Abstract
The implementation of radiotherapy in the multimodal treatment of advanced head and neck cancer has greatly improved survival rates. In some patients, however, this benefit comes at the potential expense of the tissue surrounding the primary site of malignancy. Osteoradionecrosis (ORN) of the facial bones, in particular the maxilla, is a debilitating complication of radiation therapy. Exposure to ionizing radiation results in devitalization of underlying bone with necrosis of adjacent soft tissue. Controversy surrounding appropriate early intervention in ORN persists and no consensus for clinical treatment has been established. In the present article, we review the pathophysiology of maxillary ORN and discuss the role of both conservative medical therapy and reconstruction.
Keywords: maxillary osteoradionecrosis, maxillary osteonecrosis, management of osteoradionecrosis, free flap reconstruction
Osteoradionecrosis (ORN) can be both a potentially debilitating and disfiguring sequela of radiation therapy (RT) in the treatment of malignancies within the head and neck. Exposure to ionizing radiation results in devitalization of the facial skeleton and necrosis of the overlying soft tissue envelope. 1 2 Incidence of ORN varies within the primary literature, with reported rates of up to 15%, the majority of which occur within the first 3 years following exposure to RT. 2 3 There is no consensus regarding the temporal relation between exposure to RT and subsequent signs of ORN. Clinical reports describe this phenomenon occurring within months to decades following radiotherapy. 1 4
Anatomically, the head and neck is particularly susceptible to ORN. The mandible appears to be the most commonly affected osseous structure. This is postulated to be secondary to the relatively poor vascularity within this region as well as local factors including thin mucosal soft tissue coverage, added mechanical stress and remodeling within this region due to forces of mastication, and concomitant dental or periodontal disease.
Although it displays higher porosity, facilitating increased vascularity, the maxilla is the second most commonly affected region within the head and neck. 5 6 Several risk factors are believed to potentiate the development of ORN including radiation dose (> 60 Gy), irradiated bone volume, radiation modality, utilization of concomitant chemotherapy, tumor burden, dental extraction, prior infection, poor oral hygiene, malnutrition, and alcohol or tobacco abuse. Comorbidities such as hypertension, diabetes, and connective tissue disorders appear to increase risk of ORN as well, though their exact mechanism is unknown. 3 4 7 8
ORN may present with a wide range of clinical symptoms, depending on the primary site of involvement. Patients may experience localized pain, dysesthesia, bone exposure, sinocutaneous or orocutaneous fistula, trismus, and pathological fractures. 9 Clinically, the diagnosis of ORN is characterized by the exposure of previously irradiated bone with an inability to heal within at least 3 months. Notably, this must be independent of recurrent malignancy. 10 11 Although diagnosis is clinically based, radiographic imaging may further elucidate underlying pathology in equivocal cases or facilitate early identification when a patient's symptomology remains nonspecific. In cases in which there is high suspicion for ORN, imaging either in the form of computed tomography, magnetic resonance, or scintigraphy may further delineate the extent of disease. Histology is often nonspecific but may show endarteritis, hypovascularity, hyalinization, thrombosis, and generalized fibrotic change. 9 11 12
Despite recent advances in treatment and a greater understanding of the molecular pathophysiology of ORN, prevention remains of utmost importance. No clear consensus regarding an optimal treatment paradigm has been determined to date. Identification and intervention in the form of antibiotics, pharmaceuticals, hyperbaric oxygen, and sequestrectomy have shown greatest efficacy when implemented in early stages of disease. 13 Nevertheless, evidence regarding the true utility of conservative measures in management of ORN remains limited. This may be further confounded by potential bias in the literature introduced by the increased identification of early, less aggressive disease, due to growing application of intensity-modulated radiotherapy (IMRT) and local prophylactic measures. 9 10 11 12 13 However, in the presence of late findings including osseous necrosis, soft-tissue deficit, and cutaneous fistulization radical debridement is necessary. Reconstruction following extirpation of all nonviable tissue is often best achieved with microvascular free tissue transfer. 14 15 16 17
With this in mind, the purpose of this review is to review the pathophysiology, conservative management, and reconstruction of ORN within the maxilla, with a main interest in addressing the challenging reconstructive dilemmas presented within this region and unique to this pathology.
Pathophysiology of Osteoradionecrosis
There are currently two predominant contemporary theories for the pathophysiology of ORN. Marx 9 “3 H's paradigm” postulates that exposure to ionizing radiation and resultant periarteritis, endarteritis, hyperemia, fibrosis, and microthrombosis interfere with tissue homeostasis. This physiologic cascade in turn results in decreased tissue perfusion and reduced oxygen diffusion resulting ultimately in cellular apoptosis. 9 18 19 This theory provides the foundation for the use of hyperbaric therapy in ORN.
