Abstract
Malus halliana is an iron (Fe)-efficient apple rootstock growing in calcareous soil that shows obvious ‘greenness’ traits during Fe deficiency. Recent studies have shown that exogenous sugars can be involved in abiotic stress. To identify the key regulatory steps of chlorophyll (Chl) biosynthesis in M. halliana under Fe deficiency and to verify whether exogenous sucrose (Suc) is involved in Fe deficiency stress, we determined the contents of the Chl precursor and the expression of several Chl biosynthetic genes in M. halliana. The results showed that Fe deficiency caused a significant increase in the contents of protoporphyrin IX (Proto IX), Mg-protoporphyrin IX (Mg-Proto IX) and protochlorophyllide (Pchlide) in M. halliana compared to the Fe-sensitive rootstock Malus hupehensis. Quantitative real-time PCR (RT-qPCR) also showed that the expression of protoporphyrinogen oxidase (PPOX), which synthesizes Proto IX, was upregulated in M. halliana and downregulated in M. hupehensis under Fe deficiency. Exogenous Suc application prominently enhanced the contents of porphobilinogen (PBG) and the subsequent precursor, whereas it decreased the level of δ-aminolaevulinic acid (ALA), suggesting that the transformation from ALA to PBG was catalyzed in M. halliana. Additionally, the transcript level of δ-aminolevulinate acid dehydratase (ALAD) was noticeably upregulated after exogenous Suc treatment. This result, combined with the precursor contents, indicated that Suc accelerated the steps of Chl biosynthesis by modulating the ALAD gene. Therefore, we conclude that PPOX is the key regulatory gene of M. halliana in response to Fe deficiency. Exogenous Suc enhances M. halliana tolerance to Fe deficiency stress by regulating Chl biosynthesis.
Introduction
Iron (Fe) is an essential micronutrient required by all plants [1]. Fe deficiency in plants results in severe chlorosis of leaves [2]. The application of tolerant rootstocks is an effective method to prevent chlorosis in fruit production due to Fe stress [3]. Malus halliana, an indigenous apple rootstock originating from arid saline-alkali habitats in Gansu, grows very well and shows characteristics of a Fe deficiency-tolerant rootstock. The chlorosis associated with Fe deficiency was not found in the northwest Loess Plateau of China [4].
Leaf chlorosis may be caused by deficient chlorophyll (Chl) biosynthesis [5]. Chl biosynthesis plays essential roles in photosynthesis and plant growth in response to environmental change [6]. The Chl biosynthesis pathway has many steps and involves various enzymes, and a blockade in one step will affect Chl biosynthesis and cause changes in leaf color [7]. The key regulatory sites of Chl synthesis are different for each crop under external stress. A study of adzuki bean reported that the transformation of protoporphyrin IX (Proto IX) is blocked in Chl synthesis, causing etiolated seedlings [8]. Salinity-alkalinity stress disrupted Chl synthesis by blocking the conversion of URO III to Proto IX, which reduced the Chl content in tomato [9]. Another study suggested that Chl biosynthesis is blocked in a mutant at the Chl a production step, and the expression of multiple genes related to Chl biosynthesis was downregulated in pylm [10]. A study on the effect of different light qualities of LEDs on the Chl biosynthesis precursors of nonheading Chinese cabbage showed that red plus blue LEDs enhanced Chl biosynthesis precursors [11]. Remarkably, Fe deficiency directly affected Chl synthesis [12]. Research in poplar revealed that Chl synthesis was inhibited under Fe-deficient conditions [13]. Spiller et al. attempted to study the effect of Fe on the Chl biosynthetic pathway, and the results indicated that Fe deficiency leads to the accumulation and excretion of intermediates in the tetrapyrrole biosynthetic pathway, particularly coproporphyrin [14]. Moreover, an investigation was initiated to locate possible sites where a deficiency of Fe might limit Chl synthesis of cowpea plants [2]. However, the responses of Chl biosynthesis to Fe deficiency stress and the key regulatory sites in M. halliana are still unknown.
Sucrose (Suc) is the major sugar that plants assimilate in photosynthesis and transport to various nonphotosynthetic tissues; it was not only originally recognized as an energy source for metabolism but also functions as a signaling molecule involved in the regulation of various physiological processes in plants [15–18]. Increased accumulation of Suc is a critical requirement for the adaptation of plants to stresses [19, 20]. Higher accumulation of soluble sugars in roots increases the resistance of maize plants to salt-induced osmotic stress [21]. Increased Suc accumulation is required for the regulation of Fe deficiency responses in Arabidopsis plants [22]. However, little is known about how exogenous Suc regulates the response of M. halliana to Fe deficiency through Chl synthesis.
Studies of Chl biosynthesis have focused on various aspects [23, 24], whether biochemical [25, 26] or genetic [27, 28]. However, gaps remain in the knowledge of Chl biosynthesis and the related genes in apple rootstocks. Therefore, it is important to elucidate the Chl biosynthetic molecular responses of M. halliana to Fe deficiency. In this article, we characterized the Chl biosynthetic pathway and gene expression patterns of protoporphyrin IX and porphobilinogen precursor formation in M. halliana under Fe deficiency and exogenous Suc. This study provides a foundation for improved understanding of Fe tolerance responses in apples and gives insights into the functional characterization of Fe resistance genes.
