figure 1.
Trial profile (CONSORT); CONSORT, Consolidated Standards of Reporting Trials; NPWT, Negative Pressure Wound Therapy; SMWC, Standard Moist Wound Care.
Demographics and relevant baseline characteristics of the DFU are presented in table 1 and online supplementary table 1. Baseline characteristics of the patients in the NPWT and the SMWC arm are similar in the ITT population without any relevant difference between the treatment arms.
Table 1.
Demographics and baseline characteristics of the ITT population
| Demographics of the study population and baseline parameters of the DFU in the ITT population |
Total n=345 (100%) |
NPWT n=171 (49.6%) |
SMWC n=174 (50.4%) |
| Male Female |
267 of 345 (77.4%) 78 of 345 (22.6%) |
133 of 171 (77.8%) 38 of 171 (22.2%) |
134 of 174 (77.0%) 40 of 174 (23.0%) |
| Age (years) (n=345), mean (SD) | 67.8 (11.9) | 67.6 (12.3) | 68.1 (11.5) |
| Height (n=340) (in cm), mean (SD) | 174.1 (12.4) | 173.4 (14.6) | 174.8 (9.9) |
| Weight (n=335) (in kg), mean (SD) | 93.3 (22) | 92.7 (21.5) | 93.8 (22.6) |
| Localisation of the ulcer | |||
| Regio calcanea Dorsum pedis Planta pedis Metatarsalia Phalanges distales Phalanges mediales Phalanges proximales Hallux Digitus pedis II Digitus pedis III Digitus pedis IV Digitus minimus |
39 (11.3%) 20 (5.8%) 56 (16.2%) 147 (42.6%) 64 (18.6%) 28 (8.1%) 40 (11.6%) 42 (12.2%) 22 (6.4%) 14 (4.1%) 20 (5.8%) 25 (7.2%) |
17 (9.9%) 13 (7.6%) 30 (17.5%) 73 (42.7%) 31 (18.1%) 14 (8.2%) 21 (12.3%) 24 (14%) 10 (5.8%) 7 (4.1%) 7 (4.1%) 12 (7.0%) |
22 (12.6%) 7 (4.0%) 26 (14.9%) 74 (42.5%) 33 (19%) 14 (8.0%) 19 (10.9%) 18 (10.3%) 12 (6.9%) 7 (4.0%) 13 (7.5%) 13 (7.5%) |
| Type of ulcer | |||
| Primary ulcer Recurrence |
279 of 342 (80.9%) 63 of 342 (18.3%) |
136 of 170 (79.5%) 34 of 170 (19.9%) |
143 of 172 (82.2%) 29 of 172 (16.7%) |
| Duration of ulcer (days) | |||
| n Mean (SD) Median (IQR) Min – Max |
335 189.7 (360.2) 83 (136) 0–4468 |
168 217.1 (458.1) 81 (140) 0–4468 |
167 162.1 (220) 85 (132) 0–1826 |
| Wound surface area at randomisation (mm2) | |||
| Mean (SD) Median (IQR) Min-Max |
1101 (2543) 491 (1079) 12–40 773 |
1060 (1536) 550 (1217) 20–13 188 |
1141 (3247) 471 (1007) 12–40 773 |
| Wound surface area at randomisation for small wounds (mm2) | |||
| n Mean (SD) Median (IQR) Min-Max |
173 213 (136) 188 (220) 12–484 |
83 212 (138) 176 (220) 20–484 |
90 213 (135) 196 (222) 12–471 |
| Wound surface area at randomisation for large wounds (mm2) | |||
| n Mean (SD) Median (IQR) Min-Max |
172 1995 (3377) 1276 (1482) 491–40773 |
88 1860 (1805) 1364 (1242) 520–13188 |
84 2135 (4474) 1242 (1708) 491–40773 |
Data are number (n) and percentage (%), mean and standard deviation (SD), median and interquartile range (IQR), and minimum – maximum (min – max). ‘n=’ is stating the number of patients with actual available information. Based on the median wound surface area of all included patients, the wounds were divided into an a priori planned subgroup of large (median wound surface area ≤484 mm² and a subgroup of small wounds (median wound surface area >484 mm²).
DFU, diabetic foot ulcer; ITT, intention to treat; NPWT, negative pressure wound therapy; SMWC, standard moist wound care.
bmjopen-2018-026345supp001.pdf (177KB, pdf)
The baseline of the identified factors possibly influencing wound closure is shown in table 2.
Table 2.
Baseline of the identified factors possibly influencing wound closure in the ITT population
| Confounders at baseline in the ITT population |
Total n=345 |
NPWT n=171 |
SMWC n=174 |
| Presence of neuropathy (sensation loss according to the PEDIS classification system) | 250 of 334 (72.5%) | 125 of 166 (73.1%) | 125 of 168 (71.8%) |
| Presence of a diabetic neuropathic osteoarthropathy | 61 (17.7%) | 30 (17.5%) | 31 (17.8%) |
| Wagner grading of the ulcer 1: superficial ulcer of skin or subcutaneous tissue 2: ulcers extend into tendon, bone, or capsule 3: deep ulcer with osteomyelitis, or abscess 4: gangrene of toes or forefoot 5: midfoot or hindfoot gangrene |
6 (1.7%) 225 (65.2%) 85 (24.6%) 26 (7.5%) 3 (0.9%) |
2 (1.2%) 110 (64.3%) 45 (26.3%) 13 (7.6%) 1 (0.6%) |
4 (2.3%) 115 (66.1%) 40 (23%) 13 (7.5%) 2 (1.1%) |
| Peripheral arterial occlusive disease (PAOD) PAOD with critical limb ischaemia* |
244 of 345 (70.7%) 26 of 243 (10.7%) |
121 of 171 (70.8%) 15 of 121 (12.4%) |
123 of 174 (70.7%) 11 of 122 (9.0%) |
| No chronic venous insufficiency (CVI) CVI Widmer I CVI Widmer II CVI Widmer III |
259 of 302 (75.1%) 25 of 302 (7.2%) 12 of 302 (3.5%) 6 of 302 (1.7%) |
132 of 150 (77.2%) 11 of 150 (6.4%) 3 of 150 (1.8%) 4 of 150 (2.3%) |
127 of 152 (73.0%) 14 of 152 (8.0%) 9 of 152 (5.2%) 2 of 152 (1.1%) |
| Presence of extreme foot deformities and malpositions of toes, foot or the entire limb | 59 of 342 (17.1%) | 26 of 170 (15.2%) | 33 of 172 (19.0%) |
| Untreated or therapy-refractory inflammation in the wound area | 15 of 343 (4.3%) | 7 of 170 (4.1%) | 8 of 173 (4.6%) |
| Presence of a heel necrosis | 23 of 342 (6.7%) | 10 of 168 (5.8%) | 13 of 174 (7.5%) |
| No lymphoedema Primary lymphoedema Secondary lymphoedema |
282 of 340 (81.7%) 12 of 340 (3.5%) 46 of 340 (13.3%) |
139 of 167 (81.3%) 5 of 167 (2.9%) 23 of 167 (13.5%) |
143 of 173 (82.2%) 7 of 173 (4.0%) 23 of 173 (13.2%) |
| Clinical signs of inflammation (suspected infection) | 159 of 344 (46.1%) | 83 of 170 (48.5%) | 76 of 174 (43.7%) |
| Local wound swab as part of the clinical routine | 248 of 343 (71.9%) | 126 of 170 (73.7%) | 122 of 173 (70.1%) |
| Detection of germs within the local wound swab | 205 of 247 (59.4%) | 104 of 125 (60.8%) | 101 of 122 (58.0%) |
| Haemoglobin n Mean (SD) |
177 of 345 9.5 (3.2) |
86 of 171 9.6 (3.1) |
91 of 174 9.4 (3.3) |
| Haemoglobin A1c n Mean (SD) |
32 of 345 15.6 (18.3) |
13 of 171 16.8 (16.7) |
19 of 174 14.7 (19.6) |
| Requiring dialysis | 29 of 343 (8.4%) | 15 of 170 (8.8%) | 14 of 173 (8.0%) |
| Application of skin or dermal substitutes and with living cells that produce growth factors | 0 of 341 (0%) | 0 of 169 (0%) | 0 of 172 (0%) |
Findings, diagnoses and procedures documented by the investigators are presented. Data are number (N), percentage (%), mean and standard deviation (SD), and minimum – maximum (min – max). *Critical limb ischemia was defined as persistant pain at rest with regular analgesia for a period of two weeks while nerve function is maintained or the occurence of ulceration or gangrene of the foot or toes with a systolic blood pressure of the ankle below 50 mmHg or a systolic toe pressure below 30 mmHg or tcPO2<20 mmHg.
