Abstract
Maxillary defects commonly present following surgical resection of oncologic processes. The use of rotational and free flaps has largely replaced the use of prosthetic options for hard palate and maxillary reconstruction, but prostheses remain a useful tool. Prosthetic devices may be invaluable in patients considered poor candidates for surgical reconstruction secondary to poor vascularity, need for postoperative radiation, or medical comorbidities that place them at high risk for healing following reconstruction. Obturators may also be considered over soft tissue options if oncologic surveillance via direct visualization of the surgical site is warranted.
Keywords: facial reconstruction, prosthetics, prosthesis, maxilla, craniofacial
Maxillary defect classification has been described by many surgeons including Brown, Amarya, and most recently Okay. 1 2 3 The Brown classification system will be discussed here as it is most commonly referenced and is currently utilized by the Arbeitsgemeinschaft für Osteosynthesefragen Foundation to describe maxillary defects ( Fig. 1 ). Class I defects include alveolar defects without oronasal or oroantral fistulae as well as defects in the palatal bone without an alveolar defect. Class II defects describe alveolar and antral wall defects, not including the orbital floor or rim. Class III defects constitute alveolar and antral wall defects that include the orbital floor. Periorbita may or may not be involved. Class IV defects include the alveolus, antral wall, orbital floor, and orbital contents with or without involvement of the skull base. Classes II–IV can be more specifically described by utilizing the designations a, b, and c which denote unilateral, bilateral (incomplete), and complete involvement of the alveolus and hard palate, respectively.
Fig. 1.

Maxillectomy defect classification. (Adapted with permission from Brown et al. 1 )
While most defects can be addressed with an obturator, historically, Brown classification levels less than II have been addressed with obturation. 4 Recent studies have demonstrated that the horizontal extent of resection is a critical component in obturation success, with resection of more than half or even one-fourth of the hard palate resulting in poor overall obturator function. 3 5 6 Similarly, patients with resection of the premaxilla and bilateral canines are poor candidates for prosthetic reconstruction. 3 With this in mind, patient and defect selection are integral in optimal prosthetic implementation.
Goals of Treatment
The goal of the obturator is to separate the oral cavity from the nasal cavity. This allows for separation of food and oral floral from the nasal cavity, preventing contamination of the surgical site and simultaneously allowing it to heal. The obturator also allows for increased speech intelligibility as well as improvement in swallowing function, potentially obviating the need for nasogastric or gastric tube placement. 7 8 9
Pretreatment Evaluation
Paramount to successful obturation is clear communication between the resecting surgeon, the prosthodontist, and the patient. The fine balance between oncologic control and availability of sufficient structures for prosthetic reconstruction should be recognized by all team members. As many teeth should be retained as possible, specifically the maxillary canine. 3 Its long tooth root provides the ideal structural integrity to act as an abutment. Osteoradionecrosis (ORN) is often cited as a reason to remove teeth prior to radiation. Some authors recommend dental retention if obturation is planned, as ORN is less likely to occur in the maxilla when compared with the mandible. 10 11 Bony maxillectomy cuts should be made through tooth sockets. Bony cuts made adjacent to tooth sockets place the adjacent tooth at risk of devitalization and destabilization due to thin bone that significantly diminishes osseous support. The maxillary tuberosity and anterior alveolar ridge should also be retained when possible as they provide solid structures to secure the obturator.
Split-thickness skin graft application to the maxillary defect serves several functions. First, it provides a keratinized surface to support the prosthesis which is less irritated than respiratory epithelium. Skin grafts also produce less secretions than respiratory epithelium which can become hardened and make cleaning more difficult. Additionally, the resulting scar band will later provide an undercut to support definitive obturation. The medial aspect of the defect benefits from mucosal coverage by increasing comfort and compliance with the obturator.
Structures whose removal may increase the success of obturation include the inferior portion of the vomer and the inferior turbinate by allowing the obturator bulb to engage an undercut above the palatal shelf. 12 Some authors describe removal of the coronoid process if more than half of the soft palate has been resected, as the anterior and medial movement of the coronoid may cause pain and discomfort if irritated by the prosthesis. 13 Adjustment of the prosthesis to decrease pain may result in a decreased seal, resulting in nasopharyngeal reflux and hypernasal speech. 13 Candid, clear communication with the patient with regards to the procedures involved in obturation, expectations in voice and swallowing function, and oral and nasal hygiene also improve patient and surgeon satisfaction.
