Abstract
A 69-year-old woman presented with headaches and visual disturbance in the context of marked hypertension secondary to non-compliance with antihypertensive medications. She developed seizures and hyperreflexia, and MRI brain showed changes consistent with posterior reversible encephalopathy syndrome (PRES). She was treated with antihypertensives with the resolution of symptoms. Over the following week, she developed progressive distal sensory loss, weakness and areflexia. The cerebrospinal fluid examination demonstrated albuminocytologic dissociation, and electrophysiological findings were in keeping with a diagnosis of Guillain-Barré syndrome (GBS). She was treated with intravenous immunoglobulin with gradual recovery. The co-occurrence of PRES and GBS has only been described in a handful of cases. In the majority of these, the dysautonomia of GBS leads to profound hypertension and subsequently PRES. This is a rare case of PRES preceding and possibly even triggering the onset of GBS. In this report, we review the literature and discuss the potential pathogenic mechanisms for this unusual association.
Keywords: peripheral nerve disease, neurology
Background
Posterior reversible encephalopathy syndrome (PRES) is a clinico-radiological syndrome characterised by the presence of encephalopathy, headache, visual symptoms and seizures. It is thought to be a disorder of cerebral autoregulatory failure causing cerebral hyperperfusion and release of damaging cytokines, leading to the breakdown of blood–brain barrier (BBB) and vasogenic oedema. This occurs most commonly in the setting of hypertensive crisis, pre-eclampsia or treatment with chemotherapeutic agents, however, a wide range of medical conditions have been implicated.1
Guillain-Barré syndrome (GBS) is an immune-mediated inflammatory polyradiculoneuropathy characterised clinically by rapidly progressive flaccid areflexic weakness and variable sensory deficits. Dysautonomia, which affects approximately 70% of patients with GBS leading to paroxysmal hypertension, has been reported to rarely cause the syndrome of PRES.2 3 Autonomic dysfunction in GBS typically occurs after other neurological deficits such as weakness are already present. Rarely, it can be the presenting feature.4
Case presentation
A 69-year-old woman was admitted to our unit with a 1-week history of occipital headaches and visual disturbance. She had been unwell with a diarrhoeal illness 2 weeks prior while travelling abroad. She had a past medical history of hypertension, however, had temporarily discontinued her antihypertensives while travelling.
She had presented to another hospital a week earlier with an occipital headache of 1 day duration and mild gait unsteadiness, along with accelerated hypertension with a blood pressure of 210/100 mm Hg. A CT brain at the time was within normal limits. She was given symptomatic treatment and discharged. The following day, she developed visual disturbance, described as blurred vision with impaired perception of depth and distance, resulting in impaired mobility and falls. She reported seeing multiple yellow stripes across her field of vision. She had also noticed intermittent paraesthesia affecting her hands and feet.
On examination in our hospital, her blood pressure was 209/93 mm Hg and she was afebrile. Visual acuity was reduced bilaterally to 6/18 and she complained of bilateral horizontal diplopia, however, ocular fundi showed no evidence of papilloedema. She had mild unsteadiness of gait, which was attributed to her visual symptoms. The rest of the neurological examination was within normal limits including normally elicitable deep tendon reflexes and there were no meningeal signs. She was commenced on amlodipine and prazosin, in addition to continuing her previous dose of valsartan to lower blood pressure. She developed witnessed generalised tonic-clonic seizures on the second day of hospital admission, which was treated with intravenous levetiracetam. She recovered consciousness after a brief postictal period and remained fully alert without any progressive encephalopathy, focal neurological deficits or involuntary movements for the next few days.
