Abstract
Objectives
Patients with type 2 diabetes mellitus (T2DM) exhibit strong insulin resistance or abnormal insulin production. Probiotics, which are beneficial live micro-organisms residing naturally in the intestinal tract, play indispensable roles in the regulation of host metabolism. However, the detailed mechanisms remain unclear. Here, we evaluate the mechanisms by which probiotic strains mediate glycemic regulation in the host. The findings should enable the development of a safe and natural treatment for patients with T2DM.
Research designs and methods
Sugar consumption by more than 20 strains of Lactobacillus species was first evaluated. The probiotic strains that exhibited high efficiency of sugar consumption were further coincubated with Caco-2 cells to evaluate the regulation of sugar absorption in gut epithelial cells. Finally, potential probiotic strains were selected and introduced into a T2DM animal model to study their therapeutic efficacy.
Results
Among the tested strains, Lactobacillus salivarius AP-32 and L. reuteri GL-104 had higher monosaccharide consumption rates and regulated the expression of monosaccharide transporters. Glucose transporter type-5 and Na+-coupled glucose transporter mRNAs were downregulated in Caco-2 cells after AP-32 and GL-104 treatment, resulting in the modulation of intestinal hexose uptake. Animal studies revealed that diabetic mice treated with AP-32, GL-104, or both showed significantly decreased fasting blood glucose levels, improved glucose tolerance and blood lipid profiles, and attenuated diabetes-mediated liver and kidney injury.
Conclusion
Our data elucidate a novel role for probiotics in glycemic regulation in the host. L. salivarius AP-32 and L. reuteri GL-104 directly reduce monosaccharide transporter expression in gut cells and have potential as therapeutic probiotics for patients with T2DM.
Keywords: type 2 diabetes, hexokinase, nutrient regulation, microbiology
Significance of this study.
What is already known about this subject?
Type 2 diabetes mellitus (T2DM) is a serious metabolic disorder in the modern world that needs lifelong controls of diets and medications. Previous systematic reviews and meta-analyses, including different sets of trials, have concluded an overall beneficial effect of probiotics in patients with T2DM.
What are the new findings?
Our animal studies revealed that diabetic mice treated with probiotics AP-32, GL-104, or both could significantly decrease fasting blood glucose levels, improve glucose tolerance and blood lipid profiles, and attenuate diabetes-mediated liver and kidney injury.
How might these results change the focus of research or clinical practice?
Our results provide detailed mechanisms for the beneficial effect of probiotics in T2DM, which may provide clinical application of probiotics accompanied by present antidiabetes prevention and treatment modality.
Introduction
Type 2 diabetes mellitus (T2DM) is a serious metabolic disorder that is promoted by obesity and inflammation1 and is a major health concern globally: approximately 325 million patients are expected to suffer from T2DM in 2044.2 As a result of insulin resistance and abnormal insulin production, patients with T2DM cannot sufficiently control their blood sugar, which ultimately leads to complications, including chronic kidney disease, cardiovascular diseases, glaucoma, cataracts, and leg edema.3 Therefore, glycemic levels in such patients must be carefully controlled and monitored.
Dietary modification and regular exercise can help to control or prevent the development of T2DM.4 5 Low-carbohydrate, ketogenic, vegan, and Mediterranean diets have been shown to improve glycemic levels in patients with T2DM.4 Numerous antidiabetic drugs may be used to maintain blood glucose at normal levels by decreasing sugar absorption, enhancing insulin production, inhibiting glucose reabsorption, and facilitating glucose excretion.6
Lifestyle modification and antidiabetic drugs may not be always suitable and convenient for all patients with T2DM, particularly those who are elderly, disabled, or who suffer from drug allergies. Additionally, the side effects of antidiabetic agents, including lactic acidosis and arthralgia, can significantly decrease quality of life for patients.7 8 Although insulin injections are relatively safe, hypokalemia remains a major side effect of this therapeutic option because it increases potassium uptake.9 Supplementation with probiotics, in addition to lifestyle modification and drug treatments, may represent an alternative option to manage blood glucose in patients with T2DM.10
As beneficial microbes residing in the gastrointestinal tract, probiotics function to regulate the immune system, modulate metabolic activities, maintain the gut microbiota, and prevent infection by pathogens.1 Dysbiosis of the intestinal microbiota is associated with T2DM, as evidenced by the significantly lower stool probiotic counts in patients with T2DM than in healthy individuals.11 12 Clinical trials of probiotic therapies performed by Razmpoosh et al13 and Khalili et al14 revealed positive effects on glycemic control in patients with T2DM. Fasting plasma glucose levels and insulin resistance in probiotic-treated groups were significantly lower than those in the placebo group.13 14
Supplementation with probiotics and synbiotics can significantly improve fasting plasma glucose, insulin resistance, insulin sensitivity, and glycated hemoglobin levels in adults with pre-diabetes.15 These clinical studies have demonstrated that probiotics are beneficial for patients with T2DM and prevent the development of this disease in healthy individuals. The mechanisms through which probiotics regulate blood glucose in patients with T2DM may be related to the control of intestinal permeability, increased insulin sensitivity, increased glycogen synthesis, and modulation of inflammatory reactions through reversal of the dysbiosis of gut microbiota.10 These mechanisms are highly dependent on the specific probiotic species and strains. Thus, more studies are required to identify functional probiotic strains and investigate, in detail, the mechanisms involved in probiotic-mediated glycemic regulation.
In this study, we screened Lactobacillus strains for hexose consumption and regulation of sugar transporter proteins. We then evaluated their effects on fasting blood glucose levels and glucose tolerance in diabetic (db/db) mice. Overall, our study provided insights into the potential therapeutic application of probiotic Lactobacillus strains in T2DM.
Materials and methods
Mice
C57BL/6 J-db/db (db/db; 6 weeks of age, male) mice and C57BL/6 J-db/m (db/m; 6 weeks of age, male) were purchased from the National Laboratory Animal Center (Tainan City, Taiwan). The mice were housed in sterilized cages and fed sterilized food and water. The housing environment was strictly monitored and maintained (12 hours light/dark cycle, 22±2°C, and 62%±5% humidity). Animal experiments and protocols were in compliance with the Laboratory Animal Care and Use Guidelines published by the Taiwan government.
