Abstract
The midface is a complex anatomic structure that is fundamental to many physiologic and homeostatic functions. It may be involved in many pathologic processes that require partial or complete removal. When this happens, reconstruction is mandatory to improve cosmetic outcome with its effect on social interaction as well as to provide an opportunity for complete orodental rehabilitation with restoration of all physiologic functions. This article will review the different reconstructive options available for complex defects of the maxillofacial complex. It will highlight the surgical options available to maximize functional restoration. Finally, it will discuss computer modeling to optimize reconstructive planning.
Keywords: free tissue transfer, surgical reconstruction, maxilla, maxillofacial reconstruction, dental rehabilitation
The midface is a complex anatomic structure that is fundamental to many physiologic and homeostatic functions. The maxilla is the keystone of the facial skeleton supporting the overlying soft tissue of the face and contributing heavily to one's appearance and hence self-perception. Beyond its contribution to facial contour, the maxilla anchors the upper dentition, supports the orbital contents, bridges and transmits forces between the jaw and skull base, and serves as the anchor for many of the muscles of facial expression and mastication that permit nonverbal communication, functional speech, and deglutition. The two maxillae are the centerpiece in a complex articulation with nine bones. The cavity of the maxilla forms an irregularly shaped, six walled structure—the so-called hexahedron. Diseases that originate from the orbital contents, nasal cavity, palate, paranasal sinuses, oral cavity, and facial skin often require surgical resection that includes various degrees of maxillectomy. Surgical disruption of the maxilla requires a well-considered restoration to mitigate interruption of normal vision, speech, swallowing, hygiene, and self-image ( Fig. 1 ). The priorities for reconstruction depend on patient expectations and available resources but optimally include the following—a healed wound; separation of the oral, nasal, and intracranial cavities; restoration of the horizontal and vertical buttresses; replacement of dentition; functional mastication; orbital restoration; and resuspension of the facial soft tissue and contour. 1 2
Fig. 1.
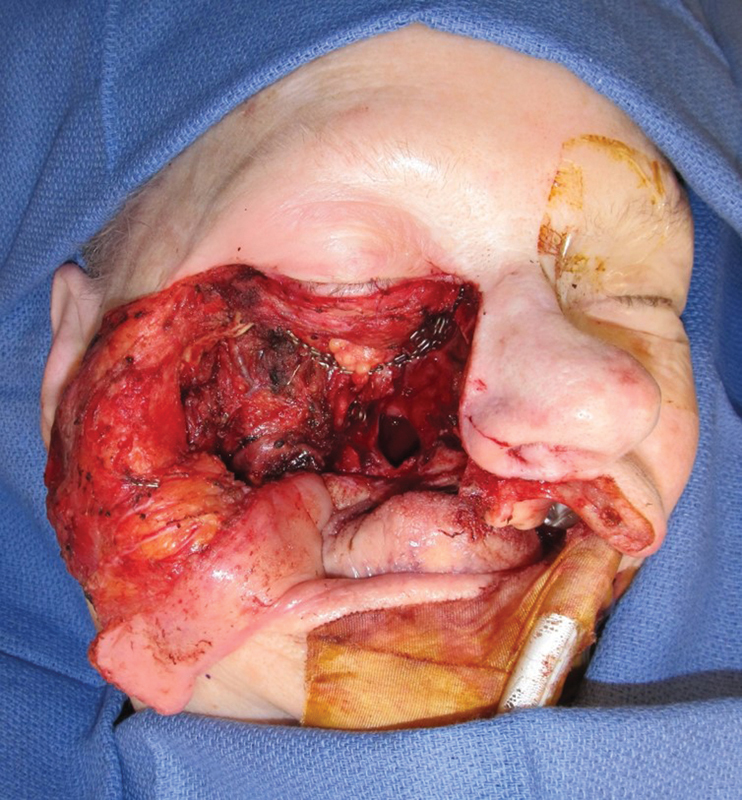
This photograph demonstrates a total maxillectomy defect.