The radiation-induced fibroatrophic (RIF) theory suggests that the progression of ORN is secondary to the deregulation of fibroblast activity resulting in tissue atrophy. 19 Since its introduction, the RIF theory has gained broad recognition and has lead to the establishment of antioxidant and antifibrotic therapeutic protocols in the treatment of ORN. 19 20 21 Overall, the unifying mechanism underlying ORN continues to be unclear. However, both of the above mechanisms likely play a complementary role in the pathophysiologic process ( Fig. 1 ).
Fig. 1.
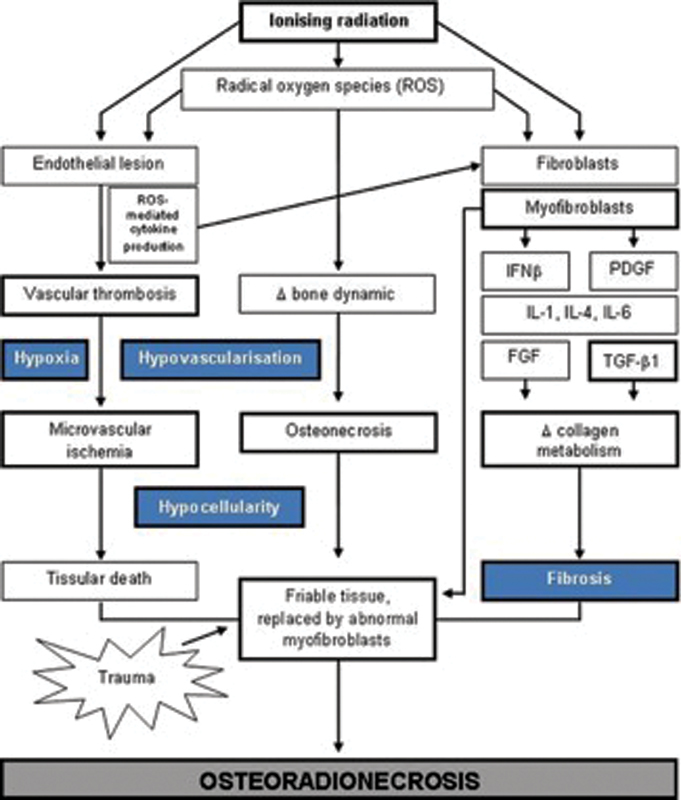
Pathophysiologic mechanisms in osteoradionecrosis (ORN). (Adapted with permission from Costa et al. 21 )
Medical Management
Preventive Measures
Given the aforementioned inciting factors in ORN, the primary reductive component may be the prevention of localized tissue trauma, including dental extraction or implantation procedures. Detailed dental examination should be stressed, addressing any areas of carious disease that may later serve as oral septic foci, prior to implementation of RT. 22 Dental extraction is best performed at least 3 to 4 weeks prior to RT. 1 23 24 Adequate oral hygiene and risk reduction including abstinence from alcohol and tobacco are crucial in decreasing the risk of ORN. 23 25 Although a known risk factor for osteonecrosis, 26 there is limited data suggesting steroid use may conversely serve a protective function in ORN. 23 27
IMRT has demonstrated a lower incidence of ORN when compared with conventional RT. This is postulated to be secondary to its ability to provide more precise radiation dosage to areas of malignancy with concomitant radiation reduction to surrounding tissue. 28 Volume reduction of areas exposed to greater than 50 to 60 Gy may help further decrease the incidence of ORN. 29 30
Hyperbaric Oxygen Therapy
Hyperbaric oxygen therapy (HBOT) is based upon inhalational exposure to 100% O 2 within an environment in which the atmospheric pressure is greater than that at sea level (1 atmosphere absolute). Sessions, or “dives,” are performed within a hermetically sealed hyperbaric chamber. Within this hyperbaric environment, oxygen supply is preferentially shunted through selective vasoconstriction of hyperoxic tissues and redistribution of oxygen to hypoxic tissues (the Robin Hood effect). 31 Furthermore, HBOT prompts fibroblast proliferation with a resultant increase in collagen deposition, increases angiogenesis, and prevents infection via both bactericidal and bacteriostatic mechanisms. 11 However, the current body of literature, examining the role of HBOT in treatment of ORN, is limited due to the heterogeneity of study design and variability in employed regimens and resulting outcomes. 32 33
Interested readers are referred to the Cochrane meta-analysis which further delineates the limited objective data reviewing the role of HBOT in ORN and justifies its use in select patients. 34 Currently, institutional guidelines reference the paucity of data, particularly prospective studies and overall expense of treatment, in avoiding its routine use except in high-risk patients with ORN refractory to medical and procedural intervention. 35
Less is known regarding the role of HBOT in maxillary ORN as it is underreported due to its more benign clinical course, in contradistinction to mandibular ORN. 24 To date, only two studies have evaluated the utility of HBOT in maxillary ORN. Both are limited by their retrospective nature, heterogeneity of patient population, and small sample size. However, decreased treatment failure was reported with use of HBOT indicating that there may be treatment benefit within the maxilla. 36 37 Presently, several studies are evaluating the role of HBOT in conjunction with multimodal medical and staged treatment protocols for ORN. 38
Medicative Treatment Modalities
Antioxidant and antifibrotic pharmaceutical agents have become increasingly implemented in the treatment of ORN as the RIF theory has garnered increased support. These drugs include tocopherol (TCP), pentoxifylline (PTX), and clodronate (CLO). 19 PTX is a methylxanthine derivative that is classified as a hemorrheologic agent, reducing blood viscosity through modulation of erythrocyte deformability and vasodilation thereby mitigating the risk for microthrombus formation. PTX has demonstrated inhibitory function of fibroblast proliferation, extracellular matrix production, and has anti-inflammatory properties. 18 20 39 TCPs have vitamin E activity and are therefore liposoluble antioxidants. Due to this capacity as a free radical scavenger, TCPs function to mitigate the risk of cell membrane damage through prevention of lipid peroxidation. 