Abstract
COVID-19 pandemic outbreak is the most astounding scene ever experienced in the XXI century. In this opinionated review, we underscore the crucial role of biosensing to handle with such situations. As a matter of fact, testing accelerates life-saving decisions on treatment and isolation of COVID-19 patients in an early stage, and thereby, decelerating or even preventing the spread of such emerging infectious diseases. Meanwhile, it is also proven that a timely and broad application of testing leads to lower mortality rates in countries like Germany or South Korea. Besides, biosensors are also powerful tools for effective assessment of clinical progress and to provide alertness on severity or critical trends of infection. In view hereof, we critically discuss the state-of-the-art biosensing devices for COVID-19 testing. We spot the urgent needs and highlight innovative diagnostic approaches for targeting various COVID-19 related biomarkers. Finally, we outline our recommendations on biosensors and biosensing-related issues towards pandemic outbreaks.
Keywords: On-site testing, Emerging infectious diseases, CRISPR, PCR, Serological tests, Lateral flow assays, Biosensors
Graphical abstract
Highlights
-
•
The crucial role of diagnostics to handle with infectious diseases is highlighted.
-
•
Strengths and weaknesses of standard technologies targeting COVID-19 are discussed.
-
•
Novel biosensing devices for fighting the next pandemics are discussed.
-
•
COVID-19-related biomarkers are underscored.
-
•
Recommendations related to biosensing towards pandemic outbreaks are provided.
Announced as pandemic on 13 March, severe acute respiratory-related coronavirus 2 (SARS-CoV-2) is not only leading to an unexpected public health crisis worldwide (Fauci et al., 2020), but it is also becoming an unprecedented socio-economic burden (Schoch-Spana, 2020). In this context, World Health Organization (WHO) was urging the international community to perform massive diagnostic testing to fight transmission of the virus and decrease number of undetected cases, since testing is also a valuable tool, aiding researchers to learn the epidemiology of the disease. Moreover, diagnostics play a decisive role in making timely decisions on treatment and isolation of infected people, thereby, slowing or stopping the spread of the infectious diseases.
Testing along with the risk management and the healthcare system is a pivotal response in all outbreaks. Extraordinary examples of this response are occurring in a few countries; for instance, South Korea is now able to test around 20,000 citizens per day using aggressive testing campaigns enabled by drive-through and “phone booths” tests (South Korean hospital, 2020, South Korea’s “drive-through,” 2020; Testing times, 2020), and German laboratories are currently carrying out about 400,000 coronavirus tests every week (Becker, 2020), which may be contributing to the lowest mortality rates due to COVID-19 in the world. However, most of countries have a paucity of massive testing given supply problems (for example, specialized swabs, face masks and reagents) (Thomas, 2020) or a disparity in terms of politics and public health measurements (Apuzzo and Gebrekidan, 2020; Dewan et al., 2020.; Wittenberg-Cox, 2020).
The technology behind testing is biosensing, which aims to detect biological and (bio)chemical agents employing a biologically derived or a biomimetic recognition element while either undergoing a (bio)chemical reaction (for example, enzyme-based biosensors) or binding the target molecule (i.e. analyte) in a highly specific way. Such a binding event can be then transduced to a measurable signal either directly (for example, by means of impedance measurements or surface plasmon resonance), or employing signaling molecules (i.e. labels) including enzymes, fluorophores or electrochemically/optically active compounds (Dincer et al., 2019).
Once China published the coronavirus genetic code in early January, polymerase chain reaction (PCR)-based tests were developed around the world and COVID-19 is conventionally being diagnosed by means of this gold standard method (Chu et al., 2020; Read Online-Handbook, 2020). PCR tests are also crucial in order to take decisions related to contact tracing and isolation of specific citizens. It is worth mentioning that thoracic imaging, via computer tomography scans, portable chest X-rays and flexible bronchoscopy, is also valuable in diagnosis, therapy monitoring and discharge assessment related to COVID-19 infection since as far as it is known, it is mainly a respiratory illness (“COVID-19 and Coagulopathy - Hematology.org, 2020”; Read Online-Handbook, 2020; Udugama et al., 2020).
