Abstract
Multiple anterior surgical approaches are available to obtain access to the nasopharynx, clivus, and craniocervical junction. These include the direct and transoral robotic surgery transpalatal, maxillary swing, and endoscopic endonasal approaches. In this article, we describe the indications for these techniques, surgical steps, and associated morbidities. This article is a PubMed literature review. A review of the literature was conducted to assess the techniques, surgical steps, and associated morbidities with transpalatal approaches to the skull base and nasopharynx. The transpalatal approach has been traditionally utilized to obtain surgical access to the nasopharynx, clivus, and craniocervical junction. Morbidity includes velopalatine insufficiency due to shortening of the soft palate from scar contraction or neuromuscular damage, thus leading to hypernasal speech and dysphagia. Middle ear effusion and oronasal or oronasopharyngeal palatal fistula are additional potential morbidities. The choice of surgical approach depends on a variety of factors including the disease location and extent, surgeon experience, and available resources.
Keywords: transpalatal approach, maxillary swing, skull base surgery
Traditionally, transpalatal approaches have been utilized to obtain surgical access to the clivus and craniocervical junction. 1 2 According to the area of the skull base that is the intended surgical target, the approach may be achieved via transoral transection of the soft and/or hard palate. Patients undergoing these approaches are at risk of developing velopalatine insufficiency (VPI) due to shortening of the soft palate from scar contraction or neuromuscular damage, associated with hypernasal speech and dysphagia, middle ear effusion, and oronasal or oronasopharyngeal palatal fistulas. 3 Furthermore, in some instances the ensuing edema and potential postoperative bleeding associated with the approach mandates securing the airway with a tracheostomy. 1 However, use of a direct transpalatal technique has significantly decreased with advances in endoscopic endonasal and transoral robotic techniques. An expanded endonasal approach (EEA) may be utilized to access the entire length of the clivus down to C1 and the odontoid process, and in some cases the superior aspect of C2. 4 5 An EEA may be combined with a transoral robotic approach to access disease that may extend inferiorly. 6 7 In contrast to a traditional transpalatal approach, an extended maxillectomy or maxillary swing approach is still required for the resection of extensive recurrent nasopharyngeal disease. 8 9 Other alternative approaches, following a lateral to medial line-of-sight (e.g., transtemporal or subtemporal approaches), are also possible. In this article, we describe the indication for transpalatal techniques, including surgical steps and associated morbidities.
Transpalatal Approach
As aforementioned, transoral transpalatal approaches are commonly used to access lesions involving the nasopharynx, clivus, and craniocervical junction. As with any transoral procedure, access is largely dependent on the patient's ability to open their mouth. 2 Thus, a patient with significant trismus may not be a candidate for this approach or may require a mandibular or maxillary osteotomies. 1 Furthermore, transpalatal approaches are best avoided if a large dural resection or cerebrospinal fluid leak is anticipated, given the challenges with achieving a watertight dural seal and the subsequent risk of meningitis due to oral contamination. 1 10
Advanced retractors allow exposing much of the craniocervical junction transorally without incising the palate. 2 However, disease that extends superiorly to the upper clivus or nasopharynx may require transection of the soft and/or hard palate to access transorally. 1 2 11
To perform the transpalatal approach, the patient is placed in a supine position. If cervical instability is present preoperatively, or possible as a result of the surgery, the head is stabilized and intraoperative nerve monitoring is used. 1 A tracheostomy is commonly utilized to secure the airway during surgery and postoperatively. 1 A mouth retractor (e.g., Dingman, KH, or those manufactured by MedRobotic) aids to displace the tongue, buccal, and pharyngeal tissues to enhance the surgical access. The soft palate and mucosa overlying the hard palate are then infiltrated with 1% lidocaine with 1:100000 epinephrine. There is some variability in terms of where the soft palate incision is placed. 1 2 Incisions through the mucoperiosteum of the hard palate are typically made laterally in an effort to reduce the risk of palatal fistula associated with a midline incision. The mucoperiosteum is then elevated off the palatine and premaxillary bones with care to preserve at least one of the paired greater palatine neurovascular bundles. 1 The hard palate is then removed with either a drill or oscillating saw. 1 2 After completion of resection of the skull base or craniocervical junction lesion, the soft palate as well as the mucosa overlying the hard palate is closed carefully with interrupted sutures in a multilayered fashion. 1
In addition to a traditional transpalatal approach to the skull base, an approach to the parapharyngeal space via the soft palate has been described. 12 This approach can be combined with an EEA or other approaches for resection of lesions with extent to the parapharyngeal space. To perform this approach, the lateral soft palate is infiltrated with 1% lidocaine with 1:100000 epinephrine. An incision is then made at the lateral soft palate ( Fig. 1A ). Dissection then proceeds through the submucosal and muscular layers ( Fig. 1B-D ). This dissection continues until the parapharyngeal space is identified ( Fig. 1E ). The lesion can then be removed ( Fig. 1F ) and the muscular and mucosal layers can be closed in multilayer fashion.
Fig. 1.