Materials and methods
Plant materials and treatment of iron deficiency
Seeds of M. halliana and M. hupehensis (provided by Lanzhou, Gansu Province, China) were surface-sterilized in 0.2% KMnO4 for 30 min and then washed with running water for 12 h. Seeds were subsequently stratified at 4°C sand for 40 d, and germinated seeds were directly sown into plastic pots filled with substrates. Seedlings with eight true leaves were employed as test materials. Uniform seedlings were transferred to foam boxes filled with half-strength Han’s nutrient solution [29] for preculture. The nutrient solution was aerated and renewed every 7 d. After 14 d, the seedlings were transferred to Han’s nutrient solution that contained either 4 μM (-Fe) or 40 μM Fe (Ⅲ)-EDTA (CK). After 0, 0.5, 3, 6 and 12 d of Fe deficiency, the leaves were used to measure Chl precursors. However, according to transcriptome data analysis [30], the relative expression of related genes was assayed after 0, 0.5 and 3 d of Fe deficiency.
Exogenous sucrose treatments
Eight-true-leaf M. halliana seedlings were uniformly transferred to foam boxes with half-strength Han’s nutrient solution for 14 d to adapt to the environment for hydroponic cultivation. After 14 d of Fe deficiency, the uniform seedlings were transferred to Han’s nutrient solution containing 4 μM Fe, 40 μM Fe, 2 mM Suc [22] and 40 μM Fe mixed with 2 mM Suc. Therefore, this part of the experiment included six treatments: T1 (-Fe 0 d), T2 (-Fe 14 d), T3 (-Fe 21d), T4 (-Fe 14 d +Fe 7 d), T5 (-Fe 14 d +Suc 7 d), T6 (-Fe 14 d + (Fe +Suc) 7 d). Leaf samples were collected at different times, frozen immediately in liquid nitrogen, and stored in the refrigerator at -80°C until needed.
Determination of chlorophyll precursors
δ-aminolaevulinic acid (ALA) was determined as described by Morton [31]. Porphobilinogen (PBG) and Uroorphyinogen III (URO III) were measured according to Bogorad [32]. Protoporphyrin IX (Proto IX), Mg-protoporphyrin IX (Mg-Proto IX), and Protochlorophyllide (Pchlide) were assayed following the method of Hodgins and Van Huystee [33].
RNA isolation and real-time PCR
Total RNA was isolated using a TRIzol kit (Invitrogen, Carlsbad, CA, USA). After extraction, the RNA quality was measured by gel electrophoresis using 1% agarose gel. For real-time PCR (RT-qPCR) analysis, cDNA was synthesized from total RNA using the PrimeScriptTM RT reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Dalian, China) according to the manufacturer's instructions. RT-qPCR was performed with a DNA Engine Opticon System using a Light Cycler® 96 Instrument (Roche, Shanghai, China). GAPDH was used as a reference gene. Measurements for each plate were replicated three times. The relative gene expression levels were calculated using the 2-ΔΔCt method. RT-qPCR primer pairs are tabulated in Table 1.
Table 1. Primers used for RT-qPCR.
| Gene name | Gene ID | Primer sequence (5'-3') | |
|---|---|---|---|
| Forward primer | Reverse primer | ||
| ALAD | 103423306 | GCGTTGTCATGGAGTCCTGATGG | CCAGTTGGCGACCACTTCAGC |
| PBGD | 103423833 | CCTTGCAACCTTCCGCGAGAG | TCAGCCGTGTCTGGACGTTACC |
| UROS | 103455996 | CCACCTTCTTGTCCGCCACTTC | TTGCTGTTCTTGCCGTGCTCTC |
| UROD | 103444879 | AGGTAGAAGGCGACTGGGAC | CCCTCTACCGGCTTTCCTCA |
| PPOX | 103444480 | TTCTGTTGACTGCGTGGTGGTG | GGTGTCGCCGTGCTTGGTAG |
| CHLH | 103456287 | AATACCAAAGCCTAACTCC | AACAGCAGCCTCATCG |
| MgPMT | 103454888 | AAAACCTACCACCCTAAA | CTTCACCACCTCCTTGT |
| MPE | 103454703 | CTTTGCTCTGCGTTGT | GCTGTGGTGCGATTT |
Statistical analysis
All data were analyzed with SPSS version 22.0 for Windows (IBM, Armonk, NY, USA). Graphs were generated using the Origin 9.0 software (Origin Lab, Hampton, MA, USA). The results are presented as the means of three independent experiments.
Statistical analysis of parameters was tested by analysis of variance and mean comparison was performed with a Duncan's test (P <0.05)
Results
Effects of iron deficiency on the chlorophyll precursor contents of M. halliana and M. hupehensis
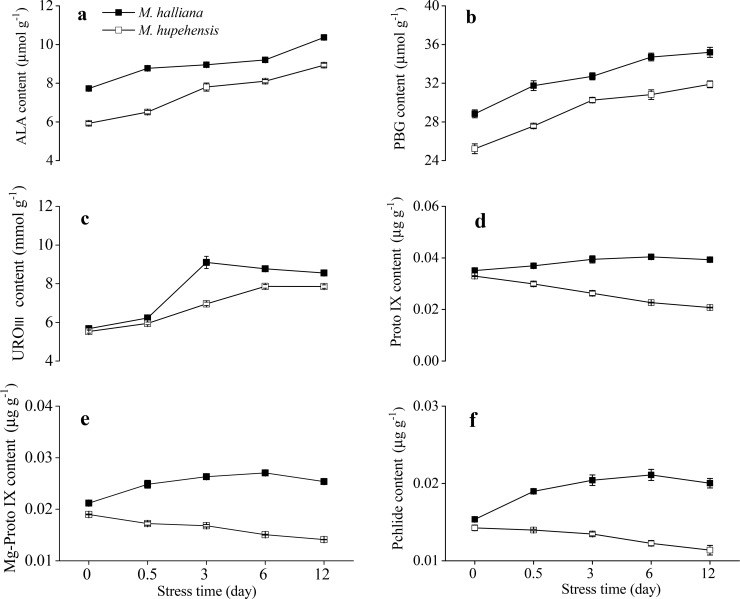
As shown in Fig 1, the contents of six precursors in M. halliana were noticeably higher than those in M. hupehensis. From 0 to 12 d of Fe deficiency, the contents of ALA, PBG and URO III of the two apple rootstocks increased (Fig 1A, 1B and 1C). However, the levels of Proto IX, Mg-Proto IX, and Pchlide in M. halliana were generally increased but decreased in M. hupehensis. Moreover, the levels in M. halliana were approximately 2-fold those in M. hupehensis (Fig 1D, 1E and 1F). Therefore, we hypothesized that the regulatory site of Chl biosynthesis in M. halliana affected Fe deficiency was the transformation from URO III to Proto IX.