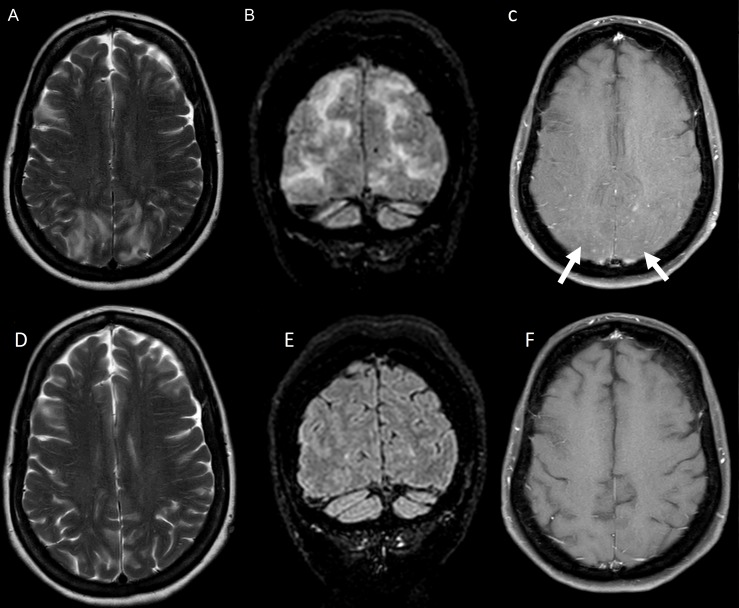
Brain MRI showed bilateral symmetrical subcortical hyperintense foci on T2 and fluid-attenuated inversion recovery (FLAIR) sequences, predominantly in a parieto-occipital distribution with associated nodular enhancement without restricted diffusion or mass effect (figure 1A–C). MRI spine was normal. Full blood picture, electrolytes, liver and renal function studies were normal. Clinical presentation with headache, visual symptoms and generalised seizures associated with accelerated hypertension and parieto-occipital white matter changes were thought to be consistent with a diagnosis of PRES. Nodular contrast enhancement was thought to be due to BBB breakdown as a consequence of accelerated hypertension. Differential diagnosis considered but felt to be less likely was postinfective acute disseminated encephalomyelitis (ADEM) and infective or autoimmune encephalitis. Electroencephalogram showed bilateral slowing with rare left temporal epileptiform abnormalities. Cerebrospinal fluid (CSF) examination showed no cells and a mildly elevated protein of 0.67 g/L and was negative for Gram stain, India ink stain, cryptococcal antigen, viral PCRs and malignant cytology.
Figure 1.
MRI brain imaging performed when symptoms initially started (A–C) and at 2-week follow-up assessment (D–F). (A) and (B) Patchy bilateral symmetrical parieto-occipital subcortical T2 and fluid-attenuated inversion recovery (FLAIR) hyperintensities. (C) Subtle foci of nodular postcontrast enhancement on T1-weighted imaging. (D–F) Follow-up brain and spine imaging show resolution of initial change.
By day 4 of hospitalisation, she reported marked improvement in headaches with improvement in her vision. She began to ambulate normally, however, complained of ongoing paraesthesia in her hands and feet. Neurological examination at this time showed normal motor power with elicitable deep tendon reflexes. By day 7, she had developed symmetrical proximodistal weakness of both upper and lower limbs. Deep tendon reflexes were now absent in the lower limbs and sluggish in the upper limbs. There was a glove and stocking distribution of sensory loss up to the wrists in the upper limbs and mid-shin level in the lower limbs. There was no facial, bulbar or respiratory muscle involvement. Her blood pressure was labile, with marked postural drops in excess of 50 mm Hg. Overall features at this time were consistent with an areflexic symmetrical proximodistal weakness of upper and lower limbs along with distal sensory involvement suggestive of an acute radiculoneuropathy. Ascending myelitis was considered unlikely in view of the absence of upper motor signs, truncal sensory level and bladder or bowel involvement.
