Abstract
Follicular dendritic cell sarcoma (FDCS) is a rare and unusual cancer that arises from sustentacular cells of the lymph node that present antigen to B cells, rather than lymphocytes themselves. While surgery for primary disease is still paramount in primary management, for unresectable, recurrent and metastatic tumours, FDCS is frequently treated with anthracycline-based lymphoma chemotherapy regimens. In recent years, it is clear that Programmed Cell Death 1 (PD1)-directed immune checkpoint inhibitors (ICIs) are active in Hodgkin lymphoma, but significantly less active in non-Hodgkin’s lymphoma. These data raised the question of whether FDCS respond to ICI therapy. We present two patients with FDCS who were treated with nivolumab and ipilimumab with evidence of tumour response. These cases also highlight the difficulty in arriving at a proper diagnosis, emphasising the need for expert review of pathology to optimise treatment for these and other patients with sarcoma.
Keywords: cancer intervention, oncology, immunology
Background
Follicular dendritic cell sarcoma (FDCS) was first described by Monda et al in 1986 as a non-lymphocytic tumour arising from the lymph nodes.1 It is clearer now that the function of follicular dendritic cells (FDCs) is to present antigen to B cells, by virtue of the presence of complement receptors that serve as antigen traps for circulating antigens from pathogens.
Fewer than 1000 cases of FDCS have been reported in the literature.2 The median age of diagnosis of FDCS, also called FDC tumour, is in the fifth decade. There is no gender difference; however, a form resembling inflammatory pseudotumour (which itself is also termed inflammatory myofibroblastic tumour) is more common in women.3
FDCS, interdigitating dendritic cell sarcoma, (true) histiocytic sarcoma and Langerhans cell histiocytosis (LCH) comprise histiocytic and dendritic cell neoplasms, with morphological and pathological features of sarcoma rather than haematological malignancies or carcinoma. FDCS appear to arise from FDCs or their precursors, which can be traced to a mesenchymal lineage,4 5 and are found in the primary and secondary follicles in the lymph nodes. FDC plays an important role in antigen presentation to B cells, while interdigitating dendritic cells present antigens to T cells.
All of these extremely rare tumours typically present as masses in lymph nodes or extranodal tissues, only infrequently with systemic symptoms such as fever, episodic diaphoresis and weight loss. The natural history of FDCS is variable, with a predisposition towards local–regional recurrence.
One of the first case series of FDCS described several prognostic factors for outcome of FDCS.6 Intra-abdominal primary site, high mitotic count, coagulative necrosis and significant cellular atypia indicated more aggressive behaviour. Metastatic potential is underestimated; at least 40% of patients die from this disease. Given the rarity of this tumour, there is no standardised treatment. Surgical resection, followed by adjuvant chemotherapy or radiation all have been employed. Adjuvant chemotherapy choices are also controversial; experts often recommend an anthracycline-based regimen based on high-grade lymphoma or sarcoma therapy as an initial approach.7 8 Although different treatment modalities are used to prevent recurrences, half or more of patients relapse. After failure of anthracycline-based therapy, treatment is undefined.
Immune checkpoint inhibitors (ICIs) are increasingly recognised as therapeutic agents in a variety of cancers. In terms of tumours arising in lymph nodes, ICIs have shown more benefit in initial trials of Hodgkin lymphoma than in non-Hodgkin’s lymphoma, at least in a subtype agnostic basis.9 10 In particular, PD-1 inhibitors demonstrate promising efficacy in newly diagnosed classic Hodgkin lymphoma.11–13 However, the situation for the use of ICI in FDCS is less clear. Programmed cell death 1 ligand (PD-L1) staining has been reported to be positive in 50%–80% of FDCS, and provides a rationale to use immunomodulatory therapy in patients with FDCS.14 15 However, to date, few people with histiocytic and dendritic cell neoplasms have been treated with ICI. One young adult with histiocytic sarcoma has been described who responded to ICI therapy, and none as of early 2020 with FDCS or with LCH who have responded to such treatment.16 A case report describes a patient with FDCS receiving nivolumab for liver metastases, without success.17
We herein present two patients with recurrent FDCS, each receiving ICI as part of therapy for metastatic disease, demonstrating such therapy as a treatment option to examine further in this class of malignancies.
