Graphical abstract
Abbreviations: ACE2, angiotensin converting enzyme 2; AKI, acute kidney injury; ARB, AT1R blocker; AT2, type II alveolar cells; AT1R, angiotensin II type 1 receptor; 3CLpro, 3C like protease; COVID-19, coronavirus disease; DM, diabetes mellitus; IF3, interferon 3; IL-17, interleukin 17; MERS, middle east respiratory syndrome; NF-κB, nuclear factor kappa B; PLpro, papain like protease; RAS, renin angiotensin system; RdRp, RNA dependent RNA polymerase; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ScRNA-seq, single cell RNA sequence; TMPRSS2, transmembrane protease serine 2; WHO, world health organization
Keywords: COVID-19, Clinical trials, Pandemic, SARS, SARS-CoV-2
Highlights
-
•
Explores the pandemic nature, morphology and viral hijacking of COVID-19 against host cells.
-
•
Highlights the possibilities of organ failure due to the attack of SARS-CoV2.
-
•
Illustrates the risk for COVID-19 attack in comorbid patients and their prognosis under medications.
-
•
Discusses the possible therapeutic strategies for the COVID-19.
Abstract
The current pandemic outbreak of COVID-19 originated from Wuhan, China. It is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with significant mortality and morbidity rate. The severe risk factors are commonly detected in patients of older age and with medical comorbidities like cancer and diabetes. Scientists and doctors have scrambled to gain knowledge about the novel virus and its pathophysiology in order to discover possible therapeutic regimens and vaccines for COVID-19. The therapeutic strategies like targeting the viral genome emphasize the promising approach to target COVID-19. Additionally, blocking the receptor, ACE2 via the neutralizing antibodies for viral escape that prevents it from entering into the cells provides another therapeutic regimen. In this review article, we have presented the effect of SARS-CoV-2 infection in comorbid patients and discussed organ failure caused by this virus. Based on the data available from the scientific literature and ongoing clinical trials, we have focused on therapeutic strategies. We hope that we would fill the gaps that puzzled the researchers and clinicians with the best of our knowledge collected for the betterment of the patients for the coming future.
1. Introduction
The SARS-CoV-2 is the frightful pathogen responsible for the outbreak of COVID-19 [1]. The disease emerged in Wuhan City, China, in late December 2019 [2] and is highly contagious with rapid human-to-human transmission [3]. The WHO declared the COVID-19 outbreak a global emergency [1]. The confirmed cases rose to 2,101,164 with 140,773 deaths and approximately 532,830 recoveries in almost 210 countries (reported by CSSE). Organizations around the world debated potential therapeutic strategies for treating COVID-19 patients and public health strategies. This pandemic also threatened the economy of the entire world. The complete role and severity of SARS-CoV-2 remain undefined. In this review article, we have focused on determining the relationship between SARS-CoV-2 infection and organ failure. Additionally, we focused on the comorbid population that includes patients with diabetes, and/or cardiovascular disease, who are COVID-19.
It is well established that morphologically, a virus is composed of genetic material surrounded by a protein capsid, which in some animal viruses is then surrounded by a lipid bilayer [4]. This small pathogen can cause a pandemic, raising global alarms. Looking back through history, many viruses have triggered pandemics similar to COVID-19, including smallpox, tuberculosis, plague, Spanish flu, HIV/AIDS, and H1N1 flu. Table 1 shows a list of viruses that have infected humans and had non-human host species.
Table 1.
Previous major outbreaks of viruses with cross-species transmission.
| Disease | Year | Host | Country |
|---|---|---|---|
| SWINE FLU/H1N1 | 1919 | Pig | Uncertain |
| HIV/AIDS | 1920 | Chimpanzee, Monkey | Democratic Republic of the Congo |
| EBOLA | 1976 | Monkey | Sudan and Zaire* *Currently DRC |
| BIRD FLU | 1997 | Water fowl | Hong Kong |
| SARS | 2002 | Civet cat | China |
| MERS | 2012 | Camel | South Arabia (Multiple Countries) |
| SARS-CoV-2 | 2019 | ? (Rat: Primary host Intermediate host: Pangolin) | China |
SARS-CoV-2 belongs to the family Coronaviridae, which includes viruses responsible for diseases from the cold to MERS and SARS [1]. SARS coronavirus, MERS coronavirus, 229E, and OC43 primarily infect humans. SARS-CoV-2 shares many similarities with SARS: they both have a crown or halo-like appearance and a glycoprotein-studded envelope.
2. Structure
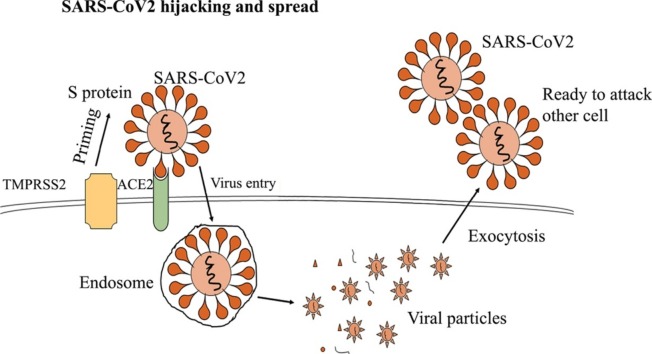
The SARS-CoV-2 virus varies structurally from other viruses that have transmembrane crown-like spiked glycoproteins [5]. It has 4 structural proteins, including envelope, spike, nucleocapsid and membrane. They are comprised of the functional subunits S1 and S2, which are responsible for detecting ACE2 receptors present on the host cells. ACE2 receptors are utilized by the virus to enter the host cells [[6], [7], [8]]. ACE2 expression is detected on type I and type II alveolar epithelium, upper respiratory system, heart, kidney tubular epithelium, pancreas, endothelial cells and enterocytes. The external spike protein determines the infectious nature and host specificity of SARS-CoV-2. The host cells (ACE2) allow the entry of the virus through the process called endocytosis. Moreover, the transmembrane proximal serine protease 2 (TMPRSS2) is the host protein that facilitates the entry of the virus through the S protein [5,9]. Additionally, it is involved in priming the S protein and potentiates its cleavage (Fig. 1 ).
Fig. 1.
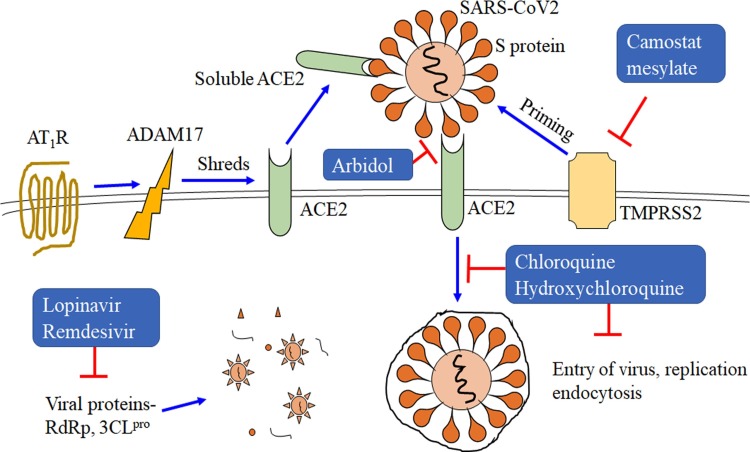
Inhibition of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry, replication, and endocytosis. Angiotensin type I receptor (AT1R) upregulates ADAM metallopeptidase domain 17 (ADAM17), that potentiates the shredding of angiotensin, converting enzyme 2 (ACE2) through ADAM17. Soluble ACE2 prevents the binding of SARS-CoV-2 with transmembrane bound ACE2. This could reduce the viral spread. Lopinavir and remdesivir inhibit RNA-dependent RNA polymerase (RdRp) and coronavirus main proteinase (3CLpro). Arbidol inhibits the interaction between ACE2 of host and S protein membrane of SARS-CoV-2. Chloroquine and hydroxychloroquine inhibit entry, replication, and endocytosis of SARS-CoV-2. Camostat inhibits transmembrane serine protease 2 (TMPRSS2), which is important for the SARS-CoV-2 infection. TMPRSS2 is the host protein, and activates the spike proteins (S-protein) of SARS-CoV-2 by priming.