Stages of Obturation
Obturation occurs via three stages to allow for healing, size, and geometric changes to the defect. They are surgical, interim, and definitive. The timing, goals, and techniques specific to each stage are described in the following sections.
Surgical Obturation
Surgical obturation occurs immediately following creation of the maxillectomy defect. It involves placing a temporary obturator over the defect to separate the nasal from oral cavity, improve contact of the skin graft to the wound edge, and holds surgical packing in place until follow up ( Fig. 2 ). As mentioned previously, surgical obturation permits the patient to speak and swallow, which may obviate the need for a nasogastric tube. Communicative dysfunction is often negatively judged by the community, resulting in decreased quality of life. 14 15 Obturation allows for intelligible speech immediately following surgery which may improve these perceptions. Facial, lip, and cheek support are also provided by the obturator. If the patient has their own dentures they can be used as a surgical obturator. Otherwise, an obturator will be created prioritizing: (1) simplicity, so that it can be easily modified; (2) low-weight for patient comfort; and (3) avoidance of posterior occlusion to prevent transmission of pressure through the surgical packing to nearby healing tissue. 16 17 18
Fig. 2.

Example of prefabricated surgical obturators. (Reproduced with permission from Haug. 13 )
Prior to surgery, impressions are taken and a cast of the native palate is made. If possible, the ablative surgeon marks the margins of resection on the cast ( Fig. 3 ). The oncologic abnormality may be removed with a bur so that the obturator represents normal palatal contours. The surgical obturator is then fabricated with polymethyl methacrylate (PMMA) and sterilized for use in the operating suite. Porous (autopolymerizing) or light-polymerized composite resins can be used but are generally avoided because they are thought to be less hygienic and brittle. If a skin graft is employed, the surgical obturator should ideally end before the skin graft–mucosal junction to allow for adequate healing and scar formation. Teeth can be included into the surgical obturator. The advantage of not including dentition is reduction in forces transmitted through the wound. In cases where the final size of the defect is unclear, multiple surgical obturators of different sizes can be fabricated ( Fig. 1 ).
Fig. 3.

( A ) Cast with proposed margins outlined. ( B ) Prepared cast for fabrication of obturator. (Reproduced with permission from Shah et al. 27 )
During surgery, the obturator is secured in place via clasps incorporated into the prosthesis specifically to encircle abutment teeth ( Fig. 4 ). If clasps are not incorporated into the obturator, the prosthesis can be perforated to allow for passage of 18 to 24-gauge, prestretched, stainless steel wire, which is secured interdentally to the abutment teeth. In edentulous patients, wires can also be placed through the obturator and into and around the remaining zygomatic process or alveolar bone. Note that 10- to 17-mm screws can also be used to secure the prosthesis to the hard palate. Placing the screws at an angle for easy removal during removal of packing allows for increased patient comfort. Suture is another option to hold the surgical packing in place which decreases surgical and anesthetic time during initial surgery. Disadvantages include increased discomfort, inability to clean the area after meals, and possible aspiration risk if the packing becomes dislodged. The surgical obturator remains in place for 5 to 10 days.
Fig. 4.

( A ) Interim obturator with cusps around teeth. ( B ) Interim prosthesis with the obturator portion formed from resilient lining material (white). (Reproduced with permission from Shah et al. 27 )
Osseointegrated implants can also be used to secure the prosthesis into place. Implants offer increased stability and retention of obturators. While successful implantation into irradiated bone can have success rates as high as 92%, the literature describes higher implant failure rates in irradiated bone with success rates for nonirradiated bone nearing 100%. 19 20 21 22 In general, the irradiated maxilla is at higher risk of implant failure when compared with the irradiated mandible. 23 Radiation doses > 70 Gy as well as implantation into grafted bone have been associated with implant failure. 23 As such, in treatment planning early implantation into native bone is recommended. Implants can be placed at the time of initial surgery. In general, no less than four implants should be placed in a nonlinear fashion to sufficiently distribute load-bearing forces. A stress-breaking bar splinted to four or five implants can also be utilized for improved stability and support. Implants can be placed in the native alveolus anterior to the surgical site, on the native canine region, and two posteriorly. Zygomatic implants have also been described. These longer implants ideally require bilateral placement and an intact infraorbital rim on the defect side to provide stability. 24 25 In cases where insufficient alveolar bone exists for implant placement, a zygomatic implant ipsilateral to the defect can be placed to stabilize the prosthesis. 24 This requires preoperative imaging to ensure adequate zygomatic bone volume.