Reconstruction of the midface and maxilla historically has been managed nonoperatively with a maxillary prosthesis, which can effectively restore the natural separation between the oral and nasal cavities necessary for speech and swallow. Dentition can be included with the prosthesis to improve appearance and to allow mastication. Prosthesis can be effective even in cases of complex defects that include the maxilla, nose, orbit, and eye, assuming there is sufficient soft tissue, native bone, and remnant dentition to serve as an anchor ( Fig. 2 ). The major advantage of a prosthesis is the shorter and less invasive surgical plan, which generally translates to a shorter recovery time with restoration of function. In terms of cancer surveillance, there is a theoretical advantage with prostheses in that it allows for direct visual surveillance; however, when advanced imaging is available, as is the case in the developed world, this potential benefit has not been proven and should not be a factor in the reconstructive algorithm. 3 4 While a maxillary prosthesis either alone or paired with soft tissue may be adequate, it is critical to understand the shortcomings of prostheses to predict when a prosthesis may not restore function as well as a composite free tissue transfer. First and foremost, prostheses require daily care and must be removed and cleaned regularly; this is more often an issue with the elderly or those with vision impairment, as it can pose a formidable challenge. Moreover, if the prosthesis does not fit perfectly, leakage around the prosthesis can be at best a nuisance and at worst contribute to deteriorating function. In addition, one should keep in mind that surgery is far more complicated as a secondary procedure versus surgical reconstruction with free tissue transfer at the time of the initial resection.
Fig. 2.
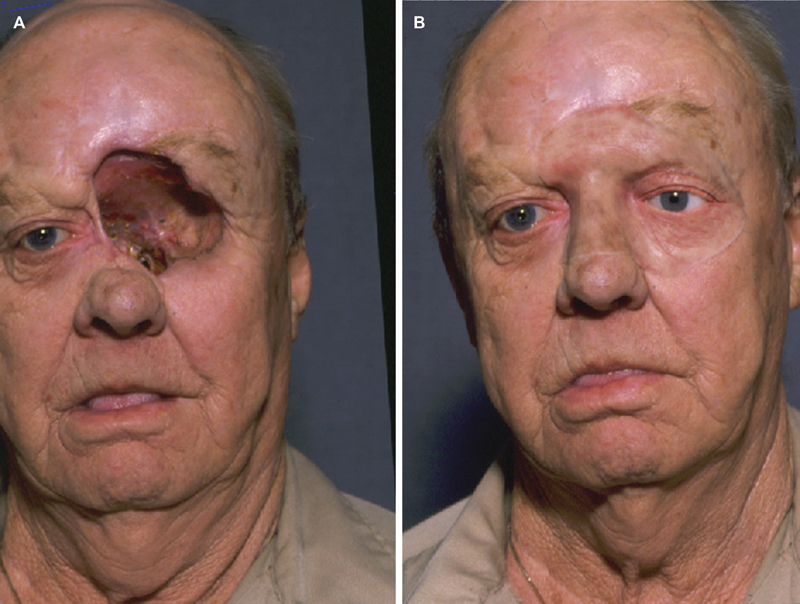
( A ) This patient has had a partial maxillectomy with orbital exenteration. ( B ) An extensive maxillofacial prosthesis demonstrates reconstruction with this technology.
In the 1960s and 1970s, the available surgical solutions to address maxillectomy defects included palatal and pharyngeal flaps for small defects and variants of the cervicofacial and myocutaneous flaps from the neck and chest for larger defects. The issues with these variants of the cervicopectoral flap were the inadequate restoration of function as well as poor aesthetic results. 5 6 7 With advancements in both technology and surgical technique in the 1980s, the consensus regarding extensive maxillary defects shifted to incorporate the many advantages both in function and aesthetics of a composite free tissue transfer at the time of the initial resection either in combination with or as an alternative to a prosthesis. 8 The question then remains whether soft tissue transfer or regional flap is the best choice for a given maxillectomy defect.
