Abstract
The transition of a metastable liquid (supersaturated solution or supercooled melt) occurring from the intermediate stage (where the crystals nucleate and grow) to the concluding stage (where the larger particles evolve at the expense of the dissolution of smaller particles) is theoretically described, with allowance for various mass transfer mechanisms (reaction on the interface surface, volume diffusion, grain-boundary diffusion, diffusion along the dislocations) arising at the stage of Ostwald ripening (coalescence). The initial distribution function (its ‘tail’) for the concluding stage (forming as a result of the evolution of a particulate assemblage during the intermediate stage) is taken into account to determine the particle-size distribution function at the stage of Ostwald ripening. This modified distribution function essentially differs from the universal Lifshitz–Slyozov (LS) solutions for several mass transfer mechanisms. Namely, its maximum lies below and is shifted to the left in comparison with the LS asymptotic distribution function. In addition, the right branch of the particle-size distribution lies above and is shifted to the right of the LS blocking point. It is shown that the initial ‘tail’ of the particle-size distribution function completely determines its behaviour at the concluding stage of Ostwald ripening. The present theory agrees well with experimental data.
This article is part of the theme issue ‘Patterns in soft and biological matters’.
Keywords: nucleation, crystal growth, Ostwald ripening, phase transformations, particle-size distribution function
1. Introduction
The processes of phase and structural transformations in metastable liquids completely determine the nonlinear dynamics of the system, the evolving patterns in it, the evolution of polydisperse particulate assemblages as well as the final state and microstructure of the material. Such processes take place in different areas of applied science ranging from condensed matter physics, materials science and geophysics to chemical industry, biophysics and life science [1–19].
Generally speaking, the phase transformation processes in metastable liquids occur in several stages, each of which is described by the corresponding mathematical model. So, for example, at the initial stage, the supercooling (supersaturation) of the liquid practically does not change, and the solid phase particles nucleate on small impurity inclusions. At the intermediate stage of the phase transformation, the nucleation of crystals of a new phase and their further growth occur. As this takes place, the growing particles release the latent heat of crystallization (in the case of crystallization processes in supercooled melts) or absorb impurities from solutions (in the case of crystallization process in supersaturated solutions). This process reduces the liquid metastability (its supercooling or supersaturation) and leads to the formation of a polydisperse ensemble of particles evolving in a metastable liquid. Note that at this stage, the particles grow almost without interacting with each other. When the supercooling (supersaturation) of the liquid becomes very small, and the number of crystals becomes sufficiently large, the process of phase transformation goes to the final stage. Here such transformations as Ostwald ripening, agglomeration and disintegration of particles are capable to occur. In the case of Ostwald ripening, smaller crystals dissolve and larger crystals continue to grow at the expense of this dissolution process.
The theory of evolution of particulate assemblages at the intermediate stage with allowance for the diffusion mechanism of the particle-size distribution function in the space of particle radii was recently developed in articles [20–22]. This theory enables us to find an unsteady-state behaviour of the distribution function as well as the liquid supercooling (supersaturation) as a function of time. In 1978, Slezov developed a theory of Ostwald ripening (coalescence) process [23] taking into account the initial particle-size distribution function that forms to the beginning of ripening stage. He showed that this initial distribution function depends on the power-law ‘tail’ that forms to the beginning of this phase transition stage. This power-law dependence can be found from the exact analytical solutions that describe the final states of the intermediate stage from the analytical solutions [20–22]. Thus, a transition between the intermediate and ripening stages becomes described analytically. The present article develops such a theory for several types of mass transfer mechanisms occurring at the stage of Ostwald ripening.
This article is organized as follows. Section 2 is devoted to the theory of the intermediate stage, where the model equations and their analytical solutions are written out. The ‘tail’ of the particle-size distribution function that forms to the concluding phase of the intermediate stage (or to the beginning of the Ostwald ripening stage) is analytically found in §3. Keeping in mind this power-law ‘tail’ of the distribution function, new analytical solutions for the Ostwald ripening stage are constructed in §4. Our concluding remarks are formulated in §5. Two appendices give more detailed information on the theory presented in §4.