Case presentations
We treated two patients with FDCS with ipilimumab (Ipi) and nivolumab (Nivo) in the regulatory authority-approved doses and schedule. We opted for dual checkpoint inhibitor treatment over a PD1 inhibitor alone, owing to the aggressiveness of the relapses the patients experienced, after shared decision-making with the patients. Response to therapy was evaluated between weeks 7–10 by contrast enhanced CT or positron emission tomography (PET)-CT, as well as by physical examination at every treatment visit. One patient with FDCS experienced a complete radiological response as part of multimodality therapy; another patient had a Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1 partial response.
Patient 1
A woman in her early 50s presented with abdominal pain and night sweats increasing in frequency and intensity over 4 years, eventually leading to CT of abdomen and pelvis as well as MRI showing a 74×61 mm mass in the portacaval space displacing the portal vein and bile duct anteriorly along with enlarged and mediastinal and retroperitoneal lymph nodes. The initial diagnosis made from a biopsy at an outside institution was leiomyosarcoma. She subsequently underwent complete resection of a 10 cm retroperitoneal FDC tumour with five lymph nodes, which were all involved by tumour. The tumour was positive for vimentin, CD21, CD23, CD35, CD4 and inhibin. PD-L1 staining was positive, with a combined tumour score of 60%–70%.
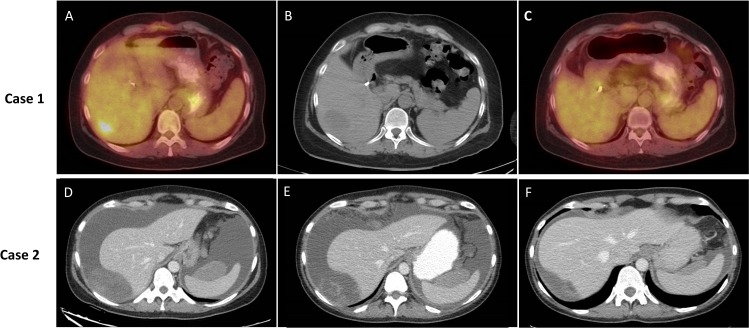
Given the high risk of recurrence and evidence of unresected mediastinal and retroperitoneal nodal disease at time of presentation, she was given adjuvant vincristine–doxorubicin–cyclophosphamide (VAdrC) for five cycles. Next-generation sequencing was performed on the tumour. She demonstrated no evidence of disease on imaging during treatment. However, 5 weeks after completion of chemotherapy, she developed recurrent diaphoresis and fevers, and was found to have a 3 cm liver mass, though there was no evidence of increased adenopathy (figure 1A–C). She underwent stereotactic radiation to the liver lesion (five fractions, total 25 Gy), and was started on Nivo/Ipi. Follow-up scans showed no 18F-fluorodeoxyglucose (FDG) avidity on PET-CT scans and no longer visible small lesions in the liver, thus achieving RECIST 1.1 complete response from combined modality therapy; her diaphoresis and fevers abated after the first two cycles of Nivo/Ipi. She was continued on therapy with Nivo 9 months after its initiation, without disease recurrence.
Figure 1.
Imaging of two patients with follicular dendritic cell sarcoma on treatment. (A–C) Case 1. (D–F) Case 2. (A) 18F FDG positron emission tomography (PET)-CT 2 weeks before biopsy of liver lesion. SUVmax of lesion was 6.2. (B) Non-contrast CT scan of liver lesion on day of biopsy showing somewhat larger tumour. (C) 18F FDG PET-CT after 5 months of nivolumab–ipilimumab therapy. (D) Intravenous and oral contrast enhanced CT scan of abdomen just before therapy showing peritoneal tumour deposits abutting liver and spleen. (E) Similar CT imaging after 6 weeks of treatment. (F) Similar CT imaging after 10 weeks of treatment. Some lesions grew in size but decreased in density at the 6-week scan, prompting continuation of therapy; lesions were improved in the week 10 imaging.