PCR involves amplification of minute traces of genetic material; i.e., the ribonucleic acid (RNA) of the virus, leading to highly specific and sensitive detection. However, PCR tests are generally performed in centralized diagnostic services by highly skilled personnel and their results may take from 4 hours up to 3 days. Apart from this, PCR tests are prone to the following limiting factors: (i) Sampling error: Nasopharyngeal swab is suitably performed to take mucus from the ventilatory system; however, this may give rise to false negatives as optimal sampling moment is still ambiguous. (ii) Besides, as a sample preparation (including cell lysis and nucleic acid purification) for PCR analysis is required, not only the number of the tests but also the number of extraction kits is a limiting factor for ramping up COVID-19 testing. (Shortage of RNA, 2020) (iii) Generally, PCR samples require specialized handling and transportation. Genetic material may be denatured during inadequate transportation, bringing also about false negatives. (iv) Quality of reagents utilized by different PCR kit manufacturers may also affect consistency among results. (v) Standard PCR methods may lack of sensitivity, potentially giving rise to false negative results in COVID-19 patients with unapparent clinical symptoms. For patients in recovery, this resulted in the heatedly debated issue with the “re-infection”, or more accurately “re-detectable positive” patients, as confirming tests with more-sensitive methods delivered again positive results (An et al., 2020). (vi) For recovered patients, even weeks after full recovery, PCR tests can report the presence of genetic material of the dead SARS-CoV-2, thereby, resulting in false positive outcomes (not particularly reinfections) (Bo-gyung, 2020). (vii) Because of the nucleic acid amplification employed, special primers and probes for each target are necessary which limits PCR's flexibility of scaling up for other nucleic acids in an easy and rapid manner. (viii) This previous point could even be a problem for the same target since SARS-CoV-2 RNA is likely to undergo mutations (Kupferschmidt K., 2020). Therefore, sequencing of genetic material of the virus should be continuously monitored and confirmed to ensure accuracy of this method. In this regard, as discussed below, other biosensing technologies offer a complementary approach to standard PCR-based tests not only in terms of sensitivity, but also in terms of diagnostics and therapeutic decisions. Moreover, the biosensing community is actively working to improve portability, time and cost of PCR-based SARS-CoV-2 detection (Nunez-Bajo et al., 2020) as well as to create easy-to-use PCR-based microfluidic devices with an integrated sample preparation (Hahn-Schickard, 2020 ). Interestingly, an international company envisions an alternative approach by detecting COVID-19 within the exhaled breath condensate using the combination of PCR and lateral flow assay (LFA) technology since it is an airborne droplet infection (The detection of COVID-19, 2020). In contrast to PCR, isothermal amplification of genetic material (at constant temperature) enables to develop point-of-care devices for the rapid detection of nucleic acids. A remarkable example of an approved technology for COVID-19 determination is shown by the company Abbott, which delivers results in around 13 min (Abbott Launches, 2020).
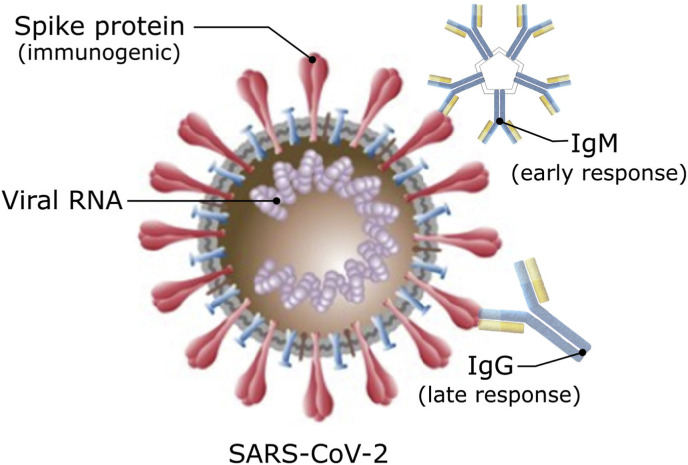
The possible ways to target SARS-CoV-2 via different biomolecules are summarized in Fig. 1 . For the COVID-19 testing, apart from the aforementioned viral RNA, novel coronavirus exhibits spike proteins which are immunogenic (Grifoni et al., 2020); hence, immune system is able to produce immunoglobulins to trigger an immune response against the pathogen. Importantly, these immunoglobulins are not only valuable to detect COVID-19, but also for its potential therapy (Chen et al., 2020). Immunoglobulin M (IgM) antibodies are produced during the onset of the infectious disease (between 4 and 10 days), whereas immunoglobulin G (IgG) response is produced later (around 2 weeks). (Immunoglobulin M Antibody, 2020; Read Online-Handbook, 2020)
Fig. 1.
Schematic illustrating the structure of SARS-CoV-2 and related targeting sites (biomolecules) that can be used for the COVID-19 detection. Not to scale.