2. Analytical solutions describing the intermediate stage of a phase transition process
Let us first formulate a mathematical model describing a phase transformation process in a metastable liquid that occurs at its intermediate stage. A metastable medium is supercooled (or supersaturated) to a great degree so that nucleation and growth of solid-phase crystals are capable to occur. Their evolution is described by an integro-differential model of kinetic and balance equations that govern the dynamics of the particle-radius distribution function f(r, t) and liquid supercooling (or supersaturation) Δ(τ) of the form [20–22]
| 2.1 |
and
| 2.2 |
where r and τ are the spherical coordinate and time, dr/dτ is the particle growth rate, D is the coefficient of mutual Brownian diffusion of particles, is the radius of critical nuclei, Δ0 is the initial supercooling (or supersaturation) and b = 4πLV/(3ρmcmΔ0), if the phase transition occurs in a single-component supercooled melt (or b = 4πCp/(3Δ0) if the phase transition occurs in a supersaturated solution). Here, ρm and cm represent the density and specific heat of a metastable mixuture, and LV and Cp are the latent heat parameter and concentration at saturation. Note that the coefficient of mutual Brownian diffusion is defined by an ‘Einstein relation’ in the r space [24]. Generally speaking, its exact determination is a difficult task of statistical physics. Therefore, for the sake of simplicity, let us use the following simple approximation , where and d1 stand for the kinetic coefficient and a pertinent factor.
The boundary and initial conditions to the model equations (2.1) and (2.2) can be written out in the form [20–22]
| 2.3 |
Note that the first boundary condition (2.3) defines the flux of particles passing through a critical nucleation barrier. The nucleation rate I(Δ) should be calculated theoretically or determined from experimental data. Let us use here the frequently used expression known as the Meirs nucleation rate [25–32] , where p and represent empirical parameters.
Let us now introduce the following dimensionless variables and parameters:
| 2.4 |
where F(s, t) and w(t) are the dimensionless particle-radius distribution function and metastability degree (supercooling or supersaturation), and s and t are the dimensionless spatial and time variables.
The model (2.1)–(2.3) was analytically solved in the case of Meirs and Weber–Volmer–Frenkel–Zeldovich nucleation kinetics in our previous papers [20–22]. Let us write down here a simplest analytical solution that describes the Meirs kinetics as [21]
| 2.5 |
and
| 2.6 |
where
| 2.7 |
The analytical solutions (2.5)–(2.7) found in a parametric form (x is the parameter playing the role of modified time variable) describe the particle-radius distribution function F(s, x) and the metastability degree w(x) at the intermediate stage of a phase transformation process. To study a behaviour of the metastable system at its concluding (coalescence) stage, we should determine the ‘tail’ of the distribution function F(s, x) that forms at the end of intermediate stage or at the beginning of Ostwald ripening stage.
3. The ‘tail’ of the particle-size distribution function
In 1978, Slezov showed that the ‘tail’ of the particle-radius distribution function that is formed at the beginning of the Ostwald ripening stage completely characterizes the system states during the concluding (coalescence) stage of a phase transformation process [23]. To find this ‘tail’, let us use the analytical solutions (2.5)–(2.7) at the end of the intermediate stage.
First of all, we take into account that the complementary error function entering in the analytical solutions (2.5) and (2.7) has an asymptotic expansion at small values of the system parameter u0 ≪ 1 [20–22], i.e.
| 3.1 |
Now combining (2.5) and (3.1), we estimate the particle-size distribution function as
| 3.2 |
The integral contribution (3.2) can be estimated by means of the saddle-point method [33–36]. So, introducing
we estimate the distribution function (3.2) as
| 3.3 |
where yo = s is the minimum point of S(y). Substitution of yo and S′′(yo) = 2/s into formula (3.3) leads to
| 3.4 |
Now estimating functions h(y) and H(x) from (2.7) at u0 ≪ 1, we arrive at the following simple expressions
| 3.5 |
Substituting (3.5) into (2.6) and (3.4), we finally obtain the ‘tail’ of the distribution function, which reads as
| 3.6 |
If we put, for example, p = 3 (or p = 4), we get n = 8 (or n = 6). An important point is that the ‘tail’ (3.6) of the distribution function completely characterizes the relaxation process of the distribution function at the concluding stage of Ostwald ripening from its initial state described by the distribution (3.6) to the universal distribution function derived by Lifshitz and Slyozov (Slezov) for different mass transfer mechanisms [37–41]. Expression (3.6) shows that the ‘tail’ of the distribution function represents the power-dependent distribution predicted for the first time by Slezov [23]. Note that his approach was developed for unknown parameters A and n. The present theory taking into account the intermediate stage of a phase transformation phenomenon enables us to find these parameters and describe the concluding stage more precisely.