Later, in the cytoplasm, the endosome exposes single-stranded RNA, the virus’ genetic material. The genome of the virus encodes various non-structural proteins like papain-like protease (PLpro), RNA-dependent RNA polymerase (RdRp) and the coronavirus main protease, 3C-like protease (3CLpro) [10,11]. The virus then hijacks the machinery of the cell to synthesize the viral polypeptides that encode for the replicase transcriptase complex. The active virus produces RNA through RdRp. PLpro actively deubiquitinases certain immune regulator cells like IF3 and NF-κB to suppress the immune response [11,12]. It uses the endoplasmic reticulum to synthesize M and S proteins, which are essential for its outer capsule. The viral proteinases 3CLpro and PLpro more effectively cleave the viral polyproteins with the help of the host translation machinery [10,11]. They produce new spikes and glycoproteins that are assembled into numerous copies of the virus. After replication of the genetic material, the golgi bodies exocytose the viruses, which then attack other cells. The created stress on the endoplasmic reticulum by the virus also induces apoptosis of the healthy host cells after releasing millions of viral copies. The viruses continue to attack other cells or end up as droplets and enter the lungs [13]. As an immune response, a fever is generated as the host’s immune system fights to clear the virus out of the body. Pro-inflammatory chemokines are activated to produce inflammatory cells. CD4 + T helper cells develop immunity against SARS-CoV-2 by producing IFN-γ and IL-17 [14]. SARS-CoV-2 also targets these circulating immune cells and induces apoptosis of CD3, CD8 and CD4 cells, causing lymphocytopenia [[15], [16], [17], [18]]. This results in the overproduction of cytokines, causing a cytokine storm as it is released from the inhibition of innate immunity. The cytokine storm results in hyper inflammation, ultimately causing failure of multiple organs [[19], [20], [21]]. For instance, under severe conditions, a patient’s immune system can attack the lung cells. This results in fluid filling the lungs and cell apoptosis, causing difficulty in breathing. In some cases, this leads to death.
3. Symptoms
The typical signs of COVID-19 infection are fatigue, cough, fever, myalgia, and some patients have also developed dyspnoea. Respiratory symptoms like cough, shortness of breath, acute respiratory syndrome and organ injury are also detected as serious complications [2,13]. The patients also experience lung alterations, reduced circulating lymphocytes and platelet counts. Human-to-human transmission usually occurs during the incubation phase or when the patient exhibits symptoms [5]. However, sometimes, a person in the asymptomatic phase is also found to be contagious. Such individuals are referred to as super-spreaders. For these cases, the transmission route was skin to skin or touching inanimate objects mediated via the nose, mouth or eyes [5]. Exhaled respiratory aerosols droplets are airborne and can remain in the air for long periods. Thus, direct inhalation of these aerosol droplets or touching the surface of infected objects like latex, steel, aluminium, or surgical gloves are possible modes of transmission [5]. Thus viruses like SARS-CoV-2 virus remain active outside the host body [22]. Furthermore, faecal and sewage transmission are also possible modes of transmission [22]. Similarly, the virus in sewage was found to be active for a few days to a week [23].
As of now, there are 400 SARS-CoV-2 genomes available from the NCBI database. The sequence analysis of these available genomes could form the platform for vaccine development. Sardar et al. [24] compared genomes of SARS-CoV-2 from diverse geographical origins including Wuhan (China), Italy, India, the USA, and Nepal by performing an integrated phylogenetic analysis using bioinformatics tools. Additionally, they also predicted that unique antiviral host miRNAs may target SARS-CoV-2 genes. They related past research studies about the inhibitory nature of has-miR-27b-3p in the ACE2 cascade [15]. Moreover, they also determined that has-miR-27b is complimentary to the spike of the Indian strain of SARS-CoV-2. Previous research data also suggested that miR-27b inhibits the replication of HIV-1 [25]. However, they could not provide any experimental evidence related to the strain of SARS-CoV-2, and there is no other research data available as of now.
4. Epidemic to pandemic
The life-threatening pneumonia outbreak started as an epidemic within Wuhan City, China. On December 31, 2019, the Wuhan Municipal Health Commission reported critically ill cases of viral pneumonia. Later, the International Committee on Taxonomy of Viruses (ICTV) identified the virus as SARS-CoV-2 [26]. The Chinese government then mandated a quarantine to control the epidemic stage and to determine the effectiveness of a quarantine against the virus [27]. However, SARS-CoV-2 was transmitted rapidly, quickly crossing borders of various countries. Thus, the number of established cases climbed quickly, causing the WHO to announce COVID-19 a global pandemic disease on March 11, 2020. Initially, China had the most confirmed cases, followed by North America and Europe. Italy and the US, however, surpassed China in terms of the number of established cases. Thus, at this stage of vigorous spread, it is essential to determine the mechanism of viral infection to control and treat the disease. Until now, it was believed that bat, especially Rhinolophus affinis, was the natural host of the virus [7]. It is still uncertain how this virus was transmitted from bat to human, although some research has suggested that pangolin (Manis javanica) could be the intermediate host [14,28,29]. The aggressive intervention steps were taken by governments, such as isolating and quarantining latent patients, have reduced the contact rate and effectively decreased the peak for COVID-19 cases.
5. COVID effect on organ failure
In addition to the symptoms discussed previously, there are other clinical manifestations observed with COVID-19 infection, including the failure of multiple organs. SARS-CoV and 2019-nCov/ SARS-CoV-2 were found to share a common progenitor with HKU9−1, the bat coronavirus. The similarity in the spike structure proteins of SARS-CoV-2 showed a higher affinity with ACE2 as discussed earlier [5]. ACE2 receptors are present on the target cells, making the cells highly susceptible to the entry of SARS-CoV-2 and subsequent pathogenesis. For instance, type II alveolar cells (AT2) of the lungs are believed to have higher ACE2 expression and are the primary targets for SARS-CoV-2 [7]. In this study, also examined different cell types from different organs that showed ACE2 expression using single-cell-RNA seq (ScRNA-seq) data to determine the effect of COVID-19 for the first time. We tabulated their results for different organs showing the type of cells tested, the proportion of cells expressing ACE2, and the risk of organ failure in Table 2 . The expression levels of ACE2 mRNA and protein in different organs were specified with the presence of gene transcription. They further strengthened their work by analysing ACE2 expression levels in various human organs and comparing the results with the Human Protein Atlas, databases like Uniprot and a few works in the literature. Their analysis of various published data demonstrated that ACE2 proteins are expressed in the epithelial cells of lung alveolar cells, myocardial cells, gastrointestinal cells and renal tubules [6,[30], [31], [32], [33]]. Additionally, myocardial infarctions increase ACE2 expression, increasing the likelihood of cardiac injury [34]. This contributes to the understanding of the effect of COVID-19 on various organs.
Table 2.
