Graphical abstract
Keywords: COVID-19, Ruxolitinib, TKIs, Begelomab, Baricitinib, Tocilizumab, GVHD, MAS
Abstract
COVID-19 is a medical emergency, with 20 % of patients presenting with severe clinical manifestations. From the pathogenetic point of view, COVID-19 mimics two other well-known diseases characterized by cytokine storm and hyper-activation of the immune response, with consequent organ damage: acute graft-versus-host disease (aGVHD) and macrophage activation syndrome (MAS). Hematologists are confident with these situations requiring a prompt therapeutic approach for switching off the uncontrolled cytokine release; here, we discuss pros and cons of drugs that are already employed in hematology in the light of their possible application in COVID-19. The most promising drugs might be: Ruxolitinib, a JAK1/2 inhibitor, with a rapid and powerful anti-cytokine effect, tyrosine kinase inhibitors (TKIs), with their good anti-inflammatory properties, and perhaps the anti-Cd26 antibody Begelomab. We also present immunological data from gene expression experiments where TKIs resulted effective anti-inflammatory and pro-immune drugs. A possible combined treatment algorithm for COVID-19 is here proposed.
1. Introduction
Coronavirus disease 2019 (COVID-19), sustained by the new Coronavirus SARS-CoV-2, started in China in December 2019 in the province of Hubei and then rapidly overspread over the world, becoming a “pandemic”. The 22 April 2020, the European Centre for Disease Prevention and Control reported 2,520,522 infected subjects around the world, with 176,786 deaths [https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases]; 1,101,681 people were infected in Europe and 825,041 in USA, with 107,453 and 45,063 deaths, in Europe and USA, respectively [https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html].
This great number of infected subjects is requiring an enormous worldwide effort for hospitalizing and caring all patients who have to receive firstly an adequate diagnostic approach (chest X-ray or CT, viral genome identification and quantitation, serology), then the best possible therapies that might avoid the more severe phase of disease. From the clinical point of view, the majority of patients remains asymptomatic or presents mild symptoms; Mizumoto et al. conducted an epidemiologic study on the 3711 people who remained on board of the Diamond Princess cruise ship, blocked in Japan after identification of a SARS-CoV-2-positive passenger; these authors estimated that 17.9 % of all infected cases remained asymptomatic during quarantine [1]. Another group estimated that the rate of symptomatic cases was 101/10,000, after a median incubation time of 14 days [2]. Moreover, the Italian COVID-19 Surveillance Group, during the peak of infection, reported 460 deaths on 85,308 infected individuals (9.9 %), with an overall case-fatality rate around 7.2 %, substantially higher than in China (2.3 %), thus highlighting the compelling need for more effective approaches. The median age of infected subjects was 62 years, 85 % of deaths occurring in patients between 70 and 89 years. Moreover, only 1.2 % of infected patients presented at the hospitalization without comorbidities, while 23.5 % had one, 26.6 % two, and 48.6 % three or more comorbidities. The most frequent concomitant diseases resulted: previous ischemic heart attack or stroke, atrial fibrillation, hypertension, diabetes, dementia, a recent history of cancer, chronic liver disease or renal failure. Only 7.5 % of patients did not present any symptom at the hospital admission, 12.7 % were pauci-symptomatic, 37.9 % and 19.6 % manifested mild and severe symptoms, respectively, while 4.4 % were critical [https://www.epicentro.iss.it/coronavirus/]. In the international scenario, the most frequent clinical manifestations were fever and dyspnea, whilst cough, diarrhea and hemoptysis were less common; acute respiratory distress syndrome (ARDS) was observed in 96 % of severe cases, followed by acute renal failure in one third of them; super-infections were documented in 8.5 % of critical cases where septic shock and the macrophage activation syndrome (MAS) were the most frequent cause of death [3,4]. From the early stages of infection patients develop lymphopenia and neutrophilia; in the more advanced cases, lymphocyte further reduce, liver failure appears with hypoalbuminemia, and the hyper-inflammatory status, characterized by high levels of reactive protein C, ferritin, d-dimer, LDH, troponin and N-terminal fragment of the B-type natriuretic peptide (NT-proBNP), is demonstrable [5,6].
The pathogenesis of this “hyper-inflammation” have been recently revised: chemokines, such as MCP-1, IL2, IL-7, IL-10, G-CSF, IP-10, MIP-1A and IL6 are highly expressed, whereas TNF-alpha seems to be only moderately up-regulated. Cytotoxic CD8+ and exhausted T cells, together with an abnormal balance between Th1 and Th2 lymphocytes, mirror the onset of a severe immune dysfunction [7]. Consequently, several approaches able to switch off inflammation by maintaining at the same time the host’s antiviral immunocompetence have been rapidly designed and tempted: Chloroquine, already employed in rheumatological diseases, inhibiting the attack of the SARS-CoV-2 to the ACE2 receptors (that represent one of the two virus receptors) resulted quite effective [8], alone or in combination with azithromycin [9]. Tocilizumab, an anti-IL6 antibody, already used both in rheumatoid arthritis [10,11] and in the cytokines release syndrome after infusion of CAR-T in patients affected by acute lymphoblastic leukemia or aggressive lymphomas [12,13], has been employed with success in COVID-19. Recently, an Italian group proposed a new treatment algorithm whose backbone is represented by Chloroquine; Tocilizumab is used precociously in all patients with high levels of IL6 and d-dimer, including those, especially the elderly cases, with hypoxemia without severe dyspnea [14]. Other possible options from the “rheumatological” background are the anti-IL1 monoclonal antibody Anakinra, already effective in the MAS [15], and the JAK1/2 inhibitors, such as Baricitinib, already employed in rheumatoid arthritis [16], used alone or in combination with intravenous Immunoglobulins [14]. In 22 April 2020 a clinical trial aimed to assess the Baricitinib effectiveness in severe COVID-19 has been authorized by the Italian Drug Agency (AIFA) [https://www.aifa.gov.it/sperimentazioni-cliniche-covid-19]. Finally, anti-TNF alpha antibodies, such as Adalimumab, prescribed for the treatment of psoriasis [17] and Behcet’s disease [18], have been proposed as possible furher therapeutic tools for COVID-19 pandemic [7].
In this apocalyptic scenario, some authors already observed that this “rheumatological” approach, notwithstanding a clear fast and positive anti-inflammatory effect, could impair the immunological control of neoplasms in patients receiving chemotherapy or immunotherapy for cancer. Indeed, cancer patients showed a higher rate of severe events after SARS-CoV-2 infection in comparison with patients without cancer (39 % vs 8%) [19]. This epidemiological observation, in addition to the consideration that the majority of reported comorbidities in patients with critical COVID-19 were diseases characterized by a pro-inflammatory profile, underlines once again the need of identifying further drugs exerting a significant anti-inflammatory action but without losing their anti-tumor effect. On the basis of these considerations, we decided to review literature and what hematologists know about the relationship between hematological drugs, inflammation and immunity, in order to help the scientific community to definitively fight the COVID-19.
2. COVID-19 challenge: what hematologists learnt from hematological diseases
2.1. Two good “hematological” COVID-19-like models: the graft-versus-host disease and the MAS
In hematology, we have a well-known similar condition that mimics the hyper-inflammation caused by the new Coronavirus: the graft-versus-host disease, in its acute (aGVHD) and chronic forms (cGVHD). GVHD, which interests about half of transplanted patients, can appear by or after 100 days from the allogeneic stem cell infusion, with a prevalence that ranges from 35 % to 55 %, according to donor type, conditioning regimen, disease status at transplant and prophylactic approach [20,21]. GVHD is the consequence of a misleading attack by donor T lymphocytes of several recipient’s antigens recognized as outsiders, with consequent damage of his/her liver, lungs, gastrointestinal tract, eyes, vagina, muscles and joints. Allogeneic T and B lymphocytes sustain this hyper-inflammation that causes tissue damage and fibrosis, both by increasing production of inflammatory cytokines (IL-1, IL-2R, IL-6, TNF alpha) and by the deposition of immune complexes. The intestinal epithelium damage releases bacteria and modifies the gut microbiome, further increasing the immune response: T CD8+ lymphocytes are especially activated by the recipient hematopoietic antigen-presenting cells (APC), whereas donor T CD4+ cells can be activated by other APC types, principally in the gut. The participation of other immunocompetent cells, such as NK, macrophages, monocytes and neutrophils, makes GVHD a hyper-inflammatory dangerous condition that well recapitulates what occurs in COVID-19, where the rapid Coronavirus replication impairs the IFN-induced immune response, with rapid increase of M1-oriented macrophages and pro-inflammatory cytokines [7]. Moreover, clinical manifestations, especially of the aGVHD, are similar to those observed in COVID-19: skin rash, diarrhea, elevated bilirubin, infections, pulmonary leak syndrome, eye and mouth damage and, in the chronic form, also fasciitis, myositis and fibrosis that mimic the systemic scleroderma [22,23].
Another COVID-19-like condition that hematologists and rheumatologists have to deal with is the Macrophage activation syndrome (MAS), an acute hyper-inflammatory condition characterized by activation and expansion of T cells and hemophagocytic macrophages, with the consequent cytokine storm, with increased levels of proinflammatory cytokines, such as IL-1, IL-6, IL-18, TNF alpha, and IFN gamma [24]. MAS is reported to interest about 4% of patients with juvenile idiopathic arthritis and systemic lupus erythematosus, but it can also represent a complication of hematological neoplasms or infections, with a mortality higher than 40 %, that makes MAS a real medical emergency [25]. From the diagnostic point of view, MAS is a febrile condition characterized by hyperferritinemia, multilineage cytopenia, coagulopathy, transaminitis, high levels of triglycerides, hypofibrinogenemia and splenomegaly. Classically, MAS is treated with high steroid doses and etoposide [26], but in the era of new biological drugs promising results derived from the use of anti-IL1 and anti-IL6 antibodies, like Anakinra, Canakinumab, Rilonacept and Tocilizumab [27].
3. The hematological approach to COVID-19: pros and cons
Hematologists are already confident with GVHD and MAS, that require a rapid intervention for switching off the cytokine storm and controlling the exaggerated immune response. In the following, we’ll discuss positive and negative aspects of drugs employed for treating GVHD and MAS, in the light of their possible employ in the COVID-19 war.
3.1. Immunosuppressive agents
In aGVHD, treatment includes topic or systemic corticosteroids, anti-thymocyte immunoglobulins, cyclosporine, mycophenolate mofetil for appropriate management of acute phase. Novel approaches also include mesenchymal stem cells, etanercept and infliximab (anti-TNF alfa), daclizumab (anti-IL2) or vedolizumab (anti-a4b7), but results are still very preliminary and not worth to be considered for translating the experience deriving from the aGVHD “new era” directly to the COVID-19 [28]. About cGVHD as “inspirating source”, also in this case the first line approach is represented by immunosuppressive agents [29] that seems to be not really effective in COVID-19 [7].
