Abstract
Context
Familial partial lipodystrophy, Dunnigan variety (FPLD2) is a rare autosomal dominant disorder resulting from LMNA causal variants, which is characterized by loss of subcutaneous fat from the extremities and predisposition to metabolic complications. The diagnostic value of various anthropometric measurements for FPLD2 remains unknown.
Objective
To determine specificity and sensitivity of anthropometric measurements for the diagnosis of FPLD2.
Methods
We measured skinfold thickness and regional body fat by dual energy X-ray absorptiometry (DXA) in 50 adult females and 6 males with FPLD2 at UT Southwestern and compared their data with the sex- and age-matched controls from the National Health and Nutrition Examination Survey (NHANES) 1999-2010. We further compared data from 1652 unaffected females from the Dallas Heart Study and 23 females with FPLD2 from the National Institutes of Health with the NHANES data.
Results
The DXA-derived lower limb fat (%) had the best specificity (0.995) and sensitivity (1.0) compared with the upper limb fat, truncal fat, the ratio of lower limb to truncal fat, and triceps skinfold thickness for adult females with FPLD2. The lower limb fat below 1st percentile of NHANES females had a false-positive rate of 0.0054 and a false negative rate of 0. The diagnostic value of anthropometric parameters could not be determined for males with FPLD2 due to small sample size.
Conclusions
The lower limb fat (%) is the best objective anthropometric measure for diagnosing FPLD2 in females. Women with below the 1st percentile lower limb fat should undergo genetic testing for FPLD2, especially if they have metabolic complications.
Keywords: Lamin A/C, familial partial lipodystrophy, Dunnigan, skinfold thickness, dual energy X-ray absorptiometry
Familial partial lipodystrophies (FPLD) are a group of monogenic (both autosomal dominant and autosomal recessive) disorders characterized by variable loss of subcutaneous (SC) fat from the extremities and trunk, and predisposition to metabolic complications such as insulin resistance, diabetes, dyslipidemia, hepatic steatosis, and premature atherosclerosis (1, 2). Among the autosomal dominant FPLD, Dunnigan variety (FPLD2) is the most common subtype and is caused by heterozygous missense disease-causing variants in lamin A/C (LMNA) gene located on chromosome 1q21 (3–6). Most FPLD2 patients (50%-75%) with “typical” severe loss of SC fat from the extremities harbor heterozygous missense LMNA variants affecting the arginine 482 residue, whereas those with other missense LMNA variants have variable (moderate to severe) loss of SC fat from the extremities and are considered to have “atypical” FPLD (7).
Other known genes for autosomal dominant FPLD include PPARG, AKT2, PLIN1, and ADRA2A; and for autosomal recessive FPLD include CIDEC, LIPE, and PCYT1A (1, 2). In addition, there are many patients with the clinical presentation of FPLD who do not have disease-causing variants in the known genes, and some of them are considered to have a polygenic inheritance (8).
FPLD2 patients reportedly have normal SC fat at birth and during early childhood. Most of the FPLD2 patients start losing SC fat from the extremities during late childhood (9). Some patients with FPLD also accumulate excess fat in the face, neck, chin, dorso-cervical, intra-abdominal, and vulvar region, which may result in a Cushingoid appearance (2, 10).
Unlike the generalized forms of lipodystrophy, the presentation of partial lipodystrophies can be subtle, with varying phenotypes and metabolic profiles. This can make the diagnosis of FPLD very challenging, especially so in the affected males, in whom the metabolic complications may be milder than those seen in affected females (11). Genetic testing is expensive; therefore, the diagnosis of FPLD is usually missed or delayed. Thus, there exists a need for an objective clinical parameter that could be used as a screening tool and can aid in the diagnosis of FPLD.
A few investigators have previously suggested the utility of dual-energy x-ray absorptiometry (DXA)-derived truncal fat to lower limb fat ratio (also called fat mass ratio) for the diagnosis of FPLD2 (12–15). However, only a small number of FPLD2 patients and control subjects were studied and there was no further validation of this ratio. Therefore, the aim of the study was to determine the predictive value of skinfold thickness measurements and various DXA-based regional body fat parameters for the diagnosis of FPLD2. Because FPLD2 is the most prevalent and presents with the most severe loss of SC fat from the extremities compared to the other subtypes (2, 11), we studied the diagnostic value of various anthropometric measures for FPLD2.
Subjects and Methods
Subjects
The study protocols were approved by the Institutional Review Boards of UT Southwestern Medical Center (UTSW), Dallas, Texas; and the National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, Maryland; and all participants gave written informed consent. The UTSW cohort consisted of a total of 56 FPLD2 adult patients, 6 males and 50 females, who had had a DXA scan for measurement of total body and regional body fat. All of these patients harbored a heterozygous, disease-causing, missense LMNA variant. Patients younger than 18 years of age were excluded. Data from the sex- and age-matched controls from the National Health and Nutrition Examination Survey (NHANES) from 4 survey cycles, 1999-2000, 2001-2002, 2003-2004, and 2005-2006 for DXA data, and 6 survey cycles including 1999-2000, 2001-2002, 2003-2004, 2005-2006, 2007-2008, and 2009-2010 for skinfold thickness data, were used for comparison (https://www.cdc.gov/nchs/nhanes/index.htm).
Predictive values of various anthropometric measurements for the diagnosis of FPLD2 were assessed by comparing the data of UTSW FPLD2 patients with the NHANES controls. Because of the small number of male FPLD2 patients in the UTSW cohort, determination of the diagnostic value of various anthropometric parameters were only possible for the female FPLD2 patients.
Following that, the data from the female participants of the Dallas Heart Study (DHS), a population-based cohort, were used to find the specificity and the false-positive rates (FPR) for the selected anthropometric parameters. Participants older than age 30 (n = 3500) in DHS provided blood samples and underwent whole-body DXA scanning for the amount and distribution of body fat (16). A total of 1695 participants were females and 1652 had DXA data, which were used for comparison with the NHANES control data (https://www.utsouthwestern.edu/research/translational-medicine/doing-research/dallas-heart/).
Furthermore, to determine the sensitivity and FPR of the selected anthropometric parameters, the DXA data from another cohort of 23 female FPLD2 patients from the National Institutes of Health (NIH), who had heterozygous LMNA disease-causing variants (17), were compared with the available NHANES control data.
Methods
Questionnaire.
Demographic data and health history were collected during either physician interview or using the lipodystrophy questionnaire.
Anthropometric measurements.