Organs and cells attacked by COVID-19 with the risk level for organ failure.
| Organ | Type of cell tested | Proportion of ACE2 | Risk for organ failure |
|---|---|---|---|
| Respiratory Tract/ Lungs/ Alveolar cells | Respiratory epithelial cells/ AT2 cells | 2% | High |
| Nasal and Bronchi | Nasal and bronchial samples | No | Low |
| Heart | Myocardial cells | 7.5 % | High |
| Ileum | Ileal epithelial cells | ∼30 % | High |
| Oesophagus | Oesophagus epithelial cells | >1% | High |
| Stomach and liver | <1% | Low | |
| Kidney | Kidney proximal tube | 4% | High |
| Urinary bladder | Bladder urothelial cells | 2.4 % | High |
Cardiac injury was the most common complication that was related to an elevated risk of disease severity in COVID-19 patients. About 23% of COVID-19 ill patients had cardiac injury [17], and 13% showed an elevation in creatinine kinase [35]. The mRNA and protein ACE2 expression levels are higher in these patients with cardiac disease, creating an increased risk for severe COVID-19 complications, including heart failure. Moreover, it was also suggested by Liang Chen et al. that the human heart infected with SARS-CoV-2 attacks pericytes, resulting in microvascular disorders [36]. This microvascular disorder causes dysfunction in capillary endothelial cells, low microvascular reactivity and high vascular permeability. The modelling and docking results also showed that SARS-CoV-2 has a receptor-binding domain that fits well with human ACE2 receptors. Moreover, the amino acid residues including 491Tyr, 441Leu, 479Gln, 487Asn, 472Phe and 480Ser of the SARS-CoV-2 mature spike protein are involved in binding with the ACE2 receptor [36]. Thus, these amino acid residues in the binding domain are important to consider in the design of potential drug treatments. Taken together, these findings show that COVID-19 in patients with cardiovascular disease often results in a critical condition and death.
The organ failure common in COVID-19 patients results in alveolar and acute respiratory failure [3]. Because there is a substantial comparison between the pathogenesis of SARS-CoV-2 and SARS, researchers and clinicians should explore the infection mechanisms of SARS-CoV-2 based on the effect of SARS. Earlier research showed that about 6.7% of SARS patients possessed acute kidney injury (AKI), leading to a mortality of almost 91.7% [37]. Thus, physicians need to consider the effect of SARS-CoV-2 on the kidney. Cheng et al. [38] explored the occurrence of AKI in COVID-19 patients to determine their association. They reported that 14.4% of the COVID-19 patients in the study showed elevated levels of serum creatinine upon admission. Most of these patients were male, older, or had other illnesses. Additionally, 13.1% had elevated blood urea nitrogen (BUN). After hospitalization (10 days), the serum creatinine peak elevated to 91 ± 67 μmol/L, and about 43.9% of the patients showed proteinuria. These patients also showed a higher incidence of AKI (11.9 %) after hospitalization; only 5.1% had AKI during admission. The in-hospital mortality recorded was 16.1%; however, those with raised baseline serum creatinine had a mortality rate of 33.7%. Furthermore, the Univariate Cox regression analysis also showed that COVID-19 patients who were male and/or older than 65 were highly prone to in-hospital death. Therefore, clinicians must be aware of kidney diseases in COVID-19 patients after hospitalization as early diagnosis and effective treatment would reduce the death rate in patients.
6. COVID-19 effect on cancer patients
Recent clinical data has shown that cancer patients receiving antineoplastic treatment are highly vulnerable to the effects of COVID-19, similar to immune-suppressed and older (>60 years) patients [39]. Patients with haematological malignancy, neutropenia or lymphopenia as well as those receiving multiple doses of chemotherapy are at higher risk for hospitalization (4 times) higher and death (10 times) higher compared with the healthy population of COVID-19 patients [40]. Moreover, cancer patients diagnosed with COVID-19 who received antitumor therapy within 14 days before diagnosis are at elevated risk for severe complications. Zhang et al. [41] also concluded that malignant patients with COVID-19 had poor prognosis. They suggested that vital COVID-19 screening should be carried out for the cancer patients receiving antitumor therapy, and cancer patients with COVID-19 should avoid using immunosuppression drugs or use a decreased dose.
The demographic data from Italy showed that among 3000 reported COVID-19 cases, 20 % of the patients who died had a medical history of malignancy in the previous 5 years [42]. Among 1524 patients joined to the Zhongnan Hospital of Wuhan University, 12 patients had COVID-19 [39]. This same study showed that the patients with NSCLC (7 patients) who were >60 years of age had the highest incidence rate for COVID-19 disease [39], followed by oesophageal (4 patients) and breast cancer (3 patients) [41]. The report also showed that these patients were more likely to suffer life-threatening complications and require ICU admission or mechanical ventilation [39]. These deaths were due to acute myocardial infarction, acute respiratory syndrome, septic shock and pulmonary embolism [41].
For these 28 cancer patients in Wuhan who also had COVID-19, the lab findings at the time of admission included low blood count (anaemic), leucopenia and lymphopenia [41]. Additionally, low levels of serum albumin, high levels of serum globulin and high lactate dehydrogenase with sensitive C-reactive protein in higher levels and erythrocyte sedimentation rate were also detected [41]. The radiological findings included ground-glass opacity, patchy consolidation [43], interlobular septal thickening, reticular appearances and fibrous strips. The patients with patchy consolidations were at greater risk for developing severe problems than those without [43]. Moreover, lung cancer patients were reported with decreased lung volume along with pneumonia [41]. It was also shown that among these malignant patients with COVID-19, 70% of the patients had stage IV cancer and are with severe complications [41]. The research and clinical data available now contribute to the proper understanding of the risk of COVID-19 occurrence in malignant patients. Thus, this helps oncologists tailor the COVID-19 clinical management for the benefit of such patients. Additionally, establishing essential guidelines and recommendations for the care of cancer patients based on a patient’s age, affected organ and stage of cancer during the COVID-19 outbreak is very crucial [44].
7. Risk of COVID-19 in patients with diabetes mellitus
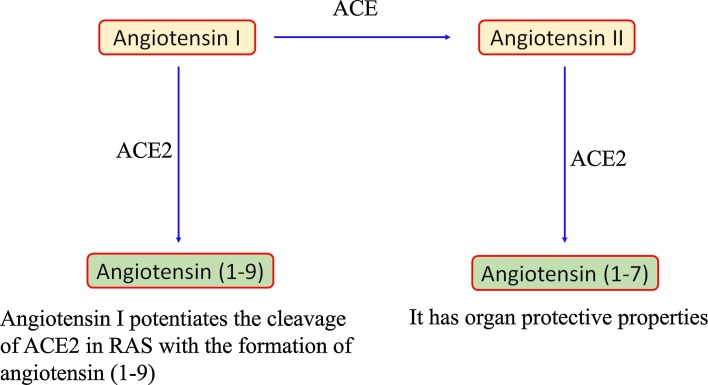
Patients with diabetes mellitus (DM), obesity and/or hypertension and COVID-19 have increased mortality and morbidity rates [17,18,35,42,[45], [46], [47]]. However, the association of DM with hypertension and cardiovascular diseases as risk factors for COVID-19 is still unknown and not clear. In one study that included 52 ICU-admitted COVID-19 patients, about 22 % of the patients were diabetic. Of the 52 admitted patients, 32 did not survive [17,44]. This suggests that DM is the predominant comorbidity with COVID-19. A few mechanisms have been suggested by researchers to explain the high susceptible of DM patients to COVID-19 pathogenesis. These include having an efficient cellular binding and easy entry of the virus, low chance for viral clearance, weakened T-cell function, highly prone to cytokine storm and hyperinflammation. Additionally, ACE2 expression weakens with the administration of insulin [48,49], whereas ACE2 expression is upregulated by hypoglycemic agents like thiazolidinediones, glucagon-like peptides-1, ACE inhibitors, angiotensin-receptor blockers, antihypertensives and statins [[50], [51], [52], [53], [54]]. The rodent DM model showed higher expression ACE2 in heart, lung, kidney and pancreas compared with normal controls [48,49]. Furthermore, Rao et al. [55]. from their Mendelian randomization study determined from the diseases and traits that the lungs of DM patient showed increased expression of ACE2. The protein levels of proteases like furin that facilitate the cleaving of the spike protein for virus entry were also found to be overexpressed in DM patients [56]. In order to understand the association between ACE2 expression in DM patients and COVID-19 infection, we must understand the mechanism of ACE2 action. ACE2 catalyses the conversion of angiotensin II to angiotensin 1–7 and angiotensin 1–9 (Fig. 2 ). These act as antioxidants and anti-inflammatory enzymes and are found to be protective against lung Acute respiratory distress syndrome (ARDs) [57]. After SARS-CoV-2 binds with ACE2, the virus degrades it, and thus the free angiotensin II induces acute lung injury [58]. Additionally, increased loss of potassium levels through urine and increased secretion of aldosterone also result after the intrusion of the virus [35]. Thus, ACE2 is unable to protect against lung injury after the entry of SARS-CoV-2 because ACE2 is degraded by the virus. The aetiology is still confusing, but it is clear that DM patients are at higher risk for severe complications with COVID-19. Thus, we have an urgent need for research studies focused on determining how hyperinsulinemia and hyperglycaemic conditions affect SARS-CoV-2 infection and how DM might affect vaccine efficacy.