Because cancer of the midface and maxilla is not common and the resultant defects and their categorization vary in the literature, there is no generalized consensus or straightforward algorithm to dictate management. The optimal reconstruction ultimately relies on a nuanced evaluation of the functional and aesthetic defects in light of the available reconstructive expertise, patient preference, resource availability, patient expectations, and prognosis.
Surgical options to address maxillectomy defects include use of a pedicled flap, soft-tissue free flap, and composite osteocutaneous free flaps. In this discussion, we will concentrate on addressing the defect from a surgical perspective, although in many cases a prosthesis with or without dentures could be considered. To facilitate a logical discussion through which to review reconstructive options, one must choose and define a classification system to categorize the defect so as to allow a more generalized discussion of the reconstructive requirements to restore function and appearance.
Memorial Sloan-Kettering Cancer Center (MSKCC) proposed a classification system in 1997 which includes type I (limited maxillectomy), type II (subtotal maxillectomy), and type III (total maxillectomy). 9 The classification has since expanded to subdivide type III (total maxillectomy) into two parts—IIIa (orbital contents are spared) and IIIb (orbital contents exenterated). In its evolution, type IV defects were added (orbitomaxillectomy) to include resection of the orbital contents and the upper five walls of the maxilla with preservation of the palate. 10 This evolution took place as different reconstructive options became more widely available.
Brown and Shaw 11 proposed a classification of maxilla and midface defects in 2010, which slightly modifies the MSKCC framework. For the purposes of this review of surgical management of the palate and maxilla, we will review the classification system of Brown and Shaw and use it for the purposes of discussion.
Defect Definition
The most recent proposed categorization of maxillectomy defects was proposed by Brown and Shaw 11 in 2010 and is based on vertical and horizontal defects as follows. Vertical defects : (I) maxillectomy without oronasal fistula, (II) maxillectomy not involving orbit with oroantral and/or oronasal fistulae, (III) maxillectomy involving the orbital adnexa with orbital retention, (IV) maxillectomy with orbital enucleation or exenteration, (V) orbitomaxillary defect with intact palate, (VI) nasomaxillary defect. Horizontal defects : (a) palatal defect without altering dental alveolus, (b) unilateral palatal defect less than one-half width of palate, (c) palatal defect less than one-half width of palate and bilateral, or defect including transverse anterior dimension of hard palate, (d) defect greater than one-half width of palate. 11
Class I
A class I defect—the so-called limited maxillectomy—spares the soft palate and often leaves a small volume/large surface area defect. The state of the patient's residual dentition and whether the patient currently uses dentures should be considered. Alveolar implants can be placed in the remnant native bone, which can then support an implant-supported prosthesis, or alternatively conventional partial prosthesis anchored to remnant teeth when available.
In a review of surgical reconstruction of the maxilla, Brown and Shaw 11 compared their own experience (Liverpool group) between 1992 and 2009 including 147 patients to a compiled group of patients collected from the literature between 1998 and 2009 totaling 736 reconstructions. This review of literature revealed 30 of 736 (4.1%) of maxillectomies were categorized as class I defects—the second least common after class VI (nasoethmoid) defects. 11 Class I defects were most commonly reconstructed with a radial forearm free flap among the Liverpool group and the compiled group. 11 Similarly in a review by Cordeiro et al 12 of 60 patients between 1992 and 1998, five of seven patients with similar defects were reconstructed with a radial forearm free flap ( Fig. 3 ).
Fig. 3.

Reconstruction of a hard palate defect completed with a radial forearm free flap.