4. Analytical solutions describing the stage of Ostwald ripening
Let us now describe the particle-size distribution function at the stage of Ostwald ripening process where larger particles grow at the expense of dissolution of smaller particles. To define the initial distribution of particles at this stage, we use the analytical distribution (3.6), which determines the initial distribution function in dimensional form as . This power-dependent function defines a transition zone in the vicinity of the Lifshitz–Slyozov (LS) blocking point u0k = k/(k − 1) [23,39–43]. Note that the blocking point depends on the mass transfer mechanism so that u0k = u02 = 2, k = 2 (reaction on the interphase surface), u0k = u03 = 3/2, k = 3 (volume diffusion), u0k = u04 = 4/3, k = 4 (grain-boundary diffusion) and u0k = u05 = 5/4, k = 5 (diffusion along the dislocations). Following articles [42,43], we introduce the scaled variables and parameters, which read as
| 4.1 |
Here, represents the critical radius of crystals, is its value at the beginning of ripening stage, Q is the total initial quantity of the material (supersaturation), ωp is the volume per atom of pure solvent, ω is the volume of a single atom in the precipitate, u and τk stand for the rescaled space and time variables and Pk(u) represents the rescaled distribution function. We use here the standard designations and scales in the theory of Ostwald ripening processes [24]. Namely, the time scale is measured in units of , where Dd stands for the diffusion coefficient of the dissolved component, and σ = 2βv′ c0∞/(kBT) (β is the interface surface tension, v′ is the volume per atom of the dissolved component, c0∞ is the equilibrium concentration at the plane boundary, kB is the Boltzmann constant and T is the absolute temperature).
In the case of different mass transfer mechanisms under consideration, the growth rates of particles take the form [42,43]
| 4.2 |
where the coefficients λk (k = 2, 3, 4, 5) are determined in table 1.
Table 1.
Parameters entering in the growth rate of particles (4.2) for several mass transfer mechanisms [42,43].
| mass transfer mechanism | k | δk | λk | u2k | u1k |
|---|---|---|---|---|---|
| reaction on the interphase surface | 2 | 1 | 3 | ||
| volume diffusion | 3 | 1.182 | 1.786 | ||
| grain-boundary diffusion | 4 | 1.183 | 1.467 | ||
| diffusion along the dislocations | 5 | 1.153 | 1.336 |
Here, functions τk(u) are defined from the following Cauchy problem [42,43]:
| 4.3 |
and
| 4.4 |
where
ν2 = 2, ν3 = 1, ν4 = 2/3 and ν5 = 1/2 [42,43]. Note that the explicit differential equations for the evaluation of functions τk(u) for several mass transfer mechanisms are presented in appendix A.
The rescaled distribution function Pk(u) entering in (4.1) is determined by means of the following law [42,43]:
| 4.5 |
where vuk is given by formulae (4.2).
