Abstract
Background/Aims
The ATP-binding cassette (ABC) transporters mediate drug biodisposition and immunological responses in the placental barrier. In vitro infective challenges alter expression of specific placental ABC transporters. We hypothesized that chorioamnionitis induces a distinct pattern of ABC transporter expression.
Methods
Gene expression of 50 ABC transporters was assessed using TaqMan® Human ABC Transporter Array, in preterm human placentas without (PTD; n=6) or with histological chorioamnionitis (PTDC; n=6). Validation was performed using qPCR, immunohistochemistry and Western blot. MicroRNAs known to regulate P-glycoprotein (P-gp) were examined by qPCR.
Results
Up-regulation of ABCB9, ABCC2 and ABCF2 mRNA was detected in chorioamnionitis (p<0.05), whereas placental ABCB1 (P-gp; p=0.051) and ABCG2 (breast cancer resistance protein-BCRP) mRNA levels (p=0.055) approached near significant up-regulation. In most cases, the magnitude of the effect significantly correlated to the severity of inflammation. Upon validation, increased placental ABCB1 and ABCG2 mRNA levels (p<0.05) were observed. At the level of immunohistochemistry, while BCRP was increased (p<0.05), P-gp staining intensity was significantly decreased (p<0.05) in PTDC. miR-331-5p, involved in P-gp suppression, was upregulated in PTDC (p<0.01) and correlated to the grade of chorioamnionitis (p<0.01).
Conclusions
Alterations in the expression of ABC transporters will likely lead to modified transport of clinically relevant compounds at the inflamed placenta. A better understanding of the potential role of these transporters in the events surrounding PTD may also enable new strategies to be developed for prevention and treatment of PTD.
Keywords: Placenta, Chorioamnionitis, Preterm delivery, ABC transporters, P-glycoprotein (P-gp), Breast cancer resistance protein (BCRP)
Introduction
Preterm delivery (PTD) is a public health problem, occurring in 5-18% of all pregnancies [1] and it is the leading cause of perinatal mortality and morbidity [2]. Intrauterine infection or chorionamnionitis are responsible for up to 40% of all PTD cases worldwide [3–7]. Important components of the pathophysiology of PTD, such as infection and/or inflammation, have been shown to influence the expression of selected ATP-binding cassette (ABC) transporters [8]. The ABC transporters comprise 50 proteins, subdivided into seven sub-families (ABCA through ABCG), that actively transport a wide array of substrates. Placental ABC transporters exert a critical role regulating steroid transport, immunological responses and drug biodisposition. Importantly, the ABC transporters namely P-glycoprotein (P-gp; encoded by ABCB1); breast cancer resistance protein (BCRP; ABCG2) and specific multidrug resistance-associated proteins (MRPs; encoded by the ABCC subset) are important components of the placental syncytiotrophoblast barrier [8, 9]. They act as “gatekeepers” protecting the fetus against accumulation of potential harmful factors that may be present in the maternal circulation [8].
Acute bacterial infection, modeled by lipopolysaccharide (LPS) exposure, impaired placental P-gp activity resulting in increased fetal drug accumulation in the mouse [10]. LPS insult of first trimester human placental explants decreased the expression of ABCB1 and ABCG2 mRNA as well as P-gp and BCRP protein, but had no effect when administered to third trimester placental explants [11], demonstrating that LPS is more likely to disrupt P-gp and BCRP expression during earlier stages of pregnancy. However, little is known as to how infection and/or inflammation impact the expression of these and other placental ABC transporters in cases of chorioamnionitis. Evidence also suggests that selected ABC transporters are key mediators of immunological responses [12] involved in labor [13, 14]. Also, P-gp transports pro-inflammatory cytokines out of cells [15, 16]. Therefore, understanding how infection and inflammation regulate the expression of ABC transporters in the placenta is key to understanding maternal-fetal transfer of steroid hormones, toxins, xenobiotics and immunological factors in pregnancies complicated by chorioamnionitis.
MicroRNAs (miRNAs) are approximately 22 nucleotides in length, single-stranded, non-coding RNAs that are currently being studied as modulators of drug metabolism and disposition via the regulation of nuclear receptors, drug-metabolizing enzymes and drug transporters [17–22]. To date, no study has comprehensively examined the ABC transporter superfamily or involved in the post-transcriptional regulation of these transporters in the human preterm placenta, and how chorioamnionitis modifies their expression. Therefore, we investigated the expression of ABC transporters and related miRNAs in preterm placenta with chorioamnionitis (PTDC) compared to preterm placentas without chorioaminionitis (PTD). We hypothesized that inflammation induces a distinct signature of placental ABC transporters potentially associated with the modulation of specific miRNAs expression in preterm pregnancies and that this signature correlates to the severity of inflammation.
Materials and Methods
Sample collection
Placental specimens were obtained from the Research Centre for Women’s and Infants’ Health (RCWH) BioBank (Mount Sinai Hospital, Toronto, CA) with informed consent and in accordance to the policies of Mount Sinai Hospital and the University of Toronto Research Ethic Boards. Placental samples were selected from pregnancies ending in PTD with histologic chorioamnionitis (PTDC; in total n=6) or PTD without histologic chorioamnionitis (PTD; in total n=6). Diagnosis of histologic chorioamnionitis (graded 0-3) was performed by Mount Sinai Hospital staff pathologists based on the presence of polymorphonuclear leukocyte infiltration within the chorionic plate and/or chorioamniotic membranes as previously described [23, 24].
In order to decrease variability, only pregnancies carrying male Caucasian fetuses were selected as an inclusion criteria, since ethnicity [25–28] and gender [29–33] can directly affect inflammatory responses and birth outcome. Exclusion criteria included samples from pregnant women experiencing PTD associated with asthma, cardiovascular diseases, cervical incompetence, diabetes, fetal growth restriction, fetal malformation, hypertension, multiple gestation, preeclampsia, sexually transmitted diseases, thyroid disease and uterine malformations [34]. The clinical profiles of all pregnancies enrolled in the study are presented in Table 1.
Table 1.
Clinical profile of the placental tissues. Results are presented as mean ± SEM. None of the parameters investigated showed statistical difference between the groups. BMI, Body Mass Index; P, positive; N, negative; U, unknown;V, vaginal; C, Caesarean section with no labor; CL, Caesarean section with labor; GBS, Group B Staphylococcus; WBC, White Blood Cells; S1, Stage1; S2, Stage2; S3, Stage3; HP, Haemorrhage+PPROM (Preterm Premature Rupture of Membranes)
| Preterm Delivery without Chorioamnionitis PTD (N=6) | Preterm Delivery with Chorioamnionitis PTDC (N=6) | P Value | |
|---|---|---|---|
| Maternal Characteristics | |||
| Age (years) | 27.7 ± 5.6 | 31.8 ± 4.1 | 0.56 |
| BMI | 22.1 ± 4.8 | 26.2 ± 4.7 | 0.65 |
| Ethnicity | Caucasian | Caucasian | |
| WBC (×109/L) | 3.0 - 10.0 | 3.0 - 10.0 | |
| Fetal Characteristics | |||
| Gestational age (weeks) | 31.6 ± 1.9 | 28.0 ± 1.7 | 0.20 |
| Mode of Delivery (V:C:CL) | 2:1:3 | 4:2:0 | |
| Neonatal birth weight (g) | 1908 ± 554 | 1185 ± 362 | 0.30 |
| Neonatal sex | Male | Male | |
| Pathology Characteristics | |||
| Chorioamnionitis (S1:S2:S3:HP) | - | 2:2:1:1 | |
| GBS status (P:N:U) | 2:2:2 | 0:5:1 | |
| Glucocorticoids treatment | yes | yes | |
Total RNA extraction and cDNA synthesis
Total RNA was extracted using the RNeasy Plus Universal Mini kit (QIAGEN, Toronto, ON, Canada). The absorbance ratios 260/280 and 260/230 were used to assess the purity of RNA and they were considered satisfactory only when ranged between 1.8 and 2.0. RNA integrity was determined by calculating the RNA Integrity Number (RIN) by capillary electrophoresis, using the Agilent Bioanalyzer 2100 and RNA 6000 Nano Labchip kit (Agilent Biotechnologies, CA, USA) according to manufacturer’s instructions. Only RNAs with RIN greater to or equal to 7 were used in our study, indicating that the RNA samples had minimal degradation products. 1 ng RNA was reverse transcribed into cDNA using the SuperScript® VILO™ cDNA Synthesis kit (Invitrogen, Grand Island, NY, USA).