Fig 1. Effects of Fe deficiency on Chl precursor contents in the leaves of M. halliana and M. hupehensis.
The seedlings underwent Fe deficiency for 0, 0.5, 3, 6, and 12 d as indicated. (a) ALA content. (b) PBG content. (c) URO III content; (d) Proto IX content. (e): Mg-Proto IX content. (f): Pchlide content. Vertical bars represent the mean ± SD value from three temporal replicates (n = 3).
Expression of genes involved in chlorophyll biosynthesis under iron deficiency
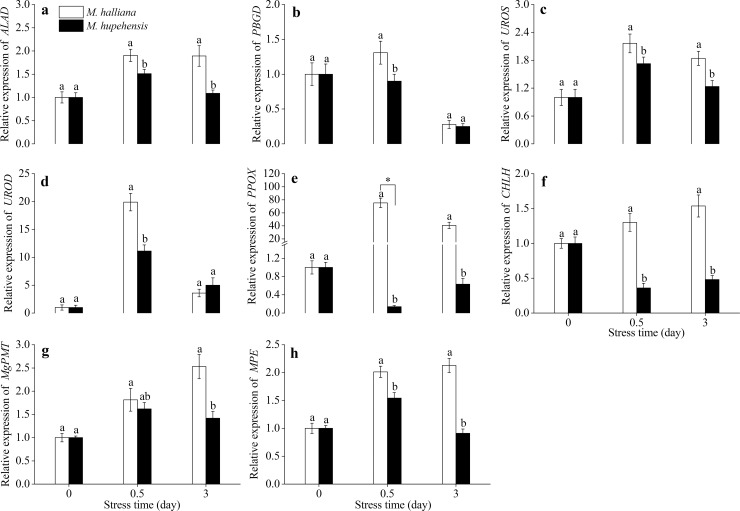
To prove the validity of the above hypothesis and gain insight into the key genes of M. halliana in response to Fe deficiency, we determined the relative expression levels of 8 genes related to Chl synthesis under Fe deficiency. Fig 2 shows that the relative levels of genes in M. halliana were dramatically higher than those in M. hupehensis. The relative levels of the δ- aminolevulinate acid dehydratase (ALAD), porphobilinogen deaminase (PBGD) and uroporphyrinogen III synthase (UROS) genes in M. halliana and M. hupehensis increased first and then dropped, peaking on 0.5 d, and the expression was no more than 3 in both groups. (Fig 2A, 2B and 2C). Thereafter, at 0.5 d, the relative levels of Uroporphyrinogen decarboxylase (UROD) and Protoporphyrinogen IX oxidase (PPOX) in M. halliana increased quickly, especially the expression of PPOX, which was approximately 75 in M. halliana instead of no more than 1 in M. hupehensis (Fig 2D and 2E). These results suggested that PPOX was responsive mainly to stresses and could improve resistance to Fe deficiency. Subsequently, the relative expression of genes in M. halliana, such as Magnesium chelatase (Subunit CHLH), Magnesium-protoporphyrin IX methyltransferase (MgPMT), and Mg-protoporphyrin IX monomethyl ester cyclase (MPEs), exhibited a gradually increasing trend during stress at 0–3 d (Fig 2F, 2G and 2H). These results were consistent with the Chl precursor analysis.
Fig 2. Expression pattern of eight genes in response to Fe deficiency in M. halliana and M. hupehensis.
(a)Expression of ALAD. (b) Expression of PBGD. (c) Expression of UROS. (d) Expression of UROD. (e) Expression of PPOX. (f) Expression of CHLH. (g) Expression of MgPMT. (h) Expression of MPE. Data are expressed as the mean ± SD (n = 3). The different letters and an asterisk show significant differences between the two apple rootstocks at each time point (P < 0.05).
Regulation of the iron deficiency phenotype by exogenous sucrose
We found that the leaves of M. halliana initially showed chlorosis symptoms after 12 d of Fe deficiency, and one of the important reasons was believed to be the loss of Suc or Fe. To investigate the effects of exogenous Suc on the phenotype of M. halliana seedlings, we conducted phenotype analysis of plants grown hydroponically in Suc-and Fe-sufficient and Fe-deficient conditions (Fig 3). The phenotype revealed that differences still existed. After 21 d of growth in Fe-deficient conditions (T3), the leaves of the plants exhibited more severe chlorosis symptoms than those of the plants treated with T4, T5, and T6. Additionally, the plants supplied with Suc and Fe (T6) showed pale green leaves compared with those under the T4 and T5 treatments. The Suc-supplied leaves (T5) were greener than the Fe-supplied leaves (T4). As a result, leaf chlorosis was ameliorated by Suc application. These results showed that the tolerance of Fe deficiency of M. halliana was positively regulated by Suc.
Fig 3. Effects of exogenous Suc on the phenotype of leaves from M. halliana.
T3: -Fe 21 d (Fe deficiency 21 d). T4: -Fe 14 d + Fe 7 d (applied Fe after 14 d of Fe deficiency). T5: -Fe 14 d + Suc 7 d (applied Suc after 14 d of Fe deficiency). T6: -Fe 14 d+ (Fe + Suc) 7 d (applied Fe and Suc after 14 d of Fe deficiency).