Height and body weight were measured with standard procedures. Skinfold thickness was measured with a Lange caliper (Cambridge Scientific Industries, Cambridge, MD) at the subscapular, triceps, and anterior thigh on the right side of the body. The mean of 3 repeat measurements at each site was calculated. For comparison, only subscapular and triceps skinfold thickness measurements were available from the NHANES database.
Genotyping.
For both the UTSW and the NIH cohorts of FPLD2 patients, the LMNA exons, including the splice site regions, were amplified in 11 segments from 50 ng of genomic DNA using the PCR and exon-specific primers pairs. The purified PCR products were sequenced using dye-terminator chemistry and an ABI 3730xl DNA analyzer. Sequence variants were verified by manually inspecting the chromatograms of both the wild-type and variant products. Pathogenicity of each variant was assessed according to genotype-phenotype segregation, functional studies, and ClinVar predictions (17).
DNA sample preparation and whole-exome sequencing for the participants in Dallas Heart Study (DHS) was performed at the Regeneron Genetics Center, as described elsewhere (18).
DXA.
For the UTSW cohort, whole-body DXA examinations were acquired on Discovery W (S/N 80502) model machine according to the procedures recommended by the manufacturer (Hologic, Inc., Bedford, MA) (19, 20). Subjects changed into paper gown and were asked to remove all jewelry and other personal effects that could interfere with the DXA study. Analysis of all scans was performed using Hologic Discovery software version 1.6.5.0. in its default configuration. The analysis of all regional body fat was performed using standard software. The regions of interest were delineated by the following lines: head, pelvis, trunk, hip, spine, leg, and groin as reported by Bazzocchi et al (21). Regional fat mass values were grouped and analyzed for the following anatomical regions: arms, legs, limbs (arms + legs), trunk (chest, axillary, abdomen), subtotal body (whole body excluding head), and whole body. Mean values of the right and left upper limb fat and right and left lower limb fat (% of regional fat) were calculated. We also calculated the ratio of the lower limb to truncal fat. DXA scanning of the participants in the DHS was performed on a Delphi W scanner (Hologic) equipped with fan beam and Discovery software (version 12.2) (22). DXA scanning of NIH female FPLD2 patients was performed on Hologic QDR 4500 (Hologic) scanner using Apex 4.0 software. DXA scanning of NHANES controls was done on Hologic QDR-4500A fan-beam densitometer (Hologic) using software version 8.26:a3* through mid-2005. In 2005, the acquisition software was updated to Hologic Discovery v12.4.
Statistical analysis
The data for the females and males were analyzed separately. To estimate NHANES percentiles for skinfold thickness and DXA variables, the appropriate complex survey design sampling weights, as well as the stratum and cluster were taken into account to obtain the nationally representative estimates because of the unequal probabilities for selection as described on the NHANES website. The descriptive analyses are reported as survey weighted mean and 95% confidence intervals (CI) for all DXA and skinfold thickness-related variables using appropriate SAS survey procedures. The mean measurements and 95% CI of adults with FPLD2 belonging to UTSW cohort were compared with corresponding sex- and age-group weighted NHANES estimates of mean and 95% CI. We considered a significant difference under the two-sided P value of 0.05 if the mean and 95% CI from FPLD2 data did not overlap with the weighted NHANES 95% CI (23).
DHS unaffected and NIH FPLD2 females at or below the 1st percentile of NHANES were identified to estimate sensitivity, specificity, FPR and false-negative rates, and corresponding 95% binomial CIs (24). All analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC). A 2-sided P value < 0.05 was the criterion for statistical significance.
Results
The mean (95% CI) age and body mass index (BMI) of the affected FPLD2 females belonging to UTSW cohort were 41.5 y (37.6-45.3 y) and 25.9 kg/m2 (24.7-27.1 kg/m2), respectively; and of the affected males were 47.7 y (35.3-60.0 y) and 28.7 kg/m2 (22.0-35.4 kg/m2), respectively Tables 1 and 2). Seventy-eight percent of the subjects were Caucasians, 13% were African Americans, 5% were Asians, and 4% were either American Indians or Hawaiians. Twenty-four patients had heterozygous p.R482W and 16 had p.R482Q LMNA variants, which are associated with “typical” FPLD2. The other heterozygous “atypical” LMNA variants included p.R582S (n = 1), p.R582H (n = 2), p.S583L (n = 3), p.R349W (n = 1), p.R25L (n = 1), p.G523R (n = 2), p.D192V (n = 1), p.L241P (n = 1), p.R62G (n = 2), and p.K515E (n = 2). Sixty-eight percent of females and all 6 affected males had “typical” FPLD2-causing LMNA variants. Hypertriglyceridemia and diabetes mellitus were present in 74% and 52% of the UTSW FPLD2 patients, respectively.
Table 1.
Comparison of Measurements and Body Fat Distribution in Females with Familial Partial Lipodystrophy, Dunnigan Type (FPLD2) (UTSW cohort) with NHANES Controls
| UTSW FPLD2 Females | NHANES Females | ||||||
|---|---|---|---|---|---|---|---|
| N | Mean | 95% CI | N | Mean | 95% CI | P Value | |
| Age | 50 | 41.5 | 37.6–45.3 | 18 370 | 46.1 | 45.4–46.7 | <0.05 |
| Height (cm) | 50 | 164.3 | 162.6–165.9 | 17 072 | 162.2 | 161.9–162.4 | <0.05 |
| Weight (kg) | 50 | 70.1 | 66.5–73.4 | 17 041 | 74.3 | 73.6–75.0 | <0.05 |
| BMI (kg/m2) | 50 | 25.9 | 24.7–27.1 | 16 963 | 28.2 | 28.0–28.5 | <0.05 |
| Thigh skinfold (mm) | 44 | 6.9 | 5.2–8.6 | NA | NA | NA | NA |
| Subscapular skinfold (mm) | 44 | 20.7 | 17.3–24.2 | 13 321 | 20.4 | 20.1–20.7 | ≥0.05 |
| Triceps skinfold (mm) | 45 | 6.4 | 5.0–7.8 | 14 903 | 23.7 | 23.4–24.0 | <0.05 |
| Upper limb fat (%)a | 49 | 21.8 | 18.7–24.8 | 9264 | 43.3 | 42.9–43.6 | <0.05 |
| Truncal fat (%)a | 50 | 27.6 | 25.5–29.6 | 9264 | 37.9 | 37.5–38.2 | <0.05 |
| Lower limb fat (%)a | 49 | 14.5 | 12.8–16.2 | 9264 | 43.4 | 43.1–43.6 | <0.05 |
| Lower limb fat/truncal fat ratioa | 49 | 0.52 | 0.49–0.55 | 9264 | 1.19 | 1.18–2.00 | <0.05 |
aFrom dual energy X-ray absorptiometry. NHANES data for the thigh skinfold were not available.