3.2. Monoclonal antibodies
Tocilizumab, anti-IL6 monoclonal antibody, has been used also for treating aGVHD, with 70 % of partial remissions (PR). Nevertheless, in a series of 11 patients, 2 developed a bacterial sepsis, one of whom died [30]. Until today, 23 trials have been registered in the “clinical trials.gov” website, thus supporting the promising use of this drug in COVID-19. Rituximab has been also used as therapeutic tool in GVHD, with 60 % of overall response rate (ORR); however, as reported by the Italian cooperative group (GITMO), 3/38 treated patients died for infections [31], and in a meta-analysis involving 111 cGVHD patients, one third of them presented pneumonitis and Herpes virus reactivation [32]. No studies involving this monoclonal antibody have still registered in the “clinical trials.gov” website. In our opinion, the use of Rituximab in the COVID19 could be not considered, either for the high rate of infections reported in the hematological context, or because Rituximab requires a too long time to be efficacious.
Begelomab, a monoclonal anti-CD26 antibody, has been recently reported to be efficacious in treatment of 69 steroid-refractory aGVHD patients. In the compassionate use, Begelomab was administered at 3 mg/m2/day for 5 days, followed by six additional doses of 3 mg/m2 at day +10, +14, +17, +21, and +24. The overall response rate at one month was 75 % in the prospective studies and 61 % in the compassionate use, with complete response rates of 11 and 12 %, respectively. Response in grade-III GVHD was higher than 70 %, and response in grade-IV GVHD cases about 60 %, with higher response rates described for skin, liver, and gut. The tolerability of treatment was good, with the most common adverse events being diarrhea, cytomegalovirus reactivation, infections, probably more linked to the GVHD and the previous steroid treatment than to the antibody itself. In the 8 complete responders there was only one late death due to infections; in the 38 partial responders, the infection rate was 10.5 % [33]. Recently, the DPP4/CD26 glycoprotein has been reported to be one of the two receptors for the spike S1 SARS-CoV2 surface protein, together with the angiotensin converting enzyme (ACE2) [34]. Once activated by SARS-CoV-2, this protease helps virus 1) to reduce autophagy, the process physiologically aimed to eliminate external microorganisms from the host cells, 2) to sustain the hyper-inflammatory status and 3) to reduce the host anti-viral immune response [35]. The hypothesis of destroying this strict link by the anti-CD26 antibodies or the DPP4 inhibitors, already employed in the diabetic patients, seems really interesting [36]. DPP4 inhibitors have been already demonstrated to be efficacious in several in vitro models of SARS [34] and, considering the 80 % of homology between old and new Coronavirus, DPP4 inhibitors might be useful also in the COVID-19 pandemic [37]. Nevertheless, no studies with Begelomab have still been registered in the “clinical trials.gov” website. Considering these novel findings about the possibility of destroying the CD26 axis connecting Coronavirus and inflammation/perturbed host immunity, in our opinion, the use of Begelomab, probably for a short time course, might be considered an interesting approach, worth to be tested in the COVID19.
3.3. BTK inhibitors
In the last two years, FDA and EMA licensed Ibrutinib as treatment for steroid-refractory cGVHD. Ibrutinib, already effective in high risk chronic lymphocytic leukemia (CLL) [34], in addition to the Bruton Kinase, also inhibits another kinase, the interleukin-2–inducible T-cell kinase (ITK), that is involved in the selective activation of T-cells that drive immune reactivity toward healthy tissues [38], and a SRC kinase, HCK, whose over-expression, in a murine model, has been reported to be responsible for extensive pulmonary inflammation and enhanced immune response, particularly in older mice [39]. In cGVHD, Ibrutinib, switching off the cytokine storm, was successful in two third of cases, with 21 % of complete and 45 % of partial responses [40], with a significant improvement of patients’ quality of life [41]. Unfortunately, this treatment is characterized by adverse events that cause treatment discontinuation in 30 % of patients; in particular, pneumonitis, fatigue and diarrhea of grade ≥3 occur in 71 % of patients in the first year and in 25 % in the second year, inducing therapy discontinuation in 40 % of cases [38]. In agreement with these results, the experience in CLL reported high infection rates: in a cohort of 378 patients, serious infections were observed in 11.4 % of cases, especially bacterial and fungal [42]. At the moment, no clinical trials using Ibrutinib in COVID-19 have been registered in the “clinical trials.gov” website; nevertheless, Treon and coworkers in the last days published in Blood an interesting report concerning the low rate of COVID-19 occurrence in patients with Waldenstrom’s macroglobulinemia (only 6 out of 300 individuals). All patients experienced cough and fever as prodromal symptoms; the 5 patients on Ibrutinib 420 mg/day experienced no dyspnea and did not require hospitalization, with a shorter disease course in comparison with the one patient receiving lower Ibrutinib dose, who, on the contrary, required the administration of Tocilizumab and i.v. immunoglobulins [43].
In our opinion, Ibrutinib, might be a potential candidate for fighting the CoV-2, but probably if used for a short time, due to the high number of infections and treatment discontinuations that usually characterize its use in the hematological scenario. Clinical trials are needed to conclude if the balance weighs more on the side of efficacy or toxicity.
3.4. JAK2 inhibitors
The other drug licensed by FDA and EMA for treatment of GVHD is Ruxolitinib, already successfully employed for reducing spleen dimension and improving quality of life and survival of patients affected by myelofibrosis [44]. Ruxolitinib, a JAK1/2-inhibitor, decreases the activity of Th1 lymphocytes, and, through modulation of the STAT pathway, the secretion of pro-inflammatory cytokines, such as TNF alpha, IL1, IL6, and IFN gamma [45]. Ruxolitinib is effective both in acute and in chronic GVHD: in 71 cases of steroid-refractory aGVHD, Ruxolitinib offered 55 % of ORR and 27 % of CR, especially in skin, gastrointestinal tract, and liver. Median duration of response was 345 days and the overall survival (OS) at 6 months 51.0 %. Cytopenias occurred in half of cases, peripheral edema in 45 %, but no significant infective toxicity has been reported [46]. In another cohort, Ruxolitinib, at a dose of 20 mg/day, offered 57.1 % of ORR; reported adverse events were anemia, thrombocytopenia, neutropenia, infections, edema, bleeding, and transaminitis [47]. In the cGVHD, Ruxolitinib has been reported to be effective in 80 % of patients; nevertheless, reactivation of CMV occurred in 15 % of patients [48]. In a meta-analysis including 414 patients with cGVHD, during treatment with Ruxolitinib infections occurred in 20 % of patients, more frequently sustained by bacteria (55 %) and CMV (39 %) [49]. The pro-infective aspect of Ruxolitinib is also evident in myelofibrosis, where cases of hepatitis B [50] and tuberculosis (in 1.4 % of cases) [51] reactivation, in addition to pneumonitis sustained by Pneumocystis jiroveci [52], have been reported. In the last weeks, 8 clinical trials with Ruxolitinib in COVID-19 started, with dose ranging from 10 to 20 mg/day. The first 11 cases treated in Italy avoided the incoming intubation, so confirming in the real life the anti-inflammatory power of this JAK1/2 inhibitor.
In our opinion, Ruxolitinib could represent a very good candidate against COVID-19 for its well-known powerful and fast anti-inflammatory effect; nevertheless, the high rate of viral and microbial reactivation observed in the hematological setting might represent a caveat in its prolonged use in the COVID-19.
3.5. Tyrosine kinase inhibitors
Another class of drugs already employed in the treatment of GVHD that could help to win the COVID-19 challenge are the tyrosine kinase inhibitors (TKIs), already successfully employed in treatment of chronic myeloid leukemia (CML), Philadelphia-positive acute lymphoblastic leukemia and stromal gastro-intestinal tumor (GIST) [53]. Imatinib has been the first TKI licensed for CML treatment, followed by Nilotinib, Dasatinib, Bosutinib (second generation TKIs) and Ponatinib (third generation TKI). All TKIs, and especially those of second and third generation, in CML offer high rates of complete hematological, cytogenetic and molecular responses [53], necessary key for treatment discontinuation (TFR), that has success in about 40 % of patients [54]. Different studies focused on TFR explored the impact of TKIs on the immunological response, showing that this class of drugs play a positive effect on NK cells whose number and activated status is fundamental for maintaining deep molecular response without treatment [55,56]. Moreover, TKIs are able to restore the immunocompromised status observed in CML patients at diagnosis by reducing myeloid-derived suppressor cells, re-activating T and NK cells, and reducing the expression of PD-1 on T and NK lymphocytes and of PD-L1 on the microenvironment and on neoplastic clone [57]. Imatinib has been employed with success also in GVHD, but mainly in its chronic form, where it was successful in about 60 % of cases [58]. From the safety point of view, in a series of 19 cases only one pneumonitis and one CNS infection by JCV have been reported [59]. In another cohort with sclerodermic GVHD Imatinib was compared to Dasatinib: one of the 4 patients receiving Imatinib had pneumonitis versus 2 of the 5 cases treated with Dasatinib [60]. Two trials proposing Imatinib in COVID-19 have been already registered in the “clinical trials.gov” website (NCT04357613, NCT04356495), both involving elderly patients. In a third study, Imatinib will be compared to hidroxicloroquine, Lopinavir/ritonavir, and Baricitinib (NCT04346147).
In our opinion, Imatinib might represent a good therapeutic possibility in the COVID-19 for its demonstrated anti-inflammatory activity added to a good safety profile, but a caveat has to be done about the delayed onset of its positive therapeutic effects.
Dasatinib has not been further used in GVHD, but the toxicities that it causes in CML might contraindicate its use in the COVID-19. In fact, about 25 % of CML patients develop pleural effusion during Dasatinib treatment [61]. Several mechanisms have been explored, from the inhibition of PDGFR beta to increased T lymphocytes in pleural fluid [62]. In multivariate analysis, a previous skin rash or history of autoimmune disease resulted as significant factors predicting pleural effusion [63]. About infective risk during Dasatinib administration, the incidence of grade 3/4 infections resulted 11 % [64]; in the DASISION trial, which compared Dasatinib with Imatinib as first-line treatment, 4.5 % of patients in the Dasatinib and lessa than 1% in the Imatinib cohort died for infections, so sustaining the high infective risk of Dasatinib in comparison to Imatinib [65]. At the moment, no studies with Dasatinib in COVID-19 have been registered in the “clinical trials.gov” website. On the basis of available data, in our opinion, Dasatinib might be not a valid candidate for the COVID-19 treatment.