Effects of exogenous sucrose on the chlorophyll precursor contents in M. halliana
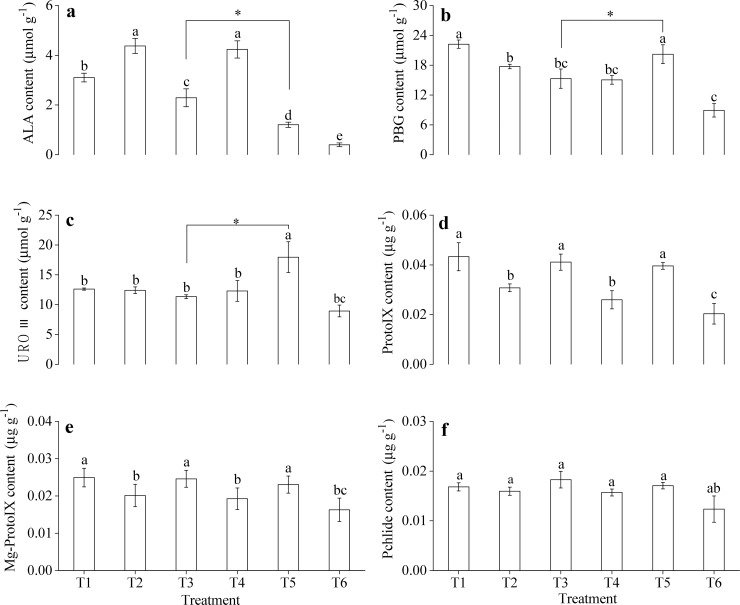
As shown in Fig 3, exogenous Suc may also be required for regulating the tolerance of Fe deficiency in M. halliana. Chlorosis was attributable to the reduction of Chl content causing defects in Chl biosynthesis. We determined the contents of the Chl precursors of the six treatments. Compared with the T3 treatment, the T5 treatment resulted in a clear decrease in the ALA level (Fig 4A) but increased the contents of PBG and URO III. (Fig 4B and 4C). The Chl intermediates Mg-Proto IX and Pchlide under T5 treatment were not significantly different from those under the other treatments (Fig 4D, 4E and 4F). The transformation of ALA to PBG and PBG to URO III was enhanced after application of Suc. As a result, after the application of exogenous Suc, the regulatory step of Chl precursor synthesis in M. halliana affected by Fe deficiency might be the conversion of ALA to PBG, suggesting this process was enhanced.
Fig 4. Effects of exogenous Suc on Chl precursor contents in the leaves of M. halliana.
(a) ALA content. (b) PBG content. (c) URO III content. (d) Proto IX content. (e) Mg- Proto IX content. (f) Pchlide content. Data are the mean ± SD (n = 3). Different letters indicate significant differences among treatments (P < 0.05).
Comparison of gene expression in M. halliana after sucrose application
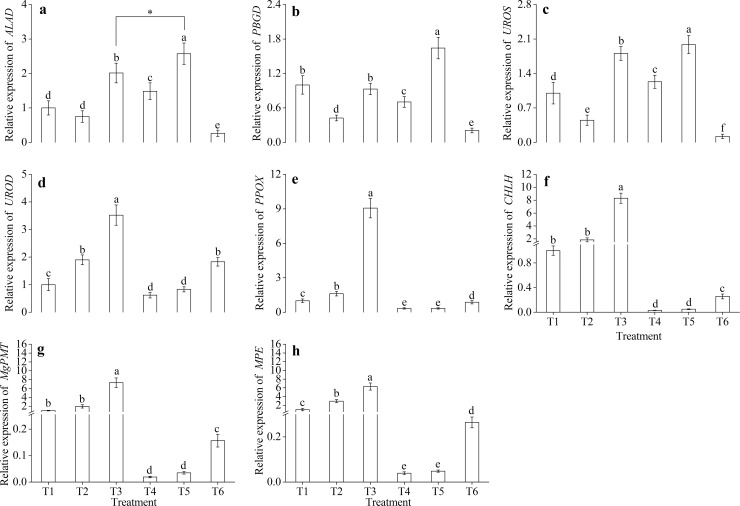
Given that exogenous Suc catalyzed the steps of Chl biosynthesis, we wanted to test the effects of exogenous Suc on the relative expression levels of related genes. As shown in Fig 5, compared with the other treatments, T5 significantly enhanced the expression of ALAD, PBGD and UROS (Fig 5A, 5B and 5C), consistent with a change in the corresponding precursor contents (Fig 4). Therefore, the expression of ALAD was significantly upregulated by the Suc treatment under Fe-deficient conditions. Nevertheless, for the other five genes, their expression levels not affected by the application of exogenous Suc, Fe or Suc and Fe. Enhanced relative levels of UROD, PPOX, CHLH, MgPMT, and MPE were associated with Fe deficiency, peaking on 21 d of Fe deficiency. Therefore, Fe deficiency caused a dramatic elevation in the relative levels of the above genes.
Fig 5. Comparison of gene expression in the leaves of M. halliana under exogenous sucrose treatment.
(a) Expression of ALAD. (b) Expression of PBGD. (c) Expression of UROS. (d) Expression of UROD. (e) Expression of PPOX. (f) Expression of CHLH. (g) Expression of MgPMT. (h) Expression of MPE. Data are expressed as the mean ± SD (n = 3). Different letters indicate significant differences between 6 treatments (P < 0.05).