Patient 2
A woman in her mid 40s presented with pelvic pain, and found to have a 12 cm adnexal mass. Biopsy of the mass yielded an initial diagnosis of juvenile granulosa cell tumour (JGCT); however, after resection, the diagnosis was changed to FDCS. She declined adjuvant chemotherapy and remained free of disease for 1 year. She then recurred in her peritoneum with multiple pelvic and other peritoneal lesions as well as large volume ascites and peripheral oedema, necessitating weekly paracentesis. She again declined systemic chemotherapy, but agreed to start Nivo/Ipi. After the third cycle of immunotherapy, frequency and amount of ascites from paracentesis decreased significantly as did night sweats. A restaging scan after week 6 of treatment showed disease worsening by size, but increased necrosis in the tumour masses and decreased ascites (figure 1D–F). Given symptomatic improvement, she continued on treatment and repeat imaging after another 4 weeks showed marked improvement in ascites and peritoneal disease. She continues on treatment with decreased disease burden, RECIST stable disease (but dimensional decrease in sum of the longest diameters) after 7 months of treatment.
Both patients had B symptoms at time of disease recurrence, that is, fevers and sweats at the time of starting therapy, which resolved once immunotherapy was undertaken, within 8–12 weeks of starting therapy.
Investigations
Tumour investigations (immunohistochemistry (IHC) and molecular testing) for both patients are indicated in table 1 and figure 2.
Table 1.
Results of tumour testing for the two patients with follicular dendritic cell sarcoma
| Patient | Institutions reviewing pathology | IHC positive staining | Molecular testing performed | TMB (Muts/Mb) or MMR testing | PD-L1 testing | Mutations observed | # of cycles of checkpoint inhibitors | Prior systemic therapy |
| 1 | A, B, D | CD21 CD35 CD23 CD4 Inhibin Vimentin |
Foundation Hem | Low (4) | Combined score 60%–70% |
ARFRP1, ATR, AURKA, CCND2, FGF23, FGF6, GNAS, KDM5A, NFKBIA, SRC, TOP1, ZNFF217 | 8 | VAdrC |
| 2 | B, C, E | CD21 CD35 CD23 CD4 Inhibin |
Caris Life Science | MMR Sufficient | 2+, combined score 10% | ‘No actionable mutations’ | 6 | None |
Pathology: institutions included: A: Mt Sinai Medical Center New York, B: Northwell Health, C: New York Presbyterian Medical Center, D: Dana Farber Cancer Institute, E: Memorial Sloan Kettering Cancer Center. No translocations were found in molecular testing of patient 1; the testing for patient 2 did not include an RNA sequencing component to investigate for translocation fusion genes.
IHC, immunohistochemistry; MMR, mismatch repair; PD-L1, programmed cell death 1 ligand; TMB, tumour mutation burden; VAdrC, vincristine, doxorubicin, cyclophosphamide.
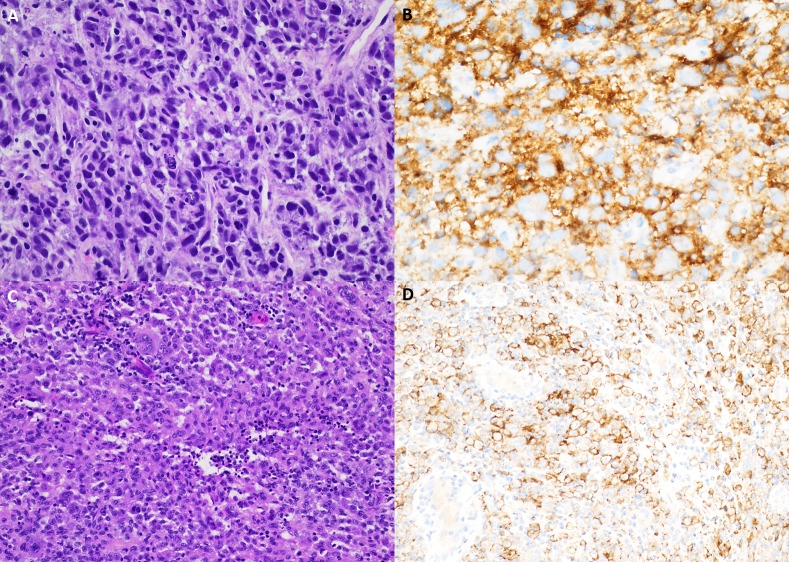
Figure 2.