Probiotic treatment
At 7 weeks of age, the animals were given a daily oral gavage administration (100 µL) with Lactobacillus salivarius AP-32, L. reuteri GL-104, or both strains for 33 days. We evaluated two different dosages (5.125×109 colony-forming unit (CFU)/kg/day, high dosage; 1.02×109 CFU/kg/day, low dosage). Non-diabetic db/m mice and untreated mice served as experimental controls. Body weights were measured on days 0, 9, 18 and 27.
Probiotic strains and cultivation
Active and dry L. salivarius (gL-28, gL-65, and AP-32), L. johnsonii (gL-24, gL84, and MH-68), L. reuteri (GL-104, gL-21, and gL-22), L. acidophilus (TYCA01, TYCA06, and gL-97), L. rhamnosus (F-1, bv-77, and gL-165), L. paracasei (GL-156, GL-105, and ET-22), L. plantarum (LPL-28 and gL-305), L. casei (gL-10 and CS-773), L. helveticus (gL-72 and RE-78), and Bifidobacterium animalis subsp lactis (CP-9) were obtained from Glac Biotech Co (Tainan, Taiwan). Lactobacillus spp were cultured with De Man, Rogosa and Sharpe (MRS) broth, and Bifidobacterium spp were incubated with MRS broth supplemented with 0.05% cysteine. The probiotics were incubated under anaerobic conditions at 37 ℃ for 20 hours and were further dried by lyophilization. Dry bacteria were plated on MRS agar. Viabilities of dry bacteria were determined by analyzing CFU.
Monosaccharide consumption assays
Probiotics (1×108 CFU/0.1 mL) were incubated with 5 mL MRS broth containing 2% glucose or a monosaccharide mixture containing 2% glucose, 2% fructose, and 2% galactose. Probiotics were cultivated for 8 hours at 37°C. After centrifugation at 12 000 rpm for 5 min, supernatants were collected. Concentrations of reducing sugars in the supernatants were evaluated by 3,5-dinitrosalicylic acid (DNS) assays.16 Then, 0.5 mL of supernatant or glucose standard solution was mixed with 0.5 mL of DNS reagent solution containing 0.63% DNS, 2.1% sodium hydroxide, 18.5% potassium sodium tartrate, 0.5% sulfite, and phenol crystals. The mixtures were reacted at 100°C for 5 min. After cooling, the absorbance was measured at 540 nm. Amounts of monosaccharides in the supernatants were determined in reference to the standard curve of glucose standard solutions. Total monosaccharide consumption of probiotics (%) was calculated as (preincubation monosaccharide value–postincubation monosaccharide value)/preincubation monosaccharide value.
In vitro coincubation of probiotic bacteria with cultured cells
Caco-2 human epithelial colorectal adenocarcinoma cells were maintained and subcultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. Cells were seeded into six-well plates (4×105/well) and treated with MEM containing 0.45% glucose or 0.45% monosaccharide mixture (0.15% glucose, 0.15% fructose, and 0.15% galactose). Cells were cultivated in an incubator overnight and then cocultured with 8×107 CFU probiotics (AP-32, GL-104, MH-68, or TYCA06) for 20 hours, after which probiotics were removed and monosaccharides and tumor necrosis factor (TNF)-α levels in the culture supernatants were analyzed. TNF-α production by Caco-2 cells was studied using ELISA kit (eBioScience, Diego, California, USA). RNA from Caco-2 cells was extracted and reverse-transcribed into cDNA using a Goscrip reverse-transcriptase kit (Promega, Madison, Wisconsin, USA). Expression levels of monosaccharide transporters (glucose transporter (GLUT) 2, Na+-coupled glucose transporter (SGLT1), and GLUT5) were analyzed by PCR (Thermal cycler S; GeneAtlas, Fukuoka, Japan). PCR gel images were analyzed using ImageJ software to quantify gene expression. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase served as an internal control. Primer sequences for PCR are provided in online supplementary table 1.
bmjdrc-2019-001028supp001.pdf (254.6KB, pdf)
Evaluation of hexose transporter protein expression
Caco-2 cells were incubated with both L. salivarius AP-32 and L. reuteri GL-104 as described previously. After coincubation, the cells were lysed with Radioimmunoprecipitation assay (RIPA) lysis buffer and sonication (Sonic 410, high mode, 10 min). The cell debris were removed by centrifugation (20 min, 1000 g), and then proteins were extracted. SGLT1, GLUT5, and GLUT2 protein expression was analyzed using ELISA kits (Human SGLT1, ABclonal, Cat: RK02278; Human GLUT5, MyBioSource, Cat:MBS9304003; Human GLUT2, Fine Test, Cat:EH3146).
Oral glucose tolerance tests and fasting blood glucose analyses
C57BL/6 J db/db mice were treated with high-dose or low-dose probiotics for 33 days and were then challenged with oral glucose (1 mg/kg). Orbital sinus blood samples were collected and glucose concentrations were measured with a blood glucose meter (Easy Touch GCU) using test strips (Bioptik Technology). Mice without probiotic treatment served as experimental controls. For fasting blood glucose assay, the blood samples were collected at days 0, 9, 18, and 27 and analyzed using a blood glucose meter.
Serum biochemistry
Blood samples were immediately centrifuged after collection and stored at −80°C until evaluation. Serum triglyceride (TG), total cholesterol (CHOL), low-density lipoprotein (LDL), high-density lipoprotein (HDL), aspartate aminotransferase, alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine (CREA) levels were evaluated using available kits (Wako Chemical USA) and analyzed by Beckman Coulter AU480.
Statistical analysis
Statistical analysis was performed using Microsoft Excel 11.0 software and GraphPad Prism software. Graphs represent means+SDs or means obtained from two or three independent experiments. Differences were evaluated using two-tailed t-tests. Results with p values of less than 0.05 were considered significant.