Fig. 2.
Angiotensin converting enzyme (ACE) catalyzes the conversion of angiotensin I to angiotensin II. ACE2 catalyses the formation of angiotensin (1-9) and angiotensin (1-7) from angiotensin I and angiotensin II respectively.
8. Therapeutic strategies for novel COVID-19
Unfortunately, therapeutic treatments are not yet available for this COVID-19 outbreak as it takes years for a drug to come into the market. However, at present, the clinical trials and sympathetic use are being expedited. Thus currently, social distancing, patient isolation and supportive medical care and monitoring are the only available approaches. The potential pharmaceutical approaches to blocking the ACE2 receptor or a viral protein to mediate the inhibition of COVID-19 are tabulated in Table 3 . These include the following potential options:
Table 3.
The role of vital proteins of host and virus during infection and the possible efficacy of the drugs against SARS-CoV-2.
| Proteins to target | The role of viral proteins during hijacking the host cells. | Drugs | Hypothesis to act against SARS-CoV-2 | Already tested against diseases | Adverse side effects | References |
|---|---|---|---|---|---|---|
| Viral Genome proteins | ||||||
| RdRp | RNA-dependent RNA polymerase- replicates genome of the virus. | ribavirin and remdesivir | A nucleotide analogue that specifically inhibits replication of virus via blocking nucleotide synthesis of the virus. | Ebola Hepatitis C, RSV infection | Elevated levels of transaminases and renal injury. | [[59], [60], [61], [62], [63]] |
| PLpro | Papain-like protease- proteolysis viral polyprotein to active viral protein. | lopinavir | Protease inhibitor | For HIV in combination with ritonavir | Nausea, vomiting, gastrointestinal disturbance, pancreatitis, cardiac abnormalities. | [60,62] |
| 3CLpro | Coronavirus protease- proteolysis viral polyprotein to active protein. | lopinavir | Protease inhibitor | HIV in combination with ritonavir | Nausea, vomiting, gastrointestinal disturbance, pancreatitis, cardiac abnormalities. | [60,62] |
| S protein | Spike protein helps in holding virus to the host ACE2 receptor. | arbidol | Prevents binding of viral S protein to the host cells and blocks its entry. | Influenza | Elevated levels of transaminases, allergic reactions and gastrointestinal disorders. | [[64], [65], [66]] |
| Host proteins | ||||||
| ACE2 | Protein receptor binds with viral S protein allowing virus to enter into the host cells. | arbidol | Prevents binding of S protein to the ACE2 receptor and blocs its entry | Influenza | Elevated levels of transaminases, allergic reactions and gastrointestinal disorders. | [[64], [65], [66]] |
| TMPRSS2 | Protease produced by the host potentiates priming of S protein of the virus, which facilitates binding. | Camostat mesylate | – | – | – | [67] |
8.1. Developing a vaccine
Developing safe and efficient vaccines during the COVID-19 pandemic stage is very crucial. The antibodies developed against viral spike proteins by the host immune system are utilized in developing vaccines. The process includes purifying the plasma that contains antibodies from a recovered COVID-19 patient. The next step is targeting these antibodies to the spike protein of the virus to neutralize it and likely to develop passive immunity against disease [68]. Moreover, structural and sequence homology of SARS-CoV-2 with the other lethal CoV viruses such as SARS and MERS would help determine the best epitope to use in the design of an anti-SARS-CoV-2 vaccine. This would essentially neutralize the viral S protein, although creating a vaccine is a slow process. The SARS-CoV and SARS-CoV-2 spike proteins are 76.5 % homologous at the amino acid level [5]. Recently, Wan et al. [69] also determined that SARS-CoV with residue 479 (lysine) and SARS-CoV-2 with glutamine 394 residue recognize and bind to lysine 31 of the ACE2 receptor in humans [70]. An alternate approach is to inject neutralizing antibodies into COVID-19-infected animals to obtain purified polyclonal antibodies from these animals [71]. This process can be expedited, but the outcome is not guaranteed as the animals might not produce the expected neutralizing antisera [72].
8.2. Inhibiting the priming activity of TMPRSS2
TMPRSS2 is a proximal serine protease present in the host that plays a crucial role in proteolytic processing called priming. It is actively involved in priming of the S protein of SARS-CoV-2 after its binding with the ACE2 receptor [73]. Thus, it is crucial for the entry and spread of SARS-CoV-2. A couple of reports recently showed that SARS-CoV-2 also enters through this mechanism, and thus the entry of SARS-CoV-2 into cells may be blocked by using inhibitors of TMPRSS2, such as camostat mesylate (Fig. 1) [7,67].
8.3. ACE2 receptor blockers
ACE2 is a mono-carboxy peptidase that hydrolyses angiotensin II. As discussed earlier, ACE2 binds with high affinity to SARS-CoV-2, thereby allowing the virus to enter the host cells. Thus, targeting the binding site of the ACE2 receptor and SARS-CoV-2 with antibodies or therapeutic drugs might provide a successful treatment strategy. Moreover, ACE2 shows similar homology with ACE, which hydrolyses angiotensin I to angiotensin II [74]. This led to a lot of confusion between ACE inhibitors and ACE2 inhibitors. Increased ACE activity and reduced ACE2 activity promote lung injury. Furthermore, there have been several reports that ACE inhibitors (ACEI) inhibit the expression of ACE2 in kidney and heart [50]. As determined histologically, ACE2 is primarily membrane-bound but is also present as a soluble form in body fluids at a very low level [75,76]. The cleavage in the membrane ADAM17 anchor by a disintegrin and metalloprotease 17 present at the membrane-bound ACE2 receptor increases the occurrence of more soluble ACE2 in the body fluids (Fig. 1). Thus, the membrane-bound ACE2 is no longer available for SARS-CoV-2 to bind with it (Fig. 1). Angiotensin II via its type 1 receptor (AT1R) induces the upregulation of ADAM17, which potentiates the cleavage and increases the soluble ACE2 concentration in the body fluids. Thus, AT1R upregulates ADAM17 and increases soluble ACE2 [77], whereas the administration of AT1R blockers (ARB) prevents the process and maintains the membrane-bound ACE2 receptors (Fig. 1). Additionally, ARB is also found to consistently alter the expression of ACE2 at the protein and mRNA levels. ACE2 expression is upregulated in the renal vasculature and cardiac tissue [[78], [79], [80]]. Therefore, using ARB to treat for pathological conditions like lung injury without any complaint of COVID-19 infection is beneficial, but it may be critical during COVID-19 infection [81,82]. Lung injury without SARS-CoV-2 infection is caused by downregulated alveolar ACE2 levels and decreased angiotensin II metabolism [83,84]. However, several researchers have proposed the use of ARB to protect against lung injury during COVID-19 infection.