On the contrary, different promising suggestions come from some in vitro and in vivo models that would support the use of Bosutinib as a powerful anti-inflammatory agent. This TKI is today indicated for treatment of CML Imatinib-intolerant or resistant patients [66]. Differing from Dasatinib, whose pro-inflammatory action is supported by the high rate of pleural effusion, Bosutinib resolved this adverse event in 17/20 cases presenting effusion during treatment with Dasatinib. Moreover, the safety of Bosutinib from the immunological point of view is supported by the quite total absence of infective adverse events [67]. Moreover, in a model of membranous glomerulonephritis, Bosutinib was able to ameliorate renal damage by reducing expression of IL2R, IL4R, and by inhibiting JAK2/JAK3 (that sustain the inflammatory pathway) [68]. In another murine model of intra-cerebral hemorrhage with brain injury caused by post-bleeding inflammation, Bosutinib once more showed its anti-inflammatory action: inhibiting SIK-2, it activates CREB and IkB, so blocking the NF-kB-derived inflammation. Moreover, Bosutinib shifted the macrophagic response from M1 to M2, and decreased pro-inflammatory cytokines production [69]. Bosutinib and Nilotinib were also used and compared in a murine model of Alzheimer’s disease (where brain plaques are considered a consequence of hyper-inflammation). In this context, both TKIs decreased inflammation by reducing TNF alpha, IL4, IL6, IL3, and IL2 levels and increasing IL10 and CX3CL1, but, in comparison with Nilotinib, Bosutinib increased IL-10 and CX3CL1 also in the peripheral blood [70]. Thus, the anti-inflammatory profile of Bosutinib is evident. About its safety, in the BEFORE trial, where Bosutinib and Imatinib were compared in 536 CML patients in first line, grade 3/4 infection rate was 3.4 % in the Bosutinib versus 4.9 % in the Imatinib arm, with only 0.4 % of upper respiratory tract infections in the cohort treated with Bosutinib [71]. All these data suggest that Bosutinib might have a relevant anti-inflammatory effect, with a good safety profile; at the moment, no studies with Bosutinib have been registered in the “clinical trials.gov” website. Nevertheless, in our opinion, Bosutinib could be considered a possible effective drug in the COVID-19. Nevertheless, no experience with this drug has been done in GVHD or MAS.
Nilotinib is a valid second-generation TKI approved for treatment of CML in first or subsequent lines [72]. Nilotinib is now in experimentation also in GVHD, on the basis of data from the preclinical studies that clearly demonstrated its anti-inflammatory power. Indeed, Nilotinib significantly reduced production of pro-inflammatory cytokines (IL-2, IFN-gamma, TNF alpha, IL-17, TGF beta), without losing the lymphocyte immunocompetence [73,74]. Nevertheless, no definitive data on Nilotinib safety in GVHD are still available; consequently, safety profile must be derived from the experience in CML. In the ENESTnd trial, comparing Nilotinib and Imatinib in 564 CML patients in first line, all grade infection rate was 17 % in the Nilotinib versus 14 % in the Imatinib arm, with grade 3/4 infections rate in the Nilotinib cohort less then 1% [75]. In conclusion, Nilotinib seems to be an anti-inflammatory agent with a good infective safe profile; these features could make it, in our opinion, a good candidate in the COVID-19 setting; nevertheless, we have to consider its high rate of cardiovascular complications seen in CML [76,77] that could be the consequence of the inflammatory endothelial damage, as shown by higher IL6 and lower IL10 levels in CML patients presenting cardiovascular events [78]. At the moment, no studies with Nilotinib in COVID-19 have been registered in the “clinical trials.gov” website. In our opinion, this pro-atherogenic aspect might made Nilotinib a sub-optimal candidate in the COVID-19 context.
3.6. Interferons
Interferons (IFNs) are old, but at the same time “evergreen” drugs, for many years used for treating different hematological diseases, from CML and Philadelphia-negative chronic myeloproliferative neoplasms (MPNs) to lymphomas and myeloma, due to their potent immune enhancing capacity that allows recognition and elimination of neoplastic cells by the patient’s immune system. In CML, Interferon has been used until the introduction of TKIs; its offered hematological and cytogenetical, but very few molecular responses. Nevertheless, for many years it represented an advantageous treatment in respect of hydroxyurea [79]. In MPNs, IFNs are still successfully employed, especially in younger people, where their discontinuation after long-term treatment may be followed by several years with normal cell counts and low-JAK2V617 F burden, that once again supports the concept that IFN-alpha is able to modulate and enhance the immune system-mediated defense against cancer [80]. In lymphomas, IFN is still the first line of treatment of hairy cell leukemia [81] and, with less fortune, has been employed as maintenance therapy in indolent lymphomas, especially after autologous transplantation [82]. In multiple myeloma, IFN has been demonstrated to reduce plasmacells growth by down-regulating the IL6 production, with a synergic action with melphalan and corticosteroids in reducing the monoclonal component. IFN has also been used as maintenance after autologous transplantation before introduction in the clinical practice of lenalidomide and bortezomib, but with doubt favorable prognostic impact [83]. Moving from the hematological context to the SARS, during the outbreak of 2002 IFNs were also tried; a meta-analysis including 54 studies with IFN was performed in 2006, with discordant results. Indeed, while the in vitro studies showed a good anti-viral power of IFNs (with IFN beta being more effective than alpha), the in vivo studies were inconclusive, with a doubtful prognostic advantage in respect of steroids [84]. At the moment, 6 studies, aimed to understand if IFNs might be useful in COVID-19, have been registered in the “clinical trials.gov” website, trying either IFN alpha/beta or lambda (NCT04344600, NCT04350671, NCT04343768, NCT04343976, NCT04254874, NCT04320238). Interestingly, one of these studies is employing the IFN alpha via aeresol, probably in order to avoid the systemic adverse events (flu-like syndrome, fatigue, hypothyroidism, creatinine increase) that frequently lead to the drug discontinuation in the hematological patients [85]. The use of IFN lambda (type III IFN), seems interesting, based on different action mechanisms that characterize tipe I and III IFNs. Indeed, for decades, type I IFNs (IFN alpha and beta) have been explored as mediators of rapid, innate antiviral protection. In 2003, a novel group of three cytokines, now known as type III IFNs (IFN lambda), have been discovered. The distinctive actions of type I and type III IFNs depend on the engagement of different receptors: type I IFNs trigger pro-inflammatory responses via the recruitment and activation of immune cells, promoting an anti-viral state in the host, while type III IFNs signal is restricted to epithelial cells and neutrophils. Therefore, type III IFN administration as a prophylactic treatment or at an early stage of COVID-19 might result in a good antiviral response localized to epithelial cells, reducing side effects and inflammation associated with the systemic action of type I IFNs [86] In our opinion, considering the actual availability of different clinical options, because of their poor tolerability, IFNs might be not good candidate in the COVID-19 therapy.
4. Our personal contribution to the COVID-19 war: the analysis of the immune transcriptome
After this analysis, we became convinced that, in addition to Ruxolitinib, Imatinib and Bosutinib would represent possible interesting therapeutic tools in the COVID-19 war. Thus, we decided to contribute to the COVID-19 challenge by confirming ex vivo the anti-inflammatory power of Imatinib and if and how it could modify the immunological profile of our patients. Thus, we used the Nanostring technology (Nanostring, Seattle, USA) for analyzing the immune transcriptome profile of 5 patients affected by CML, at diagnosis and after 6 months of treatment with Imatinib. The tested RNAs have been already stocked in our laboratory as leftovers that the respective patients donated to us for further non-profit researches after routine diagnostics. We employed the “Human nCounter Myeloid Innate Immunity panel” that measures the expression value of 770 genes involved in 19 different pathways fundamental for the innate immune response. Results were analyzed by the nCounter Advanced Analysis 2.0 software. In Fig. 1 we represented some of the up- (red squares) and down-regulated (green squares) genes by volcano plots, and in the Table 1 are listed all down- (in green) or up- (in red) regulated genes and the pathways where they are involved. In Table 2 we better detailed all genes that resulted significantly deregulated after Imatinib, their respective physiological role and their possible contribution to inflammation and immunological infection control. Overall, 40 genes resulted down- and 18 up-regulated by Imatinib; 35 of these down-regulated genes may sustain the inflammation in different autoimmune diseases, whilst 5 are anti-inflammatory. After Imatinib-induced gene expression down-regulation, the final effect was a significant reduction of pro-inflammatory cytokines and chemokines mRNAs. Unfortunately, these data are not completed by the quantification of cytokines in the serum, because of the retrospective nature of the study. On the other hand, among the 18 genes those expressions increased after Imatinib, 15 support the physiological innate immune response. More in detail, among the down-regulated ones, we found some genes that are highly expressed in autoimmune diseases: ANX4A, high in the Sjogren’s syndrome [87], CASP10, high the Crohn's disease [88], while CEACAM8 [89], CTSG [90], and IL18 [91] are overexpressed in arthritis. Moreover, CLEC5A, increased after neurogenic shock [92], CXCL2 and GRN are highly expressed in the Alzheimer’s disease [93,94], ITGAM was elevated in psoriasis [95], and PGLYRP1 had high levels in chronic gingivitis [96]. All these genes were down-regulated by Imatinib, as a demonstration of its anti-inflammatory action. At the same time, the anti-inflammatory effect exerted by Imatinib was also sustained by the reduced expression of the genes that identified the mast cells (Fig. 2 ). Our Nanostring analysis also demonstrated that, while Imatinib reduced inflammation, the patient’s immunocompetence was not lost. Indeed, Imatinib down-regulated several genes that physiologically impair the T- and NK-cell response, such as ARG1 [97], C3AR1 [98], CEACAM1 [99], GSN [100] and NECTIN1 [101]. On the contrary, this TKI up-regulated some genes that usually support the immune response, such as JAK3, able to switch the macrophagic response from M1 (pro-inflammatory) to M2 (anti-inflammatory) [102], SOCS3, which had a low expression in arthritis [103], while TLR3 displayed low levels in inflammation and during viral infections [104]. Interestingly, Imatinib on the other hand also increased expression of some genes relevant for the antiviral response: CXCL16 [105], HAVCR2 [106], IFNG [107], RNASE2 and RNASE3 [108,109]. Finally, during Imatinib treatment, an increase in T cytotoxic and activated NK cells has been observed (Fig. 2).
Fig. 1.
CML: Volcano plots of some pathways de-regulated by 6 months of treatment with Imatinib.
Some of the up- (red squares) and down- (green squares) genes de-regulated during treatment of CML patients with Imatinib are represented by volcano plots. Statistical significance (at 0.05 and 0.01) are indicated with dotted and continuous lines, respectively. In a) the Antigen presentation pathway, in b) the Cytokines pathway, in c) the FCR signaling pathway is represented.
Table 1.