Interim Obturation
After the initial healing period, the surgical obturator and packing are removed and the wound is irrigated and debrided. Coordination aids the patient in seeing the ablative surgeon before meeting with their prosthodontist the same day. The goal of the prosthodontic visit is to reline the surgical obturator with materials that best approximate the defect, provide the patient comfort, and promote healing. As such, the patient meets with their prosthodontist once every week to 2 weeks for the first month as this time represents maximal healing and contour changes. Softer materials are added to the obturator and its shape is changed according to the defect. This may include removing overextended material as the wound changes shape. Harder materials that are Candida resistant are used before the patient undergoes radiation therapy. The prosthodontist may maintain regular visits with the patient for the first 6 to 8 weeks after surgery or until the wound has stabilized.
At the initial visit, areas of overextension are trimmed and retaining clasps are adjusted to maximize stability and retention. Screw holes in the obturator can be repaired at this time with autopolymerizing acrylic resin or light-polymerized composite acrylic resin. Much like impressions taken during the creation of dentures, the second impression for obturation includes softer materials added to the surgical side of the obturator. The prosthesis is placed in the patient's mouth, making an impression of the surgical side. This process is continued until the surgical side is sufficiently obturated.
It is of utmost importance that prosthodontists and physicians educate the patient on proper care of the obturator and surgical site as well as oral hygiene. Obturator care includes removal during hygienic cleanses, irrigations, and sleep. It should be worn during waking hours. The obturator can be cleaned as one would clean a denture, that is, with a brush and nonabrasive denture cleaner or a soap and water mixture. Surgical site care includes saline (or salt and baking soda) irrigations via the nasal cavity as well as oral rinses several times per day. The patient is encouraged to maintain excellent oral hygiene of the remaining dentition.
Definitive Obturation
Definitive obturation can begin as early as 3 months following surgery or the completion of radiation surgery, although some recommend waiting longer in patients who have received radiation. The definitive obturator should extend as high as possible into the surgical defect for increased stability and retention.
Edentulous obturators consist of PMMA while partially edentulous obturators are supported by chrome-cobalt alloy frameworks. The framework for dentate and edentulous patients begins with alginate impression material. The surgical defect can be obstructed with gauze as this area will be addressed further in the fabrication process. The impression is then poured into die stone and the framework is fabricated from either acrylic resin and stainless steel clasps, or chromium alloy. 16 17 Readers are referred to landmark articles on the philosophy of the design of maxillary obturator framework written by Aramany and Parr et al. 16 17
The majority of prosthetic stability will come from impression of the surgical site. As such, it is important to incorporate as many undercuts as possible into this impression. In dentate patients, dental modeling material is placed on top of the framework with the aid of a ring-tray that allows the prosthodontist to place a finger into the defect, allowing for more precise conformation ( Fig. 5 ). The modeling compound is then trimmed to allow for the placement of impression material. The impression material is placed on the compound and the ring-tray/framework assembly is placed into the patient's mouth for impression. Impressions in edentulous patients are taken in one step via a custom ring tray made on the preliminary impression. Border molding is performed on the nonsurgical side to ensure the appropriate fit and orientation when the tray is replaced in the patient's mouth. Similar to dentate patients, compound is then added to the framework, trimmed, and impression material is added on top for impression of the surgical defect. Casts are fabricated from these impressions.
Fig. 5.