Results
Screening of Lactobacillus sp and strains for monosaccharide consumption rates
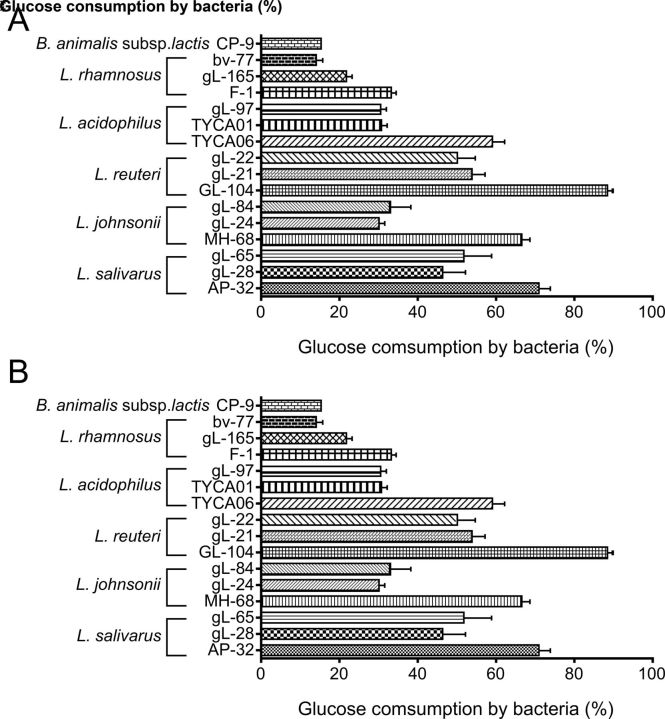
To screen potential probiotic strains for their potential in T2DM intervention, natural isolates of Lactobacillus sp were tested for monosaccharide consumption. More than 20 natural isolates of lactic acid bacteria were evaluated (figure 1 and online supplementary figure S1A). Among these strains, L. reuteri GL-104, L. salivarius AP-32, L. johnsonii MH-68, and L. acidophilus TYCA06 showed significantly higher glucose consumption rates than the others (figure 1A), suggesting that these four strains may regulate glucose absorption in the gut. L. reuteri GL-104 and L. salivarius AP-32 showed the highest sugar consumption rates. Sugar consumption assays were performed in the presence of mixtures containing 2% each of glucose, fructose, and galactose to mimic dietary sugars in the intestinal tract (figure 1B and online supplementary figure S1B). Although monosaccharide consumption rates were decreased in all strains, L. reuteri GL-104 and L. salivarius AP-32 showed higher sugar consumption rates (72% and 44%, respectively) than the other strains.
Figure 1.
Selection of Lactobacillus species and strains with high monosaccharide consumption rates. Sugar consumption rates of bacteria (108 CFU) were measured in the presence of (A) 2% glucose or (B) 6% monosaccharide mixture. Bar graphs show means+SDs of consumption rates from three individual experiments. Bifidobacterium animalis subsp lactis CP-9, which has been shown to be a low-efficiency glucose utilizer, served as an experimental control. Statistical analyses were performed for each Lactobacillus species mentioned in the text; only strains that consumed higher amounts of glucose are shown; *p<0.05, **p<0.01.
Downregulation of SGLT1 and GLUT2 and upregulation of GLUT2 in cultured human cells
To model the effects of probiotics on the expression of sugar transporter proteins in intestinal epithelial cells, we analyzed their expression in the human intestinal cell line Caco-2 in the presence of Lactobacillus strains. Cells were stimulated with 0.45% glucose and probiotic strains for 20 hours. The mRNAs encoding SGLT1 and the fructose transporter GLUT5 were downregulated in the presence of GL-104, AP-32, MH-68, and TYCA06 (figure 2A). Notably, L. salivarius AP-32 and L. reuteri GL-104 reduced SGLT1 expression by 73% and 87%, respectively, and GLUT5 expression by 84% and 95%, respectively, in Caco-2 cells (figure 2B, C). In accordance with the PCR results, proteins of the hexose transporters, SGLT1 and GLUT5, were downregulated by 80%‒95% after AP-32 and GL-104 treatments (see online supplementary figure S4A, B). GLUT2 proteins were upregulated by GL-104, whereas AP-32 significantly downregulated GLUT2 proteins in Caco-2 cells (see online supplementary figure S4C). These results indicate that these four Lactobacillus strains regulate hexose uptake in the intestinal tract by modulating the expression of SGLT1 and GLUT5.
Figure 2.
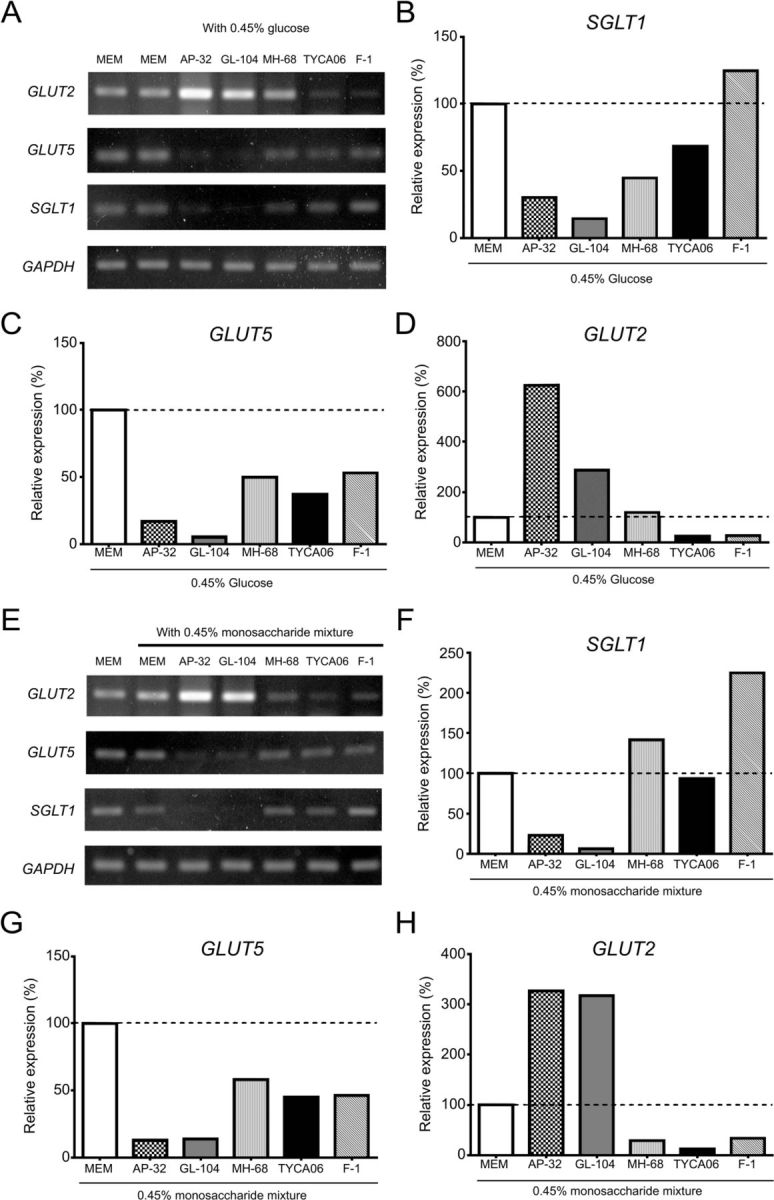