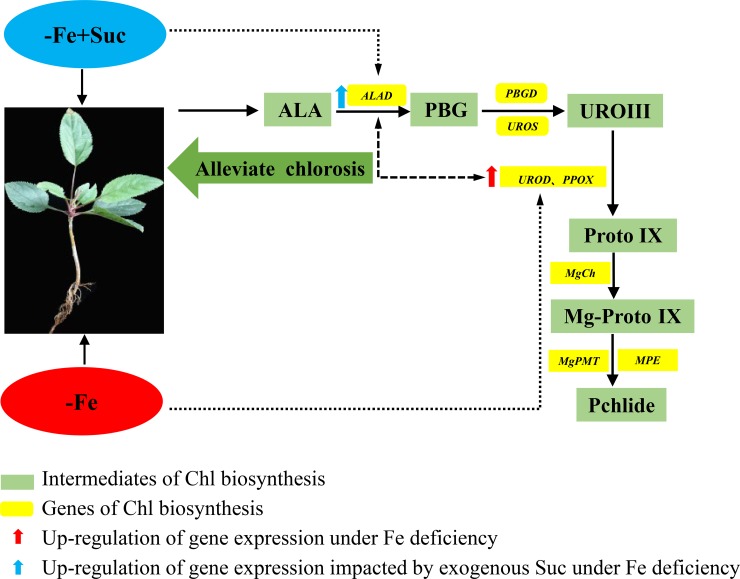
Schematic model of key genes involved in chlorophyll biosynthesis during iron deficiency and sucrose application
Overall, based on the variations in Chl precursor contents (Figs 1 and 5), gene expression (Figs 2 and 6) and phenotype characterization (Fig 3), we concluded that, under short-term stress, the key for the ‘greenness’ of M. halliana was high expression of PPOX. After long-term stress (over 12 d), M. halliana showed chlorosis symptoms.
Fig 6. The key synthetic sites of Chl biosynthesis under Fe deficiency and exogenous Suc treatment.
Results showed that (1) PPOX expression of M. halliana was enhanced to adapt to Fe-deficient condition. (2) Under Fe deficiency, application of exogenous Suc increased the expression of ALAD and further enhanced Chl synthesis.
Exogenous Suc can alleviate chlorosis. The accumulation of PBG precursor under Suc treatment enhanced the Chl synthesis, and the corresponding gene ALAD was up-regulated. Hence, PPOX and ALAD were key genes in mediating Fe-deficient and exogenous Suc-regulated Chl biosynthesis, respectively (Fig 6). Application of Suc could enhance the tolerance of M. halliana to Fe deficiency through improvement in Chl biosynthesis.
Discussion
Iron is an essential element for all living organisms, functioning in various cellular processes, such as Chl biosynthesis [34]. Fe deficiency is a nutritional disorder in plants, contributing to chlorosis by limiting Chl biosynthesis [35]. Disruption of any one step of Chl biosynthesis may lead to evident accumulation of the intermediates produced in previous steps, leading to disruption and substantial decreases in the amount of products produced in the subsequent steps. Previous research has found that seawater stress hinders the transformation of PBG to URO III in spinach [36]. The study showed that UV-B disrupts Chl synthesis at the point of ALA conversion to PBG [37]. In the present study, Chl synthesis at the step of URO III conversion to Proto IX indicated that Fe deficiency stress disrupted Chl biosynthesis in M. hupehensis, and the Chl biosynthesis of M. halliana was not blocked after Fe deficiency. The leaves of M. hupehensis exhibited chlorotic symptoms before M. halliana, consistent with the finding that the Chl precursor contents of M. hupehensis were significantly lower than those of M. halliana. Similar to a study on cabbage, M. halliana could maintain plant growth and preserve adequate chlorophyll synthesis under iron-limiting conditions, probably due to its better Fe-use efficiency than M. hupehensis [38]. The accumulation of chlorophyll intermediate metabolites can sometimes prevent adverse effects on M. halliana.
Protoporphyrinogen IX oxidase is the last enzyme in the common pathway of heme and chlorophyll synthesis and catalyzes the oxidation of protoporphyrinogen-IX to protoporphyrin IX by molecular oxygen [39, 40]. Research has suggested that the PbPPO1 gene might be involved in core browning under modified atmosphere storage in ‘Yali’ pears [41]. The PPO gene expression level in response to Aspergillus tubingensis in table grapes was enhanced with trehalose [42]. In this study, the relative expression of PPOX changed slightly in M. hupehensis but showed high expression levels in M. halliana, indicating that PPOX was the key gene in response to Fe deficiency in M. halliana, which lays the foundation for cloning genes responsive to Fe deficiency stress.
Sucrose plays a vital role in plant growth and development as well as the response to abiotic stress [43]. The application of sucrose in unripe strawberries resulted in the induction of ripening [44]. A study found that sucrose is one of most abundant metabolites in the glucose metabolic pathway, which plays an indispensable role in balancing photosynthetic activity in M. halliana [45]. In this study, Fe-deficient apple seedlings showed a typical Fe-stress phenotype, becoming yellow. The phenotypes of plants treated with Suc, Fe, and both Suc and Fe were found to be similar and showed good growth, and the Suc-supplied plants were greener than the Fe-supplied and Fe-Suc-supplied plants. This phenotype indicated that the repressions of Fe deficiency was partially reversed by exogenous Suc application. Furthermore, exogenous Suc increased the contents of PBG and subsequent precursors but decreased the ALA content in M. halliana, suggesting that the key regulatory point of Chl biosynthesis was moved forward after the application of exogenous Suc. Therefore, Suc was involved in the regulation of Chl intermediate products in M. halliana under Fe deficiency.