19 CLO, a first generation bisphosphonate, decreases osteoclastic activity thereby inhibiting bone resorption while activating osteoblasts and increasing osteosynthesis. 40 The above medications have not shown efficacy in treatment of ORN when used in isolation and their individual use is therefore not supported. Regimens employing a combination of these agents have, however, shown synergism in the treatment of ORN. 40 41 Treatment of advanced refractory ORN, following surgery and with combination therapy of PTX/TCP and CLO (PENTOCLO) has shown complete resolution within a 6- to 9-month interval. Notably, sequestrectomy was performed in the majority of these patients further accentuating the need for multimodal therapy in potentiating the healing process. 20 40 42 Although the preliminary evidence is promising, the long-term utility and side effects of PENTOCLO have yet to be determined in a randomized clinical trial.
Surgical Intervention
Despite the controversy surrounding treatment protocols in ORN, the majority of proposed treatment protocols is multimodal and accentuate the implementation of conservative measures in early-stage disease including antibiotics, debridement, or sequestrectomy. Surgical resection and reconstruction are used in the setting of severe progressive disease or in conservative treatment failure. Treatment regimens aim to avoid major debilitating surgical resections, which remain a last resort. 1 24 25 26 27
Microvascular Free Tissue Reconstruction
In the setting of advanced disease (i.e., pathologic fractures, bone exposure, or fistula formation), surgical resection is pivotal in disease control. The extent of resection is contingent on the establishment of viable tissue margins. Understandably, there is, therefore, a paucity of definitive objective clinical criteria in establishing adequate excision, likely contributing to the relatively high rates, approaching 25%, of recurrence. 43 Recurrent disease has been documented in cases with histopathologically confirmed resection margins suggesting that other contributing factors, aside from residual necrotic bone, may precipitate recurrence. 44 Due to the more indolent course of maxillary ORN, and poor surgical candidacy in afflicted patients, there remains a paucity of literature regarding clinically driven surgical decision making in maxillary ORN. 36 37 Consequently, the majority of treatment protocols are derived from literature specific to mandibular pathology. Following radical debridement and establishment of viable surrounding tissue, reconstruction is performed employing vascularized free tissue.
Reconstructive options are defect driven and therefore dependent on volume, tissue composition, and the degree of dead space. Defects with minimal osseous deficits may be reconstructed with myocutaneous flaps, such as radial forearm or anterolateral thigh. A segment of the cutaneous island can be de-epithelized to obliterate any potential dead space. Large osseous defects, particularly involving the periorbita, require composite flap reconstruction.
Goals specific to maxillary reconstruction include: separation of the oral and nasal cavities, restoration of palatal competency, reestablishment of orbital contour, obliteration of the orbital cavity in cases of exenteration, obliteration of the maxillary defect, and restoration of functional dentition and facial contour. 45 No uniform reconstructive approach has been described that achieves all the above goals. Maxillofacial prostheses may be implemented in obturation of maxillary defects. However, these are limited by need for extensive cleaning and maintenance, inadequate retention, loss of oronasal seal in cases of inadequate residual soft tissue and dentition, and nasal reflux. 46 A multimodal reconstructive effort employing prosthetics, local flaps, and free tissue transfer has been shown to optimize outcomes. 47 48 49
The use of soft tissue flaps with concomitant osseous grafts has been well documented in complex midfacial reconstruction. Cordeiro and Santamaria 50 described their use of rectus abdominis free flap with nonvascularized split calvarial or iliac crest bone grafts in composite defects. The bone grafts reconstitute the underlying skeletal framework and are draped with rectus muscle and subcutaneous fat facilitating midface contour. 50 Additional studies have illustrated the utility of nonvascularized bone grafts with myocutaneous latissimus dorsi or anterolateral thigh free flaps. 48 49 It is of paramount importance that bone grafts are enveloped in vascularized muscle or fascia with an adequate skin paddle to reline the oral cavity and provide a seal for the nasal wall, palate, and external cheek if needed.
