The novel coronavirus disease-2019 (COVID-19) has affected nearly every country worldwide. Reports note increased thromboembolic events among hospitalized patients (1,2), and there are anecdotal observations of improved outcomes with systemic anticoagulation (AC); however, the specific role of AC in disease management remains unclear (3,4). We assessed the association between administration of in-hospital AC and survival in a large cohort of hospitalized patients with COVID-19. This work was approved by the Institutional Review Board at the Icahn School of Medicine at Mount Sinai (#20-03271).
Between March 14 and April 11, 2020, 2,773 patients were hospitalized with laboratory-confirmed COVID-19 within the Mount Sinai Health System in New York City. We used a Cox proportional hazards model to evaluate the effect of treatment-dose systemic AC (including oral, subcutaneous, or intravenous forms) on in-hospital mortality. We adjusted for age, sex, ethnicity, body mass index, history of hypertension, heart failure, atrial fibrillation, type 2 diabetes, AC use prior to hospitalization, and admission date. To adjust for differential length of stay and initiation of AC treatment, AC treatment duration was used as a covariate while intubation was treated as a time-dependent variable.
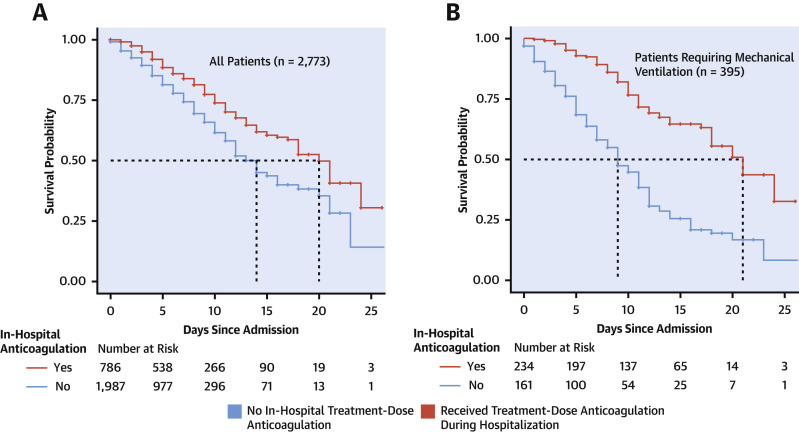
Among 2,773 hospitalized patients with COVID-19, 786 (28%) received systemic treatment-dose AC during their hospital course. The median hospitalization duration was 5 days (interquartile range [IQR]: 3 to 8 days). Median time from admission to AC initiation was 2 days (IQR: 0 to 5 days). Median duration of AC treatment was 3 days (IQR: 2 to 7 days). In-hospital mortality for patients treated with AC was 22.5% with a median survival of 21 days, compared to 22.8% and median survival of 14 days in patients who did not receive treatment-dose AC (Figure 1A ). Patients who received treatment-dose AC were more likely to require invasive mechanical ventilation (29.8% vs 8.1%; p < 0.001) as compared to those who received prophylactic dose AC or did not receive AC. Overall, we observed significantly increased baseline prothrombin time, activated partial thromboplastin time, lactate dehydrogenase, ferritin, C reactive protein, and D-dimer values among individuals who received in-hospital AC compared with those who did not. These differences were not observed, however, among mechanically ventilated patients. In patients who required mechanical ventilation (n = 395), in-hospital mortality was 29.1% with a median survival of 21 days for those treated with AC as compared to 62.7% with a median survival of 9 days in patients who did not receive treatment-dose AC (Figure 1B). In a multivariate proportional hazards model, longer duration of AC treatment was associated with a reduced risk of mortality (adjusted HR of 0.86 per day; 95% confidence interval: 0.82 to 0.89; p < 0.001).
Figure 1.
Kaplan-Meier Curve for Hospitalized Patients With COVID-19 and Those Mechanically Ventilated
Kaplan-Meier curve for hospitalized patients with COVID-19 (A) and those mechanically ventilated (B). Colors indicate treatment-dose anticoagulation. Patients hospitalized at time of data-freeze or discharged within the study period were right-censored. COVID-19 = novel coronavirus disease-2019.
We also explored the association of systemic treatment-dose AC administration with bleeding events. Major bleeding was defined as: 1) hemoglobin <7 g/dl and any red blood cell transfusion; 2) at least 2 U of red blood cell transfusion within 48 h; or 3) a diagnosis code for major bleeding including intracranial hemorrhage, hematemesis, melena, peptic ulcer with hemorrhage, colon, rectal, or anal hemorrhage, hematuria, ocular hemorrhage, and acute hemorrhagic gastritis. Among those who did not receive treatment-dose AC, 38 (1.9%) individuals had bleeding events, compared with 24 (3%) among those who received treatment-dose AC (p = 0.2). Of the 24 patients who had bleeding events on AC, 15 (63%) had bleeding events after starting AC and 9 (37%) had bleeding events before starting AC. Bleeding events were more common among intubated patients (30 of 395; 7.5%) than among nonintubated patients (32 of 2,378; 1.35%).
Although limited by its observational nature, unobserved confounding, unknown indication for AC, lack of metrics to further classify illness severity in the mechanically ventilated subgroup, and indication bias, our findings suggest that systemic treatment-dose AC may be associated with improved outcomes among patients hospitalized with COVID-19. The potential benefits of systemic AC, however, need to be weighed against the risk of bleeding and therefore should be individualized. The association of in-hospital AC and mechanical ventilation likely reflects reservation of treatment-dose AC for more severe clinical presentations. Interestingly, there was an association with AC and improved survival after adjusting for mechanical ventilation.
These data, derived from a large United States cohort, provide clinical insights for consideration in the management of patients hospitalized with COVID-19. Prospective randomized trials are needed to determine whether systemic AC confers a survival benefit in hospitalized patients with COVID-19.
Footnotes
Please note: This work was supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health. Dr. Fayad has received consulting fees from Alexion and GlaxoSmithKline; has received research funding from Daiichi-Sankyo, Amgen, Bristol-Myers Squibb, and Siemens Healthineers; and has received financial compensation as a board member and advisor to and owns equity as a co-founder of Trained Therapeutix Discovery. Dr. Nadkarni has received financial compensation as a consultant and Advisory Board member for and owns equity in RenalytixAI; is a scientific co-founder of RenalytixAI and Pensieve Health; has received operational funding from Goldfinch Bio; and has received consulting fees from BioVie Inc., AstraZeneca, Reata, and GLG consulting in the past 3 years. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. P.K. Shah, MD, served as Guest Editor-in-Chief and Guest Associate Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACCauthor instructions page.
Contributor Information
Valentin Fuster, Email: valentin.fuster@mountsinai.org.
Girish N. Nadkarni, Email: girish.nadkarni@mountsinai.org.
References
- 1.Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J Thromb Haemost. 2020;18:786–787. doi: 10.1111/jth.14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020 Apr 3 doi: 10.1007/s11239-020-02105-8. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]