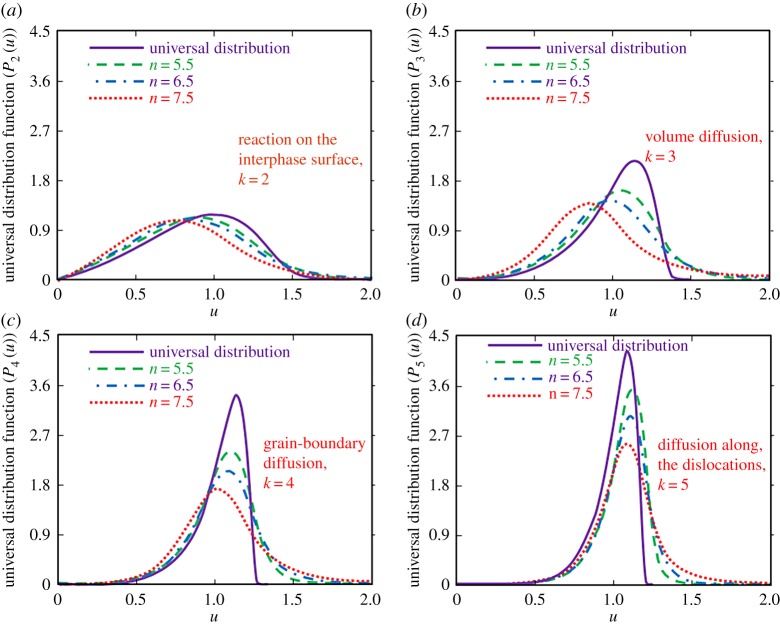
A limiting transition to the classical LS theory can be easily obtained from expressions (4.2) and (4.5) at large times τk → ∞. In this asymptotic case, one can obtain from solutions (4.2) and (4.5) the universal distribution functions Pk(u) given in table 2 for different mass transfer mechanisms k = 2, 3, 4, 5. These universal (limiting) distributions forming at large times are shown in figure 1. Note that each distribution function Pk(u) is defined to the left of its blocking point u0k. As this universal state attains only in the asymptotic limit of large times, it is important to determine the distribution function at smaller times. In this more realistic case, the solution is represented by the analytical expressions (4.2) and (4.5).
Table 2.
Universal distribution functions for several mass transfer mechanisms (these functions follow from expressions (4.2) and (4.5) at large times τk → ∞ [42,43]). Expressions for C4, C5, j(u), s, ξ, θ, η, ζ and h are presented in appendix B.
| k | Pk(u) |
|---|---|
| 2 | |
| 3 | |
| 4 | |
| 5 |
Figure 1.
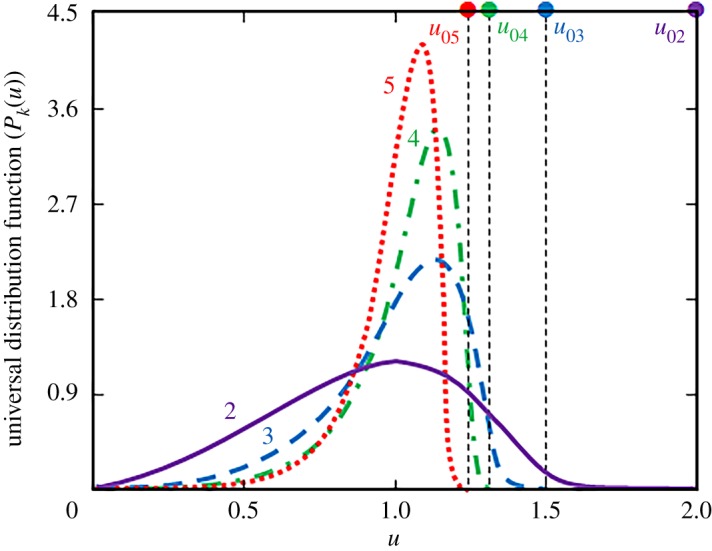
The universal distribution function (or the asymptotic state of a metastable system at the stage of Ostwald ripening) for several mass transfer mechanisms (numbers at the curves show different values of k). The mass transfer mechanisms are demonstrated by the solid (k = 2), dashed (k = 3), dash-dotted (k = 4) and dotted (k = 5) lines. The blocking points u0k for several mass transfer mechanisms are u02 = 2 (reaction on the interphase surface), u03 = 3/2 (volume diffusion), u04 = 4/3 (grain-boundary diffusion) and u05 = 5/4 (diffusion along the dislocations). (Online version in colour.)
Figure 2 shows the distribution functions (4.5) plotted for several mass transfer mechanisms with allowance for the ‘tail’ (3.6) of the initial particle-size distribution at the stage of Ostwald ripening process (here we use three shapes of the ‘tail’ (3.6) with n = 5.5, n = 6.5 and n = 7.5). Now the blocking point does not determine the behaviour of curves. The distribution function lies to the right of the corresponding blocking point and its decreasing behaviour at large values of u is determined by the transition region u2k < u < u1k for a concrete mass transfer mechanism (see, for details, table 1 and [42,43]). An important point is that the maximum point of the distribution function Pk(u) decreases and shifts to the left with increasing the exponent n of the initial ‘tail’ (3.6). As a result, the real distribution functions during the Ostwald ripening process essentially differ from the universal asymptotic distributions shown in figure 2 by the solid lines.