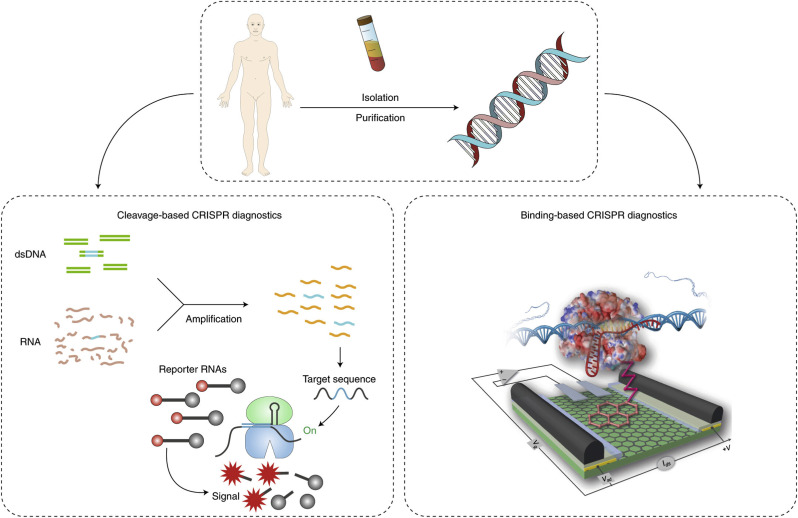
In order to overcome the aforementioned issues with PCR-based systems, researchers around the world are pushing hard to develop different methods and devices, allowing an easy, rapid, affordable and highly sensitive and selective quantification of nucleic acids in low-resource settings (such as doctors’ practices, or directly at home). For this purpose, a promising and powerful tool is the Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) technology, mainly employed in gene editing (Li et al., 2019; Zuo et al., 2017). Thanks to its different CRISPR-associated (Cas) enzymes, that can be programmed by single-guide RNAs (sgRNAs) or CRISPR RNAs (crRNAs), CRISPR technology enables to target all types of nucleic acids for a wide range of various targets, including bacteria, viruses, microRNAs and cancer mutations, in a simple and easily scalable way (only by changing the target-specific sgRNA/crRNA) (Bruch et al., 2019a (Trends in Biotechnology); Li et al., 2019). Current CRISPR-tailored approaches are based on two different detection modes, binding- or cleavage-based, shown in Fig. 2 .
Fig. 2.
Possible detection strategies for CRISPR-powered COVID-19 testing ( Bruch et al., 2019b (Nature Biomedical Engineering)). In cleavage-based approach, after the recognition event of the target gene of SARS-CoV-2, the Cas/crRNA complex gets activated and cleaves the surrounding labelled reporter RNAs (reRNA), resulting in a concentration-dependent signal change. For the binding-based CRISPR detection, a catalytically deactivated Cas/crRNA complex, specific to the target sequence of COVID-19, is immobilized on the sensor surface (for example, on a graphene-based field effect transistor as previously applied for detecting genetic mutations (Hajian et al., 2019)). Upon recognition of its target, the resulting signal can be read out via a handheld device.
Mammoth Biosciences and Sherlock Biosciences, leading companies in the field of CRISPR diagnostics, have already presented their CRISPR-powered assays to enable rapid and low-cost tests for SARS-CoV-2 employing conventional LFAs (Sheridan, 2020). Using the Cas12 effector (capable of targeting single-stranded DNAs and RNAs) and loop-mediated isothermal amplification (LAMP), Broughton and colleagues from Mammoth Biosciences have achieved a detection limit of around 10 copies per μL for the fluorescence detection of different regions of the SARS-CoV-2 viral genome (E gene and N gene) in extracted patient swab samples within 40 minutes (Broughton et al., 2020). On the other hand, CRISPR pioneer Feng Zhang and co-workers from Sherlock Biosciences have demonstrated the LFA-based colorimetric detection (only by the naked eye) of S gene and Orf1ab gene of COVID-19 with detection limits of 10 and 100 copies per μL, respectively, in less than 1 h, employing recombinase polymerase amplification (RPA) along with Cas13a enzyme (targeting single-stranded RNAs) (Zhang et al., 2020). This approach is not validated yet with patient samples.
However, these systems still employ a target amplification (such as LAMP or RPA) (Broughton et al., 2020; Shen et al., 2020); which covers up the CRISPR's great versatility as different primers and probes for each target are required. In this regard, two amplification-free biosensing systems using CRISPR technology have recently introduced: the CRISPR-Chip for electrical detection of genetic mutations using Cas9 (Hajian et al., 2019) and the electrochemical CRISPR-Biosensor for microRNA analysis employing Cas13a (Bruch et al., 2019c (Advanced Materials)). Both groups have announced their interest to extend their systems for an amplification-free on-site COVID-19 testing (Cardean Transistors, 2020; Diagnostics with molecular, 2020). Besides, CRISPR-Biosensor's group would like to target two characteristic genome sequences (E gene and RdRP gene) of SARS-CoV-2 simultaneously, using a multiplexed microfluidic chip. In principle, CRISPR-powered approaches allow for an easy adaptation to any nucleic acids and thus, can address potential mutations of SARS-CoV-2 in a timely manner. As another nucleic acid amplification-free approach, an optical biosensor combining plasmonic photothermal effect and localized surface plasmon resonance has been recently demonstrated to discriminate similar gene sequences with high analytical sensitivity (Qiu et al., 2020).