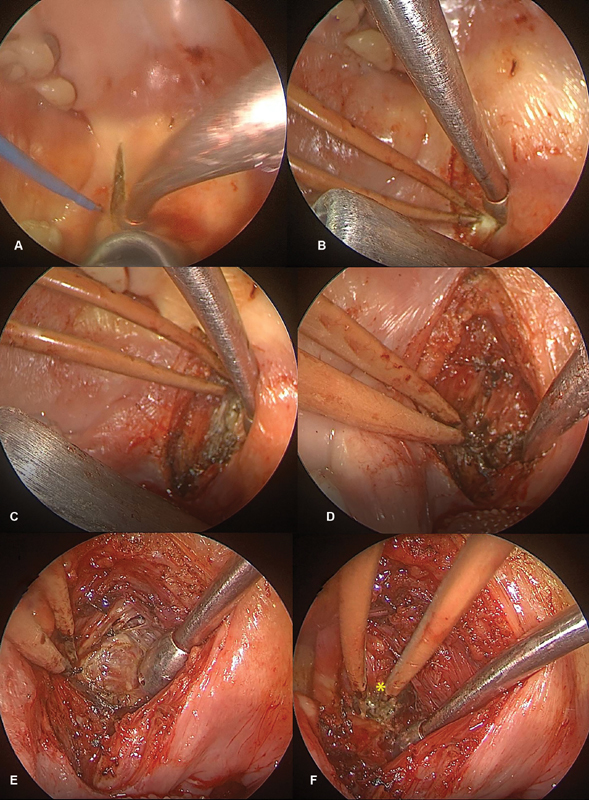
An incision is made at the lateral soft palate ( A ). Dissection then proceeds through the submucosal and muscular layers ( B–D ). This dissection continues until the parapharyngeal space is identified ( E ). The lesion can then be identified and removed ( F , asterisk indicates tumor).
The incorporation of transoral robotic surgery (TORS) in transpalatal approaches has been reported to aid in reduction in morbidity of standard transpalatal techniques. 13 Furthermore, use of TORS affords three-dimensional visualization as well as instrumentation with a high range of flexible movement. 6 A cadaveric feasibility study reported the use of TORS to access the inferior pharyngeal extent of nasopharyngeal disease after an endoscopic endonasal approach. This group reported use of this approach in two patients with nasopharyngeal disease with good results. 6 A second recent report combined use of a transpalatal approach and a TORS system to resect skull base malignancy in a series of patients with chordoma with extension to the nasopharynx. 13 To achieve access to the nasopharynx with the robotic system, the soft palate was either retracted with a suture or reflected with a mucoperiosteal incision. This group reported no intraoperative complications and limited postoperative morbidity and achieved a gross total resection in one case and a near total resection in the other two cases. 13
Maxillary Swing Approach
An extended version of the transpalatal approach, known as the maxillary swing, may be useful to obtaining wider access to the skull base and nasopharynx. However, this approach has the disadvantage of requiring multiple facial and intraoral incisions and henceforth visible scarring, facial osteotomies, as well as the potential for malocclusion and trismus. 14 This technique is still frequently utilized for nasopharyngectomy for large recurrent nasopharyngeal carcinoma due to the vast surgical access it provides. 