BMI, body mass index; CI, confidence interval; NA, not available; NHANES, the National Health and Nutrition Examination Survey.
Table 2.
Comparison of Measurements and Body Fat Distribution in Males with Familial Partial Lipodystrophy, Dunnigan Type (FPLD2) (UTSW cohort) with NHANES Controls
| UTSW FPLD2 Males | NHANES Males | ||||||
|---|---|---|---|---|---|---|---|
| N | Mean | 95% CI | N | Mean | 95% CI | P Value | |
| Age | 6 | 47.7 | 35.3–60.0 | 17 009 | 44.4 | 43.7–45.0 | ≥0.05 |
| Height (cm) | 6 | 173.3 | 168.1–178.4 | 15 886 | 176.2 | 176.0–176.4 | ≥0.05 |
| Weight (kg) | 6 | 86.5 | 65.1–107.8 | 15 860 | 87.0 | 86.4–87.6 | ≥0.05 |
| BMI (kg/m2) | 6 | 28.7 | 22.0–35.4 | 15 775 | 28.0 | 27.8–28.2 | ≥0.05 |
| Thigh skinfold (mm) | 5 | 8.0 | 3.7–12.3 | NA | NA | NA | NA |
| Subscapular skinfold (mm) | 5 | 21.8 | 17.6–26.2 | 13 082 | 18.7 | 18.4–18.9 | ≥0.05 |
| Triceps skinfold (mm) | 5 | 8.6 | 5.4–11.7 | 14 954 | 14.6 | 14.4–14.8 | <0.05 |
| Upper limb fat (%)a | 6 | 19.5 | 15.8–23.1 | 9486 | 25.6 | 25.4–25.8 | <0.05 |
| Truncal fat (%)a | 6 | 27.5 | 22.1–32.9 | 9486 | 28.8 | 28.5–29.0 | ≥0.05 |
| Lower limb fat (%)a | 6 | 16.2 | 11.3–21.0 | 9486 | 27.5 | 27.3–27.7 | <0.05 |
| Lower limb fat/truncal fat ratio a | 6 | 0.59 | 0.46–0.71 | 9486 | 0.99 | 0.98–1.00 | <0.05 |
aFrom dual energy X-ray absorptiometry. NHANES data for the thigh skinfold were not available.
BMI, body mass index; CI, confidence interval; NA, not available; NHANES, the National Health and Nutrition Examination Survey.
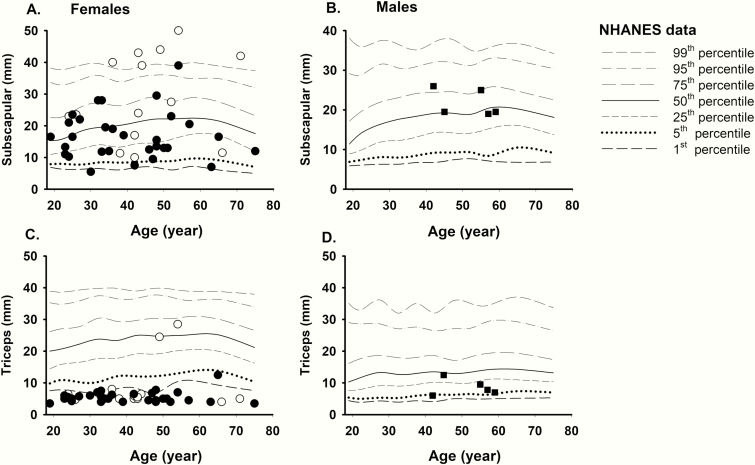
Comparison of skinfold thickness of female and male FPLD2 subjects from UTSW cohort with gender- and age-matched controls from NHANES 1999-2010 is shown in Fig. 1. The data from the NHANES controls have been drawn as a percentile chart for comparison. Most FPLD2 affected females had subscapular skinfold thickness values in the 5th to 95th percentile, except in 1 subject where the value was less than the 1st percentile. The triceps skinfold measurements were below the 1st percentile of NHANES data in all FPLD2 females except in 7. The triceps skinfold thickness of the affected females (mean 6.4 mm) was significantly lower compared with the NHANES data (mean 23.7 mm, P < 0.05). On the other hand, subscapular skinfold thickness in the affected females was not significantly different (mean 20.7 mm) compared with NHANES (mean 20.4 mm; P ≥ 0.05) (Table 1). All 5 males with FPLD2 had triceps skinfold values in 5th to 50th percentile. Male FPLD2 subject had subscapular skin folds in the 25th to 75th percentile with no specific trend. The triceps skinfold thickness of the affected males was decreased (mean 8.6 mm) compared with the NHANES data (mean 14.6 mm), and subscapular skinfold thickness increased (mean 21.8 mm) compared with the NHANES data (mean 18.7 mm), but the differences were not statistically significant (both P ≥ 0.05) (Table 2).
Figure 1.
Comparison of skinfold thickness values of FPLD2 subjects from UT Southwestern Medical Center cohort with the NHANES controls. Solid symbols signify subjects with “typical” LMNA variants vs unfilled symbols indicating “atypical” LMNA variants in females (circles) and males (squares). The data from the NHANES controls have been drawn as a percentile chart for comparison. (A) Female FPLD2 subjects have subscapular skinfold thickness values in the 5th to 95th percentile, except for 1 subject who had less than 1st percentile. (B) Male FPLD2 subject have subscapular skin folds in the 25th to 75th percentile with no specific trend. (C) The triceps skinfold measurements are <1st percentile in the majority of the female FPLD2 subjects except for 5 patients. (D) All 5 males with FPLD2 had triceps skin fold values in 5th to 50th percentile with no specific trend. FPLD2, familial partial lipodystrophy, Dunnigan variety; NHANES, National Health and Nutrition Examination Survey.
Table 1 shows that the upper and lower limb percent fat values (mean 21.8 % and 14.5 %, respectively) in UTSW FPLD2 females were significantly decreased when compared with the 1999-2006 NHANES controls (mean 43.3% and 43.4%, respectively) (P < 0.05) (Table 1). The ratio of lower limb fat/truncal fat value (mean 0.52) in the affected females was also significantly lower than the NHANES data (mean 1.19, P < 0.05). The truncal fat was also lower in UTSW FPLD2 females when compared with NHANES controls (mean 27.6%, and 37.9% respectively) (P < 0.05). The males with FPLD2 also had lower values of the upper and lower limb percent fat and the ratio of lower limb fat to truncal fat in comparison to the NHANES controls (P < 0.05)(Table 2). There was no difference in the truncal fat between the 2 groups.