ABC Transporters Low Density Array
Screening of placental ABC transporters mRNA expression was assessed using TaqMan® Human ABC Transporter Array microfluidic cards (TLDA, Applied Biosystems, Foster City, CA, USA) containing assays for 50 human ABC transporter genes in addition to 14 endogenous reference gene controls (catalog number 4378700). A total of 6 TLDA cards were used to quantify mRNA expression from PTD and PTDC placentas (n=6/group). Each card was designed to run 2 distinct samples in triplicate and contained 384 wells and 8 reservoirs in total, i.e. 192 wells per sample (64 assays in triplicate). 400 ng of total RNA reverse transcribed in cDNA was used per sample (100 ng RNA/reservoir) in the TLDA cards. qPCR was performed on the Applied Biosystems ViiA™ 7 qPCR System using the following cycling conditions: 95°C for 20s, followed by 40 cycles of 95°C for 1s and 60°C for 20s. Cycle thresholds (CTs) were assessed using Thermo Fisher Cloud online software (Life Technologies), as the average of each sample assayed in triplicate. ABC transporter mRNA levels were normalized to the geometric mean of the 4 most stable reference genes [tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (YWHAZ), beta-2-microglobulin (B2M), RNA polymerase II (POLR2A) and TATA-box binding protein (TBP)] and calculated according to the 2-MCT method [35].
qPCR
Levelsofinter/eutan (IL)-8and YWHAZmRNAwereassessed usingSensiFAST™SYBR®Hi-ROX kit(Bioline, Toronto, ON, Canada) and the CFX 96 Real-Time System C1000 Thermal Cycler (Bio-Rad, Mississauga, ON, Canada), with the following cycling conditions: 95oC for 30s and 39 cycles of 95oC for 5s and 60oC for 5s. IL-8 and YWHAZ primers sequences were F:GCAGCCTTCCTGATTTCTGCAGCT and R:CCTTGGGGTCCAGACAGAGCTCT; and F:ACTTTTGGTACATTGTGGCTTCAA and R:CCGCCAGGACAAACCAGTAT, respectively. For validation of the arrays, ABCB1 and ABCG2 mRNA expression, as well as YWHAZ expression, was measured using identical probes present in the TLDA card (Hs00184491_m1; Hs00184979_m1 and Hs00237047_m1, respectively) and Taqman® Universal Master Mix II (Applied Biosystems). In this case, the parameters used in the qPCR were: 50°C for 2min, 95°C for 10min followed by 40 cycles of 95°C for 15s and 60°C for 60s. Changes in mRNA expression were calculated according to the 2-MCT method [35].
miRNA analysis
For miRNAs evaluation, cDNA used to analyze miRNAs of interest were generated using the Taqman® miRNA reverse transcription kit (Applied Biosystems) and Taqman® assays specific for each miRNA (miR-145: ID002278; miR-331-5p: ID002233; miR-451: ID001105; miR-200c: ID406985). Expression was measured by qPCR using the Taqman® Universal Master Mix II, no Uracyl-N Glycosylase (Applied Biosystems) in a CFX 380 Real-Time system C 1000 TM Thermal Cycler (Bio-Rad), with the following parameters: 95°C for 10min, followed by 40 cycles of 95°C for 15s and 60°C for 60s. miRNA expression was normalized to the geometric mean of the small nucleolar RNAs U6 (ID001973), RNU43 (ID001095) and RNU44 (ID001094). Changes in miRNA expression were calculated according to the 2-MCT method [35].
Western Blot
Placental protein was extracted by homogenization in RIPA lysis buffer (1 mol/L Tris-HCL pH 6.8, 2% SDS, 10% glycerol, containing protease and phosphatase inhibitor cocktail; Thermo Scientific Waltham, MA, USA), followed by centrifugation (10, 000g, 10min, 4°C). The supernatant protein (30|ig/well) from each sample was separated by electrophoresis (100V, 1h) using 8% SDS-polyacrylamide gel and transferred to nitrocellulose membranes using the iBlot transfer apparatus (Invitrogen). Membranes were blocked with 5% bovine serum albumin (BSA, Sigma-Aldrich, ON, Canada) in Tris-buffered saline Tween (TBS-T, 1h) for P-gp and BCRP, and with 5% skim milk for B-actin. The membranes were then incubated at 4°C overnight, with the following primary antibodies: anti-rabbit MDR1 (1:1,000 Abcam ab170904, Toronto, ON, Canada), anti-mouse BCRP (1:500 Santa Cruz Biotechnology, 221956, Dallas, TX, USA), and anti-goat B-actin (at the same concentration relative to the respective primary antibody, Santa Cruz Biotechnology) in 5% BSA or skim milk TBS-T solution. Membranes were then washed with PBS and incubated (1h) with horseradish peroxidase conjugated secondary antibody (GE Healthcare Bio-Science, Baie D’Urf, QC, Canada) at concentrations of 1:10, 000 for P-gp or 1:15,000 for BCRP. For B-Actin, anti-goat secondary antibody (Bio-Rad, Mississauga, ON, Canada) linked to horseradish peroxidase (1:10, 000) was used. Subsequently, membranes were washed in PBS and the protein-antibody complexes were detected by incubation with Laminate™ Crescendo Western HRP Substrate (Millipore, Oak Drive, California, USA) for 3min and chemiluminescence was detected under UV using the Versa Doc system (Bio-Rad). Protein band intensity was quantified using ImageJ software (National Institutes of Health, USA) and normalized against B-actin signal, the loading control.
Immunohistochemistry
Paraffin embedded tissue sections were dewaxed and rehydrated, and the endogenous peroxidase activity was blocked using H2O2 (0.3%) in methanol (30 min). Antigen retrieval was performed by preheating the sections in sodium citrate (10mM) for 6 min and then for 3 min, leaving them in ice for 20 min after each heating. Sections were incubated in protein blocking solution (Dako, Mississauga, ON, Canada) for 1h, prior to overnight incubation with primary antibodies: anti-mouse MDR-1 (1:500, D-11, Santa Cruz Biotechnology) and anti-mouse BCRP (1:200, BXP-21, Santa Cruz Biotechnology). Mouse IgG1 was used instead of the primary antibody as a non-immune control. Sections were washed in PBS and incubated with the anti-mouse secondary antibody (1h), at the same concentration as the specific primary antibody, prior to being incubated with streptavidin-HRP (1h; Dako) and visualized with peroxidase substrate kit DAB (Dako). Slides were then counterstained with hematoxylin.