Pathology and programmed cell death 1 ligand (PD-L1) analysis. (A) A liver core needle biopsy from patient 1 demonstrates a pleomorphic spindled to histiocytoid neoplasm with prominent lymphocytic infiltrate, single-cell apoptotic bodies and mitotic figures (40×, H&E). (B) Patient 1 PD-L1 263 immunohistochemistry demonstrates membranous and cytoplasmic staining of neoplastic cells (40×). (C) Patient 2 superficial soft-tissue resection shows a histiocytoid proliferation with mild-to-moderate cytologic atypia and a prominent neutrophilic and lymphocytic infiltrate (20×, H&E). (D) Patient 2 PD-L1 263 immunohistochemistry showing cytoplasmic and membranous staining of neoplastic cells (20×).
Differential diagnosis
Both cases were reviewed in more than one centre for a variety of reasons. FDCS is a family of tumours that are extremely rare and thus consultation slides were sent out for second opinions either by the pathologist or clinician after being reviewed locally to larger academic centres.
The mistaken identification of these two cases as leiomyosarcoma and JGCT indicates how difficult it is to make a diagnosis of this rare cancer. Depending on the anatomic site, the differential diagnosis for an FDCS includes other tumours of antigen-presenting cells, including LCH, Erdheim-Chester disease, interdigitating cell sarcoma, histiocytic sarcoma, as well as lymphoma (tumours are often treated as such initially), as well as other soft-tissue tumours such as inflammatory myofibroblastic tumour, undifferentiated pleomorphic sarcoma, or even melanoma or undifferentiated carcinomas. CD21, CD35 and CXCL13 IHC are commonly positive in FDCS, and can help differentiate them from other cancers as can more specific markers of FDCs such as FDC-secreted protein and serglycin.18
Outcome and follow-up
Both patients completed Ipi/Nivo induction therapy and were continued on Nivo monotherapy. By RECIST version 1.1, scans performed 8–12 weeks after starting treatment showed complete response to the combination of radiation and ICI (case 1, figure 1A–C) and one showed tumour shrinking, but RECIST 1.1 stable disease (less than 30% decrease of the sum of the greatest diameters of target lesions) on radiographic assessment (case 2, figure 1D–F).
Discussion
Histiocytic neoplasms represent a family of very rare cancers of sustentacular cells of the immune system, that is, macrophages and dendritic cells. Langerhans cells are a special form of myeloid dendritic cell, and LCH represents the most common subtype of these disorders. In all, there are perhaps 1000 new patients with these diagnoses per year in the USA, with LCH representing the substantial majority.
Histiocytic neoplasms are sometimes lumped with lymphomas, as such tumours are still composed of immune cells, and sometimes as sarcomas, to distinguish them as non-lymphocytic tumours of immune tissue. These conditions are clearly clonal malignancies that can cause morbidity or mortality, by virtue of finding characteristic BRAF V600E mutations in patients with LCH and other histiocytic and dendritic cell neoplasms,19 with associated favourable impact of BRAF inhibitors on treatments of these tumours.20–22 These tumours have their own category of ‘histiocytic and dendritic cell neoplasms’ in the 2016 revision of WHO classification of lymphoid neoplasms.
Treatment of antigen-presenting cell tumours spans the spectrum from surgery or radiation for isolated lesions, to systemic corticosteroids and vinblastine as the backbone of treatment for LCH, to systemic chemotherapy for the most aggressive and multifocal lesions. Mortality for the non-LCH tumours is relatively high owing to the relative resistance of this family of tumours to chemotherapy. Recent basket studies indicate the subset of these neoplasms that contain BRAF V600E mutations respond well to BRAF inhibitors, such as Erdheim-Chester disease and less frequently LCH, and have generated clinical trials that are presently accruing patients.20 21 In contrast, the use of ICIs in this family of antigen-presenting cell tumours has not been systematically examined, presumably from the rare nature of these tumours.