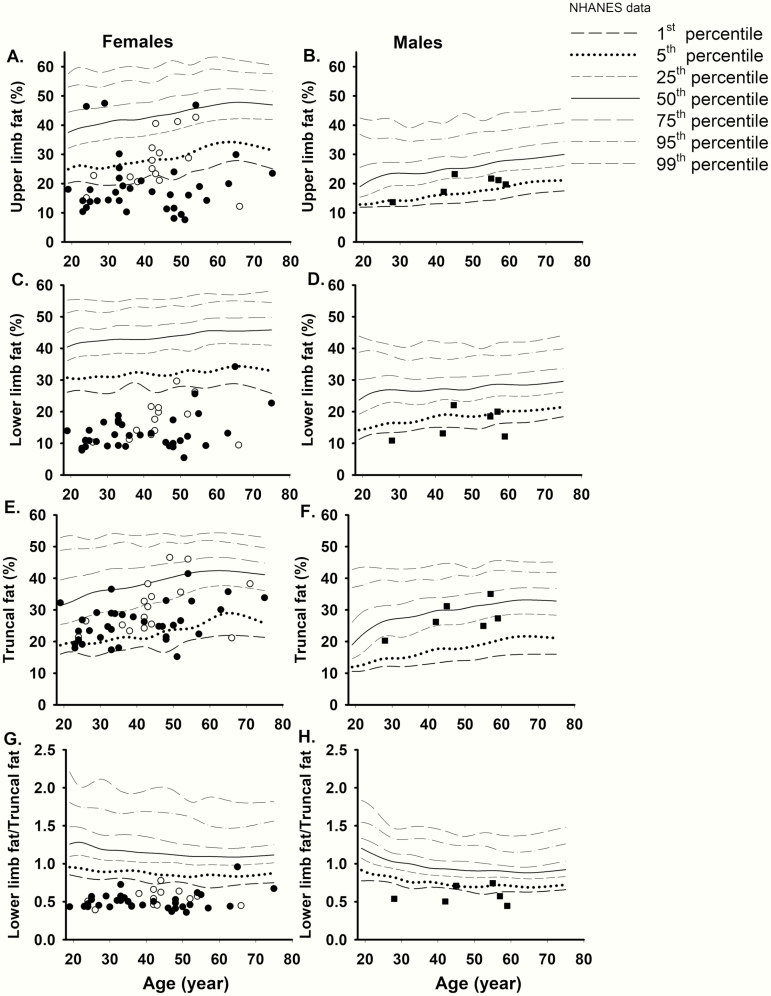
Individual values derived from regional body fat measurements by DXA in the affected females and males from the UTSW cohort are shown in Fig. 2 in comparison to the NHANES data. The upper limb fat (%) was <5th percentile in most of the affected females indicating upper limb fat loss, however, 5 patients with atypical LMNA variants and 4 with typical LMNA variants had higher values. Truncal fat ranged between 1st through 75th percentile in most affected females. All affected females, except 2, had lower limb fat (%) ≤1st percentile of NHANES. Of the 2 exceptions, 1 patient had a heterozygous LMNA p.G523R (atypical) variant; the other had a heterozygous p.R482Q (typical) variant but had excess fat accumulation in the medial part of the thighs and knees. The ratio of lower limb fat/truncal fat was ≤1st percentile (≤0.85) of NHANES in all affected females, except in the same 2 patients who did not have lower limb fat (%) ≤1st percentile of NHANES. In the affected males from the UTSW cohort, the upper limb fat (%) ranged between the 1st and 25th percentile whereas the truncal fat (%) ranged between 1st and 75th percentile. The lower limb fat and the ratio of lower limb fat/truncal fat in the affected males were both below the 25th percentile of NHANES (Fig. 2).
Figure 2.
Comparison of regional body fat (by dual-energy x-ray absorptiometry scan) of FPLD2 subjects from UTSW cohort with the NHANES controls. Solid symbols signify subjects with “typical” LMNA variants vs unfilled symbols indicate those with “atypical” LMNA variants in females (circles) and males (squares). The data from the NHANES controls have been drawn as age-specific percentile values for comparison. (A) The upper limb fat was <5th percentile in most of the affected females except in five patients with atypical LMNA variants and in four with typical LMNA variants had higher values. (B) The upper limb fat ranged between the 1st and 25th percentile in the affected males. (C) All affected females, except 2, had lower limb fat ≤1st percentile of NHANES. Of the 2 exceptions, 1 patient had a p.G523R (atypical) variant; the other had a p.R482Q (typical) variants but had excess fat accumulation in the medial part of the thighs and knees. (D) The lower limb fat in the affected males was below the 25th percentile of NHANES. (E) Truncal fat ranged between the 1st and 75th percentile in most of the affected females. (F) The truncal fat ranged between the 1st and 75th percentile in the affected males. (G) The ratio of lower limb fat/truncal fat was ≤1st percentile (≤0.85) of NHANES in all affected females, except in 1 patient with a p.G523R (atypical) variant; and the other with a p.R482Q (typical) variant. (H) The ratio of lower limb fat/truncal fat was <25th percentile of NHANES in all affected males. NHANES, National Health and Nutrition Examination Survey; UTSW, UT Southwestern Medical Center.