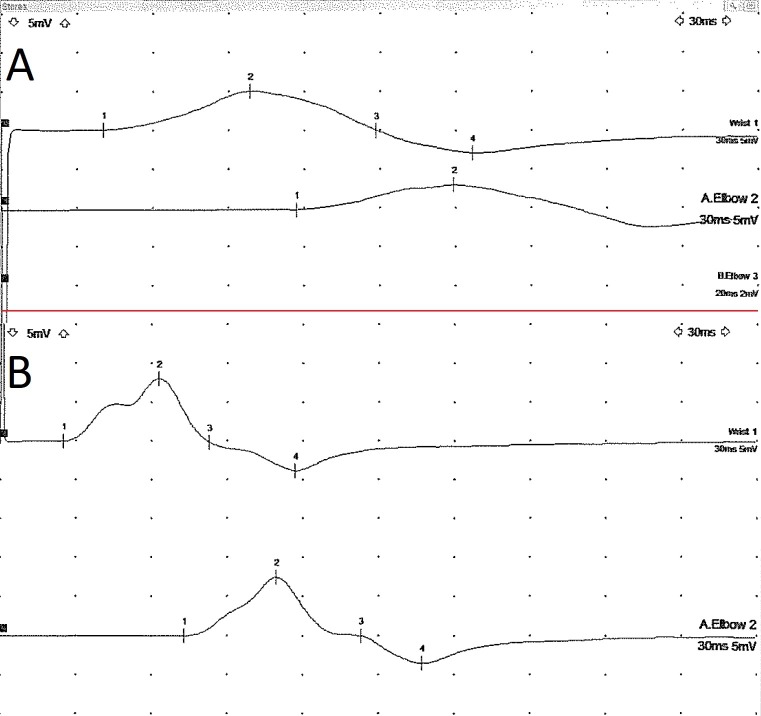
Motor nerve conduction studies on day 9 demonstrated prolonged latencies, reduced conduction velocities and dispersed compound muscle action potentials (CMAPs) from the median, ulnar and peroneal nerves, suggestive of a demyelinating neuropathy (table 1). Sensory nerve conduction studies showed dispersed responses in the upper limb, while the sural response was not elicited in the right lower limb. F-wave latencies were prolonged in the upper limb.
Table 1.
Nerve conduction studies (control values)
| Nerve | Site | Latency (ms) | Amplitude (mV) | Distance (mm) | Velocity (m/s) |
| Sensory nerve conduction studies | |||||
| R.Median—Digit II | Wrist | 4.1 (<3.5) | 6.4 (>15) | 13 | 31.7 (>56) |
| R.Ulnar—Digit V | Wrist | 3.3 (<3.5) | 15.2 (>10) | 13 | 39.3 (>56) |
| Motor nerve conduction studies | |||||
| R.Median—APB | Wrist | 15.2 (<4.2) | 2.4 (>5) | 6 | |
| Elbow | 22.2 | 1.9 | 22 | 31.3 (>49) | |
| R.Ulnar—ADM | Wrist | 4.1 (<3.5) | 5.0 (>6) | 6 | |
| Above elbow | 11.9 | 3.2 | 33 | 42.2 (>51) | |
| R.Peroneal—EDB | Ankle | 10.2 (<6.5) | 0.7 (>2) | 6 | |
| Fib. head | 19.9 | 0.2 | 35 | 35.7 (>44) | |
| F waves | |||||
| Fmin (ms) | Fmax (ms) | ||||
| R.Median | 32.8 (<31) | 33.54 | |||
| R.Ulnar | 36.9 (<32) | 37.66 | |||
ADM, abductor digiti minimi; APB, abductor pollicis brevis; EDB, extensor digitorum brevis.
Repeated CSF studies showed a raised protein of 0.72 g/L with no lymphocytes, in keeping with albuminocytologic dissociation. Repeated CSF was negative for viral PCRs, India ink stain, cryptococcal antigen, malignant cytology and oligoclonal bands. Serum testing for HIV, hepatitis B and C, syphilis, ACE level and paraprotein were negative. Faecal PCR for campylobacter, salmonella and shigella were negative. Tests for systemic autoimmune and vasculitic disorders were negative with normal serum antinuclear antibody (ANA), extractable nuclear antigens (ENA), anti-neutrophil cytoplasmic antibody (ANCA), rheumatoid factor and antithyroid antibodies. Repeat MRI spine showed subtle enhancement of the cauda equine nerve roots without any other intramedullary signal abnormality. Based on the clinical features, electrophysiological and CSF findings, a diagnosis of acute inflammatory demyelinating polyneuropathy (AIDP) was made and she was treated with 0.4 g/kg/day of intravenous immunoglobulin for 5 days.