To summarize, comorbidities including cardiovascular, hypertension diseases and diabetes 2, are generally treated with blockers such as RAS blockers, ARBs, ACE inhibitors and AR blockers. The current clinical trials performed to determine the efficacy of drugs against COVID-19 are tabulated in Table 4 . Moreover, in the most recent studies, Liu et al. [85] determined that the serum levels of angiotensin II are increased in COVID-19 patients who had pneumonia and lung injury. This indicates that SARS-CoV-2 disrupts the balance of the ratio of ACE/ACE2, resulting in increased levels of angiotensin II. This further induces inflammation, pulmonary vasoconstriction and oxidative organ damage, causing acute lung injury. Thus, the modulation of RAS by ARBs or recombinant ACE2 increases the amount of ACE2 receptors and controls the levels of angiotensin II [86]. Moreover, this also increases the level of soluble ACE2 that competitively binds with SARS-CoV-2, causing delayed entry of the virus into cells and protecting against lung injury.
Table 4.
List of current clinical trials performing to determine the efficacy of drugs.
| Drug | Title of the study | Intervention | NCT number | Phase | Posted on Date/ Sponsor |
|---|---|---|---|---|---|
| Remdesivir (RDV; GS-5734TM) | Expanded access remdesivir | Is monophosphoramidate prodrug of an adenosine analog used against viral families. Targets RdRp. | NCT04302766 | – | March 10,2020 U.S. Army Medical Research and Development Command |
| Biological: NK cells, IL15-NK cells, NKG2D CAR-NK cells, ACE2 CAR-NK cells, NKG2D-ACE2 CAR-NK cells | A phase I/II study of universal off-the-shelf NKG2D-ACE2 CAR-NK cells that secrete IL15 super agonist and GM-CSF-neutralizing ScFv for COVID-19 therapy. | The CAR-NK cells are universal, off-the-shelf NK cells enriched from umbilical cord blood and engineered genetically. | NCT04324996 | Phase I/ II | March 27, 2020 Chongqing Public Health Medical Center |
| Thiazide or Thiazide like diuretics, Calcium channel blockers, ACE inhibitor, Angiotensin receptor blocker (Antihypertensive drugs) | The COVID-19 ACE inhibitor/ARB investigation (CORONACION) | Randomized patients with primary hypertension already on ACEi/ARB medication and switch to alternative blood pressure or continue with same ACEi/ARB. | NCT04330300 | Phase IV | April 13, 2020 National University of Ireland, Galway, Ireland |
| Valsartan (Diovan) | Valsartan for prevention of ARD syndrome in SARS-CoV-2 hospitalized patients. | Placebo-controlled randomized trial in ARD syndrome and COVID patients using valsartan. | NCT04335786 | Phase IV | April 8, 2020 Radboud University |
| Camostat mesilate (CamoCo-19) | The efficacy of camostat mesilate against SARS-CoV-2. | An inhibitor of serine protease that blocks TMPRSS2 and mediates the entry of SARS-CoV-2. | NCT04321096 | Phase I Phase II | April 6, 2020 University of Aarhus |
| Lopinavir/ritonavir, Hydroxychloroquine sulphate, losartan (COVIDMED) | COVID MED trial- Comparison of therapeutics for hospitalized patients infected with COVID-19 | Comparing prognosis of the COVID-19 infection treated with hydroxychloroquine, lopinavir and losartan | NCT04328012 | Phase II Phase III | April 8, 2020 Bassett Healthcare |
| Hydroxychloroquine (COMIHY) | Treatment with hydroxychloroquine in mild COVID-19 infected patient, | To assess the efficacy of hydroxychloroquine during the mild COVID-19 symptoms and shedding of virus- taking it as a tool to reduce the risk for future community transmission. | NCT04340544 | Phase III | April 9, 2020 University Hospital Tuebingen |
| Hydroxychloroquine sulphate (COV-HCQ) | Hydroxychloroquine for COVID-19 disease | To determine the efficacy of hydroxychloroquine for clearance of virus in vivo and later perform clinical trial for post-exposure prophylaxis and therapy in COVID-19. | NCT04342221 | Phase III | April 10, 2020 University Hospital Tuebingen |
| Hydroxychloroquine sulphate, bromhexine (HCQINRLGII) | Hydroxychloroquine and bromhexine in lower dose: a novel regimen for COVID-19 infection prophylaxis in healthcare professionals. | Low dose of hydroxychloroquine and bromhexine taken as TMPRSS2 blocker | NCT04340349 | Early phase I | April 9, 2020 Instituto Nacional de Rehabilitacion |
| Biological: Recombinant novel coronavirus vaccine (Adenovirus Type 5 vector) (CTCOVID-19) | Phase I clinical trial of COVID-19 vaccine in healthy adults (CTCOVID-19) | To evaluate safety and immunogenicity of recombinant novel coronavirus vaccine. Persistent analysis of anti-S protein antibodies against SARS-CoV-2 and the vaccination dose. | NCT04313127 | Phase I | April 14, 2020 CanSino Biologics Inc |
| Biological: Recombinant novel coronavirus vaccine (Adenovirus Type 5 vector) (CTII-nCoV) | Phase II clinical trial to evaluate COVID-19 recombinant vaccine (Adenovirus vector) | To evaluate the safety and immunogenicity of Ad5-nCoV that encodes for full length S protein. To determine response of anti SARS-CoV-2 S antibody | NCT04341389 | Phase II | April 15, 2020 Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China |
| Biological: mRNA-1273. Novel lipid-based nanoparticle with encapsulated mRNA-based vaccine. mRNA-1273 encodes for full length stabilized S protein. | Study on safety and immunogenicity of 2019-nCoV vaccine (mRNA-1273) for prophylaxis COVID-19 infection | To assess the safety and immunogenicity of the mRNA-based novel vaccine | NCT04283461 | Phase I | April 13, 2020 National Institute of Allergy and Infectious Diseases |
| Clazakizumab Genetically engineered humanized IgG1 mAb. | A Randomized placebo-controlled safety and dose for the use of IL-inhibitor clazakizumab in patients with COVID-19. | To administer clazakizumab in patient with pulmonary failure as it is found that IgG1 mAb bind with human IL-6 with high affinity. | NCT04343989 | Phase II | April 14,2020 NYU Langone Health |
| Tocilizumab (COVIDOSE) | Tocilizumab to prevent clinical decompensation in hospitalized, non-critically ill COVID-19 pneumonitis patients. | To determine the effect of tocilizumab in reducing symptoms in patients. | NCT04331795 | Phase II | April 9, 2020 University of Chicago |
| Anakinra Tocilizumab (ESCAPE) | Personalized immunotherapy for COVID-19 associated with organ dysfunction | Trial for personalized immunotherapy of COVID-19 patients with organ dysfunction, immune dysregulation and macrophage activation syndrome. | NCT04339712 | Phase II | April 15, 2020 Hellenic Institute for the Study of Sepsis |
Source: https://clinicaltrials.gov/.
8.4. Additional approaches
Additionally, rapid sequencing of the SARS-CoV-2 genome has enabled epidemiological tracking, diagnosis and promotes the development of therapeutic strategies for infection prevention. The SARS-CoV-2 genome sequence has been published recently (the GenBank accession number is MN908947.3), allowing researchers to synthesize the gene and consider S protein expression as the immunogen. Understanding other viruses like MERS-CoV and SARS-CoV from the past offers novel insights into potential therapies for combating COVID-19. Researchers are now targeting different parts of the viral mechanism for infection [87].