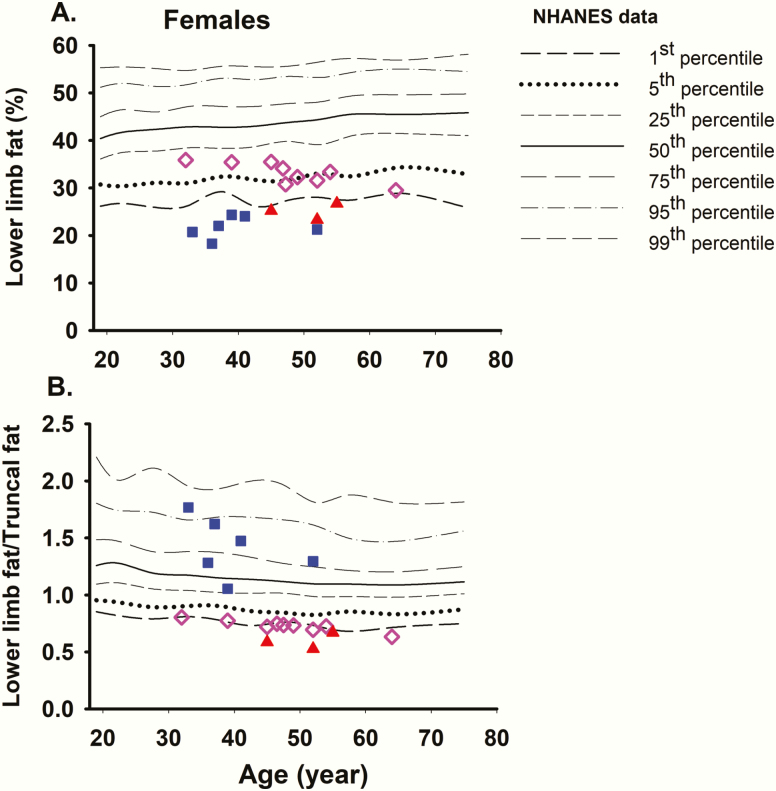
These data revealed that the lower limb fat and the lower limb fat/truncal fat ratio below the 1st percentile of NHANES controls were the best discriminants for females with FPLD2. We also combined the 2 parameters to see if that improved the discrimination. We then used the DXA data from the 1652 female participants of the DHS and the 23 FPLD2 females from the NIH for specificity and sensitivity analyses, respectively, for the 2 parameters (Figs 3 and 4).The lower limb fat %, was ≤1st percentile of NHANES in 9 DHS females with the specificity of 0.995 (95% CI, 0.989-0.998) and the FPR of 0.005 (95% CI, 0.002-0.01) (Table 3). The ratio of lower limb fat/truncal fat was ≤1st percentile of NHANES in 12 DHS females with the specificity of 0.992 (95% CI, 0.987-0.996) and the FPR of 0.007 (95% CI, 0.004-0.013) (Table 3). None of the 18 females with either lower limb fat or lower limb fat to truncal fat ratio below the 1st percentile of NHANES had FPLD2 causing LMNA variant. Only 3 females had both the lower limb fat and the ratio of lower limb fat/truncal fat below ≤1st percentile of controls with the specificity of 0.998 (95% CI, 0.995-1.00) and the FPR of 0.002 (95% CI, 0.0004-0.005) (Table 3).
Figure 3.
Comparison of regional body fat of DHS females (N = 1652) with NHANES controls. (A) The lower limb fat %, was ≤1st percentile in 9 females. Blue squares indicate females with lower limb fat ≤1st percentile but lower limb fat/truncal fat ratio >1st percentile and red triangles indicate females with both lower limb fat and lower limb fat/truncal fat ratio ≤1st percentile. The pink diamonds indicate those DHS females who had the ratio of lower limb fat/truncal fat ≤1st percentile of NHANES but had lower limb fat >1st percentile. The others are not plotted. (B) The ratio of lower limb fat/truncal fat was ≤1st percentile of controls in 12 females (9 pink diamonds and 3 red triangles). None of the 18 females with lower limb fat or lower limb fat/truncal fat ratio below the 1st percentile had any disease-causing LMNA variants. The others are not plotted. DHS, Dallas Heart Study; NHANES, National Health and Nutrition Examination Survey.
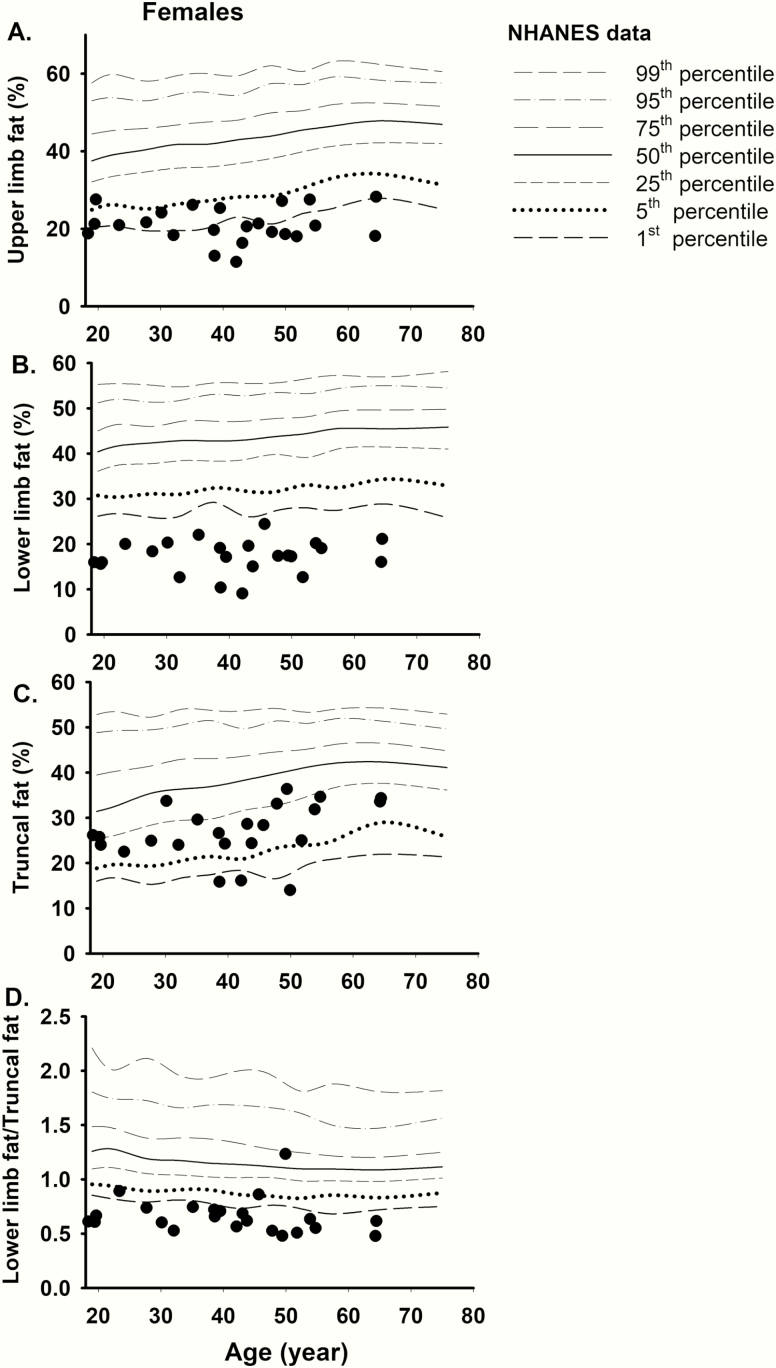
Figure 4.