CML gene expression profiling.
| Gene Id | Pathway | Gene Id | Pathway |
|---|---|---|---|
| ANXA4 | Ag present | GRN | pat resp |
| ARG1 | metabolism | GSN | pat resp |
| BTK | BCR | IL18 | cytokines |
| C3AR1 | complement | ITGAM | migration |
| CAMP | pat respo | LTA4H | metabolism |
| CASP10 | cytokines | MAP2K1 | angiogenesis |
| CDC20 | Ag present | MMP8 | ECM |
| CEACAM1 | migration | MMP9 | ECM |
| CEACAM8 | migration | MPO | pat resp |
| CLEC5A | ly activation | NECTIN1 | migration |
| COL17A1 | ECM | OLR1 | migration |
| CTSG | Ag present | PGLYRP1 | pat resp |
| CXCL2 | chemokines | PLAU | complement |
| CXCL3 | chemokines | PRG2 | pat resp |
| CYBB | Ag present | PTX3 | pat resp |
| DAGLB | metabolism | RNASE2 | pat resp |
| ELANE | ECM | RNASE3 | pat resp |
| EPX | pat resp | SPTBN1 | cytokines |
| FGFR1 | cytokines | TM7SF3 | cytokines |
| FUT4 | metabolism | TNFAIP8 | cytokines |
| Gene Id | Pathway |
|---|---|
| CCL5 | chemokines |
| CCR4 | chemokines |
| CCR5 | chemokines |
| CD28 | migration |
| CD74 | Ag presentation |
| CX3CR1 | chemokines |
| CXCL16 | chemokines |
| CXCR3 | chemokines |
| FYN | Ag presentation |
| HAVCR2 | cytokines |
| IFNG | Ag presentation |
| JAK3 | chemokines |
| NFATC2 | Ag presentation |
| PDE4A | metabolism |
| SERPINB9 | pat resp |
| SOCS3 | Ag presentation |
| STAT5A | cytokines |
| TLR3 | pat resp |
Table represents all genes that, among the 770 genes whose expression had been tested by the Nanostring “Human nCounter Myeloid Innate Immunity” panel, resulted up- (in red) and down- (in green) regulated after 6 months of treatment with Imatinib. The adopted Nanostring panel allows to classify genes in 19 different pathways. The Table reports for each gene its respective pathway of belonging.
Table 2.
CML gene expression profiling.
| GENE ID | function | output on | ref |
|---|---|---|---|
| inflammation/ | |||
| immune resp | |||
| ANXA4 | high in Sjogren | anti infl | [87] |
| ARG1 | immunosuppressive | pro immun | [97] |
| BTK | sustains GVHD | anti infl | [38] |
| C3AR1 | neutrophils chemotaxis antagonist | pro immun | [98] |
| CAMP | increased in inflammation | anti infl | [126] |
| CASP10 | increased in Chron | anti infl | [88] |
| CDC20 | increased in the adiposity inflamm model | anti infl | [127] |
| CEACAM1 | inhibits T lynf | pro immun | [99] |
| CEACAM8 | high in arthritis | anti infl | [89] |
| CLEC5A | high in neurogen shock | anti infl | [92] |
| COL17A1 | induce IL7 that sustains T & B lynf | anti immun | [128] |
| CTSG | high in rheumatic arthritis | anti infl | [90] |
| CXCL2 | high in Alzheimer | anti infl | [70] |
| CXCL3 | sustain adipogenesis | anti infl | [129] |
| CYBB | increased in inflammation | anti infl | [130] |
| DAGLB | sustains production of arachidonic acid | anti infl | [131] |
| ELANE | high in LPS inflammation | anti infl | [132] |
| EPX | high in asthma | anti infl | [133] |
| FGFR1 | high in prostatic inflammation | anti infl | [134] |
| FUT4 | increased in bacterial infections | anti infl | [135] |
| GENE ID | function | output on | ref | |
|---|---|---|---|---|
| inflammation | ||||
| GRN | high in dementhia | anti | [94] | |
| GSN | increases NK apoptosis | pro immun | [100] | |
| IL18 | high in arthritis | anti | [91] | |
| ITGAM | high in psoriasis | anti | [95] | |
| LTA4H | high after trauma | anti | [113] | |
| MAP2K1 | high in sinusitis | anti | [114] | |
| MMP8 | high in intra-amniotic infections | anti | [115] | |
| MMP9 | high in skin healing | anti | [116] | |
| MPO | high in neutrophils | anti | [117] | |
| NECTIN1 | high in Chlamidial infection | pro imm | [101] | |
| OLR1 | NFkB activator | anti | [118] | |
| PGLYRP1 | high in gengivitis | anti | [96] | |
| PLAU | high after thrombosis | anti | [119] | |
| PRG2 | eosinophils basic protein | anti | [120] | |
| PTX3 | increased by IL6 | anti | [121] | |
| RNASE2 | high in inflamm, anti-viral | anti | anti-imm | [125] |
| RNASE3 | anti viral | anti imm | [125] | |
| SPTBN1 | reduces TGFb | pro | [122] | |
| TM7SF3 | reduces nitric oxid | pro | [123] | |
| TNFAIP8 | high in inflamm | anti | [124] | |
| GENE ID | function | output on |
ref | |
|---|---|---|---|---|
| inflammation | ||||
| CCL5 | activates NK | pro immun | [138] | |
| CCR4 | high in asthma | pro | [137] | |
| CCR5 | activates NK | pro immun | [136] | |
| CD28 | inactivated by PD1 | pro immun | [139] | |
| CD74 | increases MCHII expression | pro immun | [140] | |
| CX3CR1 | high in antifungal resp | pro immun | [141] | |
| CXCL16 | high in anti-viral resp | pro-immun | [105] | |
| CXCR3 | high in T effector | pro immun | [142] | |
| FYN | high in inflamm/sustains NK | pro | pro imm | [143] |
| HAVCR2 | high in anti-viral resp | pro-immun | [106] | |
| IFNG | antiviral | pro immun | [107] | |
| JAK3 | shift from M1 to M2 resp | anti | [102] | |
| NFATC2 | increases T memory | pro immun | [144] | |
| PDE4A | low in sepsis | anti | [145] | |
| SERPINB9 | activates CD8 | pro immun | [146] | |
| SOCS3 | low in arthritis | anti | [103] | |
| STAT5A | high in colon inflamm | pro | [147] | |
| TLR3 | anti-viral/anti-inflamm | anti | pro imm | [104] |
Table represents all genes that resulted Up- (in red) and down- (in green) regulated after 6 months of treatment with Imatinib, as listed in Table 1. Table 2 in addition for each gene reports the respective physiological function (with correspondent literature references) and the final effect resulting from mRNA de-regulation made by 6 months of Imatinib, with focus on the inflammation and on the immunological infection control.
Fig. 2.
CML: Box plots representing some cellular types de-regulated by 6 months of treatment with Imatinib.
Changes of mRNAs identifying different cellular populations after Imatinib treatment are here reported. In a) cytotoxic cells (defined as GZMA+, NKG7+, CD94+), whose mRNAs resulted increased by Imatinib; in b) NK cells (CD56 bright), that increased after Imatinib treatment; in c) mast cells (defined as CPA3+, tryptase+, MSGA2+, CCL22+), whose RNAs were decreased by Imatinib; in d) RNAs characterizing neutrophils (defined as FPR1+, SIGLEC5+, CSF3R+, FCAR+), that remained unchanged in respect of diagnosis.
In conclusion, even if preliminary, our findings agree with data already published by Alves et al. that reported an increased number of NK cells and lower IL21 levels during treatment with TKIs and IFN [110], and support the hypothesis that Imatinib might be a very good candidate to fight COVID-19 due to its anti-inflammatory action in a context of a conserved and efficient immunological infection control (Fig. 3 ).
Fig. 3.
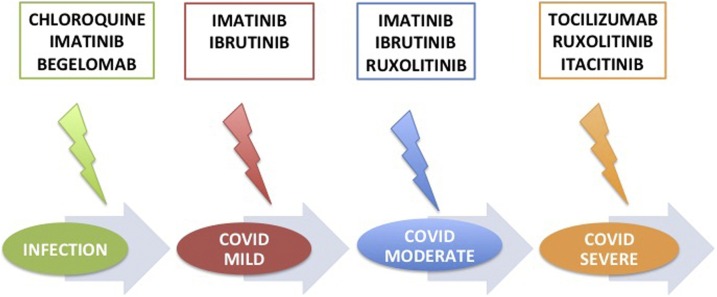
A possible therapeutic algorithm for COVID-19.
A possible “hematological-based” integrated algorithm for COVID-19 treatment, based on different disease phases, is here represented. In the early stage of SARS-CoV2 infection, chloroquine, imatinib or begelomab might be useful for blocking the attack of the viral S protein to the CD26 virus receptors, for modifying the lysosome pH or for restoring the anti-microbial autophagy. During an eventual mild COVID-19 phase, the anti-viral host reaction might be sustained by imatinib or ibrutinib, that at the same time might exert also an useful anti-inflammatory action, even if moderate. In the more severe phases of COVID-19, the anti-JAK1/2 inhibitors might be useful, alone or in combination with anti-cytokine monoclonal antibodies, such as Tocilizumab.
5. Conclusions
In Table 3 we resumed characteristics, pros and cons of drugs that, on the basis of above reported considerations, might be translated from the hematological scenario to the CoV-2 pandemic. Nevertheless, a further consideration has to be done about the costs of these possible new treatments: in 2018, a group of researchers from the Mayo Clinic performed a cost/effectiveness analysis on 1047 patients treated for cGVHD. Among the drugs that can be used against COVID-19, in that study on cGVHD the cheapest resulted chloroquine (9181 US$), followed by Imatinib (43,965 US$), and Ruxolitinib (97,807 US$) [111]. In our opinion, the final list of the “hematological” drugs that could represent promising options in the COVID-19 war might include also Ruxolitinib, Bosutinib, Imatinib and Begelomab. Ruxolitinib probably is the fastest and more powerful agent in the switching off the cytokine storm, as already shown in aGVHD and also in the first COVID-19 cases treated with this JAK1/2 inhibitor. Nevertheless, its doubtful safety from the infective point of view probably might impose at least the need of a careful observation of the immunocompetence in COVID-19 patients, also considering that super-infections have been documented in 8.5 % of them. TKIs could be tried as further options: in different models of inflammations, Bosutinib showed optimal anti-inflammatory properties, already demonstrated by its ability of reverting the pro-inflammatory effects of Dasatinib. In addition, data coming from the experience in CML sustain its good safety profile and sustain the hypothesis of a rapid efficacy also. Imatinib displays a good anti-inflammatory effect, its use is characterized by a low infection rate; it is worth to remember also that Imatinib remain the cheapest drug and probably the TKI most frequently available worldwide. Begelomab, probably for a short period of time, might also be an interesting option for its capacity of destroying the strict negative link between Coronavirus and inflammation actors.
Table 3.