Lactobacillus salivarius AP-32 and L. reuteri GL-104 regulate the expression of monosaccharide transporters in human intestinal epithelial cells. Caco-2 cells were cocultured with Lactobacillus strains AP-32, GL-104, MH-68, TYCA06, and F-1 for 20 hours in the presence of (A–D) 0.45% glucose or (E–H) 0.45% monosaccharide mixtures. mRNA was isolated from Caco-2 cells, and PCR was performed to analyze the expression of monosaccharide transporters. PCR gel images are shown in (A) and (E). Band intensities of (B, F) GLUT2, (C, G) fructose transporter (GLUT5), and (D, H) SGLT1. Data are normalized to expression levels in Caco-2 cells treated with medium alone (dashed line). The GAPDH gene served as internal control. Bar graphs show the means of two independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GLUT, glucose transporter; MEM, minimum essential medium; SGLT, sodium-glucose co-transporter 1.
Caco-2 cells cocultured with the probiotic strains were treated with a hexose mixture (0.15% glucose, 0.15% fructose, and 0.15% galactose) to mimic dietary conditions, and hexose-transporter mRNA expression was analyzed (figure 2E). The results were consistent with the data shown in figure 2A; that is, both SGLT1 and GLUT5 were downregulated by 80%–90% in response to L. salivarius AP-32 and L. reuteri GL-104 stimulation (figure 2F, G). Interestingly, GLUT2 was upregulated by L. salivarius AP-32 and L. reuteri GL-104 in Caco-2 cells (figure 2D, H). This may indicate compensation for the lack of hexose, given the dramatic reduction in the expression of both SGLT1 and GLUT5. Thus, Caco-2 cells may have expressed GLUT2 to acquire hexose from the medium, and downregulation of hexose-transporter proteins in Caco-2 cells may be mediated by the high glucose consumption rates of both probiotic strains. Indeed, L. salivarius AP-32 and L. reuteri GL-104 rapidly decreased glucose concentrations in the culture medium by 95% and 60%, respectively (see online supplementary figure S2). Interestingly, AP-32 did not induce TNF-α production in Caco-2 cells, compared with GL-104 (see online supplementary figure S5).
L. salivarius AP-32 and L. reuteri GL-104 significantly improved glucose metabolism in diabetic mice
Because both L. salivarius AP-32 and L. reuteri GL-104 may block hexose uptake by controlling transporter expression and promoting high sugar consumption rates, we used db/db diabetic mice to study the roles of both probiotic strains in the treatment of T2DM. Mice received oral L. salivarius AP-32, L. reuteri GL-104, or both strains, for 33 days. Blood glucose levels were significantly decreased in all three probiotic groups at 30 min (figure 3A, B). Treatment with both strains extended the regulation of blood glucose to 180 min (figure 3C). We monitored fasting blood glucose levels and found that they were significantly improved in the probiotic groups on days 18 and 27 relative to those in controls (figure 4). Treatment with L. salivarius AP-32 and L. reuteri GL-104 decreased fasting blood glucose levels (figure 4A, B). However, when the strains were combined, no synergistic or antagonistic effects on the maintenance of fasting blood glucose were observed (figure 4C).
Figure 3.
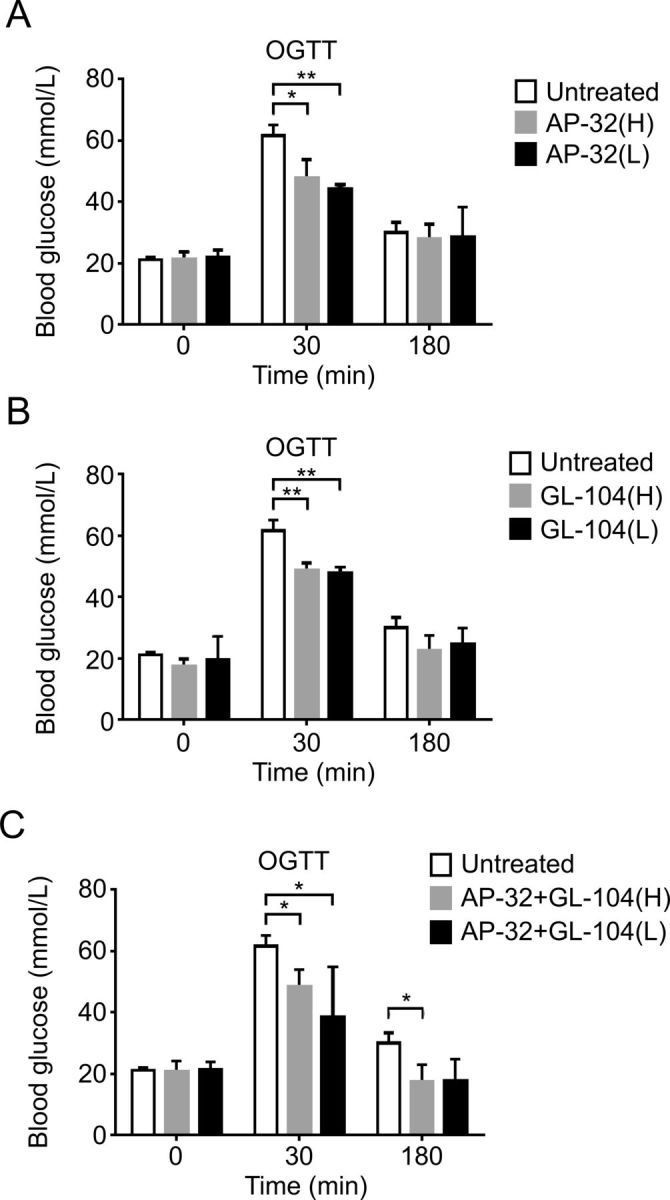
Both Lactobacillus salivarius AP-32 and L. reuteri GL-104 significantly reduced blood glucose concentrations in OGTTs. Diabetic db/db mice were challenged with glucose (1 mg/kg) after probiotic treatment. Blood glucose levels in mice treated with low-dose or high-dose (A) AP-32, (B) GL-104, or (C) both were determined. Mice without probiotic treatment were used as experimental controls. Bar graphs represent means+SDs obtained from three independent experiments; *p<0.05, **p<0.01. OGTT, oral glucose tolerance test.