Placental P-gp and BCRP scoring of stained sections was performed with adaptations as described previously [36–38]. Briefly, sections were analyzed by three independant researchers blinded to patient grouping. Staining intensity of syncytiotrophoblast, villous core was graded semiquantitatively on a scale of 0-3 arbitrary units, with 0 indicating no detectable staining, 1 = weak, 2 = moderate and 3 = strong. The area of the respective regions with positive staining was evaluated using the following scale: 0 =no detectable staining, 1 =up to 10%, 2=10-50% and 3=more than 50% of staining.
Statistical Analysis
Statistical analyses were performed using Prism software (GraphPad Software, Inc., San Diego, CA). Data were analyzed using the Kolmogorov-Smirnov normality test followed by Levene’s median test to verify equality of variances. Groups were analysed by unpaired t-test or non-parametric Mann-Whitney test. Data are expressed as mean ± SEM. Non-parametric Spearman correlation was used to correlate the relative quantities of the transporters, IL-8 and miR-331-5p with the degree of chorioamnionitis, obtained from the clinical data. Differences were considered significant when p<0.05.
Results
Clinical Data
In the current study, we made every effort to minimize the variability of our analysis, given that both ethnicity and sex may influence the development and intensity of the inflammatory process [39–42]. For this reason, we only selected placental samples from caucasian mothers carrying male fetuses. In addition, there was no significant difference in maternal age (27.7 ± 5.6 vs. 31.8 ± 4.1, p = 0.56), body mass index (BMI, 23.1 ± 4.8 vs. 26.2 ± 4.6, p = 0.65), gestational age at delivery (31.5 ± 1.9 vs. 28 ± 1.7, p = 0.20) or birth weight (1908 ± 554 vs. 1185 ± 362, p = 0.30) between the PTD and PTDC groups (Table 1).
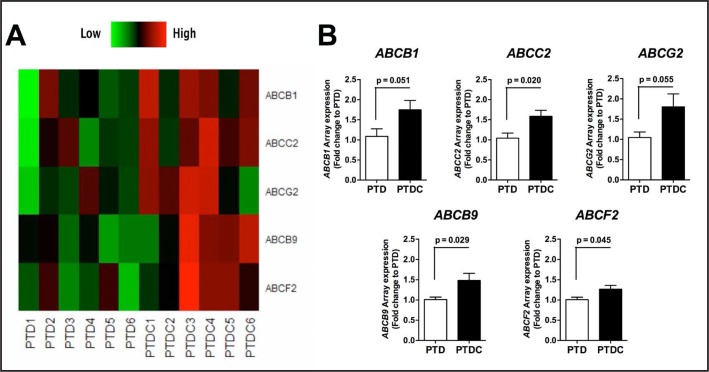
Gene expression profiling of ABC transporters in preterm placentas with chorioamnionitis Of the 50 ABC transporters assayed, 48 were detected in the preterm placenta (Table 2]. ABCG5 and ABCG8 expression were below the limit of detection. Placental ABCB9 (p = 0.029], ABCC2 (p = 0.020) and ABCF2 (p = 0.045) were significantly up-regulated in the PTDC group (Fig. 1B), whereas placental ABCB1 (P-gp; p = 0.051) and ABCG2 (BCRP; p = 0.055) approached near significant up-regulation (Fig. 1A). Further measurement of ABCB1 and ABCG2 mRNA by individual qPCR revealed a statistically significant increase of both ABCB1 (p = 0.027) and ABCG2 (p = 0.020) in the PTDC compared to PTD (Fig. 2A&B). Thus, chorioamnionitis is associated to an increased expression of 5 placental ABC transporters (Fig. 1&2).
Table 2.
Fold-change in ABC transporter expression in the presence of chorioamnionitis in the preterm human placenta. *p<0.05. Fold-change is calculated by the ratio between PTDC and PTD relative expression. A fold-change > 1.0 indicates increased expression; fold-change < 1.0 indicates decreased expression; and fold-change = 0 indicates no change in relative expression
| ABCA Gene | Fold Change | ABCB Gene | Fold Change | ABCC Gene | Fold Change | ABCD Gene | Fold Change | ABCE Gene | Fold Change |
|---|---|---|---|---|---|---|---|---|---|
| ABCA1 | 1.01 | ABCB1 | 1.61* | ABCC1 | 1.23 | ABCD1 | 0.86 | ABCE1 | 1.18 |
| ABCA2 | 0.96 | ABCB2 | 1.06 | ABCC2 | 1.52* | ABCD2 | 0.81 | ||
| ABCA3 | 1.17 | ABCB3 | 1.00 | ABCC3 | 0.95 | ABCD3 | 1.35 | ||
| ABCA4 | 2.32 | ABCB4 | 1.64 | ABCC4 | 1.13 | ABCD4 | 1.11 | ||
| ABCA5 | 0.86 | ABCB5 | 0.67 | ABCC5 | 1.21 | ||||
| ABCA6 | 0.94 | ABCB6 | 1.35 | ABCC6 | 2.10 | ||||
| ABCA7 | 0.81 | ABCB7 | 1.23 | ABCC7 | 0.91 | ABCF | Fold | ABCG | Fold |
| ABCA8 | 0.77 | ABCB8 | 1.11 | ABCC8 | 1.6 | Gene | Change | Gene | Change |
| ABCA9 | 0.83 | ABCB9 | 1.47* | ABCC9 | 1.41 | ABCF1 | 1.26 | ABCG1 | 1.00 |
| ABCA10 | 0.62 | ABCB10 | 0.99 | ABCC10 | 1.06 | ABCF2 | 1.26* | ABCG2 | 1.72* |
| ABCA11 | 0.91 | ABCB11 | 1.21 | ABCC11 | 0.60 | ABCF3 | 1.00 | ABCG4 | 0.10 |
| ABCA12 | 0.99 | ABCC12 | 0.84 | ||||||
| ABCA13 | 0.82 | ABCC13 | 0.93 |
Fig. 1.
Profile of ATP-binding cassette (ABC) different expressed transporters in the preterm placenta with chorioamnionitis. A) Heatmap derived from the Human ABC Transporters Taqman® Array showing the relative quantities (Rq) of placental ABC transporters in preterm delivery with chorioamnionitis (PTDC) and preterm delivery in the absence of chorioamnionitis (PTD). Higher expression in red, lower in green. B) Fold-change of the transporters, comparing PTD to PTDC. Statistical analysis: unpaired t-test. Data are presented as mean ± SEM (n=6/group).
Fig. 2.
qPCR of selected genes. A) ABCB1; B) ABCG2; and C) interleukin (IL)-8. Gene expression assessed by qPCR in preterm delivery with chorioamnionitis (PTDC) and preterm delivery in the absence of chorioamnionitis (PTD). Data are presented as fold-change relative to PTD group. Statistical analysis: unpaired t-test for ABCB1 and ABCG2; non-parametric Mann-Whitney test for IL-8. Data are presented as mean ± SEM (n=6/group).