Outcome and follow-up
Improvement in her weakness was noted within 1 week. Follow-up MRI brain and spine performed 2 weeks later demonstrated complete resolution of prior change (figure 1D–F).
Complete resolution of the prominent MRI changes in 2 weeks is supportive of the diagnosis of PRES rather than an infective, inflammatory or neoplastic cause. AIDP with central demyelination like ADEM would also be unlikely to have such complete resolution of MRI white matter changes in 2 weeks.
At 3-month review, she had almost normal power in both upper and lower limbs with now detectable deep tendon reflexes. A repeat nerve conduction study in the upper limbs was normal and the CMAPs were no longer dispersed (figure 2). Her visual disturbance had resolved completely.
Figure 2.
Ulnar motor nerve conduction study recorded from the abductor digiti minimi muscle showing prolonged distal latency and temporal dispersion of the compound muscle action potentialcompound muscle action potential (A). These changes resolved on repeat testing 6 months later (B).
Discussion
This case illustrates a very rare association of GBS with PRES. To the best of our knowledge, this association has only been described in 15 cases in the literature so far.5 6 Most of the reported cases attribute PRES to be a secondary phenomenon as a result of autonomic dysfunction in GBS. Other dysautonomia-related complications reported in GBS include cardiac arrhythmias and asystole, gastrointestinal dysmotility, postural hypotension and sustained hypertension causing end-organ damage and rarely intracranial complications like reversible cerebral vasoconstriction syndrome and subarachnoid haemorrhage.7 8 The sudden elevation of blood pressure that can occur in the setting of autonomic dysfunction in GBS can exceed the upper limit of cerebral autoregulation leading to hyperperfusion, vasogenic oedema and BBB breakdown.9 10 In these cases, the initial clinical presentation is consistent with GBS and they develop PRES as a complication in the acute phase. However, rarely PRES can be the presenting clinical syndrome ahead of any definite GBS symptoms, as was the case in our patient. This scenario has been reported in only three patients with coexisting GBS and PRES.11–13
Our patient had a fairly typical presentation of PRES with headaches, visual disturbance, accelerated hypertension and seizures with accompanying MRI findings. Nodular and gyriform contrast enhancement as noted in this case has been reported with PRES and is probably reflective of the degree of BBB breakdown.14 She had no objective clinical findings suggestive of GBS until day 7 of presentation. Mild intermittent subjective tingling sensations in the distal extremities in the absence of any objective abnormalities are difficult to localise definitely and could be central or peripheral in origin or could even be related to systemic factors like hyperventilation. The presence of diarrhoeal illness prior to the onset of neurological symptoms and elevated CSF protein in the initial CSF could be supportive of GBS, however CSF protein may be elevated in PRES as well.15
Variability in blood pressure as a consequence of autonomic dysfunction in the very early phase of GBS could have been the mechanism of PRES in our patient. However, considering the clinical presentation with PRES and the absence of objective GBS- related features in the first week after presentation, we would like to entertain the possibility of a reverse hypothesis—that of PRES triggering the onset of GBS. Our patient discontinued her antihypertensive medications while she was travelling abroad and this could have been a precipitating factor for PRES. Disruption of the BBB as in PRES may render the nervous system vulnerable to immune-mediated attack, leading to central and peripheral neurological dysfunction. GBS occurring in the context of head injuries, stroke and in the perioperative setting has been well described and may have a similar mechanism.16 17 Disintegration of the BBB during neurotrauma leads to the accumulation of localised T lymphocytes and macrophages, which may induce the transformation of microglial cells in the nervous system into antigen-presenting cells. Activated microglia can present post-traumatic neuronal debris to the immune system and induce B-cell-mediated production of antibodies against the myelin sheath, causing central or peripheral demyelination.18–20 It is possible that disruption of the BBB in PRES fuel activation of the immune system and subsequently accelerates or amplifies the pathogenic mechanism, increasing the chances of a more severe clinical manifestation of immune-mediated demyelination.21 22 Thus both GBS and PRES may have mutually complementary synergistic mechanisms, regardless of which happens first.