The radial forearm free flap is particularly advantageous for several reasons; the flap is very thin and pliable with a long pedicle which can be molded to fill virtually any low-volume, three-dimensional space. 12 If the oral commissure and/or upper lip are involved, a lip switch procedure is often combined with the free flap. 10 A temporalis muscle flap may be considered with class I defects particularly for patients who are not candidates for a free flap. In addition, the temporalis muscle can support a nonvascularized bone graft when the muscle is wrapped around the bone, although this scenario is more germane to class II to IV defects. 13 14
Class II
Class II defects result from a classic hemimaxillectomy or the so-called infrastructure maxillectomy. As mentioned, class II defects can be addressed well with obturation if surgery is not desired by the patient or is prohibited by health or other restraints. The requirements for surgical reconstruction include volume, surface area, and often bone to restore loss of anterior projection of the midface. Cordeiro et al described the “sandwich” free flap in 1998 with an osteocutaneous radial forearm free flap. 14 The “sandwich” flap uses radius bone to restore the alveolar arch with the skin paddle wrapped around the bone segment to provide palatal and nasal lining. The disadvantage of the osteocutaneous radial forearm free flap is insufficient bone stock to support osseointegrated implants, which can be important for restoration of maxillary dentition to optimize speech, swallowing, and appearance. 4 Double barreling of the radial forearm flap results in sufficient bone stock to support implants and should be considered ( Fig. 4 ).
Fig. 4.

( A ) A partial maxillectomy of the hard palate and infrastructure of the maxilla has resulted in this defect. ( B ) The defect repaired with a double barrel radial forearm flap to provide for dental rehabilitation and separation of the oral cavity and the sinuses.
The most commonly used composite flap for a class II defect is the fibula flap. 11 Alternatives to the fibula flap include osteocutaneous radial forearm flap, the iliac crest free flap with the internal oblique muscle, or the scapula with latissimus or serratus anterior. The disadvantage of these bony alternatives to the fibula is the low-volume bone which is typically not amenable to implantation and thus full dental rehabilitation is less likely.
In cases in which a free flap is not possible, a pedicled temporalis or temporoparietal flap can close the fistula of a class II defect but often would be paired with an obturator and/or a nonvascularized bone graft. Of note, a soft-tissue flap such as the anterior lateral thigh flap provides excellent bulk for a class II palate and alveolus defects, and if the canine remains, a sectional denture can be fashioned to restore the posterior dentition. The disadvantage of a soft-tissue flap without bone to reconstitute the anterior face of the maxilla is the gradual collapse of the midface over time if the patient does not first succumb to their disease. Class II defects were most commonly repaired in the literature review by Brown and Shaw 11 with either a radial forearm free flap or a fibula free flap. Interestingly, the Liverpool group favored the osteocutaneous radial forearm free flap and iliac crest free flap over the fibula. 11 In this sense, local expertise and surgeon preference can be determining factors.
Class III
A class III defect includes loss of the orbital floor as well as loss of the cheek and dental arch, and therefore a bulky flap is required ideally with bone sufficient to reconstitute both the orbital floor and the alveolus ( Fig. 5 ). If the orbital floor is not supported, the orbit will fall into the cheek resulting in diplopia and vision impairment. The orbital floor can be addressed with nonvascularized bone grafts provided it is contained between a healthy flap whether it be a temporalis flap or a free flap. 12 Historically, the rectus abdominis has been the free flap of choice because it provides the muscle coverage needed for free bone grafts as well as the bulk for an adequate cheek contour. In a review of 197 class III defects between 1998 and 2009, 58% were reconstituted with a soft tissue only flap—the most common being the rectus abdominis flap. 11 An alternate myocutaneous flap to the rectus flap is the latissimus dorsi free flap which has a longer pedicle and can successfully fill large cheek defects to seal the palate with bulk sufficient to restore cheek contour as well. The disadvantage of a free flap without bone for class III defects is the suboptimal dental rehabilitation and more often than not a less favorable cosmetic result.
Fig. 5.

This photograph demonstrates a class III maxillectomy with loss of the orbital floor.