ITT, intention to treat; NPWT, negative pressure wound therapy; SMWC, standard moist wound care; tcPO2, transcutaneous oxygen measurement.
Details on revascularisation performed before study start are shown in table 3.
Table 3.
Revascularisations performed in the ITT population before study start
| Revascularisation before study start in the ITT population | Total n=345 |
NPWT n=171 |
SMWC n=174 |
| Performed revascularisation before study start Percutaneous transluminal angioplasty (PTA) PTA+stent Veins-bypass Polytetrafluoroethylene bypass Thromboendarterectomy and patch plastic |
23 of 345 (6.7%) 13 of 23 (57.0%) 1 of 23 (4.0%) 5 of 23 (22.0%) 1 of 23 (4.0%) 2 of 23 (9.0%) |
9 of 171 (5.3%) 6 of 9 (67.0%) 0 of 9 (0%) 2 of 9 (22.0%) 0 of 9 (0%) 0 of 9 (0%) |
14 of 174 (8.0%) 7 of 9 (50.0%) 1 of 9 (7.0%) 3 of 9 (21.0%) 1 of 9 (7.0%) 2 of 9 (14.0%) |
| Revascularisation with influence on the wound | 22 of 23 (96.0%) | 9 of 9 (100%) | 13 of 14 (93.9.0%) |
| Sufficient revascularisation result* Insufficient revascularisation result Revascularisation result not assessable |
20 of 23 (88.0%) 2 of 23 (9.0%) 1 of 23 (4.0%) |
7 of 9 (78.0%) 1 of 9 (11.0%) 1 of 9 (11.0%) |
13 of 14 (93.0%) 1 of 14 (7.0%) 0 of 14 (0%) |
Data are n and percentage (%).
*Sufficient revascularisation result was defined as successful recanalisation of the tibial artery in which the foot lesion was located or, if it was technically impossible to recanalise the respective artery, achievement of an unhindered inflow into at least one of the tibial vessels. The evaluation of the revascularisation result was in the discretion of the attending physician.
ITT, intention to treat; NPWT, negative pressure wound therapy; SMWC, standard moist wound care.
Results for the primary outcome wound closure in the ITT population
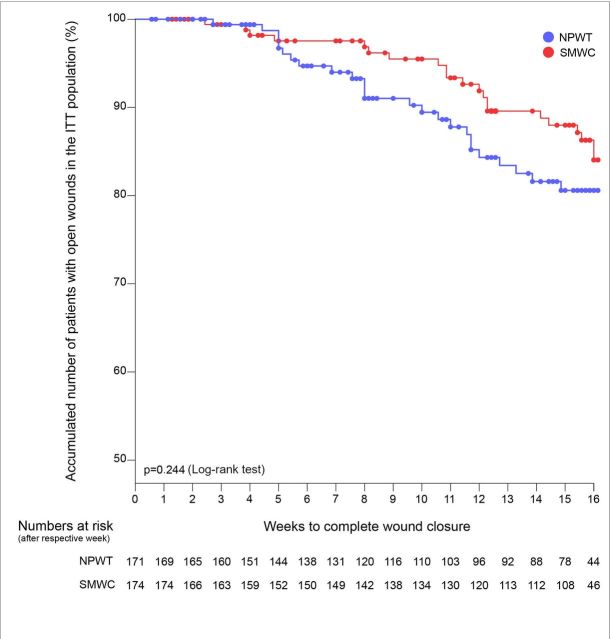
In the ITT population, there was no significant difference between the treatment arms for either wound closure rate (table 4) or time to complete wound closure (p=0.244, log-rank test; figure 2) within 16 weeks. Beginning in week 5, the number of study participants with open wounds in the NPWT arm was lower than in the SMWC arm (figure 2). However, after 16 weeks, the difference between the treatment arms was only 2.5% (95% CI −4.7% – 9.7%) (table 4). Wounds treated with NPWT were approximately at the same risk of remaining open as wounds treated with SMWC (RR 0.97 (95% CI 0.89−1.06)).
Table 4.
Study participants with wound closure (wound closure rate) and the number of participants with recurrences (recurrence rate) in the ITT population
| Wound closure and recurrence rate in the ITT population | Total n=345 |
NPWT n=171 |
SMWC n=174 |
Difference n % (95%CI) p* |
| Patients with complete, sustained and confirmed wound closure within 16 weeks | ||||
| n % (95% CI) |
46 of 345 13.3 (9.8–17.8) |
25 of 171 14.6 (9.5–21.6) |
21 of 174 12.1 (7.5–18.4) |
4 2.5 (−4.7 – 9.7) 0.53 |
| Patients with recurrence of the diabetic foot wound after complete, sustained and confirmed closure within 6 months | ||||
| n % (95% CI) |
1 of 46 2.2 (0.1–12.1) |
1 of 25 4 (0.1–22.3) |
0 of 21 0 (0.0–14.3) |
1 4 (−3.7 – 11.7) 1.00 |
Data show the number (N) of participants available for the analysis in total and for both treatment arms. Wound closures within the maximum study treatment time of 16 weeks and recurrences during the follow-up of 6 months are shown with the number (N), the percentage (%) of patients and the 95% Confidence Interval (CI).
*F=Fishers’ exact test.
ITT, intention to treat; NPWT, negative pressure wound therapy; SMWC, standard moist wound care.
Figure 2.
Time until complete, sustained and verified wound closure in the ITT population. NPWT, negative pressure wound therapy; SMWC, standard moist wound care.
Since the cumulative number of patients with open wounds was more than 70% after 16 weeks, we could not calculate medians for the time to wound closure.
Results for the secondary outcomes in the ITT population
Only one recurrence of the foot wound after complete, sustained and confirmed closure was documented for one study participant in the NPWT arm (table 4). Study participants treated with NPWT were at slightly higher risk for a recurrence than participants treated with SMWC 0.96 (95% CI 0.87−1.04).