Few, if any, patients with histiocytic and dendritic cell tumours have been reported who have received ICI for disease resistant to systemic chemotherapy.16 After the first report of a non-responder with FDCS to nivolumab,17 we report here two patients with different clinical presentations, unified by the family of diagnosis and by evidence of a radiological response to an ICI combination. There are limitations on the quality of these case-based data. We note that follow-up on treatment is short, only 6 months. In addition, for case 1, we cannot formally rule out that chemotherapy successfully treated all nodal disease in the abdomen and mediastinum, and that radiation treated the only measurable disease that recurred in the liver. However, we cannot refute that both patients demonstrated rapid resolution of B symptoms on treatment. The result for case 2 also was notable for disease worsening by size criteria, but symptomatic improvement and decrease in ascites that led to continuation of therapy and subsequent demonstration of tumour shrinking, the so-called Choi response, from the gastrointestinal stromal tumour literature.23 24
Mechanistically, we thought it rational to use an ICI on a tumour that expressed PD-L1 and also represented a source of antigen for B cells, with the assumption that target antigens also were processed in the context of class I MHC molecules and expressed on the surface of the FDC tumour for T cells. Histiocytic and dendritic cell tumours often express high levels of PD-L1, but it is not clear if this is a requirement for responsiveness to these agents. However, as ready-made troves of antigen, it is interesting to contemplate FDCs as ideal targets to enhance an antitumour immune response in a specific tumour microenvironment.
Engineering existing patient cancers to have more immunogenic antigen-presenting cells as part of their immune microenvironment is one goal of newer agents being testing in cancer immunotherapy trials. We speculate that FDCS and related tumours may represent extreme examples of cancers that have evidence of tertiary lymphoid structures,25 with T cells, B cells and immunofibroblasts, which are increasingly being recognised as important in responsiveness to ICI.26 We hope the report of these cases will raise interest in conducting prospective clinical trials of ICI in this histiocytic and dendritic cell neoplasms such as FDCS, with the expectation that a meaningful proportion of them will respond to such immune manipulation.
Learning points.
Follicular dendritic cell sarcoma (FDCS) is one of a family of cancers arising from antigen-presenting cells.
FDCS is usually primarily treated like high-grade lymphoma or sarcoma, with ~50% recurrence after definitive surgery.
Programmed cell death 1 ligand staining is observed in the majority of FDCS as a starting point for exploration of its use as a biomarker to stratify treatments.
Immune checkpoint inhibitors appear to be a meaningful treatment option in chemoresistant patients with FDCS or as perhaps even as frontline therapy.
We recommend prospective basket trials of immune checkpoint inhibitors in histiocytic and dendritic cell neoplasms to obtain more experience with these agents in this family of diagnoses.
Acknowledgments
RGM acknowledges unrestricted research support from Fondazione Enrico Pallazzo and from his patients and their families.
Footnotes
Twitter: @SarcomaRx
Contributors: RGM and M-yL were responsible for conception and design of the manuscript. All authors were involved in the reporting, acquisition of data and interpretation of data. All authors contributed to the writing of the manuscript and gave their approval of the final version of the text as prepared.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol 1986;122:562–72. [PMC free article] [PubMed] [Google Scholar]
- 2.Perkins SM, Shinohara ET. Interdigitating and follicular dendritic cell sarcomas: a SEER analysis. Am J Clin Oncol 2013;36:395–8. 10.1097/COC.0b013e31824be22b [DOI] [PubMed] [Google Scholar]
- 3.Li X-Q, Cheuk W, Lam PWY, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor of liver and spleen: granulomatous and eosinophil-rich variants mimicking inflammatory or infective lesions. Am J Surg Pathol 2014;38:646–53. 10.1097/PAS.0000000000000170 [DOI] [PubMed] [Google Scholar]