Comparison of regional body fat (by dual-energy x-ray absorptiometry scan) of FPLD2 females from the National Institutes of Health (N = 23) with the NHANES controls. (A) The upper limb fat (%) was ≤5th percentile in most of the affected females. (B) All affected females had lower limb fat (%) ≤1st percentile of NHANES. (C) Truncal fat ranged between 5th and 50th percentile in most affected females except in 3 where it was ≤1st percentile of NHANES. (D) The ratio of lower limb fat/truncal fat was ≤1st percentile of NHANES in most affected females, except in 3 patients, 1 of whom had “atypical FPLD2.” FPLD2, familial partial lipodystrophy, Dunnigan variety; NHANES, National Health and Nutrition Examination Survey.
Table 3.
Specificity and Sensitivity Analysis Using 1652 Unaffected Females from Dallas Heart Study (DHS) and 23 Genetically Proven FPLD2 Females from the National Institutes of Health (NIH)
| DHS Females (n = 1652) | FPLD2 Female NIH Cohort (n = 23) | |||||
|---|---|---|---|---|---|---|
| Region | False Positive (N) | Specificity (95% CI) | False Positive Rate (95% CI) | False Negative (N) | Sensitivity, (95% CI) | False Negative Rate (95% CI) |
| ≤1st percentile lower limb fat | 9 | 0.995 (0.989-0.998) | 0.005 (0.002-0.01) | 0 | 1.0 (0.85-1.00) | 0 (0.0-0.15) |
| ≤1st percentile lower limb fat/truncal fat ratio | 12 | 0.992 (0.987-0.996) | 0.007 (0.004-0.013) | 3 | 0.87 (0.66-0.97) | 0.13 (0.03-0.34) |
| ≤1st percentile lower limb fat and lower limb fat/truncal fat ratio | 3 | 0.998 (0.995-1.00) | 0.002(0.0004-0.005) | 3 | 0.87 (0.66-0.97) | 0.13 (0.03-0.34) |
CI, confidence interval; DHS, Dallas Heart Study; FPLD2, familial partial lipodystrophy, Dunnigan variety; NIH, National Institutes of Health.
The median (range) age and BMI of the affected females belonging to the NIH cohort were 40.8 years (18.4-64.5 y) and 25.1 kg/m2 (19.2-31.7 kg/m2), respectively. Eighty-seven percent of the subjects were Caucasians, and the rest were either Asians or Hispanics. All of these subjects had disease causing, missense LMNA variants. Twenty-one patients had either heterozygous p.R482Q (n = 15) or p.R482W (n = 6) LMNA variants, whereas 1 each had heterozygous p.R541P and c.1488 + 5G>C variant at the intron 8 consensus splice donor site (17, 25). Hypertriglyceridemia and diabetes mellitus were present in 96% and 79% of the NIH FPLD2 subjects, respectively.
Triceps skinfold thickness values were available for only 10 FPLD2 females from the NIH cohort and all values (range 3.0-6.3 mm) were below the 1st percentile of NHANES (<7.0 mm) with a sensitivity of 1.0 and false-negative rate of 0.0. Skinfold thickness values were not measured in the DHS participants. Individual values of regional body fat measurements by DXA in the NIH FPLD2 females as compared to the NHANES data are shown in Fig. 4. The upper limb fat (%) was ≤5th percentile in most of the affected females. All affected females had lower limb fat (%) ≤1st percentile of NHANES revealing a sensitivity of 1.0 (95% CI, 0.85-1.00) and false-negative rate of 0 (95% CI, 0.0-0.15) (Table 3). Truncal fat ranged between 5th and 50th percentile in most affected females except in 3 where it was less than ≤1st percentile of NHANES. The ratio of lower limb fat/truncal fat was ≤1st percentile of NHANES in most affected females, except in 3 patients, thus with the sensitivity of 0.87 (95% CI, 0.66-0.97) and the false negative rate of 0.13 (95% CI, 0.03-0.34) (Table 3). Thus, 3 FPLD2 patients from the NIH cohort also had both the lower limb fat and the ratio of lower limb fat/truncal fat below ≤1st percentile of controls.
Discussion
Overall, our data revealed that DXA derived lower limb fat (%) values below the 1st percentile of the NHANES data had the best specificity and sensitivity for the diagnosis of FPLD2 in females, compared with the upper limb fat, the ratio of lower limb fat/truncal fat and the combination of the lower limb fat, and the ratio of lower limb fat/truncal fat. The upper limb fat measurements by DXA scans may be spuriously increased by inclusion of subcutaneous fat in the axillary and shoulder region in some subjects. Because axillary fat is preserved in some FPLD2 patients (26), this may have resulted in reduced diagnostic value of upper limb fat compared with lower limb fat.
We had previously suggested that the anterior thigh skinfold thickness measurement below the 10th percentile for normal adult females (19.5 mm) and males (8 mm) can increase diagnostic suspicion for FPLD2 (1). These percentile values were derived from 279 adult females and 400 adult males from the United States (27, 28). However, because of the lack of availability of thigh skinfold thickness in the NHANES and other publicly available databases and in the NIH FPLD2 cohort, we were not able to precisely evaluate its specificity or sensitivity for the diagnosis of FPLD2. We still recommend measurement of thigh skinfold thickness as an objective parameter to increase diagnostic accuracy for FPLD2 since it is cheap, does not involve any radiation unlike DXA scan, and is likely to be particularly accurate in FPLD2 patients with values in the low range.
We were, however, able to evaluate the value of triceps skinfold thickness for diagnosis of FPLD2 and interestingly it was below the 1st percentile of NHANES data (~7 mm) in all FPLD2 females except 5. In comparison, the lower limb fat (%) from DXA was below the 1st percentile in all FPLD2 females except 2. However, triceps skinfold values were not available in the DHS and therefore we were unable to calculate specificity of this anthropometric parameter for the diagnosis of FPLD2. Only a small number of FPLD2 females from the NIH (n = 10) had triceps skinfold thickness values and the sensitivity was 1.0.