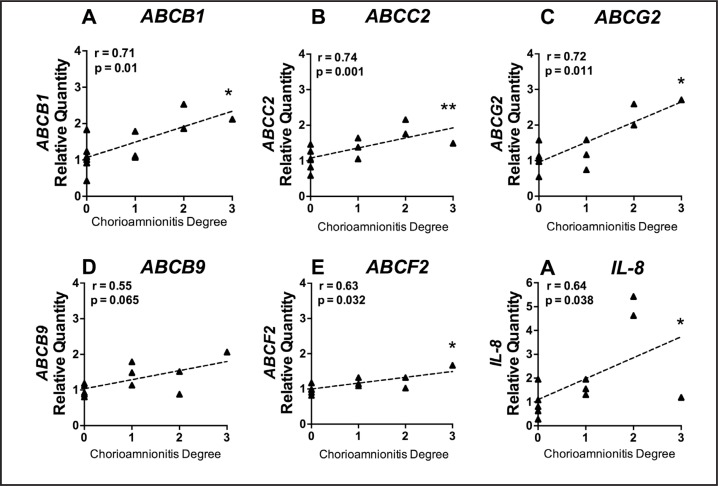
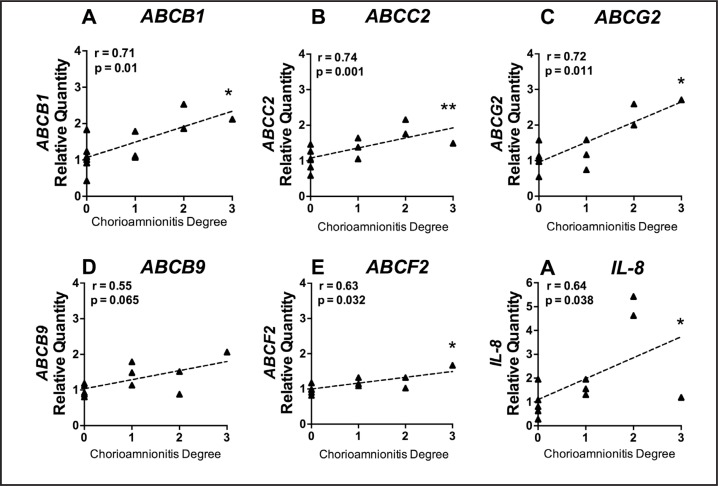
We next examined the mRNA expression of IL-8, a cytokine related to intraamniotic infection [43] (Fig. 2C). IL-8 mRNA was significantly increased in PTDC compared to PTD (p = 0.030). We also found a significant positive correlation between IL-8 expression and the severity of chorioamnionitis. Similarly, there was a significant correlation between the severity of chorioamnionitis and the increased expression of ABCB1, ABCC2, ABCF2 and ABCG2 (Fig. 3). Of note, ABCB1 and ABCG2 exhibited the higher percentage of mRNA increment (61% and 72% respectively) compared to ABCB9, ABCC2 and ABCF2 (47%, 52% and 26% respectively Fig. 1B).
Fig. 3.
Correlation between mRNA expression of selected transporters and IL-8, and the degree of chorioamnionitis in each placental sample. The degree of inflammation is presented from 0 to 3, with stages 1, 2 and 3 classified as previously described [23]. All preterm delivery placentas without chorioamnionitis (PTD) samples are considered as stage 0. Statistical analysis: non-parametric Spearman correlation (n=6/group).
Altered P-gp and BCRP protein in preterm placentas with chorioamnionitis
There were no significant differences in P-gp or BCRP (Fig. 4A) levels between groups by Western blot analysis, though there was a strong trend for increased BCRP expression in the PTDC group (Fig. 4A). This trend for an increase was corroborated by immunohistochemistal analysis demonstrating stronger BCRP staining in the syncytiotrophoblast (p = 0.030; Fig. 4B) and villous core (p = 0.009; PTD = 0.14 ± 0.04; PTDC = 0.46 ± 0.08). In contrast, P-gp staining intensity in the syncytiotrophoblast was significantly lower in the PTDC compared to PTD group (p = 0.019, Fig. 4C), with no difference in the villous core intensity (PTD = 0.53 ± 0.11; PTDC = 0.44 ± 0.06).
Fig. 4.
Protein analysis of P-gp and BCRP expression in preterm delivery with (PTDC) and in the absence of chorioamnionitis (PTD). A) Representative P-gp (150 kD), BCRP (72 kDa) and ß-actin (42 kDa) immunoblots followed by placental P-gp and BCRP expression normalized to ß-actin (internal control); B) Representative immunohistochemistry of BCRP; and C) P-gp in PTDC and PTD placentae. Semiquantitative score of the intensity of BCRP staining in the syncytiotrophoblast in PTDC compared to PTD slides. Black arrows indicate BCRP or P-gp staining, predominantly in the cytoplasm and apical membrane of the syncytiotrophoblast. Magnification bars represent 20 urn. Statistical analysis: unpaired t-test. Data are presented as mean ± SEM (n=6/group).
Chorioamnionitis-induced changes of miRNAs in human preterm placentas
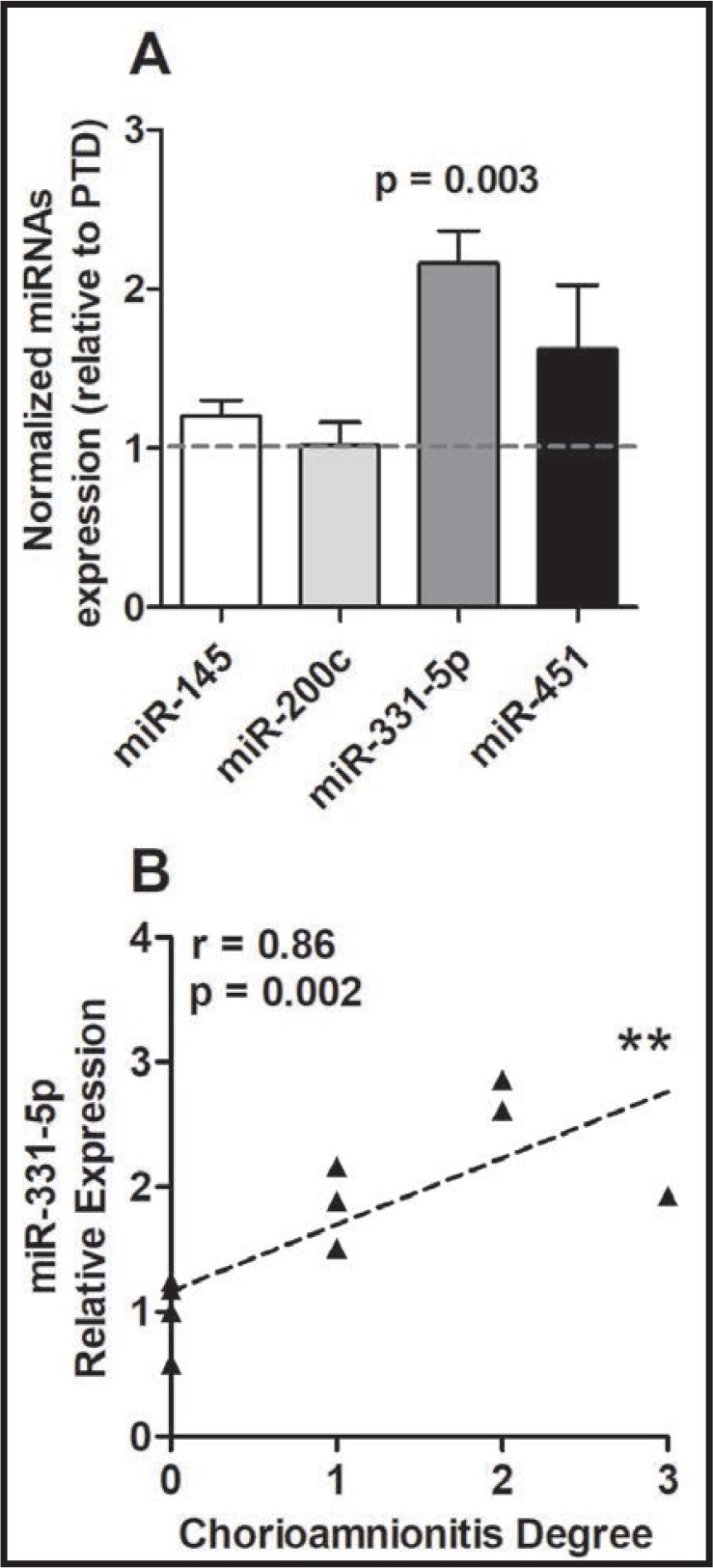
To evaluate the expression of selected miRNAs in preterm placentas with chorioaminionitis, we performed qPCR analysis of miRNAs selected on the basis of their regulatory actions on P-gp (miR-145, miR-200c, miR-331-5p and miR-451) [44]. Placental expression of miR-331-5p (p=0.003) was significantly increased in the PTDC compared to the PTD group (Fig. 5A). Importanty, miR-331-5p upregulation was positively correlated (0.77; p = 0.009) to the degree of intrauterine inflammation in preterm human placentas (Fig. 5B).