While the fibula flap is a good choice to rebuild the maxillary arch, it is much more technically demanding to address both the alveolar arch simultaneously with an orbital floor defect with a fibula flap. To rebuild both the orbital floor and alveolar arch with a fibula, three segments with sharp angulations and multiple skin paddles are required 11 15 16 ( Fig. 6 ).
Fig. 6.

( A–C ) These three views of the postoperative CT scan demonstrate the reconstruction of the defect with a fibula flap. A segment of the fibula used to reconstruct the orbital rim, the maxillary buttress, and the inferior maxilla. An orbital plate used to support the adnexal contents.
Some authors have proposed dual free flaps for class III defects, perhaps a fibula for the maxillary arch and a latissimus, anterior lateral thigh, or rectus flap with mesh or bone graft for the orbital floor and cheek contour. 17 Others argue one well-designed free flap can, in most cases, provide both the bulk and bone stock required. 1 An alternative bone-based flap to the fibula to address a class III defect is the vascularized iliac crest with the internal oblique muscle; the iliac bone can be shaped to fit the defect and the muscle serves to close the oroantral and/or oronasal fistulae. Some authors prefer the iliac bone if there is a plan for implants, as it often provides thicker bone than that typically achieved with the scapula. 11 Moreover, nonvascularized iliac bone harvested concurrently can provide volume at the alveolus if there is a gap between the en bloc iliac bone and nasal bones. 11
An alternative to the iliac flap is the scapula flap with associated muscle, most often the latissimus dorsi. 18 The scapula flap has a longer pedicle than the iliac bone flap. The soft-tissue component of the scapula flap is unique in that it can rotate around the bone with more freedom than other osteocutaneous flaps. When the angular branch of the thoracodorsal artery is dissected separately from the subscapular artery, the scapular tip and lateral border of the scapula can move independently. In this fashion, the lateral aspect of the scapula tip can articulate with the zygomatic buttress which then allows the thickest portion of the scapula to reconstitute the alveolus. 19 20 An orbital floor reconstruction plate can support the eye with coverage from the latissimus dorsi and/or serratus anterior muscle, while the scapula bone can restore maxillary projection. Recent studies have shown that the scapula can more often than not accommodate dental implants and the optimal location for the implants in the scapula can be mapped with a preoperative computed tomography (CT) of the chest. 21
Class IV
Class IV defects include a total maxillectomy with orbital exenteration or enucleation. Reconstruction of the orbital floor is not required with this defect which makes it far less complex to reconstruct. These class IV defects are often large both with respect to required volume and surface area. Either an anterior lateral thigh free flap or rectus abdominis myocutaneous flap is a good choice with one skin island needed to close the palate—and a second, if available, can be used to close the lateral nasal wall. When the external skin of the cheek is intact, there is no need for a third skin island; however, the need for a third skin island often mandates a second, concurrent free tissue transfer. In cases in which the patient prognosis is poor, the possibility of dental implants should not be a factor. An alternative to a large myocutaneous flap is the scapula flap with latissimus dorsi which provides ample muscle to close the oronasal fistula with the scapula bone to reconstitute the palate if needed. The latissimus can be used to line the bony orbit and will contract sufficiently to support a prosthesis or to seal a cerebrospinal fluid leak should one have resulted from the surgery. 11
Class V
Class V defects are defined by orbital exenteration with an intact palate. The objective in this patient population is to prepare the orbit for a prosthesis; of note, a prosthesis can often camouflage any lateral orbital bony loss if present, obviating the need for a bony graft in cases of lateral orbital bone loss. In cases in which a prosthesis is not used, an osteocutaneous radial forearm free flap can reconstitute the lateral orbital wall. The most commonly used flaps for class V defects are temporalis flaps, rectus flaps, anterior lateral thigh flaps, and radial forearm free flaps. 11 A pedicled temporalis or temporoparietal flap is a good option when the patient is not a good candidate for a free flap. When there is significant skin loss associated with the defect, a radial forearm flap or anterior lateral thigh flap is a good option to fill in the orbital defect without excessive bulk. If the goal is to obliterate the orbit without a plan for a prosthesis, a rectus or latissimus muscle flap is a good option.