Figure 2.
The particle-size distribution function Pk(u) shown accordingly to expressions (4.5) for different mass transfer mechanisms. The solid lines demonstrate universal (asymptotic) solutions presented in table 2 at large times τk → ∞. (Online version in colour.)
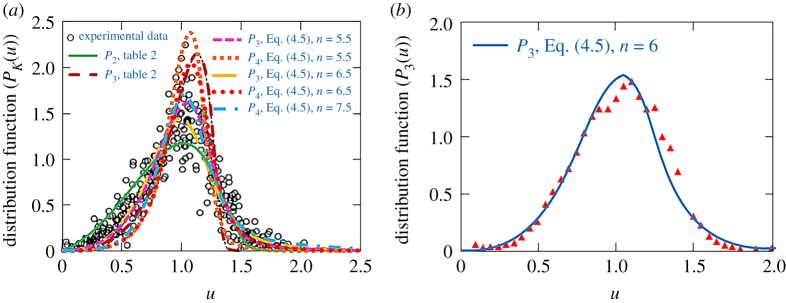
Figure 3 compares the theory under consideration with experimental data [44,45] for different mass transfer mechanisms and different shapes of the initial ‘tail’ of the particle-size distribution function. Twenty years of experiments [44] (figure 3a) confirm that the universal distribution functions P2 (u) and P3(u) given in table 2 cannot satisfactory describe experimental data: the corresponding distribution functions lie below or above of experimental points, and their behaviour is limited by the blocking points u0k. Taking into account the initial ‘tail’ (3.6) of the distribution function at the stage of Ostwald ripening we can describe experimental data. As is easily seen from figure 3, the maximum point of Pk(u) shifts closer to experimental points, its left branch has the correct inflection whereas its right branch lies above the blocking point and tends to the region of larger values of u. An important point is that this behaviour highly depends on the exponent n of the initial ‘tail’ (3.6). In other words, this ‘tail’ completely determines the particle-size distribution function at the concluding stage of Ostwald ripening process as previously predicted by Slezov [23,39].
Figure 3.
The distribution function Pk(u) (table 2 and solution (4.5)) is compared with experimental data ([44] (a) and [45] (b) for several mass transfer mechanisms, . (Online version in colour.)
5. Concluding remarks
In summary, a transition of the phase transformation process occurring in metastable liquids between the intermediate stage and the concluding stage of Ostwald ripening is theoretically described by sewing together two different analytical theories. First of all, from the analytical solutions describing the intermediate stage, we derived the initial ‘tail’ of the particle-size distribution function that forms at the beginning of the Ostwald ripening stage. Taking this ‘tail’ into account, we then found the distribution function that determines the state of a metastable system during the ripening stage when the smaller particles dissolve and the larger particles exist and evolve at the expense of this dissolution. Comparing our analytical theory with experimental data we conclude that the exponent n of the initial ‘tail’ (3.6) completely defines the behaviour of experimental data at the stage of Ostwald ripening. Namely, the shape of the distribution function changes drastically. Its maximum point becomes lower and shifts to the left side (to smaller values of u) with increasing n. As this takes place, the blocking point disappears, the right branch of the distribution function becomes higher and goes much to the right in comparison with the universal asymptotic (LS) distribution function. In addition, the left branch of the distribution function has a correct inflection that is confirmed by experimental points (if k = 2, 3, 4 this branch lies above the LS distribution curve).
Different mass transfer mechanisms at the stage of Ostwald ripening also have a decisive role in the particle-size distribution. As is easily seen from figures 1 to 3, the distribution function becomes broader and lower with decreasing the mass transfer constant k. Let us especially underline that several mass transfer mechanisms can occur simultaneously in real Ostwald ripening processes [46] (e.g. the mass transfer always happens along the grain boundaries and dislocation lines simultaneously with the volume diffusion [46]). Such a phase transformation theory represents an interesting and important task for future investigations that can be studied in the spirit of previous works [39,42,43,46]. Another important task is to combine the theories of directional and bulk crystallization (when dendritic growth, nucleation and particle growth occur simultaneously), the solution of which can be performed using the described approach and the theory of a two-phase (mushy) layer (see, among others, [47–52]).