One approach is to prevent the reproduction of SARS-CoV-2 by targeting the viral RNA polymerase, which is used to synthesize the viral RNA genome and is not produced naturally in the host body [88]. If the viral polymerase could be selectively targeted or blocked, the virus could no longer produce RNA copies and the viral infection could be stopped. For instance, remdesivir is a drug developed against the Ebola virus specifically, but it showed promising results when used to treat MERS-CoV infection [63]. Remdesivir, conceals the viral RNA polymerase and prevents the proofreading to occur via the viral exonucleases. This way it decreases the viral RNA production in the host cells (Fig. 1). Remdesivir was also suggested to give positive results when used against SARS-CoV-2, and a clinical trial sponsored by the NIH began in February 2020. However, the clinical impact of this drug against COVID-19 is still unclear and researchers are waiting for the outcome of the patient’s ongoing clinical trials.
Targeting viral processing is another possible treatment strategy, which takes advantage of the fact that viral proteases have a specific site where protein cutting occurs [89]. Therefore, drugs that fit into this specific site can block the functionality of a viral protease, thereby inhibiting viral protein production. Viral proteases are promising therapeutic targets since they are available in limited numbers in the host cells and have a specific site for catalytic activity [89]. This type of therapy has been successfully used against HIV, and now researchers are seeking to use protease inhibitors against SARS-CoV-2.
Targeted disruption of the packaging of a virus is another possible approach since, after packaging, the virus leaves the host cell through exocytosis and attacks other cells [90]. However, in coronaviruses there are no proteins specifically designed for packaging; all of the viral proteins are actively involved in viral packaging. Thus, this type of approach does not seem promising for the treatment of COVID-19.
Other potential treatments target the virulent shell. The fully matured virus particles have a shell made up of spike and membrane proteins. The membrane proteins are buried within the membrane, but the spike proteins are external. Thus, a virus can be targeted exteriorly by attacking the spike proteins with drugs. Since it is on the exterior, the spike protein is also the main target of antibodies produced by the host’s immune system [91]. This led to the development of vaccines against SARS-CoV-2, which we have already discussed earlier in this article.
8.5. Use of chloroquine and hydroxychloroquine
Chloroquine and its derivative hydroxychloroquine have long been used for the prevention and treatment of malaria and chronic inflammatory diseases like rheumatoid arthritis [92]. Both of these drugs are found to be very effective in treating dysregulations caused by a coronavirus in China [63]. It was notably reported that chloroquine inhibits the replication of HCoV-229E and SARS-CoV-1. It was also found to prevent the entry of virus by inhibiting the glycosylation of ACE2 receptors, block proteases and regulate acidification in the endosome (Fig. 1). Chloroquine and hydroxychloroquine also promote an immunomodulatory effect via the production of cytokines and suppress autophagy and lysosomal functionality in host cells [90,93]. Chloroquine was identified to suppress SARS-CoV-2 growth in vitro, showing more than a half-maximal effective concentration (EC50) with a low micromolar range [94]. There are no adverse side effects known for the proposed usage of chloroquine against COVID-19 [95]. There was a sharp increase in the prescription of these drugs for COVID-19 patients with the reference of USA President Mr Donald Trump in late March in the US (https://www.bbc.com/news/51980731). Furthermore, it was also granted by the Food and Drugs Administration (FDA) for the use of hydroxychloroquine and chloroquine drug as an emergency as authorized for COVID-19 therapy. There are more than 20 clinical trials carried in the US, UK, China and Spain on these drugs. The later reports from the clinical trials also suggested that chloroquine drug would minimise the disease duration [95]. Bur, later on, April 24, the FDA also licenced the usage of the drug issued under the warning regarding the dangerous threat for the serious heart rhythm adverse side effects in COVID-19 patients when used. However, to get a complete clearance of virus, chloroquine is combined with other drugs. For instance, hydroxychloroquine combined with azithromycin resulted in greater clearance of virus than hydroxychloroquine alone [96].
9. Conclusions
The outbreak of SARS-CoV-2 is an ongoing global public health crisis. The expedition of clinical trials for various potential therapeutic drugs is essential to evaluate their efficacy at this midpoint of the pandemic. The pressure on researchers to develop promising therapeutic drugs for COVID-19 is very high. These drugs include cytokines, bioengineered and vector-based antibodies to block the gene expression of the virus and to develop a vaccine. However, there are currently no therapeutic drugs available to treat COVID-19. Clinical symptoms caused by SARS-CoV-2 infection should be closely monitored to determine whether the virus has infected internal organs so that appropriate therapy can be performed. A patient’s demographic data and past medical history are crucial to forming a good therapeutic plan. Moreover, together, antiviral research and clinical trials will improve the effectiveness of therapy and facilitate the production of a drug or vaccine in record time. In this article we focused on the effect of COVID-19 with other diseases (comorbidities) and associated organ injury to provide researchers with a better understanding of the clinical implications of COVID-19. We also focused on the ongoing research and clinical trials conducted for the benefit of COVID-19 patients to determine the efficacy of drugs for treatment.
Contributors
GPN involved in literature search, figures, and study design, BD involved in literature search, and figures. BD and GPN drafted and finalized the paper. Both authors read, and approved this review.
Declaration of Competing Interest
None to declared.
Biographies

Begum Dariya is a PhD Scholar in the Department of Bioscience and Biotechnology, Banasthali University, Vanasthali, Jaipur, Rajasthan, India. She did her Master of Science in Biotechnology from Sri Padmavati Mahila Viswa Vidyalayam, Tirupati, AP, India. She received state award and participated in several national and international conferences. She is mainly investigating the “Evaluation and effect of resveratrol with 5-fluoropyrimidine based chemotherapy in colorectal cancer”. She published several research and review articles in reputed journals. She also published more than 15 book chapters in Springer Nature and Elsevier publications.