Fig. 5.
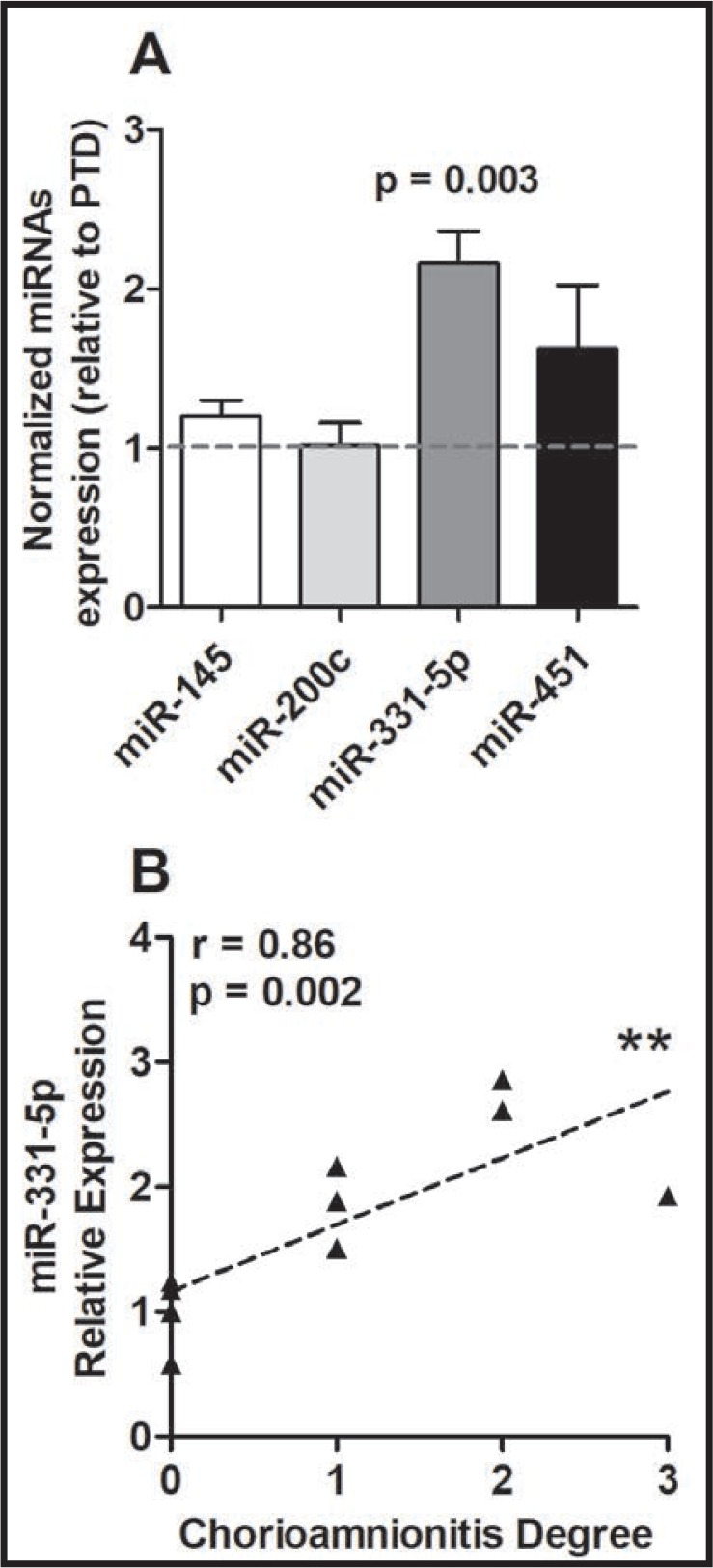
Analysis of miRNAs potentially involved in P-gp regulation by chorioamnionitis in preterm human placenta. A) Expression of selected miRNAs in preterm delivery with (PTDC) and without (PTD) chorioamnionitis placentae; B) Correlation between the miR-331-5p relative expression and the degree of chorioamnionitis of each placentae sample. MiRNA expression was normalized by the geometric mean of the internal controls U6, RNU43 and RNU44. Statistical analysis: A) unpaired t-test; B) non-parametric Spearman correlation. Data are presented as mean ± SEM (n=6/group).
Discussion
We have demonstrated that chorioamnionitis alters the placental signature of key ABC transporters, and that these effects correlate to the severity of chorioamnionitis. We have also demonstrated that chorioamnionitis modifies the expression of a specific P-gp regulatory miRNA, the miR-331-5p. Such changes have the potential to modify fetal accumulation of a large number of compounds related to obstetric care.
Out of the 50 ABC transporters analyzed by low density array, placental ABCB9, ABCC2 and ABCF2 were significantly up-regulated in the PTDC group, whereas the near significant up-regulation observed in ABCB1 and ABCG2 was posteriorly validated by individual qPCR, demonstrating that this technique is more sensitive to detect significant changes in gene expression than the low density array, possibly due to the amount of RNA transcribed into cDNA used in the separate reactions, ie. 2 ng of for the arrays, compared to 10 ng for the individual qPCR. Importantly, these changes were directly related to the degree of tissue inflammatory response, as demonstrated by the compelling positive correlation between the relative expression of four out of five up-regulated ABC transporters and the severity of chorioamnionitis in each placental sample.
Since ABCB1 and ABCG2 exhibited the largest increase in expression and the strongest correlation with the intrauterine inflammation, and because they are the most well-characterized placental ABC transporters, we further investigated whether these changes extended to the level of protein. The increase in ABCG2 mRNA in PTDC placentas was mirrored by a trend towards an increase in BCRP protein and a significant increase of BCRP immunostaining in the syncytiotrophoblast and villous core. We have previously demonstrated that LPS treatment, a common model of gram-negative bacterial infection, impairs ABCG2 and BCRP expression in first trimester human placental explants, but had no effect when administered to third trimester placental explants [11]. ABCG2 and BCRP are time-depedently expressed in the placenta [1, 37], and are modulated by infection and inflammation in a gestational-age dependent manner [9]. Our previous studies were undertaken using short-term in vitro models of infection, whereas the current results were obtained from placentas following preterm delivery in the presence or absence of chorioamnionitis. Thus, this represents quite a different situation from the in vitro studies, and this may account for the differences in the ABCG2/BCRP responses that we observed. In the present study, there is likely considerable etiological heterogeneity given the multiple possible causes of the inflammatory process, i.e., the type of pathogen involved, if it is a monomicrobial or polymicrobial infection or indeed, non-infective (i.e. sterile chorioamnionitis) [24, 45–47]. Future studies should examine how different infective agents impact the expression of ABC transporters. In the present study, we were unable to trace-back the causes of chorioamnionitis because the rate of microbiologically-proven intra-amniotic infection is still extremely low [24].