Another convenient way of sensing is the use of substrates (i.e. sensor material) functionalized with recombinant viral antigens which can be exploited to bind the aforementioned immunoglobulins. With this, it is possible to detect the presence of the novel coronavirus (via IgM) or to provide a history of past infections (via IgG) as these antibodies are much longer present in the blood (Vogel, 2020). In this regard, LFAs, well-known as at-home pregnancy and ovulation tests, offer an equipment free, user-friendly and rapid platform for optical on-site testing and are the masterpiece of biosensing and nanotechnology, so far (Marquez and Morales-Narváez, 2019). Moreover, as LFA utilizes cellulose-based materials (such as paper), it is amenable to mass production at very low costs (Morales-Narváez et al., 2017). Hence, commercial LFAs targeting the aforementioned immunoglobulins even simultaneously have already been developed and approved around the world, including Australia, China, Germany, Singapore, South Korea and USA (Sheridan, 2020). These devices are now a valuable tool to monitor the course of SARS-CoV-2 infection using a drop of human serum, plasma or even whole blood, offering qualitative results within 15 min at the point of care; for example, at home or primary care (Sheridan, 2020). Interestingly, collective positive tests facilitated by LFAs could lead to spot herd immunity regions, thereby possibly abandoning lockdowns. Moreover, Germany is planning to issue “immunity certificates” allowing citizens with positive antibody tests to stop their lockdown early (Bienkov, 2020). However, highly sensitive approaches enabling a quantitative testing are also demanded since the measurement of the virus-related immunoglobulin levels can be employed as diagnostic criteria for suspected cases with negative PCR results (Read Online-Handbook, 2020). In addition, determination of breadth and kinetics of immune responses can be meaningful to study non-severe cases of novel coronavirus, thereby forecasting infection outcome (Thevarajan et al., 2020). Although, employment of serological tests to determine COVID-19 immunity is still under debate (Lovelace Jr. and Feuer, 2020; Mallapaty, 2020), — likewise, the protection capability of face masks was cautiously discussed by WHO (Devlin and Campbell, 2020) — we believe that serological test will be a crucial tool to execute serosurveys (Stadlbauer et al., 2020), thereby, offering relevant information to take measures aimed at ceasing lockdowns and getting back to common life gradually.
Other biosensing strategies using antibodies to selectively capture the whole virus exist. A very recent example, CONVAT project, supported by the European Union, aims at the optical detection of the whole virus in 30 minutes to determine the viral load (viral content per mL) in saliva samples, whose result would be then followed via PCR in the same platform (Lechuga, 2020). Similarly, graphene-based field-effect transistors, enabling portable devices, have been engineered to determine COVID-19 viral load in clinical nasopharyngeal samples, using a specific antibody against its spike protein (Seo et al., 2020). Viral load, which is conventionally determined using quantitative real-time PCR, is not only important to determine the severity of the infectious disease, but also to monitor viral dynamics in infected patients and a therapy response (Pan et al., 2020). Hence, viral load determination may help to facilitate decisions driven by personalized medicine, thereby, effective clinical procedures can be applied. Nevertheless, antibody-based approaches mainly suffer from their relatively slow (typically a few weeks) development when compared with nucleic acid tests against a new infection as well as from their time-consuming and expensive production. Depending on the specificity of the employed antibodies, they may also suffer from false positives caused by potential cross-reactivity with other coronaviruses. Importantly, existing literature summarizes current commercialized technologies focused on COVID-19 diagnosis and compare their strengths and weaknesses (Mallapaty, 2020; Sheridan, 2020).
Detection of additional biomarkers, different than immunoglobulins, is another approach to perform effective assessment of clinical progress and provide alertness on severity or critical trends of COVID-19 infection. They are also valuable as a blueprint to formulate therapeutic procedures. The First Affiliated Hospital, Zhejiang University School of Medicine, a top hospital in China with zero fatalities, recommends the surveillance of the following inflammatory response biomarkers for the SARS-CoV-2 infection: C-reactive protein, procalcitonin, ferritin, D-dimer, IL-4, IL-6, IL-10, TNF-α and INF-γ. It is worth mentioning that low total number of lymphocytes at the onset of the disease is also an indicator of poor prognosis, whereas severe infections display a gradually decreased number of peripheral blood lymphocytes (Read Online-Handbook, 2020). Hence, lymphocyte analysis is also an effective resource in this emerging infectious disease. The clinical relevance of some of these biomarkers are discussed in Table 1 . In this context, the biosensing community is urged to create affordable, rapid, sensitive and multiplexed systems amenable to mass production in order to detect these biomarkers simultaneously. Besides, biosensing devices incorporating artificial antibodies (such as molecularly imprinted polymers) (Zamora-Gálvez et al., 2017) or other innovative antibodies (for example, shark antibodies) (Matz and Dooley, 2019) would be of great value in emerging infectious disease diagnostics, since they are amenable to withstand harsh conditions during handling and delivery.