Appendix A. Explicit differential equations for the functions τk(u)
The boundary-value problem for these differential equations is formulated by means of the initial condition (4.4) at u = 0 for a concrete mass transfer mechanism.
Appendix B. Coefficients used in the universal distribution functions (table 2)
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed equally to the present research article.
Competing interests
The authors declare that they have no competing interests.
Funding
This work was supported by the Russian Science Foundation(grant no. 18-19-00008).
Reference
- 1.Schlichtkrull J. 1957. Insulin crystals. V. The nucleation and growth of insulin crystals. Acta Chem. Scand. 11, 439–460. ( 10.3891/acta.chem.scand.11-0439) [DOI] [Google Scholar]
- 2.Schlichtkrull J. 1957. Insulin crystals. VII. The growth of insulin crystals. Acta Chem. Scand. 11, 1248–1256. ( 10.3891/acta.chem.scand.11-1248) [DOI] [Google Scholar]
- 3.Alexandrov DV, Zubarev AY. 2019. Heterogeneous materials: metastable and non-ergodic internal structures. Phil. Trans. R. Soc. A 377, 20180353 ( 10.1098/rsta.2018.0353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skripov VP. 1974. Metastable liquids. New York, NY: John Wiley & Sons. [Google Scholar]
- 5.Herlach D, Galenko P, Holland-Moritz D. 2007. Metastable solids from undercooled melts. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 6.Kelton KF, Greer AL. 2010. Nucleation in condensed matter: applications in materials and biology. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 7.Alexandrova IV, Alexandrov DV. 2020. Dynamics of particulate assemblages in metastable liquids: a test of theory with nucleation and growth kinetics. Phil. Trans. R. Soc. A 378, 20190425 ( 10.1098/rsta.2019.0425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexandrov DV, Malygin AP. 2011. Coupled convective and morphological instability of the inner core boundary of the Earth. Phys. Earth Planet. Inter. 189, 134–141. ( 10.1016/j.pepi.2011.08.004) [DOI] [Google Scholar]
- 9.Dubrovskii VG. 2014. Nucleation theory and growth of nanostructures. Berlin, Germany: Springer. [Google Scholar]
- 10.Frenkel J. 1955. Kinetic theory of liquids. New York, NY: Dover. [Google Scholar]
- 11.Galenko PK, Alexandrov DV. 2018. From atomistic interfaces to dendritic patterns. Phil. Trans. R. Soc. A 376, 20170210 ( 10.1098/rsta.2017.0210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexandrov DV, Nizovtseva IG. 2019. On the theory of crystal growth in metastable systems with biomedical applications: protein and insulin crystallization. Phil. Trans. R. Soc. A 377, 20180214 ( 10.1098/rsta.2018.0214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin S, Kauffman P. 1974. The evolution of under-ice melt ponds, or double diffusion at the freezing point. J. Fluid Mech. 64, 507–527. ( 10.1017/S0022112074002527) [DOI] [Google Scholar]
- 14.Ootaki M, Endo S, Sugawara Y, Takahashi T. 2009. Crystal habits of cubic insulin from porcine pancreas and evaluation of intermolecular interactions by macrobond and EET analyses. J. Cryst. Growth 311, 4226–4234. ( 10.1016/j.jcrysgro.2009.07.025) [DOI] [Google Scholar]
- 15.Alexandrov DV. 2014. Nucleation and growth of crystals at the intermediate stage of phase transformations in binary melts. Phil. Mag. Lett. 94, 786–793. ( 10.1080/09500839.2014.977975) [DOI] [Google Scholar]
- 16.Kurz W, Fisher DJ. 1989. Fundamentals of solidification. Aedermannsdorf, Switzerland: Trans Tech Publications. [Google Scholar]
- 17.Libbrecht K. 2004. Snowflakes. Minneapolis, MN: Voyageur Press. [Google Scholar]