After 6 months, the number of study participants with closed wounds was higher in the SMWC arm than in the NPWT arm (36 of 174 (20.7 %) vs 24 of 171 (14.0 %)), but the difference was not significant (p=1.00).
The time until optimal preparation of the wound for further treatment to achieve a complete epithelialisation (min 95% granulation tissue) was significantly shorter for patients treated with NPWT (p=0.008) (table 5).
Table 5.
Time until optimal preparation of the wound for further treatment (min 95% granulation tissue) in the ITT population
| Time until optimal preparation of the wound bed (min 95% granulation tissue) within 16 weeks in the ITT population Navailable values |
Total n=183 |
NPWT n=100 |
SMWC n=83 |
Mean difference (95% CI) p* |
| Mean (SD) | 42.7 (39.0) | 35.6 (34.6) | 51.4 (42.6) | 15.8 (4.6−27.0) 0.008 |
| Median (IQR) | 31 (64) | 22.0 (48.0) | 49.0 (53.6) | |
| Min–Max | 0–127 | 0–127 | 0–115 |
Data show the number (N) of participants available for the analysis in total and for both treatment arms. Time until optimal preparation of the wound is described with mean and standard deviation (SD); median and interquartile range (IQR); and minimum (min) and maximum (max).
*Student’s t-test.
ITT, intention to treat; NPWT, negative pressure wound therapy; SMWC, standard moist wound care.
In the ITT population, wound surface area and wound volume were similar at baseline (table 1) and decreased continuously during the study treatment time of 16 weeks in both treatment arms (online supplementary tables 2 and 3). The values are largely scattered. Measurements derived from the blinded photo analysis using the W.H.A.T. were smaller than the values documented by the clinical investigators.
Wound tissue composition (online supplementary table 4) was similar in both treatment arms at baseline. Granulation tissue values increased during the study treatment period of 16 weeks and fibrin values decreased, with clinically documented values showing only minor differences between treatment arms. The values for necrotic tissue were very low and did not differ relevantly between the treatment arms. The results of the W.H.A.T. evaluation for granulation and fibrin deviate markedly from the values documented by the clinical investigators.
Patients treated with NPWT were approximately at the same risk of undergoing an amputation or resection like patients treated with SMWC (RR 0.99 (95% CI 0.65−1.50)) (table 6).
Table 6.
Study participants with amputations/resections and the number of amputations/resections performed in the ITT population
| Amputations and resections in the ITT population |
Total n=345 |
NPWT n=171 |
SMWC n=174 |
Difference (95% CI) p |
| Study participants with amputation or resection | ||||
| n (%) (95% CI) |
71 (20.6%) (16.3−24.8) |
35 (20.5%) (14.4 to 26.5) |
36 (20.7%) (14.7−26.7) |
1 (0.2%) (−19.0 − 18.6) 1.00 (F) |
| Total number of amputations and resections | 102 | 45 | 57 | 12 0.89 (U) |
| Number of amputations and resections per study participant, n (%) | ||||
| One event Two events Three events Four events Five events |
49 (14.2%) 16 (4.6%) 4 (1.2%) 1 (0.3%) 1 (0.3%) |
25 (14.6% 10 (5.8%) 0 (0% 0 (0%) 0 (0%) |
24 (13.8%) 6 (3.4%) 4 (2.3%) 1 (0.6%) 1 (0.6%) |
1 (0.8%) 4 (2.4%) 4 (2.3%) 1 (0.6%) 1 (0.6%) |
| Study participants with minor amputation | 69 (20.0%) | 33 (19.3%) | 36 (20.7%) | 3 (1.4%) 0.79 (F) |
| Study participants with major amputation | 2 (0.6%) | 2 (1.2%) | 0 (0%) | 2 (1.2%) 0.25 (F) |
Data show the number (N) of participants, the percentage with the 95% CI, or the number of events accompanied with the respective percentage values in total and for both treatment arms.
F, Fishers' exact test; ITT, intention to treat; NPWT, negative pressure wound therapy; SMWC, standard moist wound care; U, Mann-Whitney U test.
Overall, pain levels were very low and decreased further during the study treatment time (online supplementary table 5). The values hardly differ between the treatment arms at any observation time point.
At baseline, QoL (EQ5D) was significantly limited in both treatment arms (online supplementary table 6). EQ5D levels were improved in both study participants reaching end of therapy as well as EOMT. On follow-up after 6 months, all patients still showed increased EQ5D levels in both treatment arms.
Safety results
The number of study participants with AEs was significantly higher in the NPWT arm (96 (56.1%)) than in the SMWC arm (72 (41.4%)) (p=0.007) but only 16 (10.2%) of the AEs in the NPWT arm were decided by the investigators to have a definite relation to the medical device (table 7). The number of study participants with at least one AE documented to be serious (SAE) was not significantly different between the treatment arms (NPWT n=63 (36.8%); SMWC n=58 (33.3%); p=0.50) (table 7). None of the SAEs in the NWPT arm was documented as definitely or possibly related to the medical device by the investigators. Nine of 171 (5.3%) study participants in the NPWT arm and 6 of 174 (3.5%) study participants in the SMWC arm died during the study.
Table 7.
Study participants with adverse events (AEs) and serious adverse events (SAEs) and the number of AEs and SAEs in the ITT population
| AEs and SAEs in the ITT population |
Total n=345 |
NPWT n=171 |
SMWC n=174 |
Difference (95% CI) p |
| Study participants with at least one AE | ||||
| n (%) (95% CI) |
168 (48.7%) (43.4−54.0) |
96 (56.1%) (48.7−63.6) |
72 (41.4%) (34.1−48.7) |
24 (14.7%) (4.3−25.1) p=0.007 (F) |
| Study participants with one AE (n) Study participants with two or more AEs (n) |
103 65 |
54 42 |
49 23 |
5 19 |
| Total number of AEs (n) | 269 | 167 | 102 | 65 |
| AEs with relationship to the medical device | ||||
| navailable
Yes, n (%) Possible, n (%) No, n (%) Not assessable, n (%) |
257 16 (6.2%) 13 (5.1%) 211 (82.1%) 17 (6.6%) |
157 16 (10. 2%) 11 (7.0%) 117 (74.5%) 13 (8.3%) |
100 0 (0%) 2 (2.0%)* 94 (94.0%) 4 (4.0%) |
57 16 (10.2%) 9 (5%) 23 (19.5%) 9 (4.3%) |
| AEs with relationship to SMWC | ||||
| navailable
Yes, n (%) Possible, n (%) No, n (%) Not assessable, n (%) |
185 2 (1.1%) 5 (2.7%) 163 (88.1%) 15 (8.1%) |
110 0 (0%) 5 (4.5%) 96 (87.3%) 9 (8.2%) |
75 2 (2.7%) 0 (0%) 67 (89.3%) 6 (8.0%) |
35 2 (2.7%) 5 (4.5%) 29 (2.0%) 3 (0.2%) |
| AEs with relationship to the treatment procedure | ||||
| navailable
Yes, n (%) Possible, n (%) No, n (%) Not assessable, n (%) |
244 10 (4.1%) 17 (7.0%) 191 (78.3%) 26 (10.7%) |
148 6 (4.1%) 15 (10.1%) 111 (75.0%) 16 (10.8%) |
96 4 (4.2%) 2 (2.1%) 80 (83.3%) 10 (10.4%) |
52 2 (0.1%) 13 (8%) 31 (8.3%) 6 (0.4%) |
| Study participants with at least one SAE | ||||
| n (%) (95% CI) |
121 (35.1%) (30.0−40.1) |
63 (36.8%) (29.6−44.1) |
58 (33.3%) (26.3−40.3) |
5 (3.5%) (−6.6 − 13.6) p=0.50 (F) |
| Study participants with one SAE (n) Study participants with two or more SAEs (n) |
90 31 |
45 18 |
45 13 |
0 5 |
| Total number of SAEs (n) | 163 | 87 | 76 | 11 |
| SAEs with relationship to the medical device | ||||
| navailable
Yes, n (%) Possible, n (%) No, n (%) Not assessable, n (%) |
161 0 (0%) 0 (0%) 154 (95.7%) 7 (4.3%) |
85 0 (0%) 0 (0%) 79 (92.9%) 6 (7.1%) |
76 0 (0%) 0 (0%) 75 (98.7%) 1 (1.3%) |
9 0 (0%) 0 (0%) 4 (5.8%) 5 (5.8%) |
| SAEs with relationship to SMWC | ||||
| n available
Yes, n (%) Possible No, n (%) Not assessable, n (%) |
121 1 (0.8%) 1 (0.8%) 113 (93.4%) 6 (5.0%) |
64 0 (0%) 1 (1.6%) 57 (89.1%) 6 (9.4%) |
57 1 (1.8%) 0 (0%) 56 (98.2%) 0 (0%) |
7 1 (1.8%) 1 (1.6%) 1 (9.1%) 6 (9.4%) |
| SAEs with relationship to the treatment procedure | ||||
| navailable
Yes, n (%) Possible No, n (%) Not assessable, n (%) |
156 4 (2.6%) 2 (1.3%) 140 (89.7%) 10 (6.4%) |
84 0 (0%) 2 (2.4%) 74 (88.1%) 8 (9.5%) |
72 4 (5.6%) 0 (0%) 66 (91.7%) 2 (2.8%) |
12 4 (5.6%) 2 (2.4%) 8 (10.6%) 6 (6.7%) |
Data show the number (n) and the percentage (%) in total and for both treatment arms.