Class VI
Class VI defects—nasoethmoid defects—require reconstruction only if the nasal bone is removed—in which case some authors recommend an osteocutaneous radial forearm free flap ideally with a glabellar or paramedian forehead flap such that the skin of the free flap can line the nasal mucosa. Class VI defects are repaired most commonly with either a radial forearm free flap or an osteocutaneous radial forearm free flap. 11
Postoperative Function
In a review of 58 patients who underwent free flap reconstruction after maxillectomy, the following postoperative complications occurred: flap failure (1/58), partial flap failure (3/58), flap salvage surgery (5/58), wound infection/dehiscence (3/58), donor-site hematoma (1/58), neck hematoma/seroma (3/58), postoperative pneumonia (3/58), postoperative meningitis (1/58), and postoperative death (2/58). 1 Of the 56 patients alive 6 months after maxilla/palate reconstruction surgery, 37 tolerated a regular diet and 19 tolerated a soft diet. All of the patients had successful separation of the oral and nasal cavities and were able to speak well enough to be understood over the telephone at 6-month follow-up. Regarding dental restoration at 6 months, 9/56 had implant-borne prostheses, 30/56 had a conventional partial prosthesis, and 17/56 had no dental restoration. 1 Reconstruction of the maxilla can require significant pedicle length and the reconstructive surgeon should be prepared to harvest a vein graft. Vein grafts were required in 9/12 subtotal and total palate defects. 1 In a similar review of 27 patients who underwent a fibula flap for reconstruction of at least 50% of the tooth-bearing portion of the maxilla, 9/27 patients required a vein graft. At 6-month follow-up, 14/27 tolerated a regular diet and 13/27 tolerated a soft diet; moreover, all 27 patients had intelligible speech at 6 months during a telephone interview. 15
Three-Dimensional Modeling
When reconstruction of the maxilla requires a bone-based free flap as with class II, III, and IV defects, computer-assisted maxilla reconstruction (CAMR) is very helpful. CAMR involves the following steps: (1) virtual surgical planning, (2) design and fabrication of the cutting guides and reconstruction plate, (3) ablative surgery and osseous donor-site harvest, (4) reconstruction, and (5) rehabilitation. The process begins with a high-resolution CT scan of the face with 1 mm or less thickness as well as a CT angiography of the donor site. The DICOM (Digital Imaging and Communication in Medicine) data are sent to the modeling company and a three-dimensional rendering generated with the use of a virtual planning software. A web-based conference then takes place with the ablative and reconstructive surgeons, prosthodontist, and engineers from a third-party vendor so as to generate customized cutting jigs and reconstruction plates which then guide osteotomies and points of fixation. During the virtual surgery, the surgeons and biomedical engineers can discuss the delineations and angulations (typically 45 degrees to maximize bone contact) of the proposed resection/osteotomies with ample margins, laterality of the donor site, number of bony segments (each > 2.0 cm), plate location, number and location of screw holes, and shape/position of the cutting guides ( Fig. 7a, b ). When possible, the CT angiography may give the surgeon an idea of the skin perforators and an osteotomy should be avoided directly over a perforator. In practice, the largest amount of bone possible should be harvested to allow sufficient flexibility to position the cutting guides in the most favorable location relative to the skin paddle and associated skin perforator/s.
Fig. 7.

( A ) A three-dimensional rendering is used to plan the osteotomies for the ablation. ( B ) The fibula bone used to reconstruct the various bony buttresses. Osteotomies made virtually.