Although nonvascularized bone grafts are extensively described within the literature, many authors contend that composite free flaps containing a vascularized osseous component remain the best option for single-stage maxillary and midface reconstruction when a considerable bony deficit is encountered. The osseocutaneous radial forearm free flap has been implemented in reconstruction of limited and subtotal maxillectomy defects. A “sandwich” technique in which the cutaneous paddle is draped over the radius, recreating the maxillary arch while relining both nasal and oral cavities, may be utilized. 51 However, the bone stock provided in this reconstructive technique is inadequate to support osseointegrated dental implants and patients therefore require dentures. 49 51
The osteocutaneous scapular system provides a reconstructive option with two separate bipedicled osseous flaps. The lateral scapular border retains its vascular supply from the circumflex scapular artery and maybe harvested with surrounding muscle and a large skin paddle. 52 53 A second scapular tip osseous flap can also be harvested from the angular artery, either from the serratus anterior branch or directly off of the thoracodorsal artery. 54 The scapular tip offers an ideal osseous conformation for maxillary reconstruction ( Fig. 2 ) and may be harvested with multiple skin paddles and the teres major muscle, facilitating reconstitution of soft tissue and lining of both oral and nasal cavities. 53 54 The osseous stock of this flap also allows for successful dental implantation. Additionally, the midsegment of the scapular body displays a curve similar to that of the orbital floor and may be utilized as a nonvascularized bone graft if additional reconstruction of the floor is required. Limitations to this flap are few, namely that it cannot be harvested in a two-team approach and requires patient positioning that adds difficulty to the initial ablative surgical component. 52 53 54
Fig. 2.
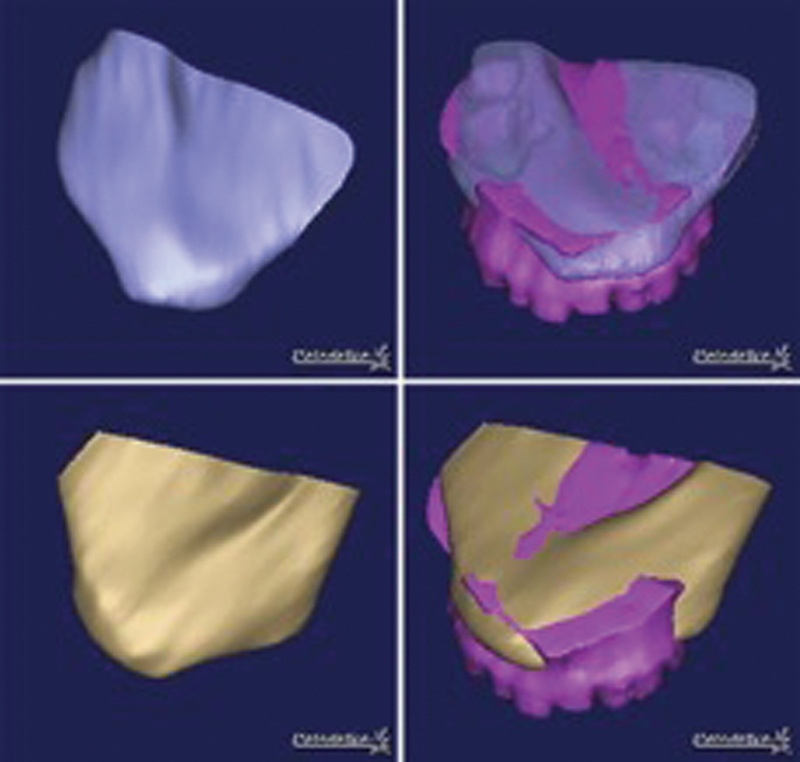
Computed tomography (CT) conformance studies showing similar contour of scapular tip with that of native palatal shape. Upper row: conformance of patient's left scapular tip. Lower row: conformance with right scapular tip. Mean reported conformance distance 2.04 mm indicating near perfect reconstructive contour. (Reproduced with permission from Shrime et al. 53 )
The myoosseous iliac crest free flap addresses many of the limitations, with respect to bone stock, of the previously mentioned flaps. It may be harvested with a significant volume of attached internal oblique muscle. Its orientation can be modified to reconstruct palatal, maxillary alveolar, or zygomaticomaxillary buttress defects ( Fig. 3 ). The associated muscular component may be implemented in palatal reconstruction or obliteration of the orbital cavity. 55 Although it provides significant bone stock for osseous reconstruction, limitations of this flap include its large bulk, limited muscle and cutaneous paddle mobility, and prohibitively short pedicle (4–5 cm).
Fig. 3.