*No treatment change to NPWT has been documented. F=Fisher’s exact test (alpha=0.05).
ITT, intention to treat; NPWT, negative pressure wound therapy; SMWC, standard moist wound care.
Secondary analyses and subgroups
Of the factors possibly influencing the outcomes identified during study planning, the covariate POAD was found to have significant influence on the endpoint time until wound closure (p=0.026, log rank test). The covariate clinical signs of inflammation (suspected infection) had a significant influence on the wound closure rate (p=0.012, χ2 test) in the univariate analysis of the primary endpoints. However, both covariates were almost equally represented in both treatment arms. Thus, the comparison of the treatment arms was not influenced by these confounders. Furthermore, the covariate suspected infection was found to be significantly associated with both wound closure rate (logistic regression; p=0.027) and time until wound closure (Cox regression; p=0.037) in the multivariate confounder analysis. Wound closure was significantly less likely in wounds with suspected infection (OR 0.38).
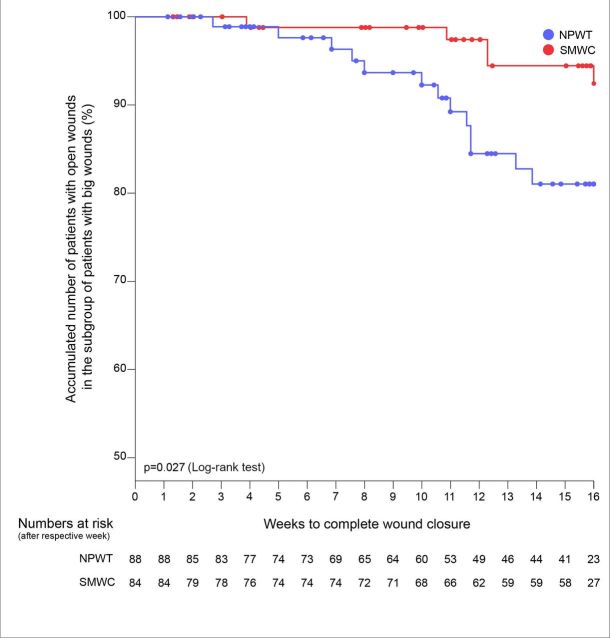
In the subgroup of large wounds (wound surface area at randomisation shown in table 1), wound closure rate within 16 weeks was significantly higher in the NPWT arm (13 of 88 (14.8 (7.4 to 22.2)%)) than in the SMWC arm (5 of 84 (6.0 (0.9 - 11.0)%)) (difference: n=8 (8.8 (−0.2 to 17.8)%), p=0.08). Study participants with large wounds had a lower risk of not achieving wound closure within 16 weeks when treated with NPWT (RR 0.91 (95% CI 0.82−1.0)) and achieved wound closure significantly faster in the NPWT arm than in the SMWC arm (p=0.027) (figure 3). The only recurrence occurred in the subgroup of large wounds. Both major amputations were performed in study participants with large wounds treated with NPWT.
Figure 3.
Time until complete, sustained and verified wound closure for the subgroup of large wounds. NPWT, negative pressure wound therapy; SMWC, standard moist wound care.
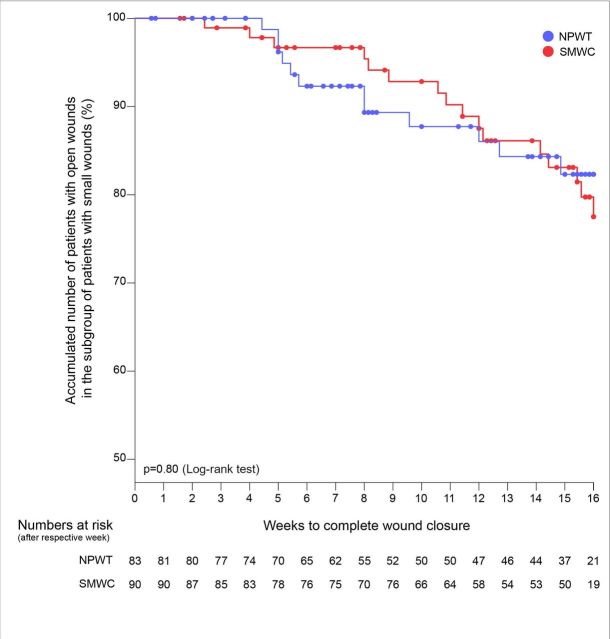
In the subgroup of small wounds (wound surface area at randomisation shown in table 1), the time to reach 95% granulation tissue was significantly shorter for the patients treated with NPWT than for those treated with SMWC (p=0.005), but wound closure rate and time until wound closure within 16 weeks were not significantly different between the treatment arms (figure 4). Further details of the subgroup analyses are presented in the online supplementary tables 7 and 8.
Figure 4.
Time until complete, sustained and verified wound closure for the subgroup of small wounds. NPWT, negative pressure wound therapy; SMWC, standard moist wound care.