8 14 15 Primary nasopharyngeal carcinoma often responds well to current management of intensity-modulated radiation therapy with or without chemotherapy. Indeed, one study by the Hong Kong Nasopharyngeal Cancer Study Group of 3,328 patients noted local recurrence or persistent disease in 14% of patients. 16 Re-irradiation is an option for recurrent cases but can be hampered by toxicities including mucosal necrosis or massive hemorrhage. 17 18 Thus, endoscopic or open approaches such as the maxillary swing have been reported in cases where negative margins can be achieved. 17 Another reported use of the maxillary swing technique has been for angiofibroma. 19
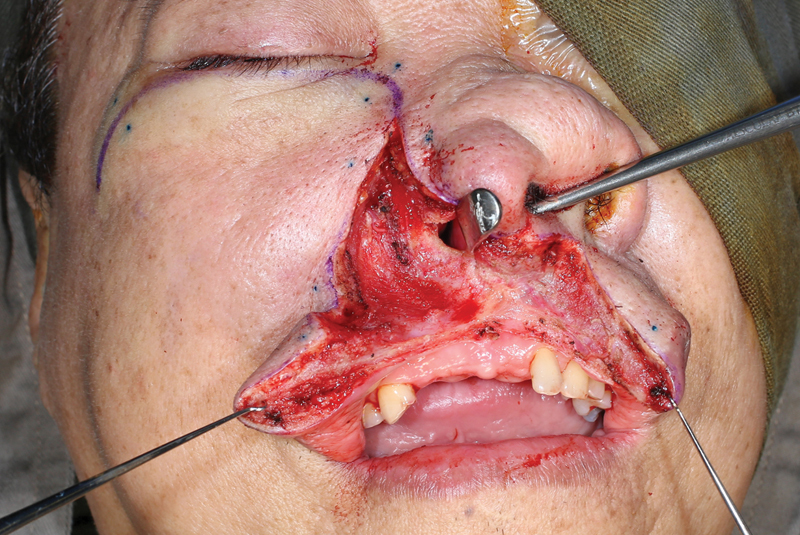
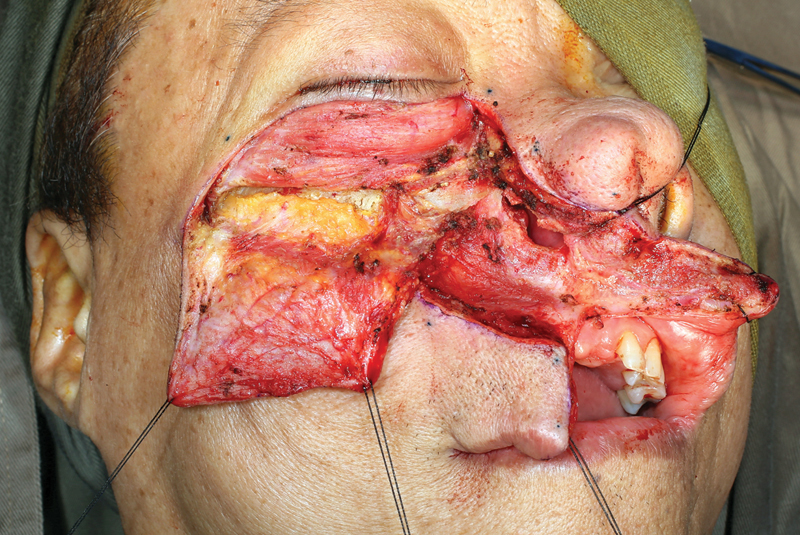
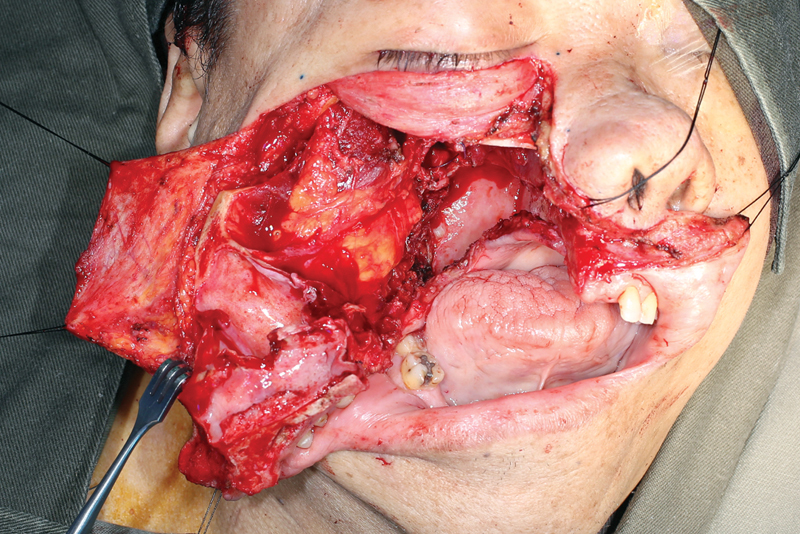
To perform this technique, the patient is placed in a supine position and tracheostomy is commonly performed. A Weber–Ferguson incision, or one of its many variants, is carried to extend between the central incisors ( Figs. 2 3 4 ). The intraoral incision then proceeds in a para-alveolar manner such that the palatal mucoperiosteal incision does not overlap with the intended midline palatal bony cut ( Figs. 5 6 ). This helps to reduce the risk of palatal fistula. 15 20 21 22 Preplating is advocated prior to completing the maxillary osteotomies to hasten the reconstructive phase and prevent postoperative malocclusion ( Fig. 7 ). 19 The planning of the osteotomies varies according to the preference of the surgical group and idiosyncrasies of the patient's anatomy or extent of the lesion but includes cuts through the frontal process of the maxilla, either the inferior orbital floor or the anterior maxillary wall, and laterally through either the zygoma or the zygomatic arch. To ultimately allow the maxilla to swing laterally, it needs to be released from the pterygoid plates ( Fig. 8 ). Once the resection is completed, the maxilla is returned to its anatomical position and plated. The skin and intraoral incisions are then closed in a multilayered fashion ( Figs. 9 10 11 12 ). 19
Fig. 2.
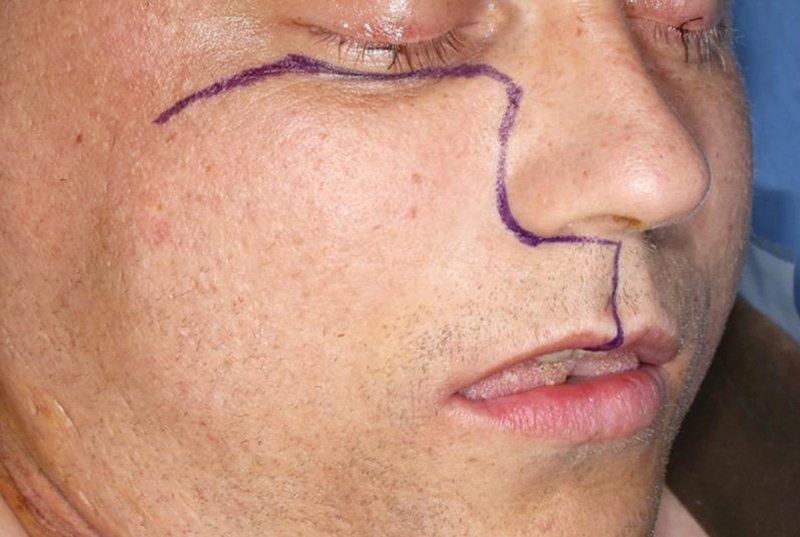
Design of the Weber–Ferguson incision.
Fig. 3.
Commencement of the Weber–Ferguson incision.
Fig. 4.
Continuation of flap elevation.
Fig. 5.