δ-Aminoleuvulinate acid dehydratase catalyzes the formation of PBG from two ALA molecules via the formation of two successive Schiff base intermediates [46, 47]. The ALAD gene, encoding δ-aminolevulinic acid dehydratase, is the rate-limiting enzyme of Chl biosynthesis [48]. In this study, after exogenous Suc application, increased expression of ALAD in M. halliana suggested that the accumulated intermediates of the PBG may have been efficiently utilized and enhanced the Chl biosynthetic pathway. As a result, in response to Fe deficiency, Suc may act as a signaling molecule to regulate the upregulation of the expression of related genes [49–51].
Conclusion
Our experimental results have showed that up regulation of PPOX gene enhanced Chl biosynthesis of M. halliana. Suc positively regulated the responses to Fe deficiency in M. halliana via Chl biosynthesis. Thus, PPOX is the key regulatory gene of M. halliana in response to Fe deficiency. Exogenous Suc application on apple seedlings could ameliorate the adverse effects caused by Fe deficiency. In future work, cloning and functional characterization of the key genes for Chl biosynthesis of M. halliana in response to Fe deficiency will be performed.
Supporting information
(XLSX)
Abbreviations
- Fe
Iron
- M. halliana
Malus Halliana
- M. hupehensis
Malus hupehensis
- Chl
Chlorophyll
- Suc
Sucrose
- ALA
δ-aminolaevulinic acid
- PBG
Porphobilinogen
- URO III
Uroorphyinogen III
- Proto IX
Protoporphyrin IX
- Mg-Proto IX
Mg-protoporphyrin IX
- Pchlide
Protochlorophyllide
- ALAD
δ- aminolevulinate acid dehydratase
- PBGD
Porphobilinogen deaminase
- UROS
Uroporphyrinogen III synthase
- UROD
Uroporphyrinogen decarboxylase
- PPOX
Protoporphyrinogen IX oxidase
- MgCh
Magnesium chelatase
- MgPMT
Magnesium-protoporphyrin IX methyltransferase
- MPE
Mg-protoporphyrin IX monomethyl ester cyclase
- RT-qPCR
Quantitative Real-time PCR
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by Gansu Agricultural University Youth Postgraduate Tutor Support Fund Project (project No. GAU-2NDS-201710).
References
- 1.Zargar SM, Agrawal GK, Rakwal R, Fukao Y. Quantitative proteomics reveals role of sugar in decreasing photosynthetic activity due to Fe deficiency. Frontiers in Plant Science. 2015;6:592 10.3389/fpls.2015.00592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh HV, Matrone G, Evans HJ. Investigations of role of iron in chlorophyll metabolism .2. effect of iron deficiency on chlorophyll synthesis. Plant Physiology. 1963;38(6):632–642. 10.1104/pp.38.6.632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rombola AD, Tagliavini M. Iron nutrition of fruit tree crops. Iron Nutrition in Plants and Rhizospheric Microorganisms. 2006;61–83. 10.1007/1-4020-4743-6_3 [DOI] [Google Scholar]
- 4.Wang YX, Hu Y, Zhu YF, Baloch AW, Jia XM, Guo AX. Transcriptional and physiological analyses of short-term Iron deficiency response in apple seedlings provide insight into the regulation involved in photosynthesis. BMC Genomics. 2018;19(1):461 10.1186/s12864-018-4846-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan S, Liu ZL, Liu WJ, Lei T, Luo MH, Du JB, et al. A chlorophyll-less barley mutant "NYB" is insensitive to water stress. Zeitschrift Fur Naturforschung C-a Journal of Biosciences. 2007;62(5–6):403–409. 10.1515/znc-2007-5-614 [DOI] [PubMed] [Google Scholar]
- 6.Li ZY, Mo WP, Jia LQ, Xu YC, Tang WJ, Yang WQ, et al. Rice fluorescent1 is involved in the regulation of chlorophyll. Plant and Cell Physiology. 2019;60(10): 2307–2318. 10.1093/pcp/pcz129 [DOI] [PubMed] [Google Scholar]
- 7.Jin XQ, Liu T, Xu JJ, Gao ZX, Hu XH. Exogenous GABA enhances muskmelon tolerance to salinity-alkalinity stress by regulating redox balance and chlorophyll biosynthesis. BMC Plant Biol. 2019;19:48 10.1186/s12870-019-1660-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He N, Wang XY, Cao LZ, Cao DW, Luo Y, Jiang LZ, et al. Effects of photoperiods and temperatures on physiological characteristics and chlorophyll synthesis precursors of adzuki bean seedlings. Acta Agronomica Sinica. 2019;45(3):460–468. 10.3724/SP.J.1006.2019.84002 [DOI] [Google Scholar]
- 9.Li JM, Hu LP, Zhang L, Pan XB, Hu XH. Exogenous spermidine is enhancing tomato tolerance to salinity-alkalinity stress by regulating chloroplast antioxidant system and chlorophyll metabolism. BMC Plant Biol. 2015;15:303 10.1186/s12870-015-0699-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang K, Liu ZY, Shan XF, Li CY, Tang XY, Chi MY, et al. Physiological properties and chlorophyll biosynthesis in a Pak-choi (Brassica rapa L. ssp chinensis) yellow leaf mutant, pylm. Acta Physiologiae Plantarum. 2017;39(1):22 10.1007/s11738-016-2321-5 [DOI] [Google Scholar]
- 11.Fan XX, Zang J, Xu ZG, Guo SR, Jiao XL, Liu XY, et al. Effects of different light quality on growth, chlorophyll concentration and chlorophyll biosynthesis precursors of non-heading Chinese cabbage (Brassica campestris L.). Acta Physiologiae Plantarum. 2013;35(9):2721–2726. 10.1007/s11738-013-1304-z [DOI] [Google Scholar]