Results for the primary and secondary outcomes in the PP population
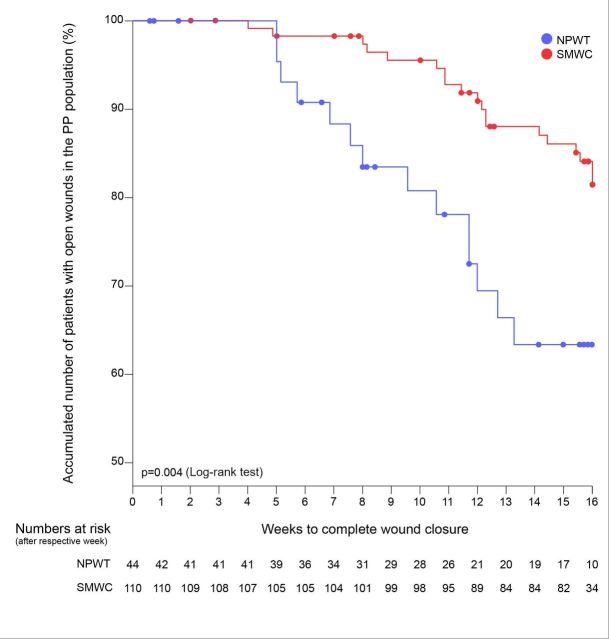
Demographics, relevant baseline characteristics and the results of the revascularisation before study start of the PP population are presented in online supplementary table 9. In the PP population, 14 of 44 study participants (31.8% (95% CI 18.1%−45.6%)) treated with NPWT and 19 of 110 participants (17.3% (95% CI 10.2%−24.3%)) treated with SMWC achieved complete, sustained and verified wound closure within 16 weeks, but the difference was not significant (5 (14.5% (95% CI −1.0% − 30.0%); p=0.053). Wounds treated with NPWT had a lower risk of remaining open after 16 weeks (RR 0.82 (95% CI 0.66−1.03)) than wounds treated with SMWC. Time to wound closure in the NPWT arm was significantly shorter than in the SMWC arm (p=0.004) (figure 5). After 6 months, wound closure rate in the SMWC arm (30 of 110 (27.3% (95% CI 18.9%−35.6%))) was higher than in the NPWT arm (11 of 44 (25.0% (95% CI 12.2−37.8))), but the difference was not significant (n=19 (2.3% (95% CI −13.0 − 17.6)); p=0.84). As in the ITT population, optimal wound bed preparation was achieved significantly faster in study participantss receiving NPWT (p<0.001). No recurrences occurred after complete, sustained and confirmed wound closure in the PP population. Neither the number of participants with amputations or resections nor the number of amputations or resections performed differed significantly between the treatment arms. No major amputations were performed in the PP population. Further details on the results for the PP population are presented in the online supplementary tables 10–16.
Figure 5.
Time until complete, sustained and verified wound closure in the PP population; NPWT, negative pressure wound therapy; SMWC, standard moist wound care.
Treatment compliance
Twenty-nine (17.0%) participants in the NPWT arm had a temporary therapy change to SMWC (mean duration 20.5±21.6 days). In the SMWC arm, 17 (9.8%) participants had a temporary therapy change to NPWT (mean duration 28.9±21.6 days). For only 2 of the 29 NPWT participants (6.9%) with a temporary therapy change to SMWC, the wound closure was achieved within 16 weeks, whereas 16.2% (23 von 142) of the wounds of the NPWT participants without therapy change were completely closed.
A total of 57.3% (98 of 171) of the participants randomised to NPWT completed treatment before achieving a granulation surface of the wound of at least 95%. Fewer participants with this premature end of NPWT (4.7%, n=8) achieved a complete wound closure than participantss with no premature end of therapy (9.9, n=17). Mean NPWT duration until premature end of therapy was 28.5 days (SD 24.1), while a mean granulation area of 59.6% (SD 30. 5) was achieved. For 131 participants (76. 6 %) in the NPWT arm less than the required three dressing changes per week were documented. Nineteen participants (14. 5 %) with this protocol violation achieved a complete wound closure. Six (15.4%) of the 39 NPWT participants who received at least three therapy changes per week achieved a complete wound closure.
Documentation quality
In the NPWT arm, 52 study participants and in the SMWC arm 43 participants were excluded from the PP population due to missing documentation until the EOMT or at wound closure confirmation (figure 1). In the eCRF, wound closure was documented for 96 patients (NPWT 56 of 171; SMWC 40 of 174), but only 46 participants (NPWT 25; SMWC 21) met all criteria for a complete, verified and sustained wound closure. For the wound closure visit, seven wound photographs (NPWT 7; SMWC 0) and for the wound closure confirmation visit four photographs (NPWT 3; SMWC 1) were missing. In addition, two of the existing wound photographs for wound closure (NPWT 0; SMWC 2) and two photographs for wound closure confirmation (NPWT 1; SMWC 3) were not assessable by the blinded observers due to serious quality issues. Furthermore, 23 (NPWT 15; SMWC 8) existing and assessable wound photographs were not able to confirm wound closure and 3 (NPWT 1; SMWC 2) photographs were not able to confirm wound closure after 14 days.
Discussion
The DiaFu study did not demonstrate significant superiority in wound closure rate or time to complete wound closure for neither NPWT nor SMWC. Wound closure rates were higher in the NPWT arm but did not significantly differ from those in the SMWC arm. Time to wound healing in the NPWT arm was lower than in the SMWC arm, while the difference between the treatment arms becomes statistically significant only in the PP population. Thus, with this study, we were not able to confirm our hypothesis that wound closure can be achieved more often and faster with NPWT than with SMWC when used in German real-life clinical practice. Previous RCTs, which were the basis for sample size calculation, showed a higher rate and a significant superiority in healing when using NPWT on amputation and chronic wounds,16 17 but the populations of these studies were different. Other than the Armstrong study, the DiaFu study did not exclude patients with venous insufficiency and included more than twice as many patients. The studies of Armstrong and Blume excluded patients with Wagner stage 4; active Charcot; uncontrolled hyperglycemia and therapy with glucocorticoids, immunosuppressants or chemotherapy; and required proof of adequate perfusion. The DiaFu study did not exclude patients with impaired perfusion but required adequate therapy of the circulatory disorder according to clinical practice guidelines. In the DiaFu study, we were able to show that the presence of PAOD at randomisation had a significant influence on the time to wound closure but not on the overall wound closure rate within the maximum study treatment time. The number of patients with critical limb ischaemia at baseline was low and differed only slightly between the treatment arms. As in clinical practice, in the DiaFu study, adequate treatment of concomitant diseases was mandatory. Invasive therapy of PAOD could be performed before initiation of wound therapy as well as during the study treatment period, if the wound needed pretreatment as a basis for the revascularisation procedure or if new or recurrent critical ischaemia occured.
The presence of clinical signs of inflammation (suspected infection) at randomisation had a significant effect on both, time to wound closure and wound closure rate within 16 weeks. Both covariates were equally represented in the treatment arms, thus the differences in time until wound closure and wound closure rate were not affected by these confounders.
However, the probably most serious factors negatively influencing treatment and outcome are documentation deficiencies and deviations from treatment guidelines. Temporary therapy changes and premature therapy cessation negatively impacted the patient relevant treatment outcome wound closure in study participants treated with NPWT. Missing study visits resulting in low numbers of complete endpoint documentations strongly affected the proof of the outcome wound closure in both, the NPWT arm and the SMWC arm.