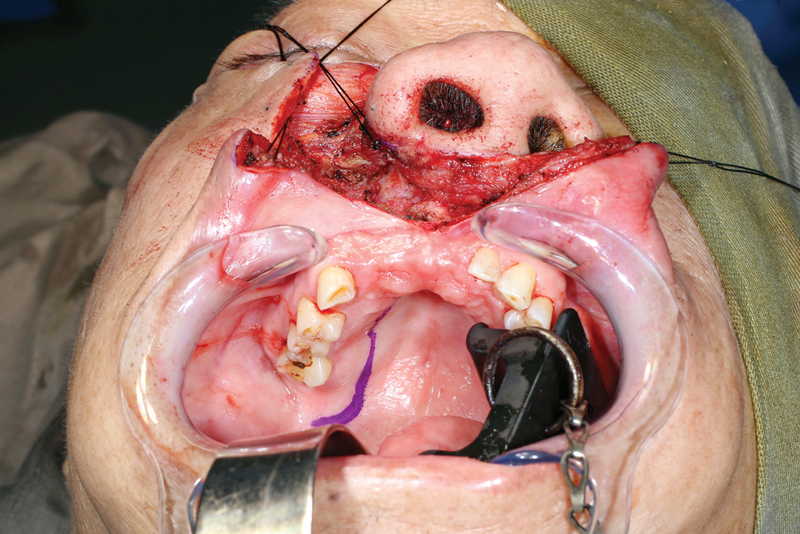
Design of the intraoral incision.
Fig. 6.
Incision proceeds in a para-alveolar manner.
Fig. 7.
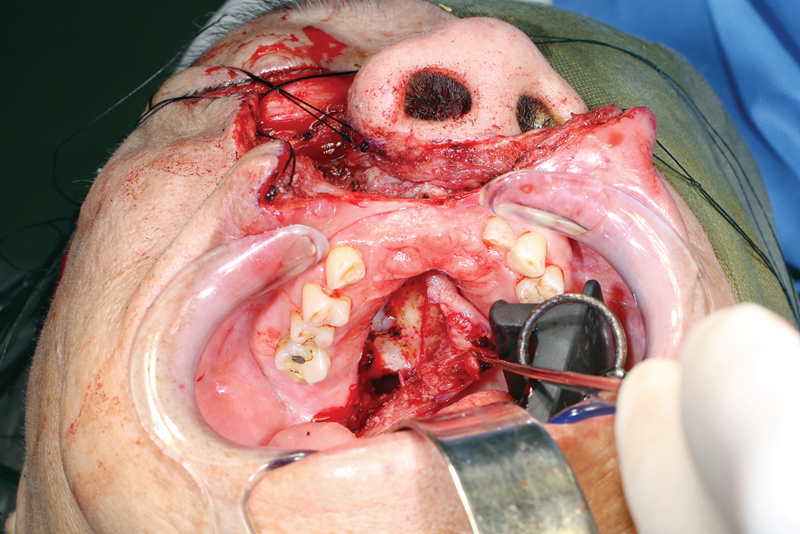
Preplating is performed prior to osteotomies.
Fig. 8.
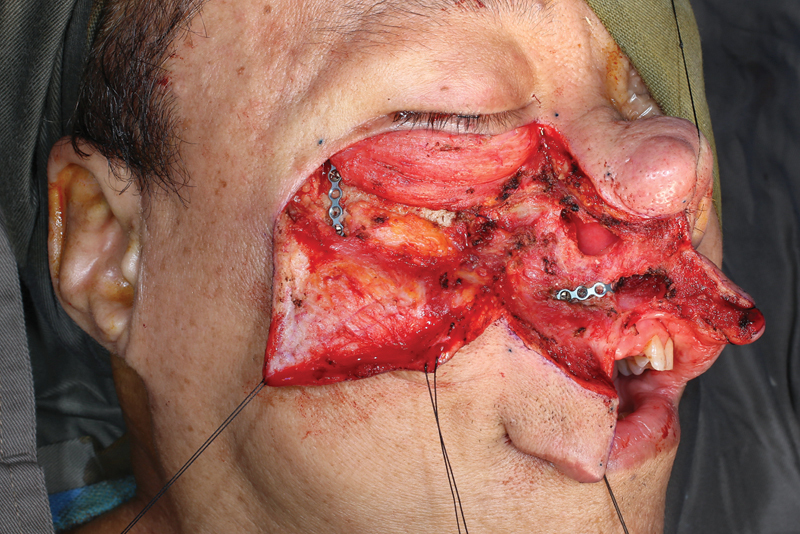
The maxillary swing allows for wide access for open nasopharyngectomy.
Fig. 9.
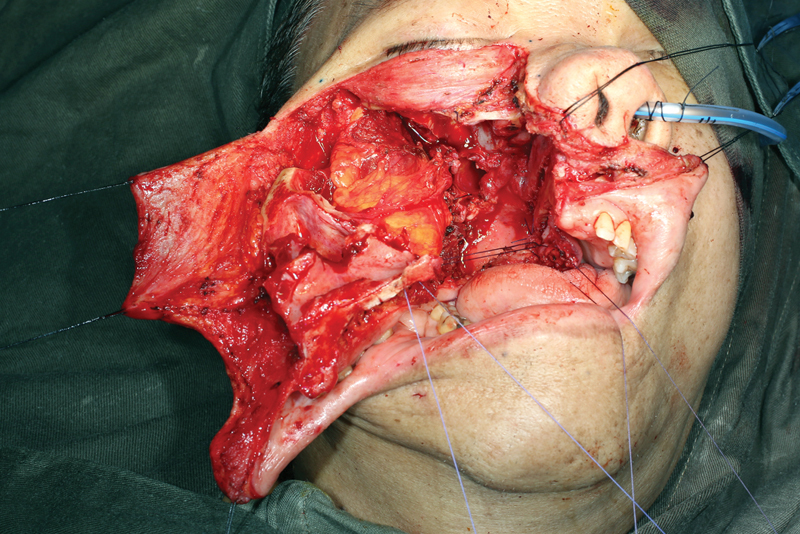
The soft tissue is closed in a multilayer fashion.
Fig. 10.