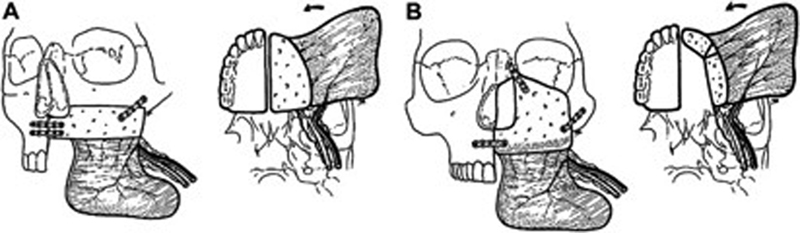
( A ) Reconstruction of inferior maxillectomy defect using iliac crest free flap oriented horizontally. ( B ) Reconstruction of “middle-height” maxillectomy defect implementing vertical orientation of iliac crest. Internal oblique rotated medially to close palatal defect. (Reproduced with permission from Brown. 60 )
The fibula free flap has long remained a workhorse in maxillofacial osseous reconstruction. This is particularly true within the midface where it provides more than adequate osseous volume required for palatal and midface structural support ( Fig. 4 ). Several studies have documented its osseous integrity, ease of flap harvest, large caliber pedicle, capacity for pedicle lengthening, and pliable skin paddle. 56 57 58 59 The available bone stock facilitates osseointegrated dental implantation allowing dental appliance retention and rehabilitation. 56 57 Although the fibular flap is an ideal candidate in reconstruction of the inferior maxilla, composite osseous defects involving the orbit and zygomaticomaxillary complex pose a reconstructive dilemma potentially requiring additional free tissue transfer or nonvascularized bone grafts.
Fig. 4.
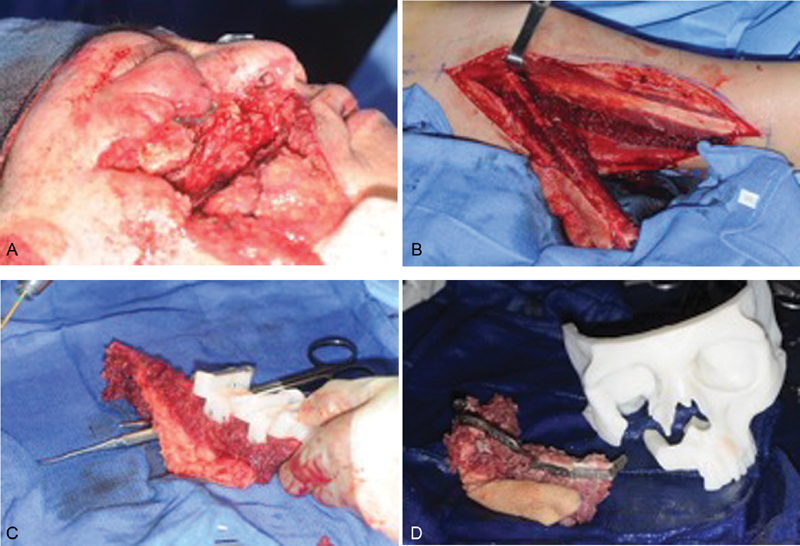
( A ) Left subtotal maxillectomy defect following ablation. ( B ) In situ right harvested fibula free flap. ( C ) Harvested fibular flap with cutting guide for segmented osteotomies. ( D ) Patient-specific three-dimensional (3D)-printed facial skeleton with fibula free flap following osteotomies and placement of reconstructive plate.
Conclusion
ORN, particularly when involving the maxilla, can be a potentially devastating complication following radiotherapy in head and neck cancer patients. Given its complex pathophysiology, no gold standard treatment modality or consensus guidelines have been established. A combination of therapeutic modalities should be implemented based upon the severity of the disease. Early-stage disease may be treated with local control, via sequestrectomy, antibiotics, and meticulous oral hygiene. However, early surgical intervention is recommended in the setting of disease progression. Although preliminary data are promising, the role of HBOT and medical therapeutic agents has yet to be delineated with progressive randomized trials, some of which are currently ongoing. Aggressive radical resection followed by free flap reconstruction should be reserved for advanced or refractory disease.
Footnotes
Conflicts of Interest None.