Figure 4.
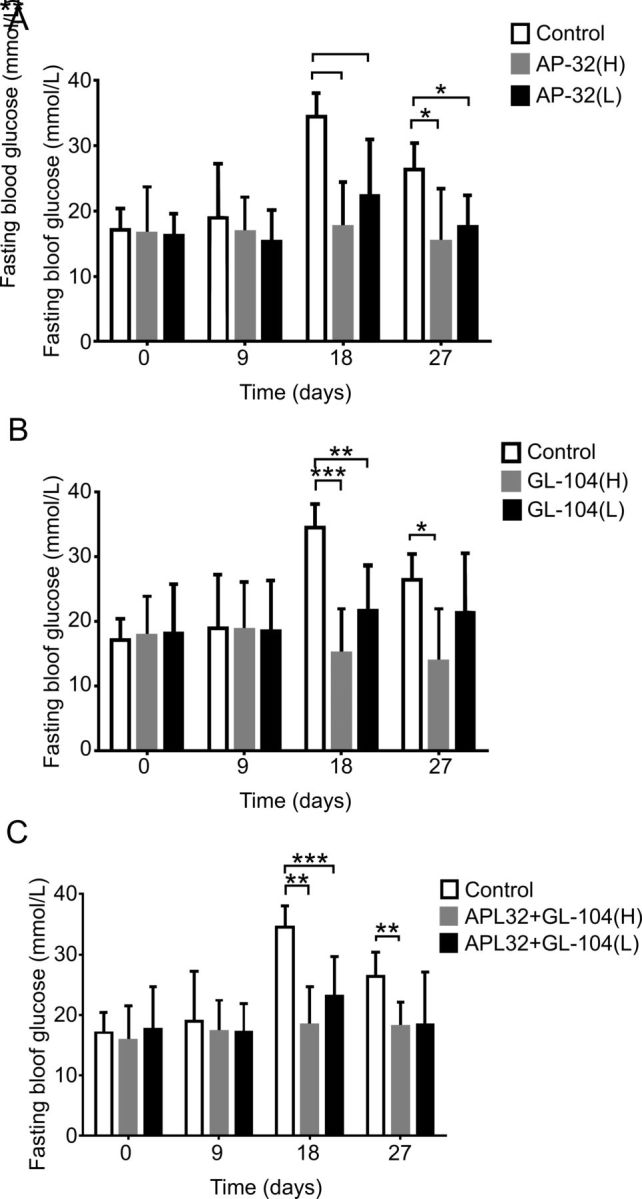
Lactobacillus salivarius AP-32 and L. reuteri GL-104 treatment modulated fasting blood glucose in diabetic mice. Fasting blood glucose analyses were performed on mice treated with low-dose or high-dose (A) AP-32, (B) GL-104, or (C) both. Untreated mice were used as experimental controls. Bar graphs represent means+SDs obtained from three independent experiments; *p<0.05, **p<0.01, ***p<0.005.
Blood lipid levels and content in diabetic mice are improved by probiotic treatment
T2DM may be complicated by fat and lipid metabolism disorders. Therefore, we further analyzed blood lipid levels in db/db mice supplemented with L. salivarius AP-32 and L. reuteri GL-104 for 33 days. Significant changes in blood TG and CHOL levels were not observed following probiotic treatment. TG and CHOL levels were reduced in the low-dose probiotic group; however, this reduction was not significant. Significant differences were observed only in mice supplemented with high-dose GL-104 or with both strains (figure 5A, B), suggesting that longer reaction times or higher probiotic doses were required to elicit significant effects in terms of the reduction of TG and CHOL levels. Interestingly, the HDL/LDL ratio was dramatically increased from 6 to 10 in response to probiotic intervention (figure 5C). HDL levels in db/db mice were not altered, whereas LDL was reduced by 25%–30% (data not shown). These results indicate that both probiotics improved blood lipid profiles in db/db mice.
Figure 5.