Table reports the comparison of several features (hematological indication, safety, cost) in different hematological drugs that might have a role in the COVID-19. Abbreviations: MPN = chronic myeloproliferative neoplasms; MAS = macrophage activation syndrome; CML = chronic myeloid leukemia; ALL = acute lymphoblastic leukemia; GIST = stromal gastro-intestinal tumor; GVHD = graft-versus-host disease; LNH = non Hodgkin’s lymphoma; MM = multiple myeloma.
| Ruxolitinib | Imatinib | Dasatinib | Nilotinib | Bosutinib | Ibrutinib | Begelomab | IFN | ||
|---|---|---|---|---|---|---|---|---|---|
| Drug | os | os | os | os | os | os | IV | os | |
| formulation | |||||||||
| Clinical use in | |||||||||
| GVHD | XX | X | X | X | – | XX | XX | – | |
| Use in | MPNs | CML | CML | CML | CML | CLL | GVHD | CML | |
| hematological | MAS | ALL | ALL | ALL | MCL | MPN | |||
| diseases | GIST | LNH | |||||||
| MM | |||||||||
| Infection rate | 20 % | 5% | 11 % | 17 % | 4% | 71 % | 10 % | na | |
| Estimated | ++ | + | + | + | + | +++ | +++ | + | |
| costs |
Thus, all considered, in a hypothetical “hematological-driven” algorithm (see graphical abstract), we could imagine using Begelomab for blocking the first steps of infection, Ruxolitinib to rapidly switch off the cytokine storm in the severe/hyperacute phase, and, then to sustain immunity (that Ruxolitinib is not able to do) and the required long-term anti-inflammatory effect by TKIs. On the other hand, the combination of Ruxolitinib with Nilotinib has already been adopted in a phase-I study in CML patients with unsatisfactory molecular response, without significant infections occurrence [112]. In the last few weeks many trials with some of the above mentioned drugs started and will gave us soon fundamental information; indeed, the war against SARS-CoV-2 has to be continued: rethinking drugs use with a multidisciplinary approach could be a possible improvement for the final victory.
Author contribution
S. Galimberti and C. Baldini wrote the manuscript; all authors revised and approved it.
Declaration of Competing Interest
S. Galimberti, C. Baratè and M. Petrini were speakers in the events supported by Novartis, Pfizer, Celgene/BMS, Janssen, Roche, Incyte and members of advisory boards for Novartis and Janssen; A. Di Paolo was a speaker for Medac GmbH, Novartis, Roche, Incyte, and was an advisory board member for Novartis; C. Baldini and F. Ferro do not have any conflict of interest.
Acknowledgments
The experimental part of the work has been supported by University of Pisa with PRA 2018 grant (PI, Prof. Petrini). This article is dedicated to all patients and all physicians that every day have to fight COVID-19 and to all people that died during the pandemic.
References
- 1.Nicastri E., D’Abramo A., Faggioni G., De Santis R., Mariano A., Lepore L. Coronavirus disease (COVID-19) in a paucisymptomatic patient: epidemiological and clinical challenge in settings with limited community transmission, Italy, 2020. Euro Surveill. 2020;25(11) doi: 10.2807/1560-7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020 doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young B.E., Ong SWX Kalimuddin S., Low J.G., Tan S.Y., Loh J. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020 doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, Korea Centers for Disease Control and Prevention. Early Epidemiological and Clinical Characteristics of 28 Cases of Coronavirus Disease in South Korea. Osong Public Health Res Perspect. 11(1) (2020) 8-14. doi: 10.24171/j.phrp.2020.11.1.03. [DOI] [PMC free article] [PubMed]
- 6.Shi F., Yu Q., Huang W., Tan C. Novel coronavirus (COVID-19) pneumonia with Hemoptysis as the initial symptom: CT and clinical features. Korean J. Radiol. 2019;2020 doi: 10.3348/kjr.2020.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarzi-Puttini P., Giorgi V., Sirotti S., Marotto D., Ardizzone S., Rizzardini G. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin. Exp. Rheumatol. 2020;38(2):337–342. Epub 2020 Mar 22. [PubMed] [Google Scholar]
- 8.Duan Y.J., Liu Q., Zhao S.Q., Huang F., Ren L., Liu L., Zhou Y.W. The trial of chloroquine in the treatment of corona virus disease 2019 (COVID-19) and its research progress in forensic toxicology. Fa Yi Xue Za Zhi. 2020;36(2) doi: 10.12116/j.issn.1004-5619.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Maihle Morgane. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Scott L.J. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77(17):1865–1879. doi: 10.1007/s40265-017-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biggioggero M., Crotti C., Becciolini A., Favalli Eg. Tocilizumab in the treatment of rheumatoid arthritis: an evidence-based review and patient selection. Drug Des. Devel. Ther. 2018;13:57–70. doi: 10.2147/DDDT.S150580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hay K.A. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br. J. Haematol. 2018;183(3):364–374. doi: 10.1111/bjh.15644. [DOI] [PubMed] [Google Scholar]
- 13.Kotch C., Barrett D., Teachey D.T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev. Clin. Immunol. 2019;(8):813–822. doi: 10.1080/1744666X.2019.1629904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferro F., Elefante E., Baldini C., Bartoloni E., Puxeddu I., Talarico R. COVID-19: the new challenge for rheumatologists. Clin. Exp. Rheumatol. 2020;38(2):175–180. [PubMed] [Google Scholar]
- 15.Lind-Holst M., Hartling U.B., Christensen A.E. High-dose anakinra as treatment for macrophage activation syndrome caused by refractory Kawasaki disease in an infant. BMJ Case Rep. 2019;12(8) doi: 10.1136/bcr-2019-229708. pii: e229708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Salama Z.T., Scott L.J. Baricitinib: a review in rheumatoid arthritis. Drugs. 2018;78(7):761–772. doi: 10.1007/s40265-018-0908-4. [DOI] [PubMed] [Google Scholar]
- 17.Blauvelt A., Shi N., Burge R., Malatestinic Wn, Lin Cy, Lew Cr. Comparison of real-world treatment patterns among psoriasis patients treated with ixekizumab or adalimumab. Patient Prefer. Adherence. 2020;14:517–527. doi: 10.2147/PPA.S233993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ono T., Iwasaki T., Terada Y., Abe K., Lee J., Mochizuki M., Miyata K. Serum KL-6 elevation in a uveitis patient with Behçet’s disease treated with adalimumab. Am. J. Ophthalmol. Case Rep. 2020;18 doi: 10.1016/j.ajoc.2020.100660. 100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy. 2020 doi: 10.2217/imt-2020-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nassereddine S., Rafei H., Elbahesh E. Tabbara I. Acute graft versus host disease: a comprehensive review. Anticancer Res. 2017;37(4):1547–1555. doi: 10.21873/anticanres.11483. [DOI] [PubMed] [Google Scholar]
- 21.Kerep A.Z., Broome J., Pirsl F., Curtis L.M., Steinberg S.M., Mitchell S.A. Impact of the 2014 NIH chronic graft-versus-host disease scoring criteria modifications assessed in a large cohort of severely affected patients. Bone Marrow Transplant. 2019;54(1):76–84. doi: 10.1038/s41409-018-0224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald K.P., Blazar B.R., Hill G.R. Cytokine mediators of chronic graft-versus-host disease. J. Clin. Invest. 2017;127(7):2452–2463. doi: 10.1172/JCI90593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McManigle W., Youssef A., Sarantopoulos S. B cells in chronic graft-versus-host disease. Hum. Immunol. 2019;80(6):393–399. doi: 10.1016/j.humimm.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulert G.S., Grom A.A. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu. Rev. Med. 2015;66:145–159. doi: 10.1146/annurev-med-061813-012806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moradinejad M.H., Ziaee V. The incidence of macrophage activation syndrome in children with rheumatic disorders. Minerva Pediatr. 2011;63(6):459–466. [PubMed] [Google Scholar]
- 26.Yildiz H., Van Den Neste E., Defour J.P., Danse E., Yombi J.C. Adult haemophagocytic lymphohistiocytosis: a Review. QJM. 2020 doi: 10.1093/qjmed/hcaa011. pii: hcaa011. [DOI] [PubMed] [Google Scholar]
- 27.Grom A.A., Horne A., De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat. Rev. Rheumatol. 2016;12(5):259–268. doi: 10.1038/nrrheum.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nassereddine S., Rafei H., Elbahesh E. Tabbara I. Acute graft versus host disease: a comprehensive review. Anticancer Res. 2017;37(4):1547–1555. doi: 10.21873/anticanres.11483. [DOI] [PubMed] [Google Scholar]
- 29.Sarantopoulos S., Cardones A.R., Sullivan K.M. How I treat refractory chronic graft-versus-host disease. Blood. 2019;133(11):1191–1200. doi: 10.1182/blood-2018-04-785899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kattner A.S., Holler E., Holler B., Klobuch S., Weber D., Martinovic D. IL6-receptor antibody tocilizumab as salvage therapy in severe chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Ann. Hematol. 2020;99(4):847–853. doi: 10.1007/s00277-020-03968-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaja F., Bacigalupo A., Patriarca F., Stanzani M., Van Lint M.T., Filì C. Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant. 2007;40(3):273–277. doi: 10.1038/sj.bmt.1705725. [DOI] [PubMed] [Google Scholar]
- 32.Kharfan-Dabaja M.A., Mhaskar A.R., Djulbegovic B., Cutler C., Mohty M., Kumar A. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol. Blood Marrow Transplant. 2009;15(9) doi: 10.1016/j.bbmt.2009.04.003. 1005-13. [DOI] [PubMed] [Google Scholar]
- 33.Bacigalupo A., Angelucci E., Raiola A.M., Varaldo R., Di Grazia C., Gualandi F. Treatment of steroid resistant acute graft versus host disease with an anti-CD26 monoclonal antibody-Begelomab. Bone Marrow Transplant. 2020 doi: 10.1038/s41409-020-0855-z. [DOI] [PubMed] [Google Scholar]