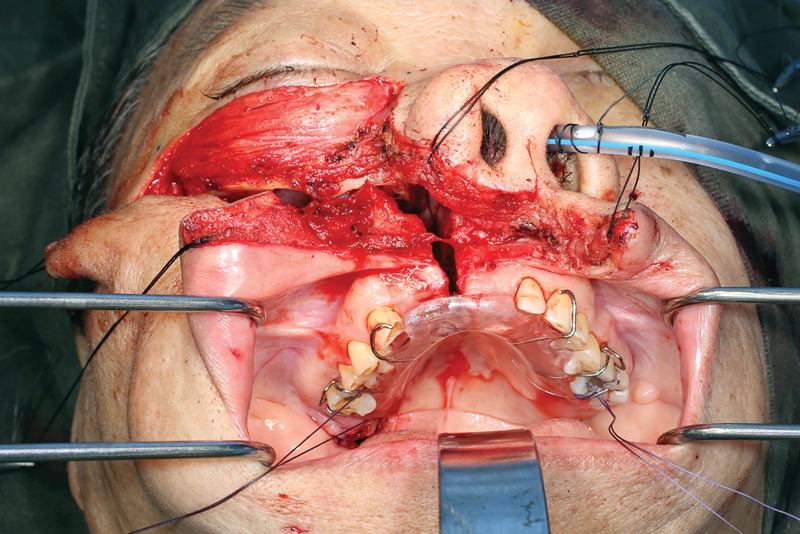
The maxillary swing is reapproximated.
Fig. 11.
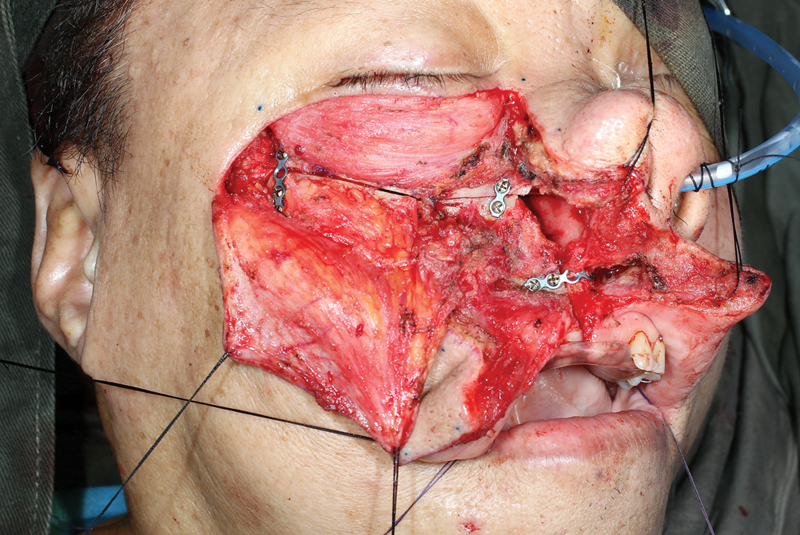
Plating is performed for repair of osteotomies.
Fig. 12.
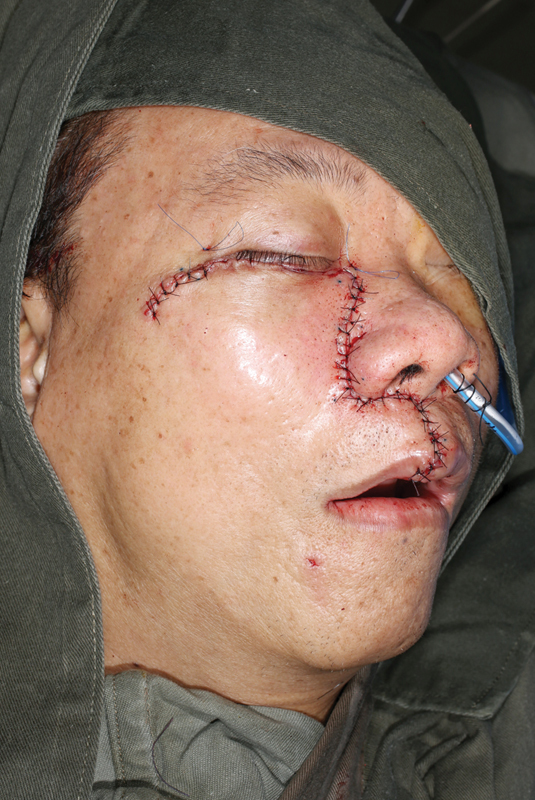
The skin and soft tissue are closed in a multilayer fashion.
The morbidity profile after nasopharyngectomy using a maxillary swing approach has been reported in one study of 338 patients. 14 In this study, the most frequently observed morbidity was middle ear effusion (40.8%). However, middle ear effusion is related to the resection of the Eustachian tube rather than the maxillary swing procedure itself. Trismus and palatal fistula were more frequent in their earlier study group, but in the more recent study group from 2002 to 2012 improved to 9.2 and 3.7%, respectively. 14 The incidence in nasal blockage increased in the more recent study group to 14.7%, although it was likely related to the increased use of free tissue transfer for defect reconstruction. 14 Another study of 62 patients that underwent maxillary swing noted palatal fistula in one patient as well as mild nasal mucosa atrophy in one patient. 19
Endoscopic Endonasal Approach
Rapid advances in endoscopic instrumentation and EEA techniques have significantly improved surgical access to the clivus and craniocervical junction. This allows for resection of lesions arising from or near the clivus and craniocervical junction such as chordoma, ecchordosis physaliphora, and meningiomas. 23 24 Furthermore, EEA can be utilized in certain cases to resect recurrent nasopharyngeal carcinoma. 25 26 Therefore, an endoscopic technique may be useful to reduce morbidities seen with transpalatal approaches.