Valerio et al (13). reported a significantly higher fat mass ratio (ratio of the percentage of truncal fat mass to the percentage of the lower limb fat mass) using DXA scanning in 13 women (age 14-51 years) with FPLD2 compared with 16 age- and BMI-matched healthy women (1.86 ± 0.43 vs. 0.93 ± 0.10, respectively, P < 0.001). Using 1.2 as a fat mass ratio cutoff value, the sensitivity and specificity for the diagnosis of FPLD2 were 0.889 and 0.938, respectively. However, they did not compare the sensitivity and specificity of fat mass ratio with the lower limb or upper limb fat. Recently, Ajluni et al (15) also suggested the ratio of truncal fat (%) to leg fat (%) as a discriminant for FPLD2 patients. However, it was based on only 7 females with heterozygous, disease-causing LMNA variants. Both these studies did not have any validation cohorts of FPLD2 subjects or of normal healthy females. We used a large population-based cohort of adult females from the DHS and an additional cohort of FPLD2 patients from the NIH to evaluate the specificity and sensitivity of various anthropometric measures and concluded that the lower limb fat (%) was a better diagnostic measure than the ratio of lower limb fat to truncal fat (inverse of fat mass ratio). The DHS cohort was chosen because all of them have had whole exome sequencing, and we were able to ascertain any disease-causing LMNA variants in those below the 1st percentile of lower limb fat or lower limb fat to truncal fat ratio. None of them were found to have a disease-causing LMNA variant.
Recently, Meral et al (29) used a DXA-based fat shadow method and reported that FPLD was differentiated from control subjects with 0.85 sensitivity (95% CI, 0.72-0.93) and 0.96 specificity (95% CI, 0.91-0.99) FPLD2 patients were reported to have a consistent body fat distribution pattern, with absence of SC fat throughout the body but with excess SC fat in the neck and pubic region. However, these conclusions were based on a small number of 22 females with FPLD2 and 90 control female subjects. The control subjects did not undergo genotyping for LMNA. Interestingly, only 1 of the 18 FPLD2 females from the University of Michigan cohort had lower limb fat % more than the 1st percentile of NHANES (false-negative rate 0.056) and only 1 of the 90 healthy controls had % lower limb fat less than 1st percentile (FPR 0.01). Thus, the data of Meral et al (29). provide further confirmation of our conclusion that lower limb fat (%) is the best diagnostic evaluation method for FPLD2. The 1st percentile values for lower limb fat (%) for various age groups among the adult female NHANES participants range from 25.8% to 29.3% (Table 4). Therefore, we propose that adult females with <1st percentile lower limb fat should be further evaluated for the diagnosis of FPLD2, especially if presenting with metabolic complications, such as diabetes, hypertriglyceridemia, hepatic steatosis, and/or polycystic ovarian syndrome. Such patients should be advised to consider genetic testing for confirmation of the diagnosis of FPLD.
Table 4.
The 1st Percentile Values for Lower Limb Fat (%) for Various Age Groups Among the Adult Female NHANES Participants
| Age Range | Mean Age | First Percentile Values for Lower Limb Fat (%) for NHANES Adult Females |
|---|---|---|
| 18–19.9 | 19 | 26.2 |
| 20–24.9 | 22.5 | 26.7 |
| 25–29.9 | 27.5 | 25.9 |
| 30–34.9 | 32.5 | 26.4 |
| 35–39.9 | 37.5 | 29.3 |
| 40–44.9 | 42.5 | 26.1 |
| 45–49.9 | 47.5 | 27.4 |
| 50–54.9 | 52.5 | 28.0 |
| 55–59.9 | 57.5 | 27.5 |
| 60–69.9 | 65 | 28.9 |
| 70-above | 75 | 25.8 |
NHANES, the National Health and Nutrition Examination Survey.
Our study, however, has a few limitations. First, we did not have adequate sample of adult male FPLD2 participants to evaluate the utility of lower limb fat for diagnosing males with FPLD2. Clearly, the lower limb fat value was below the 1st percentile of NHANES in only 3 of the 6 FPLD2 males and below the fifth percentile in 5 of 6 FPLD2 males. Thus, it is likely that for diagnosing FPLD2 in males, a cutoff of fifth percentile of NHANES may be more appropriate; however, it may lower the specificity. A multicenter effort to collect data on larger number of males with FPLD2 will be required in future to evaluate the utility of various anthropometric measures. Second, whether our data will be applicable to postpubertal children with FPLD2 remains unknown. Third, the percentile values derived from the NHANES data from United States may not be applicable to other countries with different rates of adiposity. Fourth, different models of Hologic to measure DXA-derived regional body fat at different sites may have introduced some bias. There are no data available regarding cross-calibration of different models of Hologic and their software for measurement of regional body fat. Last, whether the suggested criteria will be useful for other subtypes of FPLD remains to be determined.
In conclusion, our data reveal that the lower limb fat (%) derived from the whole-body DXA scan is the best objective anthropometric measure for diagnosing adult females with FPLD2.
Acknowledgments
The authors thank the families for participation in the study; Elaine Cochrane from NIDDK, NIH, Bethesda, USA and from UT Southwestern, Claudia Quittner for help with evaluation of patients; Carmel Tovar for LMNA sequencing; Julia Kozlitina for Dallas Heart Study data; Yulun Liu, Ph.D for her suggestions about statistical analysis; and UTSW McDermott Center Sequencing Core for sequencing at the UT Southwestern Medical Center, Dallas, TX.
Financial Support: This work was supported by grants from the National Institutes of Health, R01-DK105448, and UL1TR001105 from the National Center for Advancing Translational Sciences, National Institutes of Health, and by the intramural research programs of the National Institute of Diabetes and Digestive and Kidney Disease and the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The funding sources were not involved in study design, analysis and interpretation of data, writing of the paper, and in the decision to submit the article for publication. REDCap was used for data storage (30).