- 34.Vankadari N., Wilce J.A. Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg. Microbes Infect. 2020;9(1):601–604. doi: 10.1080/22221751.2020.173956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deretic V., Levine B. Autophagy balances inflammation in innate immunity. Autophagy. 2018;14(2):243–251. doi: 10.1080/15548627.2017.1402992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drucker D.J. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr. Rev. 2020 doi: 10.1210/endrev/bnaa011. pii: bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iacobellis G. COVID-19 and diabetes: Can DPP4 inhibition play a role? Diabetes Res. Clin. Pract. 2020;162 doi: 10.1016/j.diabres.2020.108125. 108125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miklos D., Cutler C.S., Arora M., Waller E.K., Jagasia M., Pusic I. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017;130(21):2243–2250. doi: 10.1182/blood-2017-07-793786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ernst M., Inglese M., Scholz G.M., Harder K.W., Clay F.J., Bozinovski S. Constitutive activation of the SRC family kinase Hck results in spontaneous pulmonary inflammation and an enhanced innate immune response. J. Exp. Med. 2002;196(5):589–604. doi: 10.1084/jem.20020873. PMID:12208875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waller E.K., Miklos D., Cutler C., Arora M., Jagasia M.H., Pusic I. Ibrutinib for chronic graft-versus-Host disease after failure of prior therapy: 1-Year update of a phase 1b/2 study. Biol. Blood Marrow Transplant. 2019;25(10):2002–2007. doi: 10.1016/j.bbmt.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 41.King-Kallimanis B.L., Wroblewski T., Kwitkowski V., De Claro R.A., Gwise T., Bhatnagar V. FDA review summary of patient-reported outcome results for ibrutinib in the treatment of chronic graft versus host disease. Qual. Life Res. 2020 doi: 10.1007/s11136-020-02448-y. [DOI] [PubMed] [Google Scholar]
- 42.Varughese T., Taur Y., Cohen N., Palomba M.L., Seo S.K., Hohl T.M., Redelman-Sidi G. Serious infections in patients receiving ibrutinib for treatment of lymphoid Cancer. Clin. Infect. Dis. 2018;67(5):687–692. doi: 10.1093/cid/ciy175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Treon S.P., Castillo J., Skarbnik A.P., Soumerai J.D., Ghobrial I.M., Guerrera M.L. The BTK-inhibitor ibrutinib may protect against pulmonary injury in COVID-19 infected patients. Blood. 2020 doi: 10.1182/blood.2020006288. pii: blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verstovsek S., Gotlib J., Mesa R.A., Vannucchi A.M., Kiladjian J.J., Cervantes F. Long-term survival in patients treated with ruxolitinib for myelofibrosis: COMFORT-I and -II pooled analyses. J. Hematol. Oncol. 2017;10(1):156. doi: 10.1186/s13045-017-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Albeituni S., Verbist K.C., Tedrick P.E., Tillman H., Picarsic J., Bassett R., Nichols K.E. Mechanisms of action of ruxolitinib in murine models of hemophagocytic lymphohistiocytosis. Blood. 2019;134(2):147–159. doi: 10.1182/blood.2019000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jagasia M., Perales M.A., Schroeder M.A., Ali H., Shah N.N., Chen Y.B. Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label, phase 2 trial. Blood. 2020 doi: 10.1182/blood.2020004823. pii: blood.2020004823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hui L., Qi L., Guoyu H., Xuliang S., Meiao T. Ruxolitinib for treatment of steroid-refractory graft-versus-host disease in adults: a systematic review and meta-analysis. Expert Rev. Hematol. 2020:1–11. doi: 10.1080/17474086.2020.1738214. [DOI] [PubMed] [Google Scholar]
- 48.Zeiser R., Burchert A., Lengerke C., Verbeek M., Maas-Bauer K., Metzelder S.K. Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia. 2015;29(10):2062–2068. doi: 10.1038/leu.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hui L., Qi L., Guoyu H., Xuliang S., Meiao T. Ruxolitinib for treatment of steroid-refractory graft-versus-host disease in adults: a systematic review and meta-analysis. Expert Rev. Hematol. 2020:1–11. doi: 10.1080/17474086.2020.1738214. [DOI] [PubMed] [Google Scholar]
- 50.Caocci G., Murgia F., Podda L., Solinas A., Atzeni S., La Nasa G. Reactivation of hepatitis B virus infection following ruxolitinib treatment in a patient with myelofibrosis. Leukemia. 2014;28(1):225–227. doi: 10.1038/leu.2013.235. [DOI] [PubMed] [Google Scholar]
- 51.Khalid F., Damlaj M., AlZahrani M., Abuelgasim K.A., Gmati G.E. Reactivation of tuberculosis following ruxolitinib therapy for primary myelofibrosis: case series and literature review. Hematol. Stem Cell Ther. 2020 doi: 10.1016/j.hemonc.2020.02.003. pii: S1658-3876(20)30032-30037. [DOI] [PubMed] [Google Scholar]
- 52.Lee S.C., Feenstra J., Georghiou P.R. Pneumocystis jiroveci pneumonitis complicating ruxolitinib therapy. BMJ Case Rep. 2014 doi: 10.1136/bcr-2014-204950. pii: bcr2014204950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jain P., Kantarjian H., Cortes J. Chronic myeloid leukemia: overview of new agents and comparative analysis. Curr. Treat. Options Oncol. 2013;14(2):127–143. doi: 10.1007/s11864-013-0234-8. [DOI] [PubMed] [Google Scholar]
- 54.Fava C., Rege-Cambrin G., Dogliotti I., Cerrano M., Berchialla P., Dragani M. Observational study of chronic myeloid leukemia Italian patients who discontinued tyrosine kinase inhibitors in clinical practice. Haematologica. 2019;20104(8):1589–1596. doi: 10.3324/haematol.2018.205054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dumas P.Y., Bérard E., Bréal C., Dulucq S., Réa D., Nicolini F. Killer immunoglobulin-like receptor genotypes and chronic myeloid leukemia outcomes after imatinib cessation for treatment-free remission. Cancer Med. 2019;8(11):4976–4985. doi: 10.1002/cam4.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caocci G., Martino B., Greco M., Abruzzese E., Trawinska M.M., Lai S. Killer immunoglobulin-like receptors can predict TKI treatment-free remission in chronic myeloid leukemia patients. Exp. Hematol. 2015;43(12):1015–1018. doi: 10.1016/j.exphem.2015.08.004. e1. [DOI] [PubMed] [Google Scholar]
- 57.Hughes A., Yong A.S.M. Immune effector recovery in chronic myeloid leukemia and treatment-free remission. Front. Immunol. 2017;8:469. doi: 10.3389/fimmu.2017.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alsuliman T., Magro L., Coiteux V., Gauthier J., Srour M., Lionet A. The concurrent administration of imatinib with extracorporeal photopheresis leads to complete and durable responses in patients with refractory sclerotic type chronic graft-versus-host disease. Curr. Res. Transl. Med. 2019 doi: 10.1016/j.retram.2019.10.001. pii: S2452-3186(19)30041-30048. [DOI] [PubMed] [Google Scholar]
- 59.Olivieri A., Locatelli F., Zecca M., Sanna A., Cimminiello M., Raimondi R. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood. 2009;114(3):709–718. doi: 10.1182/blood-2009-02-204156. [DOI] [PubMed] [Google Scholar]
- 60.Sánchez-Ortega I., Parody R., Servitje O., Muniesa C., Arnan M., Patino B. Imatinib and dasatinib as salvage therapy for sclerotic chronic graft-vs-host disease. Croat. Med. J. 2016;57(3):247–254. doi: 10.3325/cmj.2016.57.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iurlo A., Galimberti S., Abruzzese E., Annunziata M., Bonifacio M., Latagliata R. Pleural effusion and molecular response in dasatinib-treated chronic myeloid leukemia patients in a real-life Italian multicenter series. Ann. Hematol. 2018;97(1):95–100. doi: 10.1007/s00277-017-3144-1. [DOI] [PubMed] [Google Scholar]
- 62.Bergeron A., Réa D., Levy V., Picard C., Meignin V., Tamburini J. Lung abnormalities after dasatinib treatment for chronic myeloid leukemia: a case series. Am. J. Respir. Crit. Care Med. 2007;176(8):814–818. doi: 10.1164/rccm.200705-715CR. [DOI] [PubMed] [Google Scholar]
- 63.de Lavallade H., Punnialingam S., Milojkovic D., Bua M., Khorashad J.S., Gabriel I.H. Pleural effusions in patients with chronic myeloid leukaemia treated with dasatinib may have an immune-mediated pathogenesis. Br. J. Haematol. 2008;141(5):745–747. doi: 10.1111/j.1365-2141.2008.07108.x. [DOI] [PubMed] [Google Scholar]
- 64.Maiti A., Cortes J.E., Patel K.P., Masarova L., Borthakur G., Ravandi F. Long-term results of frontline dasatinib in chronic myeloid leukemia. Cancer. 2020;126(7):1502–1511. doi: 10.1002/cncr.32627. [DOI] [PubMed] [Google Scholar]
- 65.Cortes J.E., Saglio G., Kantarjian H.M., Baccarani M., Mayer J., Boqué C. Final 5-Year study results of DASISION: the dasatinib versus imatinib study in treatment-naïve chronic myeloid leukemia patients trial. J. Clin. Oncol. 2016;34(20):2333–2340. doi: 10.1200/JCO.2015.64.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Isfort S., Crysandt M., Gezer D., Koschmieder S., Brümmendorf T.H. Wolf D. Bosutinib: a potent second-generation tyrosine kinase inhibitor. Recent Results Cancer Res. 2018;212:87–108. doi: 10.1007/978-3-319-91439-8_4. [DOI] [PubMed] [Google Scholar]
- 67.Tiribelli M., Abruzzese E., Capodanno I., Sorà F., Trabacchi E., Iurlo A. Efficacy and safety of bosutinib in chronic phase CML patients developing pleural effusion under dasatinib therapy. Ann. Hematol. 2019;98(11):2609–2611. doi: 10.1007/s00277-019-03802-y. [DOI] [PubMed] [Google Scholar]
- 68.Zhang C., Leng L., Li Z., Zhao Y., Jiao J. Identification of biomarkers and drug repurposing candidates based on an immune-, inflammation- and membranous glomerulonephritis-associated triplets network for membranous glomerulonephritis. BMC Med. Genomics. 2020;13(1):5. doi: 10.1186/s12920-019-0655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma L., Manaenko A., Ou Y.B., Shao A.W., Yang S.X., Zhang J.H. Bosutinib attenuates inflammation via inhibiting salt-inducible kinases in experimental model of intracerebral hemorrhage on mice. Stroke. 2017;48(11):3108–3116. doi: 10.1161/STROKEAHA.117.017681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lonskaya I., Hebron M.L., Selby S.T., Turner R.S., Moussa C.E. Nilotinib and bosutinib modulate pre-plaque alterations of blood immune markers and neuro-inflammation in Alzheimer’s disease models. Neuroscience. 2015;304:316–327. doi: 10.1016/j.neuroscience.2015.07.070. [DOI] [PubMed] [Google Scholar]
- 71.Cortes J.E., Gambacorti-Passerini C., Deininger M.W., Mauro M.J., Chuah C., Kim D.W. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J. Clin. Oncol. 2018;36(3):231–237. doi: 10.1200/JCO.2017.74.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sacha T., Saglio G. Nilotinib in the treatment of chronic myeloid leukemia. Future Oncol. 2019;15(9):953–965. doi: 10.2217/fon-2018-0468. [DOI] [PubMed] [Google Scholar]