Table 1.
Clinical relevance of inflammation markers in the SARS-CoV-2 diagnosis.a
| Biomolecular scenario | Clinical scenario |
|---|---|
| Procalcitonin: normal levels C-reactive protein: significantly increased levels |
Often displayed in patients with COVID-19 infection. |
| C-reactive protein: quick and significantly elevated levels | Potentiality of secondary bacterial or fungal infection. |
| D-dimer: significantly elevated levels | Severe cases of COVID-19 infection. Probability of poor prognosis. |
| IL-6 and IL-10: dramatically increased levels | Severe condition of COVID-19 infection. |
(“COVID-19 and Coagulopathy - Hematology.org, 2020; “Read Online-Handbook of COVID-19 Prevention and Treatment.pdf,” 2020).
All in all, as early career scientists and members of the biosensing community, facing the most dramatic situation ever experienced in XXI century, we believe that we will undergo other disease outbreaks caused not only by viral but also bacterial pathogens, and the world has to rethink the way we cope with such situations. Thus, we offer the following seven recommendations:
-
1.
Investment in pandemic preparedness in terms of diagnostic tools is pivotal to prevent the spread of emerging infectious diseases. This should be a joint and even an international task of different entities, including governments, funding agencies, academia and industries. We believe timely and large-scale testing based on reliable biosensing technology might lead to avoid or soften stringent measures such as lockdowns, border closing and travel bans, as shown in the example of South Korea or Iceland.
-
2.
Collaborative networks should be formed and involve not only experts in biosensing but also in biosecurity and their corresponding facilities, as well as robust biotechnology companies ready for mass production. Actually, getting their central innovation opportunities in testing not dominated by already established methods (like PCR), the biosensing community will play a critical role to control, slow, hinder or prevent such pandemic outbreaks.
-
3.
In case of stringent measures such as border closings, each country should be prepared (i.e. sustainable and independent) in terms of manufacture of biosensing technology and protection equipment. For instance, Germany develops various kind of machines for the production of protection equipment but even Germany, itself, has a limited activity to fabricate its own protection materials such as masks and protective clothing, leading to shortages of these items.
-
4.
The aforementioned collaborative networks should be ready to develop new diagnostic tools or adapt the existing ones rapidly and the governments should offer fast-track approval programs (such as the Food and Drug Administration - FDA) to examine those tools rapidly.
-
5.
Biosensing devices for testing infectious diseases should rigorously satisfy the following requirements: They must be (i) disposable, offering reliable mass production, (ii) accessible and affordable, allowing large-scale population screening, (iii) easy-to-use by minimally trained users or even by the patients themselves, (iv) equipment-free or operated with an inexpensive and portable readout unit, (v) rapid, enabling short sample-to-result times (less than an hour), (vi) able to work with low sample volumes and easily accessible samples (for example, blood from a finger prick or non-invasive swab samples), (vii) highly selective and sensitive (if possible, without target amplification), delivering accurate results in accordance with central laboratory findings, (viii) with an integrated sample preparation (including lysis and isolation for nucleic acid testing, or plasma separation for proteomics), (ix) easily scalable and flexible to detect different targets using the same platform (such as CRISPR-powered systems), and (x) capable of multiplexing, simultaneous detection of different analytes including controls (Dincer et al., 2017). Ideally, these systems should also be able to perform multiomics simultaneously (for instance, the detection of nucleic acids along with immunoglobulins)
-
6.
Internet-of-Things, connecting sensors via databases with healthcare workers to realize a decentralized healthcare, is also an important tool to provide valuable insights in terms of emerging infectious disease scenarios and dynamics, determination of herd immunity regions, as well as epidemiology and big data. In order to secure all these information, Blockchain technology, known from cryptocurrencies like bitcoin, could be applied for Internet-of-Things.
-
7.
General population should be trained in advance to perform effective self-sampling (for example, to collect nasal swabs) as well as self-testing (especially, for LFA-based immunity testing in order to estimate population coverage). In this regard, it would be helpful to have some classes in the high or secondary school, or seminars in companies where the general public gets familiar with issues like sample collection and performing self-tests (similar to fire drills).
CRediT authorship contribution statement
Eden Morales-Narváez: Conceptualization, Investigation, Writing - original draft, Writing - review & editing, Funding acquisition. Can Dincer: Conceptualization, Investigation, Writing - original draft, Writing - review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
E.M.-N. acknowledges financial support by SICES (Guanajuato, Mexico, Grants CMTEVAL-18-S01-03 and SICES/CONV/252/2019). C.D. would like to thank the German Research Foundation (DFG) for partially funding this work under grant numbers 421356369 and 404478562.
Contributor Information
Eden Morales-Narváez, Email: eden@cio.mx.
Can Dincer, Email: dincer@imtek.de.