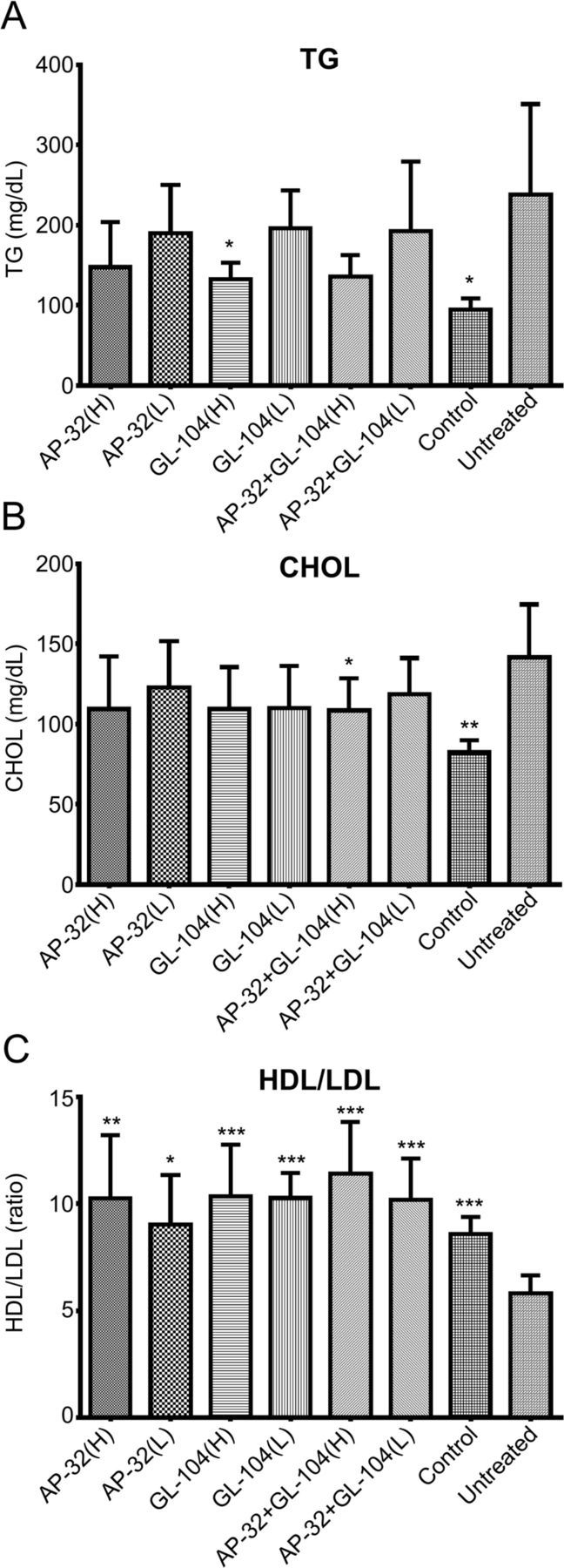
Probiotic treatment significantly decreased TG and CHOL levels and increased HDL/LDL ratios in diabetic mice. (A) TG levels, (B) total CHOL levels, and (C) HDL/LDL ratios were determined. Nondiabetic db/m mice served as a blank control. Untreated db/db mice were used as an experimental control. Bar graphs show means+SDs of three independent experiments. Statistical analyses show significant differences from controls; *p<0.05; **p<0.01; ***p<0.005. CHOL, cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, tricylglyceride.
Probiotic treatment attenuates liver injury and kidney diseases associated with T2DM
High blood-glucose levels in patients with T2DM can lead to organ injury. Therefore, we evaluated liver and kidney function in db/db mice after probiotic treatment. ALT and BUN levels were significantly decreased after L. salivarius AP-32 and L. reuteri GL-104 administration, whereas AST and CREA levels in serum were slightly downregulated, although the difference was not significant (see online supplementary table 2). Moreover, body weights did not change in response to probiotic treatment (see online supplementary figure S3). Taken together, these results suggest that probiotics gradually attenuate liver and kidney injuries mediated by T2DM.
Discussion
The development of effective and safe T2DM treatments is critical. Here, we aimed to identify potential probiotic strains with potential therapeutic application in T2DM. We hypothesized that superior probiotic strains use and metabolize monosaccharides as carbon sources more efficiently, allowing them to modulate glucose concentrations and uptake in the intestinal tract.
Both L. salivarius AP-32 and L. reuteri GL-104 had high hexose consumption efficiency, suggesting a simple strategy to modulate glycemic levels. These strains efficiently reduced monosaccharide content in the gut lumen of diabetic mice and downregulated SGLT1 and GLUT5 mRNAs and proteins in cultured human intestinal epithelial cells. SGLT1 and GLUT5 are essential hexose transporters expressed in the brush border membrane.17 Mice lacking SGLT1 show dramatically reduced glucose absorption in intestinal tissues and low glycemic levels.18 GLUT5 deficiency leads to impaired fructose transportation and gluconeogenesis-related enzyme expression.19 20 Thus, downregulation of SGLT1 and GLUT5 by probiotics may reduce fasting blood sugar levels and improve glucose tolerance in diabetic humans, consistent with their effects in this diabetic mouse model.
Regulation of the expression of both SGLT1 and GLUT5 is mediated by TNF-α expression and hexose abundance in the lumen.21 22 GL-104 induced TNF-α production in Caco-2, indicating that GL-104 may regulate hexose transporter expression through a TNF-α-dependent pathway. In contrast, AP-32 did not induce TNF-α expression, although it triggers an attenuated inflammatory reaction in Caco-2 cells after pathogenic stimulation,23 suggesting that AP-32 may not regulate SGLT1 through TNF-α signaling pathways. GL-104 may trigger TNF-α expression via pattern-recognition receptors. These receptors recognize conserved molecular structures of lactic acid bacteria, such as lipoproteins or muramyl dipeptides, stimulating inflammatory signal transduction pathways and eventually triggering TNF-α production to downregulate SGLT1 expression.24 Using monosaccharides as carbon sources, L. salivarius AP-32 and L. reuteri GL-104 rapidly and efficiently consume hexose in culture medium. Low hexose concentrations may contribute to GLUT5 downregulation.22 Interestingly, in contrast to GL-104, GLUT2 mRNA was increased in the presence of L. salivarius AP-32, while its protein was downregulated. GLUT2 is thought to allow basolateral intracellular glucose exit, showing little or no effect on hexose uptake from the intestine.25 Although L. salivarius AP-32 and L. reuteri GL-104 regulated GLUT2 protein via distinct mechanisms, both strains not only block monosaccharide uptake but also interfere with hexose translocation within host cells. Thus, the observed upregulation of GLUT2 mRNA may be compensatory, occurring in response to reductions in SGLT1 and GLUT5 expression.