Prior to performing an EEA for nasopharyngeal disease, a transcervical incision may be performed to identify and help secure the internal carotid artery. An endonasal approach is then utilized and a total ipsilateral sphenoethmoidectomy is performed. If the resection cavity can potentially be reconstructed with a nasoseptal flap rather than free tissue transfer, a contralateral nasoseptal flap is raised and preserved. This is followed by a posterior septectomy and reverse flap to cover exposed septal cartilage. A transpterygoid approach to the nasopharynx is then performed. 27 28 An ipsilateral endoscopic medial maxillectomy is achieved with the removal of the posterior two-thirds of the inferior turbinate and the medial maxillary wall. Depending on the degree of lateral extension of the disease, a Denker's modification may need to be added to allow for adequate access. The ipsilateral sphenopalatine artery is cauterized and the posterior maxillary wall is removed. 27 28 If possible, the descending palatine nerve is preserved to maintain innervation of the palate. The medial pterygoid plate is then drilled and removed to allow for resection of the cartilaginous Eustachian tube as well as the nasopharyngeal disease. After negative margins have been achieved, the contralateral nasoseptal flap can then be used to cover the resection bed. 27 28 In cases where a nasoseptal flap may not be available, alternative regional flaps such as the temporoparietal fascia flap may also be incorporated to achieve adequate vascularized tissue coverage. If free tissue transfer is required, a tunneled retropharyngeal approach may be utilized to reconstruct defects of the clivus or nasopharynx. 29
A recent anatomic study compared EEA versus maxillary swing on 10 cadaveric specimens. This study concluded that while the maxillary swing offered better access to the oropharynx and could be completed three times faster, the EEA provided precise definition of anatomic structures and a wide dissection range. 30 The EEA has been shown to have a similar overall survival rate as an open approach. 26 A recent study reported the surgical and oncological outcomes of endoscopic endonasal nasopharyngectomy for 55 patients with locally recurrent nasopharyngeal carcinoma. 25 In this study of patients ranging from rT1 to rT4, negative margins were achieved in 93% of patients with a 1-year local disease-free rate and survival rate of 93 and 98%, respectively. 25 Five patients had residual or recurrence at the surgical site and there was one case of intraoperative injury of the internal carotid artery. 25 Another study pooled the analysis of 300 patients that underwent endoscopic resection of nasopharyngeal carcinoma and reported negative margins in 90.2% of patients and an overall survival of 82.9%. 26 Thus, good surgical and oncologic results can be obtained with an endoscopic approach.
Reconstruction
Multiple techniques have been described to reconstruct palatal fistula and repair velopharyngeal insufficiency such as the sphincter pharyngoplasty, posterior pharyngeal wall, and posterior wall augmentation. 31 32 One of the most common is the posterior pharyngeal flap, which may be raised from a superior versus an inferiorly based pedicle. Multiple techniques exist for harvesting the flap as well as multiple techniques for flap inset. 33 34 The pharyngeal flap may be sutured to the palate to allow for repair and healing of the palatal fistula. 35 In the event of velopharyngeal insufficiency, this technique allows for closure of the central velopharyngeal gap with lateral ports for breathing. 35 The most common reason this technique fails is secondary to flap dehiscence. Furthermore, obstructive sleep apnea has been reported in 0 to 10% of patients as a result. 35 36
An alternative technique that has been described as a potential option when traditional techniques are unavailable for pharyngeal reconstruction is the nasoseptal flap. 37 The nasoseptal flap, which is harvested from the nasal septal mucosa with a posterior vascular pedicle, is the workhorse flap for reconstruction of skull base defects. A cadaveric study found that the nasoseptal flap could reach the upper oropharynx and could therefore serve as a potential reconstructive option for velopharyngeal insufficiency or soft palate reconstruction. 37 Another study reported use of the nasoseptal flap to create a tube mucosal lined nasopharyngeal port followed by microvascular free flap reconstruction of the soft palate. 38 Microvascular free flap reconstruction is also another option in pharyngeal defect reconstruction. This may be particularly useful when other techniques have failed or when a large tissue deficit exists. The free flap pedicle can be routed through several approaches depending on the location of the defect and whether an open or combine endoscopic technique is being utilized. 29 39 Open pharyngeal free flap reconstruction provides a multitude of options for routing the pedicle and vascular anastomosis. When combined with an endoscopic technique, the flap can be tunneled through the retropharynx or alternatively the flap may be routed through the maxillary sinus with an anastomosis to the facial artery and vein. 29 39
Conclusion
Multiple surgical approaches are available to obtain access to the nasopharynx, clivus, and craniocervical junction. These include the transpalatal, maxillary swing, and endoscopic endonasal approaches. The maxillary swing is an extension of the transpalatal approach and still commonly utilized for extensive nasopharyngectomy for recurrent nasopharyngeal carcinoma. The EEA has significantly changed utilization of the transpalatal approach and has become a viable option for experienced surgeons for nasopharyngectomy to resect lesions of the clivus and craniocervical junction.
Footnotes
Conflicts of Interest The authors have no relevant conflicts of interest to disclose.