- 4.Denton AE, Carr EJ, Magiera LP, et al. Embryonic FAP+ lymphoid tissue organizer cells generate the reticular network of adult lymph nodes. J Exp Med 2019;216:2242–52. 10.1084/jem.20181705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarjour M, Jorquera A, Mondor I, et al. Fate mapping reveals origin and dynamics of lymph node follicular dendritic cells. J Exp Med 2014;211:1109–22. 10.1084/jem.20132409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Ordoñez B, Rosai J. Follicular dendritic cell tumor: review of the entity. Semin Diagn Pathol 1998;15:144–54. [PubMed] [Google Scholar]
- 7.Perez-Ordonez B, Erlandson RA, Rosai J. Follicular dendritic cell tumor: report of 13 additional cases of a distinctive entity. Am J Surg Pathol 1996;20:944–55. 10.1097/00000478-199608000-00003 [DOI] [PubMed] [Google Scholar]
- 8.Gounder M, Desai V, Kuk D, et al. Impact of surgery, radiation and systemic therapy on the outcomes of patients with dendritic cell and histiocytic sarcomas. Eur J Cancer 2015;51:2413–22. 10.1016/j.ejca.2015.06.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manson G, Houot R. Next-generation immunotherapies for lymphoma: one foot in the future. Ann Oncol 2018;29:588–601. 10.1093/annonc/mdy032 [DOI] [PubMed] [Google Scholar]
- 10.Bröckelmann PJ, Engert A. Checkpoint Inhibition in Hodgkin Lymphoma - a Review. Oncol Res Treat 2017;40:654–60. 10.1159/000481800 [DOI] [PubMed] [Google Scholar]
- 11.Chen R, Zinzani PL, Lee HJ, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood 2019;134:1144–53. 10.1182/blood.2019000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol 2017;35:2125–32. 10.1200/JCO.2016.72.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramchandren R, Domingo-Domènech E, Rueda A, et al. Nivolumab for newly diagnosed advanced-stage classic Hodgkin lymphoma: safety and efficacy in the phase II CheckMate 205 study. J Clin Oncol 2019;37:1997–2007. 10.1200/JCO.19.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Sun HH, Fletcher CDM, et al. Expression of programmed cell death 1 ligands (PD-L1 and PD-L2) in histiocytic and dendritic cell disorders. Am J Surg Pathol 2016;40:443–53. 10.1097/PAS.0000000000000590 [DOI] [PubMed] [Google Scholar]
- 15.Agaimy A, Michal M, Hadravsky L, et al. Follicular dendritic cell sarcoma: clinicopathologic study of 15 cases with emphasis on novel expression of MDM2, somatostatin receptor 2A, and PD-L1. Ann Diagn Pathol 2016;23:21–8. 10.1016/j.anndiagpath.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 16.Bose S, Robles J, McCall CM, et al. Favorable response to nivolumab in a young adult patient with metastatic histiocytic sarcoma. Pediatr Blood Cancer 2019;66:e27491. 10.1002/pbc.27491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cingam SR, Al Shaarani M, Takalkar A, et al. Follicular dendritic sarcoma masquerading as fibrosing mediastinitis. BMJ Case Rep 2017;2017:bcr2016218889. 10.1136/bcr-2016-218889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenzi L, Döring C, Rausch T, et al. Identification of novel follicular dendritic cell sarcoma markers, FDCSP and SRGN, by whole transcriptome sequencing. Oncotarget 2017;8:16463–72. 10.18632/oncotarget.14864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Héritier S, Emile J-F, Barkaoui M-A, et al. BRAF mutation correlates with high-risk Langerhans cell histiocytosis and increased resistance to first-line therapy. J Clin Oncol 2016;34:3023–30. 10.1200/JCO.2015.65.9508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haroche J, Cohen-Aubart F, Emile J-F, et al. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. J Clin Oncol 2015;33:411–8. 10.1200/JCO.2014.57.1950 [DOI] [PubMed] [Google Scholar]
- 21.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 2015;373:726–36. 10.1056/NEJMoa1502309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donadieu J, Larabi IA, Tardieu M, et al. Vemurafenib for refractory multisystem Langerhans cell histiocytosis in children: an international observational study. J Clin Oncol 2019;37:2857–65. 10.1200/JCO.19.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamin RS, Choi H, Macapinlac HA, et al. We should desist using RECIST, at least in GIST. J Clin Oncol 2007;25:1760–4. 10.1200/JCO.2006.07.3411 [DOI] [PubMed] [Google Scholar]
- 24.Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 2007;25:1753–9. 10.1200/JCO.2006.07.3049 [DOI] [PubMed] [Google Scholar]
- 25.Nayar S, Campos J, Smith CG, et al. Immunofibroblasts are pivotal drivers of tertiary lymphoid structure formation and local pathology. Proc Natl Acad Sci U S A 2019;116:13490–7. 10.1073/pnas.1905301116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teillaud J-L, Dieu-Nosjean M-C. Tertiary lymphoid structures: an anti-tumor school for adaptive immune cells and an antibody factory to fight cancer? Front Immunol 2017;8:830. 10.3389/fimmu.2017.00830 [DOI] [PMC free article] [PubMed] [Google Scholar]