References
- 1.Reuther T, Schuster T, Mende U, Kübler A. Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients--a report of a thirty year retrospective review. Int J Oral Maxillofac Surg. 2003;32(03):289–295. doi: 10.1054/ijom.2002.0332. [DOI] [PubMed] [Google Scholar]
- 2.Thorn J J, Hansen H S, Specht L, Bastholt L.Osteoradionecrosis of the jaws: clinical characteristics and relation to the field of irradiation J Oral Maxillofac Surg 200058101088–1093., discussion 1093–1095 [DOI] [PubMed] [Google Scholar]
- 3.Vissink A, Burlage F R, Spijkervet F K, Jansma J, Coppes R P. Prevention and treatment of the consequences of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14(03):213–225. doi: 10.1177/154411130301400306. [DOI] [PubMed] [Google Scholar]
- 4.Jereczek-Fossa B A, Orecchia R. Radiotherapy-induced mandibular bone complications. Cancer Treat Rev. 2002;28(01):65–74. doi: 10.1053/ctrv.2002.0254. [DOI] [PubMed] [Google Scholar]
- 5.Delanian S, Lefaix J L. Mature bone radionecrosis: from recent physiopathological knowledge to an innovative therapeutic action [in French] Cancer Radiother. 2002;6(01):1–9. doi: 10.1016/s1278-3218(01)00142-1. [DOI] [PubMed] [Google Scholar]
- 6.Beadle B M, Liao K P, Chambers M S et al. Evaluating the impact of patient, tumor, and treatment characteristics on the development of jaw complications in patients treated for oral cancers: a SEER-Medicare analysis. Head Neck. 2013;35(11):1599–1605. doi: 10.1002/hed.23205. [DOI] [PubMed] [Google Scholar]
- 7.Rice N, Polyzois I, Ekanayake K, Omer O, Stassen L F. The management of osteoradionecrosis of the jaws--a review. Surgeon. 2015;13(02):101–109. doi: 10.1016/j.surge.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Raggio B S, Winters R. Modern management of osteoradionecrosis. Curr Opin Otolaryngol Head Neck Surg. 2018;26(04):254–259. doi: 10.1097/MOO.0000000000000459. [DOI] [PubMed] [Google Scholar]
- 9.Marx R E. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41(05):283–288. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 10.Buglione M, Cavagnini R, Di Rosario F et al. Oral toxicity management in head and neck cancer patients treated with chemotherapy and radiation: dental pathologies and osteoradionecrosis (Part 1) literature review and consensus statement. Crit Rev Oncol Hematol. 2016;97:131–142. doi: 10.1016/j.critrevonc.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Dhanda J, Pasquier D, Newman L, Shaw R. Current concepts in osteoradionecrosis after head and neck radiotherapy. Clin Oncol (R Coll Radiol) 2016;28(07):459–466. doi: 10.1016/j.clon.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Marx R E, Johnson R P. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Pathol. 1987;64(04):379–390. doi: 10.1016/0030-4220(87)90136-8. [DOI] [PubMed] [Google Scholar]
- 13.Hao S P, Chen H C, Wei F C, Chen C Y, Yeh A R, Su J L.Systematic management of osteoradionecrosis in the head and neck Laryngoscope 1999109081324–1327., discussion 1327–1328 [DOI] [PubMed] [Google Scholar]
- 14.Shaha A R, Cordeiro P G, Hidalgo D A et al. Resection and immediate microvascular reconstruction in the management of osteoradionecrosis of the mandible. Head Neck. 1997;19(05):406–411. doi: 10.1002/(sici)1097-0347(199708)19:5<406::aid-hed7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Chang Y M, Santamaria E, Wei F C et al. Primary insertion of osseointegrated dental implants into fibula osteoseptocutaneous free flap for mandible reconstruction. Plast Reconstr Surg. 1998;102(03):680–688. doi: 10.1097/00006534-199809030-00010. [DOI] [PubMed] [Google Scholar]
- 16.Celik N, Wei F C, Chen H C et al. Osteoradionecrosis of the mandible after oromandibular cancer surgery. Plast Reconstr Surg. 2002;109(06):1875–1881. doi: 10.1097/00006534-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Coskunfirat O K, Wei F C, Huang W C, Cheng M H, Yang W G, Chang Y M. Microvascular free tissue transfer for treatment of osteoradionecrosis of the maxilla. Plast Reconstr Surg. 2005;115(01):54–60. [PubMed] [Google Scholar]
- 18.Lyons A, Ghazali N. Osteoradionecrosis of the jaws: current understanding of its pathophysiology and treatment. Br J Oral Maxillofac Surg. 2008;46(08):653–660. doi: 10.1016/j.bjoms.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Delanian S, Lefaix J L. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004;73(02):119–131. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Lyons A J, Brennan P A. Pentoxifylline - a review of its use in osteoradionecrosis. Br J Oral Maxillofac Surg. 2017;55(03):230–234. doi: 10.1016/j.bjoms.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Costa D A, Costa T P, Netto E C et al. New perspectives on the conservative management of osteoradionecrosis of the mandible: a literature review. Head Neck. 2016;38(11):1708–1716. doi: 10.1002/hed.24495. [DOI] [PubMed] [Google Scholar]