While GBS is commonly associated with back and extremity pain, headache is not a typical feature, and severe headaches in these patients tend to occur in the setting of complications such as PRES or increased intracranial pressure.23 Raised intracranial pressure has been reported in patients with GBS and with markedly raised CSF protein. It is postulated that the raised protein impairs CSF reabsorption at the level of the arachnoid granulations and thus contributes to communicating hydrocephalus which can manifest with severe headaches. Treatment of hypertension is the mainstay of treatment in the majority of patients with PRES. Blood pressure should be lowered cautiously, by 25% within the first few hours.8 24 The disorder is typically rapidly reversible when the precipitating cause is eliminated, although radiological improvement may lag behind clinical recovery.25
Learning points.
Posterior reversible encephalopathy syndrome (PRES) can complicate Guillain-Barré syndrome (GBS) at any stage of the illness and clinicians must remain vigilant for this complication, particularly in those with marked dysautonomia, as early blood pressure management can prevent permanent neurological disability.
Dysautonomia can be a major issue in the early stage of GBS and can be associated with serious complications such as cardiac arrhythmias and extreme blood pressure fluctuations and is associated with increased mortality.
PRES and other aetiologies causing disruption of blood–brain barrier may confer a higher risk for developing immune-mediated dysfunction of the central or peripheral nervous system, including GBS.
It is important for clinicians to be aware of this risk as to the emergence of progressive neurological symptoms, especially involving the peripheral nervous system, maybe easily overlooked in this situation.
Footnotes
Contributors: SJ can confirm that all authors were involved equally in the design and planning of the paper. SJ and TC were primarily responsible for drafting the paper. TC and DP were responsible for the acquisition of patient data including clinical details and neurophysiology reports. JvH was responsible for the acquisition of radiology images, editing and captioning the figures.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc 2010;85:427–32. 10.4065/mcp.2009.0590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flachenecker P. Autonomic dysfunction in Guillain-Barré syndrome and multiple sclerosis. J Neurol 2007;254 Suppl 2:II96–101. 10.1007/s00415-007-2024-3 [DOI] [PubMed] [Google Scholar]
- 3.Louie J, Igbokwe E, Hinchey J. Posterior reversible encephalopathy associated with the dysautonomia of Guillain-Barré syndrome. Neurol. Bull. 2009:7–10. 10.7191/neurol_bull.2009.1001 [DOI] [Google Scholar]
- 4.Ferraro-Herrera AS, Kern HB, Nagler W. Autonomic dysfunction as the presenting feature of Guillain-Barré syndrome. Arch Phys Med Rehabil 1997;78:777–9. 10.1016/S0003-9993(97)90089-7 [DOI] [PubMed] [Google Scholar]
- 5.Nabi S, Rajput HM, Badshah M, et al. Posterior reversible encephalopathy syndrome (PRES) as a complication of Guillain-Barre’ syndrome (GBS). BMJ Case Rep 2016;2016:bcr2016216757–3. 10.1136/bcr-2016-216757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yonekura S, Anno T, Kobayashi N. Posterior reversible encephalopathy syndrome and Guillain-Barré syndrome after head injury: case report. Neurol Med Chir 2018;58:453–8. 10.2176/nmc.cr.2018-0049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaeem Z, Siddiqi ZA, Zochodne DW. Autonomic involvement in Guillain-Barré syndrome: an update. Clin Auton Res 2019;29:289–99. 10.1007/s10286-018-0542-y [DOI] [PubMed] [Google Scholar]