BCRP is highly expressed in the apical membrane of the syncytiotrophoblast [8, 48–50], and the up-regulation may promote increased efflux of BCRP substrates. Therefore, third-trimester chorioamnionitis has the potential to decrease accumulation of BCRP endogenous and exogenous substrates in the fetal compartment including folate, antibiotics and antiretrovirals.
While no change in P-gp was detected by Western blot, immunohistochemistry revealed decreased staining intensity for P-gp in the syncytiotrophoblast of PTDC group. Despite decreased P-gp immunostaining in the syncytiotrophoblast, we observed increased ABCB1 mRNA expression. This latter result is consistent with a previous study demonstrating that chorioamnionitis increased ABCB1 mRNA in the human placenta [51]. This disconnect between mRNA and protein has been previously described [49, 52], and may indicate post-transcriptional influences of inflammation. A number of miRNAs have been shown to regulate P-gp levels in cancer cells. miR-331-5p decreased P-gp protein expression in a luciferase reporter assay [53]. In the present study, we discovered that miR-331-5p is upregulated by placental chorioamnionitis and that there was correlation between this upregulation and the degree of placental inflammation. It is possible that while chorioamnionitis increases ABCB1 mRNA levels a simultaneous increase in miR-331-5p blocks P-gp protein production. Clearly further studies are warranted to investigate this novel relationship in non-malignant tissue.
A reduction of P-gp protein expression following infection and inflammation is consistent with previous studies. We have previously demonstrated that LPS treatment down-regulated P-gp and ABCB1 expression in first trimester human placental explants, while in third trimester placentas, ABCB1 expression was down-regulated by polyinosinic:polycytidylic acid (polyI:C) treatment (which models viral infection) but not by LPS [11]. A decrease in P-gp protein expression in PTDC may be associated with fetal accumulation of P-gp substrates, such as endogenous and synthetic glucocorticoids, estrogens and pro-inflammatory compounds. This possibility is supported by the evidence that LPS and polyI:C inhibited P-gp activity and increased fetal drug exposure in pregnant mice [8, 54]. P-gp also transports important classes of drugs such as angiotensin receptor blockers, antibiotics, antiepileptics, antihistamines, antiretrovirals, NSAIDs and statins1. In this context, these common medications might accumulate in the fetal compartment to a greater extent in the preterm placenta with chorioamnionitis than in PTD pregnancies without chorioamnionitis, leading to increased fetal drug exposure.
P-gp transports pro-inflammatory cytokines, including IL-2, Interferon (INF)Y, tumor necrosis factor (TNF)-a and chemokines, such as chemokine (C-C motif) ligand 2 (CCL2), into the extracellular space, hence exerting crucial role in regulation of inflammatory response [15, 16]. Simultaneously, pro-inflammatory mediators as TNF-a and IL-1ß down-regulate ABCB1 mRNA and P-gp levels in human primary placental trophoblast cells [55, 56], which in circumstances as chorioamnionitis, will likely lead to a regulatory loop consisting of P-gp suppression and progressive accumulation of inflammatory factors in the intrauterine environment. Considering that IL-6, IL-1ß e TNFa are key regulators of preterm induction in women [57, 58], the down-regulation of placental P-gp expression induced by pro-inflammatory cytokines may contribute to the induction of preterm labor in this study. In parallel, pharmacological inhibition or molecular silencing of BCRP activity in trophoblast cells led to increased susceptibility to stress-induced apoptosis, a process mediated by TNFa and INFY [56]. Thus, in the context of chorioamnionitis the increase of placental BCRP expression may be associated with enhanced placental and fetal protection against the damage induced by the inflammatory process.
ABCB9, ABCC2 and ABCF2 were also up-regulated by chorioamnionitis though very little is known concerning the potential function of these transporters in the placenta. ABCB9 is a lysosomal/endoplasmic reticulum transporter previously shown to be present in the testis, spinal cord and the brain [59, 60]. A role for ABCB9 has been proposed at the blood-testes barrier [59] and in T lymphocytes in response to infection [61]. However, its localization and function in the placental barrier, as well as its involvement to chorioamnionitis is yet to be determined. ABCC2 (MRP2) has been shown to be expressed in the apical membrane of the syncytiotrophoblast [8] and it has important physiological and pharmacological substrates, including hormones, antiretrovirals and opioids [8, 62–64]. The ABCF2 transporter has been shown to be localized to the cytoplasm and is known to be involved in breast and ovary carcinogenesis [65–67]. ABCF2 is down-regulated during chronic inflammation of the gastrointestinal tract and up-regulated by TNF-a treatment of HT29 cells [68]. Further studies investigating localization, function and relation to inflammatory processes in the placenta are warranted.
Conclusion
Inflammation results in a specific signature of ABC transporter expression in the human preterm placenta. Given that ABC transporters extrude a range of specific substrates, alterations in their expression will likely lead to modified transport of clinically relevant compounds as well as physiological factors. A better understanding of the potential role of these transporters in the events surrounding PTD may also enable new strategies to be developed for prevention and treatment of PTD.
Acknowledgements
We would like to thank Jeremy P. Landry for helping with the semi quantitative scoring and Alisa Kostaki for the basic support in this research.
Conception and design: GEI, EB, WG, TMOG, SGM. Acquisition, analysis and interpretation of data: GEI, EB, MJ, PL, AC, CD. Drafting the article and revising it for important intellectual content: GEI, EB, FMR, WG, SJL, TMOG, SGM. Final approval of the version to be published: GEI, EB, MJ, PL, AC, CD, FMR, WG, SJL, TMOG, SGM.
This study was funded by Bill & Melinda Gates Foundation (MCTI/CNPq/MS/SCTIE/Decit/Bill e Melinda Gates 05/2013], Canadian Institutes for Health Research (SGM; MOP-57746), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 304667/2016-1, 422441/2016-3, 303734/2012-4), Coordenacáo de Aperfeicoamento Pessoal de Nivel Superior (CAPES) and Fundacáo de Amparo á Pesquisa do Estado do Rio de Janeiro (FAPERJ, CNE 2015/E26/203.190/2015).
Disclosure Statement
None declared.