- 22.Levi L E, Lalla R V. Dental treatment planning for the patient with oral cancer. Dent Clin North Am. 2018;62(01):121–130. doi: 10.1016/j.cden.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Wang T H, Liu C J, Chao T F, Chen T J, Hu Y W. Risk factors for and the role of dental extractions in osteoradionecrosis of the jaws: a national-based cohort study. Head Neck. 2017;39(07):1313–1321. doi: 10.1002/hed.24761. [DOI] [PubMed] [Google Scholar]
- 24.Nabil S, Samman N. Risk factors for osteoradionecrosis after head and neck radiation: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(01):54–69. doi: 10.1016/j.tripleo.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Rivero J A, Shamji O, Kolokythas A. Osteoradionecrosis: a review of pathophysiology, prevention and pharmacologic management using pentoxifylline, α-tocopherol, and clodronate. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(05):464–471. doi: 10.1016/j.oooo.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Powell C, Chang C, Naguwa S M, Cheema G, Gershwin M E. Steroid induced osteonecrosis: an analysis of steroid dosing risk. Autoimmun Rev. 2010;9(11):721–743. doi: 10.1016/j.autrev.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldwaser B R, Chuang S K, Kaban L B, August M. Risk factor assessment for the development of osteoradionecrosis. J Oral Maxillofac Surg. 2007;65(11):2311–2316. doi: 10.1016/j.joms.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Brennan P A, Bradley K L, Brands M. Intensity-modulated radiotherapy in head and neck cancer - an update for oral and maxillofacial surgeons. Br J Oral Maxillofac Surg. 2017;55(08):770–774. doi: 10.1016/j.bjoms.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Caparrotti F, Huang S H, Lu L et al. Osteoradionecrosis of the mandible in patients with oropharyngeal carcinoma treated with intensity-modulated radiotherapy. Cancer. 2017;123(19):3691–3700. doi: 10.1002/cncr.30803. [DOI] [PubMed] [Google Scholar]
- 30.De Felice F, Musio D, Tombolini V. Osteoradionecrosis and intensity modulated radiation therapy: an overview. Crit Rev Oncol Hematol. 2016;107:39–43. doi: 10.1016/j.critrevonc.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Butler F K, Jr, Hagan C, Murphy-Lavoie H. Hyperbaric oxygen therapy and the eye. Undersea Hyperb Med. 2008;35(05):333–387. [PubMed] [Google Scholar]
- 32.Dhanda J, Hall T J, Wilkins A, Mason V, Catling J. Patterns of treatment of osteoradionecrosis with hyperbaric oxygen therapy in the United Kingdom. Br J Oral Maxillofac Surg. 2009;47(03):210–213. doi: 10.1016/j.bjoms.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 33.Kanatas A N, Lowe D, Harrison J, Rogers S N. Survey of the use of hyperbaric oxygen by maxillofacial oncologists in the UK. Br J Oral Maxillofac Surg. 2005;43(03):219–225. doi: 10.1016/j.bjoms.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Bennett M H, Feldmeier J, Hampson N B, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2016;4(04):CD005005. doi: 10.1002/14651858.CD005005.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Sultan A, Hanna G J, Margalit D N et al. The use of hyperbaric oxygen for the prevention and management of osteoradionecrosis of the jaw: a Dana-Farber/Brigham and Women's Cancer Center Multidisciplinary guideline. Oncologist. 2017;22(03):343–350. doi: 10.1634/theoncologist.2016-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng S J, Lee J J, Ting L L et al. A clinical staging system and treatment guidelines for maxillary osteoradionecrosis in irradiated nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. 2006;64(01):90–97. doi: 10.1016/j.ijrobp.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Gavriel H, Eviatar E, Abu Eta R. Hyperbaric oxygen therapy for maxillary bone radiation-induced injury: a 15-year single-center experience. Head Neck. 2017;39(02):275–278. doi: 10.1002/hed.24577. [DOI] [PubMed] [Google Scholar]
- 38.Shaw R, Forner L, Butterworth C et al. Randomised controlled trials in HBO: “A call to arms” for HOPON & DAHANCA-21. Br J Oral Maxillofac Surg. 2011;49(01):76–77. doi: 10.1016/j.bjoms.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 39.Fan H, Kim S M, Cho Y J, Eo M Y, Lee S K, Woo K M. New approach for the treatment of osteoradionecrosis with pentoxifylline and tocopherol. Biomater Res. 2014;18(01):13. doi: 10.1186/2055-7124-18-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delanian S, Chatel C, Porcher R, Depondt J, Lefaix J L. Complete restoration of refractory mandibular osteoradionecrosis by prolonged treatment with a pentoxifylline-tocopherol-clodronate combination (PENTOCLO): a phase II trial. Int J Radiat Oncol Biol Phys. 2011;80(03):832–839. doi: 10.1016/j.ijrobp.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 41.Delanian S, Porcher R, Balla-Mekias S, Lefaix J L. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol. 2003;21(13):2545–2550. doi: 10.1200/JCO.2003.06.064. [DOI] [PubMed] [Google Scholar]