Optimal preparation of the wound bed (95% granulation tissue) was achieved significantly earlier when using NPWT in the ITT and the PP population, but the overall rate of wound closures was low. Wound bed preparation and granulation tissue formation are important prerequisites for wound healing but are not a proof of treatment effectiveness and cannot serve as a basis for benefit assessment.
Although significantly more AEs were documented in the NPWT arm, only a small number of these events were related to the medical device according to the investigator’ s assessment. Mortality rates were very low in both treatment arms, and there was no significant difference between the treatment arms regarding amputations and resections performed during the study. Only two major amputations have been performed in patients with large wounds treated with NPWT. None of the treatments resulted in an additional impairment of the patients’ QoL during study treatment time or follow-up. Time until complete wound closure was significantly shorter with NPWT than with SMWC in the subgroup of large wounds, which indicates that NPWT has the potential to be a valuable treatment option for this kind of wounds.
In the DiaFu study, methods against bias were implemented whenever possible in order to avoid bias that have been described by several systematic reviews,18–21 but blinding of study participants as well as attending physicians and nurses was not possible due to the nature of NPWT.
Not addressing and analysing all factors influencing the overall treatment outcome like targeted pressure relief, continuous infection control and adequate treatment of the underlying disease during the study treatment and observation period may be seen as a limitation of this healthcare research study. Study sites have been selected based on a self-disclosure by means of a qualification checklist and cross checks using quality reports. This ensured that all prerequisites were met for guideline-compliant patient care. Nevertheless, even in the application of NPWT, there were deviations from the standards.
In order to support the decision-making process of the German G-BA on general reimbursement of NPWT in German outpatient care, the real-life clinical practice DiaFu study included patients with chronic DFUs of neuropathic and angiopathic origin regardless of whether a simple wound cleansing, tissue debridement or even amputation was necessary prior to application of wound therapy targeted to achieve complete wound closure. The study was performed without excluding concomitant diseases negatively impacting wound healing; with therapy application in the discretion of the attending physician; and with evaluation of patient relevant outcome. Thus, results can easy be generalised and applied in clinical practice settings. Anyway, shortcomings in data quality negatively impacted the study results, and statements about specific patient groups were not possible. A high number of study participants needed to be excluded from the PP population (NPWT arm: 127 of 171 (74%), SMWC arm 64 of 174 (37%)). For most of these participants, documentation was lacking until the end of the maximum treatment period (total=88, NPWT=49, SMWC=39) (figure 1). In the primary analysis based on the ITT population, it was assumed that these patients did not achieve wound closure within 16 weeks’ study treatment and observation time (using the LOCF method, the open wound status was ‘carried forward’ until the end of the maximum treatment period. This may have led to a false negative bias in the outcome wound closure in the ITT population. Due to the high loss of patients and the difference in the number of participants excluded from the treatment arms, the validity of the PP analysis is very limited.
Conclusions
NPWT was not superior to SMWC when evaluated in German real-life clinical practice. Missing compliance with therapy guidelines and poor documentation quality led to restrictions in achieving the patient-relevant endpoint complete wound closure and prevents a clear proof of effectiveness. The question if NPWT is superior to SMWC for treating diabetic foot wounds remains unanswered due to the limitations of the DiaFu study. Although the study protocol required adequate monitoring and therapy of the concomitant diseases, the presence of POAD and infection at randomisation had a significant influence on the outcome wound closure. Despite all limitations, NPWT showed a significant superiority in optimal wound bed preparation. This indicates that NPWT works according to its intended use and has a potential to be an effective treatment option. The results of the PP population suggest that without the negative impact of premature treatment cessation, temporary changes of the randomised therapy and partly incomplete documentation NPWT may be more effective for treating diabetic foot wounds than SMWC. In Germany, NPWT should be evaluated again after implementation of a sufficient, well-considered and widely accepted concept for quality control. In a future healthcare research study, the treatment outcome before and after the implementation of these quality measures should be evaluated, for which the results of this trial may serve as a basis.
Supplementary Material
Acknowledgments
The authors thank all investigators, nurses, patients and partners for supporting the study. At least one patient was included in the following facilities: HSK - Dr. Horst Schmidt Kliniken GmbH Klinik für Gefäßchirurgie Ludwig-Erhard-Straße 100 65199 Wiesbaden; Asklepios Westklinikum Hamburg Zentrum für Gefäßmedizin Suurheid 20 22559 Hamburg; Knappschaftskrankenhaus Bottrop Gefäßchirurgische Klinik Osterfelderstraße 157 46242 Bottrop; Städtisches Klinikum Karlsruhe Klinik für Gefäß- und Thoraxchirurgie Moltkestraße 90 76133 Karlsruhe; Gemeinschaftspraxis Schlotmann-Hochlenert-Zavaleta-Haberstock Merheimer Straße 217 50733 Köln; Klinikum Döbeln Abt. für Gefäßchirurgie Sörmitzer Straße 10 04720 Döbeln; Klinikum Bielefeld Mitte Klinik für Allgemeine Innere Medizin Teutoburger Straße 50 33604 Bielefeld; Klinikum Frankfurt/Oder Klinik für Gefäßchirurgie Müllroser Chaussee 7 15236 Frankfurt/Oder; Weißeritztal-Kliniken GmbH Medizinische Klinik III Bürgerstraße 7 01705 Freital; Krankenhaus Porz am Rhein Klinik für Gefäßchirurgie Urbacher Weg 19 51149 Köln; St. Remigius Krankenhaus Opladen Innere Medizin An St. Remigius 26 51379 Leverkusen; Marien Hospital Dortmund-Hombruch Klinik für Innere Medizin/Diabetologie Gablonzstraße 9 44225 Dortmund; Zentrum für Chirurgie Klinik für Gefäß- und Endovascularchirurgie Theodor-Stern-Kai 7, Haus 23C/EG 60590 Frankfurt am Main; Facharzt für Chirurgie Thorax-Kardiovaskularchirurgie Hindenburgstraße 1 86807 Buchloe; Helfenstein Klinik Geisslingen Allgemein- und Viszeralchirurgie Eybstraße 16 73312 Geislingen/Steige; Paracelsus-Klinik am Silbersee Wundzentrum Hannover Oertzeweg 24 30851 Langenhagen; Klinikum Darmstadt Chirurgische Klinik III Grafenstraße 9 64283 Darmstadt; Ortenau Klinikum Offenburg-Ebertplatz Klinik für Allgemein-, Viszeral- und Gefäßchirurgie Ebertplatz 12 77654 Offenburg; Thüringen-Kliniken "Georgius Agricola" GmbH Klinik für Gefäßchirurgie Rainweg 68 07318 Saalfeld; Klinikum Dorothea Christiane Erxleben GmbH Klinik für Allgemein-, Viszeral- und Gefäßchirurgie Ditfurter Weg 24 06484 Quedlinburg; Franziskus-Krankenhaus Berlin Abt. für Innere Medizin Budapester Straße 15-19 10787 Berlin; Hegau-Bodensee Klinikum Radolfzell (HBK) Klinik für Innere Medizin Hausherrenstraße 12 78315 Radolfzell; Diabetologische Schwerpunktpraxis Dr. med. Hansjörg Mühlen & Partner Ruhrorter Straße 195 47119 Duisburg; Kliniken Maria Hilf Mönchengladbach Klinik für Gefäßchirurgie und Angiologie Sandradstraße 43 41061 Mönchengladbach; Städtisches Klinikum München/Bogenhausen Klinik für Endokrinologie, Diabetologie und Angiologie