- 18.Nizovtseva IG, Alexandrov DV. 2020. The effect of density changes on crystallization with a mushy layer. Phil. Trans. R. Soc. A 378, 20190248 ( 10.1098/rsta.2019.0248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov AA, Alexandrov DV, Alexandrova IV. 2020. Dissolution of polydisperse ensembles of crystals in channels with a forced flow. Phil. Trans. R. Soc. A 378, 20190246 ( 10.1098/rsta.2019.0246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexandrov DV. 2014. On the theory of transient nucleation at the intermediate stage of phase transitions. Phys. Lett. A 378, 1501–1504. ( 10.1016/j.physleta.2014.03.051) [DOI] [Google Scholar]
- 21.Alexandrov DV, Malygin AP. 2014. Nucleation kinetics and crystal growth with fluctuating rates at the intermediate stage of phase transitions. Model. Simul. Mater. Sci. Eng. 22, 015003 ( 10.1088/0965-0393/22/1/015003) [DOI] [Google Scholar]
- 22.Alexandrov DV, Nizovtseva IG. 2014. Nucleation and particle growth with fluctuating rates at the intermediate stage of phase transitions in metastable systems. Proc. R. Soc. A 470, 20130647 ( 10.1098/rspa.2013.0647) [DOI] [Google Scholar]
- 23.Slezov VV. 1978. Formation of the universal distribution function in the dimension space for new-phase particles in the diffusive decomposition of the supersaturated solid solution. J. Phys. Chem. Solids 39, 367–374. ( 10.1016/0022-3697(78)90078-1) [DOI] [Google Scholar]
- 24.Lifshitz EM, Pitaevskii LP. 1981. Physical kinetics. New York, NY: Pergamon Press. [Google Scholar]
- 25.Mullin JW. 1972. Crystallization. London, UK: Butterworths. [Google Scholar]
- 26.Buyevich YuA, Mansurov VV. 1990. Kinetics of the intermediate stage of phase transition in batch crystallization. J. Cryst. Growth 104, 861–867. ( 10.1016/0022-0248(90)90112-X) [DOI] [Google Scholar]
- 27.Barlow DA. 2009. Theory of the intermediate stage of crystal growth with applications to protein crystallization. J. Cryst. Growth 311, 2480–2483. ( 10.1016/j.jcrysgro.2009.02.035) [DOI] [Google Scholar]
- 28.Barlow DA. 2017. Theory of the intermediate stage of crystal growth with applications to insulin crystallization. J. Cryst. Growth 470, 8–14. ( 10.1016/j.jcrysgro.2017.03.053) [DOI] [Google Scholar]
- 29.Alexandrov DV, Ivanov AA, Alexandrova IV. 2018. Analytical solutions of mushy layer equations describing directional solidification in the presence of nucleation. Phil. Trans. R. Soc. A 376, 20170217 ( 10.1098/rsta.2017.0217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makoveeva EV, Alexandrov DV. 2018. A complete analytical solution of the Fokker-Planck and balance equations for nucleation and growth of crystals. Phil. Trans. R. Soc. A 376, 20170327 ( 10.1098/rsta.2017.0327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makoveeva EV, Alexandrov DV. 2019. Effects of nonlinear growth rates of spherical crystals and their withdrawal rate from a crystallizer on the particle-size distribution function. Phil. Trans. R. Soc. A 377, 20180210 ( 10.1098/rsta.2018.0210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buyevich YuA, Goldobin YuM, Yasnikov GP. 1994. Evolution of a particulate system governed by exchange with its environment. Int. J. Heat Mass Trans. 37, 3003–3014. ( 10.1016/0017-9310(94)90354-9) [DOI] [Google Scholar]
- 33.Fedoruk MV. 1977. Saddle-point method. Moscow, Russia: Nauka. [Google Scholar]
- 34.Alexandrov DV, Malygin AP. 2013. Transient nucleation kinetics of crystal growth at the intermediate stage of bulk phase transitions. J. Phys. A.: Math. Theor. 45, 455101 ( 10.1088/1751-8113/46/45/455101) [DOI] [Google Scholar]