- 42.Delanian S, Depondt J, Lefaix J L. Major healing of refractory mandible osteoradionecrosis after treatment combining pentoxifylline and tocopherol: a phase II trial. Head Neck. 2005;27(02):114–123. doi: 10.1002/hed.20121. [DOI] [PubMed] [Google Scholar]
- 43.Suh J D, Blackwell K E, Sercarz J A et al. Disease relapse after segmental resection and free flap reconstruction for mandibular osteoradionecrosis. Otolaryngol Head Neck Surg. 2010;142(04):586–591. doi: 10.1016/j.otohns.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Zaghi S, Miller M, Blackwell K, Palla B, Lai C, Nabili V. Analysis of surgical margins in cases of mandibular osteoradionecrosis that progress despite extensive mandible resection and free tissue transfer. Am J Otolaryngol. 2012;33(05):576–580. doi: 10.1016/j.amjoto.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Futran N D. Primary reconstruction of the maxilla following maxillectomy with or without sacrifice of the orbit. J Oral Maxillofac Surg. 2005;63(12):1765–1769. doi: 10.1016/j.joms.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 46.Goiato M C, Pesqueira A A, Ramos da Silva C, Gennari Filho H, Micheline Dos Santos D. Patient satisfaction with maxillofacial prosthesis. Literature review. J Plast Reconstr Aesthet Surg. 2009;62(02):175–180. doi: 10.1016/j.bjps.2008.06.084. [DOI] [PubMed] [Google Scholar]
- 47.Futran N D. Improvement in the art of midface reconstruction. Curr Opin Otolaryngol Head Neck Surg. 2001;9:214–219. [Google Scholar]
- 48.Bianchi B, Ferri A, Ferrari S, Copelli C, Sesenna E. Maxillary reconstruction using anterolateral thigh flap and bone grafts. Microsurgery. 2009;29(06):430–436. doi: 10.1002/micr.20619. [DOI] [PubMed] [Google Scholar]
- 49.Coleman J J., III Osseous reconstruction of the midface and orbits. Clin Plast Surg. 1994;21(01):113–124. [PubMed] [Google Scholar]
- 50.Cordeiro P G, Santamaria E.A classification system and algorithm for reconstruction of maxillectomy and midfacial defects Plast Reconstr Surg 2000105072331–2346., discussion 2347–2348 [DOI] [PubMed] [Google Scholar]
- 51.Cordeiro P G, Bacilious N, Schantz S, Spiro R. The radial forearm osteocutaneous “sandwich” free flap for reconstruction of the bilateral subtotal maxillectomy defect. Ann Plast Surg. 1998;40(04):397–402. doi: 10.1097/00000637-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Swartz W M, Banis J C, Newton E D, Ramasastry S S, Jones N F, Acland R. The osteocutaneous scapular flap for mandibular and maxillary reconstruction. Plast Reconstr Surg. 1986;77(04):530–545. doi: 10.1097/00006534-198604000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Shrime M G, Gilbert R W. Reconstruction of the midface and maxilla. Facial Plast Surg Clin North Am. 2009;17(02):211–223. doi: 10.1016/j.fsc.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Coleman J J, III, Sultan M R. The bipedicled osteocutaneous scapula flap: a new subscapular system free flap. Plast Reconstr Surg. 1991;87(04):682–692. doi: 10.1097/00006534-199104000-00013. [DOI] [PubMed] [Google Scholar]
- 55.Maranzano M, Atzei A. The versatility of vascularized iliac crest with internal oblique muscle flap for composite upper maxillary reconstruction. Microsurgery. 2007;27(01):37–42. doi: 10.1002/micr.20307. [DOI] [PubMed] [Google Scholar]
- 56.Shokri T, Stahl L E, Kanekar S G, Goyal N. Osseous changes over time in free fibular flap reconstruction. Laryngoscope. 2019;129(05):1113–1116. doi: 10.1002/lary.27337. [DOI] [PubMed] [Google Scholar]
- 57.Futran N D, Wadsworth J T, Villaret D, Farwell D G. Midface reconstruction with the fibula free flap. Arch Otolaryngol Head Neck Surg. 2002;128(02):161–166. doi: 10.1001/archotol.128.2.161. [DOI] [PubMed] [Google Scholar]
- 58.Yim K K, Wei F C. Fibula osteoseptocutaneous free flap in maxillary reconstruction. Microsurgery. 1994;15(05):353–357. doi: 10.1002/micr.1920150513. [DOI] [PubMed] [Google Scholar]
- 59.Ducic Y, Defatta R, Wolfswinkel E M, Weathers W M, Hollier L H., Jr Tunneling technique for expedited fibula free tissue harvest. Craniomaxillofac Trauma Reconstr. 2013;6(04):233–236. doi: 10.1055/s-0033-1349208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown J S. Deep circumflex iliac artery free flap with internal oblique muscle as a new method of immediate reconstruction of maxillectomy defect. Head Neck. 1996;18(05):412–421. doi: 10.1002/(SICI)1097-0347(199609/10)18:5<412::AID-HED4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]