References
- 1.Enepekides D J, Donald P J.Transoral approaches to the clivus and nasopharynx Otolaryngol Clin North Am 200134061105–1121., ix ix. [DOI] [PubMed] [Google Scholar]
- 2.Liu J K, Couldwell W T, Apfelbaum R I. Transoral approach and extended modifications for lesions of the ventral foramen magnum and craniovertebral junction. Skull Base. 2008;18(03):151–166. doi: 10.1055/s-2007-994288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubey S P, Molumi C P. Transpalatal approach with pedicled palatal osteo-muco-periosteal flap. ANZ J Surg. 2012;82(06):439–442. doi: 10.1111/j.1445-2197.2011.05954.x. [DOI] [PubMed] [Google Scholar]
- 4.Aldana P R, Naseri I, La Corte E.The naso-axial line: a new method of accurately predicting the inferior limit of the endoscopic endonasal approach to the craniovertebral junction Neurosurgery 201271(2, Suppl Operative):ons308–ons314., discussion ons314 [DOI] [PubMed] [Google Scholar]
- 5.de Almeida J R, Zanation A M, Snyderman C H et al. Defining the nasopalatine line: the limit for endonasal surgery of the spine. Laryngoscope. 2009;119(02):239–244. doi: 10.1002/lary.20108. [DOI] [PubMed] [Google Scholar]
- 6.Carrau R L, Prevedello D M, de Lara D, Durmus K, Ozer E. Combined transoral robotic surgery and endoscopic endonasal approach for the resection of extensive malignancies of the skull base. Head Neck. 2013;35(11):E351–E358. doi: 10.1002/hed.23238. [DOI] [PubMed] [Google Scholar]
- 7.Chauvet D, Missistrano A, Hivelin M, Carpentier A, Cornu P, Hans S. Transoral robotic-assisted skull base surgery to approach the sella turcica: cadaveric study. Neurosurg Rev. 2014;37(04):609–617. doi: 10.1007/s10143-014-0553-7. [DOI] [PubMed] [Google Scholar]
- 8.Chan J YW, Wong S TS, Wei W I. Surgical salvage of recurrent T3 nasopharyngeal carcinoma: prognostic significance of clivus, maxillary, temporal and sphenoid bone invasion. Oral Oncol. 2019;91:85–91. doi: 10.1016/j.oraloncology.2019.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Muhanna N, Chan H, Qiu J et al. Volumetric analysis of endoscopic and maxillary swing surgical approaches for nasopharyngectomy. J Neurol Surg B Skull Base. 2018;79(05):466–474. doi: 10.1055/s-0037-1617432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balasingam V, Anderson G J, Gross N D et al. Anatomical analysis of transoral surgical approaches to the clivus. J Neurosurg. 2006;105(02):301–308. doi: 10.3171/jns.2006.105.2.301. [DOI] [PubMed] [Google Scholar]
- 11.Chan J Y. Surgical salvage of recurrent nasopharyngeal carcinoma. Curr Oncol Rep. 2015;17(03):433. doi: 10.1007/s11912-014-0433-x. [DOI] [PubMed] [Google Scholar]
- 12.Myatt H M, Remedios D. A transpalatal approach to the parapharyngeal space. J Laryngol Otol. 1997;111(02):159–162. doi: 10.1017/s0022215100136722. [DOI] [PubMed] [Google Scholar]
- 13.Henry L E, Haugen T W, Rassekh C H, Adappa N D, Weinstein G S, O'Malley B W., Jr A novel transpalatal-transoral robotic surgery approach to clival chordomas extending into the nasopharynx. Head Neck. 2019;41(08):E133–E140. doi: 10.1002/hed.25747. [DOI] [PubMed] [Google Scholar]
- 14.Chan J Y, Tsang R K, Wei W I. Morbidities after maxillary swing nasopharyngectomy for recurrent nasopharyngeal carcinoma. Head Neck. 2015;37(04):487–492. doi: 10.1002/hed.23633. [DOI] [PubMed] [Google Scholar]
- 15.Wei W I, Lam K H, Sham J S. New approach to the nasopharynx: the maxillary swing approach. Head Neck. 1991;13(03):200–207. doi: 10.1002/hed.2880130306. [DOI] [PubMed] [Google Scholar]
- 16.Au K H, Ngan R KC, Ng A WY et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study) Oral Oncol. 2018;77:16–21. doi: 10.1016/j.oraloncology.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Leong Y H, Soon Y Y, Lee K M, Wong L C, Tham I WK, Ho F CH. Long-term outcomes after reirradiation in nasopharyngeal carcinoma with intensity-modulated radiotherapy: a meta-analysis. Head Neck. 2018;40(03):622–631. doi: 10.1002/hed.24993. [DOI] [PubMed] [Google Scholar]
- 18.Lam J W, Chan J Y, Lui W M, Ho W K, Lee R, Tsang R K. Management of pseudoaneurysms of the internal carotid artery in postirradiated nasopharyngeal carcinoma patients. Laryngoscope. 2014;124(10):2292–2296. doi: 10.1002/lary.24721. [DOI] [PubMed] [Google Scholar]
- 19.Kalra G S, Midya M, Bedi M. Access to the skull base - maxillary swing procedure - long term analysis. Ann Maxillofac Surg. 2018;8(01):86–90. doi: 10.4103/ams.ams_5_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King W W, Ku P K, Mok C O, Teo P M. Nasopharyngectomy in the treatment of recurrent nasopharyngeal carcinoma: a twelve-year experience. Head Neck. 2000;22(03):215–222. doi: 10.1002/(sici)1097-0347(200005)22:3<215::aid-hed2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 21.Hao S P, Tsang N M, Chang C N. Salvage surgery for recurrent nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2002;128(01):63–67. doi: 10.1001/archotol.128.1.63. [DOI] [PubMed] [Google Scholar]