- 73.Marinelli Busilacchi E., Costantini A., Viola N., Costantini B., Olivieri J., Butini L. Immunomodulatory effects of tyrosine kinase inhibitor in vitro and in vivo study. Biol. Blood Marrow Transplant. 2018;24(2):267–275. doi: 10.1016/j.bbmt.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 74.Marinelli Busilacchi E., Costantini A., Mancini G., Tossetta G., Olivieri J., Poloni A. Nilotinib Treatment of Patients Affected by Chronic Graft-versus-Host Disease Reduces Collagen Production and Skin Fibrosis by Downmodulating the TGF-β and p-SMAD Pathway. Biol. Blood Marrow Transplant. 2020 doi: 10.1016/j.bbmt.2020.01.014. pii: S1083-8791(20)30046-X. [DOI] [PubMed] [Google Scholar]
- 75.Hochhaus A., Saglio G., Hughes T.P., Larson R.A., Kim D.W., Issaragrisil S. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044–1054. doi: 10.1038/leu.2016.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Caocci G., Mulas O., Annunziata M., Luciano L., Abruzzese E., Bonifacio M. Long-term mortality rate for cardiovascular disease in 656 chronic myeloid leukaemia patients treated with second- and third-generation tyrosine kinase inhibitors. Int. J. Cardiol. 2020;301:163–166. doi: 10.1016/j.ijcard.2019.10.036. [DOI] [PubMed] [Google Scholar]
- 77.Caocci G., Mulas O., Annunziata M., Luciano L., Bonifacio M., Orlandi E.M. Cardiovascular toxicity in patients with chronic myeloid leukemia treated with second-generation tyrosine kinase inhibitors in the real-life practice: Identification of risk factors and the role of prophylaxis. Am. J. Hematol. 2018;93(7):E159–E161. doi: 10.1002/ajh.25102. [DOI] [PubMed] [Google Scholar]
- 78.Bocchia M., Galimberti S., Aprile L., Sicuranza A., Gozzini A., Santilli F. Genetic predisposition and induced pro-inflammatory/pro-oxidative status may play a role in increased atherothrombotic events in nilotinib treated chronic myeloid leukemia patients. Oncotarget. 2016;7(44):72311–72321. doi: 10.18632/oncotarget.11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gale R.P., Hehlmann R., Zhang M.J., Hasford J., Goldman J.M., Heimpel H. Survival with bone marrow transplantation versus hydroxyurea or interferon for chronic myelogenous leukemia. The German CML Study Group. Blood. 1998;91(5):1810–1819. [PubMed] [Google Scholar]
- 80.Hasselbalch H.C., Holmström M.O. Perspectives on interferon-alpha in the treatment of polycythemia vera and related myeloproliferative neoplasms: minimal residual disease and cure? Semin. Immunopathol. 2019;41(1):5–19. doi: 10.1007/s00281-018-0700-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson P.A., Ravandi F. How I manage patients with hairy cell leukaemia. Br. J. Haematol. 2017;177(4):543–556. doi: 10.1111/bjh.14524]. [DOI] [PubMed] [Google Scholar]
- 82.Smyth L., Buckstein R., Pennell N., Weerasinghe R., Cheung M.C., Imrie K. Autologous stem cell transplant and combination immunotherapy of rituximab and interferon-α induces prolonged clinical and molecular remissions in patients with follicular lymphoma. Br. J. Haematol. 2019;184(3):469–472. doi: 10.1111/bjh.15118. [DOI] [PubMed] [Google Scholar]
- 83.Joshua D.E., MacCallum S., Gibson J. Role of alpha interferon in multiple myeloma. Blood Rev. 1997;11(4):191–200. doi: 10.1016/s0268-960x(97)90019-9. PMID: 9481449. [DOI] [PubMed] [Google Scholar]
- 84.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343. PMID:16968120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raanani P., Ben-Bassat I. Immune-mediated complications during interferon therapy in hematological patients. Acta Haematol. 2002;107(3):133–144. doi: 10.1159/000057631. PMID:11978934. [DOI] [PubMed] [Google Scholar]
- 86.Prokunina-Olsson L., Alphonse N., Dickenson R.E., Durbin J.E., Glenn J.S., Hartmann R. COVID-19 and emerging viral infections: the case for interferon lambda. J. Exp. Med. 2020;217(5) doi: 10.1084/jem.20200653. pii: e20200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cai W.L.A., Galtung H.K., Guerreiro E.M., Øvstebø R., Thiede B., Utheim T.P. Proteomic and histopathological characterisation of sicca subjects and primary Sjögren’s syndrome patients reveals promising tear, saliva and extracellular vesicle disease biomarkers. Arthritis Res. Ther. 2019;21(1):181. doi: 10.1186/s13075-019-1961-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li N. Shi RH. lncRNACNN3-206 activates intestinal epithelial cell apoptosis and invasion by sponging miR-212, an implication for Crohn’s disease. World J. Gastroenterol. 2020;26(5):478–498. doi: 10.3748/wjg.v26.i5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ribon M., Mussard J., Semerano L., Singer B.B., Decker P. Extracellular chromatin triggers release of soluble CEACAM8 upon activation of neutrophils. Front. Immunol. 2019;10:1346. doi: 10.3389/fimmu.2019.01346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trzybulska D., Olewicz-Gawlik A., Graniczna K., Kisiel K., Moskal M., Cieślak D. Quantitative analysis of elastase and cathepsin G mRNA levels in peripheral blood CD14(+) cells from patients with rheumatoid arthritis. Cell. Immunol. 2014;292(1-2):40–44. doi: 10.1016/j.cellimm.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 91.Bao J., Chen Z., Xu L., Wu L., Xiong Y. Rapamycin protects chondrocytes against IL-18-induced apoptosis and ameliorates rat osteoarthritis. Aging (Albany NY). 2020:12. doi: 10.18632/aging.102937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sung P.S., Chang W.C., Hsieh S.L. CLEC5A: a promiscuous pattern recognition receptor to microbes and beyond. Adv. Exp. Med. Biol. 2020;1204:57–73. doi: 10.1007/978-981-15-1580-4_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cattaneo A., Cattaneo N., Galluzzi S., Provasi S., Lopizzo N. INDIA-FBP Group. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 94.Marschallinger J., Iram T., Zardeneta M., Lee S.E., Lehallier B., Haney M.S. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat. Neurosci. 2020;23(2):194–208. doi: 10.1038/s41593-019-0566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hruska P., Kuruczova D., Vasku V., Bienertova-Vasku J. MiR-21 binding site SNP within ITGAM associated with psoriasis susceptibility in women. PLoS One. 2019;14(6) doi: 10.1371/journal.pone.0218323. e0218323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Silbereisen A., Hallak A.K., Nascimento G.G., Sorsa T., Belibasakis G.N., Lopez R., Bostanci N. Regulation of PGLYRP1 and TREM-1 during progression and resolution of gingival inflammation. JDR Clin. Trans. Res. 2019;4(4):352–359. doi: 10.1177/2380084419844937. [DOI] [PubMed] [Google Scholar]
- 97.Cai W., Dai X., Chen J., Zhao J., Xu M., Zhang L., Yang B. STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight. 2019;4(20) doi: 10.1172/jci.insight.131355. pii: 131355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brennan F.H., Jogia T., Gillespie E.R., Blomster L.V., Li X.X., Nowlan B. Complement receptor C3aR1 controls neutrophil mobilization following spinal cord injury through physiological antagonism of CXCR2. JCI Insight. 2019;4(9) doi: 10.1172/jci.insight.98254. pii: 98254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nagaishi T., Pao L., Lin S.H., Iijima H., Kaser A., Qiao S.W. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity. 2006;25(5):769–781. doi: 10.1016/j.immuni.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 100.Wątek M., Wnorowska U., Wollny T., Durnaś B., Wolak P., Kościołek-Zgódka S. Hypogelsolinemia in patients diagnosed with acute myeloid leukemia at initial stage of Sepsis. Med. Sci. Monit. 2019;25:1452–1458. doi: 10.12659/MSM.911904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Slade J.A., Hall J.V., Kintner J., Phillips-Campbell R., Schoborg R.V. Host Nectin-1 promotes chlamydial infection in the female mouse genital tract, but is not required for infection in a novel male murine rectal infection model. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0160511. e0160511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quero L., Tiaden A.N., Hanser E., Roux J., Laski A., Hall J. Kyburz D. miR-221-3p drives the shift of M2-Macrophages to a pro-inflammatory function by suppressing JAK3/STAT3 activation. Front. Immunol. 2020;10:3087. doi: 10.3389/fimmu.2019.03087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gui T., He B.S., Gan Q., Yang C. Enhanced SOCS3 in osteoarthiritis may limit both proliferation and inflammation. Biotech. Histochem. 2017;92(2):107–114. doi: 10.1080/10520295.2017.1278792. [DOI] [PubMed] [Google Scholar]
- 104.Ribes S., Arcilla C., Ott M., Schütze S., Hanisch U.K., Nessler S., Nau R. Pre-treatment with the viral Toll-like receptor 3 agonist poly(I:C) modulates innate immunity and protects neutropenic mice infected intracerebrally with Escherichia coli. J. Neuroinflammation. 2020;17(1):24. doi: 10.1186/s12974-020-1700-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Touzelet O., Broadbent L., Armstrong S.D., Aljabr W., Cloutman-Green E., Power U.F., Hiscox J.A. The secretome profiling of a pediatric airway epithelium infected with hRSV identified aberrant apical/basolateral trafficking and novel immune modulating (CXCL6, CXCL16, CSF3) and antiviral (CEACAM1) proteins. Mol. Cell Proteomics. 2020 doi: 10.1074/mcp.RA119.001546. pii: mcp.RA119.001546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liong S., Lim R., Barker G., Lappas M. Hepatitis A virus cellular receptor 2 (HAVCR2) is decreased with viral infection and regulates pro-labour mediators OA. Am J Reprod Immunol. 2017;78(1) doi: 10.1111/aji.12696. [DOI] [PubMed] [Google Scholar]
- 107.Gao P., Fan L., Du H., Xiang B., Li Y., Sun M. Recombinant duck interferon gamma inhibits H5N1 influenza virus replication in vitro and in vivo. J. Interferon Cytokine Res. 2018;38(7):290–297. doi: 10.1089/jir.2018.0034. [DOI] [PubMed] [Google Scholar]