- 8.Wei DY, Kao J, Wu TY, et al. Reversible cerebral vasoconstriction in Guillain-Barré syndrome. J Clin Neurosci 2015;22:1201–2. 10.1016/j.jocn.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 9.Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol 2015;14:914–25. 10.1016/S1474-4422(15)00111-8 [DOI] [PubMed] [Google Scholar]
- 10.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 1990;2:161–92. [PubMed] [Google Scholar]
- 11.Elahi A, Kelkar P, St Louis EK. Posterior reversible encephalopathy syndrome as the initial manifestation of Guillain-Barré syndrome. Neurocrit Care 2004;1:465–8. 10.1385/NCC:1:4:465 [DOI] [PubMed] [Google Scholar]
- 12.Van Diest D, Van Goethem JWM, Vercruyssen A, et al. Posterior reversible encephalopathy and Guillain-Barré syndrome in a single patient: coincidence or causative relation? Clin Neurol Neurosurg 2007;109:58–62. 10.1016/j.clineuro.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 13.Rigamonti A, Basso F, Scaccabarozzi C, et al. Posterior reversible encephalopathy syndrome as the initial manifestation of Guillain-Barré syndrome: case report and review of the literature. J Peripher Nerv Syst 2012;17:356–60. 10.1111/j.1529-8027.2012.00416.x [DOI] [PubMed] [Google Scholar]
- 14.Karia SJ, Rykken JB, McKinney ZJ, et al. Utility and significance of gadolinium-based contrast enhancement in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 2016;37:415–22. 10.3174/ajnr.A4563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datar S, Singh TD, Fugate JE, et al. Albuminocytologic dissociation in posterior reversible encephalopathy syndrome. Mayo Clin Proc 2015;90:1366–71. 10.1016/j.mayocp.2015.07.018 [DOI] [PubMed] [Google Scholar]
- 16.Wu Q, Liu N, Pan C, et al. Guillain-Barré syndrome and cerebral hemorrhage: two cases and literature review. Eur Neurol 2016;76:182–6. 10.1159/000450603 [DOI] [PubMed] [Google Scholar]
- 17.Jia H, Tian Y, Wu Y-M, et al. Two cases of Guillain-Barré syndrome after cerebral hemorrhage or head trauma. NN 2017;4:61–4. 10.20517/2347-8659.2016.51 [DOI] [Google Scholar]
- 18.Kanda T. Biology of the blood-nerve barrier and its alteration in immune mediated neuropathies. J Neurol Neurosurg Psychiatry 2013;84:208–12. 10.1136/jnnp-2012-302312 [DOI] [PubMed] [Google Scholar]
- 19.Morganti-Kossmann MC, Satgunaseelan L, Bye N, et al. Modulation of immune response by head injury. Injury 2007;38:1392–400. 10.1016/j.injury.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 20.Shrikant P, Benveniste EN. The central nervous system as an immunocompetent organ: role of glial cells in antigen presentation. J Immunol 1996;157:1819–22. [PubMed] [Google Scholar]
- 21.Tan IL, Ng T, Vucic S. Severe Guillain-Barré syndrome following head trauma. J Clin Neurosci 2010;17:1452–4. 10.1016/j.jocn.2009.11.037 [DOI] [PubMed] [Google Scholar]
- 22.Rivas S, Douds GL, Ostdahl RH, et al. Fulminant Guillain-Barré syndrome after closed head injury: a potentially reversible cause of an ominous examination. Case report. J Neurosurg 2008;108:595–600. 10.3171/JNS/2008/108/3/0595 [DOI] [PubMed] [Google Scholar]
- 23.Farmakidis C, Inan S, Milstein M, et al. Headache and pain in Guillain-Barré syndrome. Curr Pain Headache Rep 2015;19:40. 10.1007/s11916-015-0508-x [DOI] [PubMed] [Google Scholar]
- 24.Legriel S, Schraub O, Azoulay E, et al. And the critically III posterior reversible encephalopathy syndrome Study Group (cypress). determinants of recovery from severe posterior reversible encephalopathy syndrome. PLoS One 2012;7:e44534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roth C, Ferbert A. Posterior reversible encephalopathy syndrome: long-term follow-up. J Neurol Neurosurg Psychiatry 2010;81:773–7. 10.1136/jnnp.2009.189647 [DOI] [PubMed] [Google Scholar]