The cutting guides should take into account the size and location of the tumor such that the guides do not infringe upon the tumor. Screw holes in the maxilla cutting guides include temporary fixation points for the guide itself as well as fixation points for the laser pre-bent or milled plate. The cutting guides manage both the end cuts and the wedge osteotomies which are challenging to perform free hand ( Figs. 8 and 9 ). After the osteotomies are complete, the plate is then fixated to the donor bone segments in their proper orientation. At this point, the pedicle can be ligated and the plate can be fixated to the maxilla followed by insetting and/or vascular anastomosis. At times, some minor additional bone burring of either the native maxilla or donor bone is required. When possible, a high-resolution CT is obtained within 6 months after surgery and the data can be sent to the modeling company to measure and compare landmark points to keep records of accuracy and precision. 22
Fig. 8.

A cutting jig manufactured to guide the closing osteotomies and simplify the process of fitting the bone to the defect.
Fig. 9.

The result seen on the computer planning and fits in perfectly with the defect guided by the planning session.
Conclusion
Reconstruction of the maxilla ranges in complexity from simple to very complex, and for optimal restoration of function the expected defect should be reviewed carefully prior to the surgery to allow time for discussion of various reconstructive options and when available to utilize virtual surgical planning to minimize intraoperative improvisation, improve precision, and decrease surgical time. It is helpful to classify the maxilla defect using a familiar system for a more logical reconstructive analysis and then to incorporate as much as possible the nuances that are unique to each patient as well as hospital-specific idiosyncrasies. In addition, contingency plans should always be part of the initial reconstructive planning so as to be prepared when the need arises. And, as is the case with most surgeries, there is no substitute for experiential learning.
Footnotes
Conflict of Interest None declared.
References
- 1.Triana R J, Jr, Uglesic V, Virag M et al. Microvascular free flap reconstructive options in patients with partial and total maxillectomy defects. Arch Facial Plast Surg. 2000;2(02):91–101. doi: 10.1001/archfaci.2.2.91. [DOI] [PubMed] [Google Scholar]
- 2.Futran N D, Mendez E. Developments in reconstruction of midface and maxilla. Lancet Oncol. 2006;7(03):249–258. doi: 10.1016/S1470-2045(06)70616-7. [DOI] [PubMed] [Google Scholar]
- 3.Moreno M A, Skoracki R J, Hanna E Y, Hanasono M M. Microvascular free flap reconstruction versus palatal obturation for maxillectomy defects. Head Neck. 2010;32(07):860–868. doi: 10.1002/hed.21264. [DOI] [PubMed] [Google Scholar]
- 4.Futran N D, Haller J R. Considerations for free-flap reconstruction of the hard palate. Arch Otolaryngol Head Neck Surg. 1999;125(06):665–669. doi: 10.1001/archotol.125.6.665. [DOI] [PubMed] [Google Scholar]
- 5.Kroll S S, Reece G P, Robb G, Black J. Deep-plane cervicofacial rotation-advancement flap for reconstruction of large cheek defects. Plast Reconstr Surg. 1994;94(01):88–93. doi: 10.1097/00006534-199407000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Ariyan S. The pectoralis major myocutaneous flap. A versatile flap for reconstruction in the head and neck. Plast Reconstr Surg. 1979;63(01):73–81. doi: 10.1097/00006534-197901000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Strasnick B, Calcaterra T C.Reconstruction of full-thickness cheek defects with combined cervicopectoral and pectoralis major myocutaneous flaps Laryngoscope 198999(7, Pt 1):757–760. [DOI] [PubMed] [Google Scholar]