Englschalkingerstraße 77 81925 München; Gerhard Rothenaicher Facharzt für Chirurgie Cosimastraße 2 81927 München; Bürgerhospital Frankfurt am Main Interdisziplinäres Zentrum Diabetischer Fuß (DDG) Nibelungenallee 37- 41 60318 Frankfurt am Main; Gemeinschaftspraxis für Chirurgie und Gefäßmedizin Drs. Alter/Pourhassan/Heim Klosterstraße 12 46145 Oberhausen; Ev. KH Königin Elisabeth Herzberge gGmbH Abt. für Kardiologie, Angiologie und Diabetologie Herzbergstraße 79 10365 Berlin; Städtisches Klinikum Neunkirchen gGmbH Abt. für Gefäßchirurgie & Phlebologie Brunnenstraße 20 66538 Neunkirchen; Westküstenklinikum Heide Klinik für Viszeral- und Gefäßchirurgie Esmarchstraße 50 25746 Heide/Holstein; Chir. Praxisgemeinschaft am Bayenthalgürtel Praxis Dr. med. Gerald Engels Bayenthalgürtel 45 50968 Köln; Malteser Krankenhaus – St. Franziskus-Hospital Medizinische Klinik I, Abt. für Diabetologie Waldstraße 17 24939 Flensburg; St. Marienkrankenhaus Siegen gGmbH Klinik für Gastroenterologie Kampenstraße 51 57072 Siegen; Krankenhaus Bietigheim Klinik für Innere Medizin, Kardiologie, Endokrinologie, Diabetologie und Internistische Intensivmedizin Riedstraße 12 74321 Bietigheim-Bissingen; Asklepios Kliniken Harburg Eißendorfer Pferdeweg 52 21075 Hamburg; Diabetologikum Ludwigshafen Diabetes-Schwerpunktpraxis Ludwigsplatz 9 67059 Ludwigshafen; Mariannen-Hospital Werl Abt. für Chirurgie Unnaer Straße 15 59457 Werl; Diabetes Klinik GmbH & Co KG Theodor-Klotzbücher-Straße 12 97980 Bad Mergentheim; Institut für Diabetesforschung Münster GmbH Hohenzollernring 70 48145 Münster.
The study was initiated by a consortium of 19 statutory German health insurance funds represented by the AOK federal association (AOK-Bundesverband – AOK-BV), the association of alternative health insurance funds (Verband der Ersatzkrankenkassen – vdek) and the minors (Knappschaft). In order to guarantee outpatient care for all study participants without any restrictions, the contracting health insurance companies provided integrated care contracts for outpatient negative pressure wound therapy. A project advisory board was implemented to coordinate all processes and project partners. The board comprised two representatives each from the statutory health insurance funds, the management company and the sponsor as well as one representative each from the participating medical device manufacturers (KCI and smith & nephew). Representing the contracting authority (statutory German health insurance funds) Dr. Gerhard Schillinger (AOK-BV) and Ute Leonhard (vdek) acted as contact persons for all aspects of the project.
The management company “Gesundheitsforen Leipzig” has been entirely responsible for the logistics of the study. Central tasks of the management company included the recruitment of study sites and patients, the development of the IT infrastructure including the documentation, communication and invoicing software as well as the processing of all payments.
The manufacturers Kinetic Concepts Incorporated (KCI) (Acelity) and smith & nephew provided the NPWT devices as well as support and training for the investigators and financed the study.
The Private University of Witten/Herdecke gGmbH acted as the Sponsor of the trial and the Institute for Research in Operative Medicine with its former director Prof. E.A.M. Neugebauer, the current interim head Prof. Rolf Lefering and the head of the division for clinical research Dörthe Seidel was responsible for the scientific conception, the evaluation as well as the reporting and publication of the study. Prof. Dr. Rolf Lefering was responsible for the statistical planning and analysis. PD Dr. Peter Krüger was responsible for the data management of the study. Special thanks are going to Stefan Bauer, who supported the data management as well as the statistical analysis and reporting. We would like to thank Sophie Thorn, who checked the article as a native English speaker with regard to spelling and grammar.
Footnotes
Contributors: DS was the principal coordinating investigator. She conceived the study, reviewed the scientific literature and was responsible for study design, data analysis, data interpretation, writing and reviewing of the report. She is the lead author and takes overall responsibility for this report. She affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained. MS and HL were study investigators and contributed to study design, data collection and interpretation and reviewed the report. GW, PM, WW-R and DH were study investigators and contributed to data collection and data interpretation and reviewed the report. KS, MH, GR, TK and KZ were study investigators and contributed to data collection and reviewed the report. EN contributed to study design and data interpretation and reviewed the report. All authors approved the final version of the report.
Funding: Through a European tender, the study was initiated by a consortium of 19 statutory German health insurance funds, which provided integrated care contracts for all study participants and for up to 7000 patients with acute and chronic wounds in Germany; defined basic rules for study design based on the requirements of the German authorities; and provided a critical review of the study protocol and the final report. The study was funded by the manufacturers KCI and S&N. Both companies provided the NPWT devices and associated consumable supplies in the assigned regions of Germany as well as all necessary support and information about the used material. All authors had full access to all of the data (including statistical reports and tables) in the study and take full responsibility for the accuracy of the data analysis.
Disclaimer: The manufacturers had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Competing interests: The German statutory health insurance companies commissioned the Witten/Herdecke University (UW/H) to plan, conduct, analyse and publish the study. DS is an employee of the UW/H. The study has been financed by the manufacturers KCI (Acelity) and S&N. DS received a consulting fee for the presentation of the study during an event organised by the manufacturer Hartmann. During study planning and conduct, EN was an employee of the UW/H. He was the director of the Institut für Forschung in der Operativen Medizin. The clinical investigators MS, HL, GW, PM, DH, WW-R, KS, MH, GR, TK and KZ received a case fee of 1000€ for each patient included in the DiaFu study in order to compensate for the additional organisational and especially the documentation effort during trial conduct. Furthermore, all investigators received compensation for travelling to the investigator meetings. The institutions of the investigators used integrated care contracts for NPWT during study conduct in order to provide best practice for the study participants during outpatient care. GW and WW-R are members of the scientific advisory board of the manufacturer KCI (now Acelity).
Patient consent for publication: Not required.
Ethics approval: Ethical approval of the main ethical committee (EC): Ethical Committee of the University of Witten-Herdecke, has been fully granted without any conditions. Due to performing the trial according to § 23b MPG (German Medical Device Act), participating study sites in Germany only received a consultation for the main clinical investigator according to professional law by the respective EC. All investigators have been fully approved by the respective ECs. An evaluation of the study's content by ECs of participating study sites in Germany was not applicable. All study participants gave written informed consent prior to randomisation and any trial related procedure.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. The datasets analysed for the results presented in this article are available from the corresponding author. Datasets are available in German language.