- 108.Rosenberg H.F. Eosinophil-derived neurotoxin (EDN/RNase 2) and the mouse eosinophil-associated RNases (mEars): expanding roles in promoting host defense. Int. J. Mol. Sci. 2015;16(7):15442–15455. doi: 10.3390/ijms160715442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cao L., Wu X.M., Nie P., Chang M.X. The negative regulation of piscine CD44c in viral and bacterial infection. Dev. Comp. Immunol. 2019;96:135–143. doi: 10.1016/j.dci.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 110.Alves R., McArdle S.E.B., Vadakekolathu J., Gonçalves A.C., Freitas-Tavares P., Pereira A. Flow cytometry and targeted immune transcriptomics identify distinct profiles in patients with chronic myeloid leukemia receiving tyrosine kinase inhibitors with or without interferon-α. J. Transl. Med. 2020;18(1):2. doi: 10.1186/s12967-019-02194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yalniz F.F., Murad M.H., Lee S.J., Pavletic S.Z., Khera N., Shah N.D., Hashmi S.K. Steroid refractory chronic graft-versus-Host disease: cost-effectiveness analysis. Biol. Blood Marrow Transplant. 2018;24(9):1920–1927. doi: 10.1016/j.bbmt.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sweet K., Hazlehurst L., Sahakian E., Powers J., Nodzon L., Kayali F., Hyland K. A phase I clinical trial of ruxolitinib in combination with nilotinib in chronic myeloid leukemia patients with molecular evidence of disease. Leuk. Res. 2018;74:89–96. doi: 10.1016/j.leukres.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cheng Y., Pereira M., Raukar N.P., Reagan J.L., Quesenberry M., Goldberg L. Inflammation-related gene expression profiles of salivary extracellular vesicles in patients with head trauma. Neural Regen. Res. 2020;15(4):676–681. doi: 10.4103/1673-5374.266924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsou Y.A., Tung Y.T., Wu T.F., Chang G.R., Chen H.C., Lin C.D. Lactoferrin interacts with SPLUNC1 to attenuate lipopolysaccharide-induced inflammation of human nasal epithelial cells via down-regulated MEK1/2-MAPK signaling. Biochem. Cell Biol. 2017;95(3):394–399. doi: 10.1139/bcb-2016-0047. [DOI] [PubMed] [Google Scholar]
- 115.Yoon B.H., Romero R., Park J.Y., Oh K.J., Lee J., Conde-Agudelo A., Hong J.S. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am. J. Obstet. Gynecol. 2019;221(2):142. doi: 10.1016/j.ajog.2019.03.018. e1-142.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Funel N., Dini V., Janowska A., Loggini B., Minale M., Grieco F. Triticum vulgare extract modulates protein-kinase B and matrix metalloproteinases 9 protein expression in BV-2 cells: bioactivity on inflammatory pathway associated with molecular mechanism wound healing. Mediators Inflamm. 2020;2020 doi: 10.1155/2020/2851949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Myeloperoxidase Aratani Y. Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018;640:47–52. doi: 10.1016/j.abb.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 118.Lara-Guzmán O.J., Gil-Izquierdo Á, Medina S., Osorio E., Álvarez-Quintero R., Zuluaga N. Oxidized LDL triggers changes in oxidative stress and inflammatory biomarkers in human macrophages. Redox Biol. 2018;15:1–11. doi: 10.1016/j.redox.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shi Y., Zhang Z., Cai D., Kuang J., Jin S., Zhu C. Urokinase attenuates pulmonary thromboembolism in an animal model by inhibition of inflammatory response. J. Immunol. Res. 2018;2018 doi: 10.1155/2018/6941368. 6941368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yun Y., Kanda A., Kobayashi Y., Van Bui D., Suzuki K., Sawada S. Increased CD69 expression on activated eosinophils in eosinophilic chronic rhinosinusitis correlates with clinical findings. Allergol. Int. 2020 doi: 10.1016/j.alit.2019.11.002. pii: S1323-8930(19)30194-30197. [DOI] [PubMed] [Google Scholar]
- 121.Bottazzi B., Inforzato A., Messa M., Barbagallo M., Magrini E., Garlanda C., Mantovani A. The pentraxins PTX3 and SAP in innate immunity, regulation of inflammation and tissue remodelling. J. Hepatol. 2016;64(6):1416–1427. doi: 10.1016/j.jhep.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kitisin K., Ganesan N., Tang Y., Jogunoori W., Volpe E.A., Kim S.S. Disruption of transforming growth factor-beta signaling through beta-spectrin ELF leads to hepatocellular cancer through cyclin D1 activation. Oncogene. 2007;26(50):7103–7110. doi: 10.1038/sj.onc.1210513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Isaac R., Goldstein I., Furth N., Zilber N., Streim S., Boura-Halfon S. TM7SF3, a novel p53-regulated homeostatic factor, attenuates cellular stress and the subsequent induction of the unfolded protein response. Cell Death Differ. 2017;24(1):132–143. doi: 10.1038/cdd.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Niture S., Moore J., Kumar D. TNFAIP8: inflammation, immunity and human diseases. J. Mol. Cell. Immunol. 2019;1(2):29–34. [PMC free article] [PubMed] [Google Scholar]
- 125.Ostendorf T., Zillinger T., Andryka K., Schlee-Guimaraes T.M., Schmitz S., Marx S. Immune sensing of synthetic, bacterial, and protozoan RNA by toll-like receptor 8 requires coordinated processing by RNase T2 and RNase 2. Immunity. 2020;52(4):591–605. doi: 10.1016/j.immuni.2020.03.009. e6. [DOI] [PubMed] [Google Scholar]
- 126.Ratcliffe C., Wandschneider B., Baxendale S., Thompson P., Koepp M.J., Caciagli L. Cognitive function in genetic generalized epilepsies: insights from neuropsychology and neuroimaging. Front. Neurol. 2020;11:144. doi: 10.3389/fneur.2020.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Merhi Z., Polotsky A.J., Bradford A.P., Buyuk E., Chosich J., Phang T. Adiposity alters genes important in inflammation and cell cycle division in human cumulus granulosa cell. Reprod. Sci. 2015;22(10):1220–1228. doi: 10.1177/1933719115572484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Krześniak M., Zajkowicz A., Gdowicz-Kłosok A., Głowala-Kosińska M., Łasut-Szyszka B., Rusin M. Synergistic activation of p53 by actinomycin D and nutlin-3a is associated with the upregulation of crucial regulators and effectors of innate immunity. Cell. Signal. 2020;69 doi: 10.1016/j.cellsig.2020.109552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kusuyama J., Komorizono A., Bandow K., Ohnishi T., Matsuguchi T. CXCL3 positively regulates adipogenic differentiation. J. Lipid Res. 2016;57(10):806–1820. doi: 10.1194/jlr.M067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kröller-Schön S., Daiber A., Steven S., Oelze M., Frenis K., Kalinovic S. Crucial role for Nox2 and sleep deprivation in aircraft noise-induced vascular and cerebral oxidative stress, inflammation, and gene regulation. Eur. Heart J. 2018;39(38):3528–3539. doi: 10.1093/eurheartj/ehy333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hsu K.L., Tsuboi K., Adibekian A., Pugh H., Masuda K., Cravatt B.F. DAGLβ inhibition perturbs a lipid network involved in macrophage inflammatory responses. Nat. Chem. Biol. 2012;8(12):999–1007. doi: 10.1038/nchembio.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gu W., Wen D., Lu H., Zhang A., Wang H., Du J. MiR-608 exerts anti-inflammatory effects by targeting ELANE in monocytes. J. Clin. Immunol. 2020;40(1):147–157. doi: 10.1007/s10875-019-00702-8. [DOI] [PubMed] [Google Scholar]
- 133.Cardenas A., Sordillo J.E., Rifas-Shiman S.L., Chung W., Liang L., Coull B.A. The nasal methylome as a biomarker of asthma and airway inflammation in children. Nat. Commun. 2019;10(1):3095. doi: 10.1038/s41467-019-11058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang C., Ke Y., Liu S., Pan S., Liu Z., Zhang H. Ectopic fibroblast growth factor receptor 1 promotes inflammation by promoting nuclear factor-κB signaling in prostate cancer cells. J. Biol. Chem. 2018;293(38):14839–14849. doi: 10.1074/jbc.RA118.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Markic J., Jeroncic A., Polancec D., Bosnjak N., Markotic A., Mestrovic J., Culic V.C. CD15s is a potential biomarker of serious bacterial infection in infants admitted to hospital. Eur. J. Pediatr. 2013;172(10):1363–1369. doi: 10.1007/s00431-013-2047-y. [DOI] [PubMed] [Google Scholar]
- 136.Li F., Sheng Y., Hou W., Sampath P., Byrd D., Thorne S., Zhang Y. CCL5-armed oncolytic virus augments CCR5-engineered NK cell infiltration and antitumor efficiency. J. Immunother. Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000131. pii: e000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Matsuo K., Nagakubo D., Komori Y., Fujisato S., Takeda N., Kitamatsu M. CCR4 is critically involved in skin allergic inflammation of BALB/c mice. J. Invest. Dermatol. 2018;138(8):1764–1773. doi: 10.1016/j.jid.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 138.Li F., Sheng Y., Hou W., Sampath P., Byrd D., Thorne S., Zhang Y. CCL5-armed oncolytic virus augments CCR5-engineered NK cell infiltration and antitumor efficiency. J. Immunother. Cancer. 2020;8(1) doi: 10.1136/jitc-2019-000131. pii: e000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hui E., Cheung J., Zhu J., Su X., Taylor M.J., Wallweber H.A. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355(6332):1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang Z.Q., Milne K., Webb J.R., Watson P.H. CD74 and intratumoral immune response in breast cancer. Oncotarget. 2017;8(8):12664–12674. doi: 10.18632/oncotarget.8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Leonardi I., Li X., Semon A., Li D., Doron I., Putzel G. CX3CR1+mononuclear phagocytes control immunity to intestinal fungi. Science. 2018;359(6372):232–236. doi: 10.1126/science.aao1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Groom J.R., Luster A.D. CXCR3 in T cell function. Exp. Cell Res. 2011;317(5):620–631. doi: 10.1016/j.yexcr.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bahal D., Hashem T., Nichols K.E., SLAM-SAP-Fyn Das R. Old players with new roles in iNKT cell development and function. Int. J. Mol. Sci. 2019;20(19) doi: 10.3390/ijms20194797. pii: E4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xu T., Keller A., Martinez G.J. NFAT1 and NFAT2 differentially regulate CTL differentiation upon acute viral infection. Front. Immunol. 2019;10:184. doi: 10.3389/fimmu.2019.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lelubre C., Medfai H., Akl I., Leentjens J., Kox M., Pickkers P. Leukocyte phosphodiesterase expression after lipopolysaccharide and during sepsis and its relationship with HLA-DR expression. J. Leukoc. Biol. 2017;101(6):1419–1426. doi: 10.1189/jlb.5A0516-240R. [DOI] [PubMed] [Google Scholar]
- 146.Mangan M.S., Melo-Silva C.R., Luu J., Bird C.H., Koskinen A., Rizzitelli A. A pro-survival role for the intracellular granzyme B inhibitor Serpinb9 in natural killer cells during poxvirus infection. Immunol. Cell Biol. 2017;95(10):884–894. doi: 10.1038/icb.2017.59. [DOI] [PubMed] [Google Scholar]
- 147.Li H., Fan C., Feng C., Wu Y., Lu H., He P., Yang X. Inhibition of phosphodiesterase-4 attenuates murine ulcerative colitis through interference with mucosal immunity. Br. J. Pharmacol. 2019;176(13):2209–2226. doi: 10.1111/bph.14667. [DOI] [PMC free article] [PubMed] [Google Scholar]