T2DM can be caused by obesity. Therefore, db/db mice represent an optimal T2DM animal model for the investigation of T2DM pathogenesis and therapies.26 Diabetic db/db mice harbor a mutation in the Lepr gene encoding the leptin receptor, which results in disrupted leptin signaling and causes hyperphagic obesity, diabetic dyslipidemia, and insulin resistance.27 Both L. salivarius AP-32 and L. reuteri GL-104 significantly improved glucose tolerance and fasting blood glucose levels in db/db mice. Mice treated with either AP-32 or GL-104 showed fasting blood glucose levels comparable to those in mice treated with both strains, suggesting that these probiotics regulated glycemic levels independently. However, db/db mice treated with both strains (high dose) showed reduced glycemic levels at 180 min, indicating that supplementation with both strains might temporally extend the effects on glycemic regulation in patients with T2DM. The use of formulations containing multiple probiotic strains has recently attracted attention as a potential intervention for diabetes,28 29 given the complex factors causing diabetes and its associated complications.3 The immune modulation of L. salivarius AP-32 may contribute to reduction of chronic inflammation and insulin resistance in db/db mice.23 In addition, metabolites from L. reuteri GL-104 scavenge free radicals and show strong antioxidant effects (data not shown), potentially attenuating the symptoms of diabetes complications.
Patients with T2DM have abnormal blood lipid profiles and frequently suffer from dyslipidemia. For example, total CHOL, TG, and LDL levels in patients with T2DM are significantly higher than those in normal individuals.30 Several blood-sugar regulating strains, such as L. reuteri ADR-1, L. acidophilus La-5, and B. animalis BB-12, have been shown to improve blood lipid profiles in patients with T2DM.29 31 Consistent with these previous findings, in the present work, L. salivarius AP-32 and L. reuteri GL-104 were found to reduce CHOL and TG levels and to improve HDL/LDL ratios in db/db mice. This suggests that management of blood lipid levels may be an important characteristic of functional probiotic strains used for the management of diabetes and should be considered in the selection of glycemic regulation strains.
Patients with T2DM have a high prevalence of liver and kidney diseases,3 with the gradual loss of liver and kidney function. Here, we found that L. salivarius AP-32 and L. reuteri GL-104 significantly attenuated liver and kidney injury in an animal model. ALT and BUN levels in serum were significantly reduced, while AST and CREA concentrations were decreased, although not significantly, by probiotic treatment. AST levels in blood could be interfered by multiple factors, in comparison to ALT,32 and improvements of impaired fasting glucose or diabetes symptoms might lead to an increased serum CREA.33 These could be the factors causing the non-significant reductions we observed. Obesity is a key factor leading to T2DM in db/db mice. In this study, the body weights of db/db mice were not altered by probiotic treatment, consistent with the findings of short-term animal studies using L. rhamnosus GG, which was also shown to have positive effects on glycemic management.34 Thus, short-term intervention with probiotics may not be sufficient to reduce body weights, even at dosages of up to 1010 CFU/day,34–36 and longer-term probiotic treatments may be required.
In this study, we showed that both L. salivarius AP-32 and L. reuteri GL-104 rapidly consumed hexose and downregulated SGLT1 and GLUT5 in Caco-2 cells, suggesting that they may significantly block sugar uptake in the gut. However, direct clinical and in vivo evidence for this is insufficient and unclear; further animal or clinical studies on the expression and regulation of these hexose transporters in distinct parts of the intestinal tract are required. Because the kidneys are essential sugar absorption organs, it will be important to evaluate the effects of these probiotics on kidney sugar absorption. Although glucose tolerance, fasting blood glucose, and diabetes-related liver and kidney injuries were improved in our animal model, clinical trials of both probiotic strains are needed to determine their therapeutic efficacy in patients with T2DM. Overall, our data identified L. salivarius AP-32 and L. reuteri GL-104 as candidate probiotic strains for the clinical management of glycemic levels and attenuation of diabetes complications.
Acknowledgments
We thank the National Cheng-Kung University Animal Center for technical support and animal care.
Footnotes
Contributors: S-HH evaluated data and wrote the article, P-SH designed the experiments, H-HH designed the experiments and analysed data, Y-WK performed the experiments and evaluated the data. H-YT performed the animal experiments and evaluated the data, and J-YW designed the animal model and evaluated the data. The manuscript was critically revised and approved for submission by all the authors. All authors agreed to be accountable for the content of the work.
Funding: This research was funded by Glac Biotech Co, Ltd, Tainan City, Taiwan, and was also supported in part by Higher Education Sprout Project, Ministry of Education to the Headquarters of University Advancement at National Cheng Kung University.
Competing interests: P-SH, H-HH, S-HH and Y-WK are employers of Glac Biotech Co, Ltd, Tainan City, Taiwan.
Patient consent for publication: Not required.