References
- 1. World Health Organization Global report on diabetes, 2016. Available: http://www.who.int/diabetes/global-report/en/
- 2. International Diabetes Federation IDF diabetes atlas, 2015. Available: www.diabetesatlas.org
- 3. Yazdanpanah L, Nasiri M, Adarvishi S. Literature review on the management of diabetic foot ulcer. World J Diabetes 2015;6:37–53. 10.4239/wjd.v6.i1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Leone S, Pascale R, Vitale M, et al. [Epidemiology of diabetic foot]. Infez Med 2012;20 Suppl 1:8–13. [PubMed] [Google Scholar]
- 5. Wu L, Norman G, Dumville JC, et al. Dressings for treating foot ulcers in people with diabetes: an overview of systematic reviews. Cochrane Database Syst Rev 2015;55. 10.1002/14651858.CD010471.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norman G, Westby MJ, Rithalia AD, et al. Dressings and topical agents for treating venous leg ulcers. Cochrane Database Syst Rev 2018;6:CD012583. 10.1002/14651858.CD012583.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu SC, Marston W, Armstrong DG. Wound care: the role of advanced wound-healing technologies. J Am Podiatr Med Assoc 2010;100:385–94. 10.7547/1000385 [DOI] [PubMed] [Google Scholar]
- 8. Fleischmann W, Strecker W, Bombelli M, et al. [Vacuum sealing as treatment of soft tissue damage in open fractures]. Unfallchirurg 1993;96:488–92. [PubMed] [Google Scholar]
- 9. Argenta LC, Morykwas MJ. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 1997;38:563–77. 10.1097/00000637-199706000-00002 [DOI] [PubMed] [Google Scholar]
- 10. Morykwas MJ, Argenta LC, Shelton-Brown EI, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic Foundation. Ann Plast Surg 1997;38:553–62. 10.1097/00000637-199706000-00001 [DOI] [PubMed] [Google Scholar]
- 11. Morykwas MJ, Faler BJ, Pearce DJ, et al. Effects of varying levels of subatmospheric pressure on the rate of granulation tissue formation in experimental wounds in swine. Ann Plast Surg 2001;47:547–51. 10.1097/00000637-200111000-00013 [DOI] [PubMed] [Google Scholar]
- 12. Gregor S, Maegele M, Sauerland S, et al. Negative pressure wound therapy: a vacuum of evidence? Arch Surg 2008;143:189–96. 10.1001/archsurg.2007.54 [DOI] [PubMed] [Google Scholar]
- 13. Peinemann F, Sauerland S. Negative-Pressure wound therapy: systematic review of randomized controlled trials. Dtsch Arztebl Int 2011;108:381–9. 10.3238/arztebl.2011.0381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ubbink DT, Westerbos SJ, Evans D, et al. Topical negative pressure for treating chronic wounds. Cochrane Database Syst Rev 2008:CD001898. 10.1002/14651858.CD001898.pub2 [DOI] [PubMed] [Google Scholar]
- 15. Vikatmaa P, Juutilainen V, Kuukasjärvi P, et al. Negative pressure wound therapy: a systematic review on effectiveness and safety. Eur J Vasc Endovasc Surg 2008;36:438–48. 10.1016/j.ejvs.2008.06.010 [DOI] [PubMed] [Google Scholar]
- 16. Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. The Lancet 2005;366:1704–10. 10.1016/S0140-6736(05)67695-7 [DOI] [PubMed] [Google Scholar]
- 17. Blume PA, Walters J, Payne W, et al. Comparison of negative pressure wound therapy using vacuum-assisted closure with advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter randomized controlled trial. Diabetes Care 2008;31:631–6. 10.2337/dc07-2196 [DOI] [PubMed] [Google Scholar]
- 18. Dumville JC, Hinchliffe RJ, Cullum N, et al. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev 2013;10:CD010318. 10.1002/14651858.CD010318.pub2 [DOI] [PubMed] [Google Scholar]
- 19. Rhee SM, et al. Negative pressure wound therapy technologies for chronic wound care in the home setting. Rockville (MD): Johns Hopkins University Evidence-based Practice Center, 2014. [Google Scholar]
- 20. CADTH Rapid Response Reports Negative pressure wound therapy for managing diabetic foot ulcers: a review of the clinical effectiveness, cost-effectiveness, and guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health, 2014. [PubMed] [Google Scholar]
- 21. Liu S, He C-Z, Cai Y-T, et al. Evaluation of negative-pressure wound therapy for patients with diabetic foot ulcers: systematic review and meta-analysis. Ther Clin Risk Manag 2017;13:533–44. 10.2147/TCRM.S131193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Z, Dumville JC, Hinchliffe RJ, et al. Negative pressure wound therapy for treating foot wounds in people with diabetes mellitus. Cochrane Database Syst Rev 2018;10:CD010318. 10.1002/14651858.CD010318.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seidel D, Mathes T, Lefering R, et al. Negative pressure wound therapy versus standard wound care in chronic diabetic foot wounds: study protocol for a randomized controlled trial. Trials 2014;15:334. 10.1186/1745-6215-15-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ärztliches Zentrum für Qualität in Der Medizin (Gemeinsame Einrichtung von Bundesärztekammer und Kassenärztlicher Bundesvereinigung) Im Auftrag von BÄK, K., AWMF, Nationale VersorgungsLeitlinie (NVL) Typ-2-Diabetes Präventions- und Behandlungsstrategien für Fußkomplikationen. 2010, Bundesärztekammer (BÄK) Arbeitsgemeinschaft Der Deutschen Ärztekammern, 2010. Available: http://www.baek.de;KassenärztlicheBundesvereinigung(KBV);http://www.kbv.de;ArbeitsgemeinschaftderWissenschaftlichenMedizinischenFachgesellschaften(AWMF)http://www.awmf-online.de
- 25. Bauer H. Typ-2-Diabetes: Präventions- und Behandlungsstrategien für Fußkomplikationen Nationale Versorgungs Leitlinien; 2010. [Google Scholar]
- 26. Rüttermann M, Maier-Hasselmann A, Nink-Grebe B, et al. Local treatment of chronic wounds: in patients with peripheral vascular disease, chronic venous insufficiency, and diabetes. Dtsch Arztebl Int 2013;110:25–31. 10.3238/arztebl.2013.0025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dupont WD, Plummer WD. Power and sample size calculations. A review and computer program. Control Clin Trials 1990;11:116–28. 10.1016/0197-2456(90)90005-m [DOI] [PubMed] [Google Scholar]
- 28. Schaper NC. Diabetic foot ulcer classification system for research purposes: a progress report on criteria for including patients in research studies. Diabetes Metab Res Rev 2004;20 Suppl 1:S90–5. 10.1002/dmrr.464 [DOI] [PubMed] [Google Scholar]
- 29. Sanders LJ, Frykberg RG. Diabetic neuropathic osteoarthropathy: the Charcot foot : The high risk foot in diabetes mellitus. New York: Churchill Livingstone, 1991: 297–338. [Google Scholar]
- 30. Levin ME. Preventing amputation in the patient with diabetes. Diabetes Care 1995;18:1383–94. 10.2337/diacare.18.10.1383 [DOI] [PubMed] [Google Scholar]
- 31. Wagner FW. The diabetic foot. Orthopedics 1987;10:163–72. [DOI] [PubMed] [Google Scholar]
- 32. Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997;26:517–38. 10.1016/S0741-5214(97)70045-4 [DOI] [PubMed] [Google Scholar]
- 33. Widmer LK, Plechl SC, Leu HJ, et al. [Venous diseases in 1800 employees. Basel Studies II]. Schweiz Med Wochenschr 1967;97:107–10. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-026345supp001.pdf (177KB, pdf)