- 8.Okay D J, Genden E, Buchbinder D, Urken M. Prosthodontic guidelines for surgical reconstruction of the maxilla: a classification system of defects. J Prosthet Dent. 2001;86(04):352–363. doi: 10.1067/mpr.2001.119524. [DOI] [PubMed] [Google Scholar]
- 9.Spiro R H, Strong E W, Shah J P. Maxillectomy and its classification. Head Neck. 1997;19(04):309–314. doi: 10.1002/(sici)1097-0347(199707)19:4<309::aid-hed9>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Cordeiro P G, Santamaria E.A classification system and algorithm for reconstruction of maxillectomy and midfacial defects Plast Reconstr Surg 2000105072331–2346., discussion 2347–2348 [DOI] [PubMed] [Google Scholar]
- 11.Brown J S, Shaw R J. Reconstruction of the maxilla and midface: introducing a new classification. Lancet Oncol. 2010;11(10):1001–1008. doi: 10.1016/S1470-2045(10)70113-3. [DOI] [PubMed] [Google Scholar]
- 12.Cordeiro P G, Santamaria E, Kraus D H, Strong E W, Shah J P.Reconstruction of total maxillectomy defects with preservation of the orbital contents Plast Reconstr Surg 1998102061874–1884., discussion 1885–1887 [DOI] [PubMed] [Google Scholar]
- 13.Cordeiro P G, Wolfe S A. The temporalis muscle flap revisited on its centennial: advantages, newer uses, and disadvantages. Plast Reconstr Surg. 1996;98(06):980–987. doi: 10.1097/00006534-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Cordeiro P G, Bacilious N, Schantz S, Spiro R. The radial forearm osteocutaneous “sandwich” free flap for reconstruction of the bilateral subtotal maxillectomy defect. Ann Plast Surg. 1998;40(04):397–402. doi: 10.1097/00000637-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Futran N D, Wadsworth J T, Villaret D, Farwell D G. Midface reconstruction with the fibula free flap. Arch Otolaryngol Head Neck Surg. 2002;128(02):161–166. doi: 10.1001/archotol.128.2.161. [DOI] [PubMed] [Google Scholar]
- 16.Peng X, Mao C, Yu G Y, Guo C B, Huang M X, Zhang Y. Maxillary reconstruction with the free fibula flap. Plast Reconstr Surg. 2005;115(06):1562–1569. doi: 10.1097/01.prs.0000160691.63029.74. [DOI] [PubMed] [Google Scholar]
- 17.Freije J E, Campbell B H, Yousif N J, Matloub H S. Reconstruction after infrastructure maxillectomy using dual free flaps. Laryngoscope. 1997;107(05):694–697. doi: 10.1097/00005537-199705000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Uglesić V, Virag M, Varga S, Knezević P, Milenović A. Reconstruction following radical maxillectomy with flaps supplied by the subscapular artery. J Craniomaxillofac Surg. 2000;28(03):153–160. doi: 10.1054/jcms.2000.0137. [DOI] [PubMed] [Google Scholar]
- 19.Clark J R, Vesely M, Gilbert R. Scapular angle osteomyogenous flap in postmaxillectomy reconstruction: defect, reconstruction, shoulder function, and harvest technique. Head Neck. 2008;30(01):10–20. doi: 10.1002/hed.20649. [DOI] [PubMed] [Google Scholar]
- 20.Brown J, Bekiroglu F, Shaw R. Indications for the scapular flap in reconstructions of the head and neck. Br J Oral Maxillofac Surg. 2010;48(05):331–337. doi: 10.1016/j.bjoms.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Solis R N, Mahaney J, Mohhebali R et al. Digital imaging evaluation of the scapula for prediction of endosteal implant placement in reconstruction of oromandibular defects with scapular free flaps. Microsurgery. 2019;39(08):730–736. doi: 10.1002/micr.30466. [DOI] [PubMed] [Google Scholar]
- 22.Succo G, Berrone M, Battiston B et al. Step-by-step surgical technique for mandibular reconstruction with fibular free flap: application of digital technology in virtual surgical planning. Eur Arch Otorhinolaryngol. 2015;272(06):1491–1501. doi: 10.1007/s00405-014-3078-3. [DOI] [PubMed] [Google Scholar]