Ethics approval: The animal study was approved by the National Cheng-Kung University Animal Ethics Committee (reference approval no. 107024).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1.Hameed I, Masoodi SR, Mir SA, et al. . Type 2 diabetes mellitus: from a metabolic disorder to an inflammatory condition. World J Diabetes 2015;6:598–612. 10.4239/wjd.v6.i4.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wild S, Roglic G, Green A, et al. . Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–53. 10.2337/diacare.27.5.1047 [DOI] [PubMed] [Google Scholar]
- 3.Udler MS. Type 2 diabetes: multiple genes, multiple diseases. Curr Diab Rep 2019;19:55. 10.1007/s11892-019-1169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chester B, Babu JR, Greene MW, et al. . The effects of popular diets on type 2 diabetes management. Diabetes Metab Res Rev 2019;35:e3188. 10.1002/dmrr.3188 [DOI] [PubMed] [Google Scholar]
- 5.Sabag A, Way KL, Keating SE, et al. . Exercise and ectopic fat in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab 2017;43:195–210. 10.1016/j.diabet.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 6.Yehya A, Sadhu AR. New therapeutic strategies for type 2 diabetes CME. Methodist Debakey Cardiovasc J 2018;14:281–8. 10.14797/mdcj-14-4-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bekiari E, Rizava C, Athanasiadou E, et al. . Systematic review and meta-analysis of vildagliptin for treatment of type 2 diabetes. Endocrine 2016;52:458–80. 10.1007/s12020-015-0841-1 [DOI] [PubMed] [Google Scholar]
- 8.Dujic T, Causevic A, Bego T, et al. . Organic cation transporter 1 variants and gastrointestinal side effects of metformin in patients with type 2 diabetes. Diabet Med 2016;33:511–4. 10.1111/dme.13040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hambridge K. The management of lipohypertrophy in diabetes care. Br J Nurs 2007;16:520–4. 10.12968/bjon.2007.16.9.23428 [DOI] [PubMed] [Google Scholar]
- 10.Sun Z, Sun X, Li J, et al. . Using probiotics for type 2 diabetes mellitus intervention: advances, questions, and potential. Crit Rev Food Sci Nutr 2020;60:1–14. 10.1080/10408398.2018.1547268 [DOI] [PubMed] [Google Scholar]
- 11.Halawa MR, El-Salam MA, Mostafa BM, et al. . The gut microbiome, Lactobacillus acidophilus; relation with type 2 diabetes mellitus. Curr Diabetes Rev 2019. [DOI] [PubMed] [Google Scholar]
- 12.Wu X, Ma C, Han L, et al. . Molecular characterisation of the faecal microbiota in patients with type II diabetes. Curr Microbiol 2010;61:69–78. 10.1007/s00284-010-9582-9 [DOI] [PubMed] [Google Scholar]
- 13.Razmpoosh E, Javadi A, Ejtahed HS, et al. . The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: a randomized placebo controlled trial. Diabetes Metab Syndr 2019;13:175–82. 10.1016/j.dsx.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 14.Khalili L, Alipour B, Asghari Jafar-Abadi M, et al. . The effects of Lactobacillus casei on glycemic response, serum sirtuin1 and fetuin-A levels in patients with type 2 diabetes mellitus: a randomized controlled trial. Iran Biomed J 2019;23:68–77. 10.29252/ibj.23.1.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kassaian N, Feizi A, Aminorroaya A, et al. . The effects of probiotics and synbiotic supplementation on glucose and insulin metabolism in adults with prediabetes: a double-blind randomized clinical trial. Acta Diabetol 2018;55:1019–28. 10.1007/s00592-018-1175-2 [DOI] [PubMed] [Google Scholar]
- 16.Gusakov AV, Kondratyeva EG, Sinitsyn AP. Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities. Int J Anal Chem 2011;2011:283658. 10.1155/2011/283658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drozdowski LA, Thomson ABR. Intestinal sugar transport. World J Gastroenterol 2006;12:1657–70. 10.3748/wjg.v12.i11.1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Röder PV, Geillinger KE, Zietek TS, et al. . The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One 2014;9:e89977. 10.1371/journal.pone.0089977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel C, Douard V, Yu S, et al. . Transport, metabolism, and endosomal trafficking-dependent regulation of intestinal fructose absorption. Faseb J 2015;29:4046–58. 10.1096/fj.15-272195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel C, Douard V, Yu S, et al. . Fructose-induced increases in expression of intestinal fructolytic and gluconeogenic genes are regulated by GLUT5 and KHK. Am J Physiol Regul Integr Comp Physiol 2015;309:R499–509. 10.1152/ajpregu.00128.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrenetxe J, Sánchez O, Barber A, et al. . TNFalpha regulates sugar transporters in the human intestinal epithelial cell line Caco-2. Cytokine 2013;64:181–7. 10.1016/j.cyto.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 22.Cui X-L, Jiang L, Ferraris RP. Regulation of rat intestinal GLUT2 mRNA abundance by luminal and systemic factors. Biochim Biophys Acta 2003;1612:178–85. 10.1016/S0005-2736(03)00129-9 [DOI] [PubMed] [Google Scholar]
- 23.Hsieh P-S, An Y, Tsai Y-C, et al. . Potential of probiotic strains to modulate the inflammatory responses of epithelial and immune cells in vitro. New Microbiol 2013;36:167–79. [PubMed] [Google Scholar]
- 24.Wells JM. Immunomodulatory mechanisms of lactobacilli. Microb Cell Fact 2011;10:S17. 10.1186/1475-2859-10-S1-S17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karasov WH. Integrative physiology of transcellular and paracellular intestinal absorption. J Exp Biol 2017;220:2495–501. 10.1242/jeb.144048 [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi K, Forte TM, Taniguchi S, et al. . The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism 2000;49:22–31. 10.1016/S0026-0495(00)90588-2 [DOI] [PubMed] [Google Scholar]
- 27.Kitada M, Ogura Y, Koya D. Rodent models of diabetic nephropathy: their utility and limitations. Int J Nephrol Renovasc Dis 2016;9:279–90. 10.2147/IJNRD.S103784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, et al. . Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab Syndr 2018;12:617–24. 10.1016/j.dsx.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 29.Tonucci LB, Olbrich Dos Santos KM, Licursi de Oliveira L, et al. . Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Clin Nutr 2017;36:85–92. 10.1016/j.clnu.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 30.Ozder A. Lipid profile abnormalities seen in T2DM patients in primary healthcare in Turkey: a cross-sectional study. Lipids Health Dis 2014;13:183. 10.1186/1476-511X-13-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh M-C, Tsai W-H, Jheng Y-P, et al. . The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci Rep 2018;8:16791. 10.1038/s41598-018-35014-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGill MR. The past and present of serum aminotransferases and the future of liver injury biomarkers. Excli J 2018;15:817–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida N, Miyake T, Yamamoto S, et al. . The serum creatinine level might be associated with the onset of impaired fasting glucose: a community-based longitudinal cohort health checkup study. Intern Med 2019;58:505–10. 10.2169/internalmedicine.0760-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park K-Y, Kim B, Hyun C-K. Lactobacillus rhamnosus GG improves glucose tolerance through alleviating ER stress and suppressing macrophage activation in db/db mice. J Clin Biochem Nutr 2015;56:240–6. 10.3164/jcbn.14-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Udayappan S, Manneras-Holm L, Chaplin-Scott A, et al. . Oral treatment with Eubacterium hallii improves insulin sensitivity in db/db mice. NPJ Biofilms Microbiomes 2016;2:16009. 10.1038/npjbiofilms.2016.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yun SI, Park HO, Kang JH. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol 2009;107:1681–6. 10.1111/j.1365-2672.2009.04350.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2019-001028supp001.pdf (254.6KB, pdf)