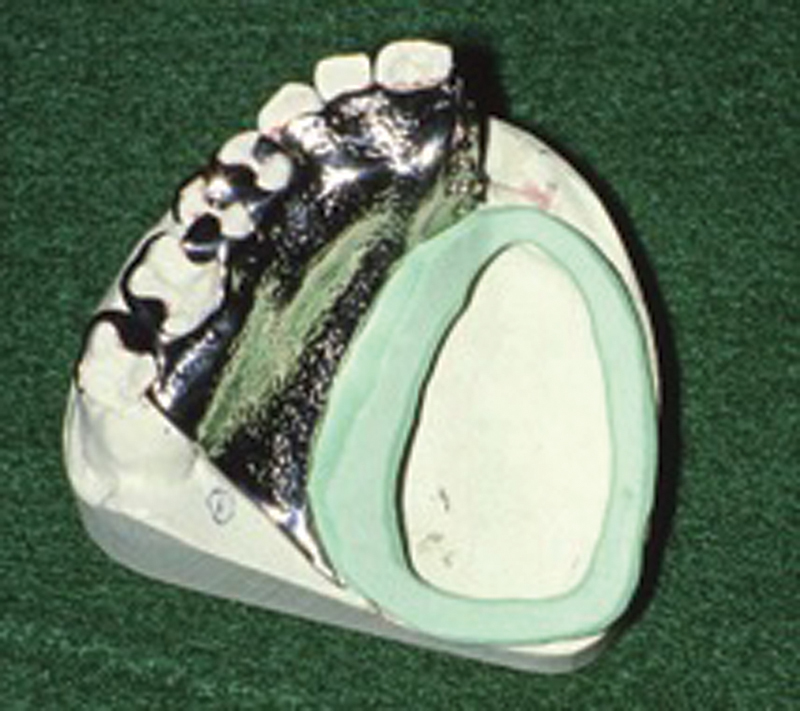
Custom ring tray on framework. (Reproduced with permission from Haug. 13 )
Record bases fabricated from heat-processed PMMA and placement of an occlusal rim are recommended. During try-in, pressure-indicated paste is used to adjust the base while maintaining as much undercut to the surgical site as possible. Prosthetic principles used for creation of full or partial dentures are then applied. Special attention should be given to deviations from norms including scar contracture that may cause cross-bite or muscle fibrosis that may require reduced vertical occlusal dimension.
The large size of the prosthetic bulb may be amenable to hollowing which allows for a lighter prosthetic that is retained more easily and does not transmit as many stresses to the remaining oral structures as those that are not hollowed. 18 In general, prosthetics greater than 45 g should be hollowed. If the bulb extends high into the nasal cavity, and/or there are decreased secretions, the bulb top may be left open. If the bulb is low in the nasal cavity or the patient has copious secretions, closure of the bulb top after hollowing allows for a more hygienic prosthesis.
Adequate time should be spent with the patient to ensure they understand how to introduce and remove their prosthesis, which may be a complicated task. Hygiene education is also encouraged. Patients are seen 24 hours, 72 hours, and 1 week after initial obturation for adjustments and then every 6 months to yearly afterwards to ensure correct fit and soft tissue health.
Soft Palate Prosthesis
Oncologic resection of the soft palate may cause velopharyngeal insufficiency. This tissue deficiency causes hypernasal speech, nasopharyngeal reflux, and difficulty swallowing.
The goals of the soft palate prosthesis, also termed “speech aids,” are to improve phonation and swallowing ability by ablating the space between the lateral and posterior nasopharyngeal musculature at the level of Passavant's ridge during swallowing ( Fig. 6 ). The prosthesis is designed to sit in the middle of the nasopharynx during quiet nasal breathing. As such, it is critical that sufficient, but not excessive, space remains between the prosthesis and the remaining nasopharyngeal musculature. Local flaps used in surgical reconstruction should maintain minimal contact with the prosthesis. A plethora of factors affect speech quality of the prosthesis including remaining tissue, scarring and fibrosis, tongue mobility, and head position. As such, continued evaluation and adjustments may be needed.
Fig. 6.