- 22.Ng R W, Wei W I. Elimination of palatal fistula after the maxillary swing procedure. Head Neck. 2005;27(07):608–612. doi: 10.1002/hed.20220. [DOI] [PubMed] [Google Scholar]
- 23.Stippler M, Gardner P A, Snyderman C H, Carrau R L, Prevedello D M, Kassam A B.Endoscopic endonasal approach for clival chordomas Neurosurgery 20096402268–277., discussion 277–278 [DOI] [PubMed] [Google Scholar]
- 24.Beer-Furlan A, Abi-Hachem R, Jamshidi A O, Carrau R L, Prevedello D M. Endoscopic trans-sphenoidal surgery for petroclival and clival meningiomas. J Neurosurg Sci. 2016;60(04):495–502. [PubMed] [Google Scholar]
- 25.Tang I P, Ngui L X, Ramachandran K et al. A 4-year review of surgical and oncological outcomes of endoscopic endonasal transpterygoid nasopharyngectomy in salvaging locally recurrent nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2019;276(09):2475–2482. doi: 10.1007/s00405-019-05522-5. [DOI] [PubMed] [Google Scholar]
- 26.Vlantis A C, Lee D L, Wong E W, Chow S M, Ng S K, Chan J Y. Endoscopic nasopharyngectomy in recurrent nasopharyngeal carcinoma: a case series, literature review, and pooled analysis. Int Forum Allergy Rhinol. 2017;7(04):425–432. doi: 10.1002/alr.21881. [DOI] [PubMed] [Google Scholar]
- 27.Al-Sheibani S, Zanation A M, Carrau R L et al. Endoscopic endonasal transpterygoid nasopharyngectomy. Laryngoscope. 2011;121(10):2081–2089. doi: 10.1002/lary.22165. [DOI] [PubMed] [Google Scholar]
- 28.Hosseini S M, McLaughlin N, Carrau R L et al. Endoscopic transpterygoid nasopharyngectomy: correlation of surgical anatomy with multiplanar CT. Head Neck. 2013;35(05):704–714. doi: 10.1002/hed.23020. [DOI] [PubMed] [Google Scholar]
- 29.London N R, Jr, Ishii M, Gallia G, Boahene K DO. Technique for reconstruction of large clival defects through an endoscopic-assisted tunneled retropharyngeal approach. Int Forum Allergy Rhinol. 2018;8(12):1454–1458. doi: 10.1002/alr.22187. [DOI] [PubMed] [Google Scholar]
- 30.Roger V, Patron V, Moreau S, Kanagalingam J, Babin E, Hitier M. Extended endonasal approach versus maxillary swing approach to the parapharyngeal space. Head Neck. 2018;40(06):1120–1130. doi: 10.1002/hed.25092. [DOI] [PubMed] [Google Scholar]
- 31.Rauso R, Tartaro G, Califano L, Rugge L, Chirico F, Colella G. Pedicled palatal flap for surgical repair of oro-nasal fistula. J Biol Regul Homeost Agents. 2018;32(06):1565–1567. [PubMed] [Google Scholar]
- 32.Rochlin D H, Mittermiller P A, Sheckter C C, Menard R M. The pushback pharyngeal flap: an 18-year experience. Plast Reconstr Surg. 2019;143(06):1246e–1254e. doi: 10.1097/PRS.0000000000005645. [DOI] [PubMed] [Google Scholar]
- 33.Ekin O, Calis M, Kulak Kayikci M E, Icen M, Gunaydin R O, Ozgur F. Modified superior-based pharyngeal flap is effective in treatment of velopharyngeal insufficiency regardless of the preoperative closure pattern. J Craniofac Surg. 2017;28(02):413–417. doi: 10.1097/SCS.0000000000003328. [DOI] [PubMed] [Google Scholar]
- 34.Emara T A, Quriba A S. Posterior pharyngeal flap for velopharyngeal insufficiency patients: a new technique for flap inset. Laryngoscope. 2012;122(02):260–265. doi: 10.1002/lary.22456. [DOI] [PubMed] [Google Scholar]
- 35.El-Anwar M W, Elsheikh E, Askar S. Single-stage repair of palatal fistula and velopharyngeal incompetence by the new L flap. J Craniofac Surg. 2018;29(01):e70–e73. doi: 10.1097/SCS.0000000000004066. [DOI] [PubMed] [Google Scholar]
- 36.Chegar B E, Shprintzen R J, Curtis M S, Tatum S A. Pharyngeal flap and obstructive apnea: maximizing speech outcome while limiting complications. Arch Facial Plast Surg. 2007;9(04):252–259. doi: 10.1001/archfaci.9.4.252. [DOI] [PubMed] [Google Scholar]
- 37.Rivera-Serrano C M, Lentz A K, Pinheiro-Neto C, Snyderman C H. Cadaveric study of the posterior pedicle nasoseptal flap: a novel flap for reconstruction of pharyngeal defects and velopharyngeal insufficiency. Plast Reconstr Surg. 2013;132(05):1269–1275. doi: 10.1097/PRS.0b013e3182a4c37b. [DOI] [PubMed] [Google Scholar]
- 38.Zenga J, Sharon J D, Gross J, Gantz J, Pipkorn P. Soft palate reconstruction after radionecrosis: combined anterolateral thigh adipofascial and nasoseptal flaps. Auris Nasus Larynx. 2018;45(04):875–879. doi: 10.1016/j.anl.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Kang S Y, Eskander A, Hachem R A et al. Salvage skull base reconstruction in the endoscopic era: vastus lateralis free tissue transfer. Head Neck. 2018;40(04):E45–E52. doi: 10.1002/hed.25094. [DOI] [PubMed] [Google Scholar]