Dr. Nagaraju GP is a faculty member in the Department of Hematology and Medical Oncology at Emory University School of Medicine. Dr. Nagaraju obtained his MSc and his PhD, both in Biotechnology, from Sri Venkateswara University in Tirupati, Andhra Pradesh, India. Dr. Nagaraju received his DSc from Berhampur University in Berhampur, Odisha, India. Dr. Nagaraju’s research focuses on translational projects related to gastrointestinal malignancies. He has published over 90 research papers in highly reputed International journals and has presented more than 50 abstracts at various national and international conferences. Dr, Nagaraju is author and editor of several published books. He serves as editorial board member of several internationally recognized academic journals. Dr. Nagaraju is an associate member of the Discovery and Developmental Therapeutics Research Program at Winship Cancer Institute. Dr. Nagaraju has received several international awards including FAACC. He also holds memberships with the ASIOA, the SICB, The Science Advisory Board, The RNA Society, The AACC and the AACR.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cytogfr.2020.05.001.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Novel C.P.E.R.E. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua liu xing bing xue za zhi= Zhonghua liuxingbingxue zazhi. 2020;41(2):145. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NAYAK D.P. Virus morphology, replication, and assembly. Viral Ecology. 2000:63. [Google Scholar]
- 5.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X., Zhong W., Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532(1–2):107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 7.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letko M.C., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 2020;5(4):562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziebuhr J., Snijder E.J., Gorbalenya A.E. Virus-encoded proteinases and proteolytic processing in the Nidovirales. J. Gen. Virol. 2000;81(4):853–879. doi: 10.1099/0022-1317-81-4-853. [DOI] [PubMed] [Google Scholar]
- 11.Báez-Santos Y.M., John S.E.S., Mesecar A.D. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee T.-W., Cherney M.M., Huitema C., Liu J., James K.E., Powers J.C., Eltis L.D., James M.N. Crystal structures of the main peptidase from the SARS coronavirus inhibited by a substrate-like aza-peptide epoxide. J. Mol. Biol. 2005;353(5):1137–1151. doi: 10.1016/j.jmb.2005.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may be at least partially responsible for the respiratory failure of COVID‐19 patients. J. Med. Virol. 2020:1–3. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahba L., Jain N., Fire A.Z., Shoura M.J., Artiles K.L., McCoy M.J., Jeong D.E. Identification of a pangolin niche for a 2019-nCoV-like coronavirus through an extensive meta-metagenomic search. bioRxiv. 2020 doi: 10.1128/mSphere.00160-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L.-J., Xu R., Yu H.-M., Chang Q., Zhong J.-C. The ACE2/apelin signaling, microRNAs, and hypertension. Int. J. Hypertens. 2015;2015 doi: 10.1155/2015/896861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;S2213-2600(20):30079–30085. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J.-j., Dong X., Cao Y.-y., Yuan Y.-d., Yang Y.-b., Yan Y.-q., Akdis C.A., Gao Y.-d. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y., Li T., Han M., Li X., Wu D., Xu Y., Zhu Y., Liu Y., Wang X., Wang L. Diagnostic Utility of Clinical Laboratory Data Determinations for Patients with the Severe COVID‐19. J. Med. Virol. 2020 doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) Medrxiv. 2020 [Google Scholar]
- 22.Weber D.J., Rutala W.A., Fischer W.A., Kanamori H., Sickbert-Bennett E.E. Emerging infectious diseases: Focus on infection control issues for novel coronaviruses (Severe Acute Respiratory Syndrome-CoV and Middle East Respiratory Syndrome-CoV), hemorrhagic fever viruses (Lassa and Ebola), and highly pathogenic avian influenza viruses, A (H5N1) and A (H7N9) Am. J. Infect. Control. 2016;44(5):e91–e100. doi: 10.1016/j.ajic.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casanova L., Rutala W.A., Weber D.J., Sobsey M.D. Survival of surrogate coronaviruses in water. Water Res. 2009;43(7):1893–1898. doi: 10.1016/j.watres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sardar R., Satish D., Birla S., Gupta D. Comparative analyses of SAR-CoV2 genomes from different geographical locations and other coronavirus family genomes reveals unique features potentially consequential to host-virus interaction and pathogenesis. bioRxiv. 2020 doi: 10.1016/j.heliyon.2020.e04658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang K., Sung T.-L., Rice A.P. Regulation of cyclin T1 and HIV-1 Replication by microRNAs in resting CD4+ T lymphocytes. J. Virol. 2012;86(6):3244–3252. doi: 10.1128/JVI.05065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King A.M., Lefkowitz E.J., Mushegian A.R., Adams M.J., Dutilh B.E., Gorbalenya A.E., Harrach B., Harrison R.L., Junglen S., Knowles N.J. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2018) Arch. Virol. 2018;163(9):2601–2631. doi: 10.1007/s00705-018-3847-1. [DOI] [PubMed] [Google Scholar]
- 27.Hou C., Chen J., Zhou Y., Hua L., Yuan J., He S., Guo Y., Zhang S., Jia Q., Zhao C. The effectiveness of the quarantine of Wuhan city against the Corona Virus Disease 2019 (COVID‐19): well‐mixed SEIR model analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25827. [DOI] [PubMed] [Google Scholar]
- 28.Lam T.T.-Y., Shum M.H.-H., Zhu H.-C., Tong Y.-G., Ni X.-B., Liao Y.-S., Wei W., Cheung W.Y.-M., Li W.-J., Li L.-F. Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China. BioRxiv. 2020 [Google Scholar]
- 29.Xiao K., Zhai J., Feng Y., Zhou N., Zhang X., Zou J.-J., Li N., Guo Y., Li X., Shen X. Isolation and characterization of 2019-nCoV-like coronavirus from Malayan pangolins. BioRxiv. 2020 [Google Scholar]
- 30.Hamming I., Timens W., Bulthuis M., Lely A., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-dos-Santos A.J., da Costa J., Zhang L., Pei Y. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417(6891):822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 32.Kowalczuk S., Broer A., Tietze N., Vanslambrouck J.M., Rasko J.E., Broer S. A protein complex in the brush-border membrane explains a Hartnup disorder allele. FASEB J. 2008;22(8):2880–2887. doi: 10.1096/fj.08-107300. [DOI] [PubMed] [Google Scholar]
- 33.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R. A novel angiotensin-converting enzyme–related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 2000;87(5):e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 34.Burrell L.M., Risvanis J., Kubota E., Dean R.G., MacDonald P.S., Lu S., Tikellis C., Grant S.L., Lew R.A., Smith A.I. Myocardial infarction increases ACE2 expression in rat and humans. Eur. Heart J. 2005;26(4):369–375. doi: 10.1093/eurheartj/ehi114. [DOI] [PubMed] [Google Scholar]
- 35.Guan W.-j., Ni Z.-y., Hu Y., Liang W.-h., Ou C.-q., He J.-x., Liu L., Shan H., Lei C.-l., Hui D.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116(6):1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu K.H., Tsang W.K., Tang C.S., Lam M.F., Lai F.M., To K.F., Fung K.S., Tang H.L., Yan W.W., Chan H.W. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67(2):698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L., Li J., Yao Y., Ge S., Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020 doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidaway P. COVID-19 and cancer: what we know so far. Nat. Rev. Clin. Oncol. 2020 doi: 10.1038/s41571-020-0366-2. 1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bitterman R., Eliakim‐Raz N., Vinograd I., Trestioreanu A.Z., Leibovici L., Paul M. Influenza vaccines in immunosuppressed adults with cancer. Cochrane Database Syst. Rev. 2018;(2) doi: 10.1002/14651858.CD008983.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., Jia P., Guan H., Peng L., Chen Y. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020;S0923-7534(20):36383–36393. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 43.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J., Fan Y., Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bitar N., Kattan J., Kourie H.R., Mukherji D., Saghir N.E. The Lebanese Society of Medical Oncology (LSMO) statement on the care of patients with cancer during the COVID-19 pandemic. Future Medicine. 2020:615–617. doi: 10.2217/fon-2020-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.CF.D. Control . Centers for Disease Control; Atlanta, GA: 2020. Prevention, National Diabetes Statistics Report, 2020; pp. 1–32. [Google Scholar]
- 46.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., Wang H., Wang Y., Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roca-Ho H., Riera M., Palau V., Pascual J., Soler M.J. Characterization of ACE and ACE2 expression within different organs of the NOD mouse. Int. J. Mol. Sci. 2017;18(3):563. doi: 10.3390/ijms18030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wysocki J., Ye M., Soler M.J., Gurley S.B., Xiao H.D., Bernstein K.E., Coffman T.M., Chen S., Batlle D. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55(7):2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- 50.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I., Gallagher P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 51.Romaní-Pérez M., Outeiriño-Iglesias V., Moya C.M., Santisteban P., González-Matías L.C., Vigo E., Mallo F. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156(10):3559–3569. doi: 10.1210/en.2014-1685. [DOI] [PubMed] [Google Scholar]
- 52.Tikoo K., Patel G., Kumar S., Karpe P.A., Sanghavi M., Malek V., Srinivasan K. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem. Pharmacol. 2015;93(3):343–351. doi: 10.1016/j.bcp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 53.Wösten‐van Asperen R.M., Lutter R., Specht P.A., Moll G.N., van Woensel J.B., van der Loos C.M., van Goor H., Kamilic J., Florquin S., Bos A.P. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin‐(1–7) or an angiotensin II receptor antagonist. J. Pathol. 2011;225(4):618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 54.Zhang W., Xu Y.-Z., Liu B., Wu R., Yang Y.-Y., Xiao X.-Q., Zhang X. Pioglitazone upregulates angiotensin converting enzyme 2 expression in insulin-sensitive tissues in rats with high-fat diet-induced nonalcoholic steatohepatitis. Sci. World J. 2014;2014 doi: 10.1155/2014/603409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rao S., Lau A., So H.-C. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of 2019-nCov: a Mendelian Randomization analysis. medRxiv. 2020 doi: 10.2337/dc20-0643. [DOI] [PubMed] [Google Scholar]
- 56.Fernandez C., Rysä J., Almgren P., Nilsson J., Engström G., Orho‐Melander M., Ruskoaho H., Melander O. Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J. Intern. Med. 2018;284(4):377–387. doi: 10.1111/joim.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wösten-van Asperen R.M., Bos A.P., Bem R.A., Dierdorp B.S., Dekker T., van Goor H., Kamilic J., van der Loos C.M., van den Berg E., Bruijn M. Imbalance between pulmonary angiotensin-converting enzyme and angiotensin-converting enzyme 2 activity in acute respiratory distress syndrome. Pediatr. Crit. Care Med. 2013;14(9):e438–e441. doi: 10.1097/PCC.0b013e3182a55735. [DOI] [PubMed] [Google Scholar]
- 58.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu W., Morse J.S., Lalonde T., Xu S. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019‐nCoV. Chembiochem. 2020;21(5):730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11(1):1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo D. Old weapon for new enemy: drug repurposing for treatment of newly emerging viral diseases. Virol. Sin. 2020:1–3. doi: 10.1007/s12250-020-00204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maxmen A. More than 80 clinical trials launch to test coronavirus treatments. Nature. 2020;578(7795):347. doi: 10.1038/d41586-020-00444-3. [DOI] [PubMed] [Google Scholar]
- 63.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kadam R.U., Wilson I.A. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc. Natl. Acad. Sci. 2017;114(2):206–214. doi: 10.1073/pnas.1617020114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.M. Bulloch, Potential Pipeline Medications May Help Patients With Novel Coronavirus, pharmacytimes.