Soft palate speech bulb prosthesis. (Reproduced with permission from Shah et al. 27 )
Soft palate prosthetics may be supported by soft tissue, teeth, or bone. If the load is tooth-borne, utilizing molar dentition for stability and support helps prevent the prosthetic succumbing to loosening forces as these teeth are near the vectors that opposes stability. Osseointegrated implants provide superior stability and can be used in combination with claps secured to teeth. While numerous surgeons use pharyngeal flaps to close the nasopharynx in these cases, advantages of the soft palate prosthetics over pharyngeal flaps include the ability to easily change the configuration of the prosthetic and ability to directly visualize the resection margin for disease surveillance. Devices incorporating soft palatal augmentation have shown to increase muscular activity, speech intelligibility, and swallowing function. 26
Conclusion
Obturation of maxillary defects requires excellent communication between the ablative surgeon and the oral maxillofacial prosthodontist to ensure adequate preoperative planning with understanding of the balance between oncologic resection and obturator success. Communication with the patient is also paramount to patient and provider satisfaction. Successful obturation separates the nasal cavity from the oral cavity, improves speech and swallowing outcomes, as well as cosmetic appearance. Implants provide the most stability for prosthetics, but careful patient selection is indicated in cases with poor bone stock or radiation history.
Footnotes
Conflicts of Interest None.
References
- 1.Brown J S, Rogers S N, McNally D N, Boyle M. A modified classification for the maxillectomy defect. Head Neck. 2000;22(01):17–26. doi: 10.1002/(sici)1097-0347(200001)22:1<17::aid-hed4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Aramany M A. Basic principles of obturator design for partially edentulous patients. Part I: classification. J Prosthet Dent. 1978;40(05):554–557. doi: 10.1016/0022-3913(78)90092-6. [DOI] [PubMed] [Google Scholar]
- 3.Okay D J, Genden E, Buchbinder D, Urken M. Prosthodontic guidelines for surgical reconstruction of the maxilla: a classification system of defects. J Prosthet Dent. 2001;86(04):352–363. doi: 10.1067/mpr.2001.119524. [DOI] [PubMed] [Google Scholar]
- 4.Andrades P, Militsakh O, Hanasono M M, Rieger J, Rosenthal E L. Current strategies in reconstruction of maxillectomy defects. Arch Otolaryngol Head Neck Surg. 2011;137(08):806–812. doi: 10.1001/archoto.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kornblith A B, Zlotolow I M, Gooen J et al. Quality of life of maxillectomy patients using an obturator prosthesis. Head Neck. 1996;18(04):323–334. doi: 10.1002/(SICI)1097-0347(199607/08)18:4<323::AID-HED3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Moreno M A, Skoracki R J, Hanna E Y, Hanasono M M. Microvascular free flap reconstruction versus palatal obturation for maxillectomy defects. Head Neck. 2010;32(07):860–868. doi: 10.1002/hed.21264. [DOI] [PubMed] [Google Scholar]
- 7.Mittal M, Sharma R, Kalra A, Sharma P. Form, function, and esthetics in prosthetically rehabilitated maxillary defects. J Craniofac Surg. 2018;29(01):e8–e12. doi: 10.1097/SCS.0000000000003985. [DOI] [PubMed] [Google Scholar]
- 8.de Carvalho-Teles V, Pegoraro-Krook M I, Lauris J R. Speech evaluation with and without palatal obturator in patients submitted to maxillectomy. J Appl Oral Sci. 2006;14(06):421–426. doi: 10.1590/S1678-77572006000600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vero N, Mishra N, Singh B P, Singh K, Jurel S K, Kumar V. Assessment of swallowing and masticatory performance in obturator wearers: a clinical study. J Adv Prosthodont. 2015;7(01):8–14. doi: 10.4047/jap.2015.7.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chronopoulos A, Zarra T, Tröltzsch M, Mahaini S, Ehrenfeld M, Otto S. Osteoradionecrosis of the mandible: a ten year single-center retrospective study. J Craniomaxillofac Surg. 2015;43(06):837–846. doi: 10.1016/j.jcms.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Perrier M, Moeller P. Osteoradionecrosis. A review of the literature [in French] Schweiz Monatsschr Zahnmed. 1994;104(03):271–277. [PubMed] [Google Scholar]