- 12.Buoso S, Pagliari L, Musetti R, Martini M, Marroni F, Schmidt W, et al. 'Candidatus Phytoplasma solani' interferes with the distribution and uptake of iron in tomato. Bmc Genomics. 2019;20(1):703 10.1186/s12864-019-6062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HM, Wang YM, Yang HL, Zeng QY, Liu YJ. NRAMP1 promotes iron uptake at the late stage of iron deficiency in poplars. Tree Physiology. 2019;39(7): 1235–1250. 10.1093/treephys/tpz055 [DOI] [PubMed] [Google Scholar]
- 14.Spiller SC, Castelfranco AM, Castelfranco PA. Effects of iron and oxygen on chlorophyll biosynthesis .1. invivo observations on iron and oxygen-defi-cient plants. Plant Physiology. 1982;69(1):107–111. 10.1104/pp.69.1.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia HF, Wang YH, Sun MZ, Li BB, Han Y, Zhao YX, et al. Sucrose functions as a signal involved in the regulation of strawberry fruit development and ripening. New Phytologist. 2013;198(2):453–465. 10.1111/nph.12176 [DOI] [PubMed] [Google Scholar]
- 16.Lilley JLS, Gee CW, Sairanen I, Ljung K, Nemhauser JL. An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiology. 2012;160(4): 2261–2270. 10.1104/pp.112.205575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohto M, Onai K, Furukawa Y, Aoki E, Araki T, Nakamura K. Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiology. 2001;127(1): 252–261. 10.1104/pp.127.1.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruan YL. Sucrose metabolism: gateway to diverse carbon use and sugar si-gnaling. Annual Review of Plant Biology. 2014;65:33–67. 10.1146/annurev-arplant-050213-040251 [DOI] [PubMed] [Google Scholar]
- 19.Hammond JP, White PJ. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. Journal of experimental botany. 2008;59(1):93–109. 10.1093/jxb/erm221 [DOI] [PubMed] [Google Scholar]
- 20.Hermans C, Hammond JP, White PJ, Verbruggen N. How do plants respond to nutrient shortage by biomass allocation?. Trends in Plant Science. 2006;11(12): 610–617. 10.1016/j.tplants.2006.10.007 [DOI] [PubMed] [Google Scholar]
- 21.Feng G, Zhang FS, Li XL, Tian CY, Tang C, Rengel Z. Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots. Mycorrhiza. 2002;12(4):185–190. 10.1007/s00572-002-0170-0 [DOI] [PubMed] [Google Scholar]
- 22.Lin XY, Ye YQ, Fan SK, Jin CW, Zheng SJ. Increased sucrose accumulation regulates iron-deficiency responses by promoting auxin signaling in Ara-bidopsis plants. Plant Physiology. 2016;170(2):907–920. 10.1104/pp.15.01598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong JL, Wang HC, Tan XY, Zhang CL, Naeem MS. 5-aminolevulinic acid improves salt tolerance mediated by regulation of tetrapyrrole and proline metabolism in Brassica napus L. seedlings under NaCl stress. Plant Physi-ology and Biochemistry. 2018;124:88–99. 10.1016/j.plaphy.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 24.Zhou M, Gong XL, Ying W, Chao L, Hong MM, Wang L, et al. Cerium relieves the inhibition of chlorophyll biosynthesis of maize caused by magn-esium deficiency. Biological Trace Element Research. 2011;143(1):468–477. 10.1007/s12011-010-8830-y [DOI] [PubMed] [Google Scholar]
- 25.Wu ZM, Zhang X, He B, Diao L, Sheng SL, Wang JL, et al. A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiology. 2007;145(1):29–40. 10.1104/pp.107.100321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Gong ZY, Yang ZF, Yuan Y, Zhu JY, Wang M, et al. Mutation of the light-induced yellow leaf 1 gene, which encodes a geranylgeranyl reductase, affects chlorophyll biosynthesis and light sensitivity in rice. PLoS ONE. 2013;8(9): e75299 10.1371/journal.pone.0075299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Cheng ZJ, Ma XD, Wu H, Liu YL, Zhou KN, et al. A knockdown mutation of yellow-green leaf2 blocks chlorophyll biosynthesis in rice. Plant Cell Rep. 2013;32(12):1855–1867. 10.1007/s00299-013-1498-y [DOI] [PubMed] [Google Scholar]
- 28.Mueller AH, Dockter C, Gough SP, Lundqvist U, von Wettstein D, Hansso-n M. Characterization of mutations in barley fch2 encoding chlorophyllide a oxygenase. Plant and Cell Physiology. 2012;53(7):1232–1246. 10.1093/pcp/pcs062 [DOI] [PubMed] [Google Scholar]
- 29.Han ZH, Wang Q, Shen T. Comparison of some physiological and bioche-mical characteristics between iron-efficient and iron-inefficient species in the genus Malus. Journal of Plant Nutrition. 1994;17(7):1257–1264. 10.1080/01904169409364803 [DOI] [Google Scholar]
- 30.Hu Y, Zhu YF, Guo AX, Jia XM, Cheng L, Zhao T, et al. Transcriptome analysis in Malus halliana roots in response to iron deficiency reveals insi-ght into sugar regulation. Molecular Genetics and Genomics. 2018; 293(6): 1523–1534. 10.1007/s00438-018-1479-5 [DOI] [PubMed] [Google Scholar]
- 31.Morton RA. Biochemical Spectroscopy. A. Hilger; 1975. [Google Scholar]