References
- Abbott Launches Molecular Point-of-Care Test to Detect Novel Coronavirus in as Little as Five Minutes - Mar 27. Abbott MediaRoom; 2020. https://abbott.mediaroom.com/2020-03-27-Abbott-Launches-Molecular-Point-of-Care-Test-to-Detect-Novel-Coronavirus-in-as-Little-as-Five-Minutes 2020 [WWW Document] (accessed 3.28.20) [Google Scholar]
- An J., Liao X., Xiao T., Qian S., Yuan J., Ye H., Qi F., Shen C., Liu Yang, Wang L., Cheng X., Li N., Cai Q., Wang F., Chen J., Liu Yingxia, Wang Y., Zhang F., Fu Y., Tan X., Liu L., Zhang Z. medRxiv; 2020. Clinical Characteristics of the Recovered COVID-19 Patients with Re-detectable Positive RNA Test. 2020.03.26.20044222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apuzzo M., Gebrekidan S. The New York Times; 2020. Can’t Get Tested? Maybe You’re in the Wrong Country.https://www.nytimes.com/2020/03/20/world/europe/coronavirus-testing-world-countries-cities-states.html (accessed 3.20.20) [Google Scholar]
- Becker A. DW; 2020. Coronavirus: Rush to Develop Rapid Tests.https://www.dw.com/en/coronavirus-rush-to-develop-rapid-tests/a-52945588 (accessed 3.29.20) [Google Scholar]
- Bienkov A. Germany Could Issue Thousands of People Coronavirus “Immunity Certificates” So They Can Leave the Lockdown Early. https://www.businessinsider.sg/coronavirus-germany-covid-19-immunity-certificates-testing-social-distancing-lockdown-2020-3?r=US&IR=T n.d, Business Insider - Business Insider Singapore [WWW Document]. URL. (accessed 3.30.2020)
- Bo-gyung K. Tests in recovered patients found false positives, not reinfections, experts say. The Korea Herald; 2020. http://www.koreaherald.com/view.php?ud=20200429000724 (accessed 5.06.20) [Google Scholar]
- Broughton J.P., Deng X., Yu G., Fasching C.L., Servellita V., Singh J., Miao X., Streithorst J.A., Granados A., Sotomayor-Gonzalez A., Zorn K., Gopez A., Hsu E., Gu W., Miller S., Pan C.-Y., Guevara H., Wadford D.A., Chen J.S., Chiu C.Y. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020:1–5. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruch R., Baaske J., Chatelle C., Meirich M., Madlener S., Weber W., Dincer C., Urban G.A. CRISPR/Cas13a-Powered electrochemical microfluidic biosensor for nucleic acid amplification-free miRNA diagnostics. Adv. Mater. 2019;31:1905311. doi: 10.1002/adma.201905311. [DOI] [PubMed] [Google Scholar]
- Bruch R., Urban G.A., Dincer C. CRISPR/Cas powered multiplexed biosensing. Trends Biotechnol. 2019;37:791–792. doi: 10.1016/j.tibtech.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Cardean TransistorsTM Made Available to Companies and Government Agencies Willing to Build Handheld Coronavirus Detection Devices. Technology Networks; 2020. https://www.technologynetworks.com/tn/product-news/cardean-transistors-made-available-to-companies-and-government-agencies-willing-to-build-handheld-332759 [WWW Document] (accessed 4.1.20) [Google Scholar]
- Bruch R., Urban G.A., Dincer C. Unamplified gene sensing via Cas9 on graphene. Nat. Biomed. Eng. 2019;3:419–420. doi: 10.1038/s41551-019-0413-4. [DOI] [PubMed] [Google Scholar]
- Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. The lancet infectious diseases 0. 2020. [DOI] [PMC free article] [PubMed]
- Chu D.K.W., Pan Y., Cheng S.M.S., Hui K.P.Y., Krishnan P., Liu Y., Ng D.Y.M., Wan C.K.C., Yang P., Wang Q., Peiris M., Poon L.L.M. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin. Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID 19 and Coagulopathy - Hematology.org 2020. https://www.hematology.org:443/covid-19/covid-19-and-coagulopathy [WWW Document], (accessed 4.21.20)
- Devlin H., Campbell D. The Guardian; 2020. WHO Considers Changing Guidance on Wearing Face Masks.https://www.theguardian.com/world/2020/apr/01/all-uk-hospital-staff-and-patients-should-wear-masks-says-doctors-group [Google Scholar]
- Diagnostics with Molecular Scissors – Is This Also Possible for On-Site COVID-19 Tests? Healthcare industry; 2020. https://www.gesundheitsindustrie-bw.de/en/article/news/Diagnostics-with-molecular-scissors-is-this-also-possible-for-on-site-COVID-19-tests [WWW Document] (accessed 4.21.20) [Google Scholar]