- 66.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Casadevall A., Pirofski L.-A. The Ebola epidemic crystallizes the potential of passive antibody therapy for infectious diseases. PLoS Pathog. 2015;11(4) doi: 10.1371/journal.ppat.1004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7):e00127–20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu K., Peng G., Wilken M., Geraghty R.J., Li F. Mechanisms of host receptor adaptation by severe acute respiratory syndrome coronavirus. J. Biol. Chem. 2012;287(12):8904–8911. doi: 10.1074/jbc.M111.325803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt R., Beltzig L.C., Sawatsky B., Dolnik O., Dietzel E., Krähling V., Volz A., Sutter G., Becker S., von Messling V. Generation of therapeutic antisera for emerging viral infections. NPJ Vaccines. 2018;3(1):1–10. doi: 10.1038/s41541-018-0082-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jahrling P., Geisbert J., Swearengen J., Jaax G., Lewis T., Huggins J., Schmidt J., LeDuc J., Peters C. Passive immunization of Ebola virus-infected cynomolgus monkeys with immunoglobulin from hyperimmune horses. Imported Virus Infections, Springer. 1996:135–140. doi: 10.1007/978-3-7091-7482-1_12. [DOI] [PubMed] [Google Scholar]
- 73.Matsuyama S., Nagata N., Shirato K., Kawase M., Takeda M., Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010;84(24):12658–12664. doi: 10.1128/JVI.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275(43):33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 75.Serfozo P., Wysocki J., Gulua G., Schulze A., Ye M., Liu P., Jin J., Bader M., Myöhänen T., García-Horsman J.A. Ang II (angiotensin II) conversion to angiotensin-(1-7) in the circulation is POP (prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension. 2020;75(1):173–182. doi: 10.1161/HYPERTENSIONAHA.119.14071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arendse L.B., Danser A.J., Poglitsch M., Touyz R.M., Burnett J.C., Llorens-Cortes C., Ehlers M.R., Sturrock E.D. Novel therapeutic approaches targeting the renin-angiotensin system and associated peptides in hypertension and heart failure. Pharmacol. Rev. 2019;71(4):539–570. doi: 10.1124/pr.118.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu J., Sriramula S., Xia H., Moreno-Walton L., Culicchia F., Domenig O., Poglitsch M., Lazartigues E. Clinical relevance and role of neuronal AT1 receptors in ADAM17-mediated ACE2 shedding in neurogenic hypertension. Circ. Res. 2017;121(1):43–55. doi: 10.1161/CIRCRESAHA.116.310509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klimas J., Olvedy M., Ochodnicka‐Mackovicova K., Kruzliak P., Cacanyiova S., Kristek F., Krenek P., Ochodnicky P. Perinatally administered losartan augments renal ACE 2 expression but not cardiac or renal Mas receptor in spontaneously hypertensive rats. J. Cell. Mol. Med. 2015;19(8):1965–1974. doi: 10.1111/jcmm.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X., Ye Y., Gong H., Wu J., Yuan J., Wang S., Yin P., Ding Z., Kang L., Jiang Q. The effects of different angiotensin II type 1 receptor blockers on the regulation of the ACE-AngII-AT1 and ACE2-Ang (1–7)-Mas axes in pressure overload-induced cardiac remodeling in male mice. J. Mol. Cell. Cardiol. 2016;97:180–190. doi: 10.1016/j.yjmcc.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 80.Soler M.J., Ye M., Wysocki J., William J., Lloveras J., Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. American Journal of Physiology-Renal Physiology. 2009;296(2):F398–F405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 81.Wysocki J., Goodling A., Burgaya M., Whitlock K., Ruzinski J., Batlle D., Afkarian M. Urine RAS components in mice and people with type 1 diabetes and chronic kidney disease. Am. J. Physiol. Renal Physiol. 2017;313(2):F487–F494. doi: 10.1152/ajprenal.00074.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bitker L., Burrell L.M. Classic and nonclassic renin-angiotensin systems in the critically ill. Crit. Care Clin. 2019;35(2):213–227. doi: 10.1016/j.ccc.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gurwitz D. Angiotensin receptor blockers as tentative SARS‐CoV‐2 therapeutics. Drug Dev. Res. 2020 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Batlle D., Wysocki J., Satchell K. Soluble angiotensin-converting enzyme 2: a potential approach for coronavirus infection therapy? Clin. Sci. 2020;134(5):543–545. doi: 10.1042/CS20200163. [DOI] [PubMed] [Google Scholar]
- 87.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 88.Lung J., Lin Y.S., Yang Y.H., Chou Y.L., Shu L.H., Cheng Y.C., Liu H.T., Wu C.Y. The potential chemical structure of anti‐SARS‐CoV‐2 RNA‐dependent RNA polymerase. J. Med. Virol. 2020 doi: 10.1002/jmv.25761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhavoronkov A., Aladinskiy V., Zhebrak A., Zagribelnyy B., Terentiev V., Bezrukov D.S., Polykovskiy D., Shayakhmetov R., Filimonov A., Orekhov P. Potential COVID-2019 3C-like protease inhibitors designed using generative deep learning approaches. Insilico Medicine Hong Kong Ltd A. 2020;307:E1. [Google Scholar]
- 90.Devaux C.A., Rolain J.-M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shanmugaraj B., Siriwattananon K., Wangkanont K., Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pac. J. Allergy Immunol. 2020;38(1):10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 92.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases. Lancet Infect. Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou D., Dai S.-M., Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J. Antimicrob. Chemother. 2020 doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14(1):72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 96.Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.