References
- 1.World Health Organization, WHO - Preterm Birth, Fact Sheet , 2017. Accessed in 14/12/2017. Retrieved from: http://www.who.int/mediacentre/factsheets/fs363/en/
- 2.Goldenberg RL: The management of preterm labor. Obstet Gynecol 2002;100:1020–1037. [DOI] [PubMed] [Google Scholar]
- 3.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, Petraglia F: Inflammation and pregnancy. Reprod Sci 2009;16:206–215. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Mazor M: Infection and preterm labor. Clin Obstet Gynecol 1988;31:553–584. [DOI] [PubMed] [Google Scholar]
- 5.Lettieri L, Vintzileos AM, Rodis JF, Albini SM, Salafia CM: Does “idiopathic” preterm labor resulting in preterm birth exist? Am J Obstet Gynecol 1993;168:1480–1485. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg RL, Culhane JF, lams JD, Romero R: Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed Al, Yoon BH, Hassan SS, Kim CJ, Yeo L: Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014;72:458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloise E, Ortiga-Carvalho TM, Reis FM, Lye SJ, Gibb W, Matthews SG: ATP-binding cassette transporters in reproduction: a new frontier. Hum Reprod Update 2016; 10.1093/humupd/dmv049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton GJ, Fowden AL, Thornburg KL: Placental Origins of Chronic Disease. Physiol Rev 2016;96:1509–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloise E, Bhuiyan M, Audette MC, Petropoulos S, Javam M, Gibb W, Matthews SG: Prenatal endotoxemia and placental drug transport in the mouse: placental size-specific effects. PLoS One 2013;8:e65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lye P, Bloise E, Javam M, Gibb W, Lye SJ, Matthews SG: Impact of bacterial and viral challenge on multidrug resistance in first- and third-trimester human placenta. Am J Pathol 2015;185:1666–1675. [DOI] [PubMed] [Google Scholar]
- 12.Kooij G, Mizee MR, van Horssen J, Reijerkerk A, Witte ME, Drexhage JA, van der Pol SM, van Het Hof B, Scheffer G, Scheper R, Dijkstra CD, van der Valk P, de Vries HE: Adenosine triphosphate-binding cassette transporters mediate chemokine (C-C motif) ligand 2 secretion from reactive astrocytes: relevance to multiple sclerosis pathogenesis. Brain 2011;134:555–570. [DOI] [PubMed] [Google Scholar]
- 13.Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, Xu Y, Dong Z, Nhan-Chang CL, Chaiworapongsa T, Lye S, Kusanovic JP, Lipovich L, Mazaki-Tovi S, Hassan SS, Mesiano S, Kim CJ: Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med 2010;38:617–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton SA, Tower CL, Jones RL: Identification of chemokines associated with the recruitment of decidual leukocytes in human labour: potential novel targets for preterm labour. PLoS One 2013;8:e56946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pawlik A, Baskiewicz-Masiuk M, Machalinski B, Safranow K, Gawronska-Szklarz B: Involvement of P-glycoprotein in the release of cytokines from peripheral blood mononuclear cells treated with methotrexate and dexamethasone. J Pharm Pharmacol 2005;57:1421–1425. [DOI] [PubMed] [Google Scholar]
- 16.Kooij G, Backer R, Koning JJ, Reijerkerk A, van Horssen J, van der Pol SM, Drexhage J, Schinkel A, Dijkstra CD, den Haan JM, Geijtenbeek TB, de Vries HE: P-glycoprotein acts as an immunomodulator during neuroinflammation. PLoS One 2009;4:e8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchiya Y, Nakajima M, Takagi S, Taniya T, Yokoi T: MicroRNA regulates the expression of human cytochrome P450 1B1 Cancer Res 2006;66:9090–9098. [DOI] [PubMed] [Google Scholar]
- 18.Yu AM: Small interfering RNA in drug metabolism and transport. Curr Drug Metab 2007;8:700–708. [DOI] [PubMed] [Google Scholar]
- 19.Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, Pogribny IP: Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther 2008;7:2152–2159. [DOI] [PubMed] [Google Scholar]
- 20.Liao R, Sun J, Zhang L, Lou G, Chen M, Zhou D, Chen Z, Zhang S: MicroRNAs play a role in the development of human hematopoietic stem cells. J Cell Biochem 2008;104:805–817. [DOI] [PubMed] [Google Scholar]
- 21.Takagi S, Nakajima M, Mohri T, Yokoi T: Post-transcriptional regulation of human pregnane X receptor by micro-RNA affects the expression of cytochrome P450 3A4. J Biol Chem 2008;283:9674–9680. [DOI] [PubMed] [Google Scholar]
- 22.Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, Yang JM: Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol 2008;76:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C, Society for Pediatric Pathology PS, A.niotic Fluid Infection Nosology Committee: Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol 2003;6:435–448. [DOI] [PubMed] [Google Scholar]
- 24.Conti N, Torricelli M, Voltolini C, Vannuccini S, Clifton VL, Bloise E, Petraglia F: Term histologic chorioamnionitis: a heterogeneous condition. Eur J Obstet Gynecol Reprod Biol 2015;188:34–38. [DOI] [PubMed] [Google Scholar]
- 25.Menon R, Velez DR, Morgan N, Lombardi SJ, Fortunato SJ, Williams SM: Genetic regulation of amniotic fluid TNF-alpha and soluble TNF receptor concentrations affected by race and preterm birth. Hum Genet 2008;124:243–253. [DOI] [PubMed] [Google Scholar]
- 26.Menon R, Dunlop AL, Kramer MR, Fortunato SJ, Hogue CJ: An overview of racial disparities in preterm birth rates: caused by infection or inflammatory response? Acta Obstet Gynecol Scand 2011;90:1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fortunato SJ, Menon R, Velez DR, Thorsen P, Williams SM: Racial disparity in maternal-fetal genetic epistasis in spontaneous preterm birth. Am J Obstet Gynecol 2008;198:666.e661–669; discussion 666. e669-610. [DOI] [PubMed] [Google Scholar]
- 28.Velez DR, Fortunato SJ, Morgan N, Edwards TL, Lombardi SJ, Williams SM, Menon R: Patterns of cytokine profiles differ with pregnancy outcome and ethnicity. Hum Reprod 2008;23:1902–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy P, Kumar A, Kaur IR, Faridi MM: Gender differences in outcomes of low birth weight and preterm neonates: the male disadvantage. J Trop Pediatr 2014;60:480–481. [DOI] [PubMed] [Google Scholar]
- 30.Kim-Fine S, Regnault TR, Lee JS, Gimbel SA, Greenspoon JA, Fairbairn J, Summers K, de Vrijer B: Male gender promotes an increased inflammatory response to lipopolysaccharide in umbilical vein blood. J Matern Fetal Neonatal Med 2012;25:2470–2474. [DOI] [PubMed] [Google Scholar]
- 31.Annibalini G, Agostini D, Calcabrini C, Martinelli C, Colombo E, Guescini M, Tibollo P, Stocchi V, Sestili P: Effects of sex hormones on inflammatory response in male and female vascular endothelial cells. J Endocrinol Invest 2014;37:861–869. [DOI] [PubMed] [Google Scholar]
- 32.Enninga EA, Nevala WK, Creedon DJ, Markovic SN, Holtan SG: Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol 2015;73:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Challis J, Newnham J, Petraglia F, Yeganegi M, Bocking A: Fetal sex and preterm birth. Placenta 2013;34:95–99. [DOI] [PubMed] [Google Scholar]
- 34.Torricelli M, Novembri R, Bloise E, De Bonis M, Challis JR, Petraglia F: Changes in placental CRH, urocortins, and CRH-receptor mRNA expression associated with preterm delivery and chorioamnionitis. J Clin Endocrinol Metab 2011;96:534–540. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 36.Bloise E, Couto HL, Massai L, Ciarmela P, Mencarelli M, Borges LE, Muscettola M, Grasso G, Amaral VF, Cassali GD, Petraglia F, Reis FM: Differential expression of follistatin and FLRG in human breast proliferative disorders. BMC Cancer 2009;9:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bloise E, Cassali GD, Ferreira MC, Ciarmela P, Petraglia F, Reis FM: Activin-related proteins in bovine mammary gland: localization and differential expression during gestational development and differentiation. J Dairy Sci 2010;93:4592–4601. [DOI] [PubMed] [Google Scholar]