- Dewan A., Pettersson H., Croker N. CNN; 2020. As Governments Fumbled Their Coronavirus Response, These Four Got it Right. Here’s How.https://www.cnn.com/2020/04/16/world/coronavirus-response-lessons-learned-intl/index.html [WWW Document] (accessed 4.19.20) [Google Scholar]
- Dincer C., Bruch R., Costa-Rama E., Fernández-Abedul M.T., Merkoçi A., Manz A., Urban G.A., Güder F. Disposable sensors in diagnostics, Food, and environmental monitoring. Adv. Mater. 2019;31:1806739. doi: 10.1002/adma.201806739. [DOI] [PubMed] [Google Scholar]
- Dincer C., Bruch R., Kling A., Dittrich P.S., Urban G.A. Multiplexed point-of-care testing – xPOCT. Trends Biotechnol. 2017;35:728–742. doi: 10.1016/j.tibtech.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A.S., Lane H.C., Redfield R.R. Covid-19 — navigating the uncharted. N. Engl. J. Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Sidney J., Zhang Y., Scheuermann R.H., Peters B., Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27(4):671–680.e2. doi: 10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajian R., Balderston S., Tran T., deBoer T., Etienne J., Sandhu M., Wauford N.A., Chung J.-Y., Nokes J., Athaiya M., Paredes J., Peytavi R., Goldsmith B., Murthy N., Conboy I.M., Aran K. Detection of unamplified target genes via CRISPR–Cas9 immobilized on a graphene field-effect transistor. Nat. Biomed. Eng. 2019;3:427–437. doi: 10.1038/s41551-019-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Detection of COVID-19 on the Breath Followed by PCR. Zimmer and Peacock; 2020. http://www.zimmerpeacocktech.com/products/electrochemical-sensors/covid-19-and-pcr-on-the-breath/ [WWW Document] (accessed 3.29.20) [Google Scholar]
- State funds the development of rapid tests for coronavirus. Hahn-Schickard; 2020. https://www.hahn-schickard.de/en/news-detail/6-millionen-landesfoerderung-fuer-corona-schnelltest (accessed 4.07.20) [Google Scholar]
- Immunoglobulin M antibody - an overview | ScienceDirect Topics. 2020. https://www.sciencedirect.com/topics/medicine-and-dentistry/immunoglobulin-m-antibody [WWW Document] (accessed 3.29.20)
- Kupferschmidt K. Science | AAAS; 2020. Mutations Can Reveal How the Coronavirus Moves—But They’re Easy to Overinterpret.https://www.sciencemag.org/news/2020/03/mutations-can-reveal-how-coronavirus-moves-they-re-easy-overinterpret [WWW Document] (accessed 3.24.20) [Google Scholar]
- Lechuga Laura. Atresmedia Corporación de Medios de Comunicación; 2020. Llevamos tiempo reclamando que hay que invertir más dinero en prevención y análisis.https://www.lasexta.com/programas/al-rojo-vivo/entrevistas/laura-lechuga-investigadora-del-csic-llevamos-tiempo-reclamando-que-hay-que-invertir-mas-dinero-en-prevencion-y-analisis_202003165e6f95d977b6810001ac0c1a.html [WWW Document] (accessed 3.26.20) [Google Scholar]
- Li Y., Li S., Wang J., Liu G. CRISPR/Cas systems towards next-generation biosensing. Trends Biotechnol. 2019;37:730–743. doi: 10.1016/j.tibtech.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Feuer W., Lovelace B., Jr. CNBC; 2020. WHO Warning: No Evidence that Antibody Tests Can Show Coronavirus Immunity.https://www.cnbc.com/2020/04/17/who-issues-warning-on-coronavirus-testing-theres-no-evidence-antibody-tests-show-immunity.html [WWW Document] (accessed 4.19.20) [Google Scholar]
- Mallapaty S. Will antibody tests for the coronavirus really change everything? Nature. 2020. [DOI] [PubMed]
- Marquez S., Morales-Narváez E. Nanoplasmonics in paper-based analytical devices. Front. Bioeng. Biotechnol. 2019;7:69. doi: 10.3389/fbioe.2019.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz H., Dooley H. Shark IgNAR-derived binding domains as potential diagnostic and therapeutic agents. Dev. Comp. Immunol. 2019;90:100–107. doi: 10.1016/j.dci.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Morales-Narváez E., Baptista-Pires L., Zamora-Gálvez A., Merkoçi A. Graphene-based biosensors: going simple. Adv. Mater. 2017;29:1604905. doi: 10.1002/adma.201604905. [DOI] [PubMed] [Google Scholar]
- Nunez-Bajo E., Kasimatis M., Cotur Y., Asfour T., Collins A., Tanriverdi U., Grell M., Kaisti M., Senesi G., Stevenson K., Guder F. bioRxiv 2020; 2020. Ultra-Low-Cost Integrated Silicon-Based Transducer for On-Site, Genetic Detection of Pathogens. 03.23.002931. [DOI] [Google Scholar]
- Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu G., Gai Z., Tao Y., Schmitt J., Kullak-Ublick G.A., Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020 doi: 10.1021/acsnano.0c02439. In press. [DOI] [PubMed] [Google Scholar]
- Read Online-Handbook of COVID-19 Prevention and Treatment.Pdf. 2020. https://www.alibabacloud.com/universal-service/pdf_reader?spm=a3c0i.14138300.8102420620.dreadnow.6df3647fwqlHUE&cdnorigin=video-intl&pdf=Read%20Online-Handbook%20of%20COVID-19%20Prevention%20and%20Treatment.pdf [WWW Document] (accessed 3.27.20) [Google Scholar]
- Schoch-Spana M. Scientific American Blog Network; 2020. COVID-19's Psychosocial Impacts.https://blogs.scientificamerican.com/observations/covid-19s-psychosocial-impacts/ [WWW Document] (accessed 3.24.20). [Google Scholar]
- Seo G., Lee G., Kim M.J., Baek S.-H., Choi M., Ku K.B., Lee C.-S., Jun S., Park D., Kim H.G., Kim S.-J., Lee J.-O., Kim B.T., Park E.C., Kim S.I. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14(4):5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- Shen M., Zhou Y., Ye J., Abdullah AL-maskri A.A., Kang Y., Zeng S., Cai S. Recent advances and perspectives of nucleic acid detection for coronavirus. J. Pharmaceut. Anal. 2020;10(2):97–101. doi: 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan C. Fast, portable tests come online to curb coronavirus pandemic. Nat. Biotechnol. 2020 doi: 10.1038/d41587-020-00010-2. [DOI] [PubMed] [Google Scholar]
- Shortage of RNA Extraction Kits Hampers Efforts to Ramp up COVID-19 Coronavirus Testing. Chemical & Engineering News; 2020. https://cen.acs.org/analytical-chemistry/diagnostics/Shortage-RNA-extraction-kits-hampers/98/web/2020/03 [WWW Document] (accessed 3.29.20) [Google Scholar]
- South Korean hospital's ‘phone booth’ coronavirus tests. 2020. https://www.youtube.com/watch?v=A-33i9B8m6E (accessed 3.23.20)
- South Korea's “drive-through” coronavirus testing stations. 2020. https://www.youtube.com/watch?v=CRTcJNry87E (accessed 3.23.20)
- Stadlbauer D., Amanat F., Chromikova V., Jiang K., Strohmeier S., Arunkumar G.A., Tan J., Bhavsar D., Capuano C., Kirkpatrick E., Meade P., Brito R.N., Teo C., McMahon M., Simon V., Krammer F. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr. Protc. Microbiol. 2020;57 doi: 10.1002/cpmc.100. e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testing times Why South Korea's COVID-19 strategy is working. 2020. https://www.youtube.com/watch?v=KdSRMrYdxVY accessed 3.23.20)
- Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Jia X., Nicholson S., Catton M., Cowie B., Tong S.Y.C., Lewin S.R., Kedzierska K. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020:1–3. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. The New York Times; 2020. The Latest Obstacle to Getting Tested? A Shortage of Swabs and Face Masks.https://www.nytimes.com/2020/03/18/health/coronavirus-test-shortages-face-masks-swabs.html [Google Scholar]
- Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C., Chen H., Mubareka S., Gubbay J., Chan W.C.W. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14(4):3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- Vogel G. Science | AAAS; 2020. New Blood Tests for Antibodies Could Show True Scale of Coronavirus Pandemic.https://www.sciencemag.org/news/2020/03/new-blood-tests-antibodies-could-show-true-scale-coronavirus-pandemic [WWW Document] (accessed 3.30.20) [Google Scholar]
- Wittenberg-Cox A. Forbes; 2020. What Do Countries with the Best Coronavirus Responses Have in Common? Women Leaders.https://www.forbes.com/sites/avivahwittenbergcox/2020/04/13/what-do-countries-with-the-best-coronavirus-reponses-have-in-common-women-leaders/ [WWW Document] (accessed 4.14.20) [Google Scholar]
- Zamora-Gálvez A., Morales-Narváez E., Mayorga-Martinez C.C., Merkoçi A. Nanomaterials connected to antibodies and molecularly imprinted polymers as bio/receptors for bio/sensor applications. Appl. Mater. Today. 2017;9:387–401. doi: 10.1016/j.apmt.2017.09.006. [DOI] [Google Scholar]
- Zhang F., Abudayyehm Gootenberg J.S. A protocol for detection of COVID-19 using CRISPR diagnostics.
- Zuo X., Fan C., Chen H.-Y. Biosensing: CRISPR-powered diagnostics. Nat. Biomed. Eng. 2017;1:1–2. doi: 10.1038/s41551-017-0091. [DOI] [Google Scholar]