Author Contributions: C.V. reviewed the medical records, collected and organized the data, performed data interpretation, drafted the initial manuscript, and approved the final manuscript as submitted. X.L. reviewed the NHANES data, carried out the statistical analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted. H.S. collected and organized the NIH data, reviewed and revised the manuscript, and approved the final manuscript as submitted. B.A.-H. reviewed the NHANES data, carried out the statistical analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted. R.J.B. performed data interpretation, reviewed and revised the manuscript, and approved the final manuscript as submitted. A.G. conceptualized and designed the study, performed data interpretation, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Glossary
Abbreviations
- CI
confidence interval
- DHS
Dallas Heart Study
- DXA
dual-energy x-ray absorptiometry
- FPLD
familial partial lipodystrophy
- FPR
false-positive rate
- NHANES
National Health and Nutrition Examination Survey
- NIH
National Institutes of Health
- SC
subcutaneous
- UTSW
UT Southwestern Medical Center
Additional Information
Disclosure Summary: A.G. consults for Amryt Pharma plc and Regeneron and has received grant support from Amryt Pharma plc, Regeneron, Quintiles, Akcea Pharmaceuticals, and Intercept Pharmaceuticals. R.J.B. has received scientific writing support from Aegerion Pharmaceuticals. The remaining authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References and Notes
- 1. Brown RJ, Araujo-Vilar D, Cheung PT, et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101(12):4500–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hussain I, Patni N, Garg A. Lipodystrophies, dyslipidaemias and atherosclerotic cardiovascular disease. Pathology. 2019;51(2):202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peters JM, Barnes R, Bennett L, Gitomer WM, Bowcock AM, Garg A. Localization of the gene for familial partial lipodystrophy (Dunnigan variety) to chromosome 1q21-22. Nat Genet. 1998;18(3):292–295. [DOI] [PubMed] [Google Scholar]
- 4. Cao H, Hegele RA. Nuclear lamin A/C R482Q mutation in Canadian kindreds with Dunnigan-type familial partial lipodystrophy. Hum Mol Genet. 2000;9(1):109–112. [DOI] [PubMed] [Google Scholar]
- 5. Speckman RA, Garg A, Du F, et al. Mutational and haplotype analyses of families with familial partial lipodystrophy (Dunnigan variety) reveal recurrent missense mutations in the globular C-terminal domain of lamin A/C. Am J Hum Genet. 2000;66(4):1192–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shackleton S, Lloyd DJ, Jackson SN, et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet. 2000;24(2):153–156. [DOI] [PubMed] [Google Scholar]
- 7. Guénantin AC, Briand N, Bidault G, et al. Nuclear envelope-related lipodystrophies. Semin Cell Dev Biol. 2014;29:148–157. [DOI] [PubMed] [Google Scholar]
- 8. Lotta LA, Gulati P, Day FR, et al. ; EPIC-InterAct Consortium; Cambridge FPLD1 Consortium Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet. 2017;49(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patni N, Li X, Adams-Huet B, Vasandani C, Gomez-Diaz RA, Garg A. Regional body fat changes and metabolic complications in children with Dunnigan lipodystrophy-causing LMNA variants. J Clin Endocrinol Metab. 2019;104(4):1099–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Akinci B, Oral EA, Neidert A, et al. Comorbidities and survival in patients with lipodystrophy: an international chart review study. J Clin Endocrinol Metab. 2019;104(11):5120–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garg A. Gender differences in the prevalence of metabolic complications in familial partial lipodystrophy (Dunnigan variety). J Clin Endocrinol Metab. 2000;85(5):1776–1782. [DOI] [PubMed] [Google Scholar]
- 12. Valerio CM, Godoy-Matos A, Moreira RO, et al. Dual-energy X-ray absorptiometry study of body composition in patients with lipodystrophy. Diabetes Care. 2007;30(7):1857–1859. [DOI] [PubMed] [Google Scholar]
- 13. Valerio CM, Zajdenverg L, de Oliveira JE, Mory PB, Moyses RS, Godoy-Matos AF. Body composition study by dual-energy x-ray absorptiometry in familial partial lipodystrophy: finding new tools for an objective evaluation. Diabetol Metab Syndr. 2012;4(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Godoy-Matos AF, Moreira RO, Valerio CM, Mory PB, Moises RS. A new method for body fat evaluation, body adiposity index, is useful in women with familial partial lipodystrophy. Obesity (Silver Spring). 2012;20(2):440–443. [DOI] [PubMed] [Google Scholar]
- 15. Ajluni N, Meral R, Neidert AH, et al. Spectrum of disease associated with partial lipodystrophy: lessons from a trial cohort. Clin Endocrinol (Oxf). 2017;86(5):698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Victor RG, Haley RW, Willett DL, et al. ; Dallas Heart Study Investigators The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93(12):1473–1480. [DOI] [PubMed] [Google Scholar]
- 17. Sekizkardes H, Cochran E, Malandrino N, Garg A, Brown RJ. Efficacy of metreleptin treatment in familial partial lipodystrophy due to PPARG vs LMNA pathogenic variants. J Clin Endocrinol Metab. 2019;104(8):3068–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abul-Husn NS, Cheng X, Li AH, et al. A protein-truncating HSD17B13 variant and protection from chronic liver disease. N Engl J Med. 2018;378(12):1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. Plos One. 2009;4(9):e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kelly TL. Advanced Body Composition Reporting and Interpretation: A Technical Discussion.2015:1–14. https://www.amslatam.com/uploads/7/7/8/0/7780386/advanced_body_composition.pdf
- 21. Bazzocchi A, Ponti F, Albisinni U, Battista G, Guglielmi G. DXA: technical aspects and application. Eur J Radiol. 2016;85(8):1481–1492. [DOI] [PubMed] [Google Scholar]
- 22. Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM. Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab. 2006;91(11):4459–4466. [DOI] [PubMed] [Google Scholar]
- 23. Cumming G. Inference by eye: reading the overlap of independent confidence intervals. Stat Med. 2009;28(2):205–220. [DOI] [PubMed] [Google Scholar]
- 24. McKenzie DP, Vida S, Mackinnon AJ, Onghena P, Clarke DM. Accurate confidence intervals for measures of test performance. Psychiatry Res. 1997;69(2-3):207–209. [DOI] [PubMed] [Google Scholar]
- 25. Morel CF, Thomas MA, Cao H, et al. A LMNA splicing mutation in two sisters with severe Dunnigan-type familial partial lipodystrophy type 2. J Clin Endocrinol Metab. 2006;91(7):2689–2695. [DOI] [PubMed] [Google Scholar]
- 26. Garg A, Vinaitheerthan M, Weatherall PT, Bowcock AM. Phenotypic heterogeneity in patients with familial partial lipodystrophy (Dunnigan variety) related to the site of missense mutations in lamin A/C gene. J Clin Endocrinol Metab. 2001;86(1):59–65. [DOI] [PubMed] [Google Scholar]
- 27. Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr. 1978;40(3):497–504. [DOI] [PubMed] [Google Scholar]
- 28. Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Med Sci Sports Exerc. 1980;12(3):175–181. [PubMed] [Google Scholar]
- 29. Meral R, Ryan BJ, Malandrino N, et al. “Fat shadows” from DXA for the qualitative assessment of lipodystrophy: when a picture is worth a thousand numbers. Diabetes Care. 2018;41(10):2255–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]