- 32.Bogorad L. Porphyrin synthesis .1. uroporphyrinogen-I synthetase. Methods in Enzymology. 1962;5:885–895. 10.1016/S0076-6879(62)05334-3 [DOI] [Google Scholar]
- 33.Hodgins RR,Vanhuystee RB. Rapid Simultaneous estimation of protoporphyrin and Mg-porphyrins in higher-plants. Journal of Plant Physiology. 1986;125(3–4):311–323. 10.1016/S0176-1617(86)80153-5 [DOI] [Google Scholar]
- 34.Israelstam GF. Anomalous growth response of bean plants in iron-deficient media to cyanide. Nature. 1968;218(5139):390–391. 10.1038/218390a0 [DOI] [Google Scholar]
- 35.Yadavalli V, Jolley CC, Malleda C, Thangaraj B, Fromme P, Subramanyam R. Alteration of proteins and pigments influence the function of photosystem I under iron deficiency from chlamydomonas reinhardtii. PLoS ONE. 2012;7(4):e35084 10.1371/journal.pone.0035084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen XB, Sun J, Guo SR, Gao P, Du J. Chlorophyll metabolism of spinach leaves under seawater stress. Acta Botanica Boreali-Occidentalia Sinica. 2012;32(9): 1781–1787. [Google Scholar]
- 37.Wang XL. Research advances about effects of enhanced UV-B radiation on plants and ecosystems. Acta Botanica Boreali-Occidentalla Sinica. 2002;22(3):670–681. 10.1088/1009-1963/11/5/313 [DOI] [Google Scholar]
- 38.Wasli H, Jelali N, Silva AMS, Ksouri R, Cardoso SM. Variation of polyphenolic composition, antioxidants and physiological characteristics of dill (An-ethum graveolens L.) as affected by bicarbonate-induced iron deficiency conditions. Industrial Crops and Products. 2018;126:466–476. 10.1016/j.indcrop.2018.10.007 [DOI] [Google Scholar]
- 39.Duke SO, Lydon J, Becerril JM, Sherman TD, Lehnen LP, Matsumoto H. Protoporphyrinogen oxidase-inhibiting herbicides. Weed Science. 1991;39(3): 465–473. 10.1017/S0043174500073239 [DOI] [Google Scholar]
- 40.Hao GF, Zhu XL, Ji FQ, Zhang L, Yang GF, Zhan CG. Understanding the mechanism of drug resistance due to a codon deletion in protoporphyrinogen oxidase through computational modeling. Journal of Physical Chemistry B. 2009; 113(14):4865–4875. 10.1021/jp807442n [DOI] [PubMed] [Google Scholar]
- 41.Cheng YD, Liu LQ, Zhao GQ, Shen CG, Yan HB, Guan JF, et al. The effects of modified atmosphere packaging on core browning and the expression patterns of PPO and PAL genes in 'Yali' pears during cold storage. Lwt-Food Science and Technology. 2015;60(2):1243–1248. 10.1016/j.lwt.2014.09.005 [DOI] [Google Scholar]
- 42.Apaliya MT, Zhang HY, Yang QY, Zheng XF, Zhao LN, Kwaw E, et al. Hanseniaspora uvarum enhanced with trehalose induced defense-related enzy-me activities and relative genes expression levels against Aspergillus tubingensis in table. Postharvest Biology and Technology. 2017;132:162–170. 10.1016/j.postharvbio.2017.06.008 [DOI] [Google Scholar]
- 43.Koch K. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Current Opinion in Plant Biology. 2004;7(3):235–246. 10.1016/j.pbi.2004.03.014 [DOI] [PubMed] [Google Scholar]
- 44.Siebeneichler TJ, Crizel RL, Camozatto GH, Paim BT, Messias RD, Rombaldi CV, et al. The postharvest ripening of strawberry fruits induced by abscisic acid and sucrose differs from their in vivo ripening. Food chemistry. 2020;317:126407 10.1016/j.foodchem.2020.126407 [DOI] [PubMed] [Google Scholar]
- 45.Zhang XY, Jia XM, Zhang R, Zhu ZL, Liu B, Gao LY, et al. Metabolic analysis in Malus halliana leaves in response to iron deficiency. Scientia Horticulturae. 2019; 258:108792 10.1016/j.scienta.2019.108792 [DOI] [Google Scholar]
- 46.Breinig S, Kervinen J, Stith L, Wasson AS, Fairman R, Wlodawer A, et al. Control of tetrapyrrole biosynthesis by alternate quaternary forms of porp-hobilinogen synthase. Nature Structural Biology. 2003;10(9):757–763. 10.1038/nsb963 [DOI] [PubMed] [Google Scholar]
- 47.Jaffe EK, Ali S, Mitchell LW, Taylor KM, Volin M, Markham GD. Characterization of the role of the stimulatory magnesium of Escherichia coli por-phobilinogen synthase. Biochemistry. 1995;34(1):244–251. 10.1021/bi00001a029 [DOI] [PubMed] [Google Scholar]
- 48.Meinecke L, Alawady A, Schroda M, Willows R, Kobayashi MC, Niyogi KK, et al. Chlorophyll-deficient mutants of chlamydomonas reinhardtii that accumulate magnesium protoporphyrin IX. Plant Molecular Biology. 2010;72(6):643–658. 10.1007/s11103-010-9604-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, et al. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science. 2003;300(5617):332–336. 10.1126/science.1080585 [DOI] [PubMed] [Google Scholar]
- 50.Rolland F, Baena-Gonzalez E, Sheen J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annual Review of Plant Biology. 2006;57: 675–709. 10.1146/annurev.arplant.57.032905.105441 [DOI] [PubMed] [Google Scholar]
- 51.Rosa M, Prado C, Podazza G, Interdonato R, Gonzalez JA, Hilal M, et al. Soluble sugars—metabolism, sensing and abiotic stress: a complex network in the life of plants. Plant signaling & behavior. 2009;4(5):388–393. 10.4161/psb.4.5.8294 [DOI] [PMC free article] [PubMed] [Google Scholar]