- 35.Ivanov AA, Alexandrova IV, Alexandrov DV. 2019. Phase transformations in metastable liquids combined with polymerization. Phil. Trans. R. Soc. A 377, 20180215 ( 10.1098/rsta.2018.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexandrov DV. 2014. Nucleation and crystal growth kinetics during solidification: the role of crystallite withdrawal rate and external heat and mass sources. Chem. Eng. Sci. 117, 156–160. ( 10.1016/j.ces.2014.06.012) [DOI] [Google Scholar]
- 37.Lifshitz IM, Slyozov VV. 1961. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 19, 35–50. ( 10.1016/0022-3697(61)90054-3) [DOI] [Google Scholar]
- 38.Slezov VV, Sagalovich VV. 1987. Diffusive decomposition of solid solutions. Sov. Phys. Usp. 30, 23–45. ( 10.1070/PU1987v030n01ABEH002792) [DOI] [Google Scholar]
- 39.Slezov VV. 2009. Kinetics of first-order phase transitions. Weinheim, Germany: Wiley, VCH. [Google Scholar]
- 40.Alexandrov DV. 2015. On the theory of Ostwald ripening: formation of the universal distribution. J. Phys. A: Math. Theor. 48, 035103 ( 10.1088/1751-8113/48/3/035103) [DOI] [Google Scholar]
- 41.Alexandrov DV. 2015. Relaxation dynamics of the phase transformation process at its ripening stage. J. Phys. A: Math. Theor. 48, 245101 ( 10.1088/1751-8113/48/24/245101) [DOI] [Google Scholar]
- 42.Alexandrov DV. 2016. On the theory of Ostwald ripening in the presence of different mass transfer mechanisms. J. Phys. Chem. Solids 91, 48–54. ( 10.1016/j.jpcs.2015.12.005) [DOI] [Google Scholar]
- 43.Alexandrov DV. 2017. Kinetics of diffusive decomposition in the case of several mass transfer mechanisms. J. Cryst. Growth 457, 11–18. ( 10.1016/j.jcrysgro.2016.03.045) [DOI] [Google Scholar]
- 44.Marder M. 1987. Correlations and Ostwald ripening. Phys. Rev. A 36, 858–874. ( 10.1103/PhysRevA.36.858) [DOI] [PubMed] [Google Scholar]
- 45.Pletcher BA, Wang KG, Glicksman ME. 2012. Experimental, computational and theoretical studies of δ′ phase coarsening in Al–Li alloys. Acta Mater. 60, 5803–5817. ( 10.1016/j.actamat.2012.07.021) [DOI] [Google Scholar]
- 46.Slezov VV, Sagalovich VV, Tanatarov LV. 1978. Theory of diffusive decomposition of supersaturated solid solution under the condition of simultaneous operation of several mass-transfer mechanisms. J. Phys. Chem. Solids 39, 705–709. ( 10.1016/0022-3697(78)90002-1) [DOI] [Google Scholar]
- 47.Alexandrov DV, Bashkirtseva IA, Ryashko LB. 2018. Nonlinear dynamics of mushy layers induced by external stochastic fluctuations. Phil. Trans. R. Soc. A 376, 20170216 ( 10.1098/rsta.2017.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alexandrov DV, Ivanov AA. 2009. The Stefan problem of solidification of ternary systems in the presence of moving phase transition regions. J. Exp. Theor. Phys. 108, 821–829. ( 10.1134/S1063776109050100) [DOI] [Google Scholar]
- 49.Worster MG. 1986. Solidification of an alloy from a cooled boundary. J. Fluid Mech. 167, 481–501. ( 10.1017/S0022112086002938) [DOI] [Google Scholar]
- 50.Kerr RC, Woods AW, Worster MG, Huppert HE. 1990. Solidification of an alloy cooled rom above. Part I. Equilibrium growth. J. Fluid Mech. 216, 323–342. ( 10.1017/S0022112090000453) [DOI] [Google Scholar]
- 51.Alexandrov DV, Bashkirtseva IA, Malygin AP, Ryashko LB. 2013. Sea ice dynamics induced by external stochastic fluctuations. Pure Appl. Geophys. 170, 2273–2282. ( 10.1007/s00024-013-0664-z) [DOI] [Google Scholar]
- 52.Alexandrov DV, Malygin AP, Alexandrova IV. 2006. Solidification of leads: approximate solutions of non-linear problem. Ann. Glaciol. 44, 118–122. ( 10.3189/172756406781811213) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.