- 12.Brown K E. Peripheral consideration in improving obturator retention. J Prosthet Dent. 1968;20(02):176–181. doi: 10.1016/0022-3913(68)90143-1. [DOI] [PubMed] [Google Scholar]
- 13.Haug S P. Maxillofacial prosthetic management of the maxillary resection patient. Atlas Oral Maxillofac Surg Clin North Am. 2007;15(01):51–68. doi: 10.1016/j.cxom.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Rieger J, Dickson N, Lemire R et al. Social perception of speech in individuals with oropharyngeal reconstruction. J Psychosoc Oncol. 2006;24(04):33–51. doi: 10.1300/J077v24n04_03. [DOI] [PubMed] [Google Scholar]
- 15.De Boer M F, McCormick L K, Pruyn J F, Ryckman R M, van den Borne B W. Physical and psychosocial correlates of head and neck cancer: a review of the literature. Otolaryngol Head Neck Surg. 1999;120(03):427–436. doi: 10.1016/S0194-5998(99)70287-1. [DOI] [PubMed] [Google Scholar]
- 16.Aramany M A. Basic principles of obturator design for partially edentulous patients. Part II: design principles. J Prosthet Dent. 1978;40(06):656–662. doi: 10.1016/0022-3913(78)90065-3. [DOI] [PubMed] [Google Scholar]
- 17.Parr G R, Tharp G E, Rahn A O. Prosthodontic principles in the framework design of maxillary obturator prostheses. J Prosthet Dent. 1989;62(02):205–212. doi: 10.1016/0022-3913(89)90315-6. [DOI] [PubMed] [Google Scholar]
- 18.Schwartzman B, Caputo A A, Beumer J. Gravity-induced stresses by an obturator prosthesis. J Prosthet Dent. 1990;64(04):466–468. doi: 10.1016/0022-3913(90)90045-e. [DOI] [PubMed] [Google Scholar]
- 19.Mancha de la Plata M, Gías L N, Díez P M et al. Osseointegrated implant rehabilitation of irradiated oral cancer patients. J Oral Maxillofac Surg. 2012;70(05):1052–1063. doi: 10.1016/j.joms.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Schoen P J, Raghoebar G M, Bouma J et al. Rehabilitation of oral function in head and neck cancer patients after radiotherapy with implant-retained dentures: effects of hyperbaric oxygen therapy. Oral Oncol. 2007;43(04):379–388. doi: 10.1016/j.oraloncology.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Korfage A, Schoen P J, Raghoebar G M, Roodenburg J L, Vissink A, Reintsema H. Benefits of dental implants installed during ablative tumour surgery in oral cancer patients: a prospective 5-year clinical trial. Clin Oral Implants Res. 2010;21(09):971–979. doi: 10.1111/j.1600-0501.2010.01930.x. [DOI] [PubMed] [Google Scholar]
- 22.Schepers R H, Slagter A P, Kaanders J H, van den Hoogen F J, Merkx M A. Effect of postoperative radiotherapy on the functional result of implants placed during ablative surgery for oral cancer. Int J Oral Maxillofac Surg. 2006;35(09):803–808. doi: 10.1016/j.ijom.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Shugaa-Addin B, Al-Shamiri H M, Al-Maweri S, Tarakji B. The effect of radiotherapy on survival of dental implants in head and neck cancer patients. J Clin Exp Dent. 2016;8(02):e194–e200. doi: 10.4317/jced.52346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salvatori P, Mincione A, Rizzi L et al. Maxillary resection for cancer, zygomatic implants insertion, and palatal repair as single-stage procedure: report of three cases. Maxillofac Plast Reconstr Surg. 2017;39(01):13. doi: 10.1186/s40902-017-0112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atalay B, Doğanay Ö, Saraçoğlu B K, Bultan Ö, Hafiz G. Clinical evaluation of zygomatic implant-supported fixed and removable prosthesis. J Craniofac Surg. 2017;28(01):185–189. doi: 10.1097/SCS.0000000000003204. [DOI] [PubMed] [Google Scholar]
- 26.de Almeida B K, Ferreira G Z, Aferri H C, Marino V CC, Dutka J CR, Pegoraro-Krook M I. Passavant's ridge during speech production with and without pharyngeal bulb. J Commun Disord. 2019;82:105939. doi: 10.1016/j.jcomdis.2019.105939. [DOI] [PubMed] [Google Scholar]
- 27.Shah J P. Philadelphia, PA: Elsevier Mosby; 2012. Oncologic dentistry, maxillofacial prosthetics and implants; pp. 752–772. [Google Scholar]