- 38.Couto HL, Buzelin MA, Toppa NH, Bloise E, Wainstein AJ, Reis FM: Prognostic value of follistatin-like 3 in human invasive breast cancer. Oncotarget 2017; 10.18632/oncotarget.15026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatia A, Sekhon HK, Kaur G: Sex hormones and immune dimorphism. ScientificWorldJournal 2014;2014:159150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu JW, Wingard JR, Logan BR, Chitphakdithai P, Akpek G, Anderlini P, Artz AS, Bredeson C, Goldstein S, Hale G, Hematti P, Joshi S, Kamble RT, Lazarus HM, O’Donnell PV, Pulsipher MA, Savani BN, Schears RM, Shaw BE, Confer DL: Race and ethnicity influences collection of granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells from unrelated donors, a Center for International Blood and Marrow Transplant Research analysis. Biol Blood Marrow Transplant 2014;21:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamil F, Pinzon I, Foreman MG: Sex and race factors in early-onset COPD. Curr Opin Pulm Med 2013;19:140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis EF, Claggett B, Parfrey PS, Burdmann EA, McMurray JJ, Solomon SD, Levey AS, Ivanovich P, Eckardt KU, Kewalramani R, Toto R, Pfeffer MA: Race and ethnicity influences on cardiovascular and renal events in patients with diabetes mellitus. Am Heart J 2015;170:322–329. [DOI] [PubMed] [Google Scholar]
- 43.Vrachnis N, Karavolos S, Iliodromiti Z, Sifakis S, Siristatidis C, Mastorakos G, Creatsas G: Review: Impact of mediators present in amniotic fluid on preterm labour. In vivo 2012;26:799–812. [PubMed] [Google Scholar]
- 44.Haenisch S, Werk AN, Cascorbi I: MicroRNAs and their relevance to ABC transporters. Br J Clin Pharmacol 2014;77:587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P: Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol 2003;189:139–147. [DOI] [PubMed] [Google Scholar]
- 46.Li J, McCormick J, Bocking A, Reid G: Importance of vaginal microbes in reproductive health. Reprod Sci 2012;19:235–242. [DOI] [PubMed] [Google Scholar]
- 47.Wang X, Buhimschi CS, Temoin S, Bhandari V, Han YW, Buhimschi IA: Comparative microbial analysis of paired amniotic fluid and cord blood from pregnancies complicated by preterm birth and early-onset neonatal sepsis. PLoS One 2013;8:e56131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iqbal M, Audette MC, Petropoulos S, Gibb W, Matthews SG: Placental drug transporters and their role in fetal protection. Placenta 2012;33:137–142. [DOI] [PubMed] [Google Scholar]
- 49.Lye P, Bloise E, Dunk C, Javam M, Gibb W, Lye SJ, Matthews SG: Effect of oxygen on multidrug resistance in the first trimester human placenta. Placenta 2013;34:817–823. [DOI] [PubMed] [Google Scholar]
- 50.Yeboah D, Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W: Expression of breast cancer resistance protein (BCRP/ABCG2) in human placenta throughout gestation and at term before and after labor. Can J Physiol Pharmacol 2006;84:1251–1258. [DOI] [PubMed] [Google Scholar]
- 51.Mason CW, Buhimschi IA, Buhimschi CS, Dong Y, Weiner CP, Swaan PW: ATP-binding cassette transporter expression in human placenta as a function of pregnancy condition. Drug Metab Dispos 2011;39:1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrovic V, Kojovic D, Cressman A, Piquette-Miller M: Maternal bacterial infections impact expression of drug transporters in human placenta. Int Immunopharmacol 2015;26:349–356. [DOI] [PubMed] [Google Scholar]
- 53.Feng DD, Zhang H, Zhang P, Zheng YS, Zhang XJ, Han BW, Luo XQ, Xu L, Zhou H, Qu LH, Chen YQ: Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J Cell Mol Med 2011;15:2164–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bloise E, Petropoulos S, Iqbal M, Kostaki A, Ortiga-Carvalho TM, Gibb W, Matthews SG: Acute Effects of Viral Exposure on P-Glycoprotein Function in the Mouse Fetal Blood-Brain Barrier. Cell Physiol Biochem 2017;41:1044–1050. [DOI] [PubMed] [Google Scholar]
- 55.Evseenko DA, Paxton JW, Keelan JA: Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab Dispos 2007;35:595–601. [DOI] [PubMed] [Google Scholar]
- 56.Evseenko DA, Murthi P, Paxton JW, Reid G, Emerald BS, Mohankumar KM, Lobie PE, Brennecke SP, Kalionis B, Keelan JA: The ABC transporter BCRP/ABCG2 is a placental survival factor, and its expression is reduced in idiopathic human fetal growth restriction. FASEB J 2007;21:3592–3605. [DOI] [PubMed] [Google Scholar]
- 57.Ogawa M, Hirano H, Tsubaki H, Kodama H, Tanaka T: The role of cytokines in cervical ripening: correlations between the concentrations of cytokines and hyaluronic acid in cervical mucus and the induction of hyaluronic acid production by inflammatory cytokines by human cervical fibroblasts. Am J Obstet Gynecol 1998;179:105–110. [DOI] [PubMed] [Google Scholar]
- 58.Jun JK, Yoon BH, Romero R, Kim M, Moon JB, Ki SH, Park JS: Interleukin 6 determinations in cervical fluid have diagnostic and prognostic value in preterm premature rupture of membranes. Am J Obstet Gynecol 2000;183:868–873. [DOI] [PubMed] [Google Scholar]
- 59.Zhang F, Zhang W, Liu L, Fisher CL, Hui D, Childs S, Dorovini-Zis K, Ling V: Characterization of ABCB9, an ATP binding cassette protein associated with lysosomes. J Biol Chem 2000;275:23287–23294. [DOI] [PubMed] [Google Scholar]
- 60.Yamaguchi Y, Kasano M, Terada T, Sato R, Maeda M: An ABC transporter homologous to TAP proteins. FEBS Lett 1999;457:231–236. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, Zhou QJ, Chen XQ, Yan BL, Guo XL, Zhang HL, Du AF: Profiling of differentially expressed genes in sheep T lymphocytes response to an artificial primary Haemonchus contortus infection. Parasit Vectors 2015;8:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keppler D: The roles of MRP2, MRP3, OATP1B1, and OATP1B3 in conjugated hyperbilirubinemia. Drug Metab Dispos 2014;42:561–565. [DOI] [PubMed] [Google Scholar]
- 63.Nies AT, Keppler D: The apical conjugate efflux pump ABCC2 (MRP2). Pflugers Arch 2007;453:643–659. [DOI] [PubMed] [Google Scholar]
- 64.Sodani K, Patel A, Kathawala RJ, Chen ZS: Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer 2012;31:58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogawa Y, Tsuda H, Hai E, Tsuji N, Yamagata S, Tokunaga S, Nakazawa K, Tamamori Y, Ogawa M, Shimizu S, Inoue T, Nishiguchi Y: Clinical role of ABCF2 expression in breast cancer. Anticancer Res 2006;26:1809–1814. [PubMed] [Google Scholar]
- 66.Nishimura S, Tsuda H, Ito K, Jobo T, Yaegashi N, Inoue T, Sudo T, Berkowitz RS, Mok SC: Differential expression of ABCF2 protein among different histologic types of epithelial ovarian cancer and in clear cell adenocarcinomas of different organs. Hum Pathol 2007;38:134–139. [DOI] [PubMed] [Google Scholar]
- 67.Tsuda H, Ito YM, Ohashi Y, Wong KK, Hashiguchi Y, Welch WR, Berkowitz RS, Birrer MJ, Mok SC: Identification of overexpression and amplification of ABCF2 in clear cell ovarian adenocarcinomas by cDNA microarray analyses. Clin Cancer Res 2005;11:6880–6888. [DOI] [PubMed] [Google Scholar]
- 68.Verma N, Ahuja V, Paul J: Profiling of ABC transporters during active ulcerative colitis and in vitro effect of inflammatory modulators. Dig Dis Sci 2013;58:2282–2292. [DOI] [